Abstract
Background
Excessive ethanol consumption remains an important health concern and effective treatments are lacking. The central oxytocin system has emerged as a potentially important therapeutic target for alcohol and drug addiction. These studies tested the hypothesis that oxytocin reduces ethanol consumption.
Methods
Male C57BL/6J mice were given access to ethanol (20% v/v) using a model of binge-like drinking (“drinking-in-the-dark”) that also included the use of lickometer circuits to evaluate the temporal pattern of intake as well as 2-bottle choice drinking in the home cage. In addition, ethanol (12% v/v) and sucrose (5% w/v) self-administration on fixed- and progressive- ratio schedules were also evaluated. A wide range of systemically administered oxytocin doses were tested (0 to 10 mg/kg) in these models.
Results
Oxytocin (0, 0.3, 1, 3 or 10mg/kg) dose-dependently reduced ethanol consumption (maximal 45% reducton) in the binge drinking model, with lower effective doses having minimal effects on general locomotor activity. Oxytocin’s effect was blocked by pretreatment with an oxytocin receptor antagonist and the pattern of contacts (licks) at the ethanol bottle suggested a reduction in motivation to drink ethanol. Oxytocin decreased 2-bottle choice drinking without altering general fluid intake. Oxytocin also reduced operant responding for ethanol and sucrose in a dose-related manner. However, oxytocin decreased responding and motivation (breakpoint values) for ethanol at doses that did not alter responding for sucrose.
Discussion
These results indicate that oxytocin reduces ethanol consumption in different models of self-administration. The effects are not likely due to a general sedative effect of the neuropeptide. Further, oxytocin reduces motivation for ethanol at doses that do not alter responding for a natural reward (sucrose). While some evidence supports a role for oxytocin receptors in mediating these effects, additional studies are needed to further elucidate underlying mechanisms. Neverthess, these results support the therapeutic potential of oxytocin as a treatment for alcohol use disorder.
Keywords: oxytocin, alcohol binge-drinking, alcohol self-administration, mouse
INTRODUCTION
Oxytocin is an endogenous neurohormone synthesized in the paraventricular nucleus (PVN) and supraoptic nucleus (SON) of the hypothalamus and released by the posterior pituitary into peripheral circulation. In addition, oxytocin is released by neurons in the PVN that project to numerous extrahypothalamic regions in the brain (e.g., cortical, limbic, basal ganglia structures) where it mediates an array of behavioral effects via interaction with G(q)-coupled oxytocin receptors (Lee et al., 2016). Aside from its known hormonal role in parturition and maternal behaviors, oxytocin also regulates a number of behaviors that involve social interactions (e.g., pair-bonding, social reward processing, aggression) and nonsocial behaviors, including anxiety and stress responses (Baskerville and Douglas, 2010; Bowen et al., 2011; Carson et al., 2013; Neumann and Landgraf, 2012).
A growing body of literature suggests that oxytocin plays a role in several neuropsychiatric disorders that involve deficits in social behaviors, including drug and alcohol addiction (Baskerville and Douglas, 2010; Lee and Weerts, 2016). For example, while alcohol use initially promotes pro-social behaviors, continued excessive drinking can lead to long-term social deficits associated with alcohol dependence, a negative consequence that may be mediated, at least in part, by adaptations in the oxytocin system (McGregor and Bowen, 2012; Stauffer and Woolley, 2014; Young et al., 2014). In a recent clinical study, Pedersen and colleagues demonstrated that intranasal oxytocin treatment attenuated alcohol withdrawal symptoms in treatment-seeking human subjects compared to placebo (Pedersen et al., 2013). Further, a number of preclinical studies provide support for a role of oxytocin in modulating alcohol preference and consumption. In a recent study, oxytocin was shown to reduce operant responding for alcohol in Sprague-Dawley rats (MacFadyen et al., 2016). Acute oxytocin treatment also produced a significant and lasting decrease in preference for an alcohol-containing solution versus a non-alcoholic sucrose solution in alcohol-preferring “P” rats (McGregor and Bowen, 2012). Additionally, Bowen and collegues demonstrated that chronic exposure to exogenous oxytocin in adolescence decreased alcohol consumption in adult rats (Bowen et al., 2011). Thus, administration of exogenous oxytocin has been shown to alter a number of alcohol effects, including its motivational effects.
Many of the oxytocin PVN projections in brain overlap with dopamine systems critically involved in processing motivationally relevant stimuli. An emerging literature suggests that oxytocin can alter dopaminergic neurotransmission within mesocorticolimbic dopamine systems, thereby altering motivational aspects of not only social and maternal behavior, but also alcohol/drug addiction (Baskerville and Douglas, 2010; Burkett and Young, 2012; Groppe et al., 2013; Love, 2014). For example, direct (intracerebroventricular) infusion of oxytocin blocked alcohol-induced increases in dopamine release in the nucleus accumbens and reduced voluntary alcohol consumption in Wistar rats (Peters et al., 2016). Additionally, viral-mediated overexpression of oxytocin receptors in the nucleus accumbens reduced alcohol-induced conditioned place preference and alcohol consumption in C57BL/6J mice (Bahi, 2015; Bahi et al., 2016). Taken together, accumulating evidence suggests that the oxytocin system may represent a promising target for the treatment of alcohol use disorders (Lee et al., 2016; Lee and Weerts, 2016).
However, few studies have examined the broad efficacy and specificity of systemically administered oxytocin in reducing voluntary ethanol consumption, and whether oxytocin influences the motivational effects of ethanol. In the present study, we investigated the effects of systemic administration of oxytocin on ethanol self-administration in a binge-like drinking model, in a two-bottle choice paradigm and in a model involving operant conditioning procedures. Specificity of oxytocin effects in the operant studies were examined by conducting parallel studies with sucrose solutions. It was hypothesized that oxytocin would selectively decrease ethanol consumption in these models at doses that would not affect sucrose consumption under similar testing conditions.
MATERIALS AND METHODS
Subjects
Adult male C57BL/6J mice (25–30g) were obtained from Jackson Laboratories (Bar Harbor, ME). Mice arrived at 9–10 weeks of age and were acclimated to the experimental housing rooms for a minimum of 1 week prior to the start of experiments. Mice were individually housed under a 12 hr reversed light/dark cycle in an AAALAC accredited facility. All testing was conducted during the dark phase of the circadian cycle. Mice were provided free access to food and water, except at the start of operant oral self-administration training. All experimental protocols were approved by the Medical University of South Carolina Institutional Animal Care and Use Committee and consistent with guidelines of the NIH Guide for the Care and Use of Laboratory Animals.
Binge-Like Ethanol Consumption
The effect of oxytocin (OT) on binge-like ethanol consumption was examined in C57BL/6J mice (n= 40) using the drinking-in-the-dark (DID) paradigm (Rhodes et al., 2005). Mice were presented in the home-cage with a single 15-ml graduated bottle of 20% (v/v) ethanol 3 hr into the dark phase of the circadian light cycle. Mice were provided access to ethanol for 2 hr/day for 3 consecutive days. On the fourth day, access to ethanol was extended to 4 hr. Vehicle injections were given 30 min prior to the 2-hr drinking sessions during the first three days to acclimate mice to handling and the injection procedure. On Day-4, separate groups of mice were injected intraperitoneally (ip.) with OT (0, 1, 3, or 10 mg/kg) 30 min prior to the 4 hr drinking session. These OT doses and the route of administration were selected based on previous work in rodents (e.g., Bahi, 2015; Bowen et al., 2011; McGregor and Bowen, 2012; Peters et al., 2013). Immediately following this test session, blood samples were collected to measure blood ethanol concentration (BEC) using an Analox Instrument analyzer (Lunenburg, MA).
Since results from the previous study indicated that the 3 and 10 mg/kg doses of OT were similarly efficacious in reducing alcohol consumption, a follow-up study was conducted to extend these findings to a lower dose range for OT. Additionally, to assess the temporal distribution of ethanol consumption during the 4 hr session, a separate group of mice (n= 24) was tested in the DID model, with the ethanol bottles fitted with lickometers as previously described (Griffin et al., 2009). Briefly, contacts (i.e., “licks”) with the ethanol tube completed a circuit grounded to the stainless steel grid floor inside of the home cage. The metal drinking spouts were covered with plastic shrink-wrap coating to limit extraneous contacts. The metal cage floor and tubes were connected through lickometer circuits (Med Associates, Inc., St. Albans, VT), and contacts were monitored and recorded by a computer outside of the testing room. Mice were acclimated to the lickometers in their home cage by providing drinking water (24 hr/day) in bottles connected to the lickometer for one week prior to DID testing. As in the previous experiment, vehicle injections were given to all mice 30 min before the 2-hr drinking sessions and on the fourth day, separate groups of mice were injected (ip.) with OT (0, 0.3, 1, or 3 mg/kg) 30 min prior to the 4-hr test session.
Finally, to investigate whether exogenous oxytocin administration was exerting its effects on ethanol consumption in the DID model via actions at the oxytocin receptor, a separate cohort of mice (n= 40) was pretreated with the selective brain penetrant, nonpeptide oxytocin receptor antagonist L-368,899 (10 mg/kg) (Borthwick, 2010; Kuteykin-Teplyakov and Maldonado, 2014; Olszewski et al., 2013) or vehicle (0.9% saline) 15 min prior to OT (1 mg/kg) or vehicle (saline) administration (ip.), which was given 30 min before the start of the 4-hr test drinking session.
Two-Bottle Choice Ethanol Drinking
Mice (N= 12) were provided unlimited daily access to two drinking bottles in the home cage containing either 20% (v/v) ethanol or tap water. Since OT has been reported to reduce general fluid intake in rats (Arletti et al., 1990), a separate group of mice (N= 9) was given two bottles containing water throughout the study to assess potential effects of OT treatment on general water intake. The position of the bottles was alternated daily to avoid a side preference. Mice were weighed weekly and fluid intake was measured at 4 hr and 24 hr. After establishing stable daily ethanol and water intake over 3 weeks, half the mice in the ethanol vs. water and water vs. water groups were injected (ip.) with 1 mg/kg OT while the remaining mice in each group received a saline injection. Following an additional 3 days when all mice were treated with saline, mice were tested again with the alternative drug (1 mg/kg OT or saline). Data were collapsed across dose condition for analyses.
Locomotor Activity
Following the DID study involving use of lickometer circuitry, mice were left undisturbed for 4 weeks and then tested for effects of oxytocin on locomotor activity. The mice (n= 24) were injected (ip.) with the same doses of OT (0, 0.3, 1, or 3 mg/kg) 30 min before the start of the test session. Injections occurred 2.5 hr into the dark phase of the circadian cycle to match the DID procedure. Locomotor activity in an open field arena was assessed using Med Associates (St. Albans, VT, USA) activity chambers (27 × 27 × 20 cm; ENV-510) situated in sound-attenuating cubicles with a ventilation fan. Animal movement was tracked through the use of 16 horizontal infrared photo beams, located on both the X and Y-axes for positional and rearing detection. Photo beam breaks were recorded and converted to distance traveled using activity-monitoring software (Activity Monitor 5.0; Med Associates).
Operant Self-Administration
Operant Conditioning Chambers
Mice were tested in standard operant chambers configured with two retractable levers, a centrally located well for liquid reinforcers, house light, and a tone generator and stimulus light above the well. The chambers were situated in sound-attenuating cubicles with a ventilation fan (Med Associates, St. Albans, VT). One lever was assigned as the “active” lever, with responses resulting in access to ethanol or sucrose reinforcement paired with a light and tone stimulus (80 dB). Ethanol or sucrose reinforcement (20 ul) was delivered to the well by an infusion pump (activated for 2 sec). Responses on the ‘inactive lever” were recorded, but did not result in any stimulus consequences or reinforcement delivery. Stimulus events and responses were controlled and monitored using Med PC, Version IV software (Med Associates, Inc., St. Albans, VT). At the end of each session, any residual fluid left in the well was collected with a pipette, measured, and subtracted from the total volume of reinforcer delivered. The corrected volume was used to calculate g/kg ethanol or ml/kg sucrose intake during the sessions.
Ethanol and Sucrose Self-Administration Procedure
Mice were trained to orally self-administer ethanol or sucrose using a post-prandial consumption procedure to facilitate acquisition of the behavior. One hour prior to the beginning of initial self-administration sessions, water bottles were removed from the cages and mice were given access to standard home cage chow for 15 min. Water bottles were returned to the home cage following the conclusion of the behavioral session. During the post-prandial manipulation (2–3 weeks), weights were monitored and food was provided after the behavioral session to maintain weight at 90–95% pre-training body weight. Mice were trained to respond on a fixed-ratio (FR)-1 schedule for access to 12% unsweetened ethanol or 5% sucrose solution in 20 min sessions. Once mice acquired stable rates of responding, the response requirement was increased to FR2 and then FR4 over 1–3 weeks. When mice demonstrated stable responding on the FR4 schedule, they were returned to ad libitum food and water access for the remainder of the study. Experiments were initiated after response rates had stabilized (<15% variability over 3 consecutive days). To assess the effects of oxytocin administration on operant self-administration of ethanol (n= 13) or sucrose (n= 18), mice were injected (ip.) with different doses of OT (0, 0.1, 0.3, or 1 mg/kg) 30 min before self-administration sessions. Each mouse received all treatment conditions in a Latin-square design, counter-balanced to avoid an order of treatment bias. Baseline levels of responding were re-established without drug pre-treatment for a minimum of 3 days between treatments.
Progressive Ratio Testing
The effect of oxytocin on motivation to seek ethanol or sucrose reward was assessed in a subset of the animals trained to self-administer ethanol (n= 10) or sucrose (n= 12) using a progressive ratio paradigm. To avoid any residual drug effects, mice were left undisturbed in the home cage for four consecutive weeks prior to re-establishing baseline lever responding. After stable baseline (FR4) responding was re-established (<15% variability over 3 consecutive days), animals were tested on a progressive ratio (PR) schedule. Mice were injected (ip.) with either OT (0.3 mg/kg) or vehicle 30 min prior to testing using a crossover design over two test sessions spaced 7 days apart. The PR schedule followed an arithmetic series, with the response requirement to obtain ethanol or sucrose reinforcement increased by four (i.e., FR 4, 8, 12, 16, 20, etc.). Breakpoint was defined as the highest ratio value reached during the session. The session was terminated following any 5-min period of inactivity on the active lever. Maximum session length was 3 hr, at which point the session was terminated.
Drugs
Synthetic human oxytocin (CellSciences, Canton, MA) and the oxytocin receptor antagonist, L-368,899 hydrochloride (Tocris Bioscience, Minneapolis, MN) were dissolved in 0.9% saline, which served as the vehicle. Injections were administered intraperitoneally at a volume of 0.01 ml/g body weight in all experiments. Ethanol (95%) was obtained from AAPER (Shelbyville, KY) and diluted with tap water to the appropriate concentration. Sucrose was also diluted to the appropriate concentration with tap water (5% w/v). The ethanol and sucrose solutions were prepared daily and presented at room temperature.
Statistical Analysis
Primary dependent variables were ethanol intake (g/kg), BEC (mg/dl), sucrose intake (mL/kg), ethanol lick contacts, and distance traveled (cm) for the binge-drinking studies. Ethanol intake (mL; g/kg), water intake (mL), total fluid intake (mL), and ethanol preference ratio (24 hr) were analyzed in the 2-bottle choice ethanol drinking study. Dependent measures for the operant studies included lever responses and ethanol/sucrose intake, as well as break points on the PR schedule. Data were analyzed by analysis of variance (ANOVA), with Dose as a between-subjects factor (DID model studies) or a within-subjects factor (2-bottle choice drinking and operant conditioning studies). The factor Time served as a repeated measure in analyses of lick responses and locomotor activity. Breakpoint data from PR testing were analyzed by t-tests. Further analyses were conducted using Newman-Keuls test, when appropriate. For all analyses, significance levels were set at p< 0.05, and data are presented as mean ± standard error of the mean (SEM).
RESULTS
Effect of Oxytocin on Binge-Like Ethanol Consumption
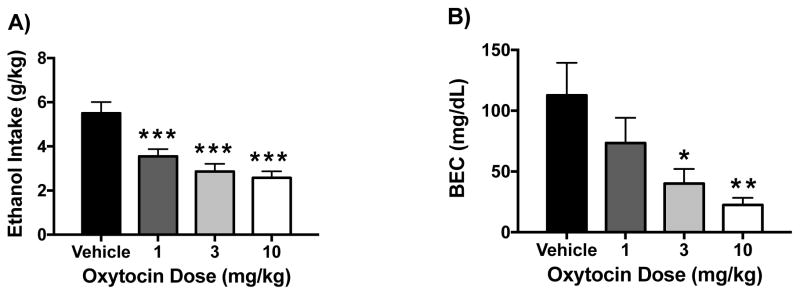
Oxytocin significantly reduced ethanol consumption and resultant blood ethanol concentrations (BEC) in a dose-related manner. ANOVA revealed a significant effect of Dose [F(3,36)= 12.31, p< 0.0001] on ethanol intake, and post hoc analysis indicated a significant decrease in consumption following 1 mg/kg, 3 mg/kg, and 10 mg/kg OT compared to vehicle (Figure 1A). Analysis of BEC data indicated a similar profile of results, with ANOVA indicating a significant effect of Dose [F(3,36)= 4.86, p< 0.01]. Further analysis indicated that 3 and 10 mg/kg OT doses significantly reduced BEC compared to the vehicle condition, with a trend for reduction at the 1 mg/kg dose (Figure 1B).
Figure 1.
Effect of oxytocin on binge-like ethanol drinking. Oxytocin reduced ethanol intake (g/kg) (A) and resulting blood ethanol concentrations (BEC) (B) in the DID model in a dose-related manner. Values are mean ± s.e.m. (N= 10/group). Significantly differs from vehicle condition: * (p< 0.05); ** (p< 0.01); *** (p< 0.001).
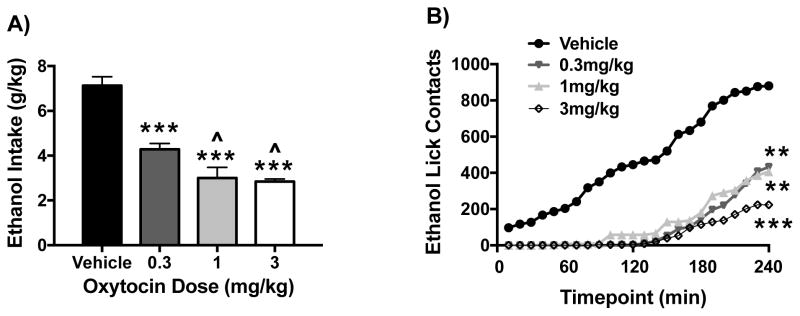
In a follow-up study with a separate cohort of mice, a lower OT dose range was examined on ethanol intake in the DID model. Additionally, lickometer circuitry was employed to examine the temporal pattern of ethanol intake in the study. As in the previous study, ANOVA revealed a significant effect of OT Dose [F(3,19)= 35.18, p< 0.0001] on ethanol consumption during the 4-hr drinking session. Post hoc analysis revealed that 0.3, 1, and 3 mg/kg OT doses significantly decreased ethanol intake compared to the vehicle condition, and the two higher doses of OT were more effective than the lowest (0.3 mg/kg) OT dose in reducing ethanol intake (Figure 2A). Further, OT administration decreased licking responses at the ethanol bottle in a dose-related manner (Figure 2B). Whereas mice treated with vehicle evidenced relatively consistent intake over the entire 4-hr session with cumulative licks increasing steadily over time, mice treated with OT exhibited significant delay in contact with the ethanol bottle, only beginning to drink 1 to 2 hours after bottles were available. This was supported by ANOVA of the lick data, which revealed significant main effects of OT Dose [F(3,18)= 30.88, p< 0.0001] and Time [F(23,414)= 57.10, p< 0.0001], and a Dose × Time interaction [F(69,414)= 5.03, p< 0.0001]. Analysis of total cumulative lick responses indicated a significant effect of OT Dose [F(3,18)= 14.39, p< 0.001] and post hoc analyses indicated that all three OT doses (0.3, 1, and 3 mg/kg) significantly decreased ethanol lick contacts compared to vehicle over the entire 4-hr drinking session. Further, there was a trend for the highest OT dose to reduce ethanol bottle contacts more than the two lower doses (p< 0.07).
Figure 2.
Effect of oxytocin on ethanol intake and lick contacts. Oxytocin reduced ethanol consumption (g/kg) (A) and decreased as well as delayed ethanol bottle lick contacts over the 4-hr drinking session (B) in the DID model in a dose-related manner. Values for ethanol intake are mean ± s.e.m. and mean lick responses are plotted as a function of 10-min intervals (N= 5–6/group). Significantly differs from the vehicle group: ** (p< 0.01); *** (p< 0.001). Significantly differs from 0.3 mg/kg OXT dose: ^ (p< 0.05).
Reversal of Oxytocin Effect on Ethanol Consumption by an Oxytocin Receptor Antagonist
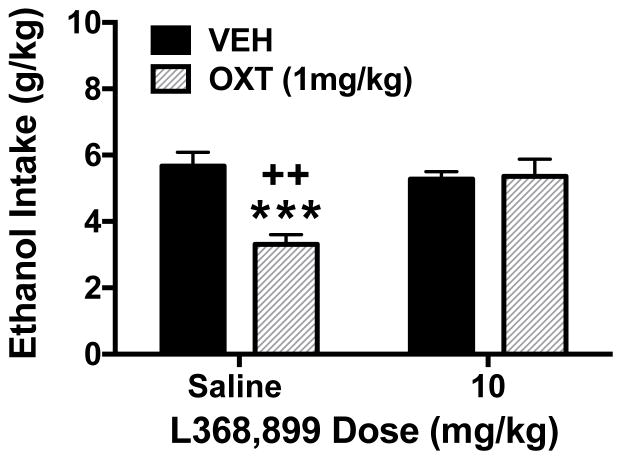
To determine whether OT reduced ethanol consumption via its receptor, separate groups of mice were treated with a selective OT receptor antagonist, L368,889, before OT or vehicle (saline) administration. As previously observed, OT (1 mg/kg) significantly reduced ethanol consumption in the DID model. However, this effect was blocked in animals that were pretreated with the OT receptor antagonist (Figure 3). These observations were supported by ANOVA, which revealed a significant main effect of OT Dose [F(1,36)= 9.08, p< 0.005] and an OT × Antagonist interaction [F(1,36)= 10.36, p< 0.003]. Post hoc analysis indicated that OT alone significantly reduced ethanol intake and that ethanol intake was significantly greater in OT-treated mice that received L-368,889 (10 mg/kg) compared to mice that received saline pretreatment. The OT receptor antagonist did not alter ethanol consumption when given alone.
Figure 3.
Oxytocin-induced reduction in binge-like ethanol consumption is blocked by pretreatment with an oxytocin receptor antagonist. Oxytocin (1 mg/kg) reduced ethanol intake in the DID model and this effect was reversed by pretreatment with the oxytocin receptor antagonist L368,889 (10 mg/kg). Values are mean ± s.e.m. (N= 10/group). Significantly differs from the vehicle-saline condition: *** (p< 0.001). Significantly differs from the L368,889-OXT group: ++ (p< 0.01).
Effect of Oxytocin on Locomotor Activity
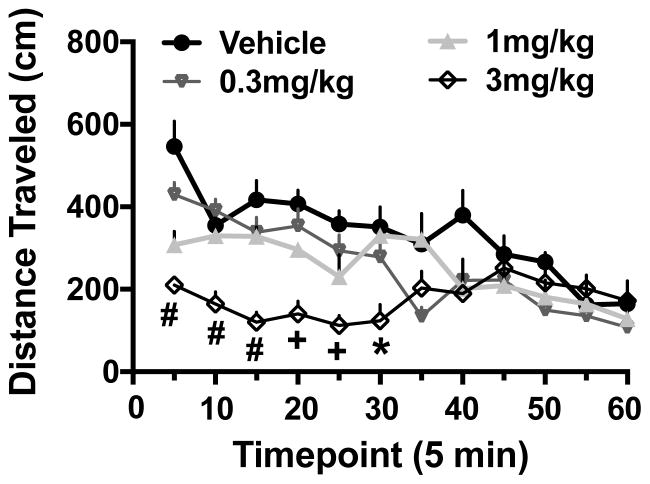
The reduction in ethanol consumption by OT in concert with the delayed temporal pattern of ethanol intake prompted additional examination of whether the OT-induced decrease in ethanol intake is due to a general sedative effect of the neurohormone. Within 5 min following administration, OT decreased locomotor activity in a dose-related manner. While this difference relative to the vehicle condition rapidly dissipated for the lower (0.3 and 1 mg/kg) OT dose groups, reduced activity produced by the highest dose tested (3 mg/kg) persisted for a longer period of time during the test session (Figure 4). ANOVA of activity data (distance traveled in the open field) indicated significant main effects of Dose [F(3,19)= 9.87, p< 0.0001] and Time [F(11,209)= 12.76, p< 0.0001] as well as the Dose × Time interaction [F(33,209)= 3.90, p< 0.0001]. Overall, activity decreased across the testing session for all groups. Further, all OT doses significantly decreased distance traveled compared to the vehicle group within the first 5 min of the test. However, while activity for mice treated with 0.3 and 1 mg/kg OT doses was similar to the vehicle group within 10 min, recovery to control (vehicle) levels required approximately 40–45 min for mice treated with 3 mg/kg OT.
Figure 4.
Effects of oxytocin on locomotor activity. Oxytocin (0.3, 1, or 3 mg/kg) or vehicle was injected (ip.) 30 minutes before locomotor activity was recorded in an open field during a 4-hr session. Shown are mean ± s.e.m. Values for distance traveled during first hour of locomotor activity measurement (N= 5–6/group). Significantly differs from the vehicle condition: * (p< 0.05); + (p< 0.01); # (p< 0.001).
Effect of Oxytocin on 2-Bottle Choice Ethanol Drinking
Oxytocin treatment reduced ethanol consumption without significantly altering total fluid intake under continuous access conditions (Table 1). At 4 hr following injection, OT (1 mg/kg) significantly reduced ethanol intake (mL: [F(1,11)= 5.92, p< 0.05] and g/kg: [F(1,11)= 6.16, p< 0.05]), while not altering total fluid intake [F1,11)< 1.0]. Similarly, OT (1 mg/kg) reduced ethanol intake over 24 hr (mL: [F(1,11)= 20.39, p< 0.001] and g/kg: [F(1,11)= 20.87, p< 0.001]). The decrease in ethanol consumption was accompanied by increased water intake [F(1,11)= 6.51, p< 0.05], resulting in no change in total fluid intake [F(1,11)< 1.0]. OT treatment also significantly reduced ethanol preference [F(1,11)= 12.80, p< 0.01] (Table 1). In animals given access to only water, OT did not alter intake at 4 hr [F(1,8)= 1.37, p> 0.1] or 24 hr [F(1,8)= 0.07, p> 0.1].
Table 1.
2-Bottle Choice Ethanol and Water Intake Following Vehicle (Sakube) or Oxytocin (1 mg/kg) Treatment
| 4-Hour Intake | 24-Hour Intake | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| EtOH (mL) | Water (mL) | Total (mL) | EtOH (g/kg) | EtOH (mL) | Water (mL) | Total (mL) | EtOH (g/kg) | EtOH Pref | ||
| EtOH vs. Water | Veh | 0.58 (0.06) | 0.43 (0.09) | 1.02 (0.08) | 3.65 (0.36) | 2.48 (0.36) | 1.23 (0.07) | 3.74 (0.17) | 15.67 (0.85) | 0.66 (0.01) |
| OT | 0.41 (0.05)* | 0.57 (0.07) | 0.98 (0.09) | 2.55 (0.31)* | 1.28 (0.16)* | 2.11 (0.03)* | 3.39 (0.18) | 8.09 (1.02)* | 0.41 (0.07)* | |
|
| ||||||||||
| Water vs. Water | Veh | 1.28 (0.10) | 3.79 (0.16) | |||||||
| OT | 1.02 (0.15) | 3.67 (0.42) | ||||||||
Effect of Oxytocin on Operant Ethanol Self-Administration
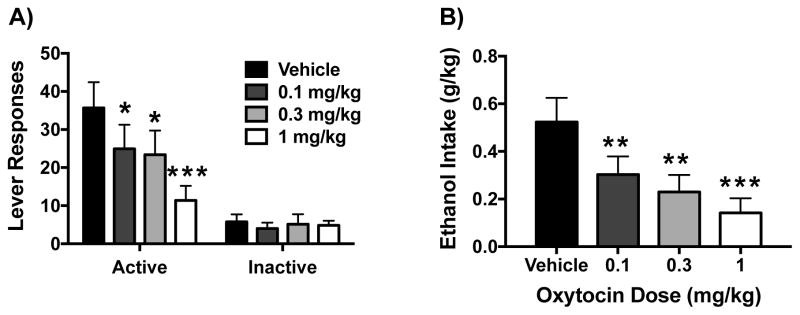
After reaching the criteria for stable responding for ethanol, the effect of OT on operant oral self-administration was assessed. For these studies, we omitted the higher doses (3 and 10 mg/kg) from the previous experiments and included a lower dose (0.1 mg/kg) of OT. Preliminary analyses indicated no significant effect of dosing order on the main dependent variables: active lever responses and ethanol (g/kg) intake [F(5,46)< 1.6 for both variables]. Thus, data was collapsed across this variable to analyze OT effects on ethanol self-administration behavior.
In a dose-related fashion OT reduced responding for ethanol on the active lever (Figure 5A) and the amount of ethanol (g/kg) self-administered (Figure 5B). These observations were supported by significant effects of OT Dose for active lever responding [F(3,36)= 7.58 p< 0.001] and ethanol intake [F(3,36)= 8.41 p< 0.001]. Post hoc analyses indicated that all doses of OT significantly reduced ethanol responding and intake compared to the vehicle condition. Analysis of inactive lever responding indicated no significant effect of OT Dose (Figure 5A).
Figure 5.
Effects of oxytocin on operant ethanol self-administration. Oxytocin reduced active lever responding (A) and ethanol consumption (g/kg) (B) in a dose-related manner. Values are mean ± s.e.m. (N= 13/group). Significantly differs from vehicle condition: * (p< 0.05); ** (p< 0.01); *** (p< 0.001).
To investigate the effect of OT on motivation to seek ethanol reinforcement, a subset of the same mice (n= 10) were challenged using a progressive ratio (PR) self-administration schedule. OT decreased overall active lever responding during the PR sessions. Further, analysis of breakpoint data indicated that OT (0.3 mg/kg) significantly reduced breakpoint ratio achieved for ethanol reward compared to vehicle [t(8)= 2.82, p< 0.03] (Figure 6).
Figure 6.
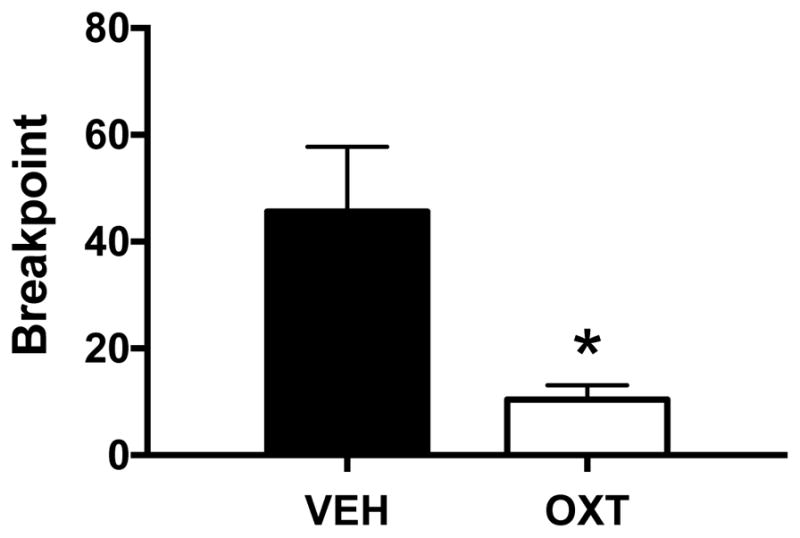
Effect of oxytocin on progressive ratio responding for ethanol. Oxytocin (0.3 mg/kg) reduced breakpoint values for progressive ratio responding. Values are mean ± s.e.m. (N=5/group). Significantly differs from vehicle condition: * (p< 0.05).
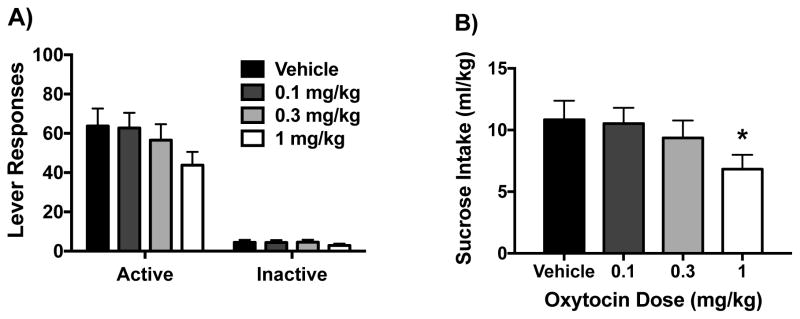
Effect of Oxytocin on Sucrose Self-Administration
To investigate whether OT administration influences self-administration of a natural reward, a separate cohort of mice was trained to respond for sucrose reinforcement. One mouse was excluded from the final analysis due to receiving incorrect doses in the Latin-square design. Preliminary analyses indicated that dosing order did not have a significant effect on active lever responding or ml/kg sucrose intake [F(4,66)< 2.4 for both dependent measures]. However, ANOVA revealed a marginal OT Dose effect on active lever responding [F(3,51)= 2.48, p< 0.07] (Figure 7A) and a significant effect on ml/kg intake [F(3,51)= 3.33, p< 0.05] (Figure 7B). Post hoc analysis revealed that the highest dose of OT (1 mg/kg) significantly reduced ml/kg intake of sucrose compared to vehicle. OT treatment did not significantly alter inactive lever responding (Figure 7A).
Figure 7.
Effects of oxytocin on operant sucrose self-administration. Oxytocin reduced active lever responding (A) and sucrose consumption (ml/kg) (B) at only the highest dose tested (1 mg/kg). Values are mean ± s.e.m. (N= 18/group). Significantly differs from vehicle condition: * (p< 0.05).
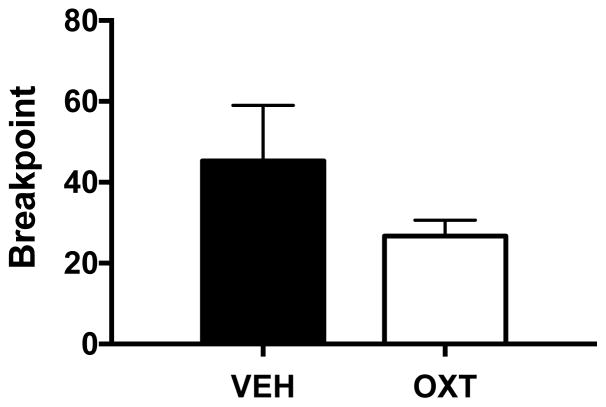
A subset of mice (n=12) from the sucrose self-administration experiment were then tested for responding under a PR schedule. OT (0.3 mg/kg) did not significantly alter responding for sucrose during PR sessions and analysis of breakpoint data indicated no significant difference between OT and vehicle conditions [t(10)= 1.31, p> 0.20] (Figure 8).
Figure 8.
Effect of oxytocin on progressive ratio responding for sucrose. Oxytocin (0.3 mg/kg) did not significantly alter breakpoint values for progressive ratio responding. Values are mean ± s.e.m. (N=6/group).
DISCUSSION
Results from the present study demonstrate that systemic administration of oxytocin reduces ethanol consumption in a binge-like drinking model, 2-bottle choice drinking paradigm, and in a model that employs operant self-administration procedures (Table 2). Oxytocin decreased ethanol consumption in a dose-related manner, and the ability of oxytocin to reduce ethanol intake did not appear to be related to nonspecific (e.g., sedation, antidipsogenic) effects. Further, oxytocin reduced motivation to respond for ethanol at doses that did not alter responding for a natural reward (sucrose). These results are generally consistent with other reports indicating that systemic administration of oxytocin reduces ethanol intake in a variety of drinking models in rats (Bowen et al., 2011; MacFadyen et al., 2016; McGregor and Bowen, 2012) and mice (Peters et al., 2013). These data also complement a recent report showing that direct (intracerebroventricular; icv.) infusion of the neuropeptide reduced ethanol consumption in Wistar rats (Peters et al., 2016).
Table 2.
Summary of oxytocin effects on ethanol behaviors.
| Behavioral Test | Measure | Oxytocin Dose (mg/kg) | |||||
|---|---|---|---|---|---|---|---|
| Veh | 0.1 | 0.3 | 1 | 3 | 10 | ||
| Drinking in the Dark (DID) | EtOH Intake |
|
|
|
|
||
| BEC |
|
|
|
|
|||
| DID with Lickometers | EtOH Intake |
|
|
|
|
||
| EtOH Licks |
|
|
|
|
|||
| DID with OT Antagonist | EtOH Intake |
|
|
||||
| Locomotor Activity | Distance |
|
|
|
|
||
| Total Fluid Intake | 4-Hr Intake |
|
|
||||
| 24-Hr Intake |
|
|
|||||
| EtOH Self-Administration | Lever Responses |
|
|
|
|
||
| EtOH Intake |
|
|
|
|
|||
| EtOH Progressive Ratio | EtOH Breakpoint |
|
|
||||
| Sucrose Self-Administration | Lever Responses |
|
|
|
|
||
| Sucrose Intake |
|
|
|
|
|||
| Sucrose Progressive Ratio | Sucrose Breakpoint |
|
|
||||
Oxytocin reduced relatively high levels of ethanol intake along with resultant blood ethanol concentrations in the binge-like DID model. Use of lickometers provided an opportunity to analyze the effects of oxytocin on the temporal pattern of ethanol drinking during the final (fourth day) of the DID procedure. Results showed vehicle-treated mice initiated licking contacts at the ethanol bottle within the first 5–10 min of the drinking period and maintained a relatively steady pace of drinking throughout the entire 4-hr session. In contrast, oxytocin treatment delayed ethanol bottle contacts until 1–2 hours into the session. This profile suggests oxytocin reduced motivation to seek and consume ethanol under these testing conditions.
Alternatively, the delay in initiating drinking produced by oxytocin could be due to a sedative effect of the neuropeptide. Oxytocin has been shown to reduce locomotor activity in rats (Klenerova et al., 2009; Uvnas-Moberg et al., 1994; Zhou et al., 2015). In the present study, oxytocin reduced locomotor actvity in an open field in a dose-related manner. However, this effect was transient, with activity returning to levels registered in vehicle-treated mice within 10 min for mice treated with low doses of oxytocin (0.3 and 1 mg/kg). In contrast, reduced activity produced by a high oxytocin dose (3 mg/kg) did not recover to control levels until at least 40–45 min into the test session. These results are congruent with other reports indicating that low-dose anxiolytic effects of oxytocin can be distinguishd from sedative effects produced by higher doses of the neuropeptide (Bahi et al., 2016; Klenerova et al., 2009). Interestingly, central (icv.) administration of oxytocin was shown to attenuate ethanol-induced sedative effects in an open-field locomotor test (Bowen et al., 2015). Taken together, it does not seem likely that the reduction in ethanol drinking produced by low doses of oxytocin can be simply attributed to a nonspecific sedative effect of the neuropeptide. Additionally, while oxytocin has been reported to produce an antidipsogenic effect in rats (Arletti et al., 1990), in the present study oxytocin did not alter general fluid intake. In fact, reduced ethanol consumption was compensated by increased water intake. Further, in mice offered only water, oxytocin treatment did not alter total fluid intake. Thus, it is unlikely that reduced ethanol consumption in the various drinking models can be simply attributed to oxytocin decreasing general fluid intake.
Using operant conditioning procedures, oxytocin was shown to reduce ethanol responding and intake in a dose-related fashion. Moreover, this effect was produced at doses that did not alter self-administration of a natural reward (sucrose). The highest oxytocin dose tested in these operant self-administration studies (1 mg/kg) reduced responding for both ethanol and sucrose. Other studies have shown oxytocin given either systemically or centrally (infused into the ventral tegmental area) reduced sucrose intake in rats (Mullis et al., 2013; Zhou et al., 2015). While the present studies were conducted in only male mice, there is some evidence that females may be more sensitive than males to the supressant effects of oxytocin on sucrose consumption (Zhou et al., 2015). Nevertheless, it is noteworthy that in the present study low doses of oxytocin reduced ethanol responding and intake without altering sucrose self-administration. The fact that responding on an “inactive” lever in both ethanol and sucrose experiments was not significantly altered by oxytocin also supports the notion that reduced ethanol self-administration is not related to a general sedative effect of the neuropeptide.
Results from progressive ratio testing further support a selective effect of oxytocin in decreasing motivation to self-administer ethanol. Oxytocin (0.3 mg/kg) reduced breakpoint value for ethanol while not altering this measure for sucrose. Similarly, a recent study in rats showed 1 mg/kg oxytocin did not alter progressive ratio responding or breakpoint for sucrose pellets (Cox et al., 2013). While it is possible that higher doses of oxytocin may reduce motivation to respond for a natural reward, results from the present study demonstrate that at a low dose, oxytocin can selectively reduce responding for ethanol. Collectively, these results align with a growing body of literature demonstrating efficacy for oxytocin to reduce alcohol/drug seeking and consumption (Lee et al., 2016).
The mechanism by which systemically administered oxytocin reduces ethanol consumption is not fully understood. Peripheral administration (ip. injection) of oxytocin was shown to produce a rapid rise in plasma and brain dialysate levels of oxytocin in mice (Neumann et al., 2013). While penetration of the neuropeptide through the blood-brain barrier is limited, passage of a small, yet physiologically relevant amount of exogenous oxytocin is thought to influence central oxytocin activity (Landgraf and Neumann, 2004; Neumann and Landgraf, 2012; Neumann et al., 2013). There is evidence indicating that oxytocin can facilitate its own release in the hypothalamus (Moos et al., 1984; Rossoni et al., 2008; Theodosis, 2002). This feed-forward loop may explain, at least in part, how acute and chronic systemically administered oxytocin can produce enduring functional and behavioral effects. For example, Bowen and colleagues showed that repeated injections of oxytocin in adolescent rats resulted in upregulated oxytocin receptor mRNA expression in the hypothalamus in adult rats, which was accompanied by reduced ethanol consumption (Bowen et al., 2011). Nevertheless, whether reduced ethanol intake produced by systemic administration of oxytocin in the present studies can be attributed to direct effects of the exogenous neuropeptide in the brain or indirect effects on release of endogenous oxytocin in brain is unknown and will require further investigation.
Oxytocin projections are known to influence mesolimbic dopamine activity in mediating the rewarding aspects of social behaviors (Young et al., 2014). The relatively high expression of oxytocin receptors within structures such as the nucleus accumbens suggests that modulation of mesolimbic dopamine pathways may underlie the ability of oxytocin to influence the rewarding effects of alcohol and other substances of abuse. In a recent study, direct (icv.) administration of oxytocin reduced ethanol consumption and ethanol-induced dopamine efflux in the nucleus accumbens of Wistar rats (Peters et al., 2016). Thus, it is possible that oxytocin reduced ethanol self-administration in the present study by blunting mesolimbic dopamine activity that ordinarily signals ethanol-related reward.
In the present study, the ablity of oxytocin to reduce binge-like drinking was blocked by pretreatment with an oxytocin receptor antagonist, suggesting that oxytocin reduced ethanol consumption via an interaction with its receptor. Few studies have examined the role of oxytocin receptors in mediating the neuropeptide’s effects on motivational actions of alcohol. Recent studies involving viral-mediated overexpression of oxytocin receptors in the nucleus accumbens core have implicated a role for these receptors in ethanol drinking and conditioned reward (Bahi, 2015; Bahi et al., 2016). However, it is noteworthy that oxytocin was shown to block ethanol-induced ataxic and sedative/hypnotic effects via an apparent direct interaction with delta-subunit containing GABA-A receptors (Bowen et al., 2015). Thus, there remains some abiguity as to the role of oxytocin receptors in mediating the neuropeptide’s effects on physiological effects of ethanol and, in particular, ethanol-related reward and self-administration behavior.
Differing by only two amino acids from the related nonapeptide arginine vasopressin, it is possible that oxytocin reduced the motivational effects of ethanol through actions at vasopressin receptors (Neumann and Landgraf, 2012). The two hypophyseal neuropeptide systems generally produce opposing physiological actions. Interestingly, the arginine vasopressin 1b (V1b) receptor antagonist SR149415 was shown to reduce ethanol drinking in genetically high alcohol-preferring rats (Zhou et al., 2011) and dependent Wistar rats (Edwards et al., 2012). However, results from a study demonstrating that the synthetic oxytocin receptor agonist carbetocin, which exhibits low affinity for V1b (and V1a) receptors compared to oxytocin receptors (Passoni et al., 2016), effectively reduced ethanol-induced conditioned reward supports a role for oxytocin receptors (Bahi, 2015). Further investigation is needed to determine the relative role of these related neuropeptide receptors in mediating the ability of oxytocin to reduce ethanol reward and drinking.
In summary, results from this study provide support and extend evidence demonstrating the ability of oxytocin to reduce the motivational effects of ethanol. The present results indicate oxytocin is effective in reducing ethanol consumption in a model of binge-like drinking, an effect reversed by pretreatment with an oxytocin receptor antagonist. Oxytocin also decreased 2-bottle choice drinking in the home cage as well as ethanol responding and intake in an oral self-administration model involving operant conditioning procedures. Further, oxytocin reduced responding for ethanol under fixed- and progressive- ratio schedules at doses that did not alter responding for a natural reward (sucrose). Collectively, these and other recent reports provide support for the theraputic potential of this neuropeptide as a treatment for alcohol use disorders. A limited number of clinical studies have shown oxytocin to have some efficacy in decreasing craving for alcohol and other drugs of abuse (Lee et al., 2016). For example, Pedersen and colleagues recently demonstrated that intranasal oxytocin attenuated alcohol withdrawal symptoms in treatment seeking human subjects compared to placebo (Pedersen et al., 2013). Thus, the oxytocin system may be a promising target for therapeutic intervention of alcohol use disorders, though additional studies are necessary to establish its clinical efficacy and further investigate the conditions and mechanisms by which oxytocin reduces alcohol consumption.
Acknowledgments
Supported by NIAAA grants P50 AA010761, U01 AA014095, and U01 AA020929, DOD/US Army Institute for Molecular Neuroscience 803-94, and VA Medical Research. The authors wish to thank Olivia Roberson for her technical support and Dr. Marcelo F. Lopez for assistance with statistical analyses.
Footnotes
Conflict of Interest: All authors declare no conflicts of interest.
References
- Arletti R, Benelli A, Bertolini A. Oxytocin inhibits food and fluid intake in rats. Physiol Behav. 1990;48:825–830. doi: 10.1016/0031-9384(90)90234-u. [DOI] [PubMed] [Google Scholar]
- Bahi A. The oxytocin receptor impairs ethanol reward in mice. Physiol Behav. 2015;139:321–327. doi: 10.1016/j.physbeh.2014.11.046. [DOI] [PubMed] [Google Scholar]
- Bahi A, Al Mansouri S, Al Maamari E. Nucleus accumbens lentiviral-mediated gain of function of the oxytocin receptor regulates anxiety- and ethanol-related behaviors in adult mice. Physiol Behav. 2016;164:249–258. doi: 10.1016/j.physbeh.2016.06.009. [DOI] [PubMed] [Google Scholar]
- Baskerville TA, Douglas AJ. Dopamine and oxytocin interactions underlying behaviors: potential contributions to behavioral disorders. CNS Neurosci Ther. 2010;16:e92–123. doi: 10.1111/j.1755-5949.2010.00154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borthwick AD. Oral oxytocin antagonists. J Med Chem. 2010;53:6525–6538. doi: 10.1021/jm901812z. [DOI] [PubMed] [Google Scholar]
- Bowen MT, Carson DS, Spiro A, Arnold JC, McGregor IS. Adolescent oxytocin exposure causes persistent reductions in anxiety and alcohol consumption and enhances sociability in rats. PLoS One. 2011;6:e27237. doi: 10.1371/journal.pone.0027237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen MT, Peters ST, Absalom N, Chebib M, Neumann ID, McGregor IS. Oxytocin prevents ethanol actions at delta subunit-containing GABAA receptors and attenuates ethanol-induced motor impairment in rats. Proc Natl Acad Sci U S A. 2015;112:3104–3109. doi: 10.1073/pnas.1416900112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkett JP, Young LJ. The behavioral, anatomical and pharmacological parallels between social attachment, love and addiction. Psychopharmacology (Berl) 2012;224:1–26. doi: 10.1007/s00213-012-2794-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox BM, Young AB, See RE, Reichel CM. Sex differences in methamphetamine seeking in rats: impact of oxytocin. Psychoneuroendocrinology. 2013;38:2343–2353. doi: 10.1016/j.psyneuen.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards S, Guerrero M, Ghoneim OM, Roberts E, Koob GF. Evidence that vasopressin V1b receptors mediate the transition to excessive drinking in ethanol-dependent rats. Addict Biol. 2012;17:76–85. doi: 10.1111/j.1369-1600.2010.00291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin WC, 3rd, Lopez MF, Yanke AB, Middaugh LD, Becker HC. Repeated cycles of chronic intermittent ethanol exposure in mice increases voluntary ethanol drinking and ethanol concentrations in the nucleus accumbens. Psychopharmacology (Berl) 2009;201:569–580. doi: 10.1007/s00213-008-1324-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groppe SE, Gossen A, Rademacher L, Hahn A, Westphal L, Grunder G, Spreckelmeyer KN. Oxytocin influences processing of socially relevant cues in the ventral tegmental area of the human brain. Biol Psychiatry. 2013;74:172–179. doi: 10.1016/j.biopsych.2012.12.023. [DOI] [PubMed] [Google Scholar]
- Klenerova V, Krejci I, Sida P, Hlinak Z, Hynie S. Oxytocin and carbetocin effects on spontaneous behavior of male rats: modulation by oxytocin receptor antagonists. Neuro Endocrinol Lett. 2009;30:335–342. [PubMed] [Google Scholar]
- Kovacs GL, Sarnyai Z, Szabo G. Oxytocin and addiction: a review. Psychoneuroendocrinology. 1998;23:945–962. doi: 10.1016/s0306-4530(98)00064-x. [DOI] [PubMed] [Google Scholar]
- Kuteykin-Teplyakov K, Maldonado R. Looking for prosocial genes: ITRAQ analysis of proteins involved in MDMA-induced sociability in mice. Eur Neuropsychopharmacol. 2014;24:1773–1783. doi: 10.1016/j.euroneuro.2014.08.007. [DOI] [PubMed] [Google Scholar]
- Landgraf R, Neumann ID. Vasopressin and oxytocin release within the brain: a dynamic concept of multiple and variable modes of neuropeptide communication. Front Neuroendocrinol. 2004;25:150–176. doi: 10.1016/j.yfrne.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Lee MR, Rohn MC, Tanda G, Leggio L. Targeting the Oxytocin System to Treat Addictive Disorders: Rationale and Progress to Date. CNS Drugs. 2016;30:109–123. doi: 10.1007/s40263-016-0313-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MR, Weerts EM. Oxytocin for the treatment of drug and alcohol use disorders. Behav Pharmacol. 2016;27:640–648. doi: 10.1097/FBP.0000000000000258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love TM. Oxytocin, motivation and the role of dopamine. Pharmacol Biochem Behav. 2014;119:49–60. doi: 10.1016/j.pbb.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacFadyen K, Loveless R, DeLucca B, Wardley K, Deogan S, Thomas C, Peris J. Peripheral oxytocin administration reduces ethanol consumption in rats. Pharmacol Biochem Behav. 2016;140:27–32. doi: 10.1016/j.pbb.2015.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor IS, Bowen MT. Breaking the loop: oxytocin as a potential treatment for drug addiction. Horm Behav. 2012;61:331–339. doi: 10.1016/j.yhbeh.2011.12.001. [DOI] [PubMed] [Google Scholar]
- Moos F, Freund-Mercier MJ, Guerne Y, Guerne JM, Stoeckel ME, Richard P. Release of oxytocin and vasopressin by magnocellular nuclei in vitro: specific facilitatory effect of oxytocin on its own release. J Endocrinol. 1984;102:63–72. doi: 10.1677/joe.0.1020063. [DOI] [PubMed] [Google Scholar]
- Mullis K, Kay K, Williams DL. Oxytocin action in the ventral tegmental area affects sucrose intake. Brain Res. 2013;1513:85–91. doi: 10.1016/j.brainres.2013.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann ID, Landgraf R. Balance of brain oxytocin and vasopressin: implications for anxiety, depression, and social behaviors. Trends Neurosci. 2012;35:649–659. doi: 10.1016/j.tins.2012.08.004. [DOI] [PubMed] [Google Scholar]
- Neumann ID, Maloumby R, Beiderbeck DI, Lukas M, Landgraf R. Increased brain and plasma oxytocin after nasal and peripheral administration in rats and mice. Psychoneuroendocrinology. 2013;38:1985–1993. doi: 10.1016/j.psyneuen.2013.03.003. [DOI] [PubMed] [Google Scholar]
- Olszewski PK, Waas JR, Brooks LL, Herisson F, Levine AS. Oxytocin receptor blockade reduces acquisition but not retrieval of taste aversion and blunts responsiveness of amygdala neurons to an aversive stimulus. Peptides. 2013;50:36–41. doi: 10.1016/j.peptides.2013.09.008. [DOI] [PubMed] [Google Scholar]
- Passoni I, Leonzino M, Gigliucci V, Chini B, Busnelli M. Carbetocin is a functional selective Gq agonist that does not promote oxytocin receptor recycling after inducing beta-arrestin-independent internalization. J Neuroendocrinol. 2016:28. doi: 10.1111/jne.12363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen CA, Smedley KL, Leserman J, Jarskog LF, Rau SW, Kampov-Polevoi A, Casey RL, Fender T, Garbutt JC. Intranasal oxytocin blocks alcohol withdrawal in human subjects. Alcohol Clin Exp Res. 2013;37:484–489. doi: 10.1111/j.1530-0277.2012.01958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters S, Slattery DA, Flor PJ, Neumann ID, Reber SO. Differential effects of baclofen and oxytocin on the increased ethanol consumption following chronic psychosocial stress in mice. Addict Biol. 2013;18:66–77. doi: 10.1111/adb.12001. [DOI] [PubMed] [Google Scholar]
- Peters ST, Bowen MT, Bohrer K, McGregor IS, Neumann ID. Oxytocin inhibits ethanol consumption and ethanol-induced dopamine release in the nucleus accumbens. Addict Biol. 2016 doi: 10.1111/adb.12362. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Best K, Belknap JK, Finn DA, Crabbe JC. Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiol Behav. 2005;84:53–63. doi: 10.1016/j.physbeh.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Rossoni E, Feng J, Tirozzi B, Brown D, Leng G, Moos F. Emergent synchronous bursting of oxytocin neuronal network. PLoS Comput Biol. 2008;4:e1000123. doi: 10.1371/journal.pcbi.1000123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stauffer CS, Woolley JD. Can we bottle psychosocial treatments for addiction? The role of oxytocin. J Clin Psychiatry. 2014;75:1028–1029. doi: 10.4088/JCP.14ac09437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodosis DT. Oxytocin-secreting neurons: A physiological model of morphological neuronal and glial plasticity in the adult hypothalamus. Front Neuroendocrinol. 2002;23:101–135. doi: 10.1006/frne.2001.0226. [DOI] [PubMed] [Google Scholar]
- Uvnas-Moberg K, Ahlenius S, Hillegaart V, Alster P. High doses of oxytocin cause sedation and low doses cause an anxiolytic-like effect in male rats. Pharmacol Biochem Behav. 1994;49:101–106. doi: 10.1016/0091-3057(94)90462-6. [DOI] [PubMed] [Google Scholar]
- Young KA, Liu Y, Gobrogge KL, Wang H, Wang Z. Oxytocin reverses amphetamine-induced deficits in social bonding: evidence for an interaction with nucleus accumbens dopamine. J Neurosci. 2014;34:8499–8506. doi: 10.1523/JNEUROSCI.4275-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Ghee SM, See RE, Reichel CM. Oxytocin differentially affects sucrose taking and seeking in male and female rats. Behav Brain Res. 2015;283:184–190. doi: 10.1016/j.bbr.2015.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Colombo G, Carai MA, Ho A, Gessa GL, Kreek MJ. Involvement of arginine vasopressin and V1b receptor in alcohol drinking in Sardinian alcohol-preferring rats. Alcohol Clin Exp Res. 2011;35:1876–1883. doi: 10.1111/j.1530-0277.2011.01532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]