Abstract
Objectives
To assess and compare the sentinel lymph node (SLN) detection rate with indocyanine green (ICG) and near-infrared (NIR) fluorescence imaging versus blue dye using the robotic platform in patients with uterine cancer.
Methods
We identified all patients with uterine cancer undergoing SLN mapping using ICG or blue dye on the robotic platform from 1/2011–12/2013. Our institutional SLN algorithm and pathologic processing protocol were adhered to uniformly. We compared detection rates of SLNs stratified by dye used. Appropriate statistical tests were used.
Results
Four hundred seventy-two patients were identified. ICG was used in 312 patients (66%) and blue dye in 160 (34%). Successful mapping was achieved in 425 (90%) of 472 patients. Mapping was bilateral in 352 patients (75%) and unilateral in 73 (15%); 47 patients (10%) did not map. Successful mapping was achieved in 295 (95%) of 312 patients using ICG compared with 130 (81%) of 160 patients using blue dye (p<0.001). Mapping was bilateral in 266 (85%) of 312 patients in the ICG group compared with 86 (54%) of 160 in the blue dye group (p<0.001). Additional lymph node dissection beyond removal of the SLNs was performed in 122 (39%) of the patients mapped with ICG compared with 98 (61%) of those mapped with blue dye (p<0.001).
Conclusions
The SLN detection rate was superior when mapping with ICG rather than blue dye. Importantly, bilateral mapping was significantly improved, resulting in a lower rate of additional lymphadenectomy.
Keywords: uterine cancer, sentinel lymph nodes, indocyanine green, near-infrared fluorescence imaging, robotic surgery
Introduction
The use of sentinel lymph node (SLN) mapping in combination with pathologic ultrastaging has gained increasing acceptance in the nodal assessment of patients with endometrial carcinoma. The techniques involved in the SLN approach with regard to injection site and preferred dye are still debatable and evolving. A successful SLN mapping is considered one in which SLN(s) are identified in both hemipelvises (i.e., “bilateral mapping”). SLN detection rates of approximately 50% have been reported (1–3). Failed mapping will often lead to more extensive lymphadenectomy. Therefore, maximizing detection rates is important. A bilateral SLN mapping rate of 69% was achieved when using both radioisotope and colorimetric tracers in the Senti-Endo trial (1). More recently, How et al reported a bilateral mapping rate of 65% with indocyanine green (ICG) and technetium (4). The use of radioisotope (e.g., technetium) can be painful for the patient, is associated with logistical issues, and adds cost. The added value of technetium is debatable, and we have abandoned its use in favor of intraoperative dye-based–only SLN mapping techniques. We initially utilized blue dyes (isosulfan blue or methylene blue) and subsequently moved on to fluorescent dyes. The primary objective of this study was to assess and compare the SLN detection rate for uterine cancer using ICG and near-infrared (NIR) fluorescent imaging versus blue dye with the robotic platform at a single institution.
Methods
Institutional review board approval was obtained for this retrospective study. We identified all patients with complex atypical hyperplasia and uterine cancer undergoing SLN mapping with either ICG or blue dye on the robotic platform from 1/2011–12/2013. All dyes were injected similarly, into the cervix only. The concentration used for ICG was 1.25 mg/mL. In other words, a 25 mg vial with ICG powder was diluted into 20 cc of aqueous sterile water. Four cc of this ICG solution was then injected into the cervix; 1 cc deep into the stroma and 1 cc submucosally at the 3- and 9-o’clock positions once the patient was prepped and draped. Isosulfan blue was injected using the same method. The cervix was injected prior to the insertion of any uterine manipulator. Our previously published institutional SLN algorithm (5) and pathologic processing protocol (6) were adhered to uniformly. The institutional SLN algorithm at Memorial Sloan Kettering Cancer Center (MSK) is as follows: Upon entry into the abdominal cavity, peritoneal and serosal evaluation and washings are performed. This is followed by a retroperitoneal evaluation and excision of all mapped SLNs. Any suspicious nodes must be removed regardless of mapping. If there is no mapping of a hemi-pelvis, an ipsilateral lymph node dissection is performed. A para-aortic lymph node dissection is performed at the attending’s discretion. As part of the SLN algorithm, excised lymph nodes are submitted for ultrastaging. An initial examination is performed using hematoxylin and eosin (H&E) staining; if the H&E assessment is negative and myometrial invasive carcinoma is found, then serial sectioning and ultrastaging are performed.
Demographic, surgical, and pathologic data were abstracted. We compared the overall detection rates of SLN, bilateral detection rates of SLN, SLN time, and operative (OR) time stratified by dye used. We also looked at additional lymph node dissection performed and anatomic distribution of detected SLNs. Median age, estimated blood loss (EBL), and SLN counts were noted. We also investigated the accuracy of lymphatic tissue identified in reported SLNs, and whether the number of SLNs resected affected SLN time. Characteristics influencing absence of mapping for patients injected with ICG were also investigated. The Fisher exact test or Wilcoxon Rank Sum test was used to test the two-way associations depending on whether a continuous variable was included or not. Kruskal-Wallis test was employed to investigate the relationship between EBL and Body Mass Index (BMI) category. The lymph node dissection, SLN mapping, and histology three-way association was tested using the Cochran Mantel Haenszel test. The relationship between SLN time and SLN number was evaluated using Pearson correlation coefficient. SAS 9.2 was used to perform all statistical tests.
Results
Four hundred seventy-two patients were identified. ICG was used in 312 patients (66%) and blue dye in 160 (34%). The ICG and blue dye groups were similar in clinical demographics and intraoperative characteristics (Table 1). Successful mapping was achieved in 425 (90%) of all 472 patients. Mapping was bilateral in 352 (75%) and unilateral in 73 (15%); 47 patients (10%) did not map. Successful mapping was achieved in 295 (95%) of 312 patients using ICG compared with 130 (81%) of 160 patients using blue dye (p<0.001). Bilateral mapping occurred in 266 (85%) of 312 patients in the ICG group compared with 86 (54%) of 160 patients in the blue dye group (p<0.001). Additional lymph node dissection beyond the removal of the SLNs was performed in 122 patients (39%) mapped with ICG compared with 98 (61%) mapped with blue dye (p<0.001) (Table 2). Patients with unsuccessful mapping in the ICG sub-group had a significantly higher BMI than those who mapped (median, 36.6 vs 30.3; p=0.015); they also had a significantly greater EBL (median, 100 cc vs 50 cc; p≤0.001). When examining the relationship between BMI and EBL in these patients, they were not independent factors for unsuccessful mapping (p=0.014).
Table 1.
Clinical demographics and intraoperative characteristics
| All N = 472 (%) |
ICG N = 312 (%) |
Blue dye N =160 (%) |
P | |
|---|---|---|---|---|
|
Age at surgery, years Median (range) |
62 (30–90) | 62 (30–90) | 62 (34–87) | 1.0* |
|
BMI, kg/m2 Median (range) |
30.4 (16.3–65.3) | 30.6 (17.7–65.3) | 30 (16.3–52.3) | 0.5* |
|
EBL, cc Median (range) |
50 (0–1000) | 50 (5–1000) | 50 (0–500) | 0.1* |
| Histology | ||||
| CAH/Endometrioid | 387 (82%) | 257 (82%) | 130 (81%) | 0.8 |
| Other histology | 85 (18%) | 55 (18%) | 30 (19%) | |
| FIGO 2009 stage | ||||
| I/II/IV/CAH | 416 (88%) | 269 (86%) | 147 (92%) | 0.097 |
| IIIC | 56 (12%) | 43 (14%) | 13 (8%) | |
|
Operative time, minutes Median (range) |
242 (141–498) | 240 (145–444) | 248 (141–498) | 0.045* |
|
SLN operative time, minutes Median (range) |
26 (3–98) | 26 (3–98) | 26 (6–90) | 1.0* |
ICG, indocyanine green; BMI, body mass index; EBL, estimated blood loss; CAH, complex atypical hyperplasia; FIGO, International Federation of Gynecology and Obstetrics; SLN, sentinel lymph node
obtained using the Wilcoxon-rank sum test, others by the Fisher exact test
Table 2.
Mapping by dye used
| All N = 472 (%) |
ICG N = 312 (%) |
Blue dye N =160 (%) |
P | |
|---|---|---|---|---|
| Overall mapping | 425 (90%) | 295 (95%) | 130 (81%) | <0.001 |
| Bilateral mapping | 352 (75%) | 266 (85%) | 86 (54%) | <0.001 |
| Additional lymph node sampling | 220 (47%) | 122 (39%) | 98 (61%) | <0.001 |
ICG, indocyanine green
One hundred ninety-one patients had both SLNs and additional lymph nodes submitted for pathology review; among these, 97% of SLNs (185/191) were identified as lymphatic tissue compared with 94% (179/191) of additional LNs (p=0.16). For the 413 patients in whom an SLN was detected, the median SLN count was 3 (range, 1–15). For the 59 patients in whom an SLN was not detected, the median number of lymph nodes other than SLNs was 3 (range, 0–46). In regards to anatomic distribution of SLNs, 490 (36%) of 1368 SLNs were located in the hypogastric basin, 453 (33%) in the external iliac basin, 313 (23%) in the obturator basin, 83 (6%) in the common iliac basin, 25 (2%) in the aortic basin, and 4 (0.3%) in the presacral basin. Twenty-three (92%) of the 25 aortic SLNs were detected using ICG.
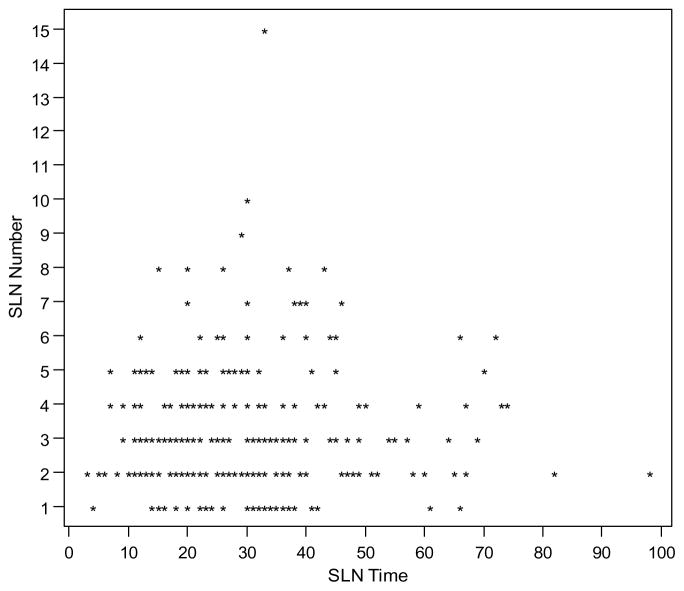
The median OR time was 242 minutes (range, 141–498) for the entire cohort, 240 (range, 145–444) for the ICG group, and 248 (range, 141–498) for the blue dye group (p=0.045). Of the 425 patients who had successful intraoperative mapping, OR time spent specifically on SLN dissection was available for 279 patients (66%). Median SLN times were 26 minutes for both the ICG and blue dye groups (p=1). A scatterplot for SLN time versus SLN number is depicted in Figure 1. The Pearson correlation coefficient for the SLN time and SLN number was 0.07, which means there was no relationship between the two variables.
Figure 1.
Scatter plot for sentinel lymph node (SLN) time versus SLN number
The Pearson correlation coefficient for the SLN time and SLN number for the 264 patients is 0.0702.
When stratifying additional lymph node dissection by SLN mapping and histology for patients with bilateral mapping, 70 (27%) of 260 patients with complex atypical hyperplasia or grade 1/2 disease of endometrioid histology (CAH/EA) underwent additional lymph node dissection compared with 66 (72%) of 92 patients with grade 3 disease of endometrioid histology or high-risk histology (EA/HRH) (three-way test p<0.001). A subset analysis showed that for the CAH/EA group, there was a significant association between additional lymph node dissection and mapping (p<0.001), whereas for the EA/HRH group, there was not a significant association between additional lymph node dissection and mapping (p=0.39) (Table 3).
Table 3.
Additional lymph node dissection by sentinel lymph node mapping and histology
| Histology | Additional LND when no mapping | Additional LND when unilateral mapping | Additional LND when bilateral mapping | P* |
|---|---|---|---|---|
| CAH/Endometrioid G1 and G2 | 18/34 (53%) | 37/52 (71%) | 70/260 (27%) | <0.001 |
| Endometrioid G3/high-risk histologies | 11/13 (85%) | 18/21 (86%) | 66/92 (72%) | 0.39 |
p value obtained using the Fisher exact test
LND, lymph node dissection; CAH, complex atypical hyperplasia; G, grade
Discussion
With the emerging acceptance of an SLN approach to the surgical staging of uterine cancer, it is important to ensure that we use techniques that are reliable, feasible, and acceptable to patients. Previous publications have reported bilateral detection rates of approximately 50% with blue dye (1– 3), with the remaining 50% requiring additional lymph node dissection in either one or both hemi-pelvises. We previously reported on our experience of SLN detection with ICG alone compared with ICG and blue dye in 227 cases with endometrial and cervical malignancies (7). In the current study, we sought to compare the detection rates using blue dye alone or ICG alone. We limited our patient selection to only include patients who had undergone surgical staging of uterine cancer with the robotic platform.
Our institution introduced the SLN approach for endometrial and cervical cancer patients in 2003, and has since developed an algorithm decreasing the false-negative rate from 15% to 2% (5). Our attending surgeons are experienced in this approach, and had all performed more than the estimated 30-case learning curve (8) prior to the inclusion period. The difference observed between dye used is not likely attributed to an insufficient experience during the earlier period of this study when blue dye alone was used.
In this study, we observed a significant difference in both overall and bilateral detection rates in favor of ICG. Successful bilateral mapping occurred in 85% of the patients in the ICG group compared with 54% in the blue dye group. When investigating the need for additional lymph node removal, we found that additional lymph node dissection beyond removal of the SLNs occurred in 39% of ICG cases versus 61% of cases with blue dye. The purpose of the SLN algorithm is to reliably stage endometrial cancer while sparing patients the morbidity associated with complete lymphadenectomy (9). Incorporating a cervical injection of ICG in combination with NIR fluorescent imaging significantly contributes to this goal. How et al (4) reported an overall detection rate of 92% and bilateral detection rate of 76% when using blue dye, ICG, and 99mTc (4). ICG and 99mTc-SC had similar SLN detection rates in both overall (87% vs. 88%) and bilateral (71% vs. 65%) detection, respectively. The use of radioisotope presents a logistical challenge, added pain and discomfort for the patient, and an additional cost. In our study, we found that ICG alone provides a bilateral detection rate similar to that of the overall ICG and 99mTc-SC detection rate reported by How et al (4). Based on our findings, we cannot justify the added cost and patient discomfort associated with the use of radioisotope, and we suggest using ICG alone when this technique is available.
When investigating clinical factors that could have influenced the absence of mapping in patients in whom ICG was used, we found that BMI and EBL were significantly higher in those patients with unsuccessful mapping. On further investigation, there was a significant correlation, where patients with increased BMI had a greater EBL. We recommend gentle dissection to minimize rupture of lymphatic channels and bleeding, to avoid ICG contamination, which in turn can compromise the identification of the SLNs.
There was no difference in the median OR times or SLN times between ICG and blue dye in this study. There was no correlation between SLN time and SLN number (Figure 1). This may suggest that it is the time spent opening the anatomic spaces and searching for the lymphatic channels to guide the surgeon to the SLNs that takes time. The mere extraction of the individual SLNs, once identified, is not time consuming. This is in line with our previously reported findings that there is a correlation between SLN time and increasing body mass index (BMI) (10). One of the potential benefits of transitioning from complete lymphadenectomy to an SLN approach is reducing OR time. It is, however, of utmost importance to do a systematic search for lymphatic channels and nodes to ensure that the correct nodes are identified and extracted. This part of the procedure should not be rushed.
One of the concerns raised with regard to implementing an SLN approach in favor of complete lymphadenectomy is the omission of para-aortic lymph node removal and assessment, thus possibly not detecting metastatic disease in this region. In this study, 25 (2%) of 1368 SLNs were located in the aortic basin, of which 23 (92%) were detected using ICG. Although nodal metastasis to the para-aortic nodes is rare when there is no metastasis to the pelvis (11, 12), these findings further support the use of ICG and NIR fluorescence imaging over blue dye.
When investigating the accuracy of lymphatic tissue identified by pathology review, the submitted SLNs were identified as such more frequently than were the additional lymph nodes. This did not reach statistical significance; however, it further highlights the accuracy of the SLN approach and the benefit of increased successful mapping with ICG.
A subset analysis showed no significant association between additional lymph node dissection and mapping for patients with high-risk histology. From this we can conclude that our surgeons performed additional lymph node sampling in these high-risk patients irrespective of mapping. This is reasonable and reflects the lack of prospective data in this area and is also in line with the current National Comprehensive Cancer Network (NCCN) guidelines (13). Retrospective data comparing an SLN algorithm to lymphadenectomy using Mayo criteria in intermediate-and high-risk patients found that in intermediate-risk patients, defined as those with disease of endometrioid histology of any grade and >50% myometrial invasion, there was a similar detection rate of stage IIIC disease between the SLN and LND cohorts, however with more stage IIIC1 disease detected in the SLN cohort and more stage IIIC2 disease noted in the LND cohort (14). For high-risk patients, defined as those with disease of serous and clear cell histologies, there were similar detection rates of stage IIIC1 and IIIC2 disease with both approaches. Overall, stage IIIC disease was diagnosed at similar rates in each cohort, with fewer lymph nodes removed in the SLN group. Once stage IIIC is detected, these patients will receive adjuvant therapy based on local guidelines. It is therefore uncertain if the removal of more lymph nodes will impact survival. Although they have been critiqued, the results from two randomized trials on lymphadenectomy in endometrial cancer indicate that the omission of a complete lymphadenectomy does not affect survival (15, 16). Further studies are needed with regard to oncologic outcomes when applying an SLN algorithm in patients with high-risk histology to guide strategy in the surgical staging of this patient population.
In conclusion, SLN detection rate is superior when using ICG rather than blue dye. Importantly, bilateral mapping is significantly improved using ICG and NIR fluorescence imaging, resulting in a lower rate of additional lymphadenectomy, and the detection of para-aortic SLNs is significantly increased when using ICG. These data favor the use of ICG cervical injection over blue dye in the surgical staging of uterine cancer.
Acknowledgments
Financial Support
This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748.
References
- 1.Ballester M, Dubernard G, Lécuru F, et al. Detection rate and diagnostic accuracy of sentinel-node biopsy in early stage endometrial cancer: a prospective multicenter study (SENTI-ENDO) Lancet Oncol. 2011;12:469–476. doi: 10.1016/S1470-2045(11)70070-5. [DOI] [PubMed] [Google Scholar]
- 2.Abu-Rustum NR. Update on sentinel node mapping in uterine cancer: 10-year experience at Memorial Sloan-Kettering Cancer Center. J Obstet Gynaecol Res. 2014;40:327–334. doi: 10.1111/jog.12227. [DOI] [PubMed] [Google Scholar]
- 3.Sinno AK, Fader AN, Long Roche K, et al. A comparison of colorimetric versus fluorometric sentinel lymph node mapping during robotic surgery for endometrial cancer. Gynecol Oncol. 2014;134:281–286. doi: 10.1016/j.ygyno.2014.05.022. [DOI] [PubMed] [Google Scholar]
- 4.How J, Gotlieb WH, Press JZ, et al. Comparing indocyanine green, technetium, and blue dye for sentinel lymph node mapping in endometrial cancer. Gynecol Oncol. 2015;137:436–442. doi: 10.1016/j.ygyno.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 5.Barlin JN, Khoury-Collado F, Kim CH, et al. The importance of applying a sentinel lymph node mapping algorithm in endometrial cancer staging: beyond removal of blue nodes. Gynecol Oncol. 2012;125:531–535. doi: 10.1016/j.ygyno.2012.02.021. [DOI] [PubMed] [Google Scholar]
- 6.Kim CH, Soslow RA, Park KJ, et al. Pathologic ultrastaging improves micrometastasis detection in sentinel lymph nodes during endometrial cancer staging. Int J Gynecol Cancer. 2013;23:964–970. doi: 10.1097/IGC.0b013e3182954da8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jewell EL, Huang JJ, Abu-Rustum NR, et al. Detection of sentinel lymph nodes in minimally invasive surgery using indocyanine green and near-infrared fluorescence imaging for uterine and cervical malignancies. Gynecol Oncol. 2014;133:274–277. doi: 10.1016/j.ygyno.2014.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khoury-Collado F, Glaser GE, Zivanovic O, et al. Improving sentinel lymph node detection rates in endometrial cancer: how many cases are needed? Gynecol Oncol. 2009;115:453–455. doi: 10.1016/j.ygyno.2009.08.026. [DOI] [PubMed] [Google Scholar]
- 9.Dowdy SC, Borah BJ, Bakkum-Gamez JN, et al. Prospective assessment of survival, morbidity, and cost associated with lymphadenectomy in low-risk endometrial cancer. Gynecol Oncol. 2012;127:5–10. doi: 10.1016/j.ygyno.2012.06.035. [DOI] [PubMed] [Google Scholar]
- 10.Eriksson AG, Montovano M, Beavis A, et al. Impact of obesity on sentinel lymph node mapping in patients with newly diagnosed uterine cancer undergoing robotic surgery. Ann Surg Oncol. 2016;23:2522–2528. doi: 10.1245/s10434-016-5134-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abu-Rustum NR, Gomez JD, Alektiar KM, et al. The incidence of isolated paraaortic nodal metastasis in surgically staged endometrial cancer patients with negative pelvic lymph nodes. Gynecol Oncol. 2009;115:236–238. doi: 10.1016/j.ygyno.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 12.Fotopoulou C, El-Balat A, du Bois A, et al. Systematic pelvic and paraaortic lymphadenectomy in early high-risk or advanced endometrial cancer. Arch Gynecol Obstet. 2015;292:1321–1327. doi: 10.1007/s00404-015-3746-6. [DOI] [PubMed] [Google Scholar]
- 13.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. [Accessed September 22, 2016];Uterine Neoplasms Version 2. 2016 https://www.nccn.org/professionals/physician_gls/pdf/uterine.pdf.
- 14.Ducie JA, Eriksson AGZ, Ali N, et al. Multicenter study assessing the detection of stage IIIC endometrial cancer in intermediate- and high-risk tumors between a contemporary sentinel node mapping versus historical comprehensive lymphadenectomy approach. Gynecol Oncol. 2015;137(suppl 1):7. [Google Scholar]
- 15.ASTEC study groupl. Kitchener H, Swart AM, Qian Q, et al. Efficacy of systematic pelvic lymphadenectomy in endometrial cancer (MRC ASTEC trial): a randomised study. Lancet. 2009;373:125–136. doi: 10.1016/S0140-6736(08)61766-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Benedetti Panici P, Basile S, Maneschi F, et al. Systematic pelvic lymphadenectomy vs. no lymphadenectomy in early-stage endometrial carcinoma: randomized clinical trial. J Natl Cancer Inst. 2008;100:1707–1716. doi: 10.1093/jnci/djn397. [DOI] [PubMed] [Google Scholar]