Abstract
Background
Because many people who inject drugs (PWID) are at high risk of hepatitis C virus (HCV) and have poor access to medical care, many HCV-infected PWID remain undiagnosed and unaccounted for in surveillance systems. Syringe exchange programs (SEPs) are an under-utilized resource for collecting information missing from surveillance systems. Partnerships with public health agencies represent a potentially innovative approach to studying the HCV epidemic for PWID. The goal of this study was to characterize the HCV care continuum for a cohort of PWID using database linkages.
Methods
Data needed to describe the HCV care continuum for 235 PWID were collected from surveillance data provided by the Wisconsin Division of Public Health, a computerized survey administered by SEP staff, and a follow-up interview delivered by academic research staff. When possible, we attempted to confirm each individual’s position in the HCV care continuum with at least 2 of the 3 data sources.
Results
Participants ranged in age from 18–63 years, 60% self-identified as non-Hispanic white, and 77% were male. Overall, we determined that 208 (89%) of the 235 participants had ever been tested for HCV and 72 (31%) had ever tested positive. Of those 72, 46 (64%) had been linked to care, 14 (19%) received pre-treatment evaluation, and 4 (6%) reported initiating treatment. Confirmation by at least 2 data sources ranged from 14–57% of cases for each stage of HCV care.
Conclusion
Available data sources show a large degree of variability when used to characterize the HCV care continuum. New strategies to enhance the quality and completeness of these data sources could substantially improve ongoing efforts to monitor the HCV care continuum among PWID.
Keywords: Hepatitis C virus, Syringe Exchange Program, Surveillance
Introduction
Increased incidence of hepatitis c virus (HCV) infection has been recently detected in many nonurban areas of the U.S. (Centers for Disease Control and Prevention [CDC], 2012; Conrad et al., 2015; Zibbell et al., 2015) Driven primarily by injection drug use, this acceleration of the HCV epidemic has come during a period of rapid improvement in the efficacy and tolerability of anti-HCV therapy. Despite projections that HCV incidence could be markedly reduced with scale-up of direct-acting antiviral treatment, uptake of treatment among people who inject drugs (PWID) has remained low at an estimated 2–4%. (Echevarria et al., 2015; Harris and Rhodes, 2013) Developing strategies to optimize the so called “HCV care continuum,” wherein HCV-infected individuals are screened, receive RNA confirmatory testing, get linked to care, undergo pretreatment evaluation, initiate antiviral treatment and are ultimately cured (Holmberg, Spradling, Moorman, & Denniston, 2013), is an important public health priority.
Viral hepatitis surveillance plays an important role in understanding the burden of HCV in communities. (CDC, 1998) Requirements for clinicians and laboratories to report diagnoses of HCV to state and local health departments serve as the backbone of HCV surveillance. Surveillance data allow jurisdictions to estimate the prevalence of known HCV cases and to detect trends in incidence, but have important limitations. Of the estimated 3.2 million persons chronically infected with HCV in the U.S., approximately half remain undiagnosed, and are consequently unaccounted for in surveillance systems. (American Association for the Study of Liver Diseases and the Infectious Diseases Society of America [AASLD and IDSA], 2015) Because many individuals at high risk of HCV, especially young PWID, have poor access to medical care, surveillance programs’ reliance on clinician and laboratory-based reporting may systematically under-represent key populations.
Syringe exchange programs (SEPs) are an under-utilized resource for collecting information about the HCV epidemic among PWID that is missing from surveillance systems. These programs often have established rapport and have earned the trust of target populations based on years of providing non-judgmental services. (Westergaard et al., 2016) Rapid screening for HCV is feasible and acceptable in SEPs (Barocas et al., 2014), and program staff is often knowledgeable and experienced in referring clients to other needed services. These features provide an opportunity to interface with difficult-to-reach populations that are both under-served and under-studied. To capitalize on this potentially rich source of information, we evaluated the HCV care continuum for PWID through a novel linkage of databases derived from a SEP, surveillance system, and community-based research project.
Methods
Study Overview
The present analysis was nested within a larger, randomized pilot intervention called the Hep-Net Study. (Westergaard et al., 2016) Hep-Net was implemented in a community-based SEP that delivers prevention and testing services across Wisconsin through 10 statewide fixed sites as well as a mobile program. SEP staff enrolled 235 adults reporting past-month injection drug use into Hep-Net, and delivered a 2 session, computerized behavioral intervention aimed at promoting safer injecting behavior, linkage to addiction treatment, and HIV/HCV testing.
Data Collection
To characterize the HCV care continuum for Hep-Net participants, we collected and analyzed data from 3 different sources. First, the computerized Hep-Net survey collected personal identifiers, demographic characteristics, risk behaviors and prior HCV testing information for all study participants. Second, we cross-matched these 235 Hep-Net participants by first and last name and date of birth to the Wisconsin Electronic Disease Surveillance System (WEDSS) using a probabilistic record matching program developed by CDC, Link Plus, to identify individuals captured by surveillance as a confirmed or suspected case of HCV. WEDSS is a secure, web-based health information system used for the reporting, investigation, and surveillance of communicable diseases in Wisconsin. (Wisconsin Department of Health Services, 2015) Per Wisconsin Statute 252.05 (2016), any health care provider with evidence that a patient has HCV is required to report it to the appropriate local health department.
Third, a university-based research assistant conducted an in-person follow-up interview with a subset of Hep-Net participants who self-reported being HCV-infected during the study. Interview questions elicited participants’ recent experience with HCV testing and care, including reasons for not engaging in HCV care (for a complete list of questions, see Supplemental Appendix). Up to 10 contact attempts were made to these individuals from June 2015 to October 2015 by phone, e-mail, direct mail, or Facebook using contact information provided at Hep-Net enrollment. Participants completed the interview with research staff at SEP sites and received $40 cash as compensation. Interview data were collected and managed using Research Electronic Data Capture (REDCap), a secure, web-based application designed to support data capture for research. (Harris et al., 2009) The protocol was reviewed and approved as a minimal risk research component of the Hep-Net Study by the Health Sciences Institutional Review Board at the University of Wisconsin-Madison.
Defining the HCV Care Continuum
For this analysis, we defined the various stages in the HCV care continuum similar to prior published literature (Holmberg et al., 2013), with several modifications based on the types of data available. Whenever possible, we attempted to confirm each individual’s position in the care continuum with at least 2 of the 3 data sources described above. Participants were considered “tested for HCV” if they self-reported a prior HCV test during the Hep-Net survey, or if they agreed to receive a rapid HCV test at the time of either Hep-Net survey session. Participants were also considered tested if a record of a prior HCV test was found in the WEDSS database. Similarly, participants were considered “tested positive for HCV” if they self-reported during the Hep-Net survey a prior HCV test that was “positive”, if the HCV test they took at either Hep-Net study session was “reactive”, or if a record of a positive test existed in WEDSS. At the time of this study, results of non-reactive HCV tests were not routinely reported to WEDSS.
“Linkage to HCV care” was defined by either self-report in the Hep-Net survey or the follow-up interview, or evidence in the WEDSS database of confirmatory nucleic acid testing (e.g. polymerase chain reaction for HCV RNA) or genotype testing after the date of the earliest positive HCV test. Both Hep-Net survey sessions asked participants whether they attended an appointment with a clinical provider that was scheduled specifically for the purpose of discussing their positive HCV test. Similarly, during the follow-up interview, participants were asked if they have attended an “appointment with a medical provider to discuss their positive HCV test”. The laboratory-based definition excludes individuals who received confirmatory HCV RNA testing on the same day they received a rapid HCV screening test because it could not be determined that such individuals actively sought care as a result of their HCV test.
The stage we refer to as “received pre-treatment evaluation” reflects individuals who have undergone at least part of the evaluation that is recommended prior to initiation of HCV therapy. Participants were considered to have reached this stage if we found results of an HCV genotype test in WEDSS, if they self-reported during the follow-up interview that a provider offered them HCV treatment, or if they self-reported in the Hep-Net survey that they were evaluated for and/or started HCV treatment. Evidence of genotype testing is an appropriate indicator for having received pre-treatment evaluation because at the time of this study, genotype testing was recommended prior to making any treatment decisions. (AASLD and IDSA, 2015) The final stage in the care continuum, “initiation of HCV treatment,” was determined by self-report at the second Hep-Net survey session and during the in-person interview.
Results
Demographics and HCV status
Of the 235 Hep-Net participants, 60% self-identified as non-Hispanic white and 28% self-identified as black or African American. The sample was 77% male and participants ranged in age from 18–63 years, with a median age of 35 years. Fifty-three percent experienced homelessness in the past year, and 73% had less than $11,500 of legal annual income. While most participants (55%) lived in the city of Milwaukee, which has a population over 500,000 residents, over one fourth (29%) lived in a city with a population less than 50,000, including 17% who resided in a city with a population under 5,000.
Based on responses to the Hep-Net survey, 46 individuals self-identified as HCV positive; an additional 26 were identified as HCV-infected through WEDSS. Five (11 %) of the 46 individuals who self-identified as HCV positive were unable to be matched to an existing record in WEDSS. There were no significant differences in any demographic variables between these 72 HCV-infected participants and those who were HCV-negative or had never been tested. We successfully re-contacted 26 of the 46 participants who self-identified as HCV-positive (56%) to complete the follow-up interview. Those who were successfully contacted and interviewed were slightly older than the overall sample (mean age of 41 years vs. 36 years, p=0.017), but were otherwise similar with respect to race, gender, education level, and frequency of injection drug use.
HCV Care Continuum
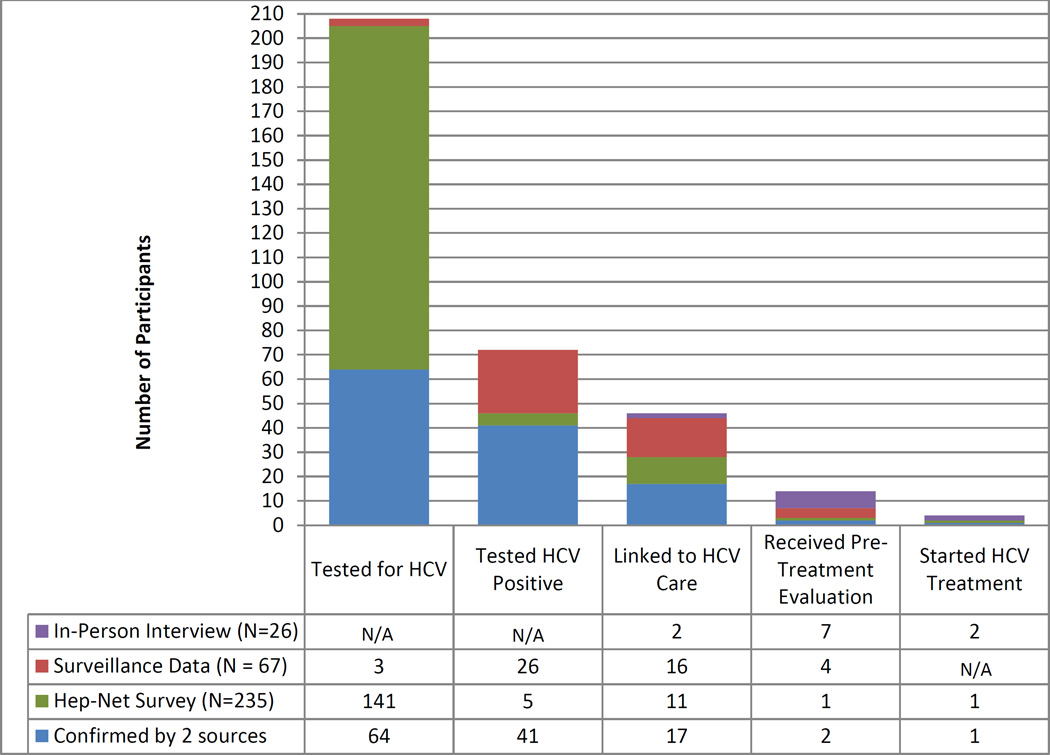
The number of participants successfully achieving the stages in the HCV care continuum, as determined by each data source, is displayed in Figure 1. Overall, we determined that 208 (89%) of the 235 participants had ever been tested for HCV and 72 (31%) had ever tested positive. Of those who were determined to be HCV-positive, 46 (64%) had been linked to care, 14 (19%) received pre-treatment evaluation, and 4 (6%) reported starting treatment. Individuals who participated in the follow-up interview commonly reported a lack of motivation, being asymptomatic, and beliefs that they cannot be treated if they are currently injecting as reasons for disengaging in the HCV care continuum.
Figure 1. The HCV care continuum for the 235 PWID by source of information.
N/A = data for that stage of the continuum was not obtained from the applicable data source
Of the 72 individuals who had ever tested positive, 11 (15%) received a RNA test the same day as their rapid HCV screening test and had no further testing, indicating this was likely a reflex test and not an active linkage. Of the 46 individuals linked to care, 33 were considered linked based off the laboratory definition. Of these 33, 2 (6%) were linked within 1 week and 17 (52%) were linked within 6 months. The remaining 14 (42%) were linked after 6 months or more.
Confirmation of individuals’ stages of HCV care using at least 2 sources of data was possible for only a minority of cases. Of the 208 individuals who we defined as having been tested for HCV, only 64 (31%) 8 had testing documented in both the Hep-Net survey and the WEDSS database. Of the 72 individuals who had tested HCV-positive, 41 (57%) had the positive test documented in both the Hep-Net survey and WEDSS. Seventeen (37%) of the 46 participants considered linked to care had this linkage documented in at least 2 of the 3 data sources. Only 2 (14%) of the 14 individuals who received pre-treatment evaluation were supported by at least 2 data sources and 1 (25%) of the 4 individuals who started treatment had documentation in both the Hep-Net survey and the follow-up interview.
Discussion
We have described the HCV care continuum for a cohort of PWID in Wisconsin using 3 different data sources and found that while most people are tested and many are linked to care, few people achieve subsequent steps in the continuum of care, resulting in low antiviral treatment uptake. Our findings highlight the need for interventions and policy reforms that aim to increase HCV care utilization among PWID.
Prior studies seeking to characterize the HCV care continuum have utilized data derived from electronic medical records (Coyle et al., 2015; Hawks, Norton, Cunningham, & Fox, 2016), which provide comprehensive information about the clinical evaluation, medications prescribed, and virologic response to treatment. However, variability in software platforms across different health care systems, and the fact that many people at high risk for HCV are under-insured and not regularly engaged in care makes reliance upon clinical data challenging on a population level and limits generalizability of these data. By leveraging several different sources of data each with unique methods for collection, our approach to describing the HCV care continuum allowed us to capture more individuals and fill in gaps that may exist in a single data source. For example, SEPs can fill an important gap in laboratory-based surveillance data by providing risk information, allowing for the identification of a cohort of PWID.
Surveillance data have important limitations when used to make inferences about utilization of health care. For example, if a surveillance system indicates an individual had a reactive screening HCV antibody test and a positive confirmatory HCV PCR several days later, one cannot discern whether the short lag time reflects prompt linkage to a medical provider for confirmatory testing, or a situation where a confirmatory test was obtained reflexively based on the reactive screen, which would have required no action on the part of the patient. Moreover, the presence of test results in the surveillance system does not necessarily imply that the individual tested received results. Finally, there is currently no requirement in Wisconsin for negative HCV tests to be reported to the surveillance system. Changing this policy to require health test providers and laboratories to report negative tests would allow for better characterization of the proportion of high-risk individuals who have been tested and know their HCV status. Whereas data captured in surveys or surveillance systems may not be able to comprehensively portray the HCV care continuum, if appropriately viewed in the context of their limitations, these data may provide useful metrics to monitor progress toward specific goals over time.
In terms of reliability and completeness of data, a strength of the computerized Hep-Net survey is that it was delivered to PWID by familiar, trusted SEP staff at a time and place convenient for the client. Contrariwise, recruitment for the follow-up interview was a limitation, as patients were contacted and interviewed by unfamiliar research staff at a scheduled time. Lack of agreement between surveillance data and survey or interview data may be due to recall bias, as both the Hep-Net survey and follow-up interview required individuals to recall what may have happened many years ago.
We observed relatively low concordance among data collected through surveillance, syringe programs, and researchers, highlighting the limitations of currently available data sources. Our findings suggest that new strategies to enhance the quality and completeness of these data sources could improve ongoing efforts to monitor the HCV care continuum among PWID. Future studies involving collaboration among organizations with access to these diverse streams of data are needed to better define which steps in the care continuum are highest priority for intervention, and to evaluate the impact of HCV control efforts.
Supplementary Material
Acknowledgments
This work was supported by the Clinical and Translational Science Award (CTSA) program, through the NIH National Center for Advancing Translational Sciences (NCATS) [grant number UL1TR000427]. RPW is supported by the National Institutes of Health [grant number K23DA032306]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Association for the Study of Liver Diseases and the Infectious Diseases Society of America. Hepatitis C guidance: AASLD-IDSA recommendations for testing, managing, and treating adults infected with hepatitis C virus. Hepatology. 2015;62(3):932–954. doi: 10.1002/hep.27950. [DOI] [PubMed] [Google Scholar]
- Barocas JA, Brennan MB, Hull SJ, Stokes S, Fangman JJ, Westergaard RP. Barriers and facilitators of hepatitis C screening among people who inject drugs: a multi-city, mixed-methods study. Harm Reduct J. 2014;11:1. doi: 10.1186/1477-7517-11-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention [CDC] Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. MMWR Morb Mortal Wkly Rep. 1998;47(Rr-19):1–39. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention [CDC] Notes from the field : hepatitis C virus infections among young adults--rural Wisconsin, 2010. MMWR Morb Mortal Wkly Rep. 2012;61(19):358. [PubMed] [Google Scholar]
- Conrad C, Bradley HM, Broz D, Buddha S, Chapman EL, Galang RR, Duwve JM. Community Outbreak of HIV Infection Linked to Injection Drug Use of Oxymorphone--Indiana, 2015. MMWR Morb Mortal Wkly Rep. 2015;64(16):443–444. [PMC free article] [PubMed] [Google Scholar]
- Coyle C, Viner K, Hughes E, Kwakwa H, Zibbell JE, Vellozzi C, Holtzman D. Identification and Linkage to Care of HCV-Infected Persons in Five Health Centers - Philadelphia, Pennsylvania, 2012–2014. MMWR Morb Mortal Wkly Rep. 2015;64(17):459–463. [PMC free article] [PubMed] [Google Scholar]
- Echevarria D, Gutfraind A, Boodram B, Major M, Del Valle S, Cotler SJ, Dahari H. Mathematical Modeling of Hepatitis C Prevalence Reduction with Antiviral Treatment Scale-Up in Persons Who Inject Drugs in Metropolitan Chicago. PLoS One. 2015;10(8):e0135901. doi: 10.1371/journal.pone.0135901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris M, Rhodes T. Hepatitis C treatment access for people who inject drugs: a review mapping the role of social factors. Harm Reduct. J. 2013;10(7) doi: 10.1186/1477-7517-10-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawks L, Norton BL, Cunningham CO, Fox AD. The Hepatitis C virus treatment cascade at an urban postincarceration transitions clinic. J Viral Hepat. 2016;23(6):473–478. doi: 10.1111/jvh.12512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmberg SD, Spradling PR, Moorman AC, Denniston MM. Hepatitis C in the United States. N Engl J Med. 2013;368(20):1859–1861. doi: 10.1056/NEJMp1302973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westergaard RP, Hull SJ, Merkow A, Stephens LK, Hochstatter KR, Olson-Streed HK, Hess TM. Computerized Tailored Interventions to Enhance Prevention and Screening for Hepatitis C Virus Among People Who Inject Drugs: Protocol for a Randomized Pilot Study. JMIR Res Protoc. 2016;5(1):e15. doi: 10.2196/resprot.4830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisconsin Department of Health Services. Wisconsin Electronic Disease Surveillance System (WEDSS) Health Care Provider Reporting of Communicable Diseases. 2015 Retrieved 20 November 2015 from https://www.dhs.wisconsin.gov/wiphin/wedss.htm.
- Wisconsin State Legislature. Chapter 252 : Communicable Diseases. 2016 Retrieved 20 May 2016 from http://docs.legis.wisconsin.gov/statutes/statutes/252.pdf.
- Zibbell JE, Iqbal K, Patel RC, Suryaprasad A, Sanders KJ, Moore-Moravian L, Holtzman D. Increases in hepatitis C virus infection related to injection drug use among persons aged </=30 years - Kentucky, Tennessee, Virginia, and West Virginia, 2006–2012. MMWR Morb Mortal Wkly Rep. 2015;64(17):453–458. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.