Abstract
Introduction
Emerging data suggests a link between calpain activation and the enhanced inflammatory response of the cardiovascular system. We hypothesize that calpain activation associates with altered inflammatory protein expression in correlation with the pro-inflammatory profile of the myocardium. Our pig hypercholesterolemic model with chronic myocardial ischemia will be treated with calpain inhibitors (CI) to establish their potentials to improve cardiac function.
Methods
Yorkshire swine, fed a high cholesterol diet (HC) for four weeks, underwent placement of an ameroid constrictor on the left circumflex artery. Three weeks later animals received either: no drug, high cholesterol control group (CON; n= 8); a low dose CI (0.12 mg/kg; LCI, n= 9); or a high dose CI (0.25 mg/kg; HCI, n= 8). The HC and CI were continued for five weeks, after which the pig was euthanized. The left ventricular myocardial tissue (ischemic and non-ischemic) was harvested and analyzed for inflammatory proteins expression. Data was statistically analyzed via the Kruskal-Wallis and Dunn’s post hoc test.
Results
CI treatment coincides with increased expression of IKB-α and decreased expression of macrophages, NFkB, IL-1 and TNF-α in the ischemic myocardial tissue as compared to the control group. NFkB array revealed decreased expression of IRF5, JNK1/2, JNK2, CD18, NFkB p65, c-Rel, Sharpin, TNF R1, TNF R2 and DR5 in the ischemic myocardium of the HCI treated group compared to the control.
Conclusions
Calpain activation in metabolic syndrome is a potential contributor to cardiac dysfunction in metabolic disorders with ischemic background. We suggest calpain inhibition down regulates NFkB signaling in the vessel walls which might be useful for improving myocardial blood flow in ischemic conditions.
Keyboard: calpain, inflammation, metabolic syndrome, myocardial ischemia
Introduction
Metabolic Syndrome is a broad term used to describe patients who have at least three of the following five risk factors for heart disease: hypertension, obesity, high fasting glucose, high triglyceride levels, low HDL levels, and hyperlipidemia. Metabolic syndrome leads to endothelial dysfunction, which causes a diminished angiogenic response to chronic coronary ischemia and leads to coronary vascular disease (CVD) [1,2]. In the US patients with CVD and concurrent metabolic syndrome have an increased rate of total mortality than patients with CVD alone [3].
Some of the metabolic syndrome associated vascular endothelial dysfunction is likely attributed to an increased inflammatory response. Inflammatory stimuli could cause disruption of the endothelial barrier facilitating migration of leukocytes through the vessel wall thus causing vascular inflammation. This inflammation may lead to atherosclerotic disease [4].
Calpain is a class of calcium dependent proteases with a wide range of targets including members of the inflammatory cascade. [5] Calpain activity has been found to promote leucocyte-endothelial rolling, change platelet function, proteolytically cleave proteins resulting in hyperaggregability, as well as modulate TNF-α and other pro-inflammatory markers. [6–10] Recent studies have reported that calpain inhibition exerts anti-inflammatory effects on cardiac tissue leading to improved function. [11]
We developed a large animal model that represents the pathologic abnormalities seen in adult patients with coronary artery disease and metabolic syndrome. [12] Using this model, we have found that calpain inhibition improves myocardial blood flow and also promotes expression of proteins that are involved in angiogenesis. [13]
To gain a better understanding of the mechanisms by which exposure to calpain inhibitors may improve myocardial blood flow, we have taken advantage of our ischemia-reperfusion metabolic syndrome model. Results reported herein demonstrate that the calpain inhibition in our metabolic syndrome pig model alters the expression of inflammatory markers in the ischemic myocardial tissue. Consequently, therapies aimed at calpain inhibition in inflammatory ischemic metabolic syndrome states might be useful for preventing inflammation and improving cardiac function.
Methods
Animal Model
The animal model used in this study has been previously described. [13–15] Briefly, to induce metabolic syndrome 25 Yorkshire Swine (E.M. Parsons and Sons, Hadley MA) were fed a high fat/ high cholesterol diet (4% cholesterol, 2.3% corn oil, 17.2% coconut oil, 1.5% sodium cholate and 75% regular chow) for four weeks (Sinclair Research, Columbia, MO). To induce chronic myocardial ischemia, the pigs then underwent a procedure to place an ameroid constrictor on their Left Circumflex artery to induce chronic coronary artery ischemia. [16] Two weeks later, animals received either: no drug, (high cholesterol control group, CON; n= 8); a low dose of the of calpain inhibitor MDL28170 (CI) drug (0.12 mg/kg daily; LCI, n= 9); or a high dose of calpain inhibitor MDL28170 (CI) (0.25 mg/kg daily; HCI, n= 8). The high cholesterol diet and calpain inhibitor were continued for five weeks. The pigs then underwent a harvest procedure and the endocardial tissue was collected from the chronically ischemic myocardium and the non-ischemic myocardium. The Rhode Island Hospital Institutional Animal Care and Use Committee approved and supervised all experiments. The Principles of Laboratory Animal Care formulated by the National Society for Medical Research and the Guide for the Care and Use of Laboratory Animals were used to ensure proper care of all animals.
Protein Expression
The western blot analysis was performed as previously described. [14] Polyvinylidene difluoride membranes (Millipore, Billerica, MA) were primed with primary antibodies against p-IkB- α(ser32), nuclear factor kappa B (NFkB (p65)), interferon gamma (IFN-γ), tumor necrosis factor alpha (TNF-α), interleukin-1 (IL-1), intercellular adhesion molecule (CD54/ICAM-1), vascular cell adhesion molecule (VCAM), inducible nitric oxide synthase (iNOS), and protein kinase C alpha (PKC-α) (Cell Signaling, Danvers, MA). Image J software was used to quantify immunological reactions as arbitrary light units. Loading error was controlled for by probing membranes with an antibody against GAPDH. GraphPad 5.0 Software (GraphPad Software Inc, Sand Diego, Ca) was used to perform Kruskal-Wallis test with Dunn’s multiple comparisons between the three groups.
Immunohistochemistry
Immunohistochemistry was performed as previously described. [13] Briefly, frozen section slides of the individual groups were incubated with antibodies to smooth muscle actin-α, NFkB p65, IkBα (Cell Signaling, Danvers, MA) and macrophage (MAC397) (Abcam, Cambridge, MA). Images for NFkB p65 and IkBα were captured at 40X magnification with a Nikon E800 Eclipse microscope (Nikon, Tokyo, Japan) at the same exposure in 5 random fields. Image J software was used to quantify mean intensity of respective antibodies. Representative images are included in the manuscript. Images for macrophage antibody (MAC397) were analyzed at 40X magnification with a Nikon E800 Eclipse microscope (Nikon, Tokyo, Japan). Macrophages were counted for each specimen (1 specimen per pig) and totals were divided by specimen area. Representative images are included in the manuscript.
Proteome Profiler
NFkB Pathway Array Kit (ARY029- R&D Systems, Minneapolis, MN) was used to evaluate the expression of inflammatory markers between calpain treated and untreated metabolic syndrome pigs by following the protocol of the supplier. Total tissue lysate samples from CON and HCI pigs were pooled prior to placement on membranes. Image J software was used to quantify mean intensity for each protein. A 2 tailed, unpaired T test was used to evaluate differences between groups.
Data Analysis
Western blot data was normalized to GAPDH for each protein tested. Results are reported as fold change values compared to the high cholesterol diet (CON) group +/− standard error of the mean. Immunohistochemistry for IkBα and NFkB are reported as average mean intensity (in pixels)/40XHPF +/−SEM. 5 random fields were analyzed for each pig. Immunohistochemistry for Macrophages are reported as average macrophage count/area +/− SEM. Western blot and immunohistochemistry data were analyzed using Kruskal Wallice ANOVA with and Dunn’s post hoc comparisons. The NFkB protein array data are reported as average mean intensity. Data was analyzed by 2 tailed, unpaired T Test All data was analyzed data using GraphPad Prism 5.0 Software (GraphPad Software Inc, Sand Diego, Ca).
Results
Ischemic Myocardial Tissue
Western Blot
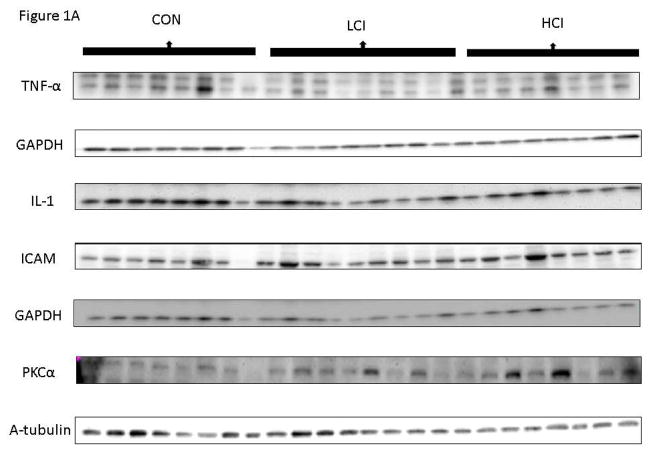
In the ischemic myocardial tissue, there was a strong trend toward decreased expression of NFk-B in both calpain inhibited groups compared to the control group (p=0.53). There was a decrease in expression of TNF-α in the low dose calpain inhibited group compared to the control group and a decrease in Il-1 in the high dose calpain inhibited group compared to the control group. There was an increase in ICAM in the low dose calpain inhibited group compared to the control group and an increase PKC-α in the high dose calpain inhibited group compared to the control group. There was a trend toward decrease in Interferon γ in the calpain inhibited groups compared to the control (p=0.13). [Figure 1]
Figure 1. Calpain Inhibition Modulates Inflammatory Proteins in Ischemic Tissue.
A: Images from Western Blot with specific antibodies as shown at left. GAPDH shows representative images for loading control B: The bar diagrams show changes in inflammatory proteins. CON-_ High Cholesterol Control, LCI- Low Dose Calpain Inhibitor, HCI- High Dose Calpain Inhibitor; tumor necrosis factor alpha (TNF-α), interleukin-1 (IL-1), intercellular adhesion molecule (ICAM), protein kinase C -alpha (PKC-α), nuclear factor kappa B (NFkB (p65)), interferon gamma (IFN-γ). *= p<0.05 by Kruskal Wallice Anova and Dunn’s Comparisons. Western blot data was normalized to GAPDH for each protein tested. Results are reported as fold change values compared to the high cholesterol diet (CON) group +/− standard error of the mean.
The expression of VCAM, p-IkB-α, and i-NOS showed no clear trends in the calpain inhibited groups compared to the control. [Table 1]
Table 1.
Calpain Inhibition Decreased NFkB Signaling Protein Expression in Ischemic Myocardial Tissue. interferon regulator factor 5 (IRF 5), c-Jun N-terminal kinase ½ (JNK ½), c-Jun N-terminal kinase 2 (JNK 2), proto-oncogene c-Rel (c-REL), tumor necrosis factor α receptor 1 (TNFR1), tumor necrosis factor α receptor 2 (TNFR 2), tumor necrosis factor- related apoptosis inducing ligand receptor 2 (TRAIL R2). Total tissue lysate samples from CON and HCI pigs were pooled prior to placement on membranes. Image J software was used to quantify mean intensity for each protein. A 2 tailed, unpaired T test was used to evaluate differences between groups.
| Protein | CON | HCI | P Value |
|---|---|---|---|
| IRF 5 | 16.5 | 9.5 | 0.04 |
| JNK 1/2 | 113 | 63 | <0.01 |
| JNK2 | 44 | 27.5 | 0.02 |
| NFkB p65 | 11 | 2 | <0.01 |
| c-Rel | 47 | 12 | 0.02 |
| Sharpin | 25 | 14.5 | 0.03 |
| TNF RI | 13 | 6.5 | 0.048 |
| TNF RII | 17 | 5.5 | 0.02 |
| TRAIL R2 | 16 | 8.5 | 0.04 |
Immunohistochemistry
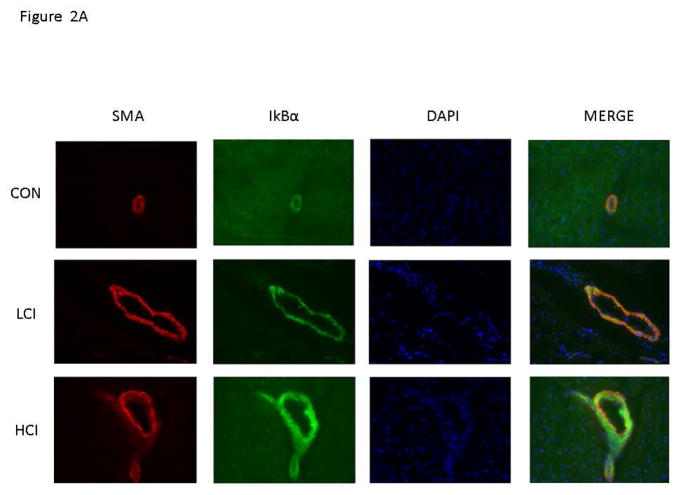
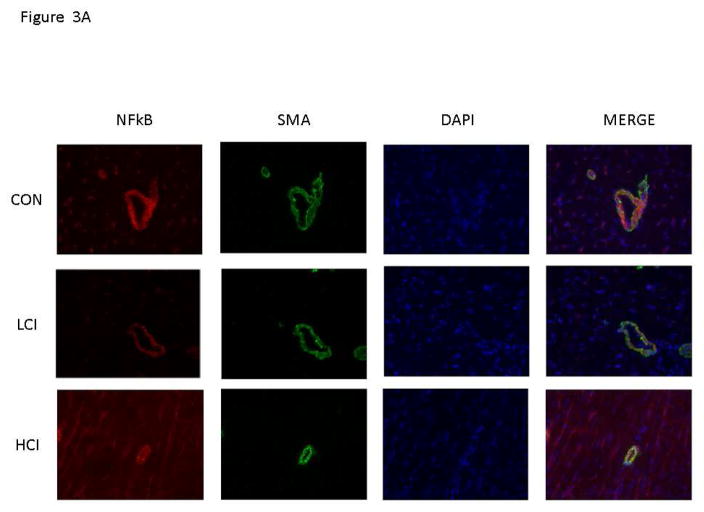
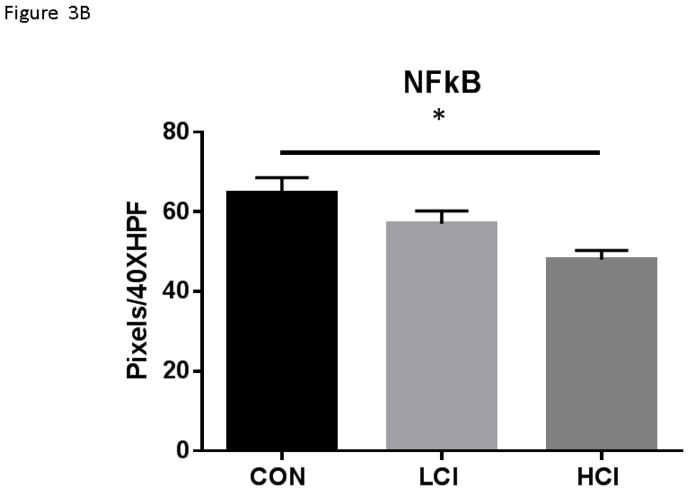
There was increased expression of IkBα in the high and low dose calpain inhibited groups compared to the control group. [Figure 2] There was decreased expression of NFkB p65 in the high dose calpain inhibited group compared to the control and a trend toward decreased expression in the low dose calpain inhibited group compared to the control. [Figure 3] Interestingly, the expression of IkBα and NFkB p65 was localized to the vessel walls (which were co stained with smooth muscle actin).
Figure 2. Calpain Inhibition Is Associated with Decreased IkBα expression in Blood Vessels.
A. Representative Images in 40XHPF: Vessels identified by smooth muscle actin (SMA) with SMA specific antibody in tissue sections (Red). IkBα expression identified by staining with NFkB antibody in tissue sections (Green). Nucleus identified with Dapi in tissue sections (Blue). B. The bar diagram shows significant increase in IkBα expression. CON-_ High Cholesterol Control, LCI- Low Dose Calpain Inhibitor, HCI- High Dose Calpain Inhibitor. *= p<0.05 by Kruskal Wallice Anova and Dunn’s Comparisons. Results are reported average intensity pixels/40HPF values +/- standard error of the mean.
Figure 3. Calpain Inhibition Is Associated with Decreased NFkB expression in Blood Vessels.
A. Representative Images in 40XHPF: Vessels identified by smooth muscle actin (SMA) with SMA specific antibody in tissue sections (Green). NFkB expression identified by staining with NFkB antibody in tissue sections (Red). Nucleus identified with Dapi in tissue sections (Blue). B. The bar diagram shows significant decrease in NFkB expression. CON-_ High Cholesterol Control, LCI- Low Dose Calpain Inhibitor, HCI- High Dose Calpain Inhibitor. *= p<0.05 by Kruskal Wallice Anova and Dunn’s Comparisons. Results are reported average intensity pixels/40HPF values +/- standard error of the mean.
NFkB Protein Array
Protein array analysis showed that in the ischemic tissue, there was decreased expression of interferon regulator factor 5 (IRF 5), c-Jun N-terminal kinase ½ (JNK ½), c-Jun N-terminal kinase 2 (JNK 2), NFkB p65, proto-oncogene c-Rel (c-REL), Shank-Associated RH domain interacting ProtIN (sharpin), tumor necrosis factor α receptor 1 (TNFR1), tumor necrosis factor a receptor 2 (TNFR 2) and tumor necrosis factor- related apoptosis inducing ligand receptor 2 (TRAIL R2) in the high dose calpain inhibited group compared to the control. [Table 1]
Macrophage Counts
In the ischemic tissue, there was increased expression of macrophage counts/mm2 in the control group compared to the high dose and low dose calpain inhibited groups. [Figure 4]
Figure 4. Calpain Inhibition Is Associated with Decreased Macrophage expression in Ischemic Myocardial Tissue.
A. Representative Images in 40XHPF: Vessels identified by smooth muscle actin (SMA) with SMA specific antibody in tissue sections (Red). Macrophage expression identified by staining with Macrophage antibody in tissue sections (Green). Nucleus identified with Dapi in tissue sections (Blue). B. The bar diagram shows significant decrease in macrophage expression. CON-_ High Cholesterol Control, LCI- Low Dose Calpain Inhibitor, HCI- High Dose Calpain Inhibitor. *= p<0.05 by Kruskal Wallice Anova and Dunn’s Comparisons. Results are reported average number of macrophage counts/area +/- standard error of the mean.
Nonischemic Myocardial Tissue
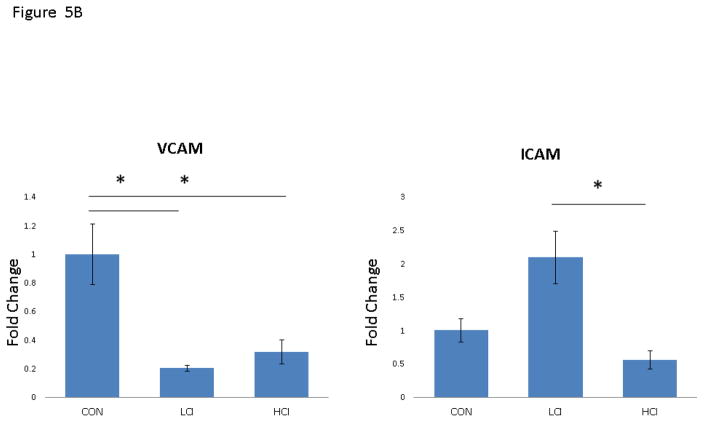
In the nonischemic myocardial tissue, there was decreased expression of VCAM in both the high and low dose calpain inhibited groups and a significant decrease in the expression of ICAM in the LCI group compared to the HCI group. However, there was no significant difference in ICAM between either calpain inhibited group and the control. [Figure 5]
Figure 5. Calpain Inhibition Modulates Inflammatory Proteins in NonIschemic Tissue.
A: Images from Western Blot with specific antibodies as shown at left. GAPDH shows representative images for loading control B: The bar diagrams show changes in inflammatory proteins. CON-_ High Cholesterol Control, LCI- Low Dose Calpain Inhibitor, HCI- High Dose Calpain Inhibitor; intercellular adhesion molecule (ICAM), vascular cell adhesion molecule (VCAM). *= p<0.05 by Kruskal Wallice Anova and Dunn’s Comparisons. Western blot data was normalized to GAPDH for each protein tested. Results are reported as fold change values compared to the high cholesterol diet (CON) group +/− standard error of the mean. All data was analyzed data using GraphPad Prism 5.0 Software (GraphPad Software Inc, Sand Diego, Ca).
Calpain Inhibition had no significant effect on the expression of Il-1, ICAM, PKC-α, Interferon Gamma, IL-1, p-IKB alpha or NFkB in the non ischemic myocardial tissue. [Table 2]
Table 2.
Protein Expression in Myocardial Tissue Unchanged by Calpain Inhibition. vascular cell adhesion molecule (VCAM), inducible nitric oxide synthase (iNOS) Comparisons. IkB-α, nuclear factor kappa B (NFkB (p65)), interferon gamma (IFN--γ), interleukin-1 (IL-1), protein kinase C alpha (PKC-α) (Cell Signaling, Danvers, MA). Western blot data was normalized to GAPDH for each protein tested. Results are reported as fold change values compared to the high cholesterol diet (CON) group +/− standard error of the mean. Western blot data were analyzed using Kruskal Wallice ANOVA with and Dunn’s post hoc comparisons. All data was analyzed data using GraphPad Prism 5.0 Software (GraphPad Software Inc, Sand Diego, Ca).
| Protein | CON | LCI | HCI | P Value |
|---|---|---|---|---|
| ISCHEMIC | ||||
| i-NOS | 1.0+/−0.15 | 1.35+/−0.27 | 0.71+/−0.23 | 0.12 |
| p-IKBα | 1.00+/−0.23 | 1.05+/−0.15 | 1.47+/−0.18 | 0.13 |
| VCAM | 1.00+/−0.20 | 0.82+/−0.14 | 0.79+/−0.15 | 0.57 |
| NONISCHEMIC | ||||
| NFkB | 1.0+/− 0.25 | 2.23+/−0.61 | 2.72+/−0.89 | 0.16 |
| p-IkBα | 1.0+/−0.11 | 1.04+/−0.10 | 0.66+/−0.14 | 0.13 |
| Interferonγ | 1.0+/−0.13 | 1.50+/−0.20 | 1.01+/−0.13 | 0.14 |
| PCKα | 1.0+/−0.31 | 1.48+/−0.35 | 2.66+/−0.91 | 0.11 |
| IL-1 | 1.0+/− 0.20 | 0.94+/−0.43 | 3.46+/−1.17 | 0.06 |
Discussion
To date, most studies directed at understanding the mechanisms that underlie the inflammatory shift during cardiac ischemia and metabolic syndrome have been performed with murine models. In the present study, we have taken advantage of the pig metabolic syndrome model and proteomics analyses to examine the inflammatory disturbances that may arise from inflammatory processes in the heart and the potential of calpain inhibitors to reverse inflammation. We hypothesized that calpain inhibition would beneficially alter inflammatory protein expression in the ischemic myocardial tissue in our pig model of diet induced metabolic syndrome. We found that in the setting of metabolic syndrome and chronic myocardial ischemia, inhibition of calpain has a beneficial effect on vessel wall NF-kB inflammatory signaling and is associated with decreased macrophage presence in ischemic myocardial tissue. Importantly, our results suggest that calpain inhibition functions by stabilizing the expression of IkBα allowing IkBα to bind to NFkB sequestering it in the cytosol. Our results were corroborated using western blot, immunohistochemistry and protein array showing that NFkB signaling is modified by calpain inhibition. This suggests that the beneficial effect seen by calpain inhibition on myocardial blood flow may be through its inhibitory effect on the inflammatory cascade.
IkBα inhibits the transcription factor activity of NFkB by binding to it and sequestering it in the cytoplasm. IkBα is inactivated by stimuli associated with stress, such as ischemia. This inactivation occurs via phosphorylation by IKK at serine-32 and serine-36, tagging the protein for degradation. IKK is a multicomponent protein complex comprised of an IKKα/a, IKKβ/2 and IKKγ. [17,18] Inactivation and degradation of IkBα thus frees NFkB allowing it to translocate to the nucleus where it promotes transcription of a number of inflammatory genes including cytokines, chemokines and adhesion molecules. [19]
Calpain activation is thought to degrade IkBα via direct cleavage. Interestingly, calpain has been found to cleave between amino acid 50 and 51. Cleavage at this site results in a liberated fragment that contains the serine and threonine residues necessary for phosphorylation by the IKK complex. In situations of stress, inhibition of calpain is associated with increased levels IkBα. [20]
Our western blot analysis showed a strong trend to decrease in NFkB and no difference in p-IkBα in calpain inhibited groups compared to the control. It is possible that we did not see a significant difference in NFkB because the western blot was showing us difference in total myocardial tissue lysate without tissue-specific localization. It is likely that we did not see a difference in p-IkBα because calpain regulates IkBα in a manner that does not involve phosphorylation. Therefore, to clarify this issue, we performed additional immunohistochemistry, in doing so, we were able to not quantify the changes in expression between of proteins between groups, but also localize the proteins in the myocardial tissue. Our immunohistochemistry analysis shows that calpain inhibition is associated with increased expression of the active form of IkBα and decreased expression of the active form of NFkB (p65). Importantly, we also found that the changes in expression of IkBα and NFkB were seen in the endothelial cells (as identified by co-staining with SMA). This suggests a mechanism through which calpain inhibition may have a beneficial effect on myocardial blood flow via an inhibitory effect on the inflammatory cascade.
Importantly, our western blot analysis shows that calpain inhibition decreases the expression of downstream targets of the transcription factor of NF-kB including the strong pro inflammatory cytokines TNF-α, IL 1 and interferon γ. This is consistent with previous research which shows that sepsis induced calpain activation leads to increased expression of TNF-α in cardiomyocytes and that calpain inhibition improves myocardial function in endotoxaemia. [11] We did not see any significant trends in VCAM or i-NOS in the calpain inhibited group compared to the control. It is possible that we did not see a significant difference because the western blot was showing us difference in total myocardial tissue lysate.
To explore the NFkB pathway more thoroughly, we performed a NFkB Protein Array. Importantly, the NFkB array showed decreased expression of NFkBp65 in the calpain inhibited ischemic myocardial tissue compared to the control. The calpain inhibited group also expressed decreased expression of proto-oncogene c-Rel which is a member of the NFkB family of transcription factors which has an important role in activation of B cells. The array also showed decreased expression of several cell surface receptors known to promote the NFkB pathway in the calpain inhibited ischemic myocardial tissue compared to the control group. These receptors include tumor necrosis factor α receptor 1 (TNFR1), tumor necrosis factor α receptor 2 (TNFR 2) and tumor necrosis factor- related apoptosis inducing ligand receptor 2 (TRAIL R2). Shank-Associated RH domain-interacting ProteIN (Sharpin) is ubiquitously expressed in various cells and tissues and is thought to play a role in inflammatory signaling by aiding in the activation of NFkB. [21]
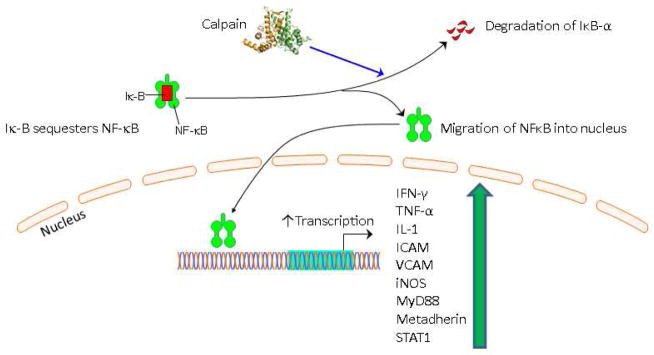
Finally, the NFkB protein array also identified decreased expression of c-Jun N-terminal kinase ½ and 2 in (JNK ½ and JNK2) in the calpain inhibited group compared to the control. JNK proteins are stress activated proteins that can translocate to the nucleus and function as a transcription factors. It is thought that JNK genes are controlled by inflammatory signals including increased reactive oxygen species. Therefore, decreased expression of JKN1/2 and 2 may indicate decreased inflammation in the ischemic myocardial tissue of the calpain inhibited group compared to the control. Together these data support the hypothesis that calpain inhibition has a beneficial effect on inflammatory signaling in ischemic myocardial tissue in the setting of metabolic syndrome. [Figure 6]
Figure 6. Schematic Showing Possible Mechanism By Which Calpain Modulates NFkB Pathway Protein Signaling.
Motifolio PPT Drawing Toolkit and the PyMOL Molecular Graphics System, Version 1.8 Schrödinger, LLC were used for designing the figure. nuclear factor kappa B (NFkB (p65)), interferon gamma (IFN--γ), vascular cell adhesion molecule (VCAM), inducible nitric oxide synthase ( iNOS), tumor necrosis factor alpha (TNF-α), interleukin-1 (IL-1), intercellular adhesion molecule (ICAM), protein kinase C alpha (PKC-α), inhibitor of nuclear factor kappa-B kinase 1 (IKK1), inhibitor of nuclear factor kappa-B kinase 2 (IKK2), myeloid differentiation primary response gene 88 (MyD88), metadherin, signal transducer and activator of transcription 1 (Stat1), c-Jun N-terminal kinase ½ (JNK ½)
Our western blot data showed that calpain inhibition lead to an increased expression of ICAM and PKC-α in the ischemic tissue. CD54/ICAM is a cell surface glycoprotein which is typically expressed on endothelial cells and cells of the immune system. The increased expression of ICAM could possibly be due to the increased presence of endothelial cells which we have previously shown to be increased in the ischemic tissue of the calpain inhibited group compared to the control. [5]PKCα is stimulated through a variety of different pathways in the cell and its increased expression in the calpain inhibited groups may have been caused by other pathways activated under stress.
In the nonischemic myocardial tissue, the only change in expression level we saw was a decrease in VCAM in the calpain inhibited groups compared to the control. VCAM is a transmembrane glycoprotein located on the endothelial cell. One explanation for the lack of effect of calpain inhibition in the non-ischemic tissue is that ischemia is thought to lead to the over activation of calpain activity and subsequent pathological angiogenesis. Moderate calpain inhibition of this over activation leads to improved neovessel formation and integration. However, complete knock out of calpain prohibits any neovessel integration and lumen formation. [23,24] This is consistent with the results of our study which found that calpain inhibition in the ischemic territories, where calpain is over activated, produces a beneficial effect on inflammatory signal expression.
Finally, we used immunohistochemistry to evaluate the presence of macrophages in the ischemic myocardial tissue of our pigs. We found that in the ischemic tissue, there were increased macrophage counts in the control group compared to the high dose and low dose calpain inhibited groups. Interestingly, in the high cholesterol control group the large number of macrophages were seen surrounding the blood vessels. Interestingly, our NFkB Protein Array showed that calpain inhibition was associated with decreased expression of interferon regulatory factor 5 (IRF 5). The interferon regulator factor family serves as a molecular switch that controls whether macrophages will promote or inhibit inflammation. Macrophages have been associated with ischemia induced atherosclerosis [25] We previously reported that calpain inhibition is associated with increased blood flow and decreased oxidative stress and cell death. Therefore, this decreased expression of macrophages in the ischemic myocardial tissue could contribute to the beneficial effects seen on blood flow associated with calpain inhibition.
Limitations
This study does not identify the optimal dose of calpain inhibition required to have a beneficial effect in the ischemic tissue and non-ischemic tissue. Future dose-response studies will be required in the future. In addition, NFkB is a transcription factor and therefore it would be interesting to use QT-PCR to evaluate mRNA expression of NFkB target genes. This data is currently being collected. Due to the high experimental costs of this study design we have not yet performed Sham operations on normal diet pigs. The nonischemic myocardial tissue is used as a baseline for all proteins of interest.
Conclusion
In the setting of metabolic syndrome and chronic myocardial ischemia, calpain inhibition modulates inflammatory protein expression in the ischemic myocardial tissue in a pig model of coronary artery disease in the setting of metabolic syndrome. We found that in the setting of metabolic syndrome and chronic myocardial ischemia, inhibition of calpain has a beneficial effect on vessel wall NF-kB inflammatory signaling and is associated with decreased macrophage counts in the ischemic myocardial tissue.
Interestingly, calpain inhibition appears to regulating NFkB possibly by increasing expression of IkB7α and decreasing expression of important pro inflammatory mediators downstream of NFkB including TNF-alpha and IL-1. Future work will need to evaluate these pathways in further detail.
Acknowledgments
Funding: Funding for this research was provided by the National Heart, Lung, and Blood Institute (R01HL46716, RO1HL128831 Dr. Sellke and Dr. Usheva); NIH/NIGMS Training Grant 2T32 GM065085 to Dr. Potz; NIH Training grant 5T32-HL094300-03, (Dr. Sabe and Dr. Elmadhun); AHA Grant-in Aid GRNT20460376 (Dr Clements).
Footnotes
Presented: AAS Paper: Presented at the 11th Annual Academic Surgical Congress, Jacksonville, Florida, and February 2-4th 2016
Disclosures: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abaci A1, Oğuzhan A, Kahraman S, Eryol NK, Unal S, Arinç HEA. Effect of Diabetes Mellitus on Formation of Coronary Collateral Vessels. Circulation. 1999;99:2239–43. doi: 10.1161/01.cir.99.17.2239. [DOI] [PubMed] [Google Scholar]
- 2.Boodhwani M, Sellke FW. Therapeutic Angiogenesis in Diabetes and Hypercholesterolemia : Influence of Oxidative Stress. Antioxid Redox Signal. 2009;11:1945–59. doi: 10.1089/ars.2009.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Malik S. Impact of the Metabolic Syndrome on Mortality From Coronary Heart Disease, Cardiovascular Disease, and All Causes in United States Adults. Circulation. 2004;110:1245–50. doi: 10.1161/01.CIR.0000140677.20606.0E. [DOI] [PubMed] [Google Scholar]
- 4.Chistiakov DA, Orekhov AN, Bobryshev YV. Endothelial Barrier and Its Abnormalities in Cardiovascular Disease. Front Physiol. 2015;6:1–11. doi: 10.3389/fphys.2015.00365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Potz BA, Sabe AA, Abid MR, Sellke FW. Calpains and Coronary Vascular Disease. Circ J. 2016;80:4–10. doi: 10.1253/circj.CJ-15-0997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stalker TJ, Skvarka CB, Scalia R. A novel role for calpains in the endothelial dysfunction of hyperglycemia. FASEB J. 2003;17:1511–3. doi: 10.1096/fj.02-1213fje. [DOI] [PubMed] [Google Scholar]
- 7.Madorin WS, Rui T, Sugimoto N, Handa O, Cepinskas G, Kvietys PR. Cardiac Myocytes Activated by Septic Plasma Promote Neutrophil Transendothelial Migration: Role of Platelet-Activating Factor and the Chemokines LIX and KC. Circ Res. 2004;94:944–51. doi: 10.1161/01.RES.0000124395.20249.AE. [DOI] [PubMed] [Google Scholar]
- 8.Pandurangan M, Hwang I, Orhirbat C, Jieun Y, Cho SH. The calpain system and diabetes. Pathophysiology. 2014;21:161–7. doi: 10.1016/j.pathophys.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 9.Tissier S, Lancel S, Marechal X, Mordon S, Depontieu F, Scherpereel A, et al. Calpain inhibitors improve myocardial dysfunction and inflammation induced by endotoxin in rats. Shock. 2004;21:352–7. doi: 10.1097/01.shk.0000116300.32648.16. [DOI] [PubMed] [Google Scholar]
- 10.Smith MA, Schnellmann RG. Calpains, mitochondria, and apoptosis. Cardiovasc Res. 2012;96:32–7. doi: 10.1093/cvr/cvs163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li X, Li Y, Shan L, Shen E, Chen R, Peng T. Over-expression of calpastatin inhibits calpain activation and attenuates myocardial dysfunction during endotoxaemia. Cardiovasc Res. 2009;83:72–9. doi: 10.1093/cvr/cvp100. [DOI] [PubMed] [Google Scholar]
- 12.Lassaletta AD, Chu LM, Robich MP, Elmadhun NY, Feng J, Burgess TA, Laham RJ, Sturek MSF. Overfed Ossabaw swine with early stage metabolic syndrome have normal coronary collateral development in response to chronic ischemia. Basic Res Cardiol. 2012;107:243–64. doi: 10.1016/j.micinf.2011.07.011.Innate. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sabe AA, Potz BA, Elmadhun NY, Liu Y, Feng J, Abid MR, et al. Calpain Inhibition Improves Collateral Dependent Perfusion in a Hypercholesterolemic Swine Model of Chronic Myocardial Ischemia. J Thorac Cardiovasc Surg. 2016;151:245–52. doi: 10.1016/j.jtcvs.2015.08.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Potz BA, Sabe AA, Elmadhun NY, Feng J, Liu Y, Mitchell H, et al. Calpain inhibition decreases myocardial apoptosis in a swine model of chronic myocardial ischemia. Surgery. 2015;158:445–52. doi: 10.1016/j.surg.2015.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feng J, Cohn WE, Parnis SM, Sodha NR, Clements RT, Sellke N, et al. New continuous-flow total artificial heart and vascular permeability. J Surg Res. 2015;199:296–305. doi: 10.1016/j.jss.2015.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sambasivarao SV. Overfed Ossabaw swine with early stage metabolic syndrome have normal coronary collateral development in response to chronic ischemia. Basic Res Cardiol. 2012;107:243–64. doi: 10.1016/j.micinf.2011.07.011.Innate. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karin M, Benneriah Y. PHOSPHORYLATION MEETS UBIQUITINATION: The Control of NFkB Activity. Annu Rev Immunol. 2000;18:621–63. doi: 10.1146/annurev.immunol.18.1.621. [DOI] [PubMed] [Google Scholar]
- 18.Karin M. How NF-kB is activated : the role of the IkB kinase (IKK) complex. Oncogene. 1999;18:6867–74. doi: 10.1038/sj.onc.1203219. [DOI] [PubMed] [Google Scholar]
- 19.Brown K, Gerstberger S, Carlson L, Franzoso G, Siebenlist U. Control of IKB-ac Proteolysis by Site-Specific , Signal-induced Phosphorylation. Science (80- ) 1995;10:1485–8. doi: 10.1126/science.7878466. [DOI] [PubMed] [Google Scholar]
- 20.Schaecher K, Goust J, Banik NL. The Effects of Calpain Inhibition on IkBalpha Degradation After Activation of PBMCs : Identification of the Calpain Cleavage Sites. Neurochem Res. 2004;29:1443–51. doi: 10.1023/b:nere.0000026410.56000.dd. [DOI] [PubMed] [Google Scholar]
- 21.Tokunaga F, Sakata S, Saeki Y, Satomi Y, Kirisako T, Kamei K, et al. Involvement of linear polyubiquitylation of NEMO in NFκ-B activation. Nat Cell Biol. 2009;11:123–32. doi: 10.1038/ncb1821. [DOI] [PubMed] [Google Scholar]
- 22.GALLARDO EDUARD, PHD, DE ANDRE’S IRENE, PHD, ISABEL ILLA M. Cathepsins Are Upregulated by IFN-gamma / STAT1 in Human Muscle Culture : A Possible Active Factor in Dermatomyositis. J Neuropathol Exp Neurol. 2001;60:847–55. doi: 10.1093/jnen/60.9.847. [DOI] [PubMed] [Google Scholar]
- 23.Hoang MV, Smith LESD. Calpain inhibitors reduce retinal hypoxia in ischemic retinopathy by improving neovascular architecture and functional perfusion. Biochim Biophys Acta. 2011;1812:997-1549-557003. doi: 10.1016/j.biotechadv.2011.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoang MV, Nagy Ja, Fox JEB, Senger DR. Moderation of calpain activity promotes neovascular integration and lumen formation during VEGF-induced pathological angiogenesis. PLoS One. 2010:5. doi: 10.1371/journal.pone.0013612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aarup A, Pedersen TX, Junker N, Christoffersen C, Bartels ED, Madsen M, et al. Hypoxia-Inducible Factor-1α Expression in Macrophages Promotes Development of Atherosclerosis. Arterioscler Thromb Vasc Biol. 2016 Jul 21; doi: 10.1161/ATVBAHA.116.307830. [DOI] [PubMed] [Google Scholar]