Abstract
Background
Intrahepatic biliary fibrosis, as seen with cholestatic liver injuries such as biliary atresia, is mechanistically distinct from fibrosis caused by hepatocyte toxicity. We previously demonstrated the expansion of cells expressing the stem/progenitor cell marker PROMININ-1 (PROM1), within regions of developing fibrosis in biliary atresia. Thus, we hypothesized that Prom1 expression is biliary fibrosis-specific.
Methods
Gene expression of Prom1 was analyzed in adult mice undergoing either cholestatic bile duct ligation (BDL) or hepatotoxic carbon tetrachloride (CCl4) administration by quantitative polymerase chair reaction. Lineage tracing of Prom1- expressing cells and Collagen-1α (Col1α)-expressing cells was performed after BDL in Prom1cre-ert2-lacz;Gfplsl and Col1αGfp transgenic mice, respectively.
Results
Prom1 expression increased significantly after BDL compared to sham (6.6±0.9 fold change at 2 weeks, p<0.05) but not with CCl4 (−0.7±0.5-fold change, NS). Upregulation of Prom1 was observed histologically throughout the liver as early as five days after BDL in Prom1cre-ert2-lacz mice by LacZ staining in non-hepatocyte cells. Lineage tracing of Prom1-expressing cells labeled prior to BDL in Prom1cre-ert2-lacz;Gfplsl mice, demonstrated increasing colocalization of GREEN FLUORESCENT PROTEIN (GFP) with biliary marker CYTOKERATIN-19 within ductular reactions up to 5 weeks after BDL consistent with biliary transdifferentiation. In contrast, rare colocalization of GFP with mesenchymal marker α-SMOOTH MUSCLE ACTIN in Prom1cre-ert2-lacz;Gfplsl mice and some colocalization of GFP with PROM1 in Col1αGfp mice, indicate minimal contribution of Prom1 progenitor cells to the pool of collagen-producing myofibroblasts.
Conclusion
During biliary fibrosis, Prom1-expressing progenitor cells transdifferentiate into cells within ductular reactions. This transdifferentiation may promote fibrosis.
Introduction
Biliary atresia (BA), a congenital progressive fibro-obliteration of the bile ducts, is the leading cause of pediatric end-stage liver disease and liver transplantation in the United States [1]. While surgical bypass of the atretic extrahepatic biliary tree is essential for survival, the extent and progression of intrahepatic biliary fibrosis are key determinants of transplant-free survival [2]. Thus, characterizing the pathogenesis of biliary fibrosis associated with biliary atresia remains essential in efforts to impact survival for infants with BA.
Prominin-1 (Prom1), or CD133, is a penta-transmembrane glycoprotein expressed by collagen-producing hepatic stellate cells and portal fibroblasts as well as by stem/progenitor cells in a variety of tissues including the liver [3]. The function of CD133 is unknown, although several studies indicate its role in maintaining the topology of the plasma membrane, in effect regulating the state of cellular differentiation [4] [5] [6]. Prom1-expressing hepatic progenitor cells possess great regenerative and malignant potential [7] and may contribute to the populating of tissue-engineered liver constructs [8]. We have also previously demonstrated the expansion of a population of cells expressing PROM1 within periportal regions of evolving biliary fibrosis in both the rodent rotavirus model of BA as well as in infants with BA [9]. These cells exhibit characteristics of progenitor cells with expression of both epithelial and mesenchymal markers such as CYTOKERATIN-19 (CK19) and α-SMOOTH MUSCLE ACTIN (αSMA), respectively.
Thus, we hypothesized that PROM1-expressing cell expansion is a biliary fibrosis-specific phenomenon. To test this hypothesis, we compared Prom1 gene expression patterns in adult mouse models of cholestasis/biliary fibrosis by bile duct ligation (BDL) and hepatocyte-specific carbon tetrachloride (CCl4) injury, as well as lineage tracing to demonstrate the progenitor cell nature of Prom1-expressing cells.
Methods
Animal experiments
Six-week old wildtype C57BL/6 mice (Jackson Laboratories, Bar Harbor, ME) were injected subcutaneously with either CCl4 (1 mL/kg body weight) in mineral oil (1:3 dilution), or control, every third day. Six-week old wildtype, Prom1cre-ert2-lacz;Gfplsl (both from Jackson Laboratories), or Col1αGfp (a gift from David Brenner, UC San Diego) [10] transgenic mice underwent BDL as previously described [11]. Double transgenic offspring bred from Prom1cre-ert2-nlacz and Gfplsl mice were treated with tamoxifen (mg/g) in olive oil 3 weeks prior to BDL. Briefly, mice underwent laparotomy under isoflurane anesthesia. The extra-hepatic bile duct was isolated and doubly ligated. The peritoneal cavity was filled with saline for fluid resuscitation and the laparotomy incisions closed. While extra-hepatic bile ducts of sham-operated controls did not receive ligation, all other procedural measures remained consistent. Thereafter, all mice were recovered and fed standard rodent chow and water ad libitum. With either liver injury model, mice were euthanized up to 6 weeks post-injury for collection of liver samples. All animal procedures were approved by the Children’s Hospital Los Angeles Saban Research Institute’s or the University of Southern California’s Institutional Animal Care and Use Committee.
Immunohistochemistry
Livers were fixed in 4% paraformaldehyde (PFA, Poly Sciences Inc., Warrington, PA), embedded in paraffin and then sectioned. Immunofluorescence imaging was performed as previously described [12] using primary antibodies listed in Supplemental Table 1 and secondary antibodies conjugated either with anti-mouse Cy3/Cy5, anti-rat Cy3/Cy5, or anti-rabbit Cy3/Cy5 (Jackson Immuno Research Lab, West Grove, PA). Images were acquired using a Leica DM5500B immunofluorescence microscope with Leica Suite Advanced Fluorescence (LAS AF) 6000 software (Wetzlar, Germany) or a LSM700 confocal system with ZEN software (Carl Zeiss Microimaging, Thornwood, NY). Bright field images were acquired using a Leica DM1000 (DFC290) microscope interfaced with Leica Application Suite, Version 2.7.1R1 (Leica Microsystems, Switzerland). Sirius red staining was performed as previously described [9].
LacZ staining
Liver tissue was fixed with 2% PFA at 4°C overnight , then incubated in 20% sucrose at 4°C overnight and finally embedded in OCT. Froze n sections were washed in phosphate buffered solution (PBS) for 5 minutes and incubated in β-gal substrate (1 mg/ml X-gal, 5 mM potassium ferricyanide, 5 mM potassium ferrocyanide, 2 mM MgCl2, 0.01% sodium deoxycholate, 0.02% NP-40 in PBS) in the dark overnight at 37°C. Sections were washed in PBS for 5 minutes and counterstained with eosin. ImageJ® was used to quantify cell positivity for LacZ staining.
Quantitative polymerase chain reaction
Total RNA was isolated from whole and non-parenchymal cell fractions of snap-frozen mouse liver tissues and FACS-sorted cells using the Qiagen RNA isolation kit (Qiagen, Valencia, CA). Complementary DNA synthesis was performed using iScript Reverse Transcription Supermix(Bio-Rad, Irvine, CA), and quantitative real-time PCR (qPCR) was performed as previously described [11, 13], using intron-spanning and gene-specific primers and probes designed using the Roche Universal Probe Library Assay Design Center (Roche, Basel, Switzerland) (see Supplemental Table 2 for primer sequences and probes).
Results
Prominin-1 expression increases after BDL but not after CCl4 induced liver injury
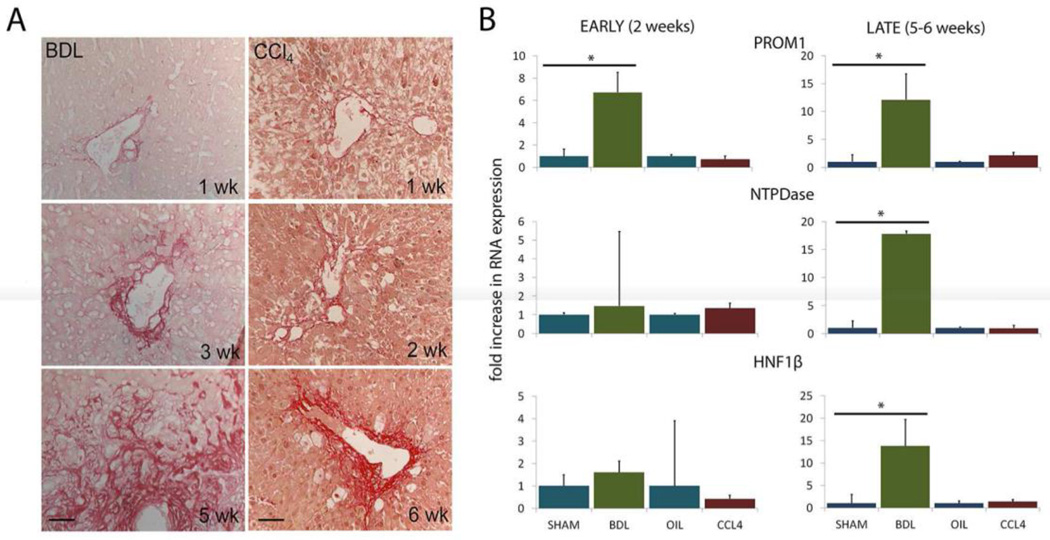
We previously reported the emergence and expansion of PROM1-expressing cells associated with evolving biliary fibrosis due to biliary atresia [9]. To further explore this observation, we compared cholestatic BDL with hepatotoxic CCl4 up to 6 weeks post-injury. Notable in both models of liver injury, there is significant destruction of liver parenchymal with loss of hepatocytes within lobules with loss of cellular definition and nuclear pyknosis, even without hematoxylin counterstaining (eosin only) (Figure 1a). By Sirius red staining, we observed comparable progression of fibrosis in both models (Figure 1a). Analysis of gene expression profiles demonstrated an increase in Prom1 expression prior to 3 weeks post-BDL 6.6±0.9 fold (p<0.05) weeks and a 12±4 fold (p<0.05) after 3 weeks post-BDL (Figure 1b). In contrast, there was no observable change in Prom1 expression with CCl4 treatment (- 0.7±0.5-fold change, NS). These data suggest a biliary-fibrosis specific upregulation of Prom1.
Figure 1.
(A) Collagen deposition demonstrated by sirius red staining with BDL and CCl4 liver injuries (counterstained with eosin only). (B) Gene expression early and later after BDL and CCL4. Scale bar = 50 µm. * denote p < 0.05.
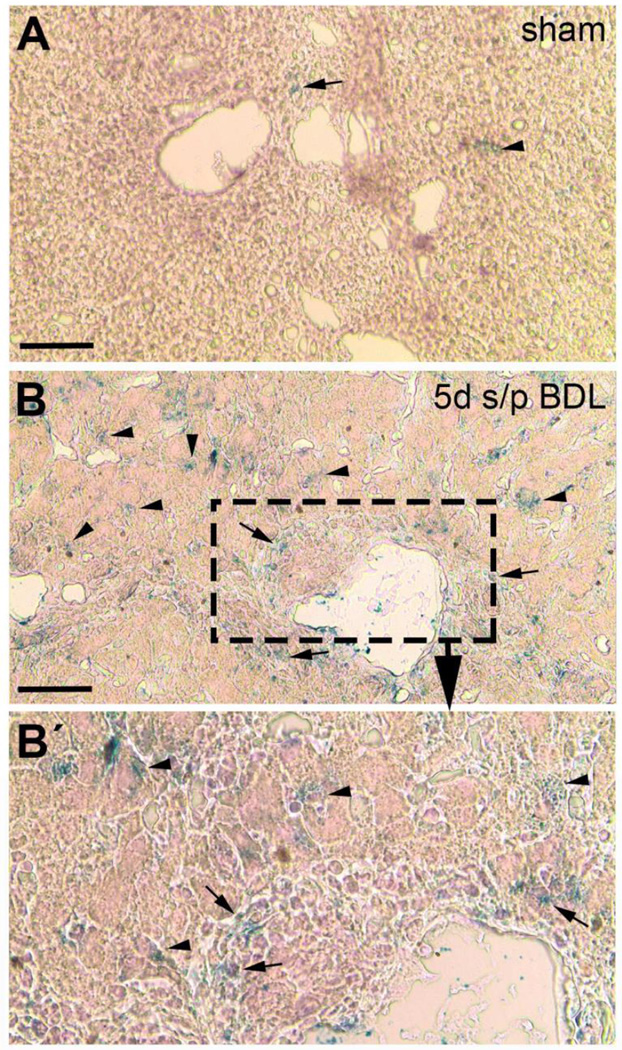
Spatial localization of Prom1 expression was then performed by LacZ staining (eosin counterstain only) of livers from Prom1cre-ert2-nlacz mice 5 days after BDL, where β-galactosidase expression marks active expression of Prom1. Rare cells positive for LacZ staining were observed 5 days after sham operation (Figure 2a). In contrast, 5 days after BDL, there were significantly more cells positive for LacZ within periportal regions near bile ducts and also within the liver parenchyma, along sinusoids where hepatic stellate cells reside (8.6 ± 1.2 vs 37.2 ± 8.8, unpaired t-test, p<0.05) (Figure 2b, 2b’). These data are consistent with the observed upregulation of Prom1 expression by qPCR (Figure 1b).
Figure 2.
LacZ staining (counterstained with eosin only) in Prom1cre-ert2-lacz mice 5 days after undergoing (A) sham operation or (B) BDL. Note the greater blue staining, which mark sites of Prom1 expression, after BDL (B, and inset B’) compared to sham. Scale bar = 50 µm.
Cell fate of Prom1-expressing cells after bile duct ligation
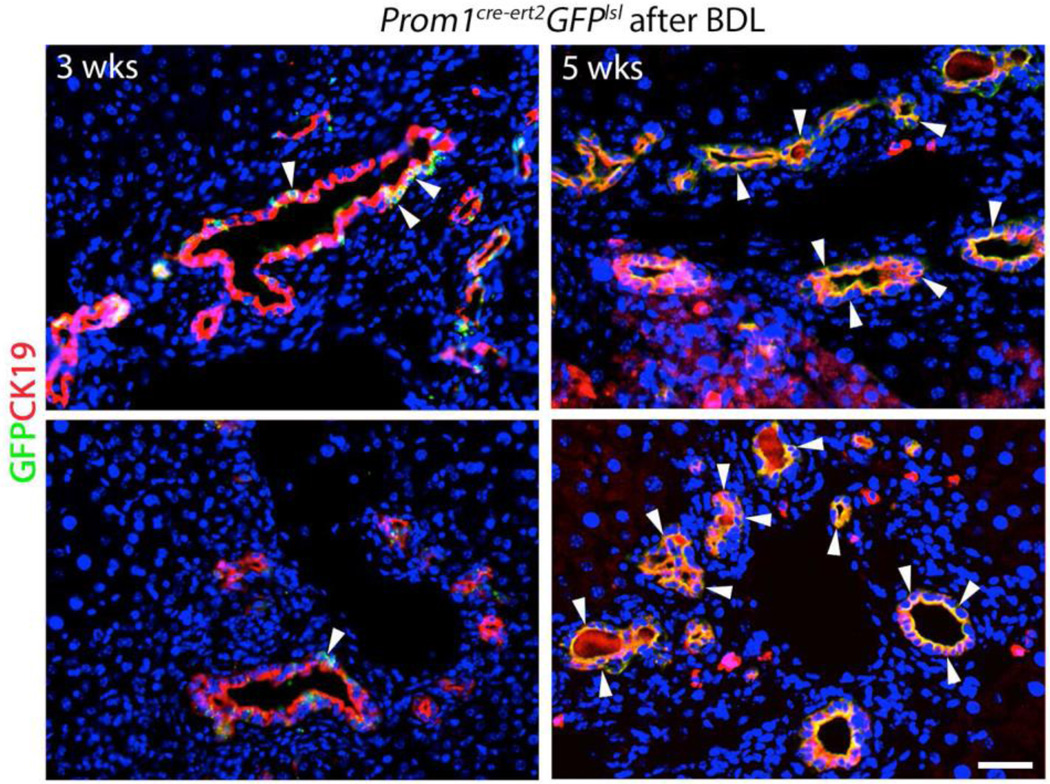
In order to determine if Prom1-expressing cells transdifferentiate during biliary fibrosis, we crossed Prom1cre-ert2-lacz mice with Gfplsl mice. Tamoxifen was administered to 4-week old Prom1cre-ert2;Gfplsl mice 3 weeks prior to BDL in order to induce expression of Prom1-expressing cell-specific Cre-driven recombination to permanently express GREEN FLUORESCENT PROTEIN (GFP) in their subsequent progeny. Three weeks after BDL, CK19positive (pos) ductular reactions were observed in the periportal regions of hepatic lobules; few CK19pos cells were co-positive for GFP, indicating derivation from cells that were originally expressing Prom1 at the time of tamoxifen administration (Figure 3). Two weeks later, at 5 weeks post-BDL, nearly all cells in CK19pos ductular reactions were GFPpos. These data suggest that over time, ductular reactions increasingly derive from Prom1-expressing precursor cells.
Figure 3.
Lineage tracing of Prom1-expressing cells labeled with Green Fluorescent Protein, via tamoxifen injection 3 weeks prior to BDL, 3 and 5 weeks after BDL in Prom1cre-ert2-lacz;GFPlsl mice. CK19 (red). Arrowheads mark GFPposCK19pos (yellow) cells. Scale bar = 25 µm.
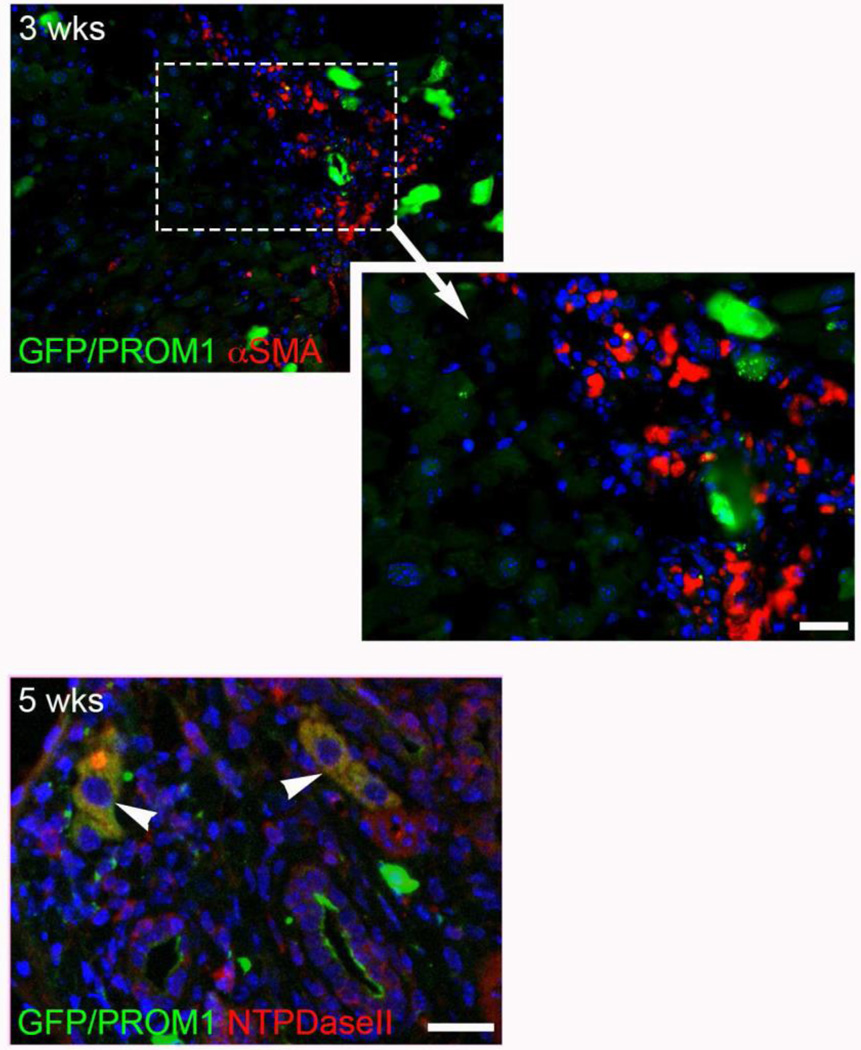
Co-positivity of GFP with α-SMOOTH MUSCLE ACTIN (αSMA) was also noted in rare fibroblast-like cells that could represent aPFs; however, most αSMApos cells were αSMAneg up to 5 weeks. At 5 weeks, co-positivity of GFP with NTPDase-II was observed in periportal regions but only within rare larger hepatocyte-like cells and not small fibroblast-like cells (Figure 4).
Figure 4.
Lineage tracing of Prom1-expressing cells labeled with Green Fluorescent Protein, via tamoxifen injection 3 weeks prior to BDL, 3 and 5 weeks after BDL in Prom1cre-ert2-lacz;GFPlsl mice. (A) NTPdase II (red), (B) αSMA (red). Arrowheads mark yellow co-positive cells. Note the presence of red single positive cells. Scale bar = 25 µm.
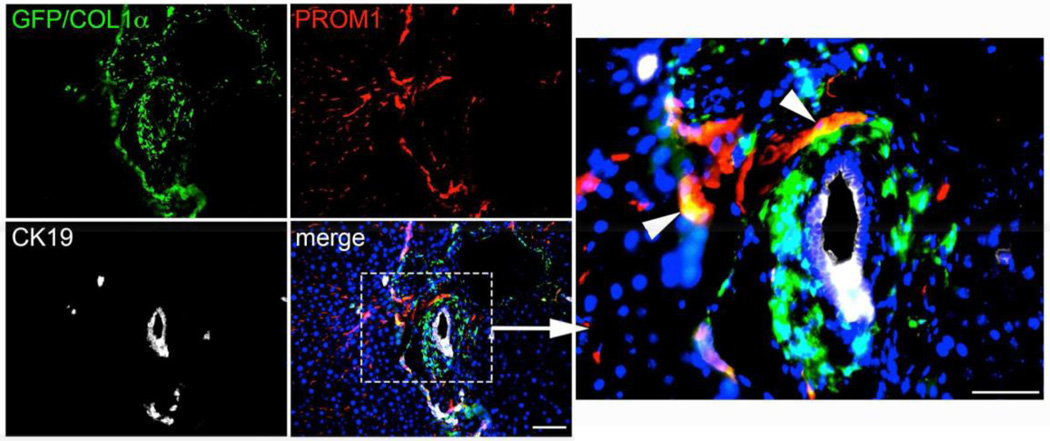
Three weeks following BDL in Col1αGfp mice, GFPpos cells, which are actively expressing Collagen-1α, were observed within regions of evolving biliary fibrosis. Some PROM1posGFPpos and PROM1posGFPneg cells were observed but PROM1posCK19pos ductular reactions were not (Figure 5). Collectively these data suggest that Prom1-expressing progenitor cells transdifferentiate predominantly into biliary ductular reactive cells and contribute minimally to the pool of collagen- producing myofibroblasts. Our data also suggest that ductular reactions which derive from Prom1-expressing progenitors, lose their expression of PROM1 over time.
Figure 5.
Immunofluorescence co-localization of GFP, CK19, and PROM1 in Col1αGfp mice 3 weeks after BDL. Arrowheads mark yellow cells co-positive for PROM1 and GFP (Collagen). Scale bar = 75 µm.
Discussion
In this study, we demonstrated a biliary fibrosis-specific upregulation of Prom1 expression after BDL. We further demonstrated evidence via histological lineage tracing, that ductular reactions within regions of evolving biliary fibrosis are increasingly comprised of cells deriving from Prom1-expressing progenitor cells. In contrast, we observed only a minimal contribution of Prom1-expressing cells to the pool of collagen producing myofibroblasts. Collectively, these data support the concept that Prom1–expressing progenitor cells transdifferentiate primarily into biliary ductular reactions, within regions of evolving biliary fibrosis, adjacent to collagen- expressing cells.
The underlying pathophysiology of biliary fibrosis, secondary to cholestatic liver injury, remains undetermined. Using Col1αGfp mice, Iwaisako et al. reported that during early biliary fibrogenesis following BDL, aPFs, which reside in periportal regions where biliary fibrogenesis originates, contribute more significantly to the population of collagen-producing myofibroblasts than hepatic stellate cells (HSCs), which reside in the hepatic sinusoids throughout the hepatic lobule [10]. Lua et al., however, observed that activated HSCs, not aPFs, are the predominant myofibroblasts following BDL [11]. Regardless of which cell population contributes most to the pool of collagen-producing myofibroblasts, our observations are consistent with Prom1-expressing progenitor cells transdifferentiating into cells comprising biliary ductular reactions within regions of biliary fibrosis during cholestatic liver injury. Furthermore, our data indicate that ductular reactions may lose expression of PROM1 over time following BDL. It is unclear how significant any downregulation of PROM1 in developing ductular reactions is, but we posit that this may be in response to terminal differentiation of a progenitor cell population.
The significance of Prom1-expressing biliary progenitor cell transdifferentiation remains unanswered by this study; however, there is evidence that Prom1- expressing hepatic progenitor cells (HPCs) may contribute to biliary fibrosis. Rountree et al. demonstrated that PROM1-expressing cells comprise ductular reactions that develop in the rodent model of cholestasis/biliary fibrosis induced by 3,5-diethoxycarbonyl-1,4-dihydrocollidine (DDC) diet [14]. Moreover, expansion of a single PROM1posCD45neg HPC clone isolated from liver tumors from AlbuminCre;Ptenloxp mice could be passaged multiply maintaining an epithelial progenitor cell phenotype. However, spontaneous transformation of a subset of these PROM1pos cells was associated with a more mesenchymal, fibroblastic phenotype and functionally with tumor metastases [15]. In this study, lineage tracing of Prom1- expressing precursor cells demonstrated qualitatively a large contribution to biliary ductular reactions within regions of collagen deposition. Although the direct contribution of Prom1-expressing progenitors to collagen-producing myofibroblasts was small, a role in biliary fibrosis cannot be excluded. Indeed, Nadler et al. demonstrated that cells within ductular reactions of biliary fibrosis in the murine model of biliary atresia express INTEGRINαvβ6, a key mediator in the activation of profibrogenic Transforming Growth Factor-β [16]. We posit that the expression of Prom1 may contribute to biliary fibrogenesis potentially, in part, through promotion of TGFβ signaling activation in downstream progeny, but further analysis is required to validate this potential link.
We had previously demonstrated colocalization of PROM1 and αSMA during fibrogenesis associated with biliary atresia [9]. In this study, we demonstrated upregulation of Prom1 gene expression within the liver following BDL but not with CCl4 injury. Interestingly, by LacZ staining of Prom1cre-ert2-nlacz mice 5 days after BDL, we observed Prom1 expression not only within the periportal regions of the hepatic lobule but also along hepatic sinusoids in a pattern consistent with that of HSCs. This spatial pattern of expression is not evident with lineage tracing of Prom1-expressing cells following BDL, suggesting not only transdifferentiation of Prom1-expressing progenitor cells, but also upregulation of Prom1 expression in existing HSCs. Further analysis is required to first determine if indeed both quiescent HSCs and portal fibroblasts upregulate Prom1 expression following BDL and second to determine the significance of Prom1 expression during fibrogenesis.
In this study, we provide qualitative, histologic evidence of Prom1-expressing cell transdifferentiation during fibrogenesis associated with cholestasis following bile duct ligation. It is likely that there is significantly more biliary ductular transdifferentiation compared to myofibroblastic transdifferentiation; however, this study does not definitively prove this point given its descriptive nature. Further analyses are required to quantify and validate this point.
In summary, we demonstrate a link between Prominin-1 and biliary fibrosis and posit that PROM1 functionally promotes biliary fibrosis in part through the expansion of profibrogenic ductular reactions. As such, Prom1-expressing cells or signaling upstream or downstream of Prom1 could provide potential targets of therapeutic intervention of biliary fibrogenesis.
Supplementary Material
Acknowledgments
This work was supported by NIH grant R01AA020753 (to K.A.), pilot project funding (to K.A.) from P50AA011999, pilot project funding (to K.A.) from P30DK048522, training program (to I.L.) from T32HD060549, K08AA016290 (from K.W.) U01DK084538 (to K.W.), and the Robert E. and May R. Wright foundation award (to K.A).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This paper was previously presented as an oral presentation abstract at the Academic Surgical Congress, Annual Meeting, Jacksonville, FL, February 3, 2016.
References
- 1.Superina R, et al. The anatomic pattern of biliary atresia identified at time of Kasai hepatoportoenterostomy and early postoperative clearance of jaundice are significant predictors of transplant-free survival. Ann Surg. 2011;254(4):577–585. doi: 10.1097/SLA.0b013e3182300950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zagory JA, Nguyen MV, Wang KS. Recent advances in the pathogenesis and management of biliary atresia. Curr Opin Pediatr. 2015;27(3):389–394. doi: 10.1097/MOP.0000000000000214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu L, et al. Prominin 1 marks intestinal stem cells that are susceptible to neoplastic transformation. Nature. 2009;457(7229):603–607. doi: 10.1038/nature07589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Corbeil D, et al. The intriguing links between prominin-1 (CD133), cholesterol-based membrane microdomains, remodeling of apical plasma membrane protrusions, extracellular membrane particles, and (neuro)epithelial cell differentiation. FEBS Lett. 2010;584(9):1659–1664. doi: 10.1016/j.febslet.2010.01.050. [DOI] [PubMed] [Google Scholar]
- 5.Marzesco AM, et al. Release of extracellular membrane particles carrying the stem cell marker prominin-1 (CD133) from neural progenitors and other epithelial cells. J Cell Sci. 2005;118(Pt 13):2849–2858. doi: 10.1242/jcs.02439. [DOI] [PubMed] [Google Scholar]
- 6.Bidlingmaier S, Zhu X, Liu B. The utility and limitations of glycosylated human CD133 epitopes in defining cancer stem cells. J Mol Med (Berl) 2008;86(9):1025–1032. doi: 10.1007/s00109-008-0357-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu L, et al. Multi-organ Mapping of Cancer Risk. Cell. 2016;166(5):1132–1146. doi: 10.1016/j.cell.2016.07.045. e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mavila N, et al. Functional Human and Murine Tissue-Engineered Liver Is Generated From Adult Stem/Progenitor Cells. Stem Cells Transl Med. 2016 doi: 10.5966/sctm.2016-0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mavila N, et al. Expansion of prominin-1-expressing cells in association with fibrosis of biliary atresia. Hepatology. 2014 doi: 10.1002/hep.27203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iwaisako K, et al. Origin of myofibroblasts in the fibrotic liver in mice. Proc Natl Acad Sci U S A. 2014;111(32):E3297–E3305. doi: 10.1073/pnas.1400062111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lua I, et al. Characterization of hepatic stellate cells, portal fibroblasts, and mesothelial cells in normal and fibrotic livers. J Hepatol. 2016;64(5):1137–1146. doi: 10.1016/j.jhep.2016.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Utley S, et al. Fibroblast Growth Factor signaling regulates the expansion of A6- expressing hepatocytes in association with AKT-dependent β-catenin activation. Journal of Hepatology. 2014;60(5):1002–1009. doi: 10.1016/j.jhep.2013.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mavila N, et al. Fibroblast growth factor receptor-mediated activation of AKT-beta- catenin-CBP pathway regulates survival and proliferation of murine hepatoblasts and hepatic tumor initiating stem cells. PLoS One. 2012;7(11):e50401. doi: 10.1371/journal.pone.0050401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rountree CB, et al. A CD133-expressing murine liver oval cell population with bilineage potential. Stem Cells. 2007;25(10):2419–2429. doi: 10.1634/stemcells.2007-0176. [DOI] [PubMed] [Google Scholar]
- 15.Ding W, et al. Epithelial-to-mesenchymal transition of murine liver tumor cells promotes invasion. Hepatology. 2010;52(3):945–953. doi: 10.1002/hep.23748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nadler EP, et al. Integrin alphavbeta6 and mediators of extracellular matrix deposition are up-regulated in experimental biliary atresia. J Surg Res. 2009;154(1):21–29. doi: 10.1016/j.jss.2008.05.023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.