Abstract
Background & aims
The reference method to study protein and arginine metabolism in critically ill children is measuring plasma amino acid appearances with stable isotopes during a short (4–8h) time period and extrapolate results to 24-hour. However, 24-hour measurements may be variable due to critical illness related factors and a circadian rhythm could be present. Since only short duration stable isotope studies in critically ill children have been conducted before, the aim of this study was to investigate 24-hour appearance of specific amino acids representing protein and arginine metabolism, with stable isotope techniques in continuously fed critically ill children.
Methods
In eight critically ill children, admitted to the pediatric (n=4) or cardiovascular (n=4) intensive care unit, aged 0–10 years, receiving continuous (par)enteral nutrition with protein intake 1.0–3.7 g/kg/day, a 24-hour stable isotope tracer protocol was carried out. L-[ring-2H5]-phenylalanine, L-[3,3-2H2]-tyrosine, L-[5,5,5-2H3]-leucine, L-[guanido-15N2]-arginine and L-[5-13C-3,3,4,4-2H4]-citrulline were infused intravenously and L-[15N]-phenylalanine and L-[1-13C]leucine enterally. Arterial blood was sampled every hour.
Results
Coefficients of variation, representing intra-individual variability, of the amino acid appearances of phenylalanine, tyrosine, leucine, arginine and citrulline were high, on average 14–19% for intravenous tracers and 23–26% for enteral tracers. No evident circadian rhythm was present. The pattern and overall 24-hour level of whole body protein balance differed per individual.
Conclusions
In continuously fed stable critically ill children, the amino acid appearances of phenylalanine, tyrosine, leucine, arginine and citrulline show high variability. This should be kept in mind when performing stable isotope studies in this population. There was no apparent circadian rhythm.
Keywords: Protein metabolism, amino acid metabolism, circadian rhythm, 24-hour pattern, critical illness, pediatric
Introduction
The acute stress response in critical illness is associated with a protein catabolic state, which in critically ill children in turn is associated with increased morbidity and mortality (1).Protein requirements to achieve anabolism in critically ill children are increased as compared to healthy children, but unfortunately not well defined (2, 3). Also, the requirements of specific amino acids may be increased. Arginine is especially of interest, because it has functions in the immune system and is the precursor of nitric oxide (NO). As sepsis and critical illness are considered arginine-deficient states, arginine supplementation might be beneficial (4). However scarce data exist on arginine metabolism and NO synthesis in critically ill children.
The reference method to study protein and arginine metabolism is considered to be stable isotope tracer techniques with measurement of amino acid appearances (4, 5). Usually an experimental protocol of 4–8 hours is used of which the results are extrapolated to 24-hour metabolism for recommendations of daily protein intake (6–9) or insight in arginine metabolism (10). Studies with 24-hour designs, which are more representative to metabolism during an entire day, have been conducted in healthy adults and comprised both a fasted and a fed state (11–13). These studies showed a circadian pattern (11–13), with e.g. 53% higher phenylalanine appearances in the fasted than in the fed state (13). In healthy neonates, a circadian rhythm is found in plasma amino acid concentrations (14), with 40% higher amino acid levels during day time as compared to night time,
However, it is not known whether appearances of amino acids, representing protein and arginine metabolism, in critically ill children during continuous feeding show a circadian rhythm. Therefore, we also do not know whether short duration tracer protocols (4–8 h) represent 24-hour protein and arginine metabolism in children. We anticipated that children at the pediatric intensive care unit (PICU) have an altered day-night cycle as a result of artificial light exposure, continuous care, sedation and hormonal changes related to the acute stress response (15, 16). We hypothesized that circadian rhythms are attenuated or completely lost in critically ill children.
The objective of the present study was to investigate the pattern of and variability in 24-hour appearance of specific amino acids, representing protein and arginine metabolism, in continuously fed critically ill children admitted to the PICU or Cardiovascular ICU (CVICU), using stable isotope tracer methodology with hourly enrichment measurements.
Materials and methods
Subjects and setting
Between May 2008 and December 2010 children aged <18 years admitted to the PICU or CVICU of Arkansas Children’s Hospital, Little Rock, AR, were included. Inclusion criteria were: arterial line and multi-lumen central venous line (or two peripheral venous catheters) in place; continuous total parenteral nutrition or continuous enteral feeding with standard nutrition appropriate for age and weight; no planned major changes or interventions (such as surgery) from enrollment to completion of study period; hemodynamic stable condition (with or without continuous inotropic medication) defined as ≤1 boluses of volume resuscitation for hypotension in 24-hour; no significant loss of plasma/blood from wounds or drains, that may influence the results of the study, no chylothorax; written informed consent by parent(s) or legally authorized representatives. Exclusion criteria were: congenital/acquired metabolic or endocrine disorders or hepatic or renal failure or anuria or oliguria; gastrointestinal obstructions or any condition that causes malabsorption; active gastro-intestinal bleeding; fluid restriction hindering ability to administer stable isotope tracer fluids.
Z-scores for weight-for-age were determined using 2000 Centers of disease control and prevention (CDC) growth charts (http://www.cdc.gov/growthcharts/zscore.htm).
Study design
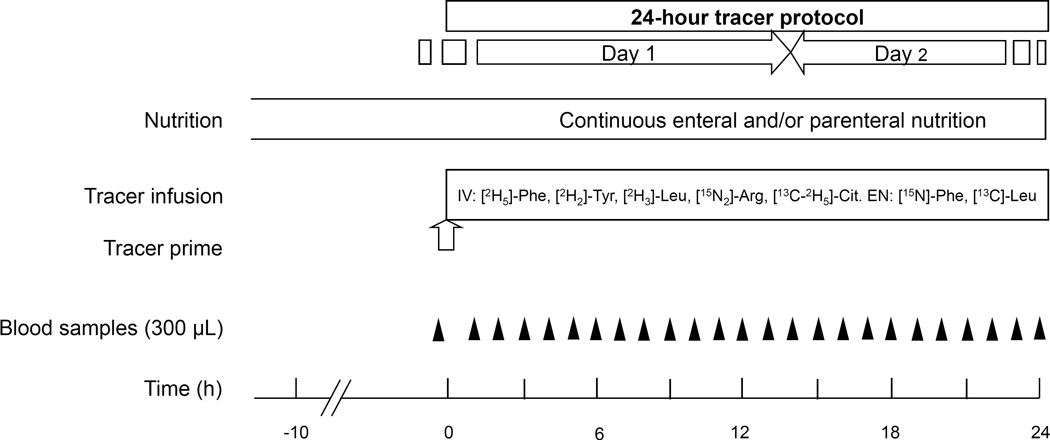
Patients were fed according to standard ICU care with commercially available, condition-specific formulas as determined by the attending medical team, by continuous enteral drip feeding and/or parenteral intravenous nutrition. The tracer protocol was started after enteral drip-feeding and/or continuous parenteral nutrition was provided at a constant and continuous rate for at least 10 hours. Nutrition was maintained at this nutrition rate and mode throughout the 24-hour tracer protocol. Stable isotope tracers were administered intravenously and enterally according to a primed, constant and continuous protocol during 24-hour, starting in the morning or afternoon. When enteral nutrition was provided via an open nutrition bag system, the enteral tracers were intermixed with nutrition and the tracer-nutrition mix was added to the nutrition bag every four hours as per standard nutrition handling for hygienic and safety issues. If enteral nutrition was given per syringe pump, the enteral tracer was administered via a Y-connector on the enteral tube, alongside nutrition using a second syringe pump. IV tracers were infused via central or peripheral venous catheters in place for medical treatment. The enteral nutrition rate was continued at the pre-experiment rate until the end of the tracer protocol. IV fat emulsions, as part of parenteral nutrition if applicable, were stopped from 12:00 am to 4:00 am, as per standard policy in our center.
Before priming the tracers, a baseline arterial blood sample was taken to determine background isotopic enrichment. During the next 24 hours 300 µl arterial blood samples were obtained every 60 minutes to determine isotopic enrichment. Samples were put on ice, transfer pipetted into heparin cups and centrifuged (12.000 × g) for 1 min. Plasma for stable isotope analysis was then deproteinized with 5% sulfosalicylic acid, kept on dry ice until the end of the experiment and subsequently stored at −80°C until analysis.
Stable isotope tracers
For assessment of whole body protein kinetics L-[ring-2H5]phenylalanine (Phe5), L-[3,3-2H2]tyrosine (Tyr2) and L-[5,5,5-2H3]leucine (Leu3) were administered intravenously. In addition of priming these tracers before continuous infusion, a bolus of L-[ring-2H4]tyrosine was infused to prime the pool of L-[ring-2H4]tyrosine coming from L-[ring-2H5]phenylalanine. For the determination of splanchnic phenylalanine and leucine extraction, L-[15N]phenylalanine (Phe1) and L-[1-13C]leucine (Leu1) were administered enterally. In order to assess arginine metabolism L-[guanido-15N2]-arginine (Arg2) and L-[5-13C-3,3,4,4-2H4]-citrulline (Cit5) tracers were infused intravenously.
Tracers (all >98% mole percent enrichment) were purchased as sterile pyrogen-free powders from Cambridge Isotope Laboratories, Andover, MA, USA. Tracer doses were calculated per subject based on weight and corrected for expected dilution by enteral nutrition (assuming splanchnic extraction of 50%) to achieve adequate enrichment levels. An external licensed pharmacist compounded the intravenous tracer solutions within 48-hour prior to administration. All tracer solutions were dispensed by the research pharmacy of Arkansas Children’s Hospital, who also compounded the enteral tracer solution the day prior to the study day. The intravenous tracers were administered through a 2µm pore filter. A sample of the intravenous tracer solution and the enteral tracer solution or tracer-nutrition mix was stored for determination of tracer concentrations.
Sample analysis
Tracer- tracee ratios and plasma amino acid concentrations
Plasma amino acid concentrations and exogenous amino acid stable isotopomer enrichments (tracer-to-tracee ratios, TTR) were simultaneously determined on a LC-ESI-MS system (QTrap 5500 MS (AB Sciex, Foster City, CA, USA) with ExpressHT Ultra LC (Eksigent Div., AB Sciex, Foster City, CA, USA). Samples were standardized with 0.1 mol/L HCl containing a stable isotopomer of every amino acid as internal standard. For internal standards stable isotopomers with a high mass were chosen (≥ m+5) to prevent contribution of overlapping isotopomer distributions to isotopomers that were used as a tracer in the experimental protocol. Then, samples were transfer-pipetted onto strong-cation-exchange drip columns. Columns were washed with water and eluted with 2.5 mol/L ammonia. Eluates were desolvated in a centrifugal evaporator. Solid residue tubes were capped for storage in the dark at room temperature. Within 3 days of LC-ESI-MS analysis samples were derivatized with 9-Fluorenylmethoxycarbonyl (Fmoc), subsequently neutralized, where after 160 nL of the solution was injected onto a 50 × 0.5 mm HALO C18 column and kept at 35° Celsius. Analyte s were eluted with a segmentally linear gradient from 35% to 85% acetonitrile in water supplemented with ammonium acetate to 10 µmol/L and 5% isopropanol. Detection was by electrospray triple quadruple tandem mass spectrometry in multiple reaction monitoring mode. Fmoc amino acid derivatives were fragmented in the collision cell for detection of either free aminoacyl anions or a fragment larger by 26 atom mass units (coming from the Fmoc derivative), whichever gave highest sensitivity. Thus monitoring occurred for each amino acid, their tracers and internal standards. Tracer-to-tracee ratios (TTR) were determined as tracer (labelled substance) / tracee (unlabelled substance). Concentrations of amino acids were calculated by using the ratio of the area under the curve of the amino acid and the area under the curve of the internal standard which was chosen for that specific amino acid (either a stable isotopomer with a high mass of that particular amino acid, or a stable isotopomer of another amino acid with a retention time close to that amino acid) and then multiplying by the known concentration of the internal standard in the sample.
Determination of amino acid concentrations in nutrition
To determine the total concentration of individual amino acids in nutrition, amino acids of the food proteins were liberated by hydrolysis. For this purpose, 3 different dilutions were made from each food sample to ensure that each amino acid concentration could be measured within the detection range of the LC-MS-MS method. An aliquot of 40 ul of a food dilution was added to 40 ul internal standard in a glass tube. For the internal standard, a mixture of stable isotopes with higher masses of each amino acid was used. The glass tube was dried down, where after 1 ml of 6 mol/L HCI was added. Closed tubes were incubated for 24-hour at 100°C. At the end of this hydrolysis period 5ml of MilliQ water was added. Concentrations of amino acids in the hydrolytes were determined using the LC-MS-MS method, as described above.
Calculations
Whole body amino acid metabolism
Whole body rate of appearance (Ra; flux or Q in µmol/kg/h) of plasma, phenylalanine, leucine, tyrosine, arginine and citrulline were calculated from arterial isotopic enrichments of [2H5]Phe, [2H3]Leu, [2H2]Tyr, [15N2]Arg and [13C-2H4]Cit using the standard steady state isotope dilution equation (17):
| (Eq1) |
where / is the rate of tracer infusion. Isotopic enrichment was calculated by correcting the measured TTRs at the plateau phase by subtracting the background TTR, which was determined in the sample obtained before the start of the tracer infusion. In case of arginine and citrulline tracers the contribution of overlapping isotopomer distributions of tracers with lower masses to the measured TTR was accounted for as previously described (18).
Whole body protein metabolism
Calculations for whole body protein metabolism during the fed state have been described before (19).
Under steady state conditions Ra of amino acids in the plasma pool is equal to the rate of disappearance (Rd; flux or Q). In the fed state Ra equals the sum of the rate at which the amino acid is released from whole body protein breakdown (WbPB) and the rate at which the amino acid enters the blood pool from the nutrition source. In case of enteral nutrition this is the rate of enteral intake, corrected for the proportion of the amino acid intake that is retained in the splanchnic area during first pass (splanchnic extraction, SPE). Rd equals the sum of the rate of oxidation or hydroxylation (in case of phenylalanine: PheOH) and the rate at which the amino acid is used for whole body protein synthesis (WbPS).
Plasma phenylalanine-to-tyrosine flux, indicating PheOH (µmol/kg/h) was calculated with the following equation:
| (Eq2) |
where QTyr is the Ra of tyrosine as calculated with equation (1) from [2H2]Tyr enrichment.
In the fed state dietary Phe via the enteral route needs to be adjusted for SPEPhe (%), as described above. Splanchnic extraction of Phe was calculated:
| (Eq3) |
where Q[2H5[Phe is the Ra of phenylalanine derived from intravenously infused [2H5]Phe and Q[15N]Phe the Ra of phenylalanine derived from enterally administered [15N]Phe.
WbPB as derived from Phe kinetics (WBPBPhe in µmol/kg/h) in the fed state was then calculated:
| (Eq4) |
WbPS as derived from Phe kinetics (WBPBPhe in µmol/kg/h) was calculated:
| (Eq5) |
Phenylalanine balance, representing whole body protein balance (WbPBalPhe) can then be calculated:
These whole body protein kinetics as derived from Phe kinetics (µmol/kg/h) were converted to whole body protein kinetics in g/kg/d (WbPBal, WbPS, WbPB) assuming an average Phe content in human proteins of 280 µmol/g protein (20).
We also used [2H3]Leu as indicator of protein metabolism. The Ra of Leu (QLeu) and leucine release from protein breakdown (WbPBLeu) were calculated in a similar fashion as described above.
Whole body arginine and citrulline metabolism
The rate of NO synthesis (µmol/kg/h) was calculated as the flux from arginine-to-citrulline (21) with the following equation:
| (Eq6) |
where QCit is the Ra of citrulline as calculated with equation (1) from L-[5-13C-3,3,4,4-2H4]citrulline enrichment.
The rate of de novo arginine production (µmol/kg/h) was calculated as the flux from citrulline-to-arginine (22) as follows:
| (Eq7) |
where QArg is the Ra of arginine as calculated with equation (1) from L-[guanido-15N2]arginine enrichment.
Statistics
Data are presented as mean ± SD or median (min-max), unless otherwise indicated. Intra-individual variation was assessed by coefficients of variation (CV): CV = 100 * (SD / mean), calculated over 24-hour. The lower the CV, the less variability there is in measurements over 24-hour, thus a specific measurement point is representative to the other measurements. We considered CV > 5% as high variability.
As variability in enteral tracer enrichment is expected to be higher when tracers are delivered into the stomach as compared to delivery into the intestine (e.g. due to gastric emptying) (23) we compared variability in enrichment of enteral tracers via intragrastric versus postpyloric nutrition modes, although groups were small. This was done by Mann Whitney U test.
Analysis was done with SPSS statistical software package (version 17; IBM, Armonk, NY, USA).
Results
Subjects
The 24-hour tracer protocol was started in 10 critically ill children. Six of them were were infants requiring extended intensive care after a congenital heart defect repair (five patients in the CVICU and one in the PICU) and the other four were older children admitted to the PICU with critical illness. One PICU subject was excluded, as the 24-hour tracer protocol was terminated after 5.5 hours because of an unstable clinical condition. One CVICU subject was excluded because a chest tube produced chyle some hours after the start of the tracer protocol.
Baseline characteristics of the 8 remaining subjects are shown in Table 1.
Table 1.
Baseline characteristics1
| Patient | ICU | M / F |
Age (y) |
Weight (kg) / z-score WFA |
Diagnosis | Admission day (AD); Post- operative day (POD) of last operation |
Mech- anical venti- lation |
Medication (Y/N)* | Start time tracers |
Nutrition mode |
Protein intake (g/kg/ d) |
Energy intake (kcal/ kg/d) |
Time on current nutrition rate (h) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | CV | M | 0.45 | 4.61 / <-2.0 |
Complex heart disease. | AD: 164 POD (cardiac surgery): 7 |
Y | Continuous sedation: Y Muscle relaxants: N Inotropics: Y Corticosteroids: Y Antieplileptics: Y Diuretics: Y Insulin: N Antibiotics: N |
9:25 | EN via TPT |
2.1 | 100 | 22 |
| S/P 3× aorta arch recon- struction and Damus-Kaye- Stansel procedure. | |||||||||||||
| S/P Aorta-pulmonary window repair. | |||||||||||||
| 2 | P | F | 0.15 | 3.70 / −1.5 |
Tetralogy of Fallot and absent pulmonary valve syndrome. |
AD: 53 POD (cardiac surgery): 54 |
Y | Continuous sedation: Y Muscle relaxants: N Inotropics: Y Corticosteroids: N Antieplileptics: Y Diuretics: Y Insulin: N Antibiotics: N |
11:32 | EN via TPT |
3.7 | 118 | 35 |
| S/P Tetralogy of Fallot repair. | |||||||||||||
| Prolonged ICU care. | |||||||||||||
| 3 | CV | M | 0.29 | 5.57 / −1.0 |
HLHS | AD: 105 POD (cardiac surgery): 8 |
N | Continuous sedation: N Muscle relaxants: N Inotropics: Y Corticosteroids: N Antieplileptics: N Diuretics: Y Insulin: N Antibiotics: N |
9:53 | EN via jejunal tube |
2.7 | 144 | 23 |
| S/P Norwood procedure with Sano shunt. | |||||||||||||
| S/P Glenn procedure | |||||||||||||
| 4 | CV | M | 0.05 | 3.45 / −0.5 |
HLHS with mitralis valve stenosis, aorta valve stenosis and coarctation. |
AD: 20 POD (cardiac surgery): 14 POD (G- tube): 9 |
N | Continuous sedation: N Muscle relaxants: N Inotropics: Y Corticosteroids: N Antieplileptics: N Diuretics: Y Insulin: N Antibiotics: N |
8:53 | EN via G-tube |
2.1 | 102 | 20 |
| S/P Norwood procedure with Sano shunt. | |||||||||||||
| 5 | P | M | 9.12 | 46.4 / +2.0 |
Respiratory distress due to pneumonia. |
AD: 3 POD: N/A |
Y | Continuous sedation: Y Muscle relaxants: N Inotropics: N Corticosteroids: Y Antieplileptics: N Diuretics: N Insulin: N Antibiotics: Y |
9:57 | EN via NG-tube |
1.2 | 40 | 24 |
| 6 | P | M | 7.87 | 25.0 / 0 |
Status epilepticus with multiple organ failure. |
AD:19 POD: N/A |
Y | Continuous sedation: Y Muscle relaxants: Y Inotropics: Y Corticosteroids: Y Antieplileptics: Y Diuretics: Y Insulin: N Antibiotics: Y |
9:45 | EN via TPT |
1.3 | 50 | 61 |
| 7 | P | F | 9.89 | 28.8 / −0.5 |
Respiratory failure due to pneumonia; sepsis. |
AD: 37 POD” N/A |
Y | Continuous sedation: Y Muscle relaxants: N Inotropics: N Corticosteroids: N Antieplileptics: Y Diuretics: N Insulin: Y Antibiotics: Y |
10:55 | EN via TPT |
1.0 | 31 | 26 |
| 8 | CV | F | 0.34 | 5.20 / −1.5 |
HLHS with hypoplastic aorta, double outlet right ventricle. |
AD: 125 POD (cardiac surgery): 7 |
Y | Continuous sedation: Y Muscle relaxants: Y Inotropics: Y Corticosteroids: Y Antieplileptics: N Diuretics: Y Insulin: N Antibiotics: Y |
12:49 | EN via G-tube + PN lipids |
1.4 | 105 | 17 |
| S/P Norwoord procedure with Sano shunt. | |||||||||||||
| 4 CV / 4 P ICU |
5 M / 3 F |
0.40 (0.0 5– 9.89 ) |
5.38 (2.9– 46.4) |
1.7 (1.0– 3.7) |
100 (31– 144) |
24 (17– 61) |
|||||||
Inotropics included milrinone and/or digoxin, no other inotropics were administered in any of the patients; Corticosteroids refer to systemic corticosteroids alone; inhalation corticosteroids were not included in this table.
AD, admission day; CV, cardiovascular intensive care unit; EN, enteral nutrition; G-tube, gastrostomy tube; HLHS, hypoplastic left heart syndrome; NG-tube, nasogastric tube; P, pediatric intensive care unit; PN, parenteral nutrition; POD, post-operative day; S/P, status post; TPT, transpyloric tube; WFA, weight-for-age
At start of the 24-hour tracer protocol all patients were at least 7 days after (cardiac) surgery, and at least 17 hours on continuous enteral drip feeding at a nutrition rate and mode that was continued throughout the tracer protocol. This rate represented full enteral nutrition as determined by the dietician, apart from patient 8 who was on parenteral lipids as well. The days before the tracer protocol enteral nutrition was either increased in a stepwise mode, e.g. after detubation, or was already administered at the target rate. Three patients received enteral nutrition intragastrically, five patients received enteral nutrition via a route beyond the stomach (Table 1). The 24-hour tracer infusion was started between 9.00 am en 1.00 pm.
In Supplementary Material 1 24-hour vital parameters and the administration of medication over 24-hour per patient is shown, to give more insight into the clinical condition of the patients and the complexity of medical care.
Measurements
Variability in tracer enrichments and plasma amino acid concentrations
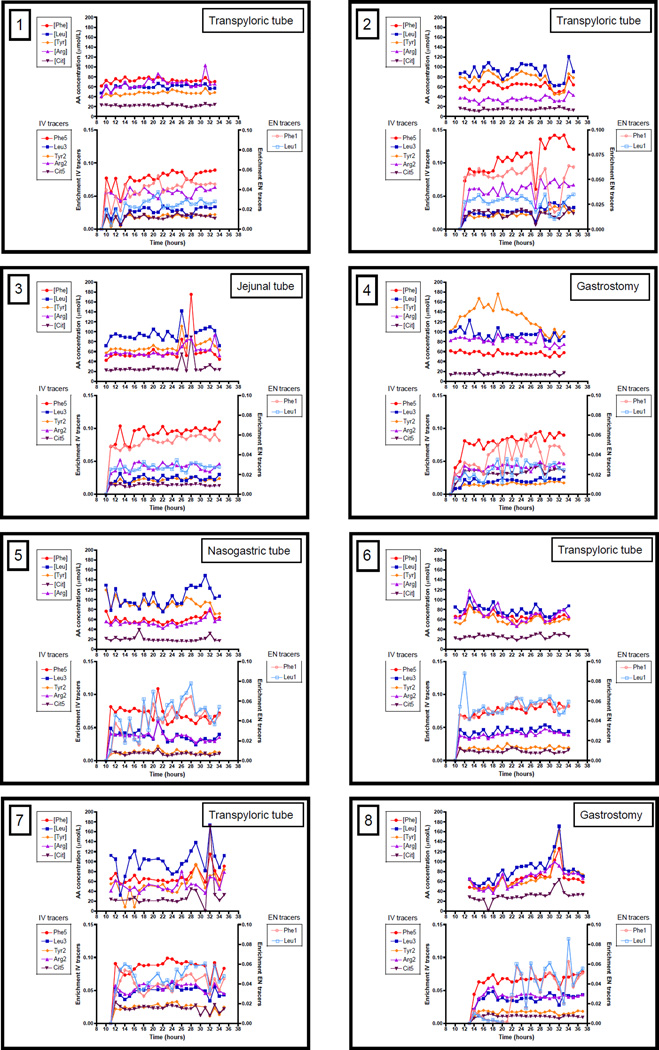
24-Hour enrichments of the infused stable isotopes are available in Figure 2, as well as the plasma amino acid concentrations of the non-isotopic equivalent. Due to a technical error, after administration of the enteral prime dose, enteral tracers were not infused in the first 8 hours in patient 8 and therefore enteral tracer enrichment and data derived from enteral tracers could not be calculated for this patient during this time period. In general, the IV tracers (Phe5, Tyr2, Leu3, Arg2, Cit5) followed approximately the same pattern and so did the enteral tracers (Phe1 and Leu1), but patterns for IV and enteral tracers differed.
Figure 2. Individual tracer enrichments and plasma amino acid concentrations over 24-hour.
Measurements over 24-hour are shown for 8 critically ill children individually per panel. Time is shown in clock hours (e.g. 8 hours = 8 am), values above 24 represent the next day. Within every panel, the upper graph shows plasma amino acid concentrations, the lower graph shows stable isotope tracer enrichments with IV tracers on the left Y-axis and EN tracers on the right Y-axis. The mode of nutrition delivery is shown in the right upper corner of the panel.
AA, amino acids; [Arg], plasma arginine concentration; Arg2, L-[15N2]-arginine; [Cit], plasma citrulline concentration; Cit5, L-[13C-2H4]]-citrulline (Cit5); EN, enteral; [Leu], plasma leucine concentration; Leu1, L-[13C]-leucine; Leu3, L-[2H3]-leucine; [Phe], plasma phenylalanine concentration; Phe1, L-[15N]-phenylalanine; Phe5, L-[ring-2H5]-phenylalanine; [Tyr], plasma tyrosine concentration; Tyr2, L-[2H2]-tyrosine.
CV, as indicator of intra-individual variation, of enrichments and plasma amino acid concentrations are shown in Table 2. To illustrate variability in clinical condition, CV of heart rate and mean arterial pressure are also shown (the pattern of vital parameters is shown in Supplementary Material 1). There was a high intra-individual variability in tracer enrichments and plasma amino acid concentrations, with mean CV’s of 14–19% for IV tracers, 23–26% for EN tracers and 17–34% for plasma amino acid concentrations. Mean CV’s for heart rate and mean arterial pressure, were somewhat lower: 8% and 13% respectively.
Table 2.
Coefficient of variation (%) of stable isotope tracer enrichments, plasma amino acid concentrations and vital parameters over 24-hours 1
| Patient | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | Mean SD | |
| IV tracers | |||||||||
| Enrichment Phe5 | 14 | 21 | 11 | 16 | 16 | 9 | 11 | 11 | 14 ± 3.8 |
| Enrichment Tyr2 | 23 | 23 | 13 | 17 | 14 | 11 | 18 | 17 | 17 ± 4.4 |
| Enrichment Leu3 | 24 | 26 | 17 | 20 | 20 | 12 | 14 | 16 | 19 ± 5.0 |
| Enrichment Arg2 | 11 | 19 | 14 | 14 | 18 | 10 | 12 | 14 | 14 ± 3.2 |
| Enrichment Cit5 | 23 | 24 | 10 | 17 | 23 | 17 | 18 | 13 | 18 ± 4.8 |
| EN tracers | 22 | 26 | 9 | 32 | 35 | 10 | 20 | 31 | 23 ± 9.8 |
| Enrichment Phe1 | 28 | 25 | 11 | 33 | 38 | 17 | 21 | 37 | 26 ± 9.7 |
| Enrichment Leu1 | |||||||||
|
Plasma AA concentrations (µmol/L) |
|||||||||
| Phenylalanine | 5 | 12 | 42 | 7 | 12 | 12 | 21 | 28 | 17 ± 12.4 |
| Tyrosine | 7 | 18 | 48 | 19 | 10 | 16 | 40 | 39 | 25 ± 15.4 |
| Leucine | 7 | 17 | 35 | 11 | 18 | 12 | 27 | 31 | 20 ± 10.2 |
| Arginine | 16 | 15 | 18 | 10 | 15 | 23 | 24 | 26 | 18 ± 5.5 |
| Citrulline | 8 | 13 | 52 | 17 | 26 | 15 | 108 | 36 | 34 ± 33.0 |
| Vital parameters | |||||||||
| Heart rate (bpm) | 7 | 6 | 6 | 7 | 8 | 12 | 9 | 8 | 8 ± 2.0 |
| Mean arterial pressure (mmHg) |
13 | 25 | 15 | 11 | 10 | 9 | 7 | 10 | 13 ± 5.4 |
Coefficient of variation in %; AA, amino acid; Arg2, L-[15N2]-arginine; Cit5, L-[13C-2H4]]-citrulline; EN, enteral; Leu1, L-[13C]-leucine; Leu3, L-[2H3]-leucine; Phe1, L-[15N]-phenylalanine; Phe5, L-[ring-2H5]-phenylalanine; Tyr2, L-[2H2]-tyrosine.
Variability of EN tracers was significantly higher when delivered intragastrically (patient 4, 5 and 8: CV of 31 ± 8.4% for Leu1 and CV 28 ± 7.1% for Phe1) as compared to delivery beyond the stomach (patient 1, 2, 3, 6, 7; CV 20 ± 7.7% for Leu1 and CV 17 ± 7.5% for Phe; p=0.036 and p=0.036 respectively).
No specific 24-hour pattern was visible in the individual enrichment plots (Figure 2). All patients seemed to have an individual pattern. Some time periods showed more variability than others. E.g. in patient 1 in the first 6 hours and in patient 7 in the last 5 hours, there was high variability in a similar pattern for IV and enteral tracer enrichments as well as plasma amino acid concentrations. On the other hand, in patient 2 in the last 8 hours, enteral tracer enrichment and plasma amino acid concentrations were decreased, whereas IV tracer enrichments were increased.
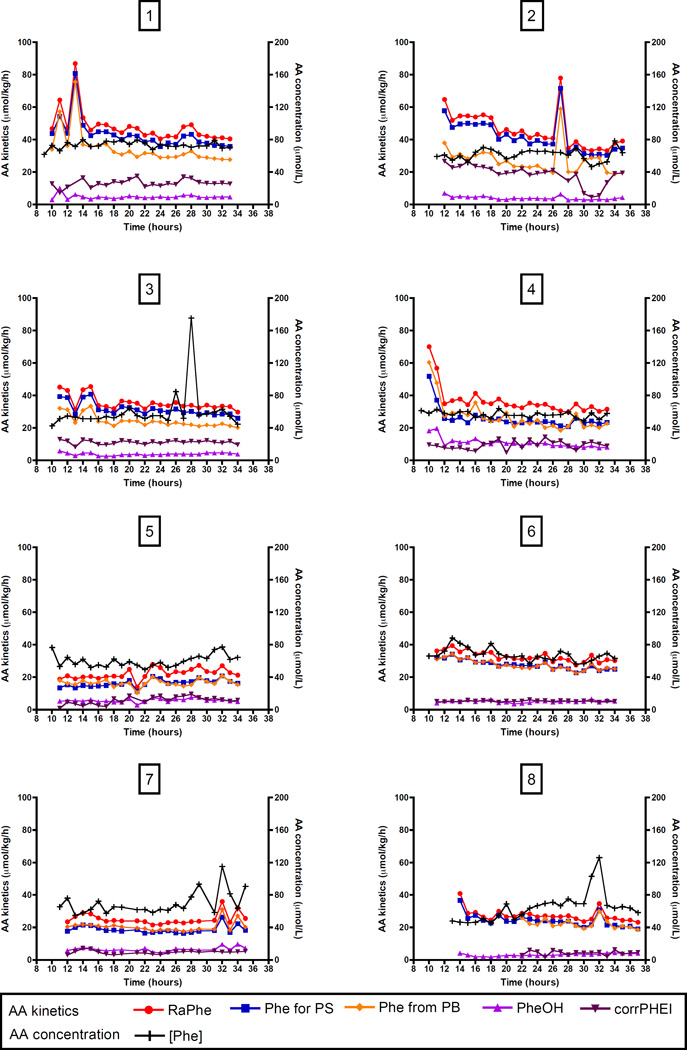
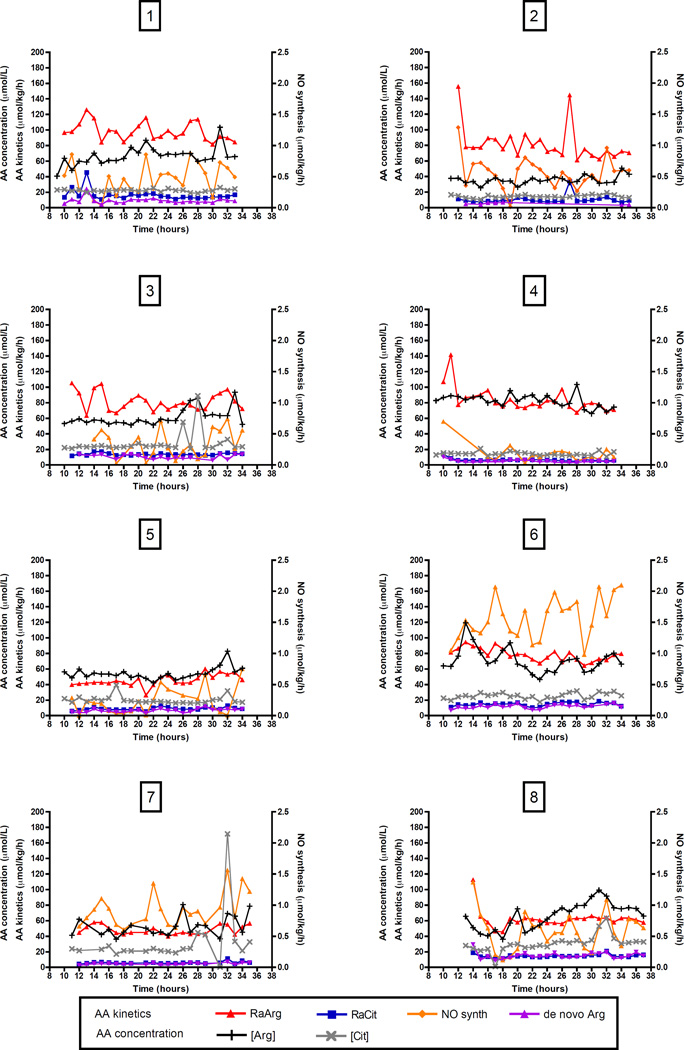
Phenylalanine and arginine kinetics
Phenylalanine kinetics of individual patients over 24-hour, representing protein metabolism as calculated from phenylalanine and tyrosine enrichments, are shown in Figure 3; arginine kinetics and NO synthesis in Figure 4. Average phenylalanine and whole body protein kinetics over 24-hour are shown in Table 3. Leucine kinetics are also included in Table 3.
Figure 3.
Figure 4. Individual phenylalanine kinetics over 24-hour.
Measurements over 24-hour are shown for 8 critically ill children individually per panel. Time is shown in clock hours (e.g. 8 hours = 8 am), values above 24 represent the next day. AA kinetics are shown on the left Y-axis, AA concentration on the right Y-axis.
AA, amino acid; [Arg], plasma arginine concentration; [Cit], plasma citrulline concentration; de novo Arg, de novo arginine synthesis; NO synth, NO synthesis; RaArg, rate of appearance of arginine; RaCit, rate of appearance of citrulline.
Table 3.
Mean amino acid and protein kinetics, and plasma amino acid concentrations over 24 hour per patient
| Patient | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | Mean ± SD | |
| Phe kinetics (µmol/kg/h) | |||||||||
| Ra Phe | 48 | 46 | 35 | 37 | 22 | 33 | 25 | 27 | 34 ± 9 |
| Phe for PS | 43 | 42 | 31 | 26 | 16 | 28 | 19 | 24 | 29 ± 10 |
| Phe from PB | 35 | 27 | 24 | 27 | 17 | 28 | 21 | 22 | 25 ± 5 |
| Phe OH | 5 | 4 | 4 | 11 | 6 | 5 | 6 | 3 | 5 ± 2 |
| Corrected Phe Intake | 13 | 19 | 11 | 10 | 6 | 5 | 5 | 4 | 9 ± 5 |
| SPE Phe (%) | 58 | 61 | 62 | 54 | 63 | 61 | 71 | 73 | 63 ± 6 |
| Leu kinetics (µmol/kg/h) | |||||||||
| Ra Leu | 139 | 175 | 154 | 160 | 82 | 115 | 90 | 98 | 127 ± 35 |
| Leu from PB | 107 | 102 | 88 | 118 | 55 | 95 | 69 | 72 | 88 ± 21 |
| SPE Leu (%) | 40 | 26 | 17 | 30 | 43 | 31 | 41 | 41 | 34 ± 9 |
| Arg kinetics (µmol/kg/h) | |||||||||
| Ra Arg | 98 | 82 | 82 | 84 | 45 | 78 | 48 | 62 | 72 ± 19 |
| Ra Cit | 16 | 10 | 14 | 7 | 9 | 15 | 6 | 15 | 11 ± 4 |
| NO synthesis | 0,59 | 0,56 | 0,35 | 0,19 | 0,23 | 1,56 | 0,92 | 0,59 | 0,62 ± 0,45 |
| de novo Arg synthesis | 9 | 5 | 10 | 5 | 6 | 12 | 4 | 15 | 8 ± 3,9 |
| Protein kinetics (g/kg/d) | |||||||||
| Wb PS | 3,7 | 3,6 | 2,7 | 2,2 | 1,4 | 2,4 | 1,6 | 2,1 | 2,5 ± 0,8 |
| Wb PB | 3,0 | 2,3 | 2,1 | 2,3 | 1,4 | 2,4 | 1,8 | 1,9 | 2,1 ± 0,5 |
| Wb PBal | 0,71 | 1,26 | 0,62 | -0,11 | -0,01 | 0,01 | -0,15 | 0,07 | 0,30 ± 0,5 |
|
Plasma AA concentrations (µmol/L) |
|||||||||
| Phenylalanine | 74 | 62 | 61 | 56 | 59 | 68 | 70 | 65 | 64 ± 6 |
| Tyrosine | 48 | 76 | 77 | 129 | 90 | 60 | 52 | 67 | 75 ± 26 |
| Leucine | 61 | 91 | 103 | 94 | 104 | 79 | 100 | 84 | 89 ± 15 |
| Arginine | 67 | 35 | 61 | 83 | 54 | 71 | 53 | 66 | 61 ± 14 |
| Citrulline | 22 | 15 | 28 | 15 | 20 | 26 | 32 | 31 | 23 ± 7 |
AA, amino acid; Arg, arginine; Cit, citrulline; corrected Phe intake, Phe intake corrected for splanchnic extraction; CV, coefficient of variation; Leu, leucine; PB, protein breakdown; PBal, protein balance; Phe from PB, phenylalanine derived from protein breakdown; Phe for PS, phenylalanine used for protein synthesis; Phe OH, phenylalanine hydroxylation; Phe, phenylalanine; PS, protein synthesis; Ra, rate of appearance; SPE, splanchnic extraction; Tyr, tyrosine; Wb, whole body;
Again, patterns differed per individual patient and were in part a reflection of the variability in enrichments (Figure 3, 4).
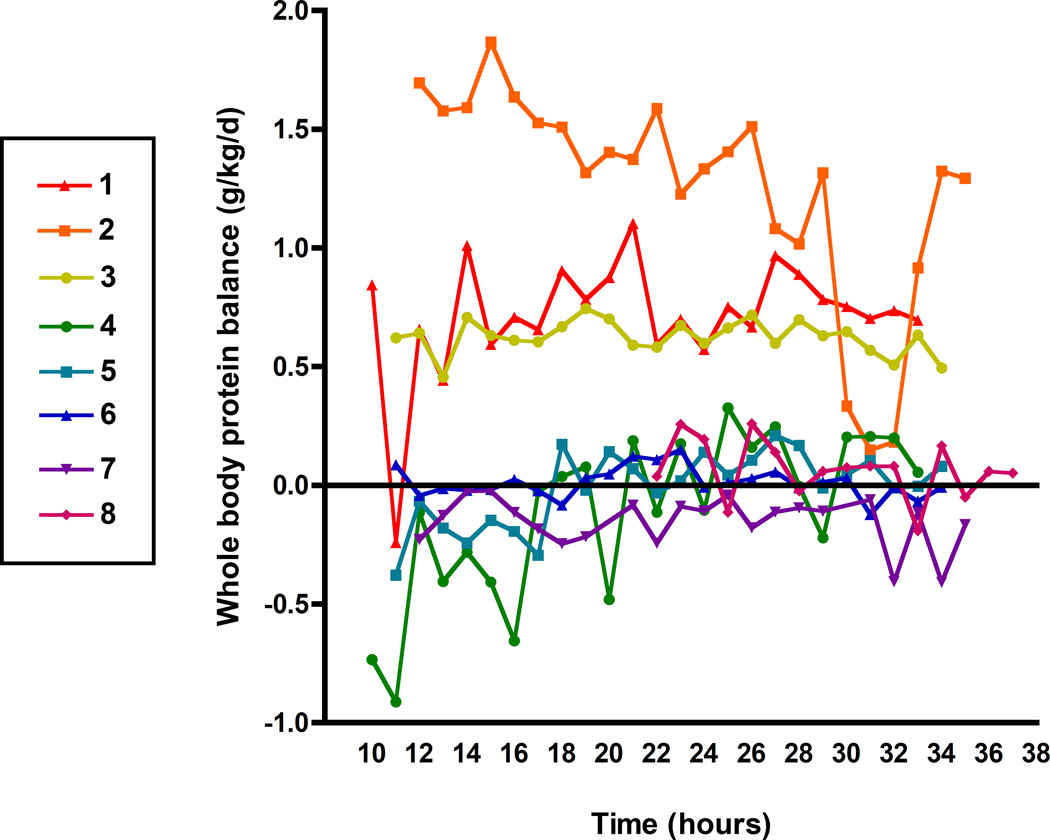
Whole body protein balance
Figure 5 depicts 24-hour whole body protein balance, as calculated from phenylalanine enrichments, per individual patient. Mean whole body protein balance is shown in Table 3. In three infants after cardiac surgery (patient 1,2,3) there was a net positive whole body protein balance, representing an anabolic state. In two infants after cardiac surgery (patient 4, 8) and two children on the PICU (patient 5, 6), who were diagnosed with pneumonia and status epilepticus, whole body protein balance over 24-hours was alternately positive and negative and overall close to zero. In the other child on the PICU with sepsis, protein balance was negative throughout the 24-hour period, in theory respresenting a catabolic state, but overall close to zero.
Figure 5. 24-hour whole body protein balance in 8 critically ill children.
Measurements over 24-hour are shown for 8 critically ill children, every line represents one patient. Time is shown in clock hours (e.g. 8 hours = 8 am), values above 24 represent the next day.
Discussion
We studied the pattern and variability in 24-hour amino acid appearances in relatively stable critically ill children receiving continuous nutrition. This information was to date not available, while it is crucial for extrapolation of short-duration studies to 24-hour metabolism. Our main finding is that there was a high variability in amino acid appearances of phenylalanine, tyrosine, leucine, arginine and citrulline and hence in calculated protein and arginine kinetics, as well as in plasma amino acid concentrations. In addition, there was no apparent circadian rhythm in amino acid appearances or plasma amino acid concentration; patterns differed per individual.
Although patients were in a relatively stable condition, we found high intra-individual variation in tracer enrichment, as well as in plasma amino acid concentrations. As heart rate and mean arterial pressure already showed mean CV’s of 6–25%, clinical condition probably in part played a role in the variation of enrichments. In a previous study of our group in 19 critically ill children, of whom six with congenital heart defects, CV’s of energy expenditure measured during 24-hour were lower (range 2–11%) (24). Variation in tracer enrichments may be the consequence of technical problems (e.g inadequate tracer priming dose, sampling and measurement errors); digestive factors (irregular gastric emptying or splanchnic uptake of enteral nutrition and tracers resulting in varying dilution in the plasma amino acid pool); disturbed metabolism; critical illness or unstable clinical condition (changes in fluid balance, necessity of inotropic support and other clinical interventions); and probably others.
In the individual plots, some periods showed more variability in enrichments than others. Priming doses in patient 2, 4 and 8 were probably too low for IV tracers; in patient 6 Leu1 was probably overprimed. High variability in a similar pattern for IV and enteral tracer enrichments as well as plasma amino acid concentrations is most likely a result of sampling or measurement issues (patient 1 first 6 hours, patient 7 last 5 hours). Periods with decreased enteral tracer enrichment and plasma amino acid concentrations with concomitantly increased IV tracer enrichments (patient 2 last 8 hours) possibly represents irregular gastric emptying or irregular splanchnic uptake of enteral nutrition and enteral tracers, resulting in decreased dilution of IV tracers in the amino acid pool. We found the highest variability in enteral tracer enrichments, especially when tracers were delivered intragastrically as compared to beyond the stomach. Crenn et al. (23) considered intragastric infusion of enteral tracers unreliable, because of CV’s of 43% versus 5% with administration via duodenal tube. Enteral tracers are needed in fed patients for assessment of splanchnic extraction, to make accurate calculations of protein metabolism. We recommend the use of postpyloric tubes to diminish variability. We postulate that the high variability in measurements are inevitable in critically ill children and we recommend that in future stable isotope tracer studies high variability should be kept in mind when interpreting study results, especially with tracer protocols of short duration.
No apparent circadian rhythm in protein and arginine metabolism was seen. Patients showed different individual patterns. In healthy volunteers, circadian rhythms in protein metabolism were found in healthy volunteers, with fasting overnight and daytime bolus meals, with differences in amino acid appearances of 40–60% between periods (11–13). The effect of 24-hour continuous feeding has not been tested in healthy controls and might blunt the circadian rhythm by itself. Other factors that normally contribute to a day-night rhythm such as daylight exposure and sleep-wake cycle are kept artificially constant in the ICU. Also, interventions might be applied continuously, e.g. ventilation and some medications. Our findings are in agreement with our previous data, which showed no circadian rhythm in 24-hour energy expenditure in critically ill children (24). A study in critically ill adults showed great disturbance of the normal 24-hour profiles of blood melatonin, cortisol, heart rate, body temperature and spontaneous motor activity (25). It is not known whether these changes are adaptive mechanisms, pathologic or a result of logistics in critical care (26). Circadian rhythms are regulated by peripheral and central clock genes (27). Disruption of clock genes and circadian de-synchrony have been associated with metabolic pathologies, increased risk for morbidities, (obesity, diabetes) (27), and may influence sepsis (28). The absence or disruption of circadian rhythms in critically ill patients is possibly unfavorable and a potential therapeutic target (28). More research into this subject is needed.
Protein balance was calculated from phenylalanine kinetics. Some patients had a relatively stable pattern over 24-hour, others had more varying patterns. Three infants were in an anabolic state as shown by positive protein balances, four patients had a protein balance close to zero and in one patient the protein balance was negative, throughout the 24-hour period, although close to zero. Since protein anabolism is needed for wound healing, recovery and growth, it is an important aim during ICU care in infants and children. All patients were fed adequately and in a relatively stable condition, therefore neutral or positive protein balances are expected. These findings are also in line with our previous randomized trial in fed critically ill infants who had either a protein balance close to zero with standard nutrition and a positive protein balance with protein-energy enriched nutrition at day 5 after admission (19).
Arginine, citrulline kinetics and NO synthesis in the current eight critically ill children, were in the range of infants fed with standard nutrition in our previous study (29), which is to date the only study on arginine kinetics in fed critically ill children. Plasma amino acid concentrations of arginine and citrulline were however higher in that population than in the current one. Sepsis and critical illness are thought to be arginine deficient states, with plasma arginine concentrations as low as 40 µmol/L (4). In the current study, arginine concentrations were above that range. Also, in children with congenital heart defects, decreased arginine availability and NO production may be associated with a higher risk of pulmonary hypertension. In a study of peri-operative citrulline supplementation in children undergoing cardiac surgery for congenital heart defects, plasma arginine and citrulline concentrations were lower than in the current study (30).
There are some limitations to this study. The character of the study was observational; we did not control the range of protein and energy intake. The sample size was small and consisted of a heterogeneous group of critically ill children. As few patients qualified as potential participant due to the necessity of lines and tubes in a relatively stable condition, it was hard to create a more homogenous group. For obvious ethical reasons, we were not able to include healthy children as control subjects.
In conclusion, 24-hour protein and arginine metabolism showed a high intra-individual variability in continuously fed critically ill children, possibly related to illness, intensive care interventions, the mode of delivery of enteral nutrition and technical issues. A circadian rhythm seemed not to be present. When performing stable isotope tracer studies in critically ill children, the high variability should be kept in mind when interpreting results. We recommend the use of postpyloric tubes for enteral tracer and nutrition delivery to minimize nutrition mode-related variability.
Supplementary Material
Figure 1. Study design.
Study design of 24-hour stable isotope tracer protocol, as used in 8 critically ill children. Black triangles indicate blood samples, the white arrow indicates administration of tracer prime.
Acknowledgments
We are grateful to the children who participated in the study and their parents or representatives. Also, our gratitude goes out to the medical and nursing staff of the PICU and CVICU of Arkansas Children’s Hospital, Little Rock, AR, USA for their support in the inclusion of patients and help in conducting the 24-hour studies. In addition we thank Wim Hop, PhD, for consultation in statistical analysis.
Funding sources
This study was financially supported by the University of Arkansas for Medical Sciences College of Medicine Children’s University Medical Group Fund Grant Program, Little Rock, Arkansas, USA, and Trustfonds Erasmus University Rotterdam, the Netherlands, and Award Number R01GM084447 from the National Institute Of General Medical and by Award Number S10RR027047 from the National Center For Research Resources to Nicolaas Deutz. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute Of General Medical Sciences or National Center For Research Resources or the National Institutes of Health.
List of abbreviations
- AA
amino acid
- Arg2
L-[guanido-15N2]-arginine
- Cit5
L-[5-13C-3,3,4,4-2H4]-citrulline
- CV
Coefficient of variation
- CVICU
Cardiovascular intensive care unit
- EN
Enteral
- IV
Intravenous
- Leu1
L-[1-13C]leucine
- Leu3
L-[5,5,5-2H3]Leucine
- Phe1
L-[15N]-phenylalanine
- Phe5
L-[ring-2H5]-phenylalanine
- PICU
Pediatric intensive care unit
- Ra
Rate of appearance
- TTR
Tracer-to-tracee ratio
- Tyr2
L-[3,3-2H2]Tyrosine
- WbPB
Whole body protein breakdown
- WbPBal
Whole body protein balance
- WbPS
Whole body protein synthesis
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Clinical trial register: NCT01511354 on clinicaltrials.gov
Statement of authorship
Author’s contributions were as follows: CTIdB designed research, conducted research, analyzed data, wrote paper, had primary responsibility for final content. XCGC conducted research and wrote paper. DAvW designed research and wrote paper. SS and KFMJ wrote paper. NEPD designed research, analyzed data and wrote paper. MPKJE designed research, conducted research, wrote paper.
Conflict of interest Statement
All authors declare no conflict of interest.
References
- 1.Pollack MM, Ruttimann UE, Wiley JS. Nutritional depletions in critically ill children: associations with physiologic instability and increased quantity of care. JPEN J Parenter Enteral Nutr. 1985;9:309–313. doi: 10.1177/0148607185009003309. [DOI] [PubMed] [Google Scholar]
- 2.Mehta NM, Compher C. A.S.P.E.N. Clinical Guidelines: nutrition support of the critically ill child. JPEN J Parenter Enteral Nutr. 2009;33:260–276. doi: 10.1177/0148607109333114. [DOI] [PubMed] [Google Scholar]
- 3.Koletzko B, Goulet O, Hunt J, Krohn K, Shamir R. Parenteral Nutrition Guidelines Working G, European Society for Clinical N, Metabolism, European Society of Paediatric Gastroenterology H, Nutrition, et al. 1. Guidelines on Paediatric Parenteral Nutrition of the European Society of Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) and the European Society for Clinical Nutrition and Metabolism (ESPEN), Supported by the European Society of Paediatric Research (ESPR) J Pediatr Gastroenterol Nutr. 2005;41(Suppl 2):S1–S87. doi: 10.1097/01.mpg.0000181841.07090.f4. [DOI] [PubMed] [Google Scholar]
- 4.de Betue CT, Deutz NE. Changes in arginine metabolism during sepsis and critical illness in children. Nestle Nutr Inst Workshop Ser. 2013;77:17–28. doi: 10.1159/000351370. [DOI] [PubMed] [Google Scholar]
- 5.Protein and amino acid requirements in human nutrition: report of a joint FAO/WHO/UNU expert consultation. Geneva, Switzerland: WHO; 2007. [Google Scholar]
- 6.Reynolds RM, Bass KD, Thureen PJ. Achieving positive protein balance in the immediate postoperative period in neonates undergoing abdominal surgery. J Pediatr. 2008;152:63–67. doi: 10.1016/j.jpeds.2007.05.042. [DOI] [PubMed] [Google Scholar]
- 7.Verbruggen SC, Coss-Bu J, Wu M, Schierbeek H, Joosten KF, Dhar A, van Goudoever JB, Castillo L. Current recommended parenteral protein intakes do not support protein synthesis in critically ill septic, insulin-resistant adolescents with tight glucose control. Crit Care Med. 2011;39:2518–2525. doi: 10.1097/CCM.0b013e3182257410. [DOI] [PubMed] [Google Scholar]
- 8.van den Akker CH, te Braake FW, Wattimena DJ, Voortman G, Schierbeek H, Vermes A, van Goudoever JB. Effects of early amino acid administration on leucine and glucose kinetics in premature infants. Pediatr Res. 2006;59:732–735. doi: 10.1203/01.pdr.0000214990.86879.26. [DOI] [PubMed] [Google Scholar]
- 9.Poindexter BB, Karn CA, Leitch CA, Liechty EA, Denne SC. Amino acids do not suppress proteolysis in premature neonates. Am J Physiol Endocrinol Metab. 2001;281:E472–E478. doi: 10.1152/ajpendo.2001.281.3.E472. [DOI] [PubMed] [Google Scholar]
- 10.Argaman Z, Young VR, Noviski N, Castillo-Rosas L, Lu XM, Zurakowski D, Cooper M, Davison C, Tharakan JF, Ajami A, et al. Arginine and nitric oxide metabolism in critically ill septic pediatric patients. Crit Care Med. 2003;31:591–597. doi: 10.1097/01.CCM.0000050291.37714.74. [DOI] [PubMed] [Google Scholar]
- 11.el-Khoury AE, Fukagawa NK, Sanchez M, Tsay RH, Gleason RE, Chapman TE, Young VR. Validation of the tracer-balance concept with reference to leucine: 24-h intravenous tracer studies with L-[1-13C]leucine and [15N-15N]urea. Am J Clin Nutr. 1994;59:1000–1011. doi: 10.1093/ajcn/59.5.1000. [DOI] [PubMed] [Google Scholar]
- 12.el-Khoury AE, Sanchez M, Fukagawa NK, Gleason RE, Young VR. Similar 24-h pattern and rate of carbon dioxide production, by indirect calorimetry vs. stable isotope dilution, in healthy adults under standardized metabolic conditions. J Nutr. 1994;124:1615–1627. doi: 10.1093/jn/124.9.1615. [DOI] [PubMed] [Google Scholar]
- 13.Sanchez M, el-Khoury AE, Castillo L, Chapman TE, Young VR. Phenylalanine and tyrosine kinetics in young men throughout a continuous 24-h period, at a low phenylalanine intake. Am J Clin Nutr. 1995;61:555–570. doi: 10.1093/ajcn/61.3.555. [DOI] [PubMed] [Google Scholar]
- 14.Feigin RD, Haymond MW. Circadian periodicity of blood amino acids in the neonate. Pediatrics. 1970;45:782–791. [PubMed] [Google Scholar]
- 15.den Brinker M, Dumas B, Visser TJ, Hop WC, Hazelzet JA, Festen DA, Hokken-Koelega AC, Joosten KF. Thyroid function and outcome in children who survived meningococcal septic shock. Intensive Care Med. 2005;31:970–976. doi: 10.1007/s00134-005-2671-8. [DOI] [PubMed] [Google Scholar]
- 16.den Brinker M, Joosten KF, Liem O, de Jong FH, Hop WC, Hazelzet JA, van Dijk M, Hokken-Koelega AC. Adrenal insufficiency in meningococcal sepsis: bioavailable cortisol levels and impact of interleukin-6 levels and intubation with etomidate on adrenal function and mortality. J Clin Endocrinol Metab. 2005;90:5110–5117. doi: 10.1210/jc.2005-1107. [DOI] [PubMed] [Google Scholar]
- 17.Wolfe RR, Chinkes DL. Isotope tracers in metabolic research: principles and practice of kinetic analysis. 2nd. Hoboken, NJ: John Wiley & Sons; 2004. [Google Scholar]
- 18.Vogt JA, Chapman TE, Wagner DA, Young VR, Burke JF. Determination of the isotope enrichment of one or a mixture of two stable labelled tracers of the same compound using the complete isotopomer distribution of an ion fragment; theory and application to in vivo human tracer studies. Biol Mass Spectrom. 1993;22:600–612. doi: 10.1002/bms.1200221008. [DOI] [PubMed] [Google Scholar]
- 19.de Betue CT, van Waardenburg DA, Deutz NE, van Eijk HM, van Goudoever JB, Luiking YC, Zimmermann LJ, Joosten KF. Increased protein-energy intake promotes anabolism in critically ill infants with viral bronchiolitis: a double-blind randomised controlled trial. Arch Dis Child. 2011;96:817–822. doi: 10.1136/adc.2010.185637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Castillo L, Yu YM, Marchini JS, Chapman TE, Sanchez M, Young VR, Burke JF. Phenylalanine and tyrosine kinetics in critically ill children with sepsis. Pediatr Res. 1994;35:580–588. [PubMed] [Google Scholar]
- 21.Castillo L, Beaumier L, Ajami AM, Young VR. Whole body nitric oxide synthesis in healthy men determined from [15N] arginine-to-[15N]citrulline labeling. Proc Natl Acad Sci U S A. 1996;93:11460–11465. doi: 10.1073/pnas.93.21.11460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Castillo L, Chapman TE, Sanchez M, Yu YM, Burke JF, Ajami AM, Vogt J, Young VR. Plasma arginine and citrulline kinetics in adults given adequate and arginine-free diets. Proc Natl Acad Sci U S A. 1993;90:7749–7753. doi: 10.1073/pnas.90.16.7749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crenn P, Thuillier F, Rakatoambinina B, Rongier M, Darmaun D, Messing B. Duodenal vs. gastric administration of labeled leucine for the study of splanchnic metabolism in humans. J Appl Physiol. 2000;89:573–580. doi: 10.1152/jappl.2000.89.2.573. [DOI] [PubMed] [Google Scholar]
- 24.Joosten KF, Verhoeven JJ, Hop WC, Hazelzet JA. Indirect calorimetry in mechanically ventilated infants and children: accuracy of total daily energy expenditure with 2 hour measurements. Clin Nutr. 1999;18:149–152. doi: 10.1016/s0261-5614(99)80004-1. [DOI] [PubMed] [Google Scholar]
- 25.Paul T, Lemmer B. Disturbance of circadian rhythms in analgosedated intensive care unit patients with and without craniocerebral injury. Chronobiol Int. 2007;24:45–61. doi: 10.1080/07420520601142569. [DOI] [PubMed] [Google Scholar]
- 26.Chan MC, Spieth PM, Quinn K, Parotto M, Zhang H, Slutsky AS. Circadian rhythms: from basic mechanisms to the intensive care unit. Crit Care Med. 2012;40:246–253. doi: 10.1097/CCM.0b013e31822f0abe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bass J, Takahashi JS. Circadian integration of metabolism and energetics. Science. 2010;330:1349–1354. doi: 10.1126/science.1195027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brainard J, Gobel M, Bartels K, Scott B, Koeppen M, Eckle T. Circadian rhythms in anesthesia and critical care medicine: potential importance of circadian disruptions. Semin Cardiothorac Vasc Anesth. 2015;19:49–60. doi: 10.1177/1089253214553066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Betue CT, Joosten KF, Deutz NE, Vreugdenhil AC, van Waardenburg DA. Arginine appearance and nitric oxide synthesis in critically ill infants can be increased with a protein-energy-enriched enteral formula. Am J Clin Nutr. 2013;98:907–916. doi: 10.3945/ajcn.112.042523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith HA, Canter JA, Christian KG, Drinkwater DC, Scholl FG, Christman BW, Rice GD, Barr FE, Summar ML. Nitric oxide precursors and congenital heart surgery: a randomized controlled trial of oral citrulline. J Thorac Cardiovasc Surg. 2006;132:58–65. doi: 10.1016/j.jtcvs.2006.02.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.