Abstract
Background and Purpose
Acute ischemic stroke (AIS) patients may have raised serum troponin (cTn) levels on admission, although it is unclear what prognostic implications this has, and whether elevated levels are associated with cardiac causes of stroke or structural cardiac disease as seen on echocardiogram. We investigated the positivity of cTn and echocardiogram testing within a large biracial AIS population, and any association with post stroke mortality.
Methods
Within a catchment area of 1.3 million we screened ED admissions from 2010 using ICD-9 discharge codes 430–436 and ascertained all physician-confirmed AIS cases by retrospective chart review. Hypertroponinemia was defined as elevation in cTn above the standard 99th percentile. Multiple logistic regression was performed, controlling for stroke severity, history of cardiac disease and all other stroke risk factors.
Results
Of 1999 AIS cases, 1706 (85.3%) had a cTn drawn and 1590 (79.5%) had echocardiograms. Hypertroponinemia occurred in 353/1706 (20.7%). 160/1590 (10.1%) had echocardiogram findings of interest. Among 1377 who had both tests performed, hypertroponinemia was independently associated with echocardiogram findings (OR 2.9 95% CI 2–4.2). When concurrent myocardial infarctions (MI, 3.5%) were excluded, hypertroponinemia was also associated with increased mortality at 1 year (35%, OR 3.45 95% CI 2.1–5.6) and 3 years (60%, OR 2.91 95% CI 2.06–4.11).
Conclusions
Hypertroponinemia in the context of AIS without concurrent MI was associated with structural cardiac disease and long-term mortality. Prospective studies are needed to determine if further cardiac evaluation might improve the long-term mortality rates seen in this group.
Background
Acute myocardial infarction (AMI) and acute ischemic stroke (AIS) are frequent indications for acute hospitalization in the US.1 Shared risk factors mean they can present contemporaneously; cardioembolic AIS accounts for 20% of all AIS, and is a recognized complication of cardiac disease.2 Similarly, AMI is a common complication of AIS; approximately 4% of AIS patients will die from myocardial infarction in the 3 months following stroke and 19% will suffer a serious cardiac event.3 Thereafter, stroke patients live with an annual myocardial infarction risk of 2.2%.4
In recognition of this strong association the American Heart Association has published guidelines that recommend emergent non-invasive cardiac testing of all patients admitted with AIS, including cardiac enzyme levels.5 Despite this, data is scarce defining the prevalence of this testing in a community population of AIS patients. It is possible that compliance with AHA recommendations is less than perfect because routine cardiac testing in this population can lead to results that are nonspecific and of questionable clinical utility. Spurred by demands for greater precision by the AHA,6 the improvement in performance of cTn assays has led to greater diagnostic sensitivity and earlier detection of AMI, but this comes at a cost of decreased specificity.7 AIS is now an established cause of non-AMI hypertroponiemia, with rates previously reported varying as widely as 20% to 60%8–10. We sought to reassess rates of hypertroponinemia in AIS using a range of contemporary assays in a biracial population representative of the US.
In contrast there are no formal recommendations from the AHA on the use of echocardiography in AIS.5 Both transthoracic (TTE) and transesophageal (TEE) echocardiograms have been used to determine whether a cardiac source of emboli (CSE) exists. CSEs represent a potential stroke mechanism and possible indication for escalation from antiplatelet therapy to anticoagulation, so their detection is crucial for long term management decisions. However, the reported positivity of echocardiography in AIS varies widely due to how CSEs and other “management altering” findings are defined. TTE has a reported positivity from 2% to 37%,11,12 for TEE the diagnostic yield is even more unclear as its higher purported sensitivity13 is often an artifact of findings (such as patent foramen ovale) with unclear embolic potential. Given the rapidly changing landscape14–16 regarding which cardiac findings indicate escalation in treatment in order to reduce future stroke risk, revisiting the prevalence of “management altering” echocardiogram findings in AIS seems relevant.
However as AIS cohorts suffer a disproportionately high cardiac event rate it is possible to defend routine echocardiography in AIS on the basis of screening for occult ischemic cardiomyopathy. Amerenco et al17 found 26% of 315 AIS patients with no known cardiac history had silent obstructive coronary artery disease on angiography. Hypertroponinemia in AIS, as evidence of underlying demand ischemia, has been linked to cardiac dyskinesias detectable on echocardiography.18 We sought to define the therapeutic yield of cTn and echocardiogram testing in our AIS population within this context, and the association these findings had on long-term patient mortality.
Methods
The Greater Cincinnati/Northern Kentucky (GCNK) region includes 2 southwestern Ohio counties and 3 contiguous Kentucky counties bordering the Ohio River. Its biracial population of 1.3 million is largely representative of the United States in terms of median age, proportion of black subjects, and economic status19. There were 15 hospitals active in this area in 2010. While residents of surrounding counties may seek care at these hospitals, only residents of the 5 study counties were eligible for this study. It has been shown that residents of these 5 counties who have a stroke seek care at these hospitals rather than going to more distant hospitals19.
This study was approved by the Institutional Review Board of all participating hospitals. Details of the design of the Greater Cincinnati/Northern Kentucky Stroke Study (GCNKSS) have been described previously19. Briefly, in the calendar year 2010 we ascertained all cases of stroke among the 1.3 million residents of the GCNK region by identifying inpatient discharge International Classification of Diseases-Ninth Revision codes 430–436 at the 15 area hospitals. We restricted the current analysis to adult patients (age 20 years and older) with AIS who presented to an emergency department. Research nurses reviewed the medical record of all suspected stroke cases and abstracted data onto standardized case report forms. Study physicians reviewed every abstract and all available neuroimaging to adjudicate the diagnosis of AIS. Transient ischemic attacks, defined as symptoms lasting less than 24 hours without radiologic evidence of infarction, were not included in this analysis.
Medical record review included a binary determination of the presence of hypertroponinemia (defined as any troponin level above the 99th percentile of a healthy reference population during initial workup). Cardiac troponin assays used were a heterogeneous mix of T and I subunits as reflecting the expected variation in community practice. All were contemporary assays targeting a co-efficient of variation of 10% at the diagnostic cutoff as recommended by the AHA5. Values at the 99th percentile tended to range between 40 ng/L and 50 ng/L determined as per protocol in each local laboratory. In addition, the use of TTE and TEE in the diagnostic process along with their findings was recorded on patient abstracts. Prior to analysis, echocardiogram findings that could possibly impact future management decisions were identified. Also recorded on the patient abstracts was the diagnosis of AMI made by the treating physician during the index hospitalization.
Statistical analysis
Baseline clinical characteristics were summarized as mean (standard deviation) or median (25th, 75th percentiles) for continuous variables or as frequency (percentage) for categorical variables. Fisher’s Exact test was used to test whether the proportion of subtype of echocardiographic findings of interest differed by presence or absence of hypertroponinemia, adjusted using multiple logistic regression for all predefined covariates (supplementary table I.)
All-cause mortality rates were ascertained at discharge and at 1 and 3 year time points from medical record review, social security death index or the national/local obituary indices. Patients for whom a death record could not be found were presumed alive. Multiple logistic regression adjusted for age (modeled so that odds were estimated for each 10-year increase), race, sex, prior cardiac disease, history of chronic renal insufficiency, atrial fibrillation, dementia, hypertension, diabetes, current smoking (within the 3 months prior to stroke), clinical stroke severity (modeled so that the odds were estimated for each 5-point increase on the NIH stroke score) and baseline functional status (modified Rankin scale 0–1, vs ≥2). A backward elimination approach was used for model reduction, and variables either statistically significant or whose removal changed the beta coefficient by more than 10% when removed were retained in the model. Statistical significance was set at p<.05 a priori. SAS® version 9.3 (SAS Institute, Cary, NC) was used for analysis.
Results
During 2010, 1999 AIS cases among GCNK residents presented to an emergency department in the region, of whom 1706 (85.3%) had a cTn drawn and 1590 (79.5%) had an echocardiogram. 1377 (68.9%) had both tests performed (table 1). Mean age was 69 and median NIHSS was 3. 622 cases had an incomplete workup, i.e. did not have a cTc drawn, or did not have an echocardiogram, or had neither. These patients tended to be older, be current smokers or had a poorer functional status as indicated by a lower baseline modified Rankin scale or history of dementia.
Table 1.
Baseline Clinical Characteristics of AIS patient groups
| AIS with cTn and Echocardiogram | AIS with cTn no Echocardiogram | AIS with Echocardiogram no cTn | AIS with no Echocardiogram no cTn | P-value# | |
|---|---|---|---|---|---|
| N | 1377 | 329 | 213 | 80 | 1377 vs 622 |
| Age, years mean (sd) | 68.6 (14.6) | 73.8 (14.4) | 68.8 (15.5) | 67.9 (19.0) | 0.0003 |
| Female, n (%) | 744 (54.0%) | 188 (57.1%) | 122 (57.3%) | 49 (61.2%) | 0.12 |
| Black, n (%) | 300 (21.8%) | 68 (20.7%) | 44 (20.7%) | 19 (23.8%) | 0.72 |
| BMI (kg/m2), mean (std) | 28.2 (6.6) | 27.5 (6.7) | 28.3 (6.7) | 29.8 (7.4) | 0.67 |
| LDL, mean, mg/dL (std) | 107.3 (39.3) | 96.6 (34.3) | 98.7 (31.7) | 99.6 (34.5) | 0.0002 |
| TC/HDL ratio, mean (std) | 4.45 (1.75) | 4.22 (1.84) | 4.26 (1.62) | 4.23 (1.51) | 0.07 |
| Prior Cardiac Disease, n (%) | 694 (50.4%) | 186 (56.5% | 83 (39.0%) | 42 (52.5%) | 0.87 |
| Hx or Current A.fib, n (%) | 322 (23.4%) | 92 (28.0%) | 38 (17.8%) | 21 (26.2%) | 0.66 |
| Baseline mRS (0–1), n (%)* | 676 (49.1%) | 112 (34.0%) | 110 (51.6%) | 26 (32.5%) | 0.0001 |
| CKD, n (%)† | 210 (15.2%) | 52 (15.8%) | 29 (13.6%) | 12 (15.0%) | 0.86 |
| Hx hypertension, n (%) | 1110 (80.6%) | 280 (85.1%) | 167 (78.4%) | 62 (77.5%) | 0.52 |
| Hx diabetes, n (%) | 478 (34.7%) | 130 (39.5%) | 77 (36.2%) | 26 (32.5%) | 0.24 |
| Hx CAD, n (%) | 484 (35.2%) | 114 (34.6%) | 60 (28.2%) | 29 (36.2%) | 0.27 |
| Hx PVD, n (%)‡ | 161 (11.7%) | 44 (13.4%) | 23 (10.8%) | 10 (12.5%) | 0.66 |
| Current smoking, n (%)§ | 410 (29.8%) | 73 (22.2%) | 50 (23.5%) | 23 (28.8%) | 0.004 |
| Dementia, n (%) | 145 (10.5%) | 62 (18.8%) | 18 (8.4%) | 17 (21.2%) | 0.001 |
| rNIHSS score median (IQR)|| | 3 (1, 7) | 3 (1, 7) | 2 (1, 5) | 2.5 (1, 7) | 0.26 |
| Family hx of stoke or CAD, n (%) | 562 (40.8%) | 106 (32.2%) | 71 (33.3%) | 27 (33.8%) | <0.001 |
modified Rankin scale,
chronic renal disease,
peripheral vascular disease,
in past 3 months,
National Institute of Health Stroke Score,
cTn and Echo vs missing cTn or Echo
Of the 1706 who had a cTn drawn, 353 (20.7%) had a hypertroponinemia. Of the 1590 who had an echocardiogram, 160 (10.1%) had at least one finding of interest. The results were similar for the 1377 whom had both tests performed (table 2). The most frequent echocardiogram finding was cardiomyopathy with a low ejection fraction, which was found in 107 (7.8%).
Table 2.
Prevalence of specific Echocardiogram findings of interest in 1377 AIS patients with both cTn level and Echocardiogram performed
| Echocardiogram findings of interest in AIS (TTE n=1217, TEE n=73, Both n=84, Unknown n=3) |
Total (n=1377) |
Positive Troponin (n=295) |
Negative Troponin (n=1082) |
P-value† |
|---|---|---|---|---|
| Intracardiac thrombus | 12 (0.9%) | 6 (2.0%) | 6 (0.6%) | 0.03 |
| Valvular vegetation | 8 (0.6%) | 6 (2.0%) | 2 (0.2%) | 0.002 |
| Low ejection fraction ≤ 35% | 107 (7.8%) | 48 (16.3%) | 59 (5.5%) | <0.0001 |
| Akinetic wall segment | 50 (3.6%) | 23 (7.8%) | 27 (2.5%) | <0.001 |
| Left ventricular aneurysm | 1 (0.1%) | 0 (0%) | 1 (0.1%) | 0.99 |
| Spontaneous echo contrast “Smoke” | 1 (0.1%) | 0 (0%) | 1 (0.1%) | 0.99 |
| Any abnormality above* | 147 (10.7%) | 68 (23.0%) | 79 (7.3%) | <0.0001 |
Patients may have multiple abnormalities,
positive vs negative troponin
After adjustment, hypertroponinemia was independently associated with echocardiogram findings (OR 2.9, 95% CI 2.0–4.2; supplementary table I). However, given that the prevalence of echocardiogram findings was low, a negative cTn did not significantly alter the pretest odds of having echocardiogram findings (7.3% vs 10.6%; negative likelihood ratio=0.66). Echocardiogram findings were also strongly associated with a prior history of cardiac disease (OR 4.9, 95% CI 2.9–8.1). Notably though, 32.5% (96/295) of those with a hypertroponinemia and 15.6% (23/147) of those with echocardiogram findings had no prior history of cardiac disease.
Median length of hospitalization was 4 days. Despite a low median NIHSS of 3, mortality was high; 10.9% of patients died within 30 days, 23.7% within 1 year and 35.0% within 3 years. Mortality rates were higher for the 622 patients who did not complete both tests (supplementary table II). For those 1377 that had both tests, and where a hypertroponinemia was detected, mortality was 26% at 30 days, 47% at 1 year, and 60% at 3 years. Hypertroponinemia was therefore independently associated with death at all time points. This association was similar when concurrent AMIs (diagnosed by the treating physician in 49/1377 {3.5%}) were removed from the analysis (table 3).
Table 3.
Multivariate odds of death after AIS in those with complete cardiac evaluation, excluding those with concurrent AMI; N=1328/1377
| Variable | Dead by 30 days OR (95% CI) |
Dead by 1 year OR (95% CI) |
Dead by 3 years OR (95% CI) |
|---|---|---|---|
| Hypertropinemia | 3.45 (2.11–5.64)* | 3.06 (2.14–4.37)* | 2.91 (2.06–4.11)* |
| Age (per 10 year increase) | 1.59 (1.25–2.03)* | 1.80 (1.53–2.13)* | 1.96 (1.70–2.27)* |
| Atrial Fibrillation | 0.98 (0.56–1.72) | 1.34 (0.90–1.98) | 1.36 (0.95–1.94) |
| Hx of Cardiac disease | 1.46 (0.80–2.68) | 1.02 (0.69–1.50) | 1.36 (0.97–1.89) |
| Hx of Diabetes | 0.61 (0.36–1.03) | 1.04 (0.74–1.47) | 1.08 (0.80–1.47) |
| Hx of CKD | 0.99 (0.54–1.82) | 1.70 (1.13–2.56)* | 1.36 (0.92–1.99) |
| Hx of Dementia | 1.29 (0.72–2.32) | 1.25 (0.80–1.94) | 1.73 (1.11–2.69)* |
| Current smoking | 1.41 (0.73–2.71) | 1.60 (1.04–2.47)* | 2.01 (1.38–2.91)* |
| mRS 0–1 | 0.58 (0.32–1.05) | 0.51 (0.35–0.75)* | 0.47 (0.34–0.64)* |
| rNIHSS (5 point increase) | 1.91 (1.70–2.17)* | 1.55 (1.41–1.72)* | 1.41 (1.28–1.54)* |
statistically significant
Echocardiogram findings were not significantly associated with mortality at 30 days or 1 year, but were at 3 years (OR 1.86 95% CI 1.23–2.84, supplementary table III).
Discussion
We found that most AIS patients in our region received cardiac testing during their admission, and that using contemporary assays 21% of AIS patients have a hypertroponinemia and 10% have echocardiogram findings of interest. We confirmed that hypertroponinemia is independently associated with short-term mortality, and our results extend this association to at least 3 years post event. This represents the first description of the relationship between cardiac testing abnormalities and long-term mortality within a large biracial population of acute stroke patients.
Approximately 96% of our AIS population received at least one of the two cardiac tests and 69% had both. This signifies that, despite the mostly mild strokes in our population, cardiac testing was considered part of the patient’s workup. Those that did not complete both tests subsequently had a higher mortality rate, perhaps indicating less aggressive investigation in an older and more disabled patient group. Overall 85% of our population received cTn testing, and 80% had echocardiograms performed. The high rate of echocardiogram performance was somewhat unexpected considering there is no recommendation from the AHA for their use in this setting.
Most of our echocardiogram findings were based on TTE (1217 of 1377), probably because the greater sensitivity of TEE for specific findings is offset by its invasive nature and cost.20 Our results are clearly contingent on our a priori assessment of what constitutes a finding of interest. For example, patent foramen ovale, a common anatomical variant that is not associated with future stroke risk, was not included in this analysis.14 Aortic arch atheromas greater than 4mm are an indicator of vascular disease, but because treatment with anticoagulation has not been shown to reduce vascular event rates we did not include them on the basis that their presence does not alter management.15 In contrast we did include spontaneous echo contrast or “smoke” due to its strong association with suspected atrial thromboembolism as a cause of stroke.21
In fact only 1.6% of those tested had a finding of intracardiac thrombus, spontaneous echo contrast or vegetation which could lead directly to anticoagulation or other therapy altering decisions. The diagnostic yield of echocardiography for CSE, the traditional indication for this test, was therefore low. The majority of findings were wall motion dyskinesis or reduced ejection fraction, and the evidence of these being a concrete CSE that anticoagulation helps ameliorate is limited.16,22 Nevertheless, obtaining an echocardiogram to search for stroke mechanism is only one possible use, and we included these structural abnormalities in the analysis under the hypothesis that they are important for a patient’s long term health, and may indicate underlying unstable atherosclerotic disease. The presence of these echocardiogram findings did not significantly increase odds of death at 30 days, but did at 3 years (supplementary table III), which suggests that the occurrence of an ischemic stroke is a possible opportunity for cardiac screening in a vulnerable population.
Supportive of this is our finding that hypertroponinemia, a biochemical marker of cardiac ischemia, was independently associated with echocardiogram findings of interest after AIS. This was expected given the high rates of (presumably ischemic) cardiac dyskinesias in our population. However, the absence of hypertroponinemia was not useful in ruling out echocardiographic findings, as 54% of those with findings had a negative cTn. Also, 15.6% of those who had findings had no prior history of cardiac disease. Therefore a diagnostic algorithm that would select a stroke patient for echocardiogram only when hypertroponinemia is present, or when there is prior cardiac history, would miss a number of patients with prognostically relevant occult structural cardiac disease detectable on echocardiogram.
Our finding that one-fifth of our stroke patients had hypertroponinemia is in line with various single-center studies and a meta-analysis that employed a range of cTn assays with 99th percentile values ranging from 30 to 50 ng/L.9,23,24 However, our finding is substantially lower than the approximately 60% prevalence of hypertroponinemia found in two recent single center studies that used new assays with a cTn upper reference limit of 14 ng/L.8,10 These newer assays are pending FDA approval and are not yet available in the US so were not used in our population. We avoid the term “high sensitivity assay” due to ambiguity around what defines this.25 Nevertheless any study of the prevalence of hypertroponiemia must qualify that this figure may change with each new assay developed.
The mechanism of hypertroponinemia in AIS is unclear. Anecdotally “troponin leaks” in this context are often dismissed as having little clinical relevance. Certainly, AMI, the customary indication for cTn testing, was diagnosed relatively rarely in our population (3.5%, and 16.6% of those with a hypertroponinemia). However, the short term prognostic implications of hypertroponinemia on in-hospital mortality have been described.8,10,24,26 In addition, Faiz et al27, following 347 patients over a median 1.5 years, showed that quantitatively higher cTn levels were associated with longer term mortality post AIS. Given therefore the high rate of obstructive coronary plaques and subsequent cardiac events following AIS,3,4,17 we and others have postulated28 that AIS may act as a kind of proxy cardiac “stress test” in the setting of underlying coronary atherosclerotic disease. Our study confirms this “stress test” is powerfully associated with 30-day mortality, and that this association continues to 1 and 3 years, at which the rate of mortality is almost double compared to those with a normal post-stroke cTn level. Other European single center studies have not replicated this association when concurrent AMI, an important driver of mortality, was excluded.29 In our population however non-AMI hypertroponinemia appears to carry more prognostic weight then a 5-point increase in the NIHSS, 10-year age increase, or current smoking (figure 1).
Figure 1.
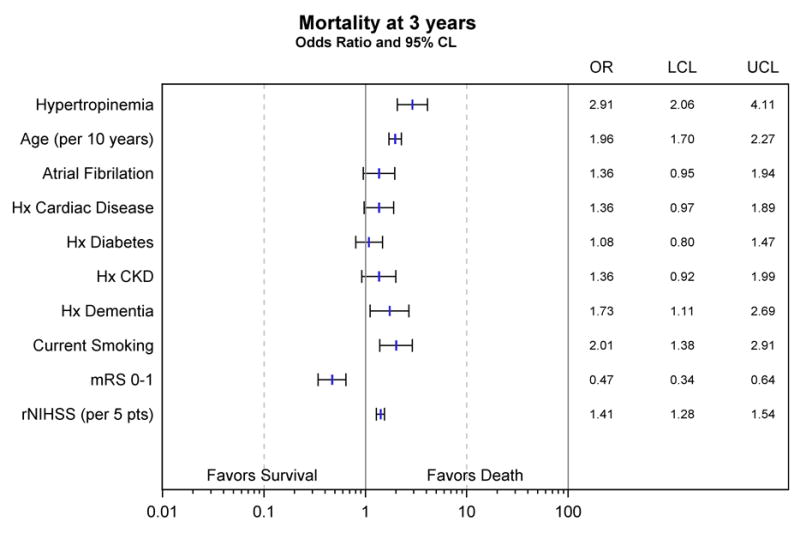
Multivariate odds of death after AIS
The 3 year mortality rate of 60% among AIS patients with hypertroponinemia is surprising given the clinically mild nature of strokes within our population. The recent TRELAS study found 24% of 29 AIS patients with hypertroponinemia had culprit lesions on coronary angiography, and 21% underwent mechanical revascularization.30. The number of culprit lesions was significantly lower than matched controls with AMI. Nonetheless the 3-year mortality rates we have demonstrated in our study imply that angiographic findings in AIS may be prognostically relevant. Prospective studies are warranted to determine if routine cardiac evaluation after hypertroponinemic AIS might confer a mortality benefit.
Limitations
Our study has several limitations. First, diagnosis of concurrent AMI was made by the treating physician and was not adjudicated by GCNKSS study physicians. Second, we did not assess for dynamic changes in cTn levels, which may have improved sensitivity for detecting an acute cardiac stress and its association with mortality. Problematically there is no agreed value change across cTn platforms that rules out normal biological variation.25 Third, we did not attempt to discriminate between cTn values quantitatively as others have.10,27 Instead we chose to dichotomize the result based on the 99th percentile value for each specific assay. This speaks to the complex methodology around detection of such tiny quantities of substrate. That we used a mix of cTn platforms is a strength of the study as it relates to common community practice. However, values from different platforms of cTn are not necessarily comparable in a linear manner, and even the same platforms may differ across sites based on local protocols and quality controls.25,31 Fourth, a possible mechanism of hypertroponinemia in AIS is neurogenic catecholamine release leading to cardiac myocyte dysfunction. Interestingly, the right insular cortex participates in sympathetic cardiac control, and its involvement in stroke has been associated with both hypertropinemia32 and mortality,33 irrespective of stroke volume or clinical severity. Unfortunately we were not able to control for right insular involvement in our study, although such a broad anatomic delineation may not respect the discrete cortical areas involved. Lastly, we did not have data on cause of death in our subjects. Thus, we cannot determine whether hypertroponinemia during AIS leads to higher long-term risk of specifically cardiac death. Given this, further study is needed to ascertain the mechanism of hypertroponinemia in stroke and how this mechanism contributes to mortality.
Conclusion
We present a study of serum troponin and echocardiogram testing in a population of acute ischemic stroke patients. We found that cardiac findings were common in our population of mostly mild strokes, even in the absence of concurrent myocardial infarction, and were independently associated with long-term mortality. Prospective studies are needed to determine whether further cardiac evaluation in this group might prevent the higher long-term mortality rates that we observed.
Supplementary Material
Acknowledgments
None.
Sources of Funding
This work was supported by National Institutes of Health, National Institute of Neurological Disorders and Stroke Division, grant R01NS30678.
Footnotes
Disclosures
Drs Kleindorfer, Kissela, Flaherty, Ferioli, Woo, Moomaw, Khoury, and K. Alwell BSN receive research support from the National Institute of Health. Drs Wrigley, De Los Rios la Rosa, Adeoye, Eckerle and Martini have no disclosures to report.
References
- 1.Elixhauser A, Owens P. HCUP Statistical Brief #2. February 2006. Agency for Healthcare Research and Quality; Rockville, Md: 2003. Reasons for Being Admitted to the Hospital through the Emergency Department. http://www.hcup-us.ahrq.gov/reports/statbriefs/sb2.pdf. Accessed September 1st 2016. [PubMed] [Google Scholar]
- 2.Bogousslavsky J, Cachin C, Regli F, Despland PA, Van Melle G, Kappenberger L. Cardiac sources of embolism and cerebral infarction-clinical consequences and vascular concomitants: The Lausanne Stroke Registry. Neurology. 1991;41:855–9. doi: 10.1212/wnl.41.6.855. [DOI] [PubMed] [Google Scholar]
- 3.Prosser J, MacGregor L, Lees K, Diener HC, Hacke W, Davis S, on behalf of the VISTA Investigators Predictors of Early Cardiac Morbidity and Mortality After Ischemic Stroke. Stroke. 2007;38:2295–2302. doi: 10.1161/STROKEAHA.106.471813. [DOI] [PubMed] [Google Scholar]
- 4.Touzé E, Varenne O, Chatellier G, Peyrard S, Rothwell PM, Mas JL. Risk of Myocardial Infarction and Vascular Death After Transient Ischemic Attack and Ischemic Stroke A Systematic Review and Meta-Analysis. Stroke. 2005;36:2748–55. doi: 10.1161/01.STR.0000190118.02275.33. [DOI] [PubMed] [Google Scholar]
- 5.Jauch EC, Saver JL, Adams HP, Bruno A, Connors JJ, Demaerschalk BM, et al. Guidelines for the Early Management of Patients With Acute Ischemic Stroke A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2013;44:870–947. doi: 10.1161/STR.0b013e318284056a. [DOI] [PubMed] [Google Scholar]
- 6.Adams R, Chimowitz MI, Alpert JS, Awad IA, Cerqueria MD, Fayad P. Coronary Risk Evaluation in Patients With Transient Ischemic Attack and Ischemic Stroke A Scientific Statement for Healthcare Professionals From the Stroke Council and the Council on Clinical Cardiology of the American Heart Association/American Stroke Association. Circulation. 2003;108:1278–90. doi: 10.1161/01.CIR.0000090444.87006.CF. [DOI] [PubMed] [Google Scholar]
- 7.Melanson SE, Conrad MJ, Mosammaparast N, Jarolim P. Implementation of a Highly Sensitive Cardiac Troponin I Assay: Test Volumes, Positivity Rates and Interpretation of Results. Clinica Chimica Acta. 2008;395:57–61. doi: 10.1016/j.cca.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 8.Faiz K, Thommessen B, Einvik G, Brekke P, Omland T, Rønning O. Determinants of High Sensitivity Cardiac Troponin T Elevation in Acute Ischemic Stroke. BMC Neurology. 14(2014):96. doi: 10.1186/1471-2377-14-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anders B, Alonso A, Artemis D, Schäfer A, Ebert A, Kablau M, et al. What Does Elevated High-Sensitive Troponin I in Stroke Patients Mean: Concomitant Acute Myocardial Infarction or a Marker for High-Risk Patients. Cerebrovascular Diseases. 2013;36:211–17. doi: 10.1159/000353875. [DOI] [PubMed] [Google Scholar]
- 10.Scheitz J, Mochmann H, Erdur H, Tütüncü S, Haeusler K, Grittner U, et al. Prognostic Relevance of Cardiac Troponin T Levels and Their Dynamic Changes Measured with a High-Sensitivity Assay in Acute Ischaemic Stroke: Analyses from the TRELAS Cohort. International Journal of Cardiology. 2014;177:886–93. doi: 10.1016/j.ijcard.2014.10.036. [DOI] [PubMed] [Google Scholar]
- 11.Sansoy V, Abbott RD, Jayaweera AR, Kaul S. Low Yield of Transthoracic Echocardiography for Cardiac Source of Embolism. The American Journal of Cardiology. 1995;75:166–69. doi: 10.1016/s0002-9149(00)80068-5. [DOI] [PubMed] [Google Scholar]
- 12.Abreu T, Mateus S, Correia J. Therapy Implications of Transthoracic Echocardiography in Acute Ischemic Stroke Patients. Stroke. 2005;36:1565–66. doi: 10.1161/01.STR.0000170636.08554.49. [DOI] [PubMed] [Google Scholar]
- 13.Lee RJ, Bartzokis T, Yeoh TK, Grogin HR, Choi D, Schnittger I. Enhanced Detection of Intracardiac Sources of Cerebral Emboli by Transesophageal Echocardiography. Stroke. 1991;22:734–39. doi: 10.1161/01.str.22.6.734. [DOI] [PubMed] [Google Scholar]
- 14.Katsanos AH, Spence D, Bogiatzi C, Parissis J, Giannopoulos S, Frogoudaki A, et al. Recurrent Stroke and Patent Foramen Ovale A Systematic Review and Meta-Analysis. Stroke. 2014;45:3352–59. doi: 10.1161/STROKEAHA.114.007109. [DOI] [PubMed] [Google Scholar]
- 15.Amarenco P, Davis S, Jones EF, Cohen AA, Heiss WD, Kaste M, et al. Clopidogrel Plus Aspirin Versus Warfarin in Patients With Stroke and Aortic Arch Plaques. Stroke. 2014;45:1248–57. doi: 10.1161/STROKEAHA.113.004251. [DOI] [PubMed] [Google Scholar]
- 16.Homma S, Thompson J, Pullicino PM, Levin B, Freudenberger RS, Teerlink JR, et al. WARCEF Investigators Warfarin and Aspirin in Patients with Heart Failure and Sinus Rhythm. The New England Journal of Medicine. 2012;366:1859–69. doi: 10.1056/NEJMoa1202299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amarenco P, Lavallée PC, Labreuche J, Ducrocq G, Juliard JM, Feldman L, et al. Prevalence of Coronary Atherosclerosis in Patients With Cerebral Infarction. Stroke. 2011;42:22–29. doi: 10.1161/STROKEAHA.110.584086. [DOI] [PubMed] [Google Scholar]
- 18.Darki A, Schneck MJ, Agrawal A, Rupani A, Barron JT. Correlation of Elevated Troponin and Echocardiography in Acute Ischemic Stroke. Journal of Stroke and Cerebrovascular Diseases. 2013;22:959–61. doi: 10.1016/j.jstrokecerebrovasdis.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 19.Broderick J, Brott T, Kothari R, Miller R, Khoury J, Pancioli A, et al. The Greater Cincinnati/Northern Kentucky Stroke Study Preliminary First-Ever and Total Incidence Rates of Stroke Among Blacks. Stroke. 1998;29:415–21. doi: 10.1161/01.str.29.2.415. [DOI] [PubMed] [Google Scholar]
- 20.Egeblad H, Andersen K, Hartiala J, Lindgren A, Marttila R, Petersen P, et al. Role of Echocardiography in Systemic Arterial Embolism: A Review with Recommendations. Scandinavian Cardiovascular Journal. 1998;32:323–42. doi: 10.1080/14017439850139780. [DOI] [PubMed] [Google Scholar]
- 21.Black IW, Hopkins AP, Lee L, Walsh WF. Left Atrial Spontaneous Echo Contrast: A Clinical and Echocardiographic Analysis. Journal of the American College of Cardiology. 1991;18:398–404. doi: 10.1016/0735-1097(91)90592-w. [DOI] [PubMed] [Google Scholar]
- 22.Le May MR, Acharya S, Wells GA, Burwash I, Chong AY, So DY, et al. Prophylactic Warfarin Therapy After Primary Percutaneous Coronary Intervention for Anterior ST-Segment Elevation Myocardial Infarction. JACC: Cardiovascular Interventions. 2015;8:155–62. doi: 10.1016/j.jcin.2014.07.018. [DOI] [PubMed] [Google Scholar]
- 23.Kerr G, Ray G, Wu O, Stott DJ, Langhorne P. Elevated Troponin after Stroke: A Systematic Review. Cerebrovascular Diseases. 2009;28:220–26. doi: 10.1159/000226773. [DOI] [PubMed] [Google Scholar]
- 24.Scheitz JF, Endres M, Mochmann HC, Audebert HJ, Nolte CH. Frequency, Determinants and Outcome of Elevated Troponin in Acute Ischemic Stroke Patients. International Journal of Cardiology. 2012;157:239–42. doi: 10.1016/j.ijcard.2012.01.055. [DOI] [PubMed] [Google Scholar]
- 25.Apple FS, Collinson PO, for the IFCC Task Force on Clinical Applications of Cardiac Biomarkers Analytical Characteristics of High-Sensitivity Cardiac Troponin Assays. Clinical Chemistry. 2012;58:54–61. doi: 10.1373/clinchem.2011.165795. [DOI] [PubMed] [Google Scholar]
- 26.Di Angelantonio E, Fiorelli M, Toni D, Sacchetti ML, Lorenzano S, Falcou A, et al. Prognostic Significance of Admission Levels of Troponin I in Patients with Acute Ischaemic Stroke. Journal of Neurology, Neurosurgery, and Psychiatry. 2005 Jan;76:76–81. doi: 10.1136/jnnp.2004.041491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Faiz KW, Thommessen B, Einvik G, Omland T, Morton Rønning O. Prognostic Value of High-Sensitivity Cardiac Troponin T in Acute Ischemic Stroke. Journal of Stroke and Cerebrovascular Diseases. 2014;23:241–48. doi: 10.1016/j.jstrokecerebrovasdis.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 28.Scheitz JF, Nolte CH, Laufs U, Endres M. Application and Interpretation of High-Sensitivity Cardiac Troponin Assays in Patients With Acute Ischemic Stroke. Stroke. 2015;46:1132–40. doi: 10.1161/STROKEAHA.114.007858. [DOI] [PubMed] [Google Scholar]
- 29.Batal O, Jentzer J, Balaney B, Kolia N, Hickey G, Dardari Z, et al. The Prognostic Significance of Troponin I Elevation in Acute Ischemic Stroke. Journal of Critical Care. 2016;31:41–47. doi: 10.1016/j.jcrc.2015.09.018. [DOI] [PubMed] [Google Scholar]
- 30.Mochmann HC, Scheitz JF, Petzold GC, Haeusler KG, Audebert HJ, Laufs U, et al. Coronary Angiographic Findings in Acute Ischemic Stroke Patients With Elevated Cardiac Troponin: The Troponin Elevation in Acute Ischemic Stroke (TRELAS) Study. Circulation. 2016;133:1264–71. doi: 10.1161/CIRCULATIONAHA.115.018547. [DOI] [PubMed] [Google Scholar]
- 31.Kavsak PA, Beattie J, Pickersgill R, Ford L, Caruso N, Clark LA. Practical Approach for the Validation and Clinical Implementation of a High-Sensitivity Cardiac Troponin I Assay across a North American City. Practical Laboratory Medicine. 2015;1:28–34. doi: 10.1016/j.plabm.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ay H, Koroshetz WJ, Benner T, Vangel MG, Melinosky C, Arsava EM, et al. Neuroanatomic Correlates of Stroke-Related Myocardial Injury. Neurology. 2006;66:1325–29. doi: 10.1212/01.wnl.0000206077.13705.6d. [DOI] [PubMed] [Google Scholar]
- 33.Colivicchi F, Bassi A, Santini M, Caltagirone C. Prognostic Implications of Right-Sided Insular Damage, Cardiac Autonomic Derangement, and Arrhythmias After Acute Ischemic Stroke. Stroke. 2005;36:1710–15. doi: 10.1161/01.STR.0000173400.19346.bd. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


