Abstract
Background
MRI studies have consistently demonstrated disproportionately smaller corpus callosa in individuals with a history of prenatal alcohol exposure but have not previously examined the feasibility of detecting this effect in infants. Tissue segmentation of the newborn brain is challenging because analysis techniques developed for the adult brain are not directly transferable, and segmentation for cerebral morphometry is difficult in neonates, due to the latter’s incomplete myelination. This study is the first to use volumetric structural MRI to investigate prenatal alcohol exposure effects in newborns using manual tracing and to examine the cross-sectional area of the corpus callosum (CC).
Methods
43 nonsedated infants born to 32 Cape Coloured heavy drinkers and 11 controls recruited prospectively during pregnancy were scanned using a custom-designed birdcage coil for infants, which increases signal-to-noise ratio almost two-fold compared to the standard head coil. Alcohol use was ascertained prospectively during pregnancy, and FASD diagnosis was conducted by expert dysmorphologists. Data were acquired using a multi-echo FLASH protocol adapted for newborns, and a knowledge-based procedure was used to hand-segment the neonatal brains.
Results
CC was disproportionately smaller in alcohol-exposed neonates than controls after controlling for intracranial volume. By contrast, CC area was unrelated to infant sex, gestational age, age at scan, or maternal smoking, marijuana, or methamphetamine use during pregnancy.
Conclusions
Given that midline craniofacial anomalies have been recognized as a hallmark of FAS in humans and animal models since this syndrome was first identified, the CC deficit identified here in newborns may support early identification of a range of midline structural impairments. Smaller CC during the newborn period may provide an early indicator of fetal alcohol-related cognitive deficits that have been linked to this critically important brain structure in childhood and adolescence.
Keywords: corpus callosum, neonatal brain MRI, manual tracing, fetal alcohol spectrum disorders, prenatal alcohol exposure, fetal alcohol syndrome
INTRODUCTION
The prevalence of fetal alcohol spectrum disorders (FASD), the most common preventable cause of neurocognitive disabilities, has been estimated at 2 to 5% of live births in the United States, Canada, and Western Europe (May et al., 2009), and as high as 13.6 to 20.9% in the Western Cape Province of South Africa (May et al., 2013). FASD are characterized by a broad range of cognitive and behavioral deficits. Fetal alcohol syndrome (FAS), the most severe FASD and the most common preventable form of mental retardation, is characterized by a distinctive pattern of craniofacial dysmorphologic features, small head circumference, and pre- and/or postnatal growth retardation (Hoyme et al., 2005). Children with alcohol-related neurodevelopmental disorder (ARND) exhibit significant neurobehavioral impairment but lack the distinctive facial anomalies.
Findings from several studies suggest that the developing corpus callosum (CC), the largest white matter (WM) tract in the human brain and critical for interhemispheric communication (Thompson et al., 2011), is particularly susceptible to the effects of prenatal alcohol exposure (PAE). The earliest autopsy studies reporting damaging effects of heavy PAE identified errors in cell migration, agenesis or thinning of the corpus callosum, and anomalies in the cerebellum and brain stem (Jones and Smith, 1973; Clarren and Smith, 1978; Wisnewski et al., 1983). These early studies suggested that the incidence of partial or complete CC agenesis among children with heavy PAE might be as high as 6.8% (Riley et al., 1995), as compared to a normal population rate of 0.3% and a developmentally delayed population rate of 2.3% (Jeret et al., 1985). Imaging studies of children with FASD have documented reduced callosal size (Archibald et al., 2001; Sowell et al., 2001; Yang et al., 2012), anterior and inferior displacement of the posterior CC regions (Sowell et al., 2001), reduced CC thickness (Yang et al., 2012), and callosal shape abnormalities in infants using ultrasound (Bookstein et al., 2002).
The structural CC deficits seen in alcohol-exposed children may lead to poorer cognitive performance, particularly on tasks designed to assess interhemispheric transfer of information (Johansen-Berg et al., 2007). Consistent with this prediction, PAE has been found to be associated with poorer performance on a finger localization task that assesses efficiency of interhemispheric transfer of tactile information in a San Diego cohort (mean age = 11.9 yr) (Roebuck et al., 2002) and in cohorts in Cape Town (mean = 10.3 yr) and Detroit (mean = 19.5 yr) (Dodge et al., 2009). In the Detroit study, quantity of alcohol consumed per occasion during pregnancy was inversely related to performance on the finger localization task. Smaller CC size was associated with poorer finger localization performance in both the San Diego and Cape Town studies. In an fMRI task assessing interhemispheric transfer of somatosensory information, dysmorphic alcohol-exposed adults showed greater neural activation in the premotor and primary motor cortices compared to nondysmorphic alcohol-exposed and control adults when pressing a button contralateral to a vibrotactile stimulus (Santhanam et al., 2007), suggesting that the dysmorphic adults were not able to perform the task as efficiently as controls.
Despite more than four decades of research, identification of alcohol-affected children continues to be challenging, especially during infancy, the optimal time to initiate remedial interventions. Early, reliable evidence of prenatal alcohol effects are needed because craniofacial dysmorphic features that characterize FASD are found in only a small proportion of children with prenatal alcohol-related neurobehavioral impairment and can be difficult to detect in infancy and childhood (Suttie et al., 2013). Given the rapid and heterochronus nature of early brain growth, volumetric measures based on MRI in the first weeks of life may detect early effects on brain structures (Nishida et al., 2006) and provide a more sensitive index of future neurological outcome than current standard newborn behavioral and neurological examinations. Due to its demonstrated vulnerability to PAE and association with cognitive function, as well as its relative reliability of definition, the CC is a likely candidate to provide a sensitive early indicator of FASD. In addition, Lebel Yang et al. (2010) have reported an association of reduced CC area and thickness with reduced palpebral fissure length, suggesting that CC size may provide a neural biomarker of FAS.
Segmentation of the newborn brain is more challenging and time-consuming than for a child or an adult due to lower contrast-to-noise ratio, small head size (which requires scanning at higher resolution) and typically short scan periods that are determined by the infant’s sleep duration during scanning and which provide less opportunity to reacquire volumes or to oversample to reduce motion artifacts (Prastawa et al., 2005). Although the brain stem and posterior limbs of the internal capsule are myelinated in the newborn with white to gray matter contrast that is similar to the adult brain, other regions are unmyelinated with the gray/white contrast inverted in T1-weighted images relative to the adult contrast. In addition, the boundaries between fully myelinated and non-myelinated regions are ambiguous, tissue types often vary markedly in levels of intensity, and there is considerable overlap between different tissues in intensity characteristics, making decisions regarding boundaries for intensity-based classifications difficult for existing automated computational methods. Given these difficulties, most newborn MRI studies to date have relied on manual tracing to delineate structures such as the CC (e.g., Thompson et al., 2011; de Macedo Rodrigues et al., 2015). In the only other study using structural MRI with prenatally exposed newborns, smaller whole brain volumes and reduced gray matter in three regions were reported in exposed newborns compared with controls, but hand tracing was not employed for segmentation and CC volume was apparently not assessed (Donald et al., 2016).
The incidence of FAS in the Cape Coloured (mixed ancestry) population in the Western Cape Province of South Africa is among the highest in the world with 68.0–89. 2 cases per 1000 (May et al., 2013). This population, composed mainly of descendants of white European settlers, Malaysian slaves, Khoi-San aboriginals, and black Africans, has historically comprised the large majority of workers in the wine-producing region of the Western Cape. The high prevalence of FAS in this community is a consequence of very heavy maternal drinking during pregnancy, which is due to poor psychosocial circumstances and the traditional dop system, in which farm laborers were paid, in part, with wine. Although the dop system has been outlawed since the 1920s, weekend binge drinking continues to be a major source of recreation for many in urban and rural Cape Coloured communities (Jacobson et al., 2006; May et al., 2013) despite numerous efforts to intervene to reduce pregnancy drinking.
In this paper, we report findings from the first study to examine effects of PAE on CC size in the newborn brain. The aims of this study were to test the following hypotheses: (1) the effect of heavy PAE on CC size can be detected in newborns; (2) this effect is not attributable to maternal food security or exposure to other potential teratogens, including maternal smoking, methamphetamine and marijuana use; and (3) reduced CC size in the newborn is observable even after adjusting for the smaller head circumference required for a diagnosis of FAS and is also seen in heavily exposed nonsyndromal children with normal overall brain size.
METHODS
Participants
The sample consisted of 43 Cape Coloured newborns: 32 prenatally alcohol exposed (17 male) and 11 healthy controls (HC; 8 male). The infants were born to women who were recruited between 2011–2013 at their first antenatal visit from two midwife obstetrical clinics that serve an economically disadvantaged Cape Coloured community. Each mother was interviewed antenatally regarding her alcohol consumption using a 2-week timeline follow-back interview (Jacobson et al., 2002), adapted to reflect how pregnant women in this community drink. The interview included information about type of beverage consumed, as well as about sharing (size of container shared by how many women) and container size (including pictures of different containers, bottles, cans, glass size), for use in the calculation of standard drinks (Jacobson et al., 2008). At recruitment the mother was interviewed regarding incidence and amount of drinking on a day-by-day basis during a typical 2-week period at time of conception. Volume was recorded for each type of beverage consumed each day and converted to oz absolute alcohol (AA) using the following weights that reflect potency of AA in Cape Town (liquor—0.4, beer—0.05, wine—0.12, cider—0.06). The mother was then asked whether her drinking had changed since conception; if so, when the change occurred and how much she drank on a day-by-day basis during the last 2 weeks. Maternal exclusionary criteria were age <18 years, HIV infection, multiple gestation pregnancy, and pharmacologic treatment for medical conditions, including diabetes, hypertension, epilepsy, or cardiac problems. Two groups of women were recruited: heavy drinkers, who consumed 14 or more standard drinks/week (≈1.0 oz AA/day) and/or engaged in binge drinking (4 or more drinks/occasion), and controls, who abstained or drank only minimally during pregnancy.
The timeline follow-back interview was repeated at 4 and 12 weeks after recruitment. Data from the three alcohol consumption interviews were averaged to provide continuous measures of drinking around time of conception and across pregnancy: average oz AA consumed/day, AA/drinking day (dose/occasion) and frequency (days/week). All women who reported drinking during pregnancy were advised to stop or reduce their intake and were offered referrals for treatment. We have previously validated this ascertainment protocol in relation to levels of fatty acid ethyl esters in meconium samples in this community (Bearer et al., 2003) and to infant outcomes (Jacobson et al., 2002).
Mothers were asked about their drug use during pregnancy. Marijuana (“dagga”), cocaine, heroin, methaqualone (“mandrax”), and methamphetamine (“tik”) were measured in days/week; and smoking as cigarettes/day. Mothers were administered the 18-item Family Food Security interview (Bickel et al., 2000), which has been widely used to assess adequacy of food availability (e.g., Health Canada, 2004). Birth weight and gestational age (GA) at birth were obtained from medical records; GA was based on early gestation ultrasound, where available, or date of last menstrual period. Infant exclusionary criteria were major chromosomal anomalies, neural tube defects, multiple births, very low birth weight (<1500 g), gestational age <32 weeks, and seizures.
Human subjects approval was obtained from the Wayne State University and University of Cape Town Faculty of Health Sciences Institutional Review Boards. All mothers provided written informed consent.
Newborn assessment
The 43 newborns were scanned at 6–40 days postpartum (mean = 17.8, SD = 9.4) or, if born preterm (<37 wk GA), at the equivalent age since conception. Of the 43 infants, 8 (18.6%) were born at GA ≤37 wk. All but 1 infant had reached 37 weeks GA before their MR scan. Each infant was scanned following a detailed standard protocol developed by PW and SW (Boston Children’s Hospital, Boston, MA) to acquire neonatal MRI data without use of sedation. The mother was instructed not to feed the baby for 3 hr prior to the scan. She and the newborn were transported to the Cape Universities Brain Imaging Centre (CUBIC) by our driver and research nurse. Upon arrival, the infant was examined by CM, a senior developmental pediatrician, who administered the Brazelton Neonatal Behavioral Assessment Scale (NBAS). The NBAS assesses the newborn’s responses to visual and auditory stimuli, reflexes, responses to stress, motor maturity, and hand-mouth coordination. Following the NBAS, weight, length, and head circumference were assessed; the infant’s diaper changed; and the infant was swaddled, wrapping the arms to reduce jerky movements and other motion. S/he was then fed by the mother and rocked until in a deep sleep. Because the NBAS and measurement procedures are tiring, their administration made it easier for the infant to fall asleep and remain asleep in the scanner without sedation.
The infant was then placed supine on an MRI-compatible vacuum cushion containing styrofoam beads (S&S X-Ray Products VacFix, Houston, TX) that fits snugly around the whole body, including the head. Sponge earplugs were inserted, and foam ear pads were placed over the ears held in place by a knitted cap to diminish noise. Air was then removed from the cushion to secure the infant’s body tightly, and the infant was moved with the pillow into the scanner. The infant’s head was positioned within the birdcage coil (described below), and his/her head was further secured with rolled towels or foam cubes to limit head and body movements and provide additional sound protection. A pulse oximeter was attached to the infant’s toe to monitor oxygen saturation. Throughout the scan, the infant was visually monitored for movement, crying, decrease in oxygen saturation, and any other signs of distress by CM or the research nurse, who remained in the scanner room, as well as the radiographer conducting the scan.
MR data acquisition
No sedation was used to obtain the MRI scans. Scanning was performed on a Siemens 3 T Allegra MR scanner (Siemens, Erlangen, Germany) using a custom-built, 170.9 mm (inner diameter) circularly polarized birdcage radiofrequency (RF) coil designed by L Wald, PhD and built by A Hess. Since the inner diameter of the coil was selected to fit a newborn head, this coil increases signal-to-noise ratio almost two-fold when compared with the standard head coil. The data acquisition used a multi-flip angle, multi-echo FLASH (MEF) protocol developed by AvdK to facilitate manual tracing (Fischl et al., 2004a). The T1 scans were acquired at two different flip angles (see below for details), allowing images to be synthesized with different relative T1 vs. proton density weightings to optimize contrast across boundaries of adjacent structures (Fig. 1). This approach provides a continuous range of contrasts rather than a single contrast to facilitate distinguishing multiple adjacent structures.
Fig. 1.
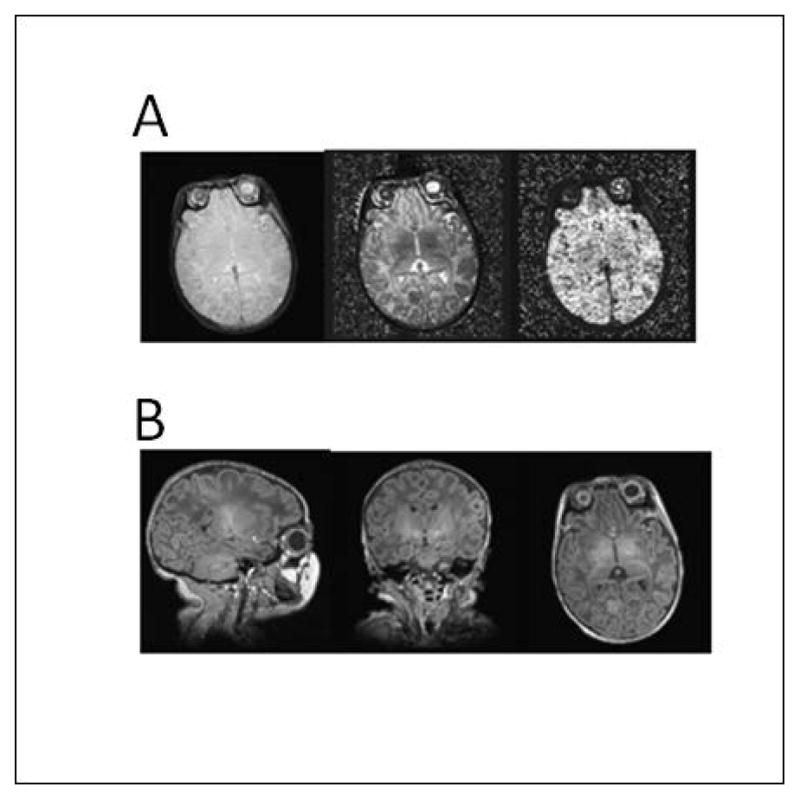
(A). Proton density (left), T1 relaxation time (middle) and T2* relaxation time (right) for newborn brain. (B) Sagittal (left), coronal (middle) and axial (right) views of a volume synthesized from tissue parameter estimates for a 24 degree flip angle.
The following imaging protocol was used: (1) Three-plane localizer; (2) MEF 20° with 3D encoding, 144 × 144 matrix, 128 sagittal slices; voxel size = 1×1×1 mm3, TR = 20 ms, 8 echoes with TE = 1.46 ms + n×1.68 ms where n = 0,..,7, bandwidth = 655 Hz/px, non-selective excitation with flip angle = 20°, acquisition time (Tacq) = 6 min 10 s; (3) MEF 5°, using identical parameters as the previous but with flip angle = 5°; (4) T2-SPACE (a T2 weighted anatomical sequence using 3D encoding), 144 × 144 matrix with 128 sagittal slices, voxel size = 1.1×1.1×1 mm3, TR/TE = 3000/355 ms, bandwidth = 655 Hz/px, non-selective excitation, Tacq = 6 min 29 s. Bandwidths were matched across MEF and T2-SPACE and had high values to minimize and match distortions due to B0 inhomogeneities across scans (van der Kouwe et al., 2008).
Image processing
MEF scans were analyzed to obtain quantitative proton density, T1 and T2* estimates, and the T2-SPACE scan provided images with T2 weighting. T1 and PD maps were reconstructed from the MEF data using the mri_ms_fitparms command in FreeSurfer, which replicates the DESPOT1 (driven equilibrium single pulse observation of T1 and T2) method (Deoni et al., 2003; Fischl et al., 2004b). Proton density and T1 are physical parameters that describe the tissue in the 3 T field and not simply weighted intensities that vary with the scanning sequence and system, facilitating comparison between studies, sequence types, and across 3 T systems. FLASH images were first preprocessed as follows, using FSL (Smith et al., 2004) and AFNI (Cox, 1996) tools: brain volumes were extracted with FSL-bet2 and corrected for non-uniform signal intensity across the image using AFNI-3dUnifize. Images across all flip angles and echo times were aligned using AFNI-3dAllineate to correct for subject motion occurring between data set acquisitions. After T1 and PD generation, an image volume was synthesized using FreeSurfer (Fischl et al, 2004b) for a flip angle of 24° to produce an optimal contrast T1-weighted image that was used for manual tracing. Tissue segmentation was performed using FSL-FAST to generate binary maps of cerebrospinal fluid (CSF), gray matter (GM) and white matter (WM) regions, from which tissue volumes were calculated.
In the present study, the CC was delineated on two mid-sagittal slices of the AC-PC aligned synthesized images, using FreeView, the visualization and editing tool of the FreeSurfer package (http://surfer.nmr.mgh.harvard.edu/). Two graduate research assistants (FW and NL) were trained and supervised at UCT by an expert neuroanatomist (CW) and at the MGH Martinos Center for Brain Imaging by K de Macedo Rodrigues, MD, a neuroradiologist trained by EG in manual segmentation. The brain was first rotated in relation to the posterior and anterior commissures by FW under the supervision of CW. The two contiguous slices on which the cerebral aqueduct is most clearly visible were identified as the mid-sagittal slices. When the cerebral aqueduct was only visible on one slice, the neighboring slice showing the CC most clearly was selected as the second mid-sagittal slice. Coronal views were also used on occasion to help clarify the pattern. FW and NL then independently traced the CC, using a knowledge-based, reproducible and highly detailed procedure adapted to neonatal brains (Nishida et al., 2006). Each segmenter averaged the areas of the two slices to generate a single value for CC area. The tracing guidelines follow procedures developed by (Caviness et al., 1989) and involve manually selecting intensity thresholds that result in continuous isointensity outlines to define specific anatomic borders. The segmenters were blind with respect to the newborn FASD diagnosis and prenatal alcohol and drug exposure. The inter-observer Dice coefficient (Pfefferbaum et al., 2006) was 0.89; the intra-class correlation was 0.83. The CC areas generated for each subject by the two segmenters were averaged to provide the measure of CC area used in the analyses reported here.
FASD Diagnosis
In September 2013 we organized a clinic in which the infants (mean age =1.0 yr, SD = 0.5; range = 0.4–1.8 yr) were examined for growth and FAS anomalies independently by two expert dysmorphologists (HE Hoyme, MD, and G De Jong, MD), using a standard protocol (Hoyme et al., 2005). They were subsequently re-examined in a second clinic (mean age = 4.0 yr, SD = 0.5; range = 3.4–4.8 yr) held in September 2016 by the same two dysmorphologists, assisted by H Bezuidenhout, MD, E Krzesinski, MD, and RC Carter, MD. Based on the revised Institute of Medicine guidelines, FAS is characterized by microcephaly, growth retardation, and a distinctive craniofacial dysmorphology, including short palpebral fissures, a flat philtrum, and a thin vermilion (upper lip). The dysmorphologists, SWJ, JLJ, and CDM subsequently conducted case conferences to reach consensus regarding which infants met criteria for FAS diagnoses. Diagnoses were obtained for all but three heavily exposed infants: one who died of SIDs and two who moved away from Cape Town after the scan but prior to the clinic.
Data Analysis
Statistical analyses were performed using SPSS software (v.22; IBM, Armonk, NY, USA). All variables were examined for normality of distribution and, where positively skewed (>3.0), were subjected to log transformation (average oz AA/day around conception and across pregnancy). Fourteen control variables were assessed as potential confounders of the effects of prenatal alcohol exposure on CC area—maternal age, parity, education, and marital status (married/unmarried); food security (secure/insecure); smoking (cigarettes/day) and marijuana and methamphetamine use (days/month) during pregnancy; and infant sex, GA at birth, birth weight, length, head circumference, and age in days at time of testing. Use of illicit drugs other than marijuana and methamphetamine was rare in this sample—only two mothers reported using methaqualone, and analyses were rerun excluding their two infants. CC was examined in relation to the continuous measures of PAE using hierarchical multiple regression analysis. Total intracranial volume (TIV; TIV = white matter + gray matter + cerebrospinal fluid) was entered as the first step to determine whether CC area was disproportionately smaller in relation to PAE than would be expected. Maternal smoking, marijuana, and methamphetamine used during pregnancy and all other control variables that were related even weakly (at p < 0.10) to CC area were entered in the second step to control for potential confounders (Jacobson and Jacobson, 2005).
RESULTS
Sample characteristics
Demographic and background characteristics are summarized in Table 1. There were no between-group differences for maternal age at delivery, parity, or marital status. Although all the women were poorly educated, PAE mothers tended to have completed fewer years of school than the controls. Almost half of the women in the PAE group reported some degree of food insecurity, compared with 18.2% of the controls; there were no group differences, however, for moderate to severe hunger.
Table 1.
Sample characteristics
| PAE (n = 32) | HC (n = 11) | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Mean or % | SD | Range | Mean or % | SD | Range | t or χ2 | p value | |
| Maternal characteristics | ||||||||
| Maternal age at delivery (yr) | 28.3 | 6.0 | 19.4 – 38.9 | 26.9 | 5.0 | 18.2 – 32.7 | 0.66 | 0.514 |
| Parity | 2.1 | 1.7 | 0.0 – 6.0 | 1.6 | 1.3 | 0.0 – 4.0 | 0.79 | 0.432 |
| Education (yr) | 9.1 | 1.7 | 5.0 – 12.0 | 10.2 | 1.3 | 9.0 – 12.0 | 1.91 | 0.064 |
| Marital status (% married) | 12.5 | 27.3 | 1.31 | 0.252 | ||||
| Food secure vs. insecure | 46.7 | 18.2 | 2.75 | 0.098 | ||||
| Alcohol consumption | ||||||||
| At conception | ||||||||
| oz AA/day | 1.6 | 2.3 | 0.3 – 12.4 | 0.02 | 0.1 | 0.0 – 0.2 | 3.96 | 0.000 |
| oz AA/occasion | 3.6 | 3.2 | 0.6 – 17.4 | 0.1 | 0.3 | 0.0 – 1.2 | 6.12 | 0.000 |
| Frequency (days/wk) | 2.4 | 1.7 | 1.2 – 7.0 | 0.1 | 0.3 | 0.0 – 1.1 | 7.07 | 0.000 |
| Across pregnancy | ||||||||
| oz AA/day | 1.1 | 1.9 | 0.4 – 1.1 | 0.01 | 0.02 | 0.0 – 0.1 | 3.40 | 0.002 |
| oz AA/occasion | 4.2 | 2.8 | 2.3 – 16.0 | 0.3 | 0.6 | 0.0 – 1.7 | 7.57 | 0.000 |
| Frequency (days/wk) | 1.5 | 1.5 | 0.8 – 6.3 | 0.04 | 0.1 | 0.0 – 0.5 | 5.60 | 0.000 |
| Smoking (cigarettes/day) | 6.3 | 4.7 | 0.0 – 20.0 | 3.2 | 2.9 | 0.0 – 10.0 | 2.07 | 0.045 |
| Marijuana (days/month) | 2.1 | 4.6 | 0.0 – 17.7 | 0.2 | 0.6 | 0.0 – 1.9 | 2.25 | 0.031 |
| Methamphetamine (days/month) | 1.3 | 3.6 | 0.0 – 17.2 | 0.7 | 1.9 | 0.0 – 6.2 | 0.51 | 0.613 |
| Infant characteristics | ||||||||
| Sex (% male) | 53.1 | 72.7 | 1.29 | 0.256 | ||||
| Gestational age at birth (wk) | 38.2 | 2.0 | 33.1 – 41.4 | 38.7 | 2.1 | 34.3 – 42.1 | 0.75 | 0.458 |
| Age at scan (wk) | 2.8 | 1.3 | 1.0 – 5.7 | 1.9 | 1.2 | 0.9 – 4.1 | 1.80 | 0.080 |
| Birth weight (g) | 2748.3 | 479.1 | 1750.0 – 3690.0 | 2977.3 | 309.3 | 2260.0 – 3350.0 | 1.48 | 0.147 |
| Total intracranial volume (cm3) | 476.5 | 68.3 | 355.1 – 650.1 | 512.1 | 59.2 | 439.0 – 605.2 | 1.54 | 0.131 |
HC = healthy control; PAE = prenatal alcohol exposure; SD = standard deviation; AA = absolute alcohol
In this heavy drinking community, women in the PAE group consumed an average of 8.4 standard drinks/occasion across pregnancy on 1–2 days/week. All but 2 of the 11 controls (81.8%) abstained from drinking during the pregnancy. When recruited into the study, one control reported no alcohol use at time of conception but subsequently reported drinking 3 drinks on 2 occasions later in pregnancy; the other drank about 2 drinks twice/month. While there were no group differences in number of women who smoked cigarettes (PAE 90.6% vs. controls 72.7%, X2(1) = 2.18, p = 0.139) or used marijuana (PAE 25.0% vs. controls 18.2%, X2(1) = 0.21, p = 0.644), mothers in the PAE group smoked more cigarettes/day and used marijuana more frequently than controls. Although 8 of the 32 alcohol users (25.0%) and 2 of the 11 controls (18.2%) used methamphetamine, there were no significant between-group differences in number of users, X2(1) = 0.21, p = 0.644, or number of days used. Other drug use was rare: two women reported using methaqualone about twice/month, and none reported using cocaine or opiates during pregnancy.
There were no between-group differences regarding infant sex, GA at birth, birth weight, or TIV, but PAE infants were about 1 week older at time of scan (Table 1). Of the 40 infants for whom FASD diagnostic data were available, 8 of the 29 (27.6%) born to the heavy drinking mothers met IOM criteria (Hoyme et al., 2005) for full FAS; they had at least two of the principal dysmorphic features (short palpebral fissures, thin upper lip, flat or smooth philtrum), small head circumference (bottom 10th percentile), and low weight or short stature (bottom 10th percentile).
Association of CC area to FASD diagnosis
CC area was first examined in relation to FASD diagnosis in an ANOVA comparing FAS, heavy exposed nonsyndromal (HE), and control groups (Table 2). As predicted, there was a significant relation between CC area and FASD diagnosis (illustrated in Fig. 2). Post-hoc comparisons showed that the CC area was significantly smaller in infants diagnosed with FAS than controls, p = 0.006. CC area was intermediate in size for the HE group (p = 0.056 between FAS and HE, although not significantly smaller than controls, p = 0.166).
Table 2.
Effects of FASD diagnosis on corpus callosum area
| N | M | SD | |
|---|---|---|---|
| FAS | 8 | 94.7 | 13.1 |
| HE | 21 | 107.7 | 11.6 |
| Controls | 11 | 116.0 | 23.2 |
F(2,37) = 4.20, p = 0.023
FAS = fetal alcohol syndrome; HE = other heavy alcohol exposed
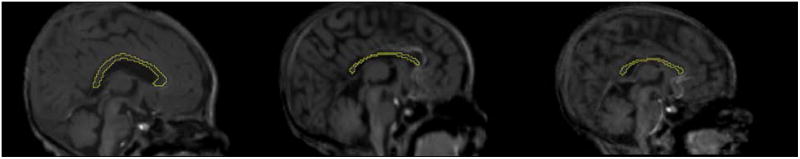
Fig 2.
Corpus callosa in three infants: Healthy control (left); newborn with FAS (middle); heavy exposed nonsyndromal (right).
Association of CC area to continuous measures of prenatal exposures
The relation of maternal demographic and newborn characteristics to CC area is summarized in Table 3. CC area was not related to maternal age at delivery, parity, marital status, or food security, and its relation to maternal education fell short of statistical significance. CC area was also not associated with maternal smoking and marijuana or methamphetamine use during pregnancy. CC area was not related to infant sex, GA at birth, or age at scan. Although CC area was not associated with birth weight, length, and head circumference, it was, as expected, positively related to TIV.
Table 3.
Relation of maternal demographics and newborn characteristics to corpus callosum area (N = 43)
| r | |
|---|---|
| Maternal characteristics | |
| Maternal age at delivery | −.01 |
| Parity | .01 |
| Education | .29† |
| Marital status | .06 |
| Food securitya | .10 |
| Smoking and drug use during pregnancy | |
| Cigarettes | −.13 |
| Marijuana | −.06 |
| Methamphetamine | −.11 |
| Newborn characteristics | |
| Sex | −.07 |
| Gestational age at birth | .02 |
| Age at scan | −.18 |
| Birth weight | .25 |
| Length | −.04 |
| Head circumference | .23 |
| Total intracranial volume | .36* |
p < .10
p < .05.
Dichotomized as food secure vs. food insecure; missing for 2 women.
CC area was next examined in relation to continuous measures of prenatal alcohol exposure (Table 4). As predicted, alcohol use both at time of conception and across pregnancy was negatively related to CC area. Moreover, the relations between PAE and CC area remained significant after adjustment for TIV. The associations between PAE and CC area were not attributable to maternal education, smoking and other drug use during pregnancy. These associations also remained unchanged after rerunning the analyses excluding the two newborns prenatally exposed to methaqualone.
Table 4.
Relation of prenatal alcohol exposure to corpus callosum area (N = 43)
| r | β1 | β2 | |
|---|---|---|---|
| Prenatal alcohol exposure | |||
| At conception | |||
| AA/day | −.40** | −.38* | −.34* |
| AA/occasion | −.31* | −.31* | −.27† |
| Frequency | −.42** | −.37* | −.34* |
| Across pregnancy | |||
| AA/day | −.39* | −.34* | −.33* |
| AA/occasion | −.32* | −.31* | −.28† |
| Frequency | −.41** | −.34* | −.34* |
p < .06
p < .05
p < .01
Values are Pearson r. β1 = standardized regression coefficient, after adjustment for total intracranial volume; β2 = standardized regression coefficient, after adjustment for total intracranial volume maternal education, prenatal exposure to smoking, marijuana, and methamphetamine.
AA = absolute alcohol
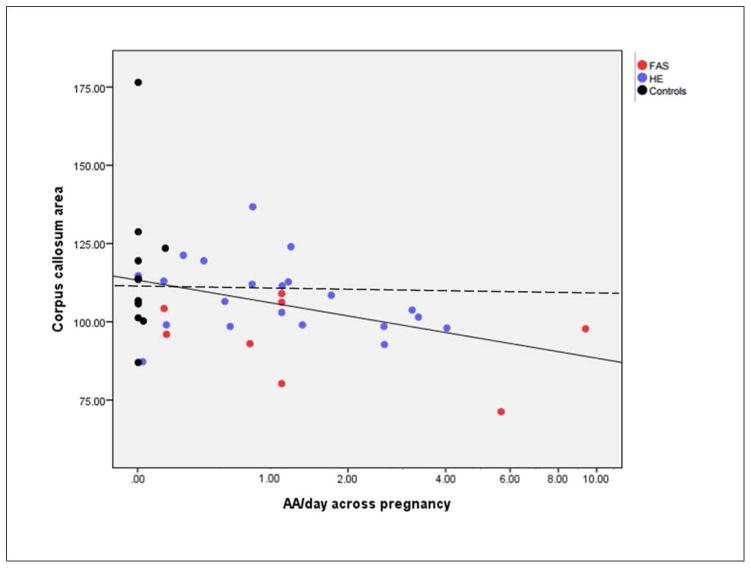
Figure 3 depicts the association between AA/day and CC area. When two bivariate outliers identified using Mahalanobis distance were removed from the analysis, the effects were virtually unchanged (e.g., the correlation for AA/day across pregnancy with CC area was −0.43, p = 0.005) and the group differences in Table 2 also remained significant (F(2,35) = 3.92, p = 0.029). Two exposed newborns appear on the scatterplot to have little or no exposure on across pregnancy AA/day measure: one was born to a woman who reported abstaining around time of conception but later reported a very heavy binge of 7 drinks; the other was born to a mother who reported drinking 7.6 drinks/occasion 6 times around time of conception but no drinking later in pregnancy. Thus, although average AA/day for these two women was relatively low, both engaged in heavy binge drinking, which animal studies have shown can lead to very severe impairment (Bonthius and West, 1990; Goodlett et al., 1990).
Fig. 3.
Relation of prenatal alcohol exposure to corpus callosum size: solid line shows linear regression line; dashed line shows median for controls.
Visual inspection of Figure 3 shows that reduction in CC size is not driven solely by smaller CC in the eight newborns with FAS, who have microcephaly and reduced TIVs. The relation of AA/day across pregnancy to CC size is reduced only modestly when the eight infants with FAS are removed from the analysis (r = −0.35, p = 0.038). As can be seen in the figure, smaller CCs are also found in the more heavily exposed newborns within the HE group. CC size was below the median for this sample in all of the infants exposed at > 1.0 oz AA/day (X2(1) = 8.84, p < 0.01).
DISCUSSION
The CC, the main interhemispheric commissure in the human brain, connects the majority of the neocortical areas (Schmahmann and Pandya, 2006) and plays a major role in sensory, motor, and higher-order communication (Thompson et al., 2011). Although the basic structure of the CC develops during the first 18–20 weeks GA, it continues to increase in size during the 3rd trimester of pregnancy and the first 2 years postpartum. To our knowledge, this is the first study to use neuroimaging to demonstrate effects of PAE on CC size in the neonatal brain and to report how exposure disrupts normal brain structure during pregnancy. A major strength of this study is the use of manual tracing, which is considered the “gold standard” for segmentation of brain regions.
Our finding of smaller callosal size in alcohol-exposed newborns is consistent with early autopsy reports of CC agenesis and with reports of disproportionately smaller CC in older children with FASD. Visual inspection of our neonatal scans by our senior neuroanatomist (CW) revealed no cases of CC agenesis in these infants who survived to term; the CC was well formed, albeit disproportionately smaller in relation to PAE. Furthermore, CC size remained significantly smaller even after adjustment for TIV, indicating that the impact of alcohol on the CC was specific and did not merely reflect overall fetal alcohol-related smaller brain size. Nor was this effect attributable to the smaller head circumference required for a diagnosis of FAS (Hoyme et al., 2005); the effect continued to be evident after the eight infants diagnosed with FAS were removed from the analysis. By contrast to the impact of PAE, CC size was not related to prenatal exposure to smoking, marijuana, and methamphetamine, indicating discriminant validity for CC area in relation to alcohol exposure during pregnancy.
As detailed above, two studies have linked smaller callosal size to poorer interhemispheric transfer of information in school-age children with FASD (Roebuck et al., 2002; Dodge et al., 2009). In addition, nine diffusion tensor imaging (DTI) studies from six independent laboratories have documented poorer microstructural integrity in the CC in children and adults with PAE, although there are inconsistencies regarding which areas of the CC are impaired. Four of these studies examined only the CC (Ma et al., 2005; Li et al., 2009; Wozniak et al., 2006, 2009), whereas the others performed whole brain analyses based on whole brain DTI (Sowell et al., 2008; Fryer et al., 2009; Fan et al., 2016), and tractography (Lebel et al., 2008). These DTI findings linking PAE to lower FA and greater diffusion indicate WM pathology due to poor myelination, less dense or coherent fiber bundles, and/or poorer axonal integrity. In the first study to perform whole brain DTI and tractography analyses in newborns, we also recently found marked alterations in WM integrity within the CC in alcohol-exposed infants in this Cape Town cohort (Taylor et al., 2015). Given that the CC is the sole region in which integrity deficits have been seen in all of the whole brain DTI studies of FASD to date, the data suggest that this structure is particularly vulnerable to PAE.
Five of the DTI studies also examined behavioral correlates of the fetal alcohol-related deficits in CC WM integrity. Using diffusion tractography, Lebel et al. (2010) found that lower FA was correlated with poorer performance on a mathematics achievement test in children with FASD. Sowell et al. (2008) found associations with poorer visual-motor performance; Wozniak et al. (2009), with poorer visual perceptual skills. In our whole brain DTI study, in which a broader range of cognitive outcomes were assessed, we found that fetal alcohol-related poorer structural integrity in two CC regions—splenium and isthmus—was associated with lower full scale IQ, verbal comprehension, and processing speed scores on the Wechsler Intelligence Scales for Children, Fourth edition (WISC-IV), poorer short-delay recall on the California Verbal Learning Test, and poorer eyeblink conditioning (Fan et al., 2016). Eyeblink conditioning is a Pavlovian classical conditioning paradigm, in which the subject learns to blink in response to the sound of a tone that has been paired to administration of an air puff to the eye. We have found that this paradigm, which assesses a basic form of learning, is particularly sensitive to prenatal alcohol exposure (Jacobson et al., 2008, 2011; Cheng et al., 2014). Using multiple regression to test statistically the degree to which effects of PAE on these cognitive outcomes are mediated by higher mean diffusivity in the CC, the two outcomes that were most clearly affected were WISC-IV information processing speed and eyeblink conditioning. Thus, these data suggest that fetal alcohol-related structural deficits already seen here in the newborn CC may later affect not only interhemispheric transfer of information but also play a role in mediating adverse effects of PAE on a range of cognitive outcomes.
Given the very heavy drinking by many pregnant women in the Cape Coloured population, this community provided a unique opportunity to prospectively recruit large numbers of newborns with FAS, which would not be possible at a single U.S. site without screening tens of thousands of pregnant women. In addition, the prospective design of this study enabled us to obtain quantitative estimates of degree of PAE based on maternal reports of drinking obtained during pregnancy. In several neuroimaging studies, we have found that, when compared with clinical diagnosis, prospective maternal reports of alcohol consumption during pregnancy more clearly predict adverse effects on brain structure (Meintjes et al., 2014; De Guio et al., 2014; Robertson et al., 2016) and function (Du Plessis et al., 2014; Woods et al., 2015). Similarly, in this study, whereas the effect of clinical diagnostic group on CC size for the nonsyndromal HE group fell short of statistical significance, the correlation of callosal size with our continuous measure of prenatal alcohol exposure continued to be significant even after adjustment for potential confounding variables.
Studies comparing very preterm newborns with typically developing fullterms have used callosal size differences to show how premature birth disrupts brain structure (Thompson et al., 2011). Because the early developing brain is particularly vulnerable to teratological insult, neonatal structural MRI also has the potential to provide early indicators of fetal alcohol-related insult before confounding postnatal socioenvironmental factors begin to impact on development. Given that the craniofacial dysmorphic features that characterize FAS are often difficult to detect in infancy, early indicators of adverse effects are needed that will contribute to understanding the ontogeny of impairment as it unfolds across development. It has been suggested that, “disrupted development of the CC may have implications for other midline structures that develop in association with the CC, such as the fornix, hippocampus, septum pellucidum, and cingulate cortex” (Thompson et al., 2011). Midline craniofacial anomalies have been recognized as a hallmark of FAS in humans and animal models, since this syndrome was first identified (Jones and Smith, 1973; Sulik et al., 1981). Given that structural deficits in other midline brain regions have also been reported in FASD (e.g., Johnson et al., 1996; Swayze et al., 1997; Meintjes et al., 2014), the CC deficit identified here in newborns may offer a unique opportunity to support early identification of a range of midline structural impairments.
The DTI evidence summarized above points to poorer WM integrity as a specific target of PAE and suggests that the CC may be particularly vulnerable. Two studies of very preterm infants have linked structural anomalies of the neonatal CC to functional impairment—one to less optimal neurological test scores at 6 weeks (Mathew et al., 2013); the other, to delayed cognitive development and impaired motor function at 2 years of age (Thompson et al., 2012). An innovative methodology indicated that degree of myelination of the CC can be assessed in infants, a technique that could be used in future studies of FASD to identify newborns at risk for the disorder (Deoni et al., 2011). Neonatal CC size has the potential to provide additional information that can be useful in identifying alcohol-affected children. For example, CC size may be one of several indicators that the infant has been exposed to alcohol prenatally in cases where mothers of children who meet criteria for FAS or PFAS do not admit drinking during pregnancy.
This study has limitations common to other longitudinal studies of prenatal exposure. Although the sample size is small and the study warrants replication, the findings were sufficiently robust to demonstrate differential effects of exposure to alcohol as contrasted to smoking and other drugs. Measurement error surrounding estimates of true alcohol exposure to the fetus may obscure some group differences, but differences between true and estimated exposure are likely small, given the validation of our pregnancy alcohol ascertainment protocol in relation to levels of fatty acid ethyl esters in meconium samples in this community (Bearer et al., 2003) and the predictive validity of the timeline follow-back interviewing techniques we used (Jacobson et al., 2002). Given the very heavy drinking in this population and the unique genetic and cultural background of the infants in our sample, these findings warrant replication in other less exposed groups using continuous measures of alcohol exposure. The absence to date of broadly accepted, well-validated automated systems for segmenting the infant brain points to the importance of using manual tracing to replicate these newborn CC findings.
Given that midline craniofacial anomalies have been identified in humans and animal models as particularly affected in FASD, the alcohol-related newborn CC deficit seen here may support early identification of a range of midline structural impairments. Use of CC size in identifying potentially affected newborns can thus facilitate early intervention, including those specifically targeted at strengthening interhemispheric connectivity. Future studies are needed to examine the degree to which smaller CC during the newborn period provides an early indicator of fetal alcohol-related cognitive deficits that have been linked to this critically important brain structure in childhood and adolescence.
Acknowledgments
Funding
National Institute on Alcohol Abuse and Alcoholism R21AA020037 (to SWJ, EMM, and AvdK); R01AA016781 (to SWJ); NRF/DST South African Research Chairs Initiative (to EMM); South African Medical Research Council; and the Lycaki/Young Fund (to SWJ and JLJ). The 2013 FASD dysmorphology clinic was supported in part by a grant from the NIAAA Collaborative Initiative on Fetal Alcohol Spectrum Disorder (U01AA014809 to T Foroud).
Portions of this research were presented at the 2014 Fetal Alcohol Spectrum Disorders Study Group, the 2015 Research Society on Alcoholism, and the 2015 Organization for Human Brain Mapping meetings. We thank L Wald, PhD, Director MRI Core, Martinos Center for Biomedical Imaging, Radiology, MGH, who designed the bird cage RF coil used in this study, and A Hess, PhD, and A Mareyam, PhD, for their work in constructing the bird cage; the Cape Universities Brain Imaging Centre radiographers N Maroof and A Siljeur; K de Macedo Rodrigues, MD, and E Ben-Avi, who trained and supervised the hand tracing of the newborn brains by FW and NL; and our UCT and WSU research staff including M September, B Arendse, M Raatz, and P Solomon. We thank H Bezuidenhout, MD, E Krzesinski, MD, P Shah, MD, R Jacquemard, MD, N Venosdel, MD, and V Kodali, who assisted in the FASD diagnostic clinics. The authors greatly appreciate the participation of the Cape Town mothers and infants in the study.
Footnotes
The authors declare no competing financial interests.
References
- Archibald SL, Fennema-Notestine C, Gamst A, Riley EP, Mattson SN, Jernigan TL. Brain dysmorphology in individuals with severe prenatal alcohol exposure. Dev Med Child Neurol. 2001;43:148–154. [PubMed] [Google Scholar]
- Bearer CF, Jacobson JL, Jacobson SW, Barr D, Croxford J, Molteno CD, Viljoen DL, Marais A, Chiodo LM, Cwik AS. Validation of a new biomarker of fetal exposure to alcohol. J Pediatr. 2003;143:463–469. doi: 10.1067/S0022-3476(03)00442-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel G, Nord M, Price C, Hamilton WL, Cook JT. Washington, DC: US Department of Agriculture, Food and Nutrition Service; 2000. Guide to Measuring Food Security in the United States: Guide to Measuring Household Food Security, Revised 2000. Available at: http://www.fns.usda.gov/fsec/files/fsguide.pdf. [Google Scholar]
- Bonthius DJ, West JR. Alcohol-induced neuronal loss in developing rats: increased brain damage with binge exposure. Alcohol Clin Exp Res. 1990;14:107–118. doi: 10.1111/j.1530-0277.1990.tb00455.x. [DOI] [PubMed] [Google Scholar]
- Bookstein FL, Streissguth AP, Sampson PD, Connor PD, Barr HM. Corpus callosum shape and neuropsychological deficits in adult males with heavy fetal alcohol exposure. Neuro Image. 2002;15:233–251. doi: 10.1006/nimg.2001.0977. [DOI] [PubMed] [Google Scholar]
- Caviness VS, Filipek PA, Kennedy DN. Magnetic resonance technology in human brain science: blueprint for a program based upon morphometry. Brain Dev. 1989;11:1–13. doi: 10.1016/s0387-7604(89)80002-6. [DOI] [PubMed] [Google Scholar]
- Cheng DT, Meintjes EM, Stanton ME, Desmond JE, Pienaar M, Dodge NC, Power JM, Molteno CD, Disterhoft JF, Jacobson JL, Jacobson SW. Functional MRI of cerebellar activity during eyeblink classical conditioning in children and adults. Hum Brain Mapp. 2014;35:1390–1403. doi: 10.1002/hbm.22261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarren SK, Smith DW. The fetal alcohol syndrome. N Eng J Med. 1978;298:1063–1067. doi: 10.1056/NEJM197805112981906. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- De Guio F, Mangin JF, Riviere D, Perrot M, Molteno CD, Jacobson SW, Meintjes EM, Jacobson JL. A study of cortical morphology in children with fetal alcohol spectrum disorders. Hum Brain Mapp. 2014;35:2285–2296. doi: 10.1002/hbm.22327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Macedo Rodrigues K, Ben-Avi E, Sliva DD, Choe M, Drottar M, Wang R, Fischl B, Grant PE, Zöllei L. A FreeSurfer-compliant consistent manual segmentation of infant brains spanning the 0–2 year age range. Front Hum Neurosci. 2015;9:21. doi: 10.3389/fnhum.2015.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deoni SC, Rutt BK, Peters TM. Rapid combined T1 and T2 mapping using gradient recalled acquisition in the steady state. Magn Res Med. 2003;49:515–526. doi: 10.1002/mrm.10407. [DOI] [PubMed] [Google Scholar]
- Deoni SC, Mercure E, Blasi A, Gasston D, Thomson A, Johnson M, Williams SCR, Murphy DG. Mapping infant brain myelination with magnetic resonance imaging. J Neurosci. 2011;31:784–791. doi: 10.1523/JNEUROSCI.2106-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge NC, Jacobson JL, Molteno CD, Meintjes EM, Bangalore S, Diwadkar V, Hoyme EH, Robinson LK, Khaole N, Jacobson SW. Prenatal alcohol exposure and interhemispheric transfer of tactile information: Detroit and Cape Town findings. Alcohol Clin Exp Res. 2009;33:1628–1637. doi: 10.1111/j.1530-0277.2009.00994.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donald KA, Fouche JP, Roos A, Koen N, Howells FM, Riley EP, Woods RP, Zar HJ, Narr KL, Stein DJ. Alcohol exposure in utero is associated with decreased gray matter volume in neonates. Metabolic Brain Disease. 2016;31:81–91. doi: 10.1007/s11011-015-9771-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Plessis L, Jacobson JL, Jacobson SW, Hess AT, Kouwe A, Avison MJ, Molteno CD, Stanton ME, Stanley JA, Peterson BS, Meintjes EM. An in vivo 1H magnetic resonance spectroscopy study of the deep cerebellar nuclei in children with fetal alcohol spectrum disorders. Alcohol Clin Exp Res. 2014;38:1330–1338. doi: 10.1111/acer.12380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, Jacobson SW, Taylor PA, Molteno CD, Dodge NC, Stanton ME, Jacobson JL, Meintjes EM. White matter deficits mediate effects of prenatal alcohol exposure on cognitive development in childhood. Hum Brain Mapp. 2016;37:2943–2958. doi: 10.1002/hbm.23218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Salat DH, van der Kouwe AJ, Makris N, Ségonne F, Quinn BT, Dale AM. Sequence-independent segmentation of magnetic resonance images. Neuro Image. 2004a;23:S69–84. doi: 10.1016/j.neuroimage.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Ségonne F, Salat DH, Busa E, Seidman LJ, Goldstein J, Kennedy D, Caviness V, Makris N, Rosen B, Dale AM. Automatically parcellating the human cerebral cortex. Cerebral Cortex. 2004b;14:11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- Fryer SL, Schweinsburg BC, Bjorkquist OA, Frank LR, Mattson SN, Spadoni AD, Riley EP. Characterization of white matter microstructure in fetal alcohol spectrum disorders. Alcohol Clin Exp Res. 2009;33:514–521. doi: 10.1111/j.1530-0277.2008.00864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodlett CR, Marcussen BL, West JR. A single day of alcohol exposure during the brain growth spurt induces brain weight restriction and cerebellar Purkinje cell loss. Alcohol. 1990;7:107–114. doi: 10.1016/0741-8329(90)90070-s. [DOI] [PubMed] [Google Scholar]
- Health Canada. Income-Related Household Food Security in Canada. Nutrition. Ottawa: Minister of Health Office of Nutrition Policy and Promotion Health Products and Food Branch; 2004. Canadian Community Health Survey Cycle 2.2. Available at: http://www.hc-sc.gc.ca/fn-an/alt_formats/hpfb-dgpsa/pdf/surveill/income_food_sec-sec_alim-eng.pdf. [Google Scholar]
- Hoyme HE, May PA, Kalberg WO, Kodituwakku P, Gossage JP, Trujillo PM, Buckley DG, Miller JH, Aragon AS, Khaole N, Viljoen DL, Jones KL, Robinson LK. A practical clinical approach to diagnosis of fetal alcohol spectrum disorders: clarification of the 1996 Institute of Medicine criteria. Pediatr. 2005;115:39–47. doi: 10.1542/peds.2004-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson JL, Jacobson SW. Methodological issues in research on developmental exposure to neurotoxic agents. Neurotoxicol Teratol. 2005;27:395–406. doi: 10.1016/j.ntt.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Jacobson JL, Jacobson SW, Molteno CD, Odenhaal H. A prospective examination of the incidence of heavy drinking during pregnancy among Cape Coloured South African women. Alcohol Clin Exp Res. 2006;30:233A. [Google Scholar]
- Jacobson SW, Chiodo LM, Sokol RJ, Jacobson JL. Validity of maternal report of prenatal alcohol, cocaine, and smoking in relation to neurobehavioral outcome. Pediatr. 2002;109:815–825. doi: 10.1542/peds.109.5.815. [DOI] [PubMed] [Google Scholar]
- Jacobson SW, Stanton ME, Molteno CD, Burden MJ, Fuller DS, Hoyme HE, Robinson LK, Khaole N, Jacobson JL. Impaired eyeblink conditioning in children with fetal alcohol syndrome. Alcohol Clin Exp Res. 2008;32:365–372. doi: 10.1111/j.1530-0277.2007.00585.x. [DOI] [PubMed] [Google Scholar]
- Jacobson SW, Stanton ME, Dodge NC, Pienaar M, Fuller DS, Molteno CD, Meintjes EM, Hoyme HE, Robinson LK, Khaole N, Jacobson JL. Impaired delay and trace eyeblink conditioning in school-age children with fetal alcohol syndrome. Alcohol Clin Exp Res. 2011;35:250–264. doi: 10.1111/j.1530-0277.2010.01341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeret JS, Serur D, Wisniewski K, Fisch C. Frequency of agenesis of the corpus callosum in the developmentally disabled population as determined by computerized tomography. Pediatr Neurosurgery. 1985;12:101–103. doi: 10.1159/000120229. [DOI] [PubMed] [Google Scholar]
- Johansen-Berg H, Della-Maggiore V, Behrens TE, Smith SM, Paus T. Integrity of white matter in the corpus callosum correlates with bimanual co-ordination skills. Neuro Image. 2007;36:T16–T21. doi: 10.1016/j.neuroimage.2007.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson VP, Swayze VW, II, Sato Y, Andreasen NC. Fetal alcohol syndrome: craniofacial and central nervous system manifestations. Am J Med Genetics. 1996;61:329–339. doi: 10.1002/(SICI)1096-8628(19960202)61:4<329::AID-AJMG6>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Jones K, Smith D. Recognition of the fetal alcohol syndrome in early infancy. Lancet. 1973;302:999–1001. doi: 10.1016/s0140-6736(73)91092-1. [DOI] [PubMed] [Google Scholar]
- Lebel C, Rasmussen C, Wyper K, Walker L, Andrew G, Yager J, Beaulieu C. Brain diffusion abnormalities in children with fetal alcohol spectrum disorder. Alcohol Clin Exp Res. 2008;32:1732–1740. doi: 10.1111/j.1530-0277.2008.00750.x. [DOI] [PubMed] [Google Scholar]
- Lebel C, Rasmussen C, Wyper K, Andrew G, Beaulieu C. Brain microstructure is related to math ability in children with fetal alcohol spectrum disorder. Alcohol Clin Exp Res. 2010;34:354–363. doi: 10.1111/j.1530-0277.2009.01097.x. [DOI] [PubMed] [Google Scholar]
- Li L, Coles CD, Lynch ME, Hu X. Voxelwise and skeleton-based region of interest analysis of fetal alcohol syndrome and fetal alcohol spectrum disorders in young adults. Hum Brain Mapp. 2009;30:3265–3274. doi: 10.1002/hbm.20747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Coles CD, Lynch ME, LaConte SM, Zurkiya O, Wang D, Hu X. Evaluation of corpus callosum anisotropy in young adults with fetal alcohol syndrome according to diffusion tensor imaging. Alcohol Clin Exp Res. 2005;29:1214–1222. doi: 10.1097/01.alc.0000171934.22755.6d. [DOI] [PubMed] [Google Scholar]
- Mathew P, Pannek K, Snow P, D’Acunto MG, Guzzetta A, Rose SE, Colditz PB, Finnigan S. Maturation of corpus callosum anterior midbody is associated with neonatal motor function in eight preterm-born infants. Neural Plasticity. 2013 doi: 10.1155/2013/359532. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Gossage JP, Kalberg WO, Robinson LK, Buckley D, Manning M, Hoyme HE. Prevalence and epidemiologic characteristics of FASD from various research methods with an emphasis on recent in-school studies. Dev Disabilities Res Rev. 2009;15:176–192. doi: 10.1002/ddrr.68. [DOI] [PubMed] [Google Scholar]
- May PA, Blankenship J, Marais AS, Gossage JP, Kalberg WO, Barnard R, De Vries M, Robinson LK, Adnams CM, Buckley D, Manning M, Jones KL, Parry C, Hoyme HE, Seedat S. Approaching the prevalence of the full spectrum of fetal alcohol spectrum disorders in a South African population-based study. Alcohol Clin Exp Res. 2013;37:818–830. doi: 10.1111/acer.12033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meintjes E, Narr K, van der Kouwe A, Molteno C, Pirnia T, Gutman B, Woods R, Thompson P, Jacobson J, Jacobson S. A tensor-based morphometry analysis of regional differences in brain volume in relation to prenatal alcohol exposure. Neuro Image Clin. 2014;5:152–160. doi: 10.1016/j.nicl.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida M, Makris N, Kennedy DN, Vangel M, Fischl B, Krishnamoorthy KS, Caviness VS, Grant PE. Detailed semiautomated MRI based morphometry of the neonatal brain: preliminary results. Neuro Image. 2006;32:1041–1049. doi: 10.1016/j.neuroimage.2006.05.020. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Adalsteinsson E, Sullivan EV. Dysmorphology and microstructural degradation of the corpus callosum: Interaction of age and alcoholism. Neurobiol Aging. 2006;27:994–1009. doi: 10.1016/j.neurobiolaging.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Prastawa M, Gilmore JH, Lin W, Gerig G. Automatic segmentation of MR images of the developing newborn brain. Med Image Analysis. 2005;9:457–466. doi: 10.1016/j.media.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Riley EP, Mattson SN, Sowell ER, Jernigan TL, Sobel DF, Jones KL. Abnormalities of the corpus callosum in children prenatally exposed to alcohol. Alcohol Clin Exp Res. 1995;19:1198–1202. doi: 10.1111/j.1530-0277.1995.tb01600.x. [DOI] [PubMed] [Google Scholar]
- Robertson FC, Narr KL, Molteno CD, Jacobson JL, Jacobson SW, Meintjes EM. Prenatal alcohol exposure is associated with thinner cortex during the preadolescent period. Cerebral Cortex. 2016;26:3083–3095. doi: 10.1093/cercor/bhv131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roebuck TM, Mattson SN, Riley EP. Interhemispheric transfer in children with heavy prenatal alcohol exposure. Alcohol Clin Exp Res. 2002;26:1863–1871. doi: 10.1097/01.ALC.0000042219.73648.46. [DOI] [PubMed] [Google Scholar]
- Santhanam P, Hu X, Peltier SJ, Li Z, Lynch ME, Coles CD. Interhemispheric transmission in adults prenatally exposed to alcohol an FMRI study. Alcohol Clin Exp Res. 2007;31:186A. [Google Scholar]
- Schmahmann JD, Pandya D. Fiber pathways of the brain. Oxford University Press; New York: 2006. [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. Neuro Image. 2004;23:S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Mattson SN, Tessner KD, Jernigan TL, Riley EP, Toga AW. Voxel-based morphometric analyses of the brain in children and adolescents prenatally exposed to alcohol. Neuro Report. 2001;12:515–523. doi: 10.1097/00001756-200103050-00018. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Johnson A, Kan E, Lu LH, Van Horn JD, Toga AW, O’Connor M, Bookheimer SY. Mapping white matter integrity and neurobehavioral correlates in children with fetal alcohol spectrum disorders. J Neurosci. 2008;28:1313–1319. doi: 10.1523/JNEUROSCI.5067-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulik KK, Johnston MC, Webb MA. Fetal alcohol syndrome: embryogenesis in a mouse model. Science. 1981;214:936–938. doi: 10.1126/science.6795717. [DOI] [PubMed] [Google Scholar]
- Suttie M, Foroud T, Wetheril L, Jacobson JL, Molteno CD, Meintjes EM, Hoyme HE, Khaole N, Robinson LK, Riley EP, Jacobson SW, Hammond P. Facial dysmorphism across the fetal across the fetal alcohol spectrum. Pediatr. 2013;131:e779–e788. doi: 10.1542/peds.2012-1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swayze VW, II, Johnson VP, Hanson JW, Piven J, Sato Y, Giedd JN, Mosnick D, Andreasen NC. Magnetic resonance imaging of brain anomalies in fetal alcohol syndrome. Pediatr. 1997;99:232–240. doi: 10.1542/peds.99.2.232. [DOI] [PubMed] [Google Scholar]
- Taylor PA, Jacobson SW, van der Kouwe A, Molteno CD, Chen G, Wintermark P, Alhamud A, Jacobson JL, Meintjes EM. A DTI-based tractography study of effects on brain structure associated with prenatal alcohol exposure in newborns. Human Brain Mapp. 2015;36:170–186. doi: 10.1002/hbm.22620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson DK, Inder TE, Faggian N, Johnston L, Warfield SK, Anderson PJ, Doyle LW, Egan GF. Characterization of the corpus callosum in very preterm and full-term infants utilizing MRI. Neuro Image. 2011;55:479–490. doi: 10.1016/j.neuroimage.2010.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson DK, Inder TE, Faggian N, Warfield SK, Anderson PJ, Doyle LW, Egan GF. Corpus callosum alterations in very preterm infants: Perinatal correlates and 2 year neurodevelopmental outcomes. Neuro Image. 2012;59:3571–3581. doi: 10.1016/j.neuroimage.2011.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Kouwe AJ, Benner T, Salat DH, Fischl B. Brain morphometry with multiecho MPRAGE. Neuro Image. 2008;40:559–569. doi: 10.1016/j.neuroimage.2007.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisniewski K, Dambska M, Sher J, Qazi Q. A clinical neuropathological study of the fetal alcohol syndrome. Neuropediatr. 1983;14:197–201. doi: 10.1055/s-2008-1059578. [DOI] [PubMed] [Google Scholar]
- Woods K, Meintjes EM, Molteno CD, Jacobson SW, Jacobson JL. Parietal dysfunction during number processing in children with fetal alcohol spectrum disorders. Neuro Image Clin. 2015;8:594–605. doi: 10.1016/j.nicl.2015.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak JR, Mueller BA, Chang PN, Muetzel RL, Caros L, aLim KO. Diffusion tensor imaging in children with fetal alcohol spectrum disorders. Alcohol Clin Exp Res. 2006;30:1799–1806. doi: 10.1111/j.1530-0277.2006.00213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak JR, Muetzel RL, Mueller BA, McGee CL, Freerks MA, Ward EE, Nelson ML, Chang PN, Lim KO. Microstructural corpus callosum anomalies in children with prenatal alcohol exposure: an extension of previous diffusion tensor imaging findings. Alcohol Clin Exp Res. 2009;33:1825–1835. doi: 10.1111/j.1530-0277.2009.01021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Phillips OR, Kan E, Sulik KK, Mattson SN, Riley EP, Jones KL, Adnams CM, May PA, O’Connor MJ. Callosal thickness reductions relate to facial dysmorphology in fetal alcohol spectrum disorders. Alcohol Clin Exp Res. 2012;36:798–806. doi: 10.1111/j.1530-0277.2011.01679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]