Abstract
Background
Academic achievement was evaluated in children with heavy prenatal alcohol exposure to determine potential strengths and weaknesses, evaluate the utility of different definitions for identifying low academic performance, and explore the neural correlates that may underlie academic performance.
Methods
Children (8–16 years) were assessed using the WIAT-II. Patterns of performance were examined in two subject groups: children with heavy prenatal alcohol exposure (n=67) and controls (n=61). A repeated-measures MANCOVA examining group differences on academic domain (reading, spelling, math) scores was conducted. Post-hoc comparisons examined within-group profiles. Numbers and percentage of children with low achievement were calculated using several criteria. In a subsample (n=42), neural correlates were analyzed using FreeSurfer-v5.3 to examine relations between cortical structure (thickness and surface area) and performance.
Results
The alcohol-exposed group performed worse than controls on all domains and had a unique academic profile, supported by a significant group × academic domain interaction (p<.001). For the alcohol-exposed group, math reasoning was significantly lower than numerical operations, which was significantly lower than spelling and word reading. Over half of the alcohol-exposed group (58.2%) demonstrated low achievement on one or more academic domains. The number and percentage of children meeting criteria for low achievement varied based on the domain and definition used. The imaging analysis identified several surface area clusters that were differentially related to math (L superior parietal and R lateral/middle occipital) and spelling (bilateral inferior and medial temporal) performance by group, with no relations for the other academic domains. Generally, scores improved as surface area decreased in controls, whereas no relation or a positive relation was observed in the alcohol-exposed group.
Conclusion
Alcohol-exposed children demonstrated deficits in academic performance across domains and definitions with a relative weakness in math functioning. Atypical brain development may contribute to these impairments in academic achievement. Understanding academic difficulties can assist in advocating effectively for alcohol-exposed children.
Keywords: Fetal alcohol syndrome (FAS), Fetal alcohol spectrum disorders (FASD), Neurobehavioral disorder associated with prenatal alcohol exposure (ND-PAE), prenatal alcohol exposure, academic achievement, brain
Introduction
For over three decades, it has been known that prenatal alcohol exposure can result in significant clinical repercussions including academic difficulties (Streissguth et al., 1990; Sampson et al., 1987) and despite greater awareness, this continues to be true (Howell et al., 2006). Prevalence estimates of FASD have been conservatively estimated around 1%, however a recent study in North America found rates as high as 4.8%, indicating a significant public health concern (May et al., 2014, 2015). Case ascertainment studies indicate the rate of prenatal alcohol exposure may be as high as 2–5% (May et al., 2014), although the majority of affected individuals are not accurately identified (Chasnoff et al., 2015). Those who are identified often come to clinical attention at school age (Streissguth et al., 2004), which limits early intervention opportunities resulting in a variety of secondary disabilities including disrupted school experiences (Streissguth et al., 1994b).
Difficulty with academic achievement overall is known to be associated with prenatal alcohol exposure. Of note, the newly updated clinical guidelines for diagnosing fetal alcohol spectrum disorders include specific learning impairment (across academic domains) as satisfying the criteria for the neurobehavioral impairment in the diagnoses (Hoyme et al., 2016). However, poor performance on math has emerged as an area of selective vulnerability for children with prenatal alcohol exposure (Coles et al., 2009, Jacobson et al., 2011, Lebel et al., 2010, Crocker et al., 2015, Howell et al., 2006, Kerns et al., 1997, Kopera-Frye et al., 1996) with less research on other academic domains (O’Leary et al., 2013, Glass et al., 2015, Streissguth et al., 1994a, Howell et al., 2006). Howell et al., (2006), compared alcohol-exposed children with and without dysmorphology to children in special education classes and those with similar socioeconomic backgrounds, and found that all children performed one to two standard deviations below the normative mean. The students in special education performed worse on reading and spelling, whereas alcohol-exposed children with dysmorphology performed worse than other groups on math. Further, studies have generally compared alcohol-exposed children to non-exposed controls even though in educational settings, children are more likely to be compared to normative cutoffs or specific criteria to determine whether performance is at a level that warrants academic or clinical attention. There is a continued need to empirically understand the profile of academic achievement deficits for alcohol-exposed individuals and determine the most effective method of identification of children in need to inform the development and dissemination of evidence-based intervention.
There has also been an effort to understand the etiology of poor academic functioning. Growing literature supports both cognitive mechanisms and specific neural correlates that underlie distinct academic pathways. Neuroimaging findings suggest that structural or functional changes in the brain may contribute to academic performance in typically developing children (Houston et al., 2014, Dehaene et al., 2003), and those with learning disorders (Ashkenazi et al., 2013). Relatively few studies have examined these relations in alcohol-exposed populations, however relations of math performance to brain structure and function have been reported (Woods et al., 2015, Lebel et al., 2010, Meintjes et al., 2010, Santhanam et al., 2009), whereas associations with reading or spelling were not assessed in these studies.
We aimed to determine the profile of academic strengths and weaknesses, explore definitional considerations of using various criteria for identification of learning difficulties, and potential neural correlates for deficits in academic performance in children with prenatal alcohol exposure. We expected to replicate previous findings demonstrating that alcohol-exposed children would perform significantly more poorly than controls on all academic domains and extend the research to demonstrate higher rates of academic difficulties across definitions. As suggested by the literature, we hypothesized alcohol-exposed children would perform significantly lower on measures of math compared to reading or spelling, and tested this by statistically comparing their performance within-subjects across academic subtests. We also expected distinct relations between neural structures and academic performance. We hypothesized that decreases in cortical structure (thickness and surface area) would be associated with increased performance in controls, as cortical reductions are often associated with the development of more mature and efficient networks during adolescence (Sowell et al., 2001). However, we expected that in alcohol-exposed children smaller surface area and thinner cortex would be related to poorer performance, as reduced cortical structure would be associated with greater alcohol teratogenesis in these children (Meintjes et al., 2014).
Methods
General Method
School-age children and their primary caregivers were recruited for participation in an ongoing research protocol, which included behavioral measures, neuropsychological assessment conducted by trained examiners blind to subject group, and a dysmorphological evaluation. A subset of children also underwent magnetic resonance imaging (MRI) to obtain T1-weighted anatomical brain scans. Subjects were recruited via schools, clinical referral, professional referrals, community outreach, advertisements, word of mouth, or other public forums. Informed consent and assent was obtained in accordance with the Institutional Review Board at San Diego State University. All participants were given a financial incentive for participation.
Subjects
Children and adolescents ages 8–16 years (M=12.69, SD=3.14) with histories of heavy prenatal alcohol exposure (AE, n=67) and with no or minimal (defined below) alcohol exposure (CON, n=61) were included in this study. All children were evaluated for fetal alcohol syndrome (FAS) by an expert dysmorphologist (KLJ). An FAS diagnosis was determined by the presence of two or more key facial features (short palpebral fissures, smooth philtrum, thin vermillion border) in addition to microcephaly (head circumference ≤10th percentile), and/or growth deficiency (≤10th percentile for height/weight) (Jones et al., 2006). Twelve (17.9%) subjects in the AE group met these criteria for FAS.
Prenatal alcohol exposure history was assessed retrospectively. If direct report was available, heavy prenatal alcohol exposure was defined as a pattern of maternal consumption of greater than 4 drinks per occasion or at least 14 drinks per week on several occasions throughout pregnancy. Due to the nature of substance abuse and the high rate of adoption or foster care in our research population, for the majority of cases, direct report was often unavailable. In the current sample, 64/67 (95%) of the AE group and 3/61 (5%) of the CON group no longer lived with their biological mothers. In these cases, records were reviewed to gain information regarding exposure history. Record review included, but was not limited to, medical history, birth, adoption, and social services records or communication with the biological mother or other relative. The presence of corroborative evidence supporting alcohol abuse or dependence during pregnancy or a diagnosis of FAS (see above) was sufficient for inclusion in the AE group. Subjects were excluded from the typically developing control group if they had greater than minimal alcohol exposure based on maternal report or record review, or if prenatal alcohol exposure was suspected or unknown. Minimal exposure was defined as no more than one drink per week on average and never more than two drinks on any occasion during pregnancy.
Exclusion criteria for both groups were: significant head trauma, loss of consciousness greater than 30 minutes, other known causes of cognitive deficiency, not fluent or primary English speakers, international adoption either two years prior to participation or after the age of five years, or severe mental, physical, or psychiatric disability that hindered study participation (e.g., active psychosis, active mania, uncontrolled movement disorders, blindness). Subjects were excluded from the imaging component if they had contraindications to the MRI procedure (e.g., irremovable metal in the body or claustrophobia).
Primary caregivers of all subjects complete a standardized, semi-structured clinical interview, the Computerized Diagnostic Interview Schedule for Children Version IV (C-DISC-4.0) (Shaffer et al., 2000). Ongoing studies at the Center for Behavioral Teratology include an overrepresentation of non-exposed children who are recruited based on symptoms of ADHD. To maintain a representative typically developing control group for the current study, subjects were included in the CON group if they did not meet criteria for ADHD on the C-DISC-4.0. In the AE group, 49 (73%) children met ADHD criteria, which is consistent with the literature (Fryer et al., 2007, Bhatara et al., 2006, Burd et al., 2003).
Procedure
Neuropsychological and Behavioral Measures
As part of the standardized neuropsychological battery, children completed four academic subtests of the Wechsler Individual Achievement Test-2nd Edition (WIAT-II) (Wechsler, 2005). The WIAT-II is normed on a national sample representative of the United States population with appropriate psychometric properties. The dependent variables used in the current analysis were the age-standardized scaled scores for word reading, spelling, numerical operations, math reasoning subtests and the math composite score, see Table 1. Full scale IQ (FSIQ) scores were calculated using the Wechsler Intelligence Scale for Children-Fourth Edition (WISC-IV; Wechsler, 2004) and a parental report of behavioral concerns was obtained through the Child Behavior Checklist (CBCL; Achenbach and Rescorla, 2001).
Table 1.
Description of Wechsler Individual Achievement Test – Second Edition (WIAT-II) subtests used to assess academic performance.
| Subtest | Description |
|---|---|
| Word Reading | Assesses single oral word reading fluency |
| Spelling | Assesses the ability to provide written spelling of dictated words used in a sentence |
| Numerical Operations | Assesses the ability to conduct paper and pencil computations of addition, subtraction, multiplication, and division |
| Math Reasoning | Assesses the ability to reason mathematically by solving word problems that are simultaneously read out loud and presented visually |
|
| |
| Math Composite | A standardized composite score of the Numerical Operations and Math Reasoning subtests on the WIAT-II. |
Neuroimaging Methodology
A subset of the total sample, those within the age range of 13–16 who could tolerate imaging and did not have any contraindications to the MRI (AE: n=26, CON: n=16) completed structural neuroimaging to assess potential brain-behavior relations for academic functioning. All MRI sessions were conducted within 180 days of the neuropsychological assessment (M: 18.27 days, SD: 22.00). A high-resolution T1-weighted anatomical image was acquired for each subject using a 3T GE Signa Excite scanner and an 8-channel head coil (TR, 8 ms; TE, 3.1 ms; flip angle 8°; matrix 256 × 256 × 192; FOV, 256 × 256 mm; acquisition time, 7 min and 24 s). Structural images were processed using FreeSurfer v5.3 software, http://surfer.nmr.mgh.harvard.edu; (Fischl et al., 2002). FreeSurfer’s technical details have been described previously (Dale et al., 1999, Fischl and Dale, 2000, Fischl et al., 1999). Briefly, the process includes removal of non-brain tissue, automated Talairach transformation, segmentation of subcortical structures, intensity normalization, tessellation of the grey/white matter boundary, topology correction, surface deformation, surface inflation, registration to a spherical atlas based on individual cortical folding patterns, parcellation of the cerebral cortex, and creation of surface based data. All images were visually inspected to ensure quality and, if necessary, manually edited to correct errors and reprocessed (n=2). Cortical structure was examined with both thickness and surface area, as these variables follow distinct developmental trajectories (Wierenga et al., 2014). This approach also prevented potential signal dilution errors that occur in measuring their product (volume) (Migliorini et al., 2015). Furthermore, recent work from our laboratory suggests that surface area is a sensitive index to the teratogenicity of prenatal alcohol exposure (Gross et al., 2016). Cortical maps were spatially smoothed with a Gaussian kernel of 10 mm full width half max and averaged across subjects using a spherical aligning method for cortical folding patterns.
Statistical Analyses
Statistical analyses were conducted using SPSS v.22. Data for the WIAT-II tasks were assessed for outliers using boxplot analysis (1.5xIQR). There was one outlier for word reading (1 AE), two outliers for spelling (2 CON), and two outliers for math reasoning (2 CON). There were also three outliers for the math composite (2 AE, 1 CON). When possible, we removed outliers from analyses only for the measure for which they were an outlier. This was not possible for the repeated measure analysis (if a subject score was an outlier on any one of the four domains included it was removed entirely, resulting in removing 3 AE and 5 CON from that analysis). For all outliers across groups, the outlier performance was below expectations, with the exception of the control subject for the math composite. Also, by chance, all outliers were independent (i.e., the same child was not an outlier on multiple subtests). The same boxplot procedure was conducted to identify outliers prior to the imaging analyses, finding two outliers for surface area (1 AE, 1 CON) and none for thickness. Subjects who were outliers based on behavioral data were also excluded form the imaging subsample (1 CON from math reasoning, 1 AE from math composite).
Demographic Information and Group Differences
Demographic information was analyzed for the total sample and the imaging subsample using chi-square statistics for categorical variables (race, ethnicity, sex, handedness) and analysis of variance (ANOVA) statistics for continuous variables [age, socioeconomic status (SES) as measured by Hollingshead (Hollingshead, 2011), FSIQ, and CBCL total behavioral problems]. Given their potential relation to academic achievement, age, race, ethnicity, SES, and sex were considered as possible covariates and included if they were significantly related to the dependent variables (WIAT-II variables or cortical structure – thickness or surface area) and did not interact with group membership. Alpha was set at p<.05 for all analyses. Significant omnibus tests were followed up with pairwise comparisons using Bonferroni correction.
Presence and Profile
To assess presence of group differences and the profile of performance across domains, a repeated-measures MANCOVA was conducted with Group (AE, CON) as the between-subjects variable and academic domain (word reading, spelling, numerical operations, math reasoning) as the within-subject variable with covariates included as appropriate. Group differences on the math composite variable were analyzed in a separate ANOVA, as it comprises numerical operations and math reasoning variables. Within the AE group, we compared performance for individuals with FAS and individuals without FAS on all academic domain variables and found no significant differences (p≥.094); therefore, all were included in a combined AE group.
Considerations for Identification
Identification of learning difficulties varies widely (i.e., cut-off scores, response to intervention, failure of standardized assessments). To compare the number of students with low achievement in the alcohol-exposed and control groups and balance sensitivity and specificity, five different criteria were selected from the literature (Fletcher et al., 2002). Cut-off scores from the normative sample for the WIAT-II standard scores of 1 SD (SS<85), 1.5 SD (SS<77.5), and 2 SD (SS<70) were utilized. The WIAT-II also offers the following mutually exclusive descriptors: Average (SS=90–109), Low Average (SS=80–89), Borderline (SS=70–79), and Extremely Low (SS≤69). The Extremely Low criterion is captured by the cutoff of 2 SDs described above. Thus, the rates of individuals meeting the WIAT-II Low Average and meeting the Borderline criteria were examined, respectively. Differences between groups in proportional rates or number of children meeting the criteria were analyzed using chi-square analyses.
Relations of Behavior and General Intelligence
The relations between academic functioning and behavior or general intelligence were tested with within-group Pearson correlations calculated between the WIAT-II subtest standard scores and both the CBCL Total Problems score and WISC-IV FSIQ.
Neural Correlates
Neural correlates were tested using FreeSurfer’s Qdec application, which fits a general linear model at each vertex. A total of 10 separate models were created, testing the relations between cortical thickness and surface area with five WIAT-II variables (word reading, spelling, numerical operations, math reasoning, and the math composite). Our primary interest was evaluating the relations between academic achievement and brain structure in alcohol-exposed versus control children. However, sex and age both can impact brain structure. Therefore, we accounted for these variables in our analyses; the discrete factors were group and sex and age was included as a nuisance factor. The right and left hemispheres were analyzed separately. All analyses were corrected for multiple comparisons using the Monte Carlo cluster-wise simulation with a threshold set at p<0.05. Significant clusters were followed-up using correlation analyses.
Results
Demographic Information
Demographic data for the total and imaging samples are presented in Table 2. For the total sample, there were no significant group differences on age, sex, SES, handedness, ethnicity, or race (ps>.152). Consistent with previous studies, the AE group had significantly lower FSIQ scores [F(1, 127)=69.771, p<.001] and significantly higher CBCL Total Problem T scores, [F(1, 126)=164.935, p<.001] than the CON group. Similar demographic results were found in the imaging subsample.
Table 2.
Descriptive data for demographic information, WIAT-II variables for children with heavy prenatal alcohol exposure (AE) and controls (CON).
| Full Sample | Imaging Subsample | |||
|---|---|---|---|---|
|
| ||||
| Variable | AE (n = 67) | CON (n = 61) | AE (n = 26) | CON (n = 16) |
| Sex [n (% Female)] | 23 (34.3) | 28 (45.9) | 7 (26.9) | 6 (37.5) |
| Handedness [n (% Right)] | 54 (80.6) | 56 (91.8) | 23 (88.5) | 15 (93.8) |
| Race [n (% White)] | 44 (65.7) | 46 (75.4) | 15 (57.7) | 9 (56.3) |
| Ethnicity [n (% Hispanic)] | 14 (20.9) | 15 (24.6) | 7 (26.9) | 3 (18.8) |
| Age [M (SD)] | 12.7 (3.23) | 12.6 (3.06) | 15.0 (1.11) | 15.2 (1.37) |
| SES [M (SD)] | 47.0 (13.30) | 49.1 (12.80) | 45.2 (13.82) | 47.9 (11.03) |
| FSIQ [M (SD)] a,b | 86.1 (15.59) | 107.7 (13.31) | 86.6 (15.66) | 102.4 (13.97) |
| ADHD [n (% Positive)] | 49 (73.1) | 0 (0.0) | 19 (73.1) | 0 (0.0) |
| CBCL [M (SD)] a,b | 65.6 (10.91) | 42.0 (9.62) | 63.8 (12.11) | 40.6 (6.80) |
| Days between test and scan [M (SD)] b | -- | -- | 11.3 (18.40) | 29.8 (23.19) |
| FAS [n (%)] | 12 (17.9) | 0 (0.0) | 6 (23.1) | 0 (0.0) |
|
| ||||
| Word Reading [M (SD)]a | 94.7 (17.32) | 105.9 (13.03) | 94.7 (18.50) | 101.6 (13.08) |
| Spelling [M (SD)]a | 93.7 (18.06) | 103.4 (14.35) | 94.6 (17.77) | 99.6 (11.51) |
| Numerical Operations [M (SD)] a,b | 88.8 (18.61) | 110.4 (15.58) | 88.3 (20.70) | 103.8 (13.45) |
| Math Reasoning [M (SD)] a,b | 85.1 (18.13) | 109.4 (15.41) | 83.3 (19.15) | 99.5 (12.80) |
|
| ||||
| Math Composite [M (SD)] a,b | 86.7 (18.10) | 110.8 (16.3) | 85.8 (19.94) | 101.5 (12.82) |
Significant group difference in the total sample (AE < CON, p < .05)
Significant group difference in the total sample (AE < CON, p < .05)
SES, Socioeconomic status, measured by Hollingshead; FSIQ, Full scale IQ composite score from the WISC-IV; ADHD, from a positive diagnosis on the the C-DISC-4.0; CBCL, Child Behavior Checklist Total Problem T-Score; FAS, Fetal Alcohol Syndrome.
Assessment of Demographic Variables and Covariates
The appropriateness of including theoretically implicated demographic variables as covariates was evaluated for all academic domains. Sex, handedness, ethnicity, and race were not correlated significantly with any academic domains (ps≥.066). SES was significantly correlated with word reading and numerical operations (ps<.009) and age was significantly associated with math reasoning (p=.028). The homogeneity of regression assumption was not violated for SES or age and therefore both were included as covariates in the repeated measures MANCOVA. The relations of FSIQ and CBCL total behavior to outcome variables were explored in additional correlation analyses.
Presence and Profile
A 2 × 4 repeated measures MANCOVA with group (AE, CON) as the between-subjects factor and academic domain (word reading, spelling, numerical operations, math reasoning) as the within-subjects factor was conducted, with SES and age included as covariates. Alcohol-exposed children performed worse than controls on all measures of academic functioning. Further, differential profiles of performance emerged by group, indicating distinct strengths and weaknesses. Descriptive data are presented in Table 2.
Using an alpha level of .001 to evaluate homogeneity assumptions, Box’s M test of homogeneity of covariance (p=.337) and Levene’s homogeneity test (ps>.018) were not statistically significant. Mauchly’s test of spherecity indicated that the sphericity assumption had been violated (p≤.001), therefore, degrees of freedom were corrected using the Greenhouse-Geisser estimate. There was not a significant interaction between SES and academic domain, nor a main effect of SES or age (ps>.064), though there was a significant interaction between age and academic domain (p=.026).
The interaction between group and academic domain was significant, F(2.397, 285.224)=21.343, p<.001, ηp2 =.152. Follow-up pairwise comparisons were conducted to assess differential profiles of functioning by group. Bonferroni correction was used to correct for multiple comparisons [(6 comparisons), alpha=0.05/6=0.0083]. Within the alcohol-exposed group, children performed significantly worse on math reasoning compared to the three other subtests (ps<.007). Performance on the numerical operations subtest was significantly higher than math reasoning subtest (p=.007), but lower than word reading and spelling (ps≤.008), which did not differ statistically (p=.409). In the control group, spelling scores were significantly lower than both math subtests (ps≤.001), with no other significant differences (ps≥.016). In addition to the significant interaction, there was a significant main effect of group, F(1, 119)=43.188, p<.001, np2 =.266. Follow up univariate ANOVA indicated that alcohol-exposed youth performed significantly lower (ps≤.001) than control children for all four subtests. Group differences on the math composite were conducted as a separate ANOVA. There was a significant effect of group, with the AE group performing significantly worse than controls, F(1, 123)=61.130, p<.001, np2 =.332.
Considerations for Identification
The number of students experiencing academic difficulties was compared using chi-square tests across groups (AE, CON) for the five criteria. Overall, there were significantly higher rates of individuals meeting criteria for impairment across criteria in the AE group compared to the CON group, though this varied based on definition and academic domain, see Table 3. The alcohol-exposed group had significantly higher percentages of individuals meeting criteria for deficits across all five criteria for the math composite (χ2s≥7.479, ps≤.006) compared to the control group. This was consistent with the higher rates for the alcohol-exposed group for math reasoning across all five definitions (χ 2s≥5.802, ps≤.016) and numerical operations, where there were significantly higher rates of impairment for alcohol-exposed individuals using all criteria (χ 2s≥9.264, ps≤.002) except Low Average (χ 2=.029, p=.864). For spelling, rates of individuals meeting the criteria were higher in the alcohol-exposed group for 1 SD and 1.5 SD cutoffs (χ 2s ≥7.375, ps≤ .043). For word reading, there was a significantly higher number of those meeting the cutoff in the alcohol-exposed group individuals compared to the control group for the 1 SD cutoff and Borderline criteria (χ 2s≥4.191, ps≤.041). More than half of the alcohol-exposed group (58.2%) demonstrated met criteria for academic difficulty based on a 1SD cutoff on one or more academic domains (word reading, spelling, numerical operations, math reasoning), which was significantly higher than the 16.4% of the control sample (z=4.861, p<.001). See Table 4.
Table 3.
Number and percentage of individuals with deficits based on various definitions for children with heavy prenatal alcohol exposure (AE) and controls (CON).
| AE | CON | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Criteria | Word Reading | Spelling | Numerical Operations | Math Reasoning | Math Composite | Word Reading | Spelling | Numerical Operations | Math Reasoning | Math Composite |
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | |
| < 1SD (SS<85) | 16 (24.2)** | 21 (31.3)** | 24 (35.8)*** | 31 (46.3)*** | 28 (43.1)*** | 4 (6.6) | 5 (19.2) | 3 (4.9) | 2 (3.4) | 4 (6.7) |
|
| ||||||||||
| < 1.5 SDs (SS<77.5) | 9 (13.6) | 11 (16.4)* | 22 (32.8)*** | 22 (32.8)*** | 19 (29.2)*** | 3 (4.9) | 3 (5.1) | 1 (1.6) | 1 (1.7) | 2 (3.3) |
|
| ||||||||||
| < 2 SDs (SS<70)1 | 4 (6.1) | 6 (9.0)* | 11 (16.4)** | 13 (19.4)*** | 10 (15.4)** | 1 (1.6) | 0 (0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
|
| ||||||||||
| WIAT-II “Low Average” (SS=80–89) | 8 (12.1) | 11 (16.4) | 6 (9.0) | 13 (19.4)* | 16 (24.6)** | 5 (8.2) | 4 (6.8) | 6 (9.8) | 3 (5.1) | 4 (6.7) |
|
| ||||||||||
| WIAT-II “Borderline” (SS=70–79) | 9 (13.6)* | 8 (11.9) | 12 (17.9)** | 12 (17.9)** | 12 (18.5)** | 2 (3.3) | 3 (5.1) | 1 (1.6) | 1 (1.7) | 2 (3.3) |
Also referred to as: “Extremely Low” for WIAT-II. Bold font indicates significant difference for AE < CON based on chi2 test.
Significant at *p< .05, **p< .01, ***p<.001. Respective outliers were removed by individual academic subtest, see text for details.
Table 4.
Number of children in the alcohol exposed group (AE) and control group (CON) that met criteria for impairment (<1SD from normative sample mean, SS<85).
| AE (n=67) | CON (n=61) | |
|---|---|---|
| Number of Domains <1SD from normative sample (SS<85) | ||
| 0 Domains [n (%)] | 28 (41.8) | 51 (83.6) |
| 1 Domain [n (%)] | 13 (19.4) | 5 (8.2) |
| 2 Domains [n (%)] | 8 (11.9) | 4 (6.6) |
| 3 Domains [n (%)] | 8 (11.9) | 0 (0.0) |
| 4 Domains [n (%)] | 10 (14.9) | 1 (1.6) |
|
| ||
| Sum (1+ domains <85) | 39 (58.2) | 10 (16.4) |
Does not include math composite as it comprises two subtests (numerical operations and math reasoning). Numbers are mutually exclusive (if a child met impaired criteria for 3 domains, they would not also meet criteria for meeting 1 domain), with exception of the sum across domains.
Relations of Behavior and General Intelligence
Pearson correlations between academic scores and FSIQ and CBCL scores are presented in Table 5. For both groups, FSIQ was strongly correlated with all variables for each group (r>.60), as expected. The CBCL total behavioral problem score was associated with math reasoning in both groups, the math composite in the AE group and word reading in the CON group. CBCL and FSIQ were not correlated for either group.
Table 5.
Correlations between WIAT-II variables and intelligence composite (FISQ) and behavioral concerns (CBCL) for children with heavy prenatal alcohol exposure (AE) and controls (CON).
| Full Scale IQ (Composite Score) | CBCL Total Score (T score) | |
|---|---|---|
| AE | ||
| Word Reading | .636*** | −205 |
| Spelling | .655*** | −166 |
| Numerical Operations | .636*** | −237 |
| Math Reasoning | .727*** | −249* |
| Math Composite | .659*** | −263* |
| CBCL Total Score | −059 | --- |
| CON | ||
| Word Reading | .656*** | −327* |
| Spelling | .605*** | −173 |
| Numerical Operations | .605*** | −169 |
| Math Reasoning | .702*** | −286* |
| Math Composite | .696*** | −221 |
| CBCL Total Score | −228 | --- |
Significant at *p< .05, **p< .01, ***p<.001. Respective outliers were removed by individual academic subtest, see text for details.
Neural Correlates
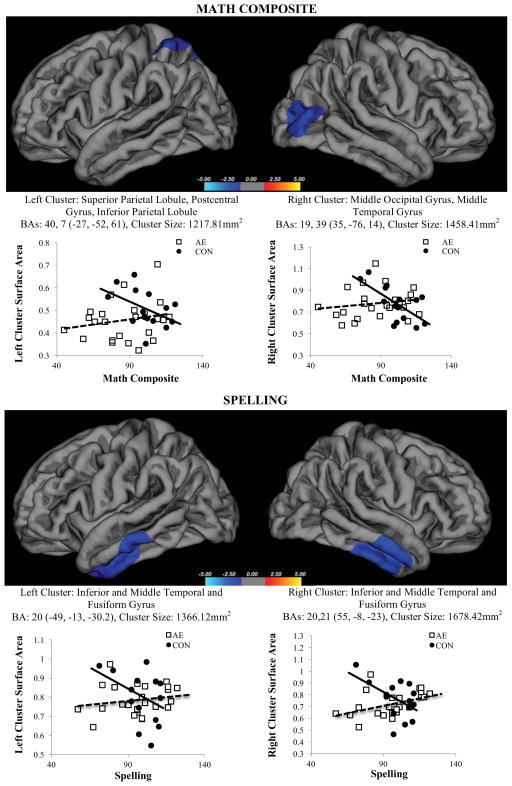
There were several surface area clusters that were differentially related to performance by group [math composite (1 RH cluster, 1 LH cluster), math reasoning (1 RH cluster), and spelling (1 RH cluster, 1 LH cluster). See Figure 1 for results. There were no clusters with significant interactions seen for word reading or numerical operations (though numerical operations had clusters that approximated the math composite, they did not withstand multiple correction). Differential relations were followed up using correlational analyses. The imaging analysis did not identify any cortical thickness clusters that were differentially related to group for any of the five WIAT-II variables. To confirm the specific neural correlates, we analyzed the coordinates for each cluster using the Talairach client (version 2.4.3) to identify the specific structures and Broadmann Area (BA) labels +/− 5 mm cube range (Lancaster et al., 2000).
Figure 1.
Two clusters (left superior parietal and right lateral/middle occipital gyrus) where there was a significant math (WIAT-II Math Composite) × group [alcohol exposure (AE), controls (CON) interaction]. Of note, the left cluster extends to the medial aspect. The right hemisphere math reasoning cluster is not shown as it approximates the right hemisphere math composite cluster. Two clusters (bilateral inferior and middle temporal, inferior gyrus) were also identified with a significant spelling (WIAT-II Spelling) × group interaction. Talairach coordinates, Broadmann area (BA) and area listed below. Follow up for each significant cluster are shown graphically.
For the math composite, there were two surface area clusters that were differentially related to performance by group, with an inverse relation in controls (better performance related to smaller surface area) and no relation present in the alcohol-exposed group. For the left cluster, the superior parietal lobule and postcentral gyrus, inferior parietal lobule (BAs 40 and 7) were identified (CON, r=−458; AE, r=.205), and for the right cluster, the middle occipital gryus, middle temporal gyrus (BAs 19 and 39) were identified (CON, r =−661; AE, r=.170).
For math reasoning, the analysis could not be conducted with the appropriate outlier (1 CON) excluded because the sample size was too small. Therefore, this analysis was run twice: both with the outlier included and with the subject’s data replaced by the group mean. In both cases the same significant cluster was identified in right hemisphere, the middle occipital gyrus and middle temporal gyrus (BAs 18, 19, 39). Relations remained the same in that cluster with the outlier included (CON, r=−401; AE, r=.182) and replaced by the mean (CON, r=−411; AE, r=.181).
For spelling, two significant clusters were identified. For the left cluster, the inferior and middle temporal and fusiform gyrus (BA 20) were identified (CON, r=−388; AE, r=.181), and for the right cluster, the inferior and middle temporal and fusiform (BAs 20, 21) were also identified (CON, r=−504; AE, r=.422).
Discussion
We aimed to better understand academic functioning in children with histories of heavy prenatal alcohol exposure. Approaching this question from multiple perspectives, we compared children with histories of alcohol-exposure to typically developing children and determined that there were unique profiles of strengths and weaknesses, with a relative weakness noted on a more complex math subtest for alcohol-exposed children. An examination of academic difficulties based on currently operationalized criteria revealed that math deficits were significantly more common for alcohol-exposed children compared to typically developing children across definitions, although there were significant group differences in other domains as well that varied based on the criteria used. Intellectual ability was related to academic performance in both groups, and behavioral concerns appeared particularly relevant for higher order math abilities. Additionally, differential relations between surface area and academic performance were identified in children with and without prenatal alcohol exposure.
Presence and Profile
Our results are consistent with previous literature which found that school-age children who have been prenatally exposed to alcohol perform significantly lower than typically developing peers across academic domains (Streissguth et al., 1990, Howell et al., 2006, O’Leary et al., 2013, Glass et al., 2015, Crocker et al., 2015). Children with prenatal exposure to alcohol demonstrated impairments on higher order mathematical reasoning as well as lower order functions such as basic numerical processing, basic calculations, proximity judgment, and cognitive estimation (Coles et al., 2009, Lebel et al., 2010, Crocker et al., 2015, Kopera-Frye et al., 1996, Meintjes et al., 2010; Jacboson et al., 2011). Using within-subjects methodology, we found that alcohol-exposed children performed worse on more complex and verbally mediated math tasks (math reasoning) compared to more simple calculations (numerical operations). This supports prior work indicating that alcohol-exposed children have greater difficultly on tasks with higher cognitive demands (Kodituwakku et al., 2006) and suggests that there is added difficulty in understanding math problems presented as word problems.
Verbal academic skills have not been as comprehensively examined. We found that alcohol-exposed children performed significantly worse than their demographically matched peers on spelling and reading tests, even after accounting for socioeconomic status and age. Prior research has found that alcohol-exposed children are impaired compared to controls on various aspects of verbal academic functioning (O’Leary et al., 2013, Streissguth et al., 1994a, Rose-Jacobs et al., 2012, Glass et al., 2015), however most studies, including ours, utilize fairly simple tasks such as single word oral word reading or basic spelling. Potentially, alcohol-exposed children may struggle more on complex or cognitively demanding tests of reading reasoning or comprehension and would show levels of impairment more similar to those observed on the more complex math measures.
Considerations for Identification
We found that across different criteria, there was a higher number and percentage of alcohol-exposed children who met criteria for academic difficulty on math subtests compared to typically developing children (almost four times the rate). If a cut-off of 1 SD is used, there are significant differences in numbers of individuals meeting criteria for all subtests, with higher rates in the alcohol-exposed group. Although the mean for a group may indicate average performance, considering the variability associated with prenatal alcohol exposure, understanding the rate of individuals meeting certain criteria may be another useful method of assessing performance. We found significant rates of academic dysfunction with over 50% of children having deficits on at least one domain, suggesting a critical area of need.
Determining the best methodology for identifying children at risk of academic failure or the rate of academic performance deficits is a convoluted question with various stakeholders who may have distinct motives, including the individual school, the parent, the teacher, national policy, and service providers. New criteria to identify learning disabilities codified in the DSM-5 (American Psychiatric Association, 2013) specify a definition using a low achievement cut-off (~1 to 2.5SD). Very few studies have examined the prevalence rates of learning disorders in children with histories of alcohol exposure, however studies report estimates of 17% – 35% of alcohol-exposed children have a diagnosis of a learning disorder, second only to the rate of ADHD which is estimated at between 40–90% in this population (Bhatara et al., 2006, Burd et al., 2003, Fryer et al., 2007). This is an important area of investigation as learning deficits are included in the criteria for the proposed diagnosis of neurobehavioral disorder associated with prenatal alcohol exposure (ND-PAE) (American Psychiatric Association, 2013) and satisfy the neurobehavioral impairment criteria in the updated clinical guidelines for diagnsoing fetal alcohol spectrum disorders (Hoyme et al., 2016).
Relations of Behavior and General Intelligence
There are complex interactions between cognitive ability, behavioral functioning, socio-emotional functioning, attention deficits, concomitant psychopathology, environmental supports, and access to services, all of which can influence academic outcomes (Kable et al., 2015). Academic functioning was highly correlated with general intelligence in both groups. While the alcohol-exposed group may have academic performance commensurate with their cognitive abilities, there is evidence of successful intervention in other neurodevelopmental populations (Allor et al., 2010) and in children with prenatal alcohol exposure (Kable et al., 2015), despite having lower intellectual functioning. Likewise, many children have behavioral concerns that contribute to disrupted school experiences (Burd et al., 2003, Kable et al., 2015, Streissguth et al., 2004). While we expected that behavioral concerns would be inversely correlated with academic performance for all domains, we found this was true for math in all children, and word reading in typically developing children. While correlation is non-directional, it may suggest that academic problems may persist even if behavioral issues are managed independently.
Neural Correlates
Consistent with the notion of math being particularly impaired in this population both in terms of the profile and increased rates of deficits, we identified several cortical surface area regions that were differentially related for controls versus alcohol-exposed children for math performance. Further, spelling was associated with two bilateral cortical regions in the temporal cortices. Reading, while impaired in alcohol-exposed children relative to controls, did not appear to have cortical surface area correlates and may relate to broader neural circuitry dysfunction or differential trajectories (Houston et al., 2014, Ashkenazi et al., 2013). Further, there were no differential associations with cortical thickness, suggesting it may not be as sensitive an indicator for prenatal alcohol exposure (Gross et al., 2016) or the use of an alcohol-exposure group may not be as sensitive in detecting cortical effects of thickness as other continuous measures of alcohol exposure (Meintjes et al., 2014, Roberston et al., 2016).
The extant literature suggests that the parietal lobe, in particular the inferior parietal sulcus and superior parietal lobule, are associated with math (Dehaene et al., 2003, Ashkenazi et al., 2013), and the temporal lobe (both the temporal-parietal junction and inferior frontal gyrus) and perisylvian cortex are associated with spelling (Heim and Keil, 2004; Cloutman et al., 2009). We found a differential relation in the superior parietal lobe for math and inferior/middle temporal and fusiform gyrus for spelling. In typically developing children, scores increased as surface area decreased in these regions. Longitudinal studies have shown that during typical adolescent development cortical surface area reductions occur as one ages, due in part to synaptic pruning and elimination that occurs during adolescence. Thus, a smaller cortical surface area may be a structural phenotype that is more mature and efficient (Wierenga et al., 2014). For math, we also found a differential relation in the lateral middle occipital gyrus, which was trending towards a similar negative relation in the control group, similar to other findings in the ventral occipitotemporal cortex (Ashkenazi et al., 2013) which may subserve more visually mediated math functioning.
In the alcohol-exposed group, the negative relations between surface area and performance seen in controls were not observed. There was generally no relation between surface area and math performance, and a positive relation for spelling performance in the right hemisphere. Relations between brain structure and function have been seen in terms of recruiting broader networks for numerical tasks and significant correlations between fractional anisotropy and math scores in the left parietal and left cerebellum for alcohol-exposed children, however these studies did not assess relations with spelling or reading (Lebel et al., 2010, Meintjes et al., 2010, Santhanam et al., 2009). It is possible that the alcohol-exposed children have a delayed or atypical trajectory of brain development in areas contributing to poor performance (Ashkenazi et al., 2013, Moore et al., 2014) or the effect size may be smaller than we have power to identify.
Limitations
While this is one of the first studies to examine academic functioning within the alcohol-exposed population in a comprehensive manner, these results must be interpreted in light of several limitations. All data were collected cross-sectionally so it is difficult to comment on the trajectory of academic deficits or expected outcomes, although we did correct for age in all analyses. Further, there are other factors that may influence academic achievement that were not explicitly assessed including quality of education, other comorbid psychiatric or medical diagnoses, home environment, history of stress or trauma, and classroom placement which are each important mediators of academic functioning and should be included in future studies.
Previous research has found family stability can be a critical protective factor for helping children with histories of prenatal alcohol exposure avoid adverse life outcomes (Streissguth et al., 2004). It is important to consider the inherent influence of environment on all outcomes, including academic performance. Additional research in this area should include methodology to investigate the influence of home environment on long-term outcomes and academic functioning. As the majority of children with prenatal alcohol exposure in our sample were no longer living with their biological parents, it is not possible to definitively separate the effects of prenatal alcohol exposure from that of adoption or foster care placements on school performance.
Many children with histories of heavy prenatal alcohol exposure also have other diagnoses, most commonly ADHD, which may also be associated with academic deficits in certain domains (Semrud-Clikeman et al., 1992). Given the study design it is difficult to disentangle the degree to which academic performance is affected by prenatal alcohol exposure or ADHD, respectively. Therefore, we are limited in concluding specific causal relations regarding the etiology of academic performance as there are a variety of confounding factors. To partially evaluate the relations with ADHD and behavioral concerns, we used the CBCL as a metric of behavioral functioning, however future studies with larger samples sizes should address the contribution of concurrent psychopathology on academic functioning by investigating heterogeneous control or comparison groups to more systematically evaluate unique influences of factors contributing to academic functioning. Information from these factors and direct comparisons with other clinical populations (both with similar behavioral and intellectual profiles) may better inform the precise etiology of academic achievement deficits and also provide insight regarding effective interventions. In addition, many states use standardized testing for all students and these measures are normed across the United States in many different settings, school districts, and classrooms, and therefore having natural variability within our AE group may facilitate generalization.
While the WIAT-II subtests are co-normed, there are inherent differences between the subtests, with the math reasoning subtest having a limited time to respond, and relying heavily on verbally mediated instructions and working memory compared to the oral word reading test. These differences may contribute to the discrepancy in performance between academic domains. The ideal method for understanding the academic profile may be to have ‘light cognitive load’ and ‘heavy cognitive load’ tasks for both reading and math to compare equivalent performances while minimizing the need for overlapping skills (word problems for math) (Moll et al., 2014).
Conclusion
This study extends previous findings of academic impairment in this population relative to controls and adds information regarding the profile of strengths and weaknesses within alcohol-exposed children by using within-subjects methodology. It substantiates the relatively sparse literature in the underlying neural correlates associated with alcohol-related dysfunction in academic achievement. As the definitions of learning disorders are implemented differently in each school district, uniformly identifying children with learning deficits may be challenging. However, regardless of the definition chosen, children with histories of prenatal alcohol exposure appear to have significantly higher rates of academic difficulties, which may necessitate additional attention and support.
Acknowledgments
Research described in this paper was supported by NIAAA grant number AA019605. Additional support was provided by F31 AA022261, K99 AA022661, and T32 AA013525. NA was supported by HD075865, DA038958, HD075489, AA019879, and HD061414. EPR was supported by U24 AA014811. SNM was supported by U01 AA014834. The authors would like to thank the families who graciously participate in our studies. The authors have no financial or other conflicts of interest.
References
- Achenbach TM, Rescorla LA. Manual for the ASEBA School-Age Forms & Profiles. University of Vermont, Research Center for Children, Youth, & Families; Burlington, VT: 2001. [Google Scholar]
- Allor JH, Mathes PG, Roberts JK, Cheatham JP, Champlin TM. Comprehensive reading instruction for students with intellectual disabilities: Findings from the first three years of a longitudinal study. Psychol Schools. 2010;47:445–466. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders - Fifth Edition, DSM-5. American Psychiatric Publishing, Inc; Arlington, VA: 2013. [Google Scholar]
- Ashkenazi S, Black JM, Abrams DA, Hoeft F, Menon V. Neurobiological underpinnings of math and reading learning disabilities. J Learn Disabil. 2013;46:549–569. doi: 10.1177/0022219413483174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatara V, Loudenberg R, Ellis R. Association of attention deficit hyperactivity disorder and gestational alcohol exposure: an exploratory study. J Atten Disord. 2006;9:515–522. doi: 10.1177/1087054705283880. [DOI] [PubMed] [Google Scholar]
- Burd L, Klug MG, Martsolf JT, Kerbeshian J. Fetal alcohol syndrome: neuropsychiatric phenomics. Neurotoxicology and Teratology. 2003;25:697–705. doi: 10.1016/j.ntt.2003.07.014. [DOI] [PubMed] [Google Scholar]
- Chasnoff IJ, Wells AM, King L. Misdiagnosis and missed diagnoses in foster and adopted children with prenatal alcohol exposure. Pediatrics. 2015;135:264–270. doi: 10.1542/peds.2014-2171. [DOI] [PubMed] [Google Scholar]
- Cloutman L, Gingis L, Newhart M, Davis C, Heidler-Gary J, Crinion J, Hillis AE. A neural network critical for spelling. Annals of Neurology. 2009;66:249–253. doi: 10.1002/ana.21693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles CD, Kable JA, Taddeo E. Math performance and behavior problems in children affected by prenatal alcohol exposure: Intervention and follow-up. J Dev Behav Pediatr. 2009;30:7–15. doi: 10.1097/DBP.0b013e3181966780. [DOI] [PubMed] [Google Scholar]
- Crocker N, Riley EP, Mattson SN. Visual-spatial abilities relate to mathematics achievement in children with heavy prenatal alcohol exposure. Neuropsychology. 2015;29:108–116. doi: 10.1037/neu0000094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. segmentation and surface reconstruction. NeuroImage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Piazza M, Pinel P, Cohen L. Three parietal circuits for number processing. Cogn Neuropsychol. 2003;20:487–506. doi: 10.1080/02643290244000239. [DOI] [PubMed] [Google Scholar]
- Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci USA. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: inflation, flattening, and a surface-based coordinate system. NeuroImage. 1999;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Fletcher JM, Foorman BR, Boudousquie A, Barnes MA, Schatschneider C, Francis DJ. Assessment of reading and learning disabilities: a research-based intervention-oriented approach. J School Psychol. 2002;40:27–63. [Google Scholar]
- Fryer SL, McGee CL, Matt GE, Riley EP, Mattson SN. Evaluation of psychopathological conditions in children with heavy prenatal alcohol exposure. Pediatrics. 2007;119:e733–e741. doi: 10.1542/peds.2006-1606. [DOI] [PubMed] [Google Scholar]
- Glass L, Graham DM, Akshoomoff N, Mattson SN. Cognitive factors contributing to spelling performance in children with prenatal alcohol exposure. Neuropsychology. 2015;29:817–828. doi: 10.1037/neu0000185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross L, Moore EM, Coles CD, Kable JA, Sowell ER, Wozniak JR, Riley EP, Mattson SN and the CIFASD. Neural correlates of verbal memory in youth with heavy prenatal alcohol exposure. 2016 doi: 10.1007/s11682-017-9739-2. Manuscript submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim S, Keil A. Large-scale neural correlates of developmental dyslexia. Eur Child Adoles Psy. 2004;13(3):125–140. doi: 10.1007/s00787-004-0361-7. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB. Four Factor Index of Social Status. Yale Journal of Sociology. 2011;8:21–51. [Google Scholar]
- Houston SM, Lebel C, Katzir T, Manis FR, Kan E, Rodriguez GG, Sowell ER. Reading skill and structural brain development. Neuroreport. 2014;25:347–352. doi: 10.1097/WNR.0000000000000121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell KK, Lynch ME, Platzman KA, Smith GH, Coles CD. Prenatal alcohol exposure and ability, academic achievement, and school functioning in adolescence: a longitudinal follow-up. J Pediatr Psychol. 2006;31:116–126. doi: 10.1093/jpepsy/jsj029. [DOI] [PubMed] [Google Scholar]
- Hoyme HE, Kalberg WO, Elliott AJ, Blankenship J, Buckley D, Marais AS, Manning MA, Robinson LK, Adam MP, Abdul-Rahman O, Jewett T. Updated clinical guidelines for diagnosing fetal alcohol spectrum disorders. Pediatrics. 2016;138:e20154256. doi: 10.1542/peds.2015-4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson JL, Dodge NC, Burden MJ, Klorman R, Jacobson SW. Number processing in adolescents with prenatal alcohol exposure and ADHD: differences in the neurobehavioral phenotype. Alcohol Clin Exp Res. 2011;35:431–442. doi: 10.1111/j.1530-0277.2010.01360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KL, Robinson LK, Bakhireva LN, Marintcheva G, Storojev V, Strahova A, Sergeevskaya S, Budantseva S, Mattson SN, Riley EP, Chambers CD. Accuracy of the diagnosis of physical features of fetal alcohol syndrome by pediatricians after specialized training. Pediatrics. 2006;118:e1734–1738. doi: 10.1542/peds.2006-1037. [DOI] [PubMed] [Google Scholar]
- Kable JA, Taddeo E, Strickland D, Coles CD. Community translation of the Math Interactive Learning Experience Program for children with FASD. Res Dev Disabil. 2015;39:1–11. doi: 10.1016/j.ridd.2014.12.031. [DOI] [PubMed] [Google Scholar]
- Kerns KA, Don A, Mateer CA, Streissguth AP. Cognitive deficits in nonretarded adults with fetal alcohol syndrome. J Learn Disabil. 1997;30:685–693. doi: 10.1177/002221949703000612. [DOI] [PubMed] [Google Scholar]
- Kodituwakku P, Coriale G, Fiorentino D, Aragon AS, Kalberg WO, Buckley D, Gossage JP, Ceccanti M, May PA. Neurobehavioral characteristics of children with fetal alcohol spectrum disorders in communities from Italy: preliminary results. Alcohol Clin Exp Res. 2006;30:1551–1561. doi: 10.1111/j.1530-0277.2006.00187.x. [DOI] [PubMed] [Google Scholar]
- Kopera-Frye K, Dehaene S, Streissguth AP. Impairments of number processing induced by prenatal alcohol exposure. Neuropsychologia. 1996;34:1187–1196. doi: 10.1016/0028-3932(96)00043-7. [DOI] [PubMed] [Google Scholar]
- Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, Kochunov PV, Nickerson D, Mikiten SA, Fox PT. Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp. 2000;10:120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C, Rasmussen C, Wyper K, Andrew G, Beaulieu C. Brain microstructure is related to math ability in children with fetal alcohol spectrum disorder. Alcohol Clin Exp Res. 2010;34:354–363. doi: 10.1111/j.1530-0277.2009.01097.x. [DOI] [PubMed] [Google Scholar]
- Mautone JA, Dupaul GJ, Jitendra AK, Tresco KE, Junod RV, Volpe RJ. The relationship between treatment integrity and acceptability of reading interventions for children with attention-deficit/hyperactivity disorder. Psychol Schools. 2009;46:919–931. [Google Scholar]
- May PA, Baete A, Russo J, Elliott AJ, Blankenship J, Kalberg WO, Buckley D, Brooks M, Hasken J, Abdul-Rahman O, Adam MP, Robinson LK, Manning M, Hoyme HE. Prevalence and characteristics of fetal alcohol spectrum disorders. Pediatrics. 2014;134:855–866. doi: 10.1542/peds.2013-3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Keaster C, Bozeman R, Goodover J, Blankenship J, Kalberg WO, Buckley D, Brooks M, Hasken J, Gossage JP, Robinson LK, Manning M, Hoyme HE. Prevalence and characteristics of fetal alcohol syndrome and partial fetal alcohol syndrome in a Rocky Mountain Region City. Drug Alcohol Depen. 2015;155:118–127. doi: 10.1016/j.drugalcdep.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meintjes EM, Jacobson JL, Molteno CD, Gatenby JC, Warton C, Cannistraci CJ, Hoyme HE, Robinson LK, Khaole N, Gore JC, Jacobson SW. An fMRI study of number processing in children with fetal alcohol syndrome. Alcohol Clin Exp Res. 2010;34:1450–1464. doi: 10.1111/j.1530-0277.2010.01230.x. [DOI] [PubMed] [Google Scholar]
- Meintjes EM, Narr KL, van der Kouwe AJW, Molteno CD, Pirnia T, Gutman B, Woods RP, Thompson PM, Jacobson JL, Jacobson SW. A tensor-based morphometry analysis of regional differences in brain volume in relation to prenatal alcohol exposure. NeuroImage: Clinical. 2014;5:152–160. doi: 10.1016/j.nicl.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliorini R, Moore EM, Glass L, Infante MA, Tapert SF, Jones KL, Mattson SN, Riley EP. Anterior cingulate cortex surface area relates to behavioral inhibition in adolescents with and without heavy prenatal alcohol exposure. Behav Brain Res. 2015;292:26–35. doi: 10.1016/j.bbr.2015.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll K, Kunze S, Neuhoff N, Bruder J, Schulte-Körne G. Specific learning disorder: prevalence and gender differences. PloS One. 2014;9:e103537. doi: 10.1371/journal.pone.0103537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore EM, Migliorini R, Infante MA, Riley EP. Fetal alcohol spectrum disorders: recent neuroimaging findings. Curr Dev Disord Rep. 2014;1:161–172. doi: 10.1007/s40474-014-0020-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Leary CM, Taylor C, Zubrick SR, Kurinczuk JJ, Bower C. Prenatal alcohol exposure and educational achievement in children aged 8–9 years. Pediatrics. 2013;132:e468–475. doi: 10.1542/peds.2012-3002. [DOI] [PubMed] [Google Scholar]
- Robertson FC, Narr KL, Molteno CD, Jacobson JL, Jacobson SW, Meintjes EM. Prenatal Alcohol Exposure is Associated with Regionally Thinner Cortex During the Preadolescent Period. Cerebral Cortex. 2016;26:3083–3095. doi: 10.1093/cercor/bhv131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose-Jacobs R, Augustyn M, Beeghly M, Martin B, Cabral HJ, Heeren TC, Richardson MA, Frank DA. Intrauterine substance exposures and Wechsler Individual Achievement Test-II scores at 11 years of age. Vulnerable Children and Youth Studies: An International Interdisciplinary Journal for Research, Policy and Care. 2012;7:186–197. [Google Scholar]
- Sampson PD, Streissguth AP, Barr HM, Bookstein FL. Neurobehavioral effects of prenatal alcohol: Part II. Partial least squares analysis. Neurotoxicology and Teratology. 1989;11:477–491. doi: 10.1016/0892-0362(89)90025-1. [DOI] [PubMed] [Google Scholar]
- Santhanam P, Li Z, Hu X, Lynch ME, Coles CD. Effects of prenatal alcohol exposure on brain activation during an arithmetic task: an fMRI study. Alcohol Clin Exp Res. 2009;33:1901–1908. doi: 10.1111/j.1530-0277.2009.01028.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semrud-Clikeman M, Biederman J, Sprich-Buckminster S, Lehman BK, Faraone SV, Norman D. Comorbidity between ADDH and learning disability: a review and report in a clinically referred sample. J Am Acad Child Psy. 1992;31:439–448. doi: 10.1097/00004583-199205000-00009. [DOI] [PubMed] [Google Scholar]
- Shaffer D, Fisher P, Lucas CP, Dulcan MK, Schwab-Stone ME. NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): description, differences from previous versions, and reliability of some common diagnoses. J Am Acad Child Psy. 2000;39:28–38. doi: 10.1097/00004583-200001000-00014. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Delis D, Stiles J, Jernigan TL. Improved memory functioning and frontal lobe maturation between childhood and adolescence: A structural MRI study. Journal of the International Neuropsychological Society. 2001;7(3):312–322. doi: 10.1017/s135561770173305x. [DOI] [PubMed] [Google Scholar]
- Streissguth AP, Barr HM, Sampson PD. Moderate prenatal alcohol exposure: effects on child IQ and learning problems at age 7 1/2 years. Alcohol Clin Exp Res. 1990;14:662–669. doi: 10.1111/j.1530-0277.1990.tb01224.x. [DOI] [PubMed] [Google Scholar]
- Streissguth AP, Barr HM, Carmichael Olson H, Sampson PD, Bookstein FL, Burgess DM. Drinking during pregnancy decreases word attack and arithmetic scores on standardized tests: adolescent data from a population-based prospective study. Alcohol Clin Exp Res. 1994a;18:248–254. doi: 10.1111/j.1530-0277.1994.tb00009.x. [DOI] [PubMed] [Google Scholar]
- Streissguth AP, Barr HM, Sampson PD, Bookstein FL. Prenatal alcohol and offspring development: the first fourteen years. Drug Alcohol Depend. 1994b;36:89–99. doi: 10.1016/0376-8716(94)90090-6. [DOI] [PubMed] [Google Scholar]
- Streissguth AP, Bookstein FL, Barr HM, Sampson PD, O’Malley K, Young JK. Risk factors for adverse life outcomes in fetal alcohol syndrome and fetal alcohol effects. J Dev Behav Pediatr. 2004;25:228–238. doi: 10.1097/00004703-200408000-00002. [DOI] [PubMed] [Google Scholar]
- Vellutino FR, Scanlon DM, Lyon GR. Differentiating between difficult-to-remediate and readily remediated poor readers: more evidence against the IQ-achievement discrepancy definition of reading disability. J Learn Disabil. 2000;33:223–238. doi: 10.1177/002221940003300302. [DOI] [PubMed] [Google Scholar]
- Wechsler D. The Wechsler Intelligence Scale for Children. 4. Pearson Assessment; London: 2004. (WISC-IV) [Google Scholar]
- Wechsler D. Wechsler Individual Achievement Test. 2. The Psychological Corporation; London: 2005. (WIAT II) [Google Scholar]
- Wierenga LM, Langen M, Oranje B, Durston S. Unique developmental trajectories of cortical thickness and surface area. NeuroImage. 2014;87:120–126. doi: 10.1016/j.neuroimage.2013.11.010. [DOI] [PubMed] [Google Scholar]
- Woods KJ, Meintjes EM, Molteno CD, Jacobson SW, Jacobson JL. Parietal dysfunction during number processing in children with fetal alcohol spectrum disorders. NeuroImage: Clinical. 2015;8:594–605. doi: 10.1016/j.nicl.2015.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]