Abstract
Over the last five decades, healthcare advances have yielded quantum improvements in life expectancy for individuals with congenital genitourinary conditions (CGCs), leading to a crisis of care. Many individuals with CGC enter adulthood unprepared to manage their condition. Pediatric CGC specialists lack training to manage adulthood-related healthcare issues, while adult genitourinary specialists lack training within the context of CGCs. To address these challenges, the National Institutes of Diabetes and Digestive and Kidney Diseases convened individuals with CGCs and experts from a variety of fields to identify research needs to improve transitional urology care. This manuscript outlines identified research needs.
Keywords: congenital genitourinary conditions, care transitions, spina bifida, neurogenic bladder, bladder exstrohpy, disorders of sex development
INTRODUCTION
Over the last five decades, advances in pediatric urology and overall healthcare have resulted in quantum improvements in life expectancy and quality of life (QoL) for individuals with congenital genitourinary conditions (CGCs). Specifically, the improved survival of people with spina bifida, bladder/cloacal exstrophy, disorders of sex development, and obstructive uropathy (i.e., posterior urethral valves) has led to a veritable "tidal wave" of individuals with CGCs reaching young adulthood.1–3 Many enter adulthood ill-prepared to manage their condition or handle issues such as sexuality and fertility.4, 5 Pediatric genitourinary specialists with expertise in complex CGCs often view sexuality and fertility as outside the scope of their practice, while adult genitourinary specialists lack specific training on these topics within the context of CGCs. This has resulted in a crisis of care in the field: individuals with CGCs who were provided excellent, often multidisciplinary care in childhood but are at risk of receiving suboptimal urologic care in adulthood.6–9
The traditional clinical approach that divides the lifespan into “pediatric,” “adult,” and “geriatric” phases has proven disadvantageous for our understanding of patients with CGCs. An increasing awareness that healthcare decisions made in childhood influence health behaviors in adulthood has pushed providers to examine how children, families and young adults are prepared for transition to an adult-care model. Broadly defined as “transitional care,” this practice has been endorsed by organizations including the American Academy of Pediatrics (AAP), American College of Physicians (ACP) and American College of Family Physicians (ACFP).5 Transition is a complex process that takes place in multiple inter-related domains: school-to-work, home-to-community, and pediatric-to-adult healthcare. One framework for transition quality improvement is composed of six elements: establishing a policy, tracking progress, administering transition readiness assessments, planning for adult care, transferring, and integrating into an adult practice (Got Transition [http://www.gottransition.org]).5
According to the World Health Organization’s (WHO) International Classification of Function, Disability and Health (ICF) model, high-quality care encompasses not only health-related outcomes, but also activities, social participation, and environmental factors to address an individual’s ability to fully function in society.10 This approach requires interdisciplinary collaboration among healthcare providers, patients, family caregivers, educators and community-based rehabilitation service providers, including occupational therapists, behavioral specialists, social workers and personal care attendants.
Interdisciplinary care is imperative for individuals with CGCs, who have disproportionate rates of infectious, renal, reproductive, and neurocognitive complications, many evolving across the lifespan and emerging or changing during adolescence and young adulthood (Figure 1), when individuals are transitioning from pediatric to adult care. During this transition, individuals may encounter issues regarding their reproductive health. A substantial number of urogenital disorders occur in the context of intellectual disability and/or developmental disability (e.g. Prader-Wili Syndrome, spina bifida). Collaboration with family caregivers, educators, and community-based habilitation service providers is particularly important for these patients.
Figure 1. Factors related to urologic care and outcomes across the lifespan.
Across several functional domains—including health, social and emotional well-being, productivity and self-management— several factors related to urological and health outcomes become increasingly relevant during the period of transition from childhood to adulthood. For example, sexuality, functional independence, and integration into the workplace all demonstrate relatively greater importance during young adulthood than they did in pediatric life. UTI: urinary tract infection.
METHODS
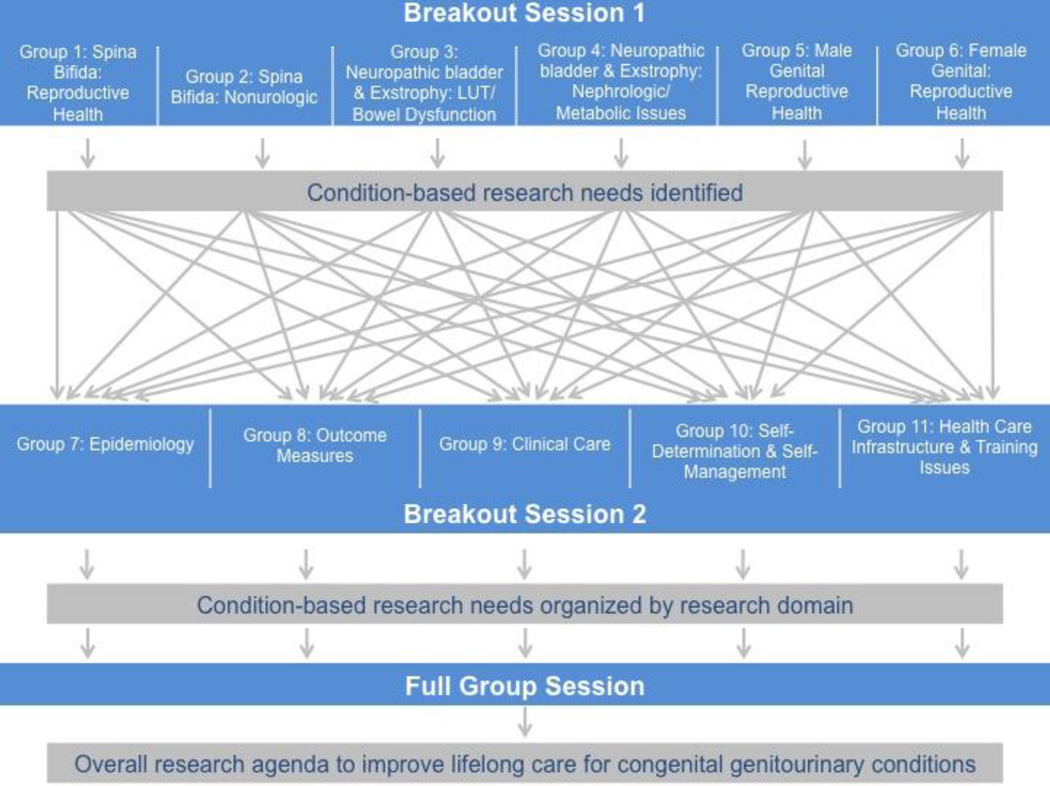
In response to the challenges to delivery of transitional urologic care, the National Institutes of Health’s National Institutes of Diabetes and Digestive and Kidney Diseases (NIDDK) convened individuals with CGCs and experts from a variety of health fields, including urology, adolescent and developmental pediatrics, nephrology, gynecology, epidemiology, nursing, and public health, to identify research needs to improve transitional care. Individuals with CGCs presented their experiences and lessons learned during their own transitions. Experts presented clinical challenges pertaining to adolescents with CGCs. The remainder of the meeting consisted of two sets of breakout sessions (Figure 2). First, six multidisciplinary groups of participants identified research areas of highest and intermediate need across six condition-based areas. Then, participants reassembled into six groups to discuss the identified condition-based needs across scientific disciplines. Afterwards, all participants reconvened for a facilitated discussion to summarize major research needs. A meeting summary is available.11
Figure 2. Schematic Diagram of Breakout Sessions by Groups and Topics of Discussion.
The meeting included two sets of breakout groups. First, six multidisciplinary groups of participants identified research areas of highest and intermediate need across six condition-based areas. Then, participants reassembled into six groups to organize the identified condition-based needs across scientific disciplines. Afterwards, all participants reconvened for a facilitated discussion to summarize major research needs. LUT: lower urinary tract; NGB: neurogenic bladder
FINDINGS AND RECOMMENDATIONS
Priority research needs identified during the meeting are presented below, organized by the elements of WHO’s ICF model (i.e., environmental factors, body structures and function, activities and participation) and the two primary domains of urologic care: the urinary tract and reproductive system.
Environmental Factors
Healthcare delivery systems to improve continuity of care
As with many populations with chronic health conditions, continuity of care is highly relevant to individuals with CGCs. Young adulthood is a critical and high-risk period, as responsibilities for self-management and health maintenance transition from the parent/caregiver to the adolescent who may be ill prepared for this role. Unhealthy behaviors are often initiated during this period, when many young adults experience a lapse in routine health maintenance previously facilitated by their parents.12 This can be exacerbated by insurance challenges as young adults transition from family to individual insurance coverage. Before the Affordable Care Act, many young adults with congenital conditions were uninsurable and failed to maintain recommended healthcare in adulthood. Access challenges may be heightened for young adults with CGCs and intellectual disability or developmental disability (ID/DD). The complexity of healthcare financing for patients with ID/DD—which requires navigation of publicly funded insurance programs—may limit access to care. Physical access to healthcare facilities also is a concern in some practice environments. Testing, validation, and comparative analyses of transitional care models and alternative payment models are needed to determine how to best organize and deliver urologic care to individuals with CGCs transitioning to adulthood.
Barriers and facilitators to successful transition
Even among established spina bifida clinics, only 68% have a written transition policy, and only half of those routinely evaluate their transition process.13 Within organizations with established transition clinics in the same location and with overlapping providers, low rates of successful transition have been reported (R. Misseri, personal communication). Barriers to successful transition have not been well-defined, but may include: (1) systemic (i.e., incompatibility in insurance coverage between providers, electronic medical records, or hospitals); (2) physical (i.e., lack of transportation or facilities ill-equipped for examination and treatment of patients with disabilities); (3) psychoemotional (i.e., resistance of the patient or pediatric provider to “let go” of the relationship); and (4) educational/training (i.e., lack of expertise in the available adult-care pool, limited opportunities for training across systems of care such as habilitation services).
Standardized healthcare pathways
Developing clinical pathways (e.g., pharmacologic treatment, behavioral treatments, diagnostic guidelines, patient education recommendations) and procedural interventions (e.g., self-intermittent catheterization, bladder irrigation, surgical treatments) to establish healthcare pathways with less variability than currently exist for management of individuals with CGCs could potentially facilitate wider and more consistent access to care and follow-up/surveillance protocols.
Specialist training
Intrinsic differences in training of pediatric versus "general" gynecologists and urologists contribute to suboptimal care of adolescents and young adults with urologic conditions requiring lifelong follow-up.14 The adult-onset health issues experienced by people with CGCs require expertise that is not yet widely available. Assessments are needed to determine the education and training needed to prepare urologic specialists to effectively manage adult-onset health issues in individuals with CGCs. Additionally, training interventions must be developed and implemented.
Individuals with ID/DD may face additional barriers, as adult urologists often lack specific information about the functional goals, habilitation needs, communication style, and decision-making abilities of patients with ID/DD. Understanding neuronal plasticity and “critical windows” or sensitivities for development of executive functioning may help clinicians better time self-management interventions as individuals with CGCs and ID/DD transition to adulthood.
Interventions to improve urology referrals
Access to healthcare and the quality of healthcare can be problematic for individuals with CGCs, especially among those who also have ID/DD. Diagnostic overshadowing (e.g., the tendency for clinicians to attribute incontinence to the disability) can result in delayed referrals to urologists and incomplete diagnostic evaluations. This limits timely access to appropriate therapeutic care. Research must be conducted to understand how best to encourage appropriate referral to urologic specialists for individuals with CGCs and ID/DD.
Urinary Tract
Body Structure and Function
Epidemiology and natural history of congenital neuropathic bladder
The rarity and heterogeneity of CGCs and the temporal limit of pediatric care, inhibit understanding of the epidemiology and natural history of CGCs. While the underlying pathologic process may be divergent for many individuals with congenital neurogenic bladder (NGB), most can be broadly characterized by a combination of storage and voiding mechanisms (Table 1). Classifying patients into these groups often permits characterization of patients for surveillance and management. However, we lack a phenotypic classification scheme for congenital NGB in adulthood with specific considerations for special populations. For example, a patient with spina bifida managed during childhood with augmentation cystoplasty may have more in common with a similarly managed patient with exstrophy compared to another patient with spina bifida who voids by Valsalva maneuvers. Epidemiological characterization of individuals with CGCs, including rates of supravesical diversion, acute kidney injury (AKI), end-stage renal disease (ESRD), bladder cancer, urolithiasis, and urinary tract infection (UTI), must be defined so that surveillance protocols and best practices can be developed. Because existing registries and databases are insufficient for understanding CGCs and their complications across the lifespan, developing new and enhancing existing registries may facilitate an increased understanding of the epidemiology of CGCs and related complications.
Table 1.
Classification schema for congenital neuropathic bladder based on mechanism of emptying and mechanism of storage
| Mechanism of emptying |
Bladder | Bladder + Augment- urethra |
Bladder + Augment- mitrofanoff |
Continent Reservoir (bowel) |
Conduit/ Vesicostomy |
|---|---|---|---|---|---|
| Void | Continent/ Incontinent |
Continent/ Incontinent |
Continent/ Incontinent |
Continent/ Incontinent |
Incontinent |
| Valsalva | Continent/ Incontinent |
Continent/ Incontinent |
Continent/ Incontinent |
Continent/ Incontinent |
Incontinent |
|
Intermittent catheter |
Continent/ Incontinent |
Continent/ Incontinent |
Continent/ Incontinent |
Continent/ Incontinent |
NA |
|
Continuous catheter |
SPT/Foley | SPT/Foley | SPT/Foley | Continent/ Incontinent |
NA |
Neurogenic bladder can be broadly characterized by a combination of storage and voiding mechanisms. Patients with congenital neurogenic bladder may migrate through several pathophysiologic mechanisms of emptying and storage. NA: not applicable; SPT: suprapubic tube.
Relationship between congenital NGB and bladder cancer
While many studies have reported an elevated risk of bladder cancer in congenital bladder diseases (i.e., bladder exstrophy and adenocarcinoma),15, 16 the absolute risk remains poorly defined.17, 18 While still rare, these cancers often impart significant morbidity and are almost universally fatal. Prevention and surveillance strategies for urinary reservoirs that contain bowel and are chronically bacteriuric have not been defined. Moreover, recurrent UTI as a potential mechanism of increased bladder cancer risk remains poorly understood. Little is known about the role chronic inflammation and/or underlying congenital bladder disease may play in bladder oncogenesis. Hypotheses that have been posed but remain unproven include concepts that UTI-induced inflammation may promote a microenvironment conducive to malignant transformation of the bladder urothelium.19 Another theory is that uropathogenic bacteria produce metabolic products, such as nitrosamines, which may facilitate urothelial carcinogenesis.20
Bladder microbiome
Molecular-based, non-culture-dependent methods of characterizing microbes in the human body have revealed a previously unappreciated microverse.21 Conventional urine cultures may only represent the "tip of the iceberg," and defining how the multitudes of bacteria may interact to prevent or cause UTI will be critical to the care of transitional patients with NGB.22 The urine microbiome, and associated UTI risk factors, may change as a function of patient age. For instance, puberty results in estrogenization of the vagina, fostering the growth of lactic acid-producing bacteria, which in turn provide a microbiological barrier to uropathogenic bacteria.23 Conversely, menopause is linked to loss of estrogenization of the vagina, and the resulting vaginal atrophy has been associated with increased UTI risk.24 Pregnant women with a history of vesicoureteral reflux during childhood appear to be at increased risk of UTI.25, 26 Interestingly, these small retrospective series also suggest that pregnant women who underwent childhood surgical repair of reflux (a set of procedures with >90% success rates) also seem to be susceptible to UTI. These initial observations need to be confirmed using prospective cohorts.
Recently described processes such as the development of intracellular bacterial communities within urothelial cells, where bacteria can "hide" from the immune system and antibiotics, may contribute to recurrent UTI in patients with NGB.27 A more comprehensive understanding of these processes may help identify new therapeutic targets for UTI in this patient population, which is particularly relevant given the high rates of antibiotic resistance among bacterial isolates derived from these patients.
Asymptomatic bacteruria versus UTI
Many patients requiring lifelong urologic care have abnormal bladders and are chronically bacteriuric due to bladder colonization.28 The distinction between asymptomatic bacteriuria and UTI, particularly in patients with sensory and/or intellectual disability that alter symptoms, is not established. Guidelines defining best practices for diagnosis and treatment of UTI in patients with congenital NGB are critically needed.
Assessing kidney function
Monitoring renal function and appropriate medical interventions are important to prevent progressive chronic kidney disease (CKD) and ESRD. In clinical practice, estimated glomerular filtration rate (eGFR), which is calculated from serum creatinine level using estimating equations, is commonly used to define kidney function.29, 30 However, creatinine-based estimates of GFR have many limitations that may be relevant for populations with CGCs, particularly spina bifida.31 These include: (1) reduced muscle mass on serum creatinine levels, which reduces accuracy of creatinine-based equations in individuals with low lean body mass, and (2) change in serum creatinine because of common CGC medications (e.g., artificial elevation of serum creatinine with antibiotics like trimethoprim-sulfamethoxazole and cefoxitin). Cystatin C-based equations for assessing eGFR may be a promising alternative in populations with CGCs.32 For renal scarring, dimercapto-succinic acid scan is the imaging method of choice.33 However, wide access to the nuclear tracer needed for this study has been problematic, limiting clinical utilization. Thus, research is needed to clarify the best means by which to evaluate kidney function in transitional urologic patients.
Etiology, prevention and management of kidney diseases
Although kidney diseases are common in CGC, prevention methods and ideal management are not clear. Potential contributors to kidney disease in CGC are diverse and vary by condition. Among children with spinal dysraphism, high-grade reflux is associated with the highest risk for renal cortical loss.34 UTI, particularly in the presence of vesicoureteral reflux, increases the risk of renal scarring, which can cause hypertension, proteinuria, pregnancy-related problems and progressive CKD, including ESRD in some patients.33, 35 Urodynamic risk factors for the development of CKD include detrusor sphincter dyssynergia, detrusor overactivity, high detrusor leak point pressure and high intravesical pressure. UTIs in childhood are also linked to renal dysfunction later in life.36, 37 In patients with diminished renal function, serum creatinine levels are known to increase during late adolescence. This may be related to disease progression, poor adherence to catheterization schedules and medications, increased bladder outlet resistance due to prostatic growth, alterations in creatinine metabolism and estrogenization of the female urethra and/or tethering of the spinal cord.38
Few studies have focused on renal outcomes in adolescents and adults with congenital NGB and vesicoureteral reflux. Recent studies suggest that death from renal failure in well-managed patients is much lower than that reported for historical cohorts.39, 40 Changes in continence, recurrent UTI, weight gain, hypertension, proteinuria, and urolithiasis may independently affect renal function. In addition, some of these conditions may indicate a change in bladder dynamics. Each may ultimately contribute to upper tract changes and renal disease. With each episode of pyelonephritis, the risk of renal scarring increases, nephrons are lost and renal failure may ensue. Antibiotic prophylaxis may be helpful in carefully selected patients. Kidney stones are also a risk factor for CKD and progression to ESRD.41 Treatment with angiotensin-converting enzyme inhibitors may prevent CKD progression.42, 43 Progressive CKD may result in metabolic complications; however, evidence is limited regarding risk factors for these complications and effective interventions to prevent them.
Activities and Participation
Assessing treatment history, patient disease state knowledge, and patient self-care
As individuals with CGCs receive medical care and often undergo surgery in childhood, they frequently rely on parents and other surrogates to act as “historian,” understand their disease state (e.g., bladder or renal function), and help with self-care. As parents/caregivers begin to withdraw from care during patient transition to adulthood, the individual with CGC may be left with severe limitations in the extent and accuracy of self-knowledge about their disease state and medical history and in their self-care abilities. Such assessments should include knowledge of the rationale for care requirements (e.g., preservation of renal function, continence, reduction of infection and stone risk), dexterity, cognitive ability and responsibility for self-management, communication skills, and the patient’s capacity for troubleshooting bladder and urinary tract management relative to equipment and technique (e.g., stomal access, prolonged retention, appliance seal, catheter occlusion). If ability for self-care is limited, the need to engage family, aides and other assistants is essential, but the optimal approaches for such engagement remain poorly defined.
Strategies to improve healthcare engagement and self-management
During adolescence and young adulthood, physical development precedes emotional maturity, and as a result, organizational skills and logical reasoning may not be optimal.44 Despite these challenges, the ability to engage the healthcare system is a necessary skill for individuals with CGCs in transition. Young adults with CGCs often face significant self-management demands, many of which may have previously been facilitated by a parent or caregiver. Additionally, engagement with the healthcare system is central to identification and management of complications. Social Learning Theory,45 which posits that learning is a cognitive process that takes place in a social context, and its concept of reciprocal determinism may provide a lens for considering adolescent health behaviors. Reciprocal determinism suggests that, just as an individual’s behavior is influenced by the environment, the environment is also influenced by the individual’s behavior. That is, the expectation of competence is essential to the development of competence. Studies have shown that children who are given household chores at an early age (“required helpfulness”) have better social outcomes in adulthood.46 Transposed to skills such as hygiene and catheterization, the expectation of competence (wellness model) may be more effective than medicalization of self-care skills (deficit model). Similarly, expectations of patient participation in decision-making may support self-determination, or the ability to make choices and decisions based on their own preferences, to monitor their own actions and to be goal-oriented and self-directing. Understanding how health care providers foster self-management is a research priority. Studies are needed to delineate how health care providers and processes for developing effective self-management behaviors during transition relate to overall health and QoL.47
For individuals with ID/DD, supported decision-making—a process in which people receive the help they need and want to understand the situations and choices they face, so they can make life decisions for themselves, without the need for undue guardianship—was introduced in 2007 at the United Nations Convention on the Rights of Persons with Disabilities.48 Self-determination and supported or shared decision-making are key concepts for promoting health literacy of patients with CGCs—especially those with ID/DD. The ability of patients to make informed decisions and participate in their own healthcare correlates with adherence, satisfaction with care, and health outcomes. Research is needed to examine the impact of hygiene and self-care skills on social participation, assess the effectiveness of wellness programs and healthcare delivery models (e.g., group visits) that encompass social learning, and evaluate self-determination and supported or shared decision-making programs adapted to the urologic care setting. Additional strategies to improve treatment adherence, self-management and engagement may include a combination of health education, parental involvement, and self-monitoring.49 Whether and how development and use of electronic communication, telephone access, and local care providers to facilitate engagement, thereby minimizing delays in treating complications, and improving the efficacy and safety of care, remains to be determined.
Screening tools to assess executive function
Adolescence is a period of rapid cognitive and executive function development. Executive function skill development may vary from person to person, affecting their ability to interact with the healthcare team and perform self-management activities. Executive function may be further complicated in individuals with ID/DD. Understanding executive function in young adults with CGCs will be critical to ensuring appropriate engagement as their healthcare responsibility increases during transition. Because neuropsychological testing is not always covered by insurance, incorporation of school-based psycho-educational reports into the medical record may be a cost-effective method to improve the understanding of IQ and adaptive functioning skills. Several tools exist that may be adapted and validated in the adolescent CGC population.50–52
Impact of aging and condition-related disability on continence management and bladder care
While an incontinent diversion such as a urinary conduit may represent “end-stage” management, patients with congenital NGB may migrate through several pathophysiologic mechanisms of emptying and storage (Table 1) throughout their lifetime as they experience age- or condition-related disability. Examples of age-related conditions that may alter bladder management are provided in Table 2. Condition-related disability that occurs with aging is particularly relevant for congenital conditions that are multi-system, like spina bifida and cerebral palsy (Table 3).
Table 2.
Acquired conditions that may effect bladder management with aging by relevant specialty
| Nephrological | Urological | Neurological | Other |
|---|---|---|---|
| Chronic kidney disease |
Pelvic organ prolapse Nocturia Overactive bladder |
Cognitive decline/cerebrovascular accidents |
Polypharmacy |
| Polyuria | Prostatic hyperplasia |
Spinal stenosis | |
| Failure to thrive in young children |
Prostate cancer | Neuropathy |
Examples of specific acquired conditions that may affect bladder management beyond childhood. Such conditions may be encountered by the treating urologist, neurologist, nephrologist, or other care providers and require prompt recognition and appropriate treatment to protect the kidneys from damage. For example, a patient who Valsalva voids through young adulthood may encounter retention, urinary tract infections, or new leakage as the prostate enlarges and creates increased outlet resistance.
Table 3.
Acquired conditions that may effect bladder management with aging by relevant condition
| Spina bifida | Cerebral Palsy | Bladder Exstrophy |
Prune Belly Syndrome |
PUV |
|---|---|---|---|---|
| Loss of upper extremity dexterity |
Progression to atonic bladder |
Pelvic organ prolapse |
Abdominal hernia | Renal graft deterioration |
| Obesity | Extremity contractures |
Urethral stricture |
Bladder deterioration |
|
| Spinal disease | ||||
| Loss of ambulatory status |
||||
| Abdominal hernia |
Examples of specific acquired conditions that may affect bladder management beyond childhood by primary diagnosis. Such conditions may impair the patient’s ability to perform bladder management or self care, and therefore place the patient at risk. For example, a spina bifida patient who self catheterizes through young adulthood may encounter increasing obesity, which would impair her ability to cleanly and safely catheterize her bladder per urethra in her chair, therefore subjecting her to risk of recurrent infections, retention, urinary incontinence and ultimately renal demise.
Effects of renal impairment on QoL
Many studies have been published on QoL consequences of progressive CKD and ESRD.53, 54 Multiple tools exist to evaluate QoL; however, further validation through appropriately designed prospective studies is necessary in adolescents with CGCs to better understand QoL outcomes in this population.
Relationships among urinary and fecal continence and self-esteem
Many patients with “continent” bladder management routines (like intermittent self-catheterization or Mitrofanoff channels) experience varying levels of urinary incontinence (UI). In a French multicenter study, urinary continence outcomes were evaluated in periadolescents and young adults with spina bifida undergoing surgical treatment for UI.55 While the results suggested higher urinary continence rates among those who underwent operations (compared with those managed medically), some level of UI remained the norm for most.55 Bowel disorders—including fecal incontinence—also are common in adolescents with NGB and are associated with social stigma in children and adults.56 Thus, despite the fact that higher levels of urinary continence have been demonstrated to result in improved self-esteem,57 relationships between self-esteem, and urinary and fecal incontinence are not well characterized in adolescents with CGCs.
Effects on social participation
Toileting issues and the need for ongoing healthcare at a greater frequency than that of their peers can uniquely influence employment and educational opportunities. Toileting issues may be particularly challenging in group housing scenarios, as commonly encountered in undergraduate and graduate education. There are likely significant knowledge deficits and misconceptions regarding the impact of clean intermittent catheterization, urostomy care and indwelling catheters among patients and society at large. In spite of broad provisions of the Americans with Disabilities Act (ADA) regarding accommodation and accessibility, concerns for bathroom access, social embarrassment, risks surrounding transmission or acquisition of infection, and physical limitations may hamper patient educational and employment opportunities. Particularly among patients who use catheterization to manage continence, these issues are exacerbated by any acute lower urinary tract symptoms associated with UTI. Coping with acute UTI symptoms may contribute to time away from school, work, family and friends. Consequently, further study and clarification of these issues is necessary to reduce obstacles to societal participation of young adults with CGCs as they transition to adulthood.
Reproductive System
Body Structures and Function
Epidemiology
Functional issues will be highly variable depending on whether the CGC is primarily genital (e.g., exstrophy-epispadias) or neurological (e.g., spina bifida) and on gender, experience and age of the individual. Knowledge of the rates of sexual dysfunction among men and women in these populations is limited at best.58 To develop reliable estimates of sexual dysfunction, validated measures of erectile, ejaculatory, sensory, orgasmic and pain symptoms associated with intercourse need to be developed. Similarly, patients with CGCs can demonstrate risk factors for hypofertility and infertility related to anatomical, physiological, functional, or endocrine-related comorbidities. For some conditions, fertility risks have been described;59 however, these include largely small, single-center studies. Among individuals with CGCs, changes in body structure and function, including gonadal failure or gonadectomy, fallopian tube damage after multiple operations, and anatomical anomalies of internal organs impact fertility. Men with CGCs also can be infertile due to a variety of issues resulting from their underlying condition. In men with Eagle Barrett syndrome, fertility is impaired because of ejaculatory dysfunction from bladder neck incompetence and megalo-urethra obstruction caused by an absent/atretic vas deferens and cryptorchidism. Men with exstrophy or spina bifida, however, often have a combination of erectile, ejaculatory, and spermatogenic defects that render them infertile. In many cases, infertility and heritability risks are either so complex or so poorly understood by the practicing urologist/gynecologist that adequate evaluation and counseling is not feasible.
Oncologic risks
Although there have been reports of increased risk for seminoma in testes of XY females, as well as development of cancers in bowel neovaginas, the exact risk is unclear. Certain disorders of sexual development are associated with increased risk of gonadal tumors, but the risk is based on case series and optimal treatment is poorly understood. Many undergo gonadectomy without clear evidence that the benefits outweigh the immediate and long-term risks.60–63 Moreover, it is unclear whether these conditions are associated with different levels of risk in certain cancers (e.g., vaginal cancer), and optimal screening for these conditions is poorly understood. Research to provide a better understanding of the processes that might put women with CGCs at risk for adult cancers and strategies to increase screening is needed.
Contraception and urological health
Addressing contraception issues for individuals with CGCs is critical, as many young adults may become sexually active during their transition. Individuals with CGCs may be at increased risk for latex sensitivity due to repeated latex exposure (e.g., latex catheters).64 Although dramatic changes have occurred over the last 20 years to reduce exposure to latex for persons with CGCs (e.g., non-latex catheters, gloves and surgical equipment), the potential for latex sensitivity, especially in adults who may have had repeated exposures, continues. Options for barrier contraception—and associated risks—for individuals with latex sensitivity need to be identified. Non-latex condoms are available and recommended for those with latex allergy, but in a review of adults without disability they were twice as likely to break or slip off during intercourse.65 In women with CGCs for whom pregnancy is possible, use of certain contraception methods may not be possible due to underlying anatomy. Some conditions may preclude the use of an intra-uterine device, one of the safest methods of contraception. Associated comorbidities, such as hypertension due to renal disease, may preclude the use of estrogen-containing contraception.
Risks related to pregnancy/delivery
Pregnancy risks and safety in women with CGCs are limited to case reports, and delivery is associated with issues resulting from the underlying anatomy.66, 67 Reports point to increased risk of UTI, early delivery and Cesarean birth.66 One observational study of pregnancy in women with cloacal/exstrophy reported a 100% Cesarean birth rate.66 If these women had had previous urological or gastrointestinal surgery, they may be at further risk during Cesarean delivery. However, the exact prevalence of pregnancy complications and negative outcomes by CGC type are unknown, and factors that may mediate risk are poorly understood.
Activities and participation
Methods to improve sexual function and satisfaction
Despite anatomical variations or sexual function limitations, many men desire sexual contact68 and tend to engage in sexual activities despite these limitations.69 For women, sexual function is an important aspect of well-being and is significantly related to psychosocial issues, including body image and feelings of attractiveness. Barriers to sexual activity include incontinence,70 cognitive impairments,71 pain,72 mobility limitations, impairments in manual dexterity, reduced penile sensation in men,71 and libido in women, which in turn, can be affected by endocrine, anatomical and psychosocial issues. Men with conditions like spina bifida become sexually active later than their peers without disabilities, possibly in part due to living in their parents’ home well into adulthood.73 They also report lower sexual satisfaction compared with women.72
Women born with differences in sexual development (DSD) often delay sexual activity, possibly owing to anxiety about genital appearance and function. Several studies have reported that—across disability groups—adolescent females with DSD and other CGCs reported fewer sexual activities, initiation or receptivity to sexual activity, romantic appeal, and satisfaction than did their peers.74–76 Issues regarding privacy, relevant to many women with CGCs who may live with their parents until at least late adulthood, play a large role in women’s sexual experiences.74, 77–79 Research is needed to better understand the barriers to sexual participation in men and women with CGCs and to develop and validate interventions to overcome these barriers.
Educational models for delivering information about sexuality
Few individuals with limitations in sexual function receive sufficient sexual education from clinicians,80 and yet affected individuals report they are interested in discussing sexuality.81 The lack of information on the impact of spina bifida on sexuality has persisted during the past decade, with >30% of patients in 2002 and 2014 reporting having limited information despite ongoing interactions with healthcare providers.76, 79 Individuals with sexual limitations tend to lack sufficient knowledge about reproductive anatomy and physiology, available treatments for erectile or ejaculatory dysfunction, or methods of contraception or protection from disease. Among those who are sexually active, <20% use contraception.82 This lack of knowledge can restrict participation in sexual relationships, especially as an individual matures. Difficulties in establishing social relationships and poor self-perceptions of body image can compound the difficulty in establishing intimate relationships.71 Uncertainty about sexual function can also result in depression.83 Finally, the need for physical assistance can introduce concerns about privacy. Research is needed to determine how best to provide individuals with timely, accurate and useful information. In the meantime, those providing urological and gynecological healthcare to this population need to continually assess the understanding of structural and functional changes as they relate to sexuality and provide in-depth education for these complex conditions.
Effects of incontinence on sexual activity
von Linstow, et al. found that fecal, but not urinary, incontinence was related to decreased sexual activity and satisfaction in men and women with spina bifida.79 By contrast, Game et al. found that UI was the major factor related to deceased sexual activity –decreased desire, arousal and receptivity – among women with myelomeningocele.74 Even the experience of wearing pads was seen as a barrier to intimacy.74 In men and women, sexual activity is associated with continence.70 However, the risk and protective factors for psychological health for those dealing with incontinence is still not well understood and merits further research.
CONCLUSION
Research and clinical care have not kept pace with millions of adolescents and young adults with CGCs who are transitioning to adulthood owing to significant improvements in life expectancy. Because many urological issues emerge or evolve during adolescence and young adulthood, these issues and their impact on health, QoL, self-management and social participation outcomes in individuals with CGCs are poorly understood. Research is needed to better equip the healthcare system as a whole, pediatric and adult urologic and gynecologic providers, and individuals with CGCs to effectively manage these conditions—particularly during the volatile period of transition from pediatric to adult care.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Michael Hsieh, Children's National Health System, George Washington University, 111 Michigan Ave NW, Washington, DC 20010, Tel: 202-476-5000, Fax: 202-476-4739, MHsieh@childrensnational.org.
Hadley M. Wood, 9500 Euclid Ave/Q10, Cleveland, OH 44195, Tel: 216-444-2146, Fax: 216-445-2267, woodh@ccf.org.
Brad E. Dicianno, University of Pittsburgh Medical Center, Dept. of Physical Medicine and Rehabilitation, Kaufmann Medical Bldg, Suite 202, 3471 5th Avenue, Pittsburgh, PA 15213, Tel: 412-648-6666, Fax: 412-692-4410, dicianno@pitt.edu.
Nienke P. Dosa, Center for Development Behavior and Genetics, Department of Pediatrics, SUNY Upstate Medical University, 750 East Adams Street, Syracuse, NY 13210, Tel: 315-464-7561, Fax: 315-464-7630, dosan@upstate.edu.
Veronica Gomez-Lobo, MedStar Washington Hospital, Center/Children's National Medical Center, Georgetown University, 110 Irving Street Room 5B41, Washington, DC 20010, Tel: 202-877-4099, Fax: 202-877-6975, veronica.gomez-lobo@medstar.net.
Tej K. Mattoo, Wayne State University School of Medicine, Pediatric Nephrology and Hypertension, Children's Hospital of Michigan, 3901 Beaubien Boulevard, Detroit, MI 48201, Tel: 313-745-5604, Fax: 313-966-0039, tmattoo@med.wayne.edu.
Rosalia Misseri, Department of Urology, Indiana University School of Medicine, Riley Hospital for Children, 705 Riley Hospital Dr, Ste 4230, Tel: 317-944-7469, Fax: 317-944-7481, rmisseri@iupui.edu.
Jenna M. Norton, Division of Kidney, Urologic and Hematologic Diseases, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, 6707 Democracy Blvd – MSC 5458, Bethesda, MD 20817, Tel: 301-451-7314, Fax: 301-480-3510, jenna.norton@nih.gov.
Kathleen J. Sawin, Self-Management Science Center, College of Nursing, University of Wisconsin-Milwaukee (UWM), Children's Hospital of Wisconsin (CHW), Tel: 414-337-0262(CHW), Fax: 414-337-6337, sawin@uwm.edu and ksawin@chw.org.
Peter Scal, Division of General Pediatrics and Adolescent Health, Department of Pediatrics, University of Minnesota Medical School, Room 353 717 Del, 1932K, 717 Delaware St SE, Minneapolis, MN 55455, Tel: 612-624-7141, Fax: 612-626-2134, scal0005@umn.edu.
James E. Wright, James Buchanan Brady Urological Institute, Johns Hopkins Medical Institution, 4940 Eastern Ave, Baltimore, MD 21224, Tel: 410-550-7739, Fax: 410-550-3341, jwright1@jhmi.edu.
Robert A. Star, Division of Kidney, Urologic and Hematologic Diseases, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, 6707 Democracy Blvd – MSC 5458, Bethesda, MD 20817, Tel: 301-496-6325, Fax: 301-480-3510, starr@mail.nih.gov.
Tamara Bavendam, Division of Kidney, Urologic and Hematologic Diseases, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, 6707 Democracy Blvd – MSC 5458, Office 615, Bethesda, MD 20817, Tel: 301-594-4733, Fax: 301-480-3510, tamara.bavendam@nih.gov.
REFERENCES
- 1.Baird AD. Exstrophy in the adolescent and young adult population. Semin Pediatr Surg. 2011;20:109. doi: 10.1053/j.sempedsurg.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 2.Berens JC, Peacock C. Implementation of an academic adult primary care clinic for adolescents and young adults with complex, chronic childhood conditions. J Pediatr Rehabil Med. 2015;8:3. doi: 10.3233/PRM-150313. [DOI] [PubMed] [Google Scholar]
- 3.Timberlake MD, Corbett ST, Costabile RA, et al. Identification of adolescent and adult patients receiving pediatric urologic care and establishment of a dedicated transition clinic. J Pediatr Urol. 2015;11:62 e1. doi: 10.1016/j.jpurol.2014.11.013. [DOI] [PubMed] [Google Scholar]
- 4.Sawin KJ, Betz CL, Linroth R. Gaps and opportunities: an agenda for further research, services, and program development in spina bifida. Pediatr Clin North Am. 2010;57:1041. doi: 10.1016/j.pcl.2010.07.020. [DOI] [PubMed] [Google Scholar]
- 5.Cooley WC, Sagerman PJ. American Academy of Pediatrics, American Academy of Family Physicians, American College of Physicians, Transitions Clinical Report Authoring Group. Supporting the health care transition from adolescence to adulthood in the medical home. Pediatrics. 2011;128:182. doi: 10.1542/peds.2011-0969. [DOI] [PubMed] [Google Scholar]
- 6.Adams MM, Greenberg F, Khoury MJ, et al. Survival of infants with spina bifida--Atlanta, 1972–1979. Am J Dis Child. 1985;139:518. [PubMed] [Google Scholar]
- 7.Oakeshott P, Hunt GM, Poulton A, et al. Expectation of life and unexpected death in open spina bifida: a 40-year complete, non-selective, longitudinal cohort study. Dev Med Child Neurol. 2010;52:749. doi: 10.1111/j.1469-8749.2009.03543.x. [DOI] [PubMed] [Google Scholar]
- 8.Shin M, Kucik JE, Siffel C, et al. Improved survival among children with spina bifida in the United States. J Pediatr. 2012;161:1132. doi: 10.1016/j.jpeds.2012.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wong LY, Paulozzi LJ. Survival of infants with spina bifida: a population study, 1979–94. Paediatr Perinat Epidemiol. 2001;15:374. doi: 10.1046/j.1365-3016.2001.00371.x. [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization. Geneva: WHO; 2001. International Classification of Function, Disability and Health. [Google Scholar]
- 11.National Institute of Diabetes and Digestive and Kidney Diseases. Meeting Summary. Presented at the Research Needs for Effective Transition in Lifelong Care of Congenital Genitourinary Conditions; February 2, 2015; Bethesda, MD. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Institute of Medicine, National Research Council. Investing in the health and well-being of young adults. Washington, DC: The National Academic Press; 2015. [Google Scholar]
- 13.Kelly MS, Thibadeau J, Struwe S, et al. Evaluation of Spina Bifida Transitional Care Practices in the United States. J Urol. 2016 doi: 10.3233/PRM-170455. (in press): [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Michaud PA, Suris JC, Viner R. Geneva, Switzerland: WHO Press, World Health Organization; 2011. The Adolescent with a Chronic Condition: Epidemiology, developmental issues and health care provision. [Google Scholar]
- 15.Di Lauro G, Iacono F, Ruffo A, et al. Presenting a case of a mucinous adenocarcinoma of an exstrophic bladder in an adult patient and a review of literature. BMC Surg. 2013;13(Suppl 2):S36. doi: 10.1186/1471-2482-13-S2-S36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xiong W, Peng R, Zhu L, et al. Bladder exstrophy-epispadias complex with adenocarcinoma in an adult patient: A case report. Exp Ther Med. 2015;10:2194. doi: 10.3892/etm.2015.2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Austin JC, Elliott S, Cooper CS. Patients with spina bifida and bladder cancer: atypical presentation, advanced stage and poor survival. J Urol. 2007;178:798. doi: 10.1016/j.juro.2007.05.055. [DOI] [PubMed] [Google Scholar]
- 18.Soergel TM, Cain MP, Misseri R, et al. Transitional cell carcinoma of the bladder following augmentation cystoplasty for the neuropathic bladder. J Urol. 2004;172:1649. doi: 10.1097/01.ju.0000140194.87974.56. [DOI] [PubMed] [Google Scholar]
- 19.Kantor AF, Hartge P, Hoover RN, et al. Urinary tract infection and risk of bladder cancer. Am J Epidemiol. 1984;119:510. doi: 10.1093/oxfordjournals.aje.a113768. [DOI] [PubMed] [Google Scholar]
- 20.Hicks RM, Gough TA, Walters CL. Demonstration of the presence of nitrosamines in human urine: preliminary observations on a possible etiology for bladder cancer in association with chronic urinary tract infection. IARC Sci Publ. 1978;465 [PubMed] [Google Scholar]
- 21.Whiteside SA, Razvi H, Dave S, et al. The microbiome of the urinary tract--a role beyond infection. Nat Rev Urol. 2015;12:81. doi: 10.1038/nrurol.2014.361. [DOI] [PubMed] [Google Scholar]
- 22.Groah SL, Perez-Losada M, Caldovic L, et al. Redefining Healthy Urine: A Cross-Sectional Exploratory Metagenomic Study of People With and Without Bladder Dysfunction. J Urol. 2016 doi: 10.1016/j.juro.2016.01.088. [DOI] [PubMed] [Google Scholar]
- 23.Borges S, Silva J, Teixeira P. The role of lactobacilli and probiotics in maintaining vaginal health. Arch Gynecol Obstet. 2014;289:479. doi: 10.1007/s00404-013-3064-9. [DOI] [PubMed] [Google Scholar]
- 24.Rahn DD, Carberry C, Sanses TV, et al. Vaginal estrogen for genitourinary syndrome of menopause: a systematic review. Obstet Gynecol. 2014;124:1147. doi: 10.1097/AOG.0000000000000526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Austenfeld MS, Snow BW. Complications of pregnancy in women after reimplantation for vesicoureteral reflux. J Urol. 1988;140:1103. doi: 10.1016/s0022-5347(17)41972-0. [DOI] [PubMed] [Google Scholar]
- 26.Mansfield JT, Snow BW, Cartwright PC, et al. Complications of pregnancy in women after childhood reimplantation for vesicoureteral reflux: an update with 25 years of followup. J Urol. 1995;154:787. doi: 10.1097/00005392-199508000-00123. [DOI] [PubMed] [Google Scholar]
- 27.Rosen DA, Hooton TM, Stamm WE, et al. Detection of intracellular bacterial communities in human urinary tract infection. PLoS Med. 2007;4:e329. doi: 10.1371/journal.pmed.0040329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vigil HR, Hickling DR. Urinary tract infection in the neurogenic bladder. Transl Androl Urol. 2016;5:72. doi: 10.3978/j.issn.2223-4683.2016.01.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 30.Schwartz GJ, Haycock GB, Spitzer A. Plasma creatinine and urea concentration in children: normal values for age and sex. J Pediatr. 1976;88:828. doi: 10.1016/s0022-3476(76)81125-0. [DOI] [PubMed] [Google Scholar]
- 31.Quan A, Adams R, Ekmark E, et al. Serum creatinine is a poor marker of glomerular filtration rate in patients with spina bifida. Dev Med Child Neurol. 1997;39:808. doi: 10.1111/j.1469-8749.1997.tb07547.x. [DOI] [PubMed] [Google Scholar]
- 32.Morgan C, Senthilselvan A, Bamforth F, et al. Correlation between cystatin C- and renal scan-determined glomerular filtration rate in children with spina bifida. Pediatr Nephrol. 2008;23:329. doi: 10.1007/s00467-007-0613-0. [DOI] [PubMed] [Google Scholar]
- 33.Mattoo TK, Chesney RW, Greenfield SP, et al. Renal Scarring in the Randomized Intervention for Children with Vesicoureteral Reflux (RIVUR) Trial. Clin J Am Soc Nephrol. 2016;11:54. doi: 10.2215/CJN.05210515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.DeLair SM, Eandi J, White MJ, et al. Renal cortical deterioration in children with spinal dysraphism: analysis of risk factors. J Spinal Cord Med. 2007;30(Suppl 1):S30. doi: 10.1080/10790268.2007.11753966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mattoo TK. Vesicoureteral reflux and reflux nephropathy. Adv Chronic Kidney Dis. 2011;18:348. doi: 10.1053/j.ackd.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Inoue K, Shitamura T, Nose K, et al. Renal function and urodynamic evaluations in adult spina bifida patients. Int Urogynecol J Pelvic Floor Dysfunct. 2011;22:S632. [Google Scholar]
- 37.Thorup J, Biering-Sorensen F, Cortes D. Urological outcome after myelomeningocele: 20 years of follow-up. BJU Int. 2011;107:994. doi: 10.1111/j.1464-410X.2010.09681.x. [DOI] [PubMed] [Google Scholar]
- 38.Woodhouse CR. Myelomeningocele in young adults. BJU Int. 2005;95:223. doi: 10.1111/j.1464-410X.2005.05374.x. [DOI] [PubMed] [Google Scholar]
- 39.Malakounides G, Lee F, Murphy F, et al. Single centre experience: long term outcomes in spina bifida patients. J Pediatr Urol. 2013;9:585. doi: 10.1016/j.jpurol.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 40.Szymanski KM, Misseri R, Whittam B, et al. Mortality after bladder augmentation in children with spina bifida. J Urol. 2015;193:643. doi: 10.1016/j.juro.2014.07.101. [DOI] [PubMed] [Google Scholar]
- 41.Rule AD, Bergstralh EJ, 3rd, Melton LJ, et al. Kidney stones and the risk for chronic kidney disease. Clin J Am Soc Nephrol. 2009;4:804. doi: 10.2215/CJN.05811108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jafar TH, Schmid CH, Landa M, et al. Angiotensin-converting enzyme inhibitors and progression of nondiabetic renal disease. A meta-analysis of patient-level data. Ann Intern Med. 2001;135:73. doi: 10.7326/0003-4819-135-2-200107170-00007. [DOI] [PubMed] [Google Scholar]
- 43.Wingen AM, Fabian-Bach C, Schaefer F, et al. Randomised multicentre study of a low-protein diet on the progression of chronic renal failure in children. European Study Group of Nutritional Treatment of Chronic Renal Failure in Childhood. Lancet. 1997;349:1117. doi: 10.1016/s0140-6736(96)09260-4. [DOI] [PubMed] [Google Scholar]
- 44.Bell LE, Sawyer SM. Transition of care to adult services for pediatric solid-organ transplant recipients. Pediatr Clin North Am. 2010;57:593. doi: 10.1016/j.pcl.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 45.Bandura A. Social Learning Theory. New York: General Learning Press; 1977. [Google Scholar]
- 46.Werner EE. The children of Kauai: resiliency and recovery in adolescence and adulthood. J Adolesc Health. 1992;13:262. doi: 10.1016/1054-139x(92)90157-7. [DOI] [PubMed] [Google Scholar]
- 47.Ryan P, Sawin KJ. The Individual and Family Self-Management Theory: background and perspectives on context, process, and outcomes. Nurs Outlook. 2009;57:217. doi: 10.1016/j.outlook.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kohn NA, Blumenthal JA, Campbell AT. Supported Decision-Making: A Viable Alternative to Guardianship? Penn State Law Review. 2013;117 Epub. [Google Scholar]
- 49.Fredericks EM, Dore-Stites D. Adherence to immunosuppressants: how can it be improved in adolescent organ transplant recipients? Curr Opin Organ Transplant. 2010;15:614. doi: 10.1097/MOT.0b013e32833d3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Achenbach TM, Howell CT, Quay HC, et al. National survey of problems and competencies among four- to sixteen-year-olds: parents' reports for normative and clinical samples. Monogr Soc Res Child Dev. 1991;56:1. [PubMed] [Google Scholar]
- 51.Korkman M, Kemp SL, Kirk U. Effects of age on neurocognitive measures of children ages 5 to 12: a cross-sectional study on 800 children from the United States. Dev Neuropsychol. 2001;20:331. doi: 10.1207/S15326942DN2001_2. [DOI] [PubMed] [Google Scholar]
- 52.Mahone EM, Cirino PT, Cutting LE, et al. Validity of the behavior rating inventory of executive function in children with ADHD and/or Tourette syndrome. Arch Clin Neuropsychol. 2002;17:643. [PubMed] [Google Scholar]
- 53.Finkelstein FO, Arsenault KL, Taveras A, et al. Assessing and improving the health-related quality of life of patients with ESRD. Nat Rev Nephrol. 2012;8:718. doi: 10.1038/nrneph.2012.238. [DOI] [PubMed] [Google Scholar]
- 54.Finkelstein FO, Wuerth D, Finkelstein SH. Health related quality of life and the CKD patient: challenges for the nephrology community. Kidney Int. 2009;76:946. doi: 10.1038/ki.2009.307. [DOI] [PubMed] [Google Scholar]
- 55.Lemelle JL, Guillemin F, Aubert D, et al. A multicenter evaluation of urinary incontinence management and outcome in spina bifida. J Urol. 2006;175:208. doi: 10.1016/S0022-5347(05)00055-8. [DOI] [PubMed] [Google Scholar]
- 56.Dicianno BE, Kurowski BG, Yang JM, et al. Rehabilitation and medical management of the adult with spina bifida. Am J Phys Med Rehabil. 2008;87:1027. doi: 10.1097/PHM.0b013e31818de070. [DOI] [PubMed] [Google Scholar]
- 57.Moore C, Kogan BA, Parekh A. Impact of urinary incontinence on self-concept in children with spina bifida. J Urol. 2004;171:1659. doi: 10.1097/01.ju.0000117865.98229.e5. [DOI] [PubMed] [Google Scholar]
- 58.Trofimenko V, Brant WO. Fertility and sexual dysfunction issues in adults with genitourinary congenital anomalies. Curr Opin Urol. 2016;26:357. doi: 10.1097/MOU.0000000000000295. [DOI] [PubMed] [Google Scholar]
- 59.Rubenwolf P, Thomas C, Thuroff JW, et al. Sexual Function and Fertility of Women with Classic Bladder Exstrophy and Continent Urinary Diversion. J Urol. 2016;196:140. doi: 10.1016/j.juro.2015.12.099. [DOI] [PubMed] [Google Scholar]
- 60.Deans R, Creighton SM, Liao LM, et al. Timing of gonadectomy in adult women with complete androgen insensitivity syndrome (CAIS): patient preferences and clinical evidence. Clin Endocrinol (Oxf) 2012;76:894. doi: 10.1111/j.1365-2265.2012.04330.x. [DOI] [PubMed] [Google Scholar]
- 61.Bouman MB, van Zeijl MC, Buncamper ME, et al. Intestinal vaginoplasty revisited: a review of surgical techniques, complications, and sexual function. J Sex Med. 2014;11:1835. doi: 10.1111/jsm.12538. [DOI] [PubMed] [Google Scholar]
- 62.Cools M, Drop SL, Wolffenbuttel KP, et al. Germ cell tumors in the intersex gonad: old paths, new directions, moving frontiers. Endocr Rev. 2006;27:468. doi: 10.1210/er.2006-0005. [DOI] [PubMed] [Google Scholar]
- 63.Steiner E, Woernle F, Kuhn W, et al. Carcinoma of the neovagina: case report and review of the literature. Gynecol Oncol. 2002;84:171. doi: 10.1006/gyno.2001.6417. [DOI] [PubMed] [Google Scholar]
- 64.Ellsworth PI, Merguerian PA, Klein RB, et al. Evaluation and risk factors of latex allergy in spina bifida patients: is it preventable? J Urol. 1993;150:691. doi: 10.1016/s0022-5347(17)35587-8. [DOI] [PubMed] [Google Scholar]
- 65.Festin MR. Non-latex versus latex male condoms for contraception. Geneva: The WHO Reproductive Health Library; World Health Organization; 2013. Apr 1, [Google Scholar]
- 66.Dy GW, Willihnganz-Lawson KH, Shnorhavorian M, et al. Successful pregnancy in patients with exstrophy-epispadias complex: A University of Washington experience. J Pediatr Urol. 2015;11:213 e1. doi: 10.1016/j.jpurol.2015.04.019. [DOI] [PubMed] [Google Scholar]
- 67.Visconti D, Noia G, Triarico S, et al. Sexuality, pre-conception counseling and urological management of pregnancy for young women with spina bifida. Eur J Obstet Gynecol Reprod Biol. 2012;163:129. doi: 10.1016/j.ejogrb.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 68.Vroege JA, Zeijlemaker BY, Scheers MM. Sexual functioning of adult patients born with meningomyelocele. A pilot study. Eur Urol. 1998;34:25. doi: 10.1159/000019673. [DOI] [PubMed] [Google Scholar]
- 69.Shiomi T, Hirayama A, Fujimoto K, et al. Sexuality and seeking medical help for erectile dysfunction in young adults with spina bifida. Int J Urol. 2006;13:1323. doi: 10.1111/j.1442-2042.2006.01559.x. [DOI] [PubMed] [Google Scholar]
- 70.Cass AS, Bloom BA, Luxenberg M. Sexual function in adults with myelomeningocele. J Urol. 1986;136:425. doi: 10.1016/s0022-5347(17)44891-9. [DOI] [PubMed] [Google Scholar]
- 71.Joyner BD, McLorie GA, Khoury AE. Sexuality and reproductive issues in children with myelomeningocele. Eur J Pediatr Surg. 1998;8:29. doi: 10.1055/s-2008-1071115. [DOI] [PubMed] [Google Scholar]
- 72.Valtonen K, Karlsson AK, Siosteen A, et al. Satisfaction with sexual life among persons with traumatic spinal cord injury and meningomyelocele. Disabil Rehabil. 2006;28:965. doi: 10.1080/09638280500404362. [DOI] [PubMed] [Google Scholar]
- 73.Game X, Moscovici J, Game L, et al. Evaluation of sexual function in young men with spina bifida and myelomeningocele using the International Index of Erectile Function. Urology. 2006;67:566. doi: 10.1016/j.urology.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 74.Game X, Moscovici J, Guillotreau J, et al. Sexual function of young women with myelomeningocele. J Pediatr Urol. 2014;10:418. doi: 10.1016/j.jpurol.2013.07.016. [DOI] [PubMed] [Google Scholar]
- 75.Sawin KJ, Brei TJ, Buran CF, et al. Factors associated with quality of life in adolescents with spina bifida. J Holist Nurs. 2002;20:279. doi: 10.1177/089801010202000307. [DOI] [PubMed] [Google Scholar]
- 76.Sawin KJ, Buran CF, Brei TJ, et al. Sexuality issues in adolescents with a chronic neurological condition. J Perinat Educ. 2002;11:22. doi: 10.1624/105812402X88579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kleinemeier E, Jurgensen M, Lux A, et al. Psychological adjustment and sexual development of adolescents with disorders of sex development. J Adolesc Health. 2010;47:463. doi: 10.1016/j.jadohealth.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 78.Kohler B, Kleinemeier E, Lux A, et al. Satisfaction with genital surgery and sexual life of adults with XY disorders of sex development: results from the German clinical evaluation study. J Clin Endocrinol Metab. 2012;97:577. doi: 10.1210/jc.2011-1441. [DOI] [PubMed] [Google Scholar]
- 79.von Linstow ME, Biering-Sorensen I, Liebach A, et al. Spina bifida and sexuality. J Rehabil Med. 2014;46:891. doi: 10.2340/16501977-1863. [DOI] [PubMed] [Google Scholar]
- 80.Cardenas DD, Topolski TD, White CJ, et al. Sexual functioning in adolescents and young adults with spina bifida. Arch Phys Med Rehabil. 2008;89:31. doi: 10.1016/j.apmr.2007.08.124. [DOI] [PubMed] [Google Scholar]
- 81.Sawyer SM, Roberts KV. Sexual and reproductive health in young people with spina bifida. Dev Med Child Neurol. 1999;41:671. doi: 10.1017/s0012162299001383. [DOI] [PubMed] [Google Scholar]
- 82.Cromer BA, Enrile B, McCoy K, et al. Knowledge, attitudes and behavior related to sexuality in adolescents with chronic disability. Dev Med Child Neurol. 1990;32:602. doi: 10.1111/j.1469-8749.1990.tb08544.x. [DOI] [PubMed] [Google Scholar]
- 83.Dorner S. Adolescents with spina bifida. How they see their situation. Arch Dis Child. 1976;51:439. doi: 10.1136/adc.51.6.439. [DOI] [PMC free article] [PubMed] [Google Scholar]