Abstract
Background and Purpose
Functional polymorphisms (Ser49Gly and Arg389Gly) in ADRB1 have been associated with cardiovascular and β-blocker response outcomes. Herein we examined associations of these polymorphisms with Major Adverse Cardiovascular Events (MACE), with and without stratification by β-blocker treatment in patients with a history of stroke.
Methods
926 participants of the Secondary Prevention of Small Subcortical Strokes (SPS3) trial’s genetic substudy with hypertension were included. MACE included stroke, myocardial infarction, and all-cause death. Kaplan-Meier and multivariable Cox regression analyses were used. Because the primary component of MACE was ischemic stroke, we tested the association of Ser49Gly with ischemic stroke among 41,475 individuals of European and African ancestry in the NINDS Stroke Genetics Network (SiGN).
Results
MACE was higher in carriers of the Gly49 allele than those with the Ser49Ser genotype (10.5% vs. 5.4%, log-rank p=0.005). Gly49 carrier status was associated with MACE (HR 1.62; 95% CI 1.00–2.68) and ischemic stroke (HR 1.81; 95% CI 1.01–3.23) in SPS3 and with small artery ischemic stroke (OR 1.14; 95% CI 1.03–1.26) in SiGN. In SPS3, β-blocker-treated Gly49 carriers had increased MACE versus non-β-blocker-treated individuals and non-carriers (HR 2.03; 95% CI 1.20–3.45). No associations were observed with the Arg389Gly polymorphism.
Conclusion
Among individuals with previous small artery ischemic stroke, the ADRB1 Gly49 polymorphism was associated with MACE, particularly small artery ischemic stroke, a risk that may be increased among β-blocker-treated individuals. Further research is needed to define β-blocker benefit among ischemic stroke patients by ADRB1 genotype.
Clinical Trial Registration-URL
http://www.clinicaltrials.gov. Unique identifier: NCT00059306.
Keywords: pharmacogenetics, ADRB1, Ser49Gly, cardiovascular disease, stroke, beta-blockers
Introduction
Stroke is the second leading cause of death worldwide after ischemic heart disease 1. Ischemic stroke, the most common stroke, is classified by three major subtypes, namely cardioembolic, large artery and small artery 2, 3. Many clinical risk factors for stroke, including hypertension, atrial fibrillation, dyslipidemia, and diabetes have been identified 4. However, not all of the risk is explained by clinical factors. It is estimated that genetic factors may explain up to 30–40% of the remaining risk 5.
Several meta-analyses of genome-wide association studies (GWAS) have identified convincing single nucleotide polymorphism (SNP) associations with overall ischemic 6–9 as well as cerebral small vessel stroke or small vessel pathology 8–10. Collectively, SNPs showing the strongest evidence for association with ischemic stroke have been subtype-specific, suggesting a potential role for targeted therapies for stroke subtypes.
Numerous cardiovascular (CV) pharmacogenetic studies with implications for stroke prevention and treatment have been conducted, with strongest evidence for anticoagulation and antiplatelet therapies 11–13. Compelling pharmacogenetic data also exist for antihypertensive agents, particularly β-blockers with ADRB1 and thiazide diuretics with NEDD4L 14.
There has been longstanding interest in genetic variants in ADRB1, the gene encoding the β1 adrenergic receptor (β1AR), and their role in CV disease and treatment response. Several studies have tested these variants with various CV phenotypes, including blood pressure (BP) 15, heart rate 16, dilated cardiomyopathy 17 and composite CV outcomes 18. However, less is known about the relation of ADRB1 variants to the pathology and pharmacogenetics of ischemic stroke. The two most common and well-studied SNPs in ADRB1 are missense SNPs rs1801252 (A>G; Ser49Gly) and rs1801253 (G>C; Arg389Gly). Pharmacogenetic studies have extensively evaluated these SNPs with different CV response phenotypes 14, 19. Among hypertensive individuals, homozygous carriers of the wild-type alleles with serine at codon 49 (Ser49) and arginine at codon 389 (Arg389) may experience greater BP lowering from β-blocker therapy than carriers of the minor allele (Gly49, Gly389), with effect size larger for codon 389 than codon 49 19. To our knowledge, one study has examined the associations of Ser49Gly and Arg389Gly with ischemic stroke 20, and no study has examined the pharmacogenetic associations of either SNP with ischemic stroke.
The previous literature has identified associations with ischemic stroke for the minor alleles Gly49 and Gly389 20. In vitro studies indicate Gly49 has greater agonist-induced receptor down-regulation, and Gly389 has reduced β1AR coupling with Gs-protein 19. Based on these findings, and the fact that in our study Major Adverse Cardiovascular Events (MACE) was primarily composed of recurrent ischemic stroke, we hypothesized that carriers of the minor allele at both loci will have a greater risk of MACE and reduced response to β-blocker therapy as reflected by a higher incidence of MACE among β-blocker-treated carriers compared to non-carriers.
Using data from the Secondary Prevention of Small Subcortical Strokes (SPS3) trial, we investigated associations of Ser49Gly and Arg389Gly with MACE, particularly ischemic stroke, among participants with recent history of small artery ischemic stroke. We also tested pharmacogenetic associations among β-blocker-treated participants. To validate the association with MACE, we tested the association of Ser49Gly with ischemic stroke using consortium data from the Stroke Genetics Network (SiGN) 21.
Methods
Study population
The data for this study come from SPS3, an international multi-center randomized controlled clinical trial that evaluated the effect of two different antihypertensive and antiplatelet therapy regimens on the rate of recurrent stroke (ClinicalTrials.gov identifier NCT00059306). SPS3 has been described elsewhere 22. Briefly, 3,020 participants ≥30 years old with recent symptomatic small artery ischemic stroke (within 180 days) were recruited and randomized in a 2×2 factorial design to an antiplatelet regimen (aspirin 325 mg daily plus clopidogrel 75 mg daily vs. aspirin 325 mg daily plus placebo) and a BP target (lower [also referred to as ‘intensive’]: systolic BP (SBP) <130 mmHg vs. higher [also referred to as ‘usual’]: SBP 130–149 mmHg). The primary outcome was recurrent stroke (ischemic or hemorrhagic). Secondary outcomes were rate of cognitive decline and major vascular events, including transient ischemic attack (TIA), acute myocardial infarction, non-central nervous system thromboembolism, and all-cause death. The mean length of follow-up was 3.9±1.9 years (median: 3.8 years). In the SPS3 Genetic Substudy SPS3-GENES, DNA was collected from saliva samples using OG-500 Oragene DNA self-collection kits (DNA Genotek Inc.) on 1,139 participants.
The NINDS SiGN consortium provided data for the validation, which was a meta-analysis study of the association with presence of ischemic stroke 21. Data on a total of 41,475 individuals of European and African ancestry in SiGN, excluding SPS3, were analyzed.
Institutional review boards at participating centers for SPS3 and SiGN approved the studies. All participants provided written informed consent.
Genotyping and imputation
DNA samples collected from SPS3-GENES participants were isolated using the prepIT-L2P kit (PT-LP2-45; DNA Genotek Inc). Genotyping for Ser49Gly (rs1801252) and Arg389Gly (rs1801253) was performed using a TaqMan assay based QuantStudio polymerase chain reaction system (Life Technologies, Carlsbad, CA). Genotyping call rate was 99% for both Ser49Gly and Arg389Gly. In total, 1,126 samples were successfully genotyped. Genotype concordance between genotyped and 1000 Genomes (phase I) imputed data, which became available later, was 99% for Ser49Gly and 96% for Arg389Gly. We tested deviation from Hardy-Weinberg Equilibrium (HWE) in each ancestry group using the χ2 test. In SiGN, Ser49Gly and Arg389Gly were imputed from a reference panel that included 1000 Genomes (phase I) and Genome of the Netherlands (GoNL). Imputation quality for Ser49Gly was 0.984–0.998 and for Arg389Gly was 0.982–0.993.
Statistical analysis
Descriptive statistics of baseline and follow-up characteristics on SPS3-GENES participants were compared between participants with and without MACE by the end of the trial. Categorical variables are presented in frequencies and differences were tested between groups (MACE vs. no MACE) using Fisher’s exact or χ2-square test, as appropriate. Continuous variables are presented as mean±standard deviation, and differences were tested between groups using Student’s t-test. Principal components analysis was conducted on high-quality, linkage disequilibrium-pruned genome-wide SNP data using EIGENSTRAT 23. Genome-wide genotyping was performed on the Illumina Omni 5M array. The top two principal components (PCs) separated self-identified Whites, African Americans/Blacks, and Hispanics.
In SPS3-GENES, associations between Ser49Gly and MACE, with and without β-blocker treatment, were tested using Kaplan-Meier survival analysis. MACE was comprised of stroke (ischemic or hemorrhagic), acute myocardial infarction, and all-cause death. Because 75% of MACE was ischemic stroke, the secondary analysis was of ischemic stroke. Participants were classified as β-blocker-treated if they used a β-blocker at any time during the study. In multivariable Cox proportional hazards regression analyses, covariates included age, sex, ancestry-specific PCs 1–2, history of myocardial infarction, history of diabetes, on-treatment SBP, and β-blocker treatment (except for the model stratified on β-blocker treatment). We selected covariates based on associations with time-to-event in univariate Cox regression analyses with p<0.20; however, age, sex, history of diabetes, and on-treatment SBP were forced into the model. Due to prior genetic associations of Ser49Gly and Arg389Gly with ischemic stroke 20, we considered two-sided p<0.05 as statistically significant for the main effects analyses. Ancestry groups were combined in all analyses given the well-documented functional evidence for these SNPs. Since no prior studies have examined the pharmacogenetic associations of Ser49Gly and Arg389Gly with ischemic stroke, we used a p-value threshold of 0.025 (0.05/2 SNPs tested) for the pharmacogenetic analyses. A dominant model of inheritance was assumed, consistent with methodology used in previous studies 18, 20. We similarly examined ischemic stroke alone. Analyses were performed using SAS 9.4 (Cary, NC) and GraphPad Prism 5.01 (San Diego, CA).
This analysis only included participants with hypertension at baseline, defined as SBP ≥140 mmHg and/or diastolic BP (DBP) ≥90 mmHg and/or taking antihypertensive medication(s) at the time of enrollment, because we anticipated β-blockers would not be expected to be prescribed in this population in the absence of hypertension. In total, 926 participants were included in the analyses.
While there was no dataset available for validation of MACE, since the majority of events were ischemic stroke, and specifically small artery ischemic stroke, we validated our findings using SiGN consortium data. Specifically, meta-analysis of 36 studies in SiGN was conducted for association with presence of ischemic stroke. The SiGN sample (with exclusion of the SPS3 stratum) included 13,449 ischemic stroke cases and 28,026 non-stroke controls. The association was tested under a dominant model by logistic regression, adjusted for sex and ancestry PCs. Stroke subtypes were classified using TOAST criteria 3. We are aware of no stroke dataset in which we can validate the pharmacogenetic finding.
Results
Description of study participants
Overall, background characteristics of SPS3-GENES participants did not differ significantly by MACE or no MACE status at the time of event or censoring (Table 1). There were more men (60.6%), and the average age was 62.2±10.3 years. Treatment with thiazide diuretics, calcium-channel blockers, angiotensin converting enzyme inhibitors, and angiotensin receptor blockers did not differ by MACE status. However, β-blocker use was significantly higher among those with MACE at follow-up (p=0.002).
Table 1.
Demographic and clinical characteristics among hypertensive participants in SPS3-GENES, N=926
| Characteristics | Outcome | p-value | ||
|---|---|---|---|---|
|
| ||||
| Overall N=926 |
MACE N=67 |
Non-MACE N=859 |
||
| Age (years) | 62.2 ± 10.3 | 61.4 ± 10.5 | 62.3 ± 10.3 | 0.49 |
| Male | 561 (60.6) | 40 (59.7) | 521 (60.6) | 0.88 |
| BMI (kg/m2) | 29.1 ± 5.9 | 29.8 ± 6.0 | 28.0 ± 5.86 | 0.33 |
| Baseline SBP (mmHg) | 145.3 ± 18.0 | 147.1 ± 17.2 | 145.1 ± 18.1 | 0.36 |
| Baseline DBP (mmHg) | 79.5 ± 10.5 | 80.2 ± 11.0 | 79.5 ± 10.5 | 0.64 |
| Lower BP target | 472 (51.0) | 29 (43.3) | 443 (51.6) | 0.19 |
| Current smoker | 166 (17.9) | 17 (25.4) | 149 (17.3) | 0.10 |
| Disease history | ||||
| Myocardial infarction | 38 (4.1) | 6 (9.0) | 32 (3.7) | 0.05 |
| Congestive heart failure | 9 (1.0) | 1 (1.5) | 8 (1.0) | 0.49 |
| Diabetes mellitus | 285 (30.8) | 27 (40.3) | 258 (30.0) | 0.10 |
| Drug exposure during study | ||||
| Thiazide diuretic | 766 (82.7) | 52 (77.6) | 714 (83.1) | 0.25 |
| Calcium-channel blocker | 528 (57.0) | 42 (62.7) | 486 (56.6) | 0.33 |
| β-blocker | 385 (41.6) | 40 (59.7) | 345 (40.2) | 0.002 |
| ACE inhibitor | 668 (72.1) | 51 (76.1) | 617 (71.8) | 0.45 |
| ARB | 432 (46.6) | 28 (41.8) | 404 (47.0) | 0.41 |
Mean ± SD or No. (%) shown; p-values derived from Student’s t-test and Fisher’s exact or χ2 tests; BMI: Body Mass Index; ACE: Angiotensin Converting Enzyme; ARB: Angiotensin II Receptor Blocker; MACE (Major Adverse Cardiovascular Events): stroke, myocardial infarction, all-cause death.
The frequency of MACE was 7.2% in the overall sample (67/926). The majority of first events at follow-up was recurrent ischemic stroke (75%), of which 86% was small artery stroke. The remaining events included acute myocardial infarction (n=12), CNS hemorrhage (n=4) and death (n=1).
The minor allele frequencies (MAFs) for Ser49Gly were 12%, 26% and 25% and for Arg389Gly were 29%, 45%, and 15% in Whites, African Americans/Blacks and Hispanics, respectively. For both polymorphisms, these are consistent with reference population values in the 1000 Genomes database. Both variants were in HWE.
SiGN participants have been described elsewhere 21, 24. Briefly, their mean age was 67 years, half (49%) were women, and White participants comprised 79%. Twenty-five percent had diabetes, 68% had hypertension, and 23% had coronary artery disease 24.
ADRB1 SNP associations with MACE
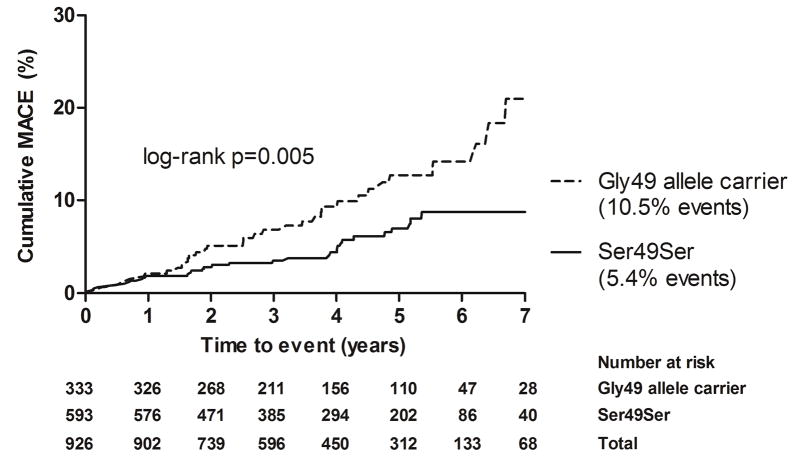
Overall, events were observed in 10.5% of Gly49 allele carriers vs. 5.4% of those with the Ser49Ser genotype (p=0.004). Among Gly389 carriers vs. non-carriers, event frequencies were 7.5% and 6.9%, respectively (p=0.707). Kaplan-Meier analysis showed that Gly49 allele carriers had a significantly higher incidence of MACE than non-carriers (log-rank p=0.005) (Figure 1). In additional analyses, Gly49 allele was associated with increased risk of ischemic stroke (log-rank p=0.007) and small artery ischemic stroke (log-rank p=0.005) (online-only Data Supplement Figures I, II).
Figure 1.
Cumulative incidence of MACE by Ser49Gly genotype among hypertensive participants in SPS3-GENES, N=926
MACE (Major Adverse Cardiovascular Events): stroke, myocardial infarction, all-cause death; 75% of events were ischemic stroke
In multivariable Cox regression analysis, which included adjustment for β-blocker use, Gly49 allele carriers compared to non-carriers had marginally significant associations with MACE (HR 1.62; 95% CI 1.00–2.68) and ischemic stroke (HR 1.81; 95% CI 1.01–3.23). In stratified analysis among non-β-blocker users (n=541), hazard ratios of Gly49 with risk crossed 1.0 for both MACE (HR 1.52; 95% CI 0.69–3.38) and ischemic stroke (HR 1.95; 95% CI 0.79–4.85). Arg389Gly was not associated with MACE in Kaplan-Meier analysis (log-rank p=0.460), or with MACE (p=0.291) or ischemic stroke (p=0.537) in adjusted Cox regression analyses.
In the SiGN validation study (n=41,475), multivariable logistic regression analysis showed that Gly49 was significantly associated with presence of small artery ischemic stroke (OR 1.14; 95% CI 1.03–1.26; p=0.012), but not other types of ischemic stroke.
Pharmacogenetic associations
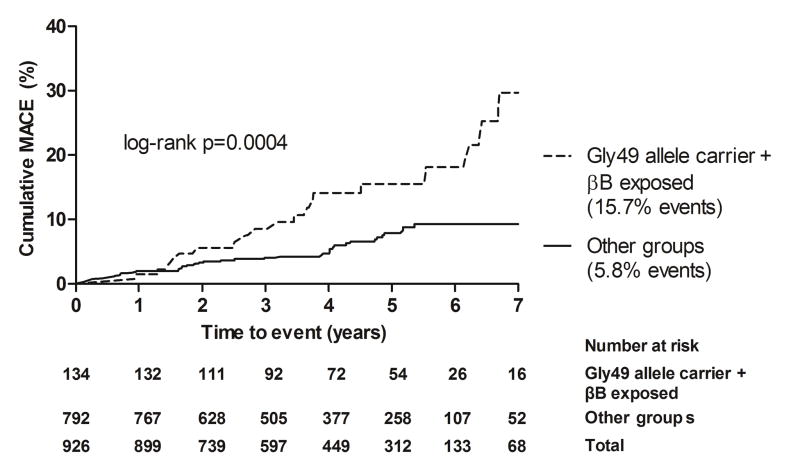
Gly49 allele carriers treated with β-blockers had a significantly higher incidence of MACE when compared to other groups (15.7% vs. 5.8%; log-rank p=0.0004) (Figure 2). Additionally, Kaplan-Meier analysis showed that among β-blocker-treated individuals, cumulative incidence of MACE was significantly higher among Gly49 allele carriers than those with Ser49Ser genotype (15.7% vs. 7.6%; log-rank p=0.018) (online-only Data Supplement Figure III). Among individuals with the Ser49Ser genotype, event rates did not differ significantly by β-blocker treatment (log-rank p=0.107).
Figure 2.
Cumulative incidence of MACE among Gly49 allele carriers treated with β-blockers compared to other groups* in SPS3-GENES, N=926
MACE (Major Adverse Cardiovascular Events): stroke, myocardial infarction, all-cause death; 75% of events were ischemic stroke
*‘Other groups’ included Gly49 allele carriers not treated with β-blockers and non-carriers (Ser49Ser) regardless of β-blocker treatment
In stratified multivariable Cox regression analysis, with Ser49Ser and no β-blocker treatment as reference, Gly49 allele carriers treated with β-blockers had significantly increased risk of MACE and ischemic stroke, whereas there were no differences in these outcomes for Ser49Ser treated with β-blockers or Gly49 carriers not treated with β-blockers (Table 2). When compared to the three other groups, β-blocker-treated Gly49 carriers had increased risk of MACE and ischemic stroke (Table 2). In parallel analyses, we did not find significant pharmacogenetic associations between Arg389Gly and outcomes (Gly389 carrier + β-blocker-treated vs. Arg389Arg + β-blocker-treated, p=0.506; Gly389 carrier + β-blocker-treated vs. other groups, p=0.408).
Table 2.
Pharmacogenetic associations of Ser49Gly with MACE and ischemic stroke stratified by β-blocker treatment among hypertensive participants in SPS3-GENES, N=926
| HR (95% CI) | p-value | |
|---|---|---|
| I. MACE | ||
| Ser49Ser + Not β-blocker-treated | Ref. | |
| Gly49 carrier + β-blocker-treated | 2.79 (1.37–5.70) | 0.005 |
| Gly49 carrier + Not β-blocker-treated | 1.51 (0.70–3.26) | 0.30 |
| Ser49Ser + β-blocker-treated | 1.64 (0.80–3.36) | 0.18 |
| II. Ischemic stroke | ||
| Ser49Ser + Not β-blocker-treated | Ref. | |
| Gly49 carrier + β-blocker-treated | 2.92 (1.24–6.84) | 0.01 |
| Gly49 carrier + Not β-blocker-treated | 1.93 (0.79–4.66) | 0.15 |
| Ser49Ser + β-blocker-treated | 1.69 (0.72–3.97) | 0.23 |
| III. Gly49 allele carrier + β-blocker-treated vs. other groups* | ||
| MACE | 2.03 (1.20–3.45) | 0.01 |
| Ischemic stroke | 1.92 (1.04–3.58) | 0.04 |
Hazard ratio (HR) and 95% confidence interval (CI) in parentheses; Cox regression models with adjustments for age, sex, principal components 1–2 for ancestry, history of myocardial infarction, history of diabetes, on-treatment systolic blood pressure
MACE (Major Adverse Cardiovascular Events): stroke, myocardial infarction, all-cause death
‘Other groups’ included Gly49 allele carriers not treated with β-blockers and non-carriers (Ser49Ser) regardless of β-blocker treatment
To assess whether achieved BP differed by Ser49Gly genotype and β-blocker use, we compared on-treatment BP, taken at the visit prior to MACE or censoring, between Gly49 allele carriers treated with β-blockers and Gly49 allele carriers treated with other antihypertensives. β-blocker-treated Gly49 allele carriers had a higher mean on-treatment SBP, but not DBP, compared to Gly49 carriers treated with other antihypertensives (SBP: 132.4 vs. 128.4 mmHg; p=0.023). Among non-carriers, on-treatment SBP did not differ by β-blocker treatment (p=0.180). Additionally, in spite of higher SBP, β-blocker-treated Gly49 carriers on average took more antihypertensive medications than Gly49 carriers treated with other antihypertensive drugs (4.2 vs. 2.5; p<0.001). In parallel analyses, the difference in on-treatment SBP between Gly389 carriers treated with β-blockers and Gly389 carriers treated with other agents was non-significant (p=0.641). On average, Gly389 carriers treated with β-blockers were treated with a higher number of antihypertensive medication classes than Gly389 carriers treated with other agents (4.1 vs. 2.7; p<0.001).
Discussion
Using data from a randomized controlled trial, we tested the association of β1AR polymorphisms with CV outcomes, composed primarily of ischemic stroke, and evaluated pharmacogenetic associations in β-blocker-treated individuals. Our study population was relatively young (mean age 62 years), which is comparable to that of similar stroke cohorts 7. Among participants with a recent history of small artery ischemic stroke, Gly49 allele carriers had an increased risk of recurrent ischemic stroke, particularly small artery stroke, and this finding was corroborated by SiGN consortium meta-analysis that showed Gly49 allele carriers were more likely to have small artery ischemic stroke, but not other types of stroke. These results are consistent with those of a previous small case-control study that reported a higher risk of ischemic stroke among Gly49 allele carriers 20. Additional analysis suggested that, in SPS3-GENES, the association between Gly49 allele and CV risk may be increased among β-blocker-treated individuals.
The mechanism by which the Gly49 allele may confer risk for ischemic stroke, especially of small arteries, is unclear. It is well-established that β2AR is important in mediating peripheral vasodilation; however, recent studies have indicated this is true only for large conductance vessels such as the aorta 25. In smaller arteries such as small mesenteric resistance arteries 25–27 and cerebral arteries 28, vasodilation may primarily depend upon β1AR stimulation. Another study showed that agonist-induced vasodilation of cerebral arteries is blunted by β1AR blockade 29. If β1AR plays a similar role in small subcortical arteries as it does in peripheral resistance arteries and cerebral arteries, it is possible that perturbing the normal functioning of β1AR may attenuate its vasodilating effects in small subcortical arteries. The Gly49 variant of β1AR might be one such perturbation that may attenuate or even reverse vasodilation due to β1AR stimulation in small subcortical arteries. Evidence from recombinant cell studies have suggested that the Gly49 allele leads to greater agonist-induced β1AR down-regulation, which means the number of receptors on the cell surface available to bind catecholamines, and thus trigger a downstream response, is reduced 19. In light of β1AR’s vasodilating effects, the Gly49 variant of β1AR may be less responsive to adrenergic stimulation, and consequently, less able to mediate vasodilation in small arteries. Collectively, these studies may in part explain our main effect finding of an association of Gly49 with ischemic stroke of small arteries.
To our knowledge, this is the first study to examine β-blocker pharmacogenetic associations with ischemic stroke, and the data suggest that the main effect finding of risk of MACE and ischemic stroke based on the Gly49 polymorphism may be slightly increased with β-blocker treatment. Specifically, when considering Ser49Ser and no β-blocker treatment as the reference, Gly49 carriers treated with β-blockers had increased risk of adverse outcomes whereas Ser49Ser participants treated with β-blockers did not. Additionally, Gly49 carriers treated with β-blockers had a 3-fold increased risk while Gly49 carriers without β-blocker treatment had a 2-fold risk, which suggests that β-blocker treatment may have an amplifying effect on the Gly49 allele. Further, analysis of on-treatment BP and number of antihypertensive drugs suggests that Gly49 participants treated with β-blockers had higher on-treatment BPs despite being treated with, on average, 1.7 more antihypertensive drugs. In contrast, non-carriers did not have different blood pressures, based on β-blocker treatment. Collectively, these data suggest Gly49 carriers may be less responsive to the antihypertensive effects of β-blockers, which is consistent with previous studies 19. A recent Cochrane Review indicated that β-blockers have significantly reduced stroke risk compared to placebo, but have increased stroke risk when compared against calcium-channel blockers and renin-angiotensin system inhibitors 30. Our data suggest this increased risk might be particularly important in Gly49 carriers treated with β-blockers. Further research is needed to provide greater clarity on the role of the Gly49 polymorphism on stroke outcomes in patients treated with β-blockers.
We did not find significant main effects or pharmacogenetic associations for Arg389Gly. While previous studies have found associations of Arg389Gly with CV and CV pharmacogenetic phenotypes 19, Arg389Gly may play a minor role in ischemic stroke pathophysiology and pharmacogenetics when compared to Ser49Gly.
This study has several strengths. In a multi-ethnic population, Ser49Gly was associated with ischemic stroke in a direction consistent with the previous literature, and we validated this association specifically with small artery ischemic stroke in over 40,000 participants in the SiGN consortium. Furthermore, we may have identified novel pharmacogenetic associations between β-blocker treatment and the ADRB1 Gly49 allele.
This study has limitations that are acknowledged. We were unable to conduct replication analyses of the pharmacogenetic findings, as currently, to our knowledge there are no other replication cohorts of patients with a history of stroke available. Future studies in participants with a previous history of small artery ischemic stroke with medication and genetic information are needed to validate the pharmacogenetic findings. Additionally, we were unable to detect significant associations for some multivariable regression analyses, but the reduced sample sizes and thus limited power in these secondary analyses should be considered when interpreting those analyses.
Conclusions
Our study provides evidence that the ADRB1 Gly49 allele may increase CV risk, particularly for small artery stroke. Additionally, these data suggest that the association between the Gly49 allele and CV risk may be enhanced by β-blocker treatment. However, because stroke risk was higher in the study population overall, it is difficult to know with certainty if β-blockers in the population are a marker for increased stroke risk, if β-blockers are increasing risk overall and should be avoided, or if indeed, β-blocker treatment enhances the apparent risk of carrying the ADRB1 Gly49 allele. This highlights the critical importance of future studies to clarify this point since it may have important clinical implications for treatment of patients with small artery stroke.
Supplementary Material
Acknowledgments
We thank the site investigators and are grateful to the participants of SPS3, SPS3-GENES and SiGN. We also thank Tushar Dave for assistance with SiGN data analysis.
Funding
This project was supported by funding from the National Institutes of Health’s National Institute of Neurological Disorders and Stroke (Grants R01 NS073346, U01 NS038529, and U01 NS069208), National Institute for General Medical Sciences (U01 GM074492), and National Institute for Diabetes, Kidney, and Digestive Disorders (P30 DK072488).
Footnotes
Disclosures
Dr. Shuldiner is employed by Regeneron Pharmaceuticals, Inc. The other authors have no conflicts of interest to declare.
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Heart disease and stroke statistics-2016 update: A report from the american heart association. Circulation. 2016;133:e38–60. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 2.Ay H, Furie KL, Singhal A, Smith WS, Sorensen AG, Koroshetz WJ. An evidence-based causative classification system for acute ischemic stroke. Ann Neurol. 2005;58:688–697. doi: 10.1002/ana.20617. [DOI] [PubMed] [Google Scholar]
- 3.Adams HP, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. Toast. Trial of org 10172 in acute stroke treatment. Stroke; a journal of cerebral circulation. 1993;24:35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 4.Meschia JF, Bushnell C, Boden-Albala B, Braun LT, Bravata DM, Chaturvedi S, et al. Guidelines for the primary prevention of stroke: A statement for healthcare professionals from the american heart association/american stroke association. Stroke; a journal of cerebral circulation. 2014;45:3754–3832. doi: 10.1161/STR.0000000000000046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bevan S, Traylor M, Adib-Samii P, Malik R, Paul NL, Jackson C, et al. Genetic heritability of ischemic stroke and the contribution of previously reported candidate gene and genomewide associations. Stroke; a journal of cerebral circulation. 2012;43:3161–3167. doi: 10.1161/STROKEAHA.112.665760. [DOI] [PubMed] [Google Scholar]
- 6.Malik R, Traylor M, Pulit SL, Bevan S, Hopewell JC, Holliday EG, et al. Low-frequency and common genetic variation in ischemic stroke: The metastroke collaboration. Neurology. 2016;86:1217–1226. doi: 10.1212/WNL.0000000000002528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Traylor M, Farrall M, Holliday EG, Sudlow C, Hopewell JC, Cheng YC, et al. Genetic risk factors for ischaemic stroke and its subtypes (the metastroke collaboration): A meta-analysis of genome-wide association studies. Lancet Neurol. 2012;11:951–962. doi: 10.1016/S1474-4422(12)70234-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Consortium NWGotCfHaARiGEC, (SiGN), SGN, (ISGC), ISGC. Identification of additional risk loci for stroke and small vessel disease: A meta-analysis of genome-wide association studies. Lancet Neurol. 2016;15:695–707. doi: 10.1016/S1474-4422(16)00102-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Network NSG, International Stroke Genetics C. Loci associated with ischaemic stroke and its subtypes (sign): A genome-wide association study. Lancet Neurol. 2015:174–184. doi: 10.1016/S1474-4422(15)00338-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kubo M, Hata J, Ninomiya T, Matsuda K, Yonemoto K, Nakano T, et al. A nonsynonymous snp in prkch (protein kinase c eta) increases the risk of cerebral infarction. Nat Genet. 2007;39:212–217. doi: 10.1038/ng1945. [DOI] [PubMed] [Google Scholar]
- 11.Meschia JF. Pharmacogenetics and stroke. Stroke; a journal of cerebral circulation. 2009;40:3641–3645. doi: 10.1161/STROKEAHA.109.562231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson JA, Cavallari LH. Pharmacogenetics and cardiovascular disease--implications for personalized medicine. Pharmacol Rev. 2013;65:987–1009. doi: 10.1124/pr.112.007252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McDonough CW, McClure LA, Mitchell BD, Gong Y, Horenstein RB, Lewis JP, et al. Cyp2c19 metabolizer status and clopidogrel efficacy in the secondary prevention of small subcortical strokes (sps3) study. J Am Heart Assoc. 2015;4:e001652. doi: 10.1161/JAHA.114.001652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cooper-DeHoff RM, Johnson JA. Hypertension pharmacogenomics: In search of personalized treatment approaches. Nat Rev Nephrol. 2016;12:110–122. doi: 10.1038/nrneph.2015.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kong H, Li X, Zhang S, Guo S, Niu W. The β1-adrenoreceptor gene arg389gly and ser49gly polymorphisms and hypertension: A meta-analysis. Mol Biol Rep. 2013;40:4047–4053. doi: 10.1007/s11033-012-2482-2. [DOI] [PubMed] [Google Scholar]
- 16.Wilk JB, Myers RH, Pankow JS, Hunt SC, Leppert MF, Freedman BI, et al. Adrenergic receptor polymorphisms associated with resting heart rate: The hypergen study. Ann Hum Genet. 2006;70:566–573. doi: 10.1111/j.1469-1809.2005.00258.x. [DOI] [PubMed] [Google Scholar]
- 17.Forleo C, Sorrentino S, Guida P, Romito R, De Tommasi E, Iacoviello M, et al. Beta1- and beta2-adrenergic receptor polymorphisms affect susceptibility to idiopathic dilated cardiomyopathy. J Cardiovasc Med (Hagerstown) 2007;8:589–595. doi: 10.2459/01.JCM.0000281710.51304.03. [DOI] [PubMed] [Google Scholar]
- 18.Pacanowski MA, Gong Y, Cooper-Dehoff RM, Schork NJ, Shriver MD, Langaee TY, et al. Beta-adrenergic receptor gene polymorphisms and beta-blocker treatment outcomes in hypertension. Clin Pharmacol Ther. 2008;84:715–721. doi: 10.1038/clpt.2008.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson JA, Liggett SB. Cardiovascular pharmacogenomics of adrenergic receptor signaling: Clinical implications and future directions. Clin Pharmacol Ther. 2011;89:366–378. doi: 10.1038/clpt.2010.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumar A, Pandit AK, Vivekanandhan S, Srivastava MV, Tripathi M, Prasad K. Association between beta-1 adrenergic receptor gene polymorphism and ischemic stroke in north indian population: A case control study. Journal of the neurological sciences. 2015;348:201–205. doi: 10.1016/j.jns.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 21.Meschia JF, Arnett DK, Ay H, Brown RD, Benavente OR, Cole JW, et al. Stroke genetics network (sign) study: Design and rationale for a genome-wide association study of ischemic stroke subtypes. Stroke; a journal of cerebral circulation. 2013;44:2694–2702. doi: 10.1161/STROKEAHA.113.001857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Benavente OR, White CL, Pearce L, Pergola P, Roldan A, Benavente MF, et al. The secondary prevention of small subcortical strokes (sps3) study. Int J Stroke. 2011;6:164–175. doi: 10.1111/j.1747-4949.2010.00573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, Reich D. Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet. 2006;38:904–909. doi: 10.1038/ng1847. [DOI] [PubMed] [Google Scholar]
- 24.Ay H, Arsava EM, Andsberg G, Benner T, Brown RD, Chapman SN, et al. Pathogenic ischemic stroke phenotypes in the ninds-stroke genetics network. Stroke; a journal of cerebral circulation. 2014;45:3589–3596. doi: 10.1161/STROKEAHA.114.007362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Flacco N, Segura V, Perez-Aso M, Estrada S, Seller JF, Jiménez-Altayó F, et al. Different β-adrenoceptor subtypes coupling to camp or no/cgmp pathways: Implications in the relaxant response of rat conductance and resistance vessels. Br J Pharmacol. 2013;169:413–425. doi: 10.1111/bph.12121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chruscinski A, Brede ME, Meinel L, Lohse MJ, Kobilka BK, Hein L. Differential distribution of beta-adrenergic receptor subtypes in blood vessels of knockout mice lacking beta(1)- or beta(2)-adrenergic receptors. Mol Pharmacol. 2001;60:955–962. doi: 10.1124/mol.60.5.955. [DOI] [PubMed] [Google Scholar]
- 27.Briones AM, Daly CJ, Jimenez-Altayo F, Martinez-Revelles S, Gonzalez JM, McGrath JC, et al. Direct demonstration of beta1- and evidence against beta2- and beta3-adrenoceptors, in smooth muscle cells of rat small mesenteric arteries. Br J Pharmacol. 2005;146:679–691. doi: 10.1038/sj.bjp.0706369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Edvinsson L, Owman C. Pharmacological characterization of adrenergic alpha and beta receptors mediating the vasomotor responses of cerebral arteries in vitro. Circ Res. 1974;35:835–849. doi: 10.1161/01.res.35.6.835. [DOI] [PubMed] [Google Scholar]
- 29.Moore CL, McClenahan SJ, Hanvey HM, Jang DS, Nelson PL, Joseph BK, et al. Beta1-adrenergic receptor-mediated dilation of rat cerebral artery requires shaker-type kv1 channels on psd95 scaffold. J Cereb Blood Flow Metab. 2015;35:1537–1546. doi: 10.1038/jcbfm.2015.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wiysonge CS, Bradley HA, Volmink J, Mayosi BM, Opie LH. Beta-blockers for hypertension. Cochrane Database Syst Rev. 2017;1:CD002003. doi: 10.1002/14651858.CD002003.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.