Abstract
Objective
To evaluate the efficacy of a small change behavioral weight loss intervention with or without a positive affect/self-affirmation (PA/SA) component on weight loss at 12 months.
Methods
Black and Hispanic adults (N=405) with body mass index 25-50 kg/m2 selected one of 10 small change eating strategies and a physical activity goal, randomly with/without positive affect/self-affirmation. Participants were followed by community health workers (CHW's) at set intervals (weekly months 1-3; biweekly months 4-9; once monthly months 10-12).
Results
There was no difference in weight loss at 12 months between participants in the small change approach alone (1.1%) vs. the small change PA/SA intervention (1.2%). During treatment, 9% of participants lost at least 7% of their initial body weight. Participants who reported more interval life events had a lower likelihood of losing weight (p <.0001). However, those randomized to the small change PA/SA intervention gained less weight (+0.3% vs. 2.3% gain; P value <.0001).
Conclusion
The small change PA/SA intervention did not lead to a significant difference in weight loss in comparison to the small change approach alone. It did however decrease the negative impact of psychosocial stressors on weight gain among participants with more interval life events.
Keywords: obesity, weight loss, lifestyle, small changes, physical activity, intervention, prevention research
Introduction
Black and Hispanic adults are disproportionally affected by the adverse health consequences of obesity including diabetes, hypertension, and heart disease (1). Studies have shown that modest amounts of weight loss can prevent complications and improve outcomes in these obesity-related health conditions (2-4). Unfortunately, the outcomes of Black and Hispanic participants in weight loss trials have been less favorable than white participants (5). Only a few obesity behavioral randomized trials have yielded modest (5-10%) weight loss among Black and Hispanic participants. The field's inability to consistently produce modest weight loss in high-risk populations calls for novel study designs. Thus, among Black and Hispanic urban dwelling adults, the objective of this proof of concept randomized trial was to evaluate the efficacy of a small change approach behavioral weight loss intervention coupled with a novel psychoeducational intervention on achieving ≥ 7 % weight loss at 12 months. The secondary outcomes were adherence to the small change eating strategies and physical activity goals. In addition, mediating factors of the intervention were evaluated.
The small change approach to weight loss is theorized to increase self-efficacy for sustainable behavior change by targeting a more achievable small daily energy deficit of about 100-200 kcal per day via reductions in nutritional intake or increases in physical activity (6). To date, the small change approach has resulted in modest weight loss and prevention of further weight gain across several populations (7-16). We developed a psychoeducational intervention that combines the constructs of positive affect and self-affirmation into a simple teachable self-directing script that has been shown to enhance positive behavior change in several populations (17).
Using this construct our hypotheses were: 1) The positive affect/self-affirmation intervention would increase the success of the small change eating and physical activity intervention by increasing self-efficacy for the goals thus leading to weight loss; 2) Participants with high perceived stress from work or from family would be less successful in sustaining eating and physical activity behavioral changes, and less likely to lose weight; and 3) The positive affect/self-affirmation intervention would lessen the adverse behavioral impact of high perceived stress on eating and physical activity behaviors.
Methods
Study Design
Details of the study design have been previously described (18). The SCALE proof of concept trial randomly assigned participants in a 2:1 ratio to a 12-month small change intervention with or without a positive affect/self-affirmation component. Community sites were cluster-randomized in order to prevent contamination. Participants were enrolled from August 2012 to September 2013 and followed for 1 year by trained CHW's at routine intervals (weekly for months 1-3; biweekly months 4-9; once monthly for months 10-12). Close-out interviews were conducted in person at 12 months. The study was approved by the Institutional Board on Human Rights in Research at the Weill Cornell Medical College, Cornell University, and Lincoln Medical and Mental Health Center of the Health and Hospital Corporations of New York City.
Setting and Participants
Eligibility criteria included age ≥ 21 years, BMI 25 to 50 kg/m2, Black and/or Hispanic race/ethnicity, and fluency in English or Spanish. Exclusion criteria were pregnancy within the year, participating in another weight loss program, weight loss surgery within the year, untreated mental illness or thyroid disease, active cancer, active eating disorder, advanced chronic obstructive pulmonary disease, renal disease on dialysis or the inability to control meal contents. Participants were recruited at clinical and community sites in Harlem and the South Bronx, New York.
Small Change Intervention
At enrollment, participants identified their specific eating challenges. CHW's used these challenges to guide participants in the selection of one of ten small change eating strategies to address them. The ten small change eating strategies were: prepare the main meal at home, take time for meals, drink water instead of sweetened beverages, eat a fruit or vegetable before snacking, eat breakfast daily, make half the main meal vegetables, turn off the television during meals, stop buying snack foods, hide snacks in an inconvenient place, and eat main meals on a 10-inch plate (19). Participants also set self-selected physical activity goals (i.e.: walk 20 minutes daily), and made a behavior contract to adhere to their goals at least 6 days per week.
Small Change Intervention with Positive Affect/Self-Affirmation Component
After goal setting, participants randomized to the PA/SA group were taught the positive affect/self-affirmation script. They were instructed to identify small things that made them feel good and asked to think about these things when they first wake up in the morning and throughout their day. For the self-affirmation component participants were asked to think of a proud moment in their life and to think of that moment when they were faced with barriers to their new behavior goals (17).
Data Collection
The following data were collected at baseline and the 12-month assessment: demographic characteristics, weight (measured using a SECA 812 High Capacity Digital Floor Scale) and height (measured using a Seca Stadiometer Model 214); the Charlson Comorbidity Index (20); fruit and vegetable consumption by the Behavioral Risk Factor Surveillance System (21); Dutch Eating Behavior Questionnaire (22); food choice coping strategies (23); home eating environment (24); perceived stress (25); trait affect (26); social support (27); life events (28) and depressive symptoms (29).
At each follow up, interval life and medical events, adherence to small change eating and physical activity goals, challenges experienced with the eating and physical activity goals, and self– efficacy for continuing the behavior were assessed. Mean adherence was measured as the number of days of the week that a participant achieved their specific goal divided by the total number of follow-ups Challenges and self –efficacy for the goals were measured on a scale of 1-10 with 10 being the most challenging or the highest confidence level. If self-efficacy for either behavior fell below 8, participants were asked to change the behavior to increase likelihood of completion in the upcoming week. Interval life and adverse events were measured by CHW's asking the following questions “Since we last spoke how have you been feeling?” and “Since we last spoke have you had any major life events and difficulties?” The details of how these interval life events were coded are being published elsewhere. The close-out interview was conducted by a CHW or research staff member who was blinded to the participant's randomization group.
Statistical analysis
The data were analyzed using SAS version 9.1, SAS Institute, Inc. and Stata 14 for Windows. For baseline comparison between the randomization groups, x2 tests and student t tests were used to investigate group differences. An intent-to-treat and completers only analysis were conducted in evaluating the primary outcome of ≥ 7 % weight loss. Percent weight change and adherence to the small change behaviors were also evaluated. Structural equation modeling (SEM) was used to assess the impact of different mechanisms of the intervention in relation to weight loss.
The SEM estimated the direct and indirect associations between exogenous (independent) variables and endogenous (dependent) variables. The total effect of the model is the sum of the direct and indirect effects of the exogenous (independent) variables on the outcome (%weight loss). SEM models are represented by path diagrams comprised of nodes and lines where a single straight arrow from one variable indicates the direction of the relationship with the connecting variable. Two straight single-headed arrows in opposing directions indicates a correlation. The SEM model was fit using Stata 14 and underwent several iterations. The model fit was assessed using the Bentler–Raykov (30) squared multiple correlation, an overall coefficient of determination, the Bentler-Freeman (31) stability index, and modification indices.
Results
Participants
A total of 405 participants were randomized, see Figure 1, of which 60% (n=73) of the control group and 62% (n=175) of the intervention group completed the study. Table 1 shows the baseline characteristics of the participants who were mostly women (89%), aged 48 ± 14.2 years old, 52% Black, 42% Hispanic and 5% both. Hispanic participants were younger than Blacks (47 vs. 54 years; P<.0001). The randomization groups differed in race and ethnicity; they were either mostly Black or Hispanic due to the natural racial and ethnic composition of the eight recruitment settings (3 clinical and 5 community) (p=.0001). The small change alone group (control) also had fewer people who lived alone (p=.01), who were insured (p=.008), and were native English speakers (p=.000).
Figure 1. SCALE CONSORT Flow Diagram.
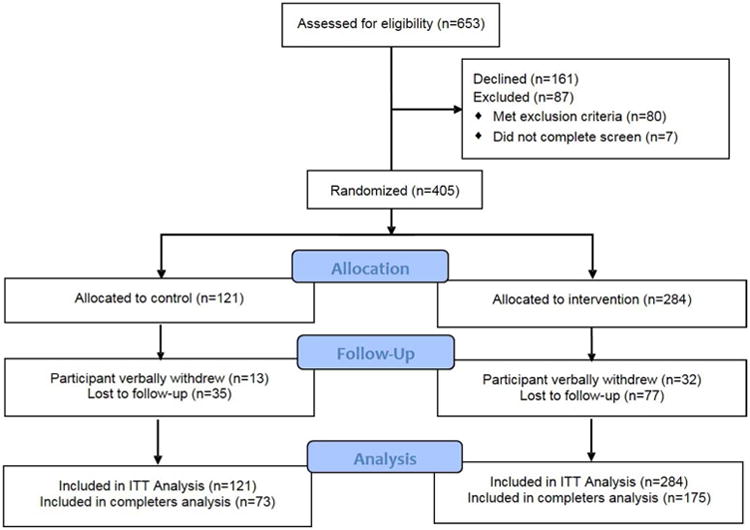
Table 1. Baseline Characteristics of SCALE Participants (n=405).
| Characteristic | Control (n=121) | Intervention (n=284) | p-value | |
|---|---|---|---|---|
| Sociodemographic | ||||
| Age, mean (SD) years | 46.3 ± 14.4 | 49.1 ± 14.1 | ||
| BMI, mean (SD) | 33.4 ± 5.7 | 34.2 ± 6.2 | ||
| Women, % | 86 | 90 | ||
| Married, % | 31 | 25 | ||
| Race/ethnicity, % | ||||
| Black | 36 | 66 | <.0001 | |
| Hispanic | 70 | 38 | ||
| English native language, % | ||||
| Black | 77 | 92 | .000 | |
| Hispanic | 12 | 22 | ||
| Completed high school or beyond, % | 77 | 79 | ||
| Currently Employed, % | 50 | 48 | ||
| Insured, % | 66 | 79 | .008 | |
| Type of Health Insurance, % | ||||
| Medicaid | 25 | 23 | ||
| Medicare | 10 | 10 | ||
| Commercial | 17 | 37 | .000 | |
| Living Conditions | ||||
| Lives alone, % | 16 | 26 | .01 | |
| Have children ≤ 18 years in the home | 46 | 46 | ||
| All or some responsibility for preparing meals in the home,% | 89 | 94 | ||
| Food Stamp Recipient,% | 39 | 39 | ||
| Clinical | ||||
| Diabetes, % | 23 | 21 | ||
| Charlson Comorbidity Index, mean (SD) | 1.1 | 1.0 | ||
| Perceived Health Status | ||||
| Excellent- Very Good | 26 | 21 | ||
| Good | 40 | 42 | ||
| Fair-Poor | 34 | 37 | ||
| Psychosocial | ||||
| Trait Positive Affect, mean (SD) | 20.3 ± 4.4 | 20.3 ± 4.2 | ||
| Trait Negative Affect, mean (SD) | 11.4 ± 3.9 | 11.9 ± 4.0 | ||
| SRRS Live Events Within the Past Year, % | 45 | 43 | ||
| Perceived Stress, mean (SD) | 15.9 ± 8.1 | 15.3 ± 7.2 | ||
| MOS Support, mean (SD) | 74 ± 17 | 75 ± 17 | ||
| Depressive Symptoms, mean (SD) | 4.0 ± 5.8 | 4.2 ± 5.5 | ||
Comparatively, non-completers (n=154) of the study were younger (44.5 vs. 50.5 years, P < 0.0001), had a higher BMI at enrollment (34.7 kg/m2 vs. 33.5 kg/m2, P = 0.05), were less involved in food preparation at home (35% vs 58%: P< .002), and were more likely to have Supplemental Nutrition Assistance Program (SNAP) benefits (48% vs. 34%; p<.005). In multivariate analysis, only younger age (P=.0003), receipt of SNAP (p=.03) and limited involvement in preparing food at home (p=.01) remained predictors of study non-completion.
Small Change Behavior Engagement
Overall, the most commonly selected eating strategies by both randomization groups were: eat a half plate of vegetables as part of their main meal (44%); take time for meals (28%); use a 10” plate for main meal (23%); drink water not sweetened drinks (23%); eat breakfast (23%) and eat a fruit before snack (21%). Other strategies included turning the television off during the main meal (14%); preparing their main meal at home (9%); not buying snacks (7%); and hiding snacks (2%). Almost half of participants chose to increase walking as their primary physical activity goal; and the remainder chose a wide variety of different activities, including dancing, exercise videos and cycling.
Weight Loss Outcomes
As shown in Table 2 there was no difference in weight loss between participants in the small change approach alone (1.1%) vs. the small change approach plus PA/SA component groups (1.2%) at 12 months. During treatment, a small minority (9%) of participants lost at least 7% of their initial body weight. However, 63% of participants lost weight overall [(mean 2.4% (4.2 ± 4.1 kg)].
Table 2. Weight Loss by Condition (n = 405).
| Intent-to-treat (n=405) | Completers (n=248) | |||
|---|---|---|---|---|
| Condition | Mean % weight loss (s.d.) | ≥7% weight loss | Mean % weight loss (s.d) | ≥7% weight loss |
| Intervention | 1.2 ± 4.3 | 8.8% | 1.8 ± 4.9 | 14% |
| Control | 1.1 ± 4.6 | 9% | 1.9 ± 5.4 | 15% |
Starting at follow-up 13 (14 weeks after enrollment), participants were instructed to self-monitor their weights on a standard scale provided by the study. At all subsequent follow-ups, home weights were recorded. At 16 weeks, the average self-reported weight loss was 2.2%. Adherence to the small change eating behavior strategy was the only significant predictor of self-reported weight loss at 16 weeks (p=.045). Being engaged in weight self-monitoring increased the likelihood of a participant completing the trial (OR=5.7; CI 3.2 to 9.8).
Small Change Behaviors Adherence
Participants had greater self-reported adherence to their eating strategies in comparison to their physical activity goals (68% vs. 36% adherence). Among non-Hispanic Blacks participants, higher physical activity participation was associated with greater self-efficacy (β =7.07, 95% CI: [1.36, 12.77]) and less negative affect (β =-0.97, 95% CI: [-1.69, -0.24]) at 12 months. In comparison, among Hispanic participants, higher physical activity participation was also associated with greater self-efficacy (β =15.05, 95% CI: [6.90, 23.19]) but less social support (β =-0.25, 95% CI: [-0.37, -0.13]) at 12 months. Less perceived stress (β =-0.54, 95% CI: [-1.09, 0.01]) was marginally associated with higher physical activity participation at 12 months, albeit not statistically significant.
Mediators of the PA/SA Intervention on Weight Loss
Life events are generally grouped by social roles. The following categories were developed from the open ended questions and were used to code the detected interval life events (ILE's) in our study: work-related events (e.g. unemployment, new job); education-related events (e.g. exams); family conflicts (e.g. children with behavioral or school problems,); other family transitions (e.g. birth); personal health problems; major health problems of close family and close friends; death and bereavement; financial and housing problems (e.g. threatened eviction); miscellaneous other events and refused to describe.
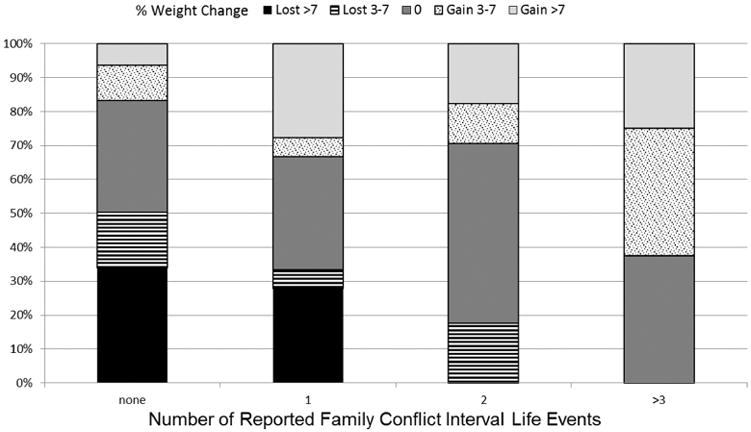
Over the course of the trial 63% of participants reported at least one ILE [(mean .19 ± .24);(range = 0 – 1.5)]. The most frequently reported event was personal health problems (231 events, 27% of participants), while the second most common was family conflict (127 events, 16% of participants). Family conflicts was the only ILE sub-category associated with significant weight gain. As the number of interval family conflicts increased, the likelihood of losing weight decreased. Moreover, as shown in figure 2, 72% of participants with three or more reported interval family conflict events gained (≥ 3%) weight during treatment. Participants with interval family conflicts who were randomized to the PA/SA intervention gained significantly less weight than those in the small change group alone (+0.3% vs. 2.3% gain; P value <.0001).
Figure 2. Impact of Interval Life Events - Family Conflicts on Weight Loss.
In the structural equation modeling we used the percentage of weight loss, rather than the primary outcome of achieving ≥ 7 % weight loss, to explore the mediating relations with the secondary outcomes. The estimation results in Table 3 provide the direct, indirect and total effects for each SEM equation. The path diagram displayed in Figure 3 depicts the mediation model and the corresponding t-tests of the direct effects (from Table 3) of key variables (challenge, adherence, self-efficacy, perceived stress, and interval life events) that mediate the effect of the PA/SA intervention on % weight loss. The significance indictors (*, **, #) in the boxes that contain the particular dependent variable names review the significance of the total effect of the PA/SA intervention and that dependent variable.
Table 3. SEM Estimation Results.
The “To” columns and “From” rows represent the left-hand side and right-hand side variables in the structural equation model. Direct, indirect, and total effects (t-tests in parenthesis) for the endogenous variables (percent of weight loss, assessment of challenge for eating behavior, and assessment of challenge for physical activity behavior, adherence to eating behavior, adherence to physical behavior, and efficacy for activity and eating behavior) and exogenous variables (age, gender, SNAP, ILE-other, ILE-family, and PSS). Controls for site and community health worker using Huber-White standard errors.
| From | To | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % Weight Loss | Challenge to Eating | Challenge to Physical Activity | Adherence to Eating | Adherence to Physical Activity | Efficacy for Eating and Physical Activity | |||||||||||||
| Direct | Indirect | Total | Direct | Indirect | Total | Direct | Indirect | Total | Direct | Indirect | Total | Direct | Indirect | Total | Direct | Indirect | Total | |
| Age | 0 (NP) | -.001 (-.14) | -.001 (-.14) | 0.005 (-1.27) | .004 (1.76) | 0.001 (-.27) | .010* (2.54) | -.001 (-.25) | .009* (2.30) | 0 (NP) | .000 (-.09) | .000 (-.09) | 0 (NP) | -.005* (-2.15) | -.005* (-2.15) | -.35 (-1.12) | 0 (NP) | -.35 (-1.12) |
| Gender | 0 (NP) | .001 (0.63) | .001 (0.63) | .131 (1.06) | .063 (.35) | .193 (.77) | .008 (.03) | .134 (.71) | .141 (.706) | 0 (NP) | -.007 (-.73) | -.007 (-.73) | 0 (NP) | -.076 (-.39) | -.076 (-.39) | -6.089 (-.32) | 0 (NP) | -6.089 (-.32) |
| SNAP | 0 (NP) | -.001 (-.55) | -.001 (-.55) | -.099 (-1.36) | -.166 (-1.16) | -.265* (-1.96) | -.192 (-.86) | -.183** (-3.48) | -.375 (1.69) | 0 (NP) | .070 (1.48) | .070 (1.48) | 0 (NP) | .183 (1.55) | .183 (1.55) | -14.51 (-1.17) | 0 (NP) | -14.51 (-1.17) |
| ILE-Other | 0 (NP) | -.001 (-.51) | -.001 (-.51) | -.025 (-.53) | .037 (1.09) | .012 (.28) | .075# (1.68) | .009 (.27) | .084 (1.48) | 0 (NP) | .012 (0.68) | .012 (0.68) | 0 (NP) | -.037 (-1.30) | -.037 (-1.30) | 10.469** (6.01) | 0 (NP) | 10.469** (6.01) |
| ILE-Family | 0 (NP) | -.001 (-.48) | -.001 (-.48) | .031 (.36) | -.101 (-1.06) | -.07 (-.84) | -.181 (-1.54) | -.048 (-.78) | -.229 (.046) | 0 (NP) | .044# (1.72) | 044# (1.72) | 0 (NP) | .124** (2.52) | .124** (2.52) | 12.47* (2.12) | 0 (NP) | 12.47* (2.12) |
| PSS | 0 (NP) | .000# (1.74) | .000# (1.74) | .132# (1.69) | .162* (2.27) | .295# (5.09) | .163 (1.43) | .204* (2.07) | .367** (3.75) | 0 (NP) | -.127** (-4.12) | -.127** (-4.12) | 0 (NP) | -.196** (-3.59) | -.196** (-3.59) | 15.108** (-7.53) | 0 (NP) | 15.108** (-7.53) |
| PA/SA | 0 (NP) | .001# (1.68) | .001# (1.68) | .157 (1.29) | .245 (1.29) | .402** (3.06) | .277 (.85) | .278 (2.87) | .555 (.031) | 0 (NP) | -.193** (-4.28) | -.193** (-4.28) | 0 (NP) | -.302* (-2.35) | -.302* (-2.35) | -32.81** (-2.59) | 0 (NP) | -32.81** (-2.59) |
| Challenge Physical Activity | 0 (NP) | .001 (1.38) | .001 (1.38) | .442* (2.22) | .195* (2.22) | .637* (2.22) | 0 (NP) | .441 (2.22) | .441 (2.22) | 0 (NP) | -.223* (-2.22) | -.223* (-2.22) | -.511 (-8.18) | -.225* (-2.22) | -.737** (-12.65) | – | – | – |
| Challenge Eating | 0 (NP) | .002** (6.15) | .002** (6.15) | 0 (NP) | .441 (2.10) | .441 (2.10) | .692 (2.10) | .305* (2.10) | .998 (2.10) | -0.35 (-5.79) | -.154* (-2.10) | -.504** (-5.50) | 0 (NP) | -.510* (-2.10) | -.510* (-2.10) | – | – | – |
| Adherence to Eating | -.005 (-1.08) | 0 (NP) | -.005 (-1.08) | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Adherence to Physical Activity | .001 (-.21) | 0 (NP) | .001 (-.21) | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Efficacy Eating & Physical Activity | 0 (NP) | -.001** (-4.22) | -.001** (-4.22) | – | – | – | – | – | – | .002** (4.30) | 0 (NP) | 002** (4.30) | .001 (1.22) | 0 (NP) | .001 (1.22) | – | – | – |
N=248 and the significance are denoted by
p<.1
p<.05
p<.01.
Figure 3. Path Model for the Effects of the Positive-Affect Self-Affirmation Intervention.
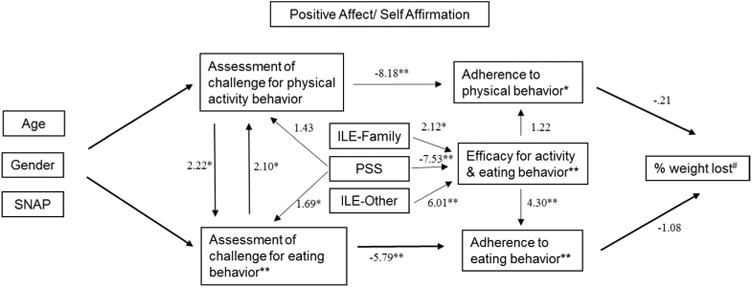
Direct effect t-statistics for the endogenous variables (percent of weight loss, adherence to eating behavior, adherence to physical behavior, efficacy for activity and eating behavior, assessment of challenge for eating behavior, and assessment of challenge for physical activity behavior) and some key exogenous variables (ILE-Family, ILE-Other, and PA/SA). The significance notations (*, **, #) within the rectangles containing the endogenous variables denote the significance of the PA/SA intervention. For the age gender and SNAP t-statistics and significance results see Table 3. Controls for site and community health worker using Huber-White standard errors. N=248 and the significance are denoted by #p<.1. * p<.05, **p<.01.
ILE-Family = Interval Life Events –Family Conflicts
ILE-Other = Interval Life Events – Other
PA/SA = Positive Affect/ Self-Affirmation Intervention
SNAP = Supplemental Nutrition Assistance Program
As shown in the path diagram, adherence to the physical activity (-.21) and eating behavior (-1.08) had an inverse direct effect on percent weight loss. Efficacy for either behavior had a stronger effect on adherence to eating (4.30) than to physical activity (1.22). Perceived stress had a direct effect on participants assessment of their challenges to their physical activity goal (1.43) and eating strategy (1.69), while it had an inverse effect (-7.53) on self-efficacy for either behavior. The ILE-family (2.12) and ILE-other (6.01) variables also had a significant direct effect on self-efficacy for either behavior.
The Bentler–Raykov squared multiple correlation (mc2), a measure of explained variance, for the endogenous variables are: percent of weight loss (mc2= .23), adherence to eating behavior (mc2= 0.51), adherence to physical behavior (mc2= 0.52), efficacy for activity and eating behavior (mc2= 0.17), assessment of challenge for eating behavior (mc2=0.43), and assessment of challenge for physical activity behavior (mc2=0.47). For the overall coefficient of determination for the model is R2=0.73. Our system is considered stable since the stability index equals 0.55. All of the model modification indices values were less than 3.84.
Discussion
We found that a small change approach to weight loss resulted in modest weight loss (≥ 7%) among a minority (9%) of overweight and obese Black and Hispanic urban dwelling adults. At 12 months there was no significant difference in weight loss as a result of the PA/SA component. Our results are consistent with the less than favorable weight loss outcomes seen in racial/ethnic minority participants enrolled in behavioral weight loss trials. Even in trials of lifestyle behavior change programs adapted for Black Americans only a small percent achieve clinically significant weight loss (32). At least 2 trials have demonstrated that patients in high-risk populations achieve clinical benefit from treatment, even if they do not exhibit a 3-kg weight loss at 6 months (33,34). This suggests that applying the same weight loss targets across populations regardless of risk may be unfavorable to expanding our understanding of the differences in treatment response and potentially the need for different targets.
As in most other behavioral weight loss intervention studies we had heterogeneous weight loss results ranging from losses of 21% to gains of 12%. In a post-hoc analysis we identified patterns indicative of more or less success in losing weight. We found the likelihood of losing weight decreased as participant's experienced more interval life events. More importantly, participants who reported three or more ILE's actually gained weight. The combined small change and positive affect/ self-affirmation intervention was most effective in preventing weight gain in this sub-cohort. Our findings support the hypothesis by Pressman and Cohen that positive affect influences health, at least in part, through its ability to buffer the adverse consequences of increased perceived stress (35). Thus the positive affect/ self-affirmation intervention may be particularly key to mitigating weight gain in individuals with higher levels of perceived stress.
Prevention of weight gain is equally important to halting the rapid rise of extreme weight categories (BMI>40 and >50). Between 2000 and 2010, the prevalence of a BMI over 40 (type III obesity) increased by 70%, the prevalence of a BMI over 50 increased even faster (36). Identifying sub-optimal responders to weight loss interventions early and adapting the treatment approach is critical to better positioning program enrollees for success.
Adaptive designs which allow for individualized treatment through empirically-supported decision rules advising when and how treatments should unfold over time have the potential to maximize weight loss interventions in high risk racial and ethnic minority populations in real-world settings. Such designs also hold great promise for improving treatment success by reducing attrition, a major cause of treatment failure in the field of obesity. Among studies that have described the factors associated with attrition, the following pre-treatment variables have had some consistency in predicting attrition: younger age at the initiation of dieting, higher number of previous weight loss attempts, low motivation, more depressive symptoms, and higher perceived stress. The two most common treatment variables associated with attrition have been practical difficulties to sustaining behavior change (37) and slow weight loss. In one study, 57% of dropouts indicated that “slow weight” loss was responsible for their withdrawal from treatment (38).
We experienced an attrition rate of 39%, which was far greater than our estimated rate at the conception of the study, and is thus a major limitation. Our losses were mostly in two of the three clinical sites due to the site ceasing operations during the study. We lost 55% of participants at those sites alone. The loss was significantly greater than in any of our other participating sites (p<.001). We found several variables to be predictive of early drop out; younger age, receipt of SNAP benefits, and having little or no responsibility for food preparation at home. In addition participants with early weight loss response were more likely to complete the trial. To our knowledge this is the first study that has found an association between receiving SNAP and study attrition in a behavioral weight loss trial. Thus, the role of food insecurity on retention in weight loss interventions warrants further evaluation.
Another modifiable factor early on in the trial was participant engagement with self-monitoring (dietary intake, physical activity and weight) activities that have all been associated with (39) greater long-term weight loss. In SCALE we introduced self-monitoring of weight at 12 weeks. There were no objective measures of eating and physical activities. Although adherence to the behaviors changes was reviewed at each follow-up (weekly months 1-3, then bi-weekly months 4-9 and then monthly 9-12) we allowed participants to determine their own methods of tracking their adherence. Thus, the use of non-standardized self-reported measures for adherence to the self-selected behaviors could have led to over-reporting; however, this bias would have been present in both groups.
Our study has several strengths, including detailed frequent psychosocial assessments for behavioral mediators of the desired goals, a scripted and standardized intervention that was not labor intensive, prospective evaluation for interval life events and the use of the SEM approach to evaluate the main effects of the positive affect/ self-affirmation intervention which would have otherwise been undetected in a typical regression model. Statistical mediation analysis can be used to improve the design of future interventions by identifying the possible mechanisms through which an existing intervention achieved its effects. Mediation analyses provide information regarding the effectiveness of various intervention components and such information can be used to tailor interventions for specific groups. Furthermore, mediation analyses allow researchers to develop more parsimonious interventions by eliminating less important components and emphasizing others. Through this model we are able to identify the characteristics of a cohort of participants who in future studies may benefit from a more intensive positive affect/ self-affirmation intervention in order to allow for greater adherence to desired healthy behavior changes. Lastly, our use of non-clinical coaches to deliver the intervention has important implications for its broad reach. There is growing evidence that the use of community health workers in behavioral interventions is a cost-effective strategy for implementing and sustaining behavior change in populations that may be otherwise hard to reach (40).
In conclusion, our main finding of clinically significant weight loss in a minority of participants is consistent with previously published reports and indicates a need to tailor interventions to the important differences that gives rise to variability in treatment outcomes in order to facilitate meaningful weight loss in high-risk populations.
What is already known about this subject?
Racial/ethnic minority populations even when included in well-powered studies with intensive interventions, experience small absolute weight losses.
Attrition is one of the major causes of treatment failure in weight loss interventions. Thus, the identification of factors leading to premature program termination is key to developing a more in depth understanding of which treatments work best in racial/ethnic minority populations.
The small change approach to weight loss reduces barriers to behavior change, contributing to increased motivation to make more small changes, and contributes directly to making small changes in the near environment. The small changes approach has been used to increase physical activity, decrease total energy intake, and prevent or reduce excessive weight gain.
What does our study add?
The small change approach to weight loss led to a 7% or more initial body weight loss at 12 months in less than 10% of participants.
The measurement of interval life events had greater predictive value than baseline stress characteristics alone in predicting long term weight loss success.
The use of structural equation modeling to analyze the mechanisms of the intervention revealed a sub-cohort of participants who may benefit from a positive-affect self-affirmation intervention for weight loss.
Acknowledgments
This study was supported by a grant from the National, Heart, Lung, and Blood Institute (NHLBI UO1 HL097843). We thank the lay leaders, administrators and community health workers of the following organizations, whose efforts in the development and tailoring of this study made its completion possible: First Baptist Church, Metropolitan Community United Methodist Church, Abyssinian Baptist Church, Iglesia Congregación Cristiana del Bronx, St Luke's Catholic Church, East Side Settlement, Northern Manhattan Perinatal Partnership, Renaissance Health Care Network, and Lincoln Medical and Mental Health Center.
Dr. Phillips reports grants from U.S. Department of Health and Human Services National Institutes of Health, National Heart, Lung, and Blood Institute, during the conduct of the study.
Study registered at ClinicalTrials.gov Identifier: NCT01198990
References
- 1.Kumanyika SK. Special issues regarding obesity in minority populations. Ann Intern Med. 1993;119:650–654. doi: 10.7326/0003-4819-119-7_part_2-199310011-00005. [DOI] [PubMed] [Google Scholar]
- 2.Wing RR, Lang W, Wadden TA, Safford M, Knowler WC, Bertoni AG, et al. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care. 2011;34:1481–1486. doi: 10.2337/dc10-2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stevens VJ, Obarzanek E, Cook NR, Lee IM, Appel LJ, Smith West D, et al. Long-term weight loss and changes in blood pressure: results of the Trials of Hypertension Prevention, phase II. Ann Intern Med. 2001;134:1–11. doi: 10.7326/0003-4819-134-1-200101020-00007. [DOI] [PubMed] [Google Scholar]
- 5.Kumanyika SK, Obarzanek E, Stevens VJ, Hebert PR, Whelton PK. Weight-loss experience of black and white participants in NHLBI-sponsored clinical trials. Am J Clin Nutr. 1991;53:1631S–1638S. doi: 10.1093/ajcn/53.6.1631S. [DOI] [PubMed] [Google Scholar]
- 6.Hill JO. Can a small-changes approach help address the obesity epidemic? A report of the Joint Task Force of the American Society for Nutrition, Institute of Food Technologists, and International Food Information Council. Am J Clin Nutr. 2009;89:477–484. doi: 10.3945/ajcn.2008.26566. [DOI] [PubMed] [Google Scholar]
- 7.Damschroder LJ, Lutes LD, Kirsh S, Kim HM, Gillon L, Holleman RG, et al. Small-changes obesity treatment among veterans: 12-month outcomes. Am J Prev Med. 2014;47:541–553. doi: 10.1016/j.amepre.2014.06.016. [DOI] [PubMed] [Google Scholar]
- 8.Cummings DM, Lutes LD, Littlewood K, DiNatale E, Hambidge B, Schulman K. EMPOWER: A randomized trial using community health workers to deliver a lifestyle intervention program in African American women with Type 2 diabetes: Design, rationale, and baseline characteristics. Contemporary Clinical Trials. 2013;36:147. doi: 10.1016/j.cct.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 9.Lutes LD, Dinatale E, Goodrich DE, Ronis DL, Gillon L, Kirsh S, et al. A randomized trial of a small changes approach for weight loss in veterans: design, rationale, and baseline characteristics of the ASPIRE-VA trial. Contemp Clin Trials. 2013;34:161–172. doi: 10.1016/j.cct.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 10.Lutes LD, Daiss SR, Barger SD, Read M, Steinbaugh E, Winett RA. Small changes approach promotes initial and continued weight loss with a phone-based follow-up: nine-month outcomes from ASPIRES II. Am J Health Promot. 2012;26:235–238. doi: 10.4278/ajhp.090706-QUAN-216. [DOI] [PubMed] [Google Scholar]
- 11.Damschroder LJ, Lutes LD, Goodrich DE, Gillon L, Lowery JC. A small-change approach delivered via telephone promotes weight loss in veterans: results from the ASPIRE-VA pilot study. Patient Educ Couns. 2010;79:262–266. doi: 10.1016/j.pec.2009.09.025. [DOI] [PubMed] [Google Scholar]
- 12.Lutes LD, Winett RA, Barger SD, Wojcik JR, Herbert WG, Nickols-Richardson SM, et al. Small changes in nutrition and physical activity promote weight loss and maintenance: 3-month evidence from the ASPIRE randomized trial. Ann Behav Med. 2008;35:351–357. doi: 10.1007/s12160-008-9033-z. [DOI] [PubMed] [Google Scholar]
- 13.Zinn C, Schofield GM, Hopkins WG. Efficacy of a “small-changes” workplace weight loss initiative on weight and productivity outcomes. J Occup Environ Med. 2012;54:1224–1229. doi: 10.1097/JOM.0b013e3182440ac2. [DOI] [PubMed] [Google Scholar]
- 14.Zinn C, Schofield GM, Hopkins WG. A “small-changes” workplace weight loss and maintenance program: examination of weight and health outcomes. J Occup Environ Med. 2012;54:1230–1238. doi: 10.1097/JOM.0b013e3182480591. [DOI] [PubMed] [Google Scholar]
- 15.Rodearmel SJ, Wyatt HR, Stroebele N, Smith SM, Ogden LG, Hill JO. Small changes in dietary sugar and physical activity as an approach to preventing excessive weight gain: the America on the Move family study. Pediatrics. 2007;120:e869–79. doi: 10.1542/peds.2006-2927. [DOI] [PubMed] [Google Scholar]
- 16.Rodearmel SJ, Wyatt HR, Barry MJ, Dong F, Pan D, Israel RG, et al. A family-based approach to preventing excessive weight gain. Obesity (Silver Spring) 2006;14:1392–1401. doi: 10.1038/oby.2006.158. [DOI] [PubMed] [Google Scholar]
- 17.Charlson ME, Wells MT, Peterson JC, Boutin-Foster C, Ogedegbe GO, Mancuso CA, et al. Mediators and moderators of behavior change in patients with chronic cardiopulmonary disease: the impact of positive affect and self-affirmation. Transl Behav Med. 2014;4:7–17. doi: 10.1007/s13142-013-0241-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Phillips-Caesar EG, Winston G, Peterson JC, Wansink B, Devine CM, Kanna B, et al. Small Changes and Lasting Effects (SCALE) Trial: The Formation of a Weight Loss Behavioral Intervention Using EVOLVE. Contemp Clin Trials. 2015;41C:118–128. doi: 10.1016/j.cct.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wansink B. From mindless eating to mindlessly eating better. Physiol Behav. 2010;100:454–463. doi: 10.1016/j.physbeh.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 20.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 21.Anonymous Behavioral Risk Factor Surveillance System Survey Questionnaire. Atlanta, Georgia: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2012. [Google Scholar]
- 22.van Strien T, Frijters JER, Bergers GPA, Defares PB. The Dutch Eating Behavior Questionnaire (DEBQ) for assessment of restrained, emotional, and external eating behavior. Int J Eat Disord. 1986;5:295–315. [Google Scholar]
- 23.Devine CM, Jastran M, Jabs J, Wethington E, Farell TJ, Bisogni CA. “A lot of sacrifices:” work-family spillover and the food choice coping strategies of low-wage employed parents. Soc Sci Med. 2006;63:2591–2603. doi: 10.1016/j.socscimed.2006.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wansink B, Hanks AS, Kaipainen K. Slim by Design: Kitchen Counter Correlates of Obesity. Health Educ Behav. 2015 doi: 10.1177/1090198115610571. [DOI] [PubMed] [Google Scholar]
- 25.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- 26.Spielberger CD, Gorsuch RL, Lushene R. Manual for the State-Trait Anxiety Inventory. Consulting Psychologists Press; Palo Alto, CA: 1970. [Google Scholar]
- 27.Sherbourne CD, Stewart AL. The MOS social support survey. Soc Sci Med. 1991;32:705–714. doi: 10.1016/0277-9536(91)90150-b. [DOI] [PubMed] [Google Scholar]
- 28.Holmes TH, Rahe RH. The Social Readjustment Rating Scale. J Psychosom Res. 1967;11:213–218. doi: 10.1016/0022-3999(67)90010-4. [DOI] [PubMed] [Google Scholar]
- 29.Kroenke K, Spitzer RL, Williams JB. The Patient Health Questionnaire-2: validity of a two-item depression screener. Med Care. 2003;41:1284–1292. doi: 10.1097/01.MLR.0000093487.78664.3C. [DOI] [PubMed] [Google Scholar]
- 30.Bentler PM, Raykov T. On measures of explained variance in nonrecursive structural equation models. J Appl Psychol. 2000;85:125–131. doi: 10.1037/0021-9010.85.1.125. [DOI] [PubMed] [Google Scholar]
- 31.Bentler P, Freeman E. Tests for stability in linear structural equation systems. Psychometrika. 1983;48:143. [Google Scholar]
- 32.West DS, Elaine Prewitt T, Bursac Z, Felix HC. Weight loss of black, white, and Hispanic men and women in the Diabetes Prevention Program. Obesity (Silver Spring) 2008;16:1413–1420. doi: 10.1038/oby.2008.224. [DOI] [PubMed] [Google Scholar]
- 33.Bennett GG, Warner ET, Glasgow RE, Askew S, Goldman J, Ritzwoller DP, et al. Obesity treatment for socioeconomically disadvantaged patients in primary care practice. Arch Intern Med. 2012;172:565–574. doi: 10.1001/archinternmed.2012.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ockene IS, Tellez TL, Rosal MC, Reed GW, Mordes J, Merriam PA, et al. Outcomes of a Latino community-based intervention for the prevention of diabetes: the Lawrence Latino Diabetes Prevention Project. Am J Public Health. 2012;102:336–342. doi: 10.2105/AJPH.2011.300357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pressman SD, Cohen S. Does positive affect influence health? Psychol Bull. 2005;131:925–971. doi: 10.1037/0033-2909.131.6.925. [DOI] [PubMed] [Google Scholar]
- 36.Sturm R, Hattori A. Morbid obesity rates continue to rise rapidly in the United States. Int J Obes (Lond) 2013;37:889–891. doi: 10.1038/ijo.2012.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grossi E, Dalle Grave R, Mannucci E, Molinari E, Compare A, Cuzzolaro M, et al. Complexity of attrition in the treatment of obesity: clues from a structured telephone interview. Int J Obes (Lond) 2006;30:1132–1137. doi: 10.1038/sj.ijo.0803244. [DOI] [PubMed] [Google Scholar]
- 38.Nackers LM, Ross KM, Perri MG. The association between rate of initial weight loss and long-term success in obesity treatment: does slow and steady win the race? Int J Behav Med. 2010;17:161–167. doi: 10.1007/s12529-010-9092-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burke LE, Wang J, Sevick MA. Self-monitoring in weight loss: a systematic review of the literature. J Am Diet Assoc. 2011;111:92–102. doi: 10.1016/j.jada.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Viswanathan M, Kraschnewski JL, Nishikawa B, Morgan LC, Honeycutt AA, Thieda P, et al. Outcomes and costs of community health worker interventions: a systematic review. Med Care. 2010;48:792–808. doi: 10.1097/MLR.0b013e3181e35b51. [DOI] [PubMed] [Google Scholar]


