Abstract
Background
The Systolic Blood Pressure Intervention Trial (SPRINT) demonstrated a 27% reduction in all-cause mortality with a systolic blood-pressure (SBP) goal of <120 mmHg versus <140 mmHg among U.S. adults at high cardiovascular disease (CVD) risk but without diabetes, stroke, or heart failure. To quantify the potential benefits and risks of SPRINT intensive goal implementation, we estimated the deaths prevented and excess serious adverse events (SAEs) incurred if the SPRINT intensive SBP treatment goal was implemented in all eligible U.S. adults.
Methods
SPRINT eligibility criteria were applied to the 1999–2006 National Health and Nutrition Examination Survey and linked with the National Death Index through December 2011. SPRINT eligibility included age ≥ 50 years, SBP of 130–180 mmHg (depending on the number of antihypertensive medications being taken), and high CVD risk. Exclusion criteria were diabetes, history of stroke, >1 gram of proteinuria, heart failure, estimated glomerular filtration rate < 20 ml/min/1.73m2, or dialysis. Annual mortality rates were calculated by dividing the Kaplan-Meier 5-year mortality by 5. Hazard ratios for all-cause mortality and heart failure and absolute risks for SAEs in SPRINT were used to estimate the number of potential deaths and heart failure cases prevented and SAEs incurred with intensive SBP treatment.
Results
The mean age was 68.6 years and 83.2% and 7.4% were non-Hispanic white and non-Hispanic black, respectively. The annual mortality rate was 2.20% (95%CI 1.91%–2.48%) and intensive SBP treatment was projected to prevent about 107,500 deaths per year (95%CI 93,300–121,200) and give rise to 56,100 (95%CI 50,800–61,400) episodes of hypotension, 34,400 (95%CI 31,200–37,600) episodes of syncope, 43,400 (95%CI 39,400–47,500) serious electrolyte disorders, and 88,700 (95%CI 80,400–97,000) cases of acute kidney injury per year. The analysis of extremes approach indicated that the range of estimated lower and upper bound number of deaths prevented per year with intensive SBP control was 34,600 to 179,600. Intensive SBP control was projected to prevent 46,100 (95%CI 41,800–50,400) cases of heart failure annually.
Conclusions
If fully implemented in eligible U.S. adults, intensive SBP treatment could prevent about 107,500 deaths per year. A consequence of this treatment strategy, however, could be an increase in SAEs.
Keywords: blood pressure, hypertension, treatment outcome, SPRINT
Journal Subject Terms: High Blood Pressure, Hypertension, Mortality/Survival
Introduction
High blood pressure (BP) is the leading modifiable cardiovascular disease (CVD) risk factor world-wide.1 Observational studies show a monotonic increase in risk of CVD beginning at a systolic blood pressure (SBP) of 115 mmHg.2 However, the optimal SBP threshold for antihypertensive medication initiation and goal attainment is unclear. Current U.S. recommendations are a SBP threshold of 140 or 150 mmHg for initiation of antihypertensive medication, depending on age and other co-existing conditions.3 Until recently, randomized trials did not provide definitive evidence supporting lower SBP goals in high-risk sub-populations.3–5
The Systolic Blood Pressure Intervention Trial (SPRINT) was designed to determine whether lowering SBP to an intensive goal of < 120 mmHg versus the standard goal of <140 mmHg resulted in reduced CVD risk in high-risk patients without a history of diabetes, stroke, or heart failure.6 SPRINT achieved a mean SBP of 121 mmHg in the intensive treatment arm and 136 mmHg in the standard treatment arm, resulting in a 27% reduction (hazard ratio[HR] 0.73 95%CI 0.60–0.90) in all-cause mortality.7 Given the mortality rate observed in SPRINT, only 90 SPRINT-eligible patients need to be treated to an intensive SBP goal to prevent one death from any cause after 3.26 years.7 However, participants in the intensive SBP treatment group experienced a higher incidence of treatment-related serious adverse events (SAEs) and the number-needed-to-harm (NNH) for any SAE definitely or probability related to intensive treatment was 45.
Implementation of SPRINT-based intensive SBP goals has the potential to greatly reduce mortality in high CVD risk patients living in the U.S. and worldwide. In order to quantify the potential benefits and risks of SPRINT intensive goal implementation, we estimated the deaths prevented and excess SAEs incurred if the SPRINT intensive SBP goal was implemented in all eligible U.S. adults.
Methods
Study Population
Data were drawn from the National Health and Nutrition Examination Survey (NHANES), a multistage stratified probability sample of non-institutionalized U.S. adults conducted by the National Center for Health Statistics (NCHS).4 To provide sufficient sample size, data were pooled from the 1999–2000, 2001–2002, 2003–2004, and 2005–2006 NHANES cycles.8 Participants who completed a medical evaluation at the NHANES mobile examination center, were 20 years of age or older and had complete information on SBP measurements and use of antihypertensive medication (n = 17,746) were included. From the 8,327 NHANES participants who were ≥ 50 years old, 4,249 met SPRINT SBP criteria (Figure 1). Next, participants who did not meet the high CVD risk criteria (n=895) or who had diabetes (n=868), history of stroke (n=263), proteinuria > 1 g/day (n=53), heart failure (n=228), estimated glomerular filtration rate (eGFR) < 20 ml/min/1.73 m2 or end-stage renal disease on dialysis (n=30) were excluded. After these exclusions, a total of 2,185 SPRINT-eligible participants were included in the current analyses. All participants provided written informed consent and the NCHS institutional review board approved each NHANES cycle.
Figure 1.
Flowchart showing the SPRINT eligibility criteria applied to the National Health and Nutrition Examination Survey 1999–2006
Baseline Data Collection
In each NHANES cycle, data were collected via a medical evaluation and participant interviews. The participant interview collected self-reported data on age, race/ethnicity, sex, smoking status, history of a diagnosis of diabetes mellitus, heart failure, hypertension, myocardial infarction, angina, coronary heart disease (CHD), or stroke as well as receipt of dialysis in the past 12 months or the use of antihypertensive or anti-diabetes medication.
The NHANES medical evaluation included measurements of height and weight that were used to calculate body mass index (BMI). A blood sample was collected for measurement of serum creatinine and glucose, and glycosylated hemoglobin (hemoglobin A1c). Urine albumin and creatinine concentrations were analyzed from spot, random urine samples. Diabetes was defined by a prior diagnosis, excluding during pregnancy, with concurrent use of insulin or oral hypoglycemic medication, or a hemoglobin A1c ≥ 6.5%, non-fasting glucose ≥ 200 mg/dL, or fasting glucose ≥ 126 mg/dL. The Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation was used to calculate eGFR.9 NHANES participants were asked to bring all prescription medications taken in the past two weeks to their NHANES medical evaluation. Trained study personnel reviewed the pill bottles and medication names were recoded into medication classes according to their generic equivalents. Use of antihypertensive medication was defined by self-report and report of taking one or more classes of antihypertensive medication identified through the pill bottle review.
Baseline BP measurement
Using an appropriately sized cuff, BP was measured with participants seated after five minutes of rest using a mercury sphygmomanometer by a trained study physician. SBP and diastolic BP (DBP) were defined as the mean of three BP measurements taken one-minute apart.
Death Ascertainment
Mortality follow-up for the NHANES participants was available through December 31, 2011. To identify vital status, probabilistic matching was used to link NHANES participants with the National Death Index. Matching was based on 12 identifiers for each participant, including data of birth, sex, and Social Security Number. The follow-up period for each participant was calculated as the interval between their NHANES evaluation and the data of death or December 31, 2011, for participants who did not die.
Statistical Analysis
Annual mortality rates in the SPRINT-eligible population overall and within subgroups defined by sex, age (< 75, and ≥ 75 years), race/ethnicity, history of CHD, SBP groups (≤132, 133–144, and ≥ 145 mmHg; pre-specified SBP strata defined in SPRINT), and by eGFR 20–59 ml/min/1.73 m2 were calculated. The Kaplan-Meier method was used to calculate the 5-year survival probability, S(5), which was then used to calculate the 5-year mortality, F(5), which is equal to 1-S(5), which amounts to the 5-year cumulative incidence (under the exponential model approximation). The annual mortality rate was then approximated by dividing this probability by 5. Population estimates for annual deaths in the overall population meeting the SPRINT eligibility criteria and in subgroups was determined by multiplying the population size for that group or subgroup by their respective annual all-cause mortality rate. The projected annual number of deaths that would occur if SPRINT intensive SBP goals were fully implemented was determined by multiplying the observed annual mortality rate and its 95% confidence intervals in the overall SPRINT-eligible NHANES population by 0.73, the observed HR for all-cause mortality with intensive SBP treatment in SPRINT compared to standard SBP treatment (i.e., usual care).7 This was done for the overall population and within subgroups defined by sex, age (< 75, and ≥ 75 years), race/ethnicity, history of CHD, SBP groups (≤132, 133–144, and ≥ 145 mmHg) and moderate stage chronic kidney disease (defined by SPRINT as eGFR 20–59 ml/min/1.73 m2). Deaths postponed were then calculated as the difference between total and expected deaths with full implementation of intensive SBP treatment. Because the oscillometric blood pressure measurement methods used in SPRINT by research personnel may result in a lower mean SBP than observed using manual auscultatory method by physicians in NHANES, we performed a sensitivity analysis including the NHANES population with a 10 and 20 mm Hg higher SBP than the SPRINT entry criteria (e.g., 140–190 mmHg and 150–200 versus 130–180 mm Hg for those on 0–1 antihypertensive medications, respectively.10 Since heart failure was the component of the primary composite outcome in SPRINT that was statistically significantly different between treatment groups, we also estimated the number of new cases of heart failure prevented by multiplying the HR for incident heart failure observed in SPRINT (0.62) by the number of heart failure cases expected with standard SBP treatment. The number of heart failure cases expected with standard SBP treatment was calculated by taking the annual rate of rate incident heart failure observed in SPRINT (0.67%/year) by the SPRINT-eligible NHANES population size and it’s 95% confidence interval overall and within subgroups.
SAEs were defined in SPRINT as an event that was fatal or life-threatening that resulted in clinically significant or persistent disability, required or prolonged a hospitalization, or judged by the investigator to represent a clinically significant hazard or harm to the participant that might require intervention to prevent another SAE. To project the number of SAEs expected with intensive SBP treatment, we multiplied the absolute risk difference for each SAE of interest reported in the main SPRINT manuscript that were statistically significantly different between intensive and standard SBP treatment arms (i.e., hypotension, syncope, bradycardia, electrolyte abnormality, and acute kidney injury) by the number of U.S. adults meeting the SPRINT eligibility criteria.7 This was done for these SAEs overall and for these SAEs classified as possibly or definitely related to the intervention. This number was then divided by 3.26 (the median follow-up in SPRINT) to yield the projected SAEs incurred per year. To account for the uncertainty in treatment effects from SPRINT, sensitivity analyses were conducted using the analysis-of-extremes methodology, where the upper and lower confidence bounds of the treatment effects for all-cause mortality, heart failure, and SAEs were used.11, 12 Since confidence bounds were not available for SAEs in SPRINT main results publication, we calculated 95% confidence intervals for SAEs from the p value (Supplemental Table 1). The number-needed-to-treat (NNT) was calculated for all-cause mortality and heart failure and the NNH was calculated for each individual SAE by taking the reciprocal of the absolute reduction in risk overall and within subgroups. SUDAAN 10.1 (Research Triangle Institute, Research Triangle Park, NC) was used for all analyses to account for the complex sampling design of NHANES.
Results
For the years between 1999 and 2006, an estimated 18.1 million (95%CI 16.4–19.8 million) U.S. adults met the SPRINT eligibility criteria, including 7.4 million (95%CI 6.5–8.3 million) and 10.7 million (95%CI 9.9–11.5 million) who were taking and not taking antihypertensive medication, respectively. The mean age in the sample was 68.6 years with 32.0% age 75 years or older (Table 1). Over half were men (53.8%) while 83.2% were non-Hispanic white and 7.4% non-Hispanic black and 3.0% Mexican American.
Table 1.
Characteristics of U.S. adults eligible for SPRINT overall and by antihypertensive medication status using NHANES 1999–2006.
| Group | Overall (n=18.1 million) | Taking Antihypertensive Medication (n=7.4 million) | Not Taking Antihypertensive Medication (n=10.7 million) | SPRINT* (n=9,361) |
|---|---|---|---|---|
| Age, mean (SE), years | 68.6 (0.26) | 68.8 (0.32) | 68.5 (0.37) | 67.9 (9.4) |
| Age group, % | ||||
| < 75 years | 68.0 | 68.1 | 67.7 | 71.8 |
| ≥ 75 years | 32.0 | 31.9 | 32.3 | 28.2 |
| Male sex, % | 53.8 | 46.1 | 53.8 | 64.4 |
| Race/ethnicity, % | ||||
| Non-Hispanic White | 83.2 | 82.9 | 83.4 | 57.7 |
| Non-Hispanic Black | 7.4 | 9.3 | 6.0 | 29.9 |
| Mexican American | 3.0 | 2.2 | 3.5 | NA |
| Other | 6.5 | 5.6 | 7.1 | 1.88 |
| Current smoker, % | 17.6 | 10.3 | 17.6 | 13.2 |
| Body mass index, mean (SE) | 28.1 (0.13) | 29.1 (0.21) | 27.5 (0.17) | 29.9 (5.7) |
| Obese, % | 31.0 | 37.2 | 26.7 | NA |
| eGFR 20 to 59 ml/min/1.73 m2, % | 23.0 | 26.1 | 20.2 | 28.3 |
| Framingham risk score groups, % | ||||
| < 5% | 0.2 | 0.0 | 0.39 | NA |
| 5.0–7.4% | 0.8 | 0.8 | 0.7 | NA |
| 7.5–9.9% | 1.6 | 1.5 | 1.6 | NA |
| 10 – 14.9% | 6.3 | 5.9 | 6.6 | NA |
| ≥ 15% | 91.1 | 91.9 | 90.6 | 61.3 |
| History of CHD, % | 12.1 | 16.9 | 8.7 | 16.7 |
| Systolic blood pressure, mmHg, % | ||||
| ≤132 | 9.9 | 7.3 | 11.7 | 33.5 |
| 133 – 144 | 40.7 | 43.6 | 38.8 | 32.5 |
| ≥145 | 49.4 | 49.1 | 49.5 | 34.0 |
| Education, % | ||||
| Less than high school | 26.0 | 23.1 | 28.0 | NA |
| High school only | 29.5 | 27.8 | 30.7 | NA |
| More than high school | 25.2 | 27.7 | 23.4 | NA |
| Completed college | 19.3 | 21.3 | 17.9 | NA |
| No insurance, % | 11.0 | 7.4 | 13.5 | NA |
Numbers in table are expressed as mean (Standard error [SE]) or percentages. Percentages are based on weighted data. CVD - cardiovascular disease, eGFR - estimated glomerular filtration rate, SPRINT - Systolic Blood Pressure Intervention Trial, CHD=coronary heart disease, NHANES= National Health and Nutrition Examination Survey, NA=not applicable.
Obesity defined as body mass index ≥ 30.0 kg/m2. Framingham risk score was calculate using the using the equation for general clinical practice.13 Use of antihypertensive medication was defined by self-report and report of taking one or more classes of antihypertensive medication identified through the pill bottle review.
Baseline characteristics from SPRINT are shown for comparisons (mean and standard deviation are shown for age and body mass index).
The overall observed annual mortality of the study population was 2.20% (95%CI 1.91%–2.48%), resulting in 398,200 projected deaths per year (95%CI 345,700–448,900) with standard SBP treatment (i.e., usual care) (Table 2). Based on the HR for all-cause mortality observed in SPRINT, intensive SBP treatment was projected to decrease annual mortality to 1.61% (95%CI 1.39%–1.81%), resulting in 290,700 deaths per year (95%CI 252,400–327,700 deaths). We estimate that 107,500 deaths (95%CI 93,300–121,200 deaths) could be prevented annually with full implementation of intensive SBP treatment in this group of SPRINT-eligible U.S. adults. After accounting for the uncertainty of the HR for all-cause mortality in SPRINT in the analysis of extremes sensitivity analysis, the range of estimated lower and upper bound number of deaths prevented per year with intensive SBP treatment was 34,600 to 179,600 (Supplemental Table 2). In sensitivity analyses, 84,000 (95%CI 73,600–95,700) and 62,700 (95% CI 55,400–71,300) deaths could be averted with intensive SBP treatment when we required SBP to be 10 mm Hg and 20 mm Hg higher than the SPRINT entry criteria, respectively (Supplemental Figures 1 and 2 and Supplemental Tables 3–6).
Table 2.
Observed and predicted annual mortality if SPRINT is fully implemented among NHANES participants who meet the SPRINT eligibility criteria.
| SPRINT-eligible U.S. adults in millions (95%CI) | Observed annual mortality among SPRINT-eligible NHANES participants | Predicted annual mortality if SPRINT fully applied | Deaths Prevented if SPRINT fully applied, thousands (95%CI) | Number needed to treat (for 3.26 years) | |||
|---|---|---|---|---|---|---|---|
| % (95%CI) | No. per year, thousands (95%CI) | % (95%CI) | No. per year, thousands (95%CI)* | ||||
| Overall | 18.1 (16.4–19.8) | 2.20 (1.91–2.48) | 398.2 (345.7–448.9) | 1.61 (1.39–1.81) | 290.7 (252.4–327.7) | 107.5 (93.3–121.2) | 52 |
| Sex | |||||||
| Men | 9.7 (8.7–10.8) | 2.53 (2.13–2.99) | 245.4 (206.6–290) | 1.85 (1.55–2.18) | 179.1 (150.8–211.7) | 66.3 (55.8–78.3) | 45 |
| Women | 8.4 (7.4–9.3) | 1.81 (1.49–2.20) | 152.0 (125.2–184.8) | 1.32 (1.09–1.61) | 111.0 (91.4–134.9) | 41.1 (33.8–49.9) | 63 |
| Age Group | |||||||
| < 75 years | 12.3 (11.2–13.5) | 1.21 (0.95–1.54) | 148.8 (116.9–189.4) | 0.88 (0.69–1.12) | 108.6 (85.3–138.3) | 40.2 (31.5–51.1) | 94 |
| ≥ 75 years | 5.8 (5.1–6.5) | 4.30 (3.74–4.93) | 249.4 (216.9–285.9) | 3.14 (2.73–3.6) | 182.1 (158.4–208.7) | 67.3 (58.6–77.2) | 26 |
| Race/ethnicity | |||||||
| Non-Hispanic White | 15.1 (13.2–16.9) | 2.18 (1.90–2.51) | 329.2 (286.9–379) | 1.59 (1.39–1.83) | 240.3 (209.4–276.7) | 88.9 (77.5–102.3) | 52 |
| Non-Hispanic Black | 1.3 (1.1–1.6) | 2.52 (1.87–3.36) | 32.8 (24.3–43.7) | 1.84 (1.37–2.45) | 23.9 (17.7–31.9) | 8.8 (6.6–11.8) | 45 |
| Mexican American | 0.05 (0.04–0.07) | 1.46 (0.95–2.24) | 0.7 (0.5–1.1) | 1.07 (0.69–1.64) | 0.5 (0.3–0.8) | 0.2 (0.1–0.3) | 78 |
| Other | 1.2 (0.8–1.5) | 2.35 (1.17–4.56) | 28.2 (14–54.7) | 1.72 (0.85–3.33) | 20.6 (10.2–39.9) | 7.6 (3.8–14.8) | 48 |
| History of CHD | 2.2 (1.7–2.6) | 3.31 (2.33–4.65) | 72.8 (51.3–102.3) | 2.42 (1.7–3.39) | 53.2 (37.4–74.7) | 19.7 (13.8–27.6) | 34 |
| Baseline SBP | |||||||
| SBP ≤132 mmHg | 1.8 (1.5–2.1) | 2.02 (1.25–3.21) | 36.4 (22.5–57.8) | 1.47 (0.91–2.34) | 26.5 (16.4–42.2) | 9.8 (6.1–15.6) | 56 |
| SBP 133–144 mmHg | 7.4 (6.7–8.1) | 1.85 (1.48–2.29) | 136.9 (109.5–169.5) | 1.35 (1.08–1.67) | 99.9 (79.9–123.7) | 37.0 (29.6–45.8) | 61 |
| SBP ≥ 145 mmHg | 8.9 (7.9–9.9) | 2.52 (2.15–2.96) | 224.3 (191.4–263.4) | 1.84 (1.57–2.16) | 163.7 (139.7–192.3) | 60.6 (51.7–71.1) | 45 |
| eGFR 20–59 ml/min/1.73m2 | 4.1 (3.5–4.7) | 2.95 (2.37–3.65) | 121.0 (97.2–149.7) | 2.15 (1.73–2.66) | 88.3 (70.9–109.2) | 32.7 (26.2–40.4) | 39 |
CHD = coronary heart disease, SBP=systolic blood pressure, SPRINT - Systolic Blood Pressure Intervention Trial, NHANES= National Health and Nutrition Examination Survey. Estimated glomerular filtration rate (eGFR) in ml/min/1.73 m2 was estimated using the CKD-EPI equation using the serum creatinine;
Calculated by multiplying the hazard ratio for all-cause mortality from SPRINT (0.73) by the number of deaths among the NHANES population meeting the SPRINT eligibility criteria
The observed annual mortality among the SPRINT-eligible population taking antihypertensive medication was 2.03% (95%CI 1.58–2.49) (Table 3). Within this group, intensive SBP treatment could reduce annual mortality to 1.48% per year (95%CI 1.15%–1.82%) resulting in a projected decrement of 40,600 deaths per year (95%CI 31,600–49,800). Among those SPRINT-eligible and not currently taking antihypertensive medication, the observed annual mortality was 2.31% (95%CI 1.89%–2.73%). Intensive SBP treatment was projected to prevent 66,700 deaths per year among this group (95%CI 54,600–78,900).
Table 3.
Observed and predicted annual mortality if SPRINT-based SBP goals are fully implemented among NHANES participants who meet the SPRINT eligibility criteria by antihypertensive medication use.
| SPRINT-eligible U.S. adults in millions (95%CI) | Observed annual mortality | Predicted annual mortality if SPRINT fully applied* | Deaths Prevented if SPRINT fully applied, thousands (95%CI) | Number needed to treat (for 3.26 years) | |||
|---|---|---|---|---|---|---|---|
| % (95%CI) | No. per year, thousands (95%CI) | % (95%CI) | No. per year, thousands (95%CI) | ||||
| Taking Antihypertensive Medication | |||||||
| Overall | 7.4 (6.5–8.3) | 2.03 (1.58–2.49) | 150.2 (116.9–184.3) | 1.48 (1.15–1.82) | 109.7 (85.4–134.5) | 40.6 (31.6–49.8) | 56 |
| Sex | |||||||
| Men | 3.4 (2.9–3.9) | 2.59 (2.01–3.33) | 88.1 (68.3–113.2) | 1.89 (1.47–2.43) | 64.3 (49.9–82.7) | 23.8 (18.5–30.6) | 44 |
| Women | 4.0 (3.4–4.6) | 1.56 (1.07–2.25) | 62.4 (42.8–90) | 1.14 (0.78–1.64) | 45.6 (31.2–65.7) | 16.8 (11.6–24.3) | 73 |
| Age Group | |||||||
| < 75 years | 5.1 (4.4–5.7) | 1.20 (0.85–1.69) | 61.2 (43.4–86.2) | 0.88 (0.62–1.23) | 44.7 (31.6–62.9) | 16.5 (11.7–23.3) | 95 |
| ≥ 75 years | 2.3 (1.9–2.7) | 3.85 (3.04–4.83) | 88.6 (69.9–111.1) | 2.81 (2.22–3.53) | 64.6 (51–81.1) | 23.9 (18.9–30) | 30 |
| Race/ethnicity | |||||||
| Non-Hispanic White | 6.1 (5.2–7.0) | 2.09 (1.62–2.68) | 127.5 (98.8–163.5) | 1.53 (1.18–1.96) | 93.1 (72.1–119.3) | 34.4 (26.7–44.1) | 54 |
| Non-Hispanic Black | 0.70 (0.52–0.86) | 2.63 (1.84–3.72) | 18.4 (12.9–26.0) | 1.92 (1.34–2.72) | 13.4 (9.4–19) | 5.0 (3.5–7) | 43 |
| Mexican American | 0.02 (0.01–0.02) | 2.20 (1.01–4.64) | 0.4 (0.2–0.9) | 1.61 (0.74–3.39) | 0.3 (0.1–0.7) | 0.1 (0.1–0.3) | 52 |
| History of CHD | 1.3 (0.92–1.6) | 2.69 (1.82–4.72) | 35.0 (23.7–61.4) | 1.96 (1.33–3.45) | 25.5 (17.3–44.8) | 9.4 (6.4–16.6) | 42 |
| Baseline SBP | |||||||
| SBP ≤132 mmHg | 0.054 (0.037–0.071) | 1.82 (0.66–4.80) | 1.0 (0.4–2.6) | 1.33 (0.48–3.50) | 0.7 (0.3–1.9) | 0.3 (0.1–0.7) | 62 |
| SBP 133–144 mmHg | 3.2 (2.6–3.7) | 2.14 (1.51–3.00) | 68.5 (48.3–96) | 1.56 (1.10–2.19) | 50.0 (35.3–70.1) | 18.5 (13–25.9) | 53 |
| SBP ≥ 145 mmHg | 3.8 (3.1–4.2) | 1.98 (1.49–2.62) | 75.2 (56.6–99.6) | 1.45 (1.09–1.91) | 54.9 (41.3–72.7) | 20.3 (15.3–26.9) | 57 |
| eGFR 20–59 ml/min/1.73 m2 | 2.0 (1.6–2.3) | 3.02 (2.18–4.16) | 60.4 (43.6–83.2) | 2.20 (1.59–3.04) | 44.1 (31.8–60.7) | 16.3 (11.8–22.5) | 38 |
| Not Taking Antihypertensive Medication | |||||||
| Overall | 10.7 (9.9–11.5) | 2.31 (1.89–2.73) | 247.2 (202.2–292.1) | 1.69 (1.38–1.99) | 180.4 (147.6–213.2) | 66.7 (54.6–78.9) | 49 |
| Sex | |||||||
| Men | 6.3 (5.8–6.9) | 2.49 (2.03–3.05) | 156.9 (127.9–192.2) | 1.82 (1.48–2.23) | 114.5 (93.4–140.3) | 42.4 (34.5–51.9) | 46 |
| Women | 4.4 (2.9–4.7) | 2.04 (1.49–2.80) | 89.8 (65.6–123.2) | 1.49 (1.09–2.04) | 65.5 (47.9–89.9) | 24.2 (17.7–33.3) | 56 |
| Age Group | |||||||
| < 75 years | 7.2 (6.7–7.7) | 1.21 (0.86–1.71) | 87.1 (61.9–123.1) | 0.88 (0.63–1.25) | 63.6 (45.2–89.9) | 23.5 (16.7–33.2) | 94 |
| ≥ 75 years | 3.5 (3.1–3.8) | 4.60 (3.84–5.49) | 161.0 (134.4–192.2) | 3.36 (2.80–4.01) | 117.5 (98.1–140.3) | 43.5 (36.3–51.9) | 25 |
| Race/ethnicity | |||||||
| Non-Hispanic White | 8.9 (8.0–9.9) | 2.25 (1.89–2.68) | 200.3 (168.2–238.5) | 1.64 (1.38–1.96) | 146.2 (122.8–174.1) | 54.1 (45.4–64.4) | 50 |
| Non-Hispanic Black | 0.64 (0.57–0.71) | 2.40 (1.54–3.68) | 15.4 (9.9–23.6) | 1.75 (1.12–2.87) | 11.2 (7.2–17.2) | 4.1 (2.7–6.4) | 47 |
| Mexican American | 0.36 (0.27–0.48) | 1.14 (0.71–1.82) | 4.1 (2.6–6.6) | 0.83 (0.52–1.33) | 3.0 (1.9–4.8) | 1.1 (0.7–1.8) | 100 |
| History of CHD | 0.98 (0.81–1.05) | 3.78 (2.52–5.57) | 37.0 (24.7–54.6) | 2.76 (1.84–4.07) | 27.0 (18.0–39.8) | 10.0 (6.7–14.7) | 30 |
| Baseline SBP | |||||||
| SBP ≤132 mmHg | 1.3 (1.1–1.4) | 2.10 (1.22–3.57) | 27.3 (15.9–46.4) | 1.53 (0.89–2.61) | 19.9 (11.6–33.9) | 7.4 (4.3–12.5) | 54 |
| SBP 133–144 mmHg | 4.2 (3.9–4.4) | 1.62 (1.21–2.16) | 68.0 (50.8–90.7) | 1.18 (0.88–1.58) | 49.7 (37.1–66.2) | 18.4 (13.7–24.5) | 70 |
| SBP ≥ 145 mmHg | 5.3 (4.9–5.8) | 2.90 (2.34–3.58) | 153.7 (124.0–189.7) | 2.12 (1.71–2.61) | 112.2 (90.5–138.5) | 41.5 (33.5–51.2) | 39 |
| eGFR 20–59 ml/min/1.73 m2 | 2.1 (1.9–2.4) | 2.88 (2.15–3.84) | 60.5 (45.2–80.6) | 2.10 (1.57–2.80) | 44.2 (33–58.9) | 16.3 (12.2–21.8) | 39 |
CHD = coronary heart disease, SBP=systolic blood pressure, SPRINT - Systolic Blood Pressure Intervention Trial, NHANES= National Health and Nutrition Examination Survey. Estimated glomerular filtration rate (eGFR) in ml/min/1.73 m2 was estimated using the CKD-EPI equation using the serum creatinine;
Calculated by multiplying the hazard ratio for all-cause mortality from SPRINT (0.73) by the annual mortality rate and the number of deaths among the NHANES population meeting the SPRINT eligibility criteria
Other race/ethnicity group not shown due to unstable estimates of mortality in stratified analyses
Use of antihypertensive medication was defined by self-report and report of taking one or more classes of antihypertensive medication identified through the pill bottle review.
The highest annual mortality rate was noted for SPRINT-eligible U.S. adults age ≥ 75 years, among whom intensive SBP treatment could prevent 67,300 (95%CI 58,600–77,200) deaths per year. SPRINT-eligible U.S. adults with eGFR 20–59 ml/min/1.73 m2 had annual mortality rates of 3.02% (95%CI 2.18%–4.16%) if taking antihypertensive medication and 2.88% (95%CI 2.15–3.84) if not taking antihypertensive medication, yielding total annual deaths of 121,000 (95%CI 97,200–149,700). Intensive SBP treatment among SPRINT-eligible U.S. adults with eGFR 20–59 ml/min/1.73 m2 was projected to prevent 32,700 (95%CI 26,200–40,400) deaths per year.
Intensive SBP treatment was projected to prevent 46,100 (95%CI 41,800–50,400) new cases of heart failure annually (Supplemental Table 7). After accounting for uncertainty in both the estimates for the population size and treatment effect on incident heart failure, intensive SBP treatment is projected to prevent between 17,600 and 73,000 new cases of heart failure per year.
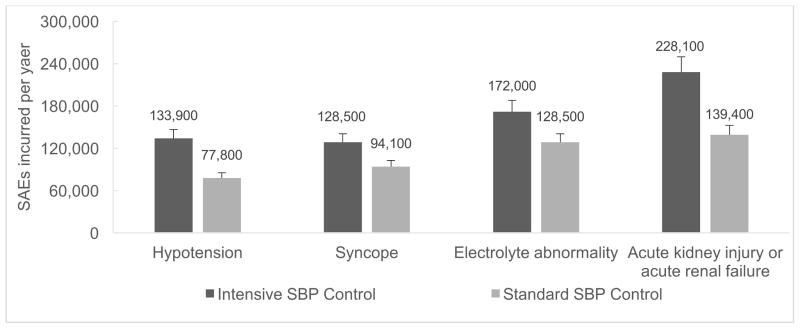
Among SPRINT-eligible U.S. adults overall, standard SBP treatment (i.e., usual care) could lead to 77,800 (95%CI 70,500–85,100) episodes of hypotension, 94,100 (95%CI 85,300–103,000) episodes of syncope, 128,500 (95%CI 116,400–140,600) electrolyte abnormalities, and 139,400 (95%CI 126,300–152,500) cases of acute kidney injury per year (Table 4 and Figure 2). In this same group, intensive SBP treatment is projected to result in 56,100 (95%CI 50,800–61,400) additional episodes of hypotension, 34,400 (95%CI 31,200–37,600) additional episodes of syncope, 43,400 (95%CI 39,400–47,500) additional electrolyte abnormalities, and 88,700 (95%CI 80,400–97,000) additional cases of acute kidney injury per year. The analysis-of-extremes sensitivity analyses for SAEs are shown in Supplemental Table 8.
Table 4.
Projected number of serious adverse events incurred per year with intensive SBP control
| Serious Adverse Events | Annual Risk Observed in SPRINT | Expected Number of SAEs per year, thousands* | SAEs incurred per year (95%CI)† | Number Needed to Harm (over 3.26 years of treatment) | |||
|---|---|---|---|---|---|---|---|
| Intensive | Standard | Risk Difference | Intensive | Standard | |||
| Hypotension | 0.74% | 0.43% | 0.31% | 133.9 (121.4–146.5) | 77.8 (70.5–85.1) | 56.1 (50.8–61.4) | 99 |
| Syncope | 0.71% | 0.52% | 0.19% | 128.5 (116.4–140.6) | 94.1 (85.3–103.0) | 34.4 (31.2–37.6) | 161 |
| Electrolyte abnormality | 0.95% | 0.71% | 0.24% | 172.0 (155.8–188.1) | 128.5 (116.4–140.6) | 43.4 (39.4–47.5) | 128 |
| Acute kidney injury or acute renal failure | 1.26% | 0.77% | 0.49% | 228.1 (206.6–249.5) | 139.4 (126.3–152.5) | 88.7 (80.4–97.0) | 63 |
| SAEs Possibly of Definitely Related to the Intervention | |||||||
| Hypotension | 0.55% | 0.25% | 0.30% | 99.6 (90.2–108.9) | 45.3 (41.0–49.5) | 54.3 (49.2–59.4) | 102 |
| Syncope | 0.43% | 0.18% | 0.25% | 77.8 (70.5–85.1) | 32.6 (29.5–35.6) | 45.3 (41.0–49.5) | 123 |
| Electrolyte abnormality | 0.46% | 0.31% | 0.15% | 83.3 (75.4–91.1) | 56.1 (50.8–61.4) | 27.2 (24.6–29.7) | 204 |
| Acute kidney injury or acute renal failure | 0.58% | 0.21% | 0.37% | 105.0 (95.1–114.8) | 38.0 (34.4–41.6) | 67.0 (60.7–73.3) | 83 |
SBP= systolic blood pressure, SAE = serious adverse event, SPRINT=Systolic Blood Pressure Intervention Trial.
Calculated by multiply the annual SAE rate by 18,100,000 and its 95%CD (16,400,000–19,800,000) which is the number of U.S. Adults meeting the sprint eligibility criteria from 1999–2006.
Calculated by multiplying the risk difference for each SAE by 18,100,000 which is the number of U.S. Adults meeting the sprint eligibility criteria from 1999–2006. Acute kidney injury or acute renal failure was defined in SPRINT as an event that occurred during a hospitalization and were reported in the hospital discharge summary as a primary or main secondary diagnosis.
An SAE was defined in SPRINT as an event that was fatal or life-threatening, that resulted in clinically significant or persistent disability, that required or prolonged a hospitalization, or that was judged by the investigator to represent a clinically significant hazard or harm to the participant that might require medical or surgical intervention to prevent another SAE
Figure 2. Potential number of serious adverse events per year with standard and intensive SBP control among NHANES participants who meet the SPRINT eligibility criteria.
Projected number of SAEs from standard and intensive SBP control were calculated by taking the observed SAE rate in SPRINT and dividing it by 3.26 (the median follow-up in SPRINT) and then multiplying it by 18,100,000 which is the number of U.S. Adults meeting the sprint eligibility criteria from 1999–2006 SAEs= Serious adverse events, SBP=systolic blood pressure, SPRINT - Systolic Blood Pressure Intervention Trial.
Vertical lines denote the upper limit of the 95% confidence interval.
Discussion
In the current analysis, we project that about 107,500 deaths could be averted annually if an intensive SBP target goal of < 120 mmHg were to be adopted among all U.S. adults meeting the SPRINT eligibility criteria. To provide context, this number represents nearly 20% of the 614,348 Americans who died of heart disease in 2014.13 Moreover, projections from the current study indicate a similar number of deaths could be prevented with intensive SBP treatment in eligible US adults as another public health strategy of dietary salt reduction in the entire US adult population.14 Benefits of intensive SBP treatment were more pronounced in high risk subgroups such as those age ≥ 75 years. Intensive SBP treatment was also projected to prevent about 46,100 new cases of heart failure per year. These benefits are tempered by a projected increase in SAEs, including about 56,100 additional episodes of hypotension, 88,700 cases of acute kidney injury, 34,400 episodes of syncope, and 43,400 episodes of electrolyte abnormalities.
The magnitude of the potential benefit estimated can be conceptualized in practical terms by recognizing that the NNT from SPRINT to prevent one death from any cause was 90 over 3.26 years.7 While varying assumptions could change our overall estimate, the combination of a low NNT (i.e., 90 over 3.26 years) and the large number of US adults meeting the SPRINT eligibility criteria supports the contention that an impact in the range of 100,000 deaths averted per year is realistic. Few other medical interventions are currently available that could have such a large and immediate public health impact on broad sectors of the U.S. adult population. Adding to the evidence supporting intensive SBP target goals in the general population, a recent meta-analysis of 34 BP lowering trials comparing intensive versus standard BP lowering including SPRINT found that the direction of effect was consistent across the component trials, and that overall more intensive SBP lowering significantly reduced risk of CVD events and stroke compared to standard SBP lowering.15
Intensive BP treatment involves the potential risk of SAEs, especially in the elderly. Both the relative and absolute CVD and all-cause mortality risk reductions were greater in SPRINT participants 75 years of age or older, regardless of frailty status at baseline.16 The HR among participants over age 75 at entry was 0.67 for all-cause mortality, and, based on the 3-year interval of the trial, only 27 patients would need to be treated with the intensive SBP control to prevent the primary SPRINT outcome of CVD, while treatment of 41 people would prevent a death.16
SPRINT provided detailed estimates of SAEs, virtually all of which were either without lasting consequence or reversible with dose de-escalation. Even in participants age 75 and older, SAEs related to hypotension were only slightly increased in the intensive group - an excess that did not reach statistical significance.16 Together with past results from the Hypertension in the Very Elderly Trial (HYVET) and the Systolic Hypertension in the Elderly Program (SHEP), the SPRINT results in elderly participants support a recommendation of the same BP treatment goals in young adults and most elderly patients.17, 18 At the same time, neither SPRINT nor NHANES studied institutionalized elderly patients; intensive goals may not be appropriate in such patients until more evidence emerges.
Reduction in CVD risk factor levels through primary and secondary prevention have accounted for the majority of the 80% decline in CVD deaths in the U.S. and many other countries over the last several decades.19, 20 Recently, however, it appears that the decline in CVD in the U.S. has slowed.21 More aggressive use of the available safe, effective, and inexpensive antihypertensive medications to reduce SBP to < 120 mmHg among eligible U.S. adults may restore the downward trend in CVD mortality that has transformed adult health in the U.S. over the last 50 years.
Intensive SBP treatment in patients with diabetes yielded more equivocal evidence in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) Trial. Therefore, there is uncertainty as to whether the benefits observed in SPRINT extend to patients with diabetes.4 However, a recent meta-analysis including ACCORD and other BP lowering trials in diabetes patients estimated a significant CVD and all-cause mortality reductions with lower SBP target goals in patients with diabetes, with no evidence of diminished risk reductions below 130 mmHg.22 On the contrary, another meta-analysis of BP trials found an attenuation of the treatment effect in people with diabetes when SBP was lowered to < 130 mmHg.23 More definitive evidence on the role of intensive BP treatment in patients with diabetes, with outcome definitions more similar to SPRINT, is urgently needed.
Implementation of the SPRINT intensive regimen will require overcoming a number of obstacles. Because SPRINT was a practice-based trial and recruited patients from clinics, it is unclear how likely intensive SBP goals will be achieved among population-based free-dwelling adults. It is likely that an additional investment will be required from providers and patients (e.g., more frequent clinic visits, lab testing and additional medications) to produce the mean SBP change achieved in SPRINT (14.8 mmHg after one year of treatment). Integrated health systems such as Kaiser Permanente of Northern California are already achieving control rates of > 90% for SBP and DBP targets goals of <140/90 mmHg.24, 25 However, consistent with the history of implementing evidence from virtually any new trial that substantially challenges established practice, reluctance to implement an SBP goal of < 120 mmHg could be encountered among some health care providers for months and possibly years, due to worry about SAEs and simple clinical inertia. Cognitive bias that weights negative consequences of a preventive therapy more heavily than long-term benefit is common in providers. Patients treated to intensive goals will require careful monitoring in order to avoid hypotension, syncope, electrolyte abnormalities, or acute kidney injury. Active involvement of patients who desire a CVD-free life despite a small risk of an SAE may be a positive force to overcome providers’ resistance to change and clinical inertia.
This report draws on several major strengths. NHANES provides accurate estimates of the target U.S. adult population and has enrolled a large sample size allowing us to conduct analyses in several subgroups. Inclusions and exclusions did not exactly match, since NHANES did not have information on some of the SPRINT eligibility criteria (i.e., the presence of reduced left ventricular ejection fraction, coronary calcium score, ankle brachial index, or left ventricular hypertrophy) or a history of medication non-adherence. SPRINT excluded individuals whose SBP was <110 mm Hg after one minute of standing. As standing BP was not obtained in NHANES, these individuals could not be excluded from the current analysis. There also was a higher percentage of black participants in SPRINT than were in the SPRINT-eligible NHANES sample in the current analysis. As there were no statistically significant interactions in pre-specified subgroups in SPRINT, we assumed no heterogeneity of treatment effect across these subgroups. The observed mortality was substantially higher in the U.S. population meeting SPRINT criteria compared to SPRINT. This is likely because volunteers for clinical trials, including SPRINT, tend to be healthier than the general population. As a result, the smaller absolute mortality reduction observed in this trial may lead to an underestimate of preventable deaths in the population. Likewise, it could have led to under-estimation of SAE rates. Both SPRINT and NHANES used the mean of three BP measurements after five minutes of seated rest, however, SPRINT used an automated device (Model 907, Omron Healthcare), whereas NHANES used a mercury sphygmomanometer. Unless an automated office BP without an observer being present is taken, usual clinic SBP could be expected to be approximately 10 mm Hg higher than usual, observed clinic BP.10, 26, 27 The results of sensitivity analysis requiring SBP levels to be 10 and 20 mm Hg higher than the SPRINT entry criteria indicated that a somewhat smaller number of deaths would be averted with intensive SBP treatment (about 63 to 84) thousand deaths averted per year). However, the projected number of deaths averted due to intensive treatment would remain high.
In conclusion, the current analysis projects that if the intensive SBP treatment studied in SPRINT were widely adopted in eligible, high CVD risk US adults, about 107,500 deaths could be prevented annually. This benefit must be balanced against increased risk of SAEs including a projected 56,100 and 88,700 additional cases per year of hypotension and acute kidney injury incurred with intensive SBP treatment.
Supplementary Material
Clinical Perspective.
What is new?
In this population based study, if fully implemented in eligible U.S. adults, intensive blood pressure treatment was projected to prevent about 107,500 deaths per year and give rise to about 56,100 episodes of hypotension, 34,400 episodes of syncope, 43,400 serious electrolyte disorders, and 88,700 cases of acute kidney injury per year compared to standard blood pressure treatment.
What are the clinical implications?
If fully implemented in eligible U.S. adults with raised blood pressure and at high risk for cardiovascular disease, intensive blood pressure treatment has the potential to prevent about 107,500 deaths per year compared to standard blood pressure treatment.
Careful patient selection and implementation are important because intensive treatment is associated with increased risk of hypotension, syncope, electrolyte abnormalities, and acute kidney injury.
Acknowledgments
Sources of Funding
Dr. Bress was supported by 1K01HL133468-01 from the National Heart, Lung, and Blood Institute, Bethesda, MD. Dr. Moran was supported by R01 HL 130500-01A1 from the National Heart, Lung, and Blood Institute. Dr. Muntner was supported by 15SFRN2390002 from the American Heart Association.
Footnotes
Drs. Bress, Kramer, Khatib, and Cooper had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis
Disclosures
APB: Receives research support from Novartis not related to the current project.
HK: No conflicts of interest to disclose
RH: No conflicts of interest to disclose
RK: No conflicts of interest to disclose
SB: No conflicts of interest to disclose
AKC: No conflicts of interest to disclose
VKB: No conflicts of interest to disclose
GC: No conflicts of interest to disclose
JY: No conflicts of interest to disclose
AEM: No conflicts of interest to disclose
RD: No conflicts of interest to disclose
PM: No conflicts of interest to disclose
RC: No conflicts of interest to disclose
References
- 1.Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, Amann M, Anderson HR, Andrews KG, Aryee M, Atkinson C, Bacchus LJ, Bahalim AN, Balakrishnan K, Balmes J, Barker-Collo S, Baxter A, Bell ML, Blore JD, Blyth F, Bonner C, Borges G, Bourne R, Boussinesq M, Brauer M, Brooks P, Bruce NG, Brunekreef B, Bryan-Hancock C, Bucello C, Buchbinder R, Bull F, Burnett RT, Byers TE, Calabria B, Carapetis J, Carnahan E, Chafe Z, Charlson F, Chen H, Chen JS, Cheng AT, Child JC, Cohen A, Colson KE, Cowie BC, Darby S, Darling S, Davis A, Degenhardt L, Dentener F, Des Jarlais DC, Devries K, Dherani M, Ding EL, Dorsey ER, Driscoll T, Edmond K, Ali SE, Engell RE, Erwin PJ, Fahimi S, Falder G, Farzadfar F, Ferrari A, Finucane MM, Flaxman S, Fowkes FG, Freedman G, Freeman MK, Gakidou E, Ghosh S, Giovannucci E, Gmel G, Graham K, Grainger R, Grant B, Gunnell D, Gutierrez HR, Hall W, Hoek HW, Hogan A, Hosgood HD, 3rd, Hoy D, Hu H, Hubbell BJ, Hutchings SJ, Ibeanusi SE, Jacklyn GL, Jasrasaria R, Jonas JB, Kan H, Kanis JA, Kassebaum N, Kawakami N, Khang YH, Khatibzadeh S, Khoo JP, Kok C, Laden F, Lalloo R, Lan Q, Lathlean T, Leasher JL, Leigh J, Li Y, Lin JK, Lipshultz SE, London S, Lozano R, Lu Y, Mak J, Malekzadeh R, Mallinger L, Marcenes W, March L, Marks R, Martin R, McGale P, McGrath J, Mehta S, Mensah GA, Merriman TR, Micha R, Michaud C, Mishra V, Mohd Hanafiah K, Mokdad AA, Morawska L, Mozaffarian D, Murphy T, Naghavi M, Neal B, Nelson PK, Nolla JM, Norman R, Olives C, Omer SB, Orchard J, Osborne R, Ostro B, Page A, Pandey KD, Parry CD, Passmore E, Patra J, Pearce N, Pelizzari PM, Petzold M, Phillips MR, Pope D, Pope CA, 3rd, Powles J, Rao M, Razavi H, Rehfuess EA, Rehm JT, Ritz B, Rivara FP, Roberts T, Robinson C, Rodriguez-Portales JA, Romieu I, Room R, Rosenfeld LC, Roy A, Rushton L, Salomon JA, Sampson U, Sanchez-Riera L, Sanman E, Sapkota A, Seedat S, Shi P, Shield K, Shivakoti R, Singh GM, Sleet DA, Smith E, Smith KR, Stapelberg NJ, Steenland K, Stockl H, Stovner LJ, Straif K, Straney L, Thurston GD, Tran JH, Van Dingenen R, van Donkelaar A, Veerman JL, Vijayakumar L, Weintraub R, Weissman MM, White RA, Whiteford H, Wiersma ST, Wilkinson JD, Williams HC, Williams W, Wilson N, Woolf AD, Yip P, Zielinski JM, Lopez AD, Murray CJ, Ezzati M, AlMazroa MA, Memish ZA. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2224–60. doi: 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R Prospective Studies C. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–13. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 3.James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, Lackland DT, LeFevre ML, MacKenzie TD, Ogedegbe O, Smith SC, Jr, Svetkey LP, Taler SJ, Townsend RR, Wright JT, Jr, Narva AS, Ortiz E. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8) JAMA. 2014;311:507–20. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 4.Cushman WC, Evans GW, Byington RP, Goff DC, Jr, Grimm RH, Jr, Cutler JA, Simons-Morton DG, Basile JN, Corson MA, Probstfield JL, Katz L, Peterson KA, Friedewald WT, Buse JB, Bigger JT, Gerstein HC, Ismail-Beigi F. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575–85. doi: 10.1056/NEJMoa1001286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benavente OR, Coffey CS, Conwit R, Hart RG, McClure LA, Pearce LA, Pergola PE, Szychowski JM. Blood-pressure targets in patients with recent lacunar stroke: the SPS3 randomised trial. Lancet. 2013;382:507–15. doi: 10.1016/S0140-6736(13)60852-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ambrosius WT, Sink KM, Foy CG, Berlowitz DR, Cheung AK, Cushman WC, Fine LJ, Goff DC, Jr, Johnson KC, Killeen AA, Lewis CE, Oparil S, Reboussin DM, Rocco MV, Snyder JK, Williamson JD, Wright JT, Jr, Whelton PK, Group SSR. The design and rationale of a multicenter clinical trial comparing two strategies for control of systolic blood pressure: the Systolic Blood Pressure Intervention Trial (SPRINT) Clin Trials. 2014;11:532–46. doi: 10.1177/1740774514537404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wright JT, Jr, Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, Reboussin DM, Rahman M, Oparil S, Lewis CE, Kimmel PL, Johnson KC, Goff DC, Jr, Fine LJ, Cutler JA, Cushman WC, Cheung AK, Ambrosius WT. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. N Engl J Med. 2015;373:2103–16. doi: 10.1056/NEJMoa1511939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mirel LB, Mohadjer LK, Dohrmann SM, Clark J, Burt VL, Johnson CL, Curtin LR. National Health and Nutrition Examination Survey: estimation procedures, 2007–2010. Vital Health Stat. 2013;2:1–17. [PubMed] [Google Scholar]
- 9.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, Ckd EPI. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–12. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Myers MG. A Short History of Automated Office Blood Pressure - 15 Years to SPRINT. J Clin Hypertens (Greenwich) 2016;18:721–4. doi: 10.1111/jch.12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chew DP, Huynh LT, Liew D, Astley C, Soman A, Brieger D. Potential survival gains in the treatment of myocardial infarction. Heart. 2009;95:1844–50. doi: 10.1136/hrt.2009.174276. [DOI] [PubMed] [Google Scholar]
- 12.Fonarow GC, Hernandez AF, Solomon SD, Yancy CW. Potential Mortality Reduction With Optimal Implementation of Angiotensin Receptor Neprilysin Inhibitor Therapy in Heart Failure. JAMA Cardiol. 2016;1:714–7. doi: 10.1001/jamacardio.2016.1724. [DOI] [PubMed] [Google Scholar]
- 13.National Center for Health Statistics. Health, United States, 2015: With Special Feature on Death and Dying. Hyattsville, Maryland: 2015. p. 107. [PubMed] [Google Scholar]
- 14.Bibbins-Domingo K, Chertow GM, Coxson PG, Moran A, Lightwood JM, Pletcher MJ, Goldman L. Projected effect of dietary salt reductions on future cardiovascular disease. N Engl J Med. 2010;362:590–9. doi: 10.1056/NEJMoa0907355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thomopoulos C, Parati G, Zanchetti A. Effects of blood pressure lowering on outcome incidence in hypertension: 7. Effects of more vs. less intensive blood pressure lowering and different achieved blood pressure levels - updated overview and meta-analyses of randomized trials. J Hypertens. 2016;34:613–22. doi: 10.1097/HJH.0000000000000881. [DOI] [PubMed] [Google Scholar]
- 16.Williamson JD, Supiano MA, Applegate WB, Berlowitz DR, Campbell RC, Chertow GM, Fine LJ, Haley WE, Hawfield AT, Ix JH, Kitzman DW, Kostis JB, Krousel-Wood MA, Launer LJ, Oparil S, Rodriguez CJ, Roumie CL, Shorr RI, Sink KM, Wadley VG, Whelton PK, Whittle J, Woolard NF, Wright JT, Jr, Pajewski NM, Group SR. Intensive vs Standard Blood Pressure Control and Cardiovascular Disease Outcomes in Adults Aged >/=75 Years: A Randomized Clinical Trial. JAMA. 2016;315:2673–82. doi: 10.1001/jama.2016.7050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP) SHEP Cooperative Research Group. JAMA. 1991;265:3255–64. [PubMed] [Google Scholar]
- 18.Beckett NS, Peters R, Fletcher AE, Staessen JA, Liu L, Dumitrascu D, Stoyanovsky V, Antikainen RL, Nikitin Y, Anderson C, Belhani A, Forette F, Rajkumar C, Thijs L, Banya W, Bulpitt CJ, Group HS. Treatment of hypertension in patients 80 years of age or older. N Engl J Med. 2008;358:1887–98. doi: 10.1056/NEJMoa0801369. [DOI] [PubMed] [Google Scholar]
- 19.Ford ES, Ajani UA, Croft JB, Critchley JA, Labarthe DR, Kottke TE, Giles WH, Capewell S. Explaining the decrease in U.S. deaths from coronary disease, 1980–2000. N Engl J Med. 2007;356:2388–98. doi: 10.1056/NEJMsa053935. [DOI] [PubMed] [Google Scholar]
- 20.Unal B, Critchley JA, Capewell S. Explaining the decline in coronary heart disease mortality in England and Wales between 1981 and 2000. Circulation. 2004;109:1101–7. doi: 10.1161/01.CIR.0000118498.35499.B2. [DOI] [PubMed] [Google Scholar]
- 21.Sidney S, Quesenberry CP, Jr, Jaffe MG, Sorel M, Nguyen-Huynh MN, Kushi LH, Go AS, Rana JS. Recent Trends in Cardiovascular Mortality in the United States and Public Health Goals. JAMA Cardiol. 2016;1:594–9. doi: 10.1001/jamacardio.2016.1326. [DOI] [PubMed] [Google Scholar]
- 22.Ettehad D, Emdin CA, Kiran A, Anderson SG, Callender T, Emberson J, Chalmers J, Rodgers A, Rahimi K. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet. 2016;387:957–67. doi: 10.1016/S0140-6736(15)01225-8. [DOI] [PubMed] [Google Scholar]
- 23.Brunstrom M, Carlberg B. Effect of antihypertensive treatment at different blood pressure levels in patients with diabetes mellitus: systematic review and meta-analyses. BMJ. 2016;352:i717. doi: 10.1136/bmj.i717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jaffe MG, Lee GA, Young JD, Sidney S, Go AS. Improved blood pressure control associated with a large-scale hypertension program. JAMA. 2013;310:699–705. doi: 10.1001/jama.2013.108769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jaffe MG, Young JD. The Kaiser Permanente Northern California Story: Improving Hypertension Control From 44% to 90% in 13 Years (2000 to 2013) The Journal of Clinical Hypertension. 2016;18:260–261. doi: 10.1111/jch.12803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sica DA, Phillips RA, White WB, Bisognano JD, Townsend RR. “Translational” medicine: transforming SPRINT findings into clinical practice. J Am Soc Hypertens. 2016;10:382–6. doi: 10.1016/j.jash.2016.03.192. [DOI] [PubMed] [Google Scholar]
- 27.Phillips RA. Current and Future Treatment of Hypertension in the SPRINT Era. Methodist Debakey Cardiovasc J. 2015;11:206–13. doi: 10.14797/mdcj-11-4-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.