Abstract
Social attitudes and cultural norms around the issue of substance abuse are shifting rapidly around the world, leading to complex and unpredictable consequences. On the positive side, efforts to more intensly disseminate the scientific evidence for the many connections between chronic substance use and the emergence of measurable and discrete brain dysfunctions, has ushered in an evolving climate of acceptance and a new era of improved access to more effective interventions, at least in the United States. On the negative side, there has been a steady erosion in the public perception of the harms associated with the use of popular drugs, especially cannabis. This worrisome trend has sprouted at the convergence of several forces that have combined, more or less fortuitously, to effectively change long-standing policies away from prohibition and toward decriminalization or legalization. These forces include the outsized popularity of the cannabis plant among recreational users, the unflagging campaign by corporate lobbyists and patient advocates to mainstream its medicinal use, and the honest realization in some quarters of the deleterious impact of the drug war and its draconian cannabis laws, in particular, on society’s most vulnerable populations.
Updating drug policies is a desirable goal, and significant changes may indeed be warranted. However, there is a real concern when policy changes are hurriedly implemented without the required input from the medical, scientific, or policy research communities. Regardless of how well intentioned, such initiatives are bound to magnify the potential for unintended adverse consequences in the form of far ranging health and social costs. To minimize this risk, science must be front and center in this important policy debate.
Here, we review the state of the science on cannabis and cannabinoid health effects, both adverse and therapeutic. We focus on the prevalence of use in different populations, the mechanisms by which cannabis exerts its effects (i.e., via the endocannabinoid system), and the double-edged potential of this system to inspire new medications, on one hand, and to cause short and long term harmful effects on the other. By providing knowledge of cannabis’ broad ranging effects, we hope to enable better decision making regarding cannabis legislation and policy implementation.
Keywords: Cannabis Policy, Cannabis Science, Medicinal Cannabis, Cannabis Effects
Introduction
Cannabis policy is evolving rapidly, not only in the United States (US) but also in many countries around the world. To date, in the US, twenty-eight states plus the District of Columbia (D.C.) have legalized medical use of cannabis while sixteen states have legalized a component of the cannabis plant called cannabidiol (CBD) (National Conference of State Legislatures, 2016). Eight states plus D.C. have legalized the recreational use of cannabis for individuals over the age of 21 (National Conference of State Legislatures, 2016). These changes in State laws have occurred largely without significant input from the medical, scientific, or policy research communities, a stance that, unfortunately, appears to follow historical tradition when it comes to crafting cannabis policy. Consequently, the degree to which the current scientific evidence base has complemented other critical domains, like social science or economic methods, to inform the implementation of those policy changes is highly variable and, at best, minimal, While there is general consensus that cannabis use has risks, particularly for children and adolescents, there is also a recognition that prohibition has been ineffective or worse; leading, for example, to a disproportionate incarceration of ethnic and racial minorities (Drucker, 1999). At the same time, there are still many areas of contention and scientific ambiguity in need of further research. This paper briefly highlights the current state of the science regarding: a) the prevalence of cannabis use; b) the endocannabinoid system where cannabis’ active ingredients exert their effects; c) the potential for cannabis/cannabinoid based therapeutic development; d) the known and suspected risks associated with cannabis use; and e) some of the research gaps that need to be addressed in order to judiciously influence policy. The paper focuses on the basic science of cannabis and the endocannabinoid system in order to complement the extant epidemiological and policy literature discussed in this special issue.
Prevalence of Cannabis Use
Cannabis is the most commonly used illicit substance in the US and globally. An estimated 22.2 million Americans aged 12 or older reported current (past month) use in 2014 and approximately 181.8 million people worldwide, ages 15–64, consumed cannabis for nonmedical reasons in 2013 (Center for Behavioral Health Statistics and Quality, 2015) (Hall, Renström, & Poznyak, 2016). Approximately 4.2 million individuals in the US met diagnostic criteria for cannabis dependence in 2014 while about 5.7 million reported daily or almost daily use in the past 12 months (300 or more days) in 2013, up from 3.1 million in 2006 (Center for Behavioral Health Statistics and Quality, 2015) (Substance Abuse and Mental Health Services Administration, 2014). Not surprisingly, according to the National Survey on Drug Use and Health (NSDUH), the highest prevalence rate is among young adults aged 18–25 (approximately 19.6% of whom reported past month use in 2014), but this rate has been relatively stable over the last 5 years.. Increases in use were reported by adults over the age of 26, where past month cannabis use rose from 4.6 percent in 2009 to 6.6 percent in 2014 (p=0.01) (Center for Behavioral Health Statistics and Quality, 2015).
Among high school students (8th, 10th, and 12th graders), use also appears to have stabilized (or decreased in 8th graders) over the past 5 years, despite a declining perception of harm associated with occasional or frequent cannabis use during the same period (Johnston, Malley, Miech, Bachman, & Schulenberg, 2016). In 2015, 31.9% of 12th graders perceived regular cannabis use as risky compared to 46.8% in 2010, and 78.6 percent in 1991 (Johnston et al., 2016). In this age group, recent (past month) cannabis use (21.3%) exceeds that of cigarettes (11.4%) and e-cigarettes (16.2%) (Johnston et al., 2016). Also, 1.2 million youth aged 12 to 17 used cannabis for the first time in 2014 (Center for Behavioral Health Statistics and Quality, 2015)
Of greatest concern is the nearly 6% of 12th graders who report daily or almost daily cannabis use (Johnston et al., 2016). This statistic likely underestimates the true percentage for this age range, since regular cannabis use is associated with higher levels of school dropout, and Monitoring the Future (the source of these data) is a school-based survey (McCaffrey, Pacula, Han, & Ellickson, 2010) (Silins et al., 2014). Among regular users, cannabis’ cognitive impairing effects outlive its intoxicating effect, lasting between one and a few days (Solowij, 2002) (H. G. Pope et al., 2003). Thus, daily users are likely performing at sub-optimal levels all or most of the time, when they should be advancing academically and developing emotional maturity and social skills.
Impact of Medical Cannabis Laws on Non-medical Use
A number of researchers have investigated the impact of changes in cannabis’ legal status on its use, particularly in young populations (Gorman & Huber Jr., 2007) (Lynne-Landsman, Livingston, & Wagenaar, 2013) (Pacula, Powell, Heaton, & Sevigny, 2014). This research has mainly focused on medical cannabis laws, since legal recreational use (for those 21 and older) has only been enacted very recently. Although not all studies have drawn the same conclusions, the evidence suggests that the passage of medical cannabis laws does not per se increase the prevalence of cannabis use among adolescents (Stolzenberg, D’Alessio, & Dariano, 2016) (Hasin, Wall, et al., 2015) (Pacula et al., 2014). In fact, among 8th graders, where use has been decreasing, perceived harmfulness has risen in states that have passed medical cannabis laws (Keyes et al., 2016). However, youth in states with medical cannabis laws do report obtaining cannabis from friends or family members that have been authorized to receive it, suggesting significant diversion of medical cannabis (Johnston et al., 2016) (Salomonsen-Sautel, Sakai, Thurstone, Corley, & Hopfer, 2012). Moreover, states that have medical cannabis laws also have higher rates of cannabis use by youth (even before legalization) than those that do not (Hasin, Wall, et al., 2015). These findings may reflect a more permissive attitude towards cannabis, which might also have contributed to its legalization within these states in the first place.
Knowledge Gaps
Whereas national surveys provide useful data tracking the prevalence of cannabis use over time, these measures only provide snapshots that often fail to capture important information, including the quantity, frequency, and potency of the cannabis being used in the community (Hall, 2015) (Solowij, Lorenzetti, & Yücel, 2016). The potency of cannabis, defined as the concentration of the plant’s main psychoactive ingredient responsible for the “high” or reinforcing effects (Δ9-tetrahydrocannabinol or THC), has been steadily increasing in the US, from approximately 3% in the 1980’s to over 12% in recent years (ElSohly et al., 2016). However, these estimates are based on the potency measured in samples seized by the US Drug Enforcement Administration (DEA) and may not reflect the potency of cannabis strains being sold in dispensaries in States with legal cannabis access or the cannabis strains, formulations, and/or routes of administration (e.g. dabbing, shatter) that purportedly contain or achieve very high concentrations of THC (e.g., 70% or more). The effects of such high levels of THC exposure on the brain are unexplored, and may be particularly hazardous for young users, whose brains are actively developing, or for new users, who are not experienced at titrating their intake and may be more prone to suffering adverse reactions (Ilan, Gevins, Coleman, ElSohly, & de Wit, 2005) (Volkow et al., 2016).
The value of current surveillance instruments is also limited because of their inadequate capacity to assess polysubstance use, especially the combination of cannabis and tobacco (e.g., “spliffs or blunts”), or cannabis and alcohol (Grucza, Abbacchi, Przybeck, & Gfroerer, 2007) (Fairman, 2015). Thus, additional research is needed to better characterize the patterns, types, and frequencies of cannabis use among various populations and their social, behavioral, and cognitive impact, while taking into account the changing legal environment of cannabis.
The Endocannabinoid System (ECS): How Cannabis Exerts its Effects
Cannabis has been used for many centuries for various reasons; however, it was not until 1964 that researchers in Israel isolated and identified THC as the main psychoactive component of cannabis (Gaoni & Mechoulam, 1964). More than two decades after the discovery of THC, researchers in the US identified the first type of cannabinoid receptor (CB1) located throughout the central nervous system, mainly on neurons and glial cells in the brain, but they can also be found in several other organs throughout the body (Devane, Dysarz III, Johnson, Melvin, & Howlett, 1988). A few years later, another group of researchers found a second type of cannabinoid receptor (CB2), which was primarily expressed in the periphery, and most prominently in cells of the immune system (Munro, Thomas, & Abu-Shaar, 1993). With the discovery of these receptors, a search for the natural (i.e., endogenous) ligands that bind to them and the role of this system in normal physiology, in animals and humans, followed (Fride & Mechoulam, 1993) (Sugiura et al., 1995) (Mechoulam & Hanus, 2000). Our growing knowledge about the endocannabinoid system (ECS) combined with intense research documenting the interaction between THC and cannabinoid receptors (CBRs) have dramatically increased our understanding of how cannabis exerts its effects.
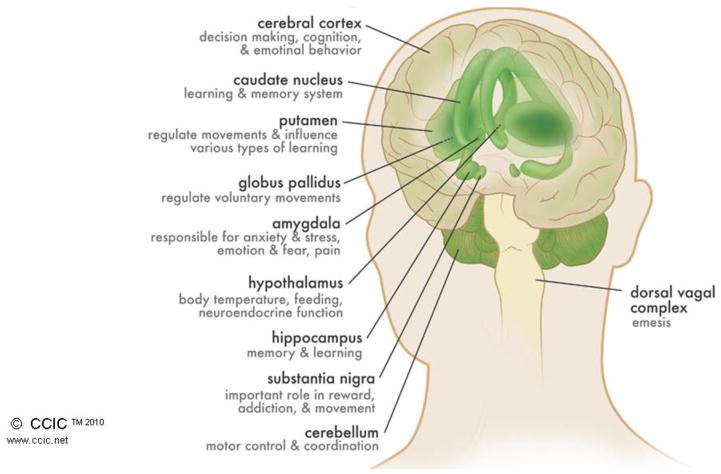
The ECS comprises the cannabinoid receptors (CB1 and CB2), their endogenous ligands (the best characterized of which are anandamide (AEA) and 2-arachidonoylglycerol (2-AG)), and the enzymes responsible for their synthesis and break down (including FAAH, MAGL, DAGL, NAPE-PLD, ABHD6, GDE1) (Piomelli, 2003). CB1 receptors are among the most ubiquitous receptors in the brain; understanding their distinct brain distribution has contributed greatly to our understanding of both ECS function and the effects of cannabis. For example, brain CB1 receptors reach their highest density in areas like the hippocampus (involved in memory formation), cerebellum and basal ganglia (involved in coordination and initiation of movement), cerebral cortex (involved in regulating executive function and enhanced sensation), hypothalamus (involved in regulating appetite), dorsal vagal complex (involved in emesis), and spinal cord (important in pain transmission/perception) (See Figure 1) (Mackie, 2008) (Herkenham et al., 1990). On the other hand, identification of brain structures with few CB1 receptors (such as those controlling respiratory function) help explain why cannabis does not typically depress respiration, the way other abused substances do (e.g., opioids) (Herkenham et al., 1990). CB2 receptors play an important role in the regulation of immune function and possibly pain, and are found in high concentrations in the spleen, gastrointestinal system, peripheral nervous system, and testes (Roche & Finn, 2010). They are also found in the brain to a lesser extent, where their function is less clear (Xi et al., 2011) (Malfitano, Basu, Maresz, Bifulco, & Dittel, 2014).
Figure 1.
Distribution of CB1 Receptors in the Brain. This figure illustrates the structures of the human brain with the highest density of CB1 receptor concentrations. Each identified brain structure is also notated with its attributed function. The figure is reproduced with permission from the Canadian Consortium for the Investigation of Cannabinoids.
The ECS is tightly regulated, with endocannabinoids being synthesized “on demand” in dynamic response to the activity state of a particular neural network (Figure 2). After being synthesized, endocannabinoids (e.g., 2-AG) travel back to the transmitting [presynaptic) neuron where they bind to cannabinoid receptors to dampen the activity of other neurotransmitter systems (e.g., GABA or glutamate) before being degraded through the action of specific hydrolytic enzymes (DAGL, in the case of 2-AG) (Piomelli, 2003). Thus, activation of cannabinoid receptors can lead to a blunting of excitation or inhibition within a circuit, depending on the types of neurons affected. While cannabinoid receptors are located on multiple neuron types (and glia cells), the most common are the glutamatergic (excitatory) and the GABAergic (inhibitory) neurons (Alger, 2012). Regional selectivity is conferred by the location of the neurons and the circuits they are a part of. This is why, when THC (or other cannabinoid agonists) activates cannabinoid receptors, it does so in a non-selective way, affecting CB receptors throughout the brain (and the body) thereby producing a non-physiological response. The enzymes that rapidly break down the endocannabinoids to halt their activity do not metabolize THC, so its effects persist rather than being under tight temporal control (Pertwee, 2008). With repeated exposure to THC, the endocannabinoid system may undergo plastic changes in an attempt to adapt to a chronically increased cannabinoid level (Hoffman & Lupica, 2013). This maladaptive process typically involves reductions in the number of CB1 receptors, which can lead to the ECS becoming less sensitive to endogenous cannabinoids and/or natural stimulation (Ceccarini et al., 2015) (Hirvonen et al., 2012). Moreover, users may experience this as tolerance, or a decrease in cannabis’ effects, which could lead to more frequent use or to the use of more potent cannabis strains. Yet, it is important to point out that these adaptations may be reversible: for example, CB1 receptor downregulation recovered following a 4 week period of monitored abstinence in daily cannabis users (Hirvonen et al., 2012). Finally, synthetic cannabinoids, like Spice, K2, and others, are considered “super agonists” because they exert a profound and long-lasting supraphysiological effect on the cannabinoid system, which can lead to severe toxic reactions, including psychosis (outlasting the drug’s intoxication effect), heart attacks, vomiting, seizures, etc. (Rosenbaum, Carreiro, & Babu, 2012).
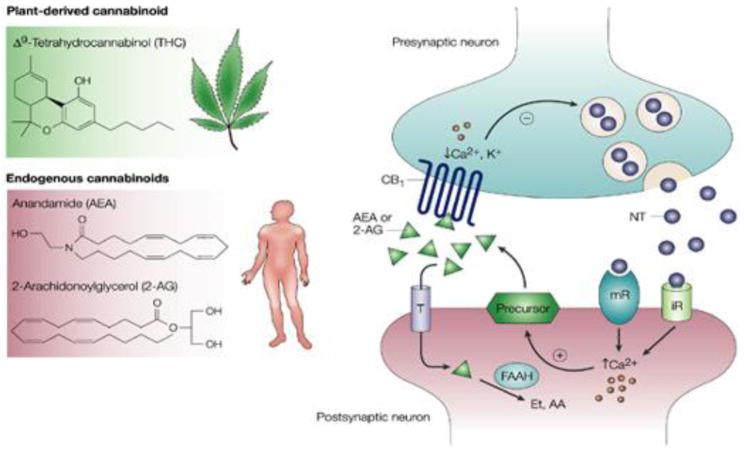
Figure 2.
The Endogenous Cannabinoid System. This figure illustrates how the endocannabinoid system transmits a signal from one neuron to another. The left panel shows the molecular structures of the plant derived (THC) and natural (AEA, 2AG) cannabinoids that bind to the CB1 cannabinoid receptor. The right panel illustrates how the endocannabinoid system works, using a schematic representation of a synaptic junction, which is where signals are passed from one neuron (presynaptic) to another (postsynaptic). When the presynaptic neuron is activated, it releases neurotransmitters (NT) that bind to receptors on the postsynaptic cell. Depending on the NT released, these can be ionotropic receptors (iR) which allow charged particles to flow directly into a cell, or metabotropic receptors (mR), which initiate a cascade of intracellular event events. In either case, intracellular calcium (Ca) is released, which stimulates the synthesis of endocannabinoids (AEA or 2AG) from precursor lipids located within the cell membrane. These endocannabinoids travel backwards to the presynaptic neuron where they bind to the CB1 receptors. Through a series of intracellular events, the endocannabinoids attenuate the subsequent release of neurotransmitter from the presynaptic neuron. Enzymes that breakdown the endocannabinoids (e.g. FAAH) are also located within this synaptic junction, enabling the rapid termination of the endocannabinoid signal. THC binds to the same CB1 receptors, displacing the natural cannabinoids, and remaining active for longer durations.
The figure is reprinted by permission from Macmillan Publishers Ltd: Nature Reviews Cancer. Guzman, M. Cannabinoids: potential anticancer agents. Nature Reviews Cancer, 3(10), 745–755., Copyright 2003.
Potential Therapeutic Applications
The ubiquity of cannabinoid receptors (type 1 and 2) and their endogenous activators in the brain and the body has led researchers to study the ECS in myriad physiological processes in both health and disease. In fact, it is nearly impossible to find a health condition in which the ECS has not been hypothesized to play some role (Pacher & Kunos, 2013). Thus, it is not surprising to find great interest in investigating ways for exploiting cannabis’ medical potential. A number of strategies are being employed to this end. For example, researchers are synthesizing and testing compounds that block the activity of the enzymes that break down the endogenous cannabinoids, such as FAAH or MAGL (Piomelli et al., 2006) (Blankman & Cravatt, 2013). This could allow for a selective response enhancing cannabinoid effects only in those neurons and circuits that happen to be active at the time of administration. FAAH inhibitors have been shown in animal models and some human studies to reduce anxiety, depression, nicotine and cannabis intake, and to improve social behavior in a model of autism spectrum disorders (Scherma et al., 2008) (Gunduz-Cinar, Hill, McEwen, & Holmes, 2013) (Marco et al., 2015) (Blankman & Cravatt, 2013). Side effects have been minimal, and tolerance (loss of efficacy following repeated administration) does not seem to occur. Another approach is to selectively activate peripheral CB2 receptors using compounds that do not cross the blood brain barrier, thus minimizing or totally avoiding undesirable psychoactive effects. Animal studies have provided evidence that such compounds can alleviate pain through their actions on the immune or peripheral nervous systems (Pertwee, 2012) (Rahn et al., 2011). There are also compounds being developed that are called ‘positive or negative allosteric modulators’ of cannabinoid receptors, which work by enhancing or reducing the cellular response to stimulation by an endogenous ligand (Morales, Goya, Jagerovic, & Hernandez-Folgado, 2016) (Baillie et al., 2013). Finally, there are receptor blockers for cannabinoid receptors that do not have any intrinsic activity on their own (Console-Bram, Marcu, & Abood, 2012). All these approaches strive for greater selectivity and physiological relevance to improve function while avoiding disruptive downstream or countering adaptations that would interfere with or even cancel any potential therapeutic benefits. Thus, while the evidence-base is still developing, the promise of therapeutics is marked.
State of the Science for the Medical Use of Cannabis or its Extracts
As a result of the current legal status of medical cannabis in many states across the US and nations around the world (e.g., Israel, Germany, the Netherlands, Canada, Mexico), various derivatives of the cannabis plant are routinely being used to treat over 50 conditions or symptoms, despite the paucity of clinical trials showing efficacy of the whole plant for any specific condition (Madras, 2015) (Whiting et al., 2015)(Koppel et al., 2014). This is further complicated by: 1) the inherent variability of botanical products stemming from genetic variations, growing conditions, use of pesticides, time of harvest, etc.; 2) the large number of compounds that comprise the plant which may or may not have significant, harmful, or even opposite biological activities (cannabis contains 400 compounds, including at least 100 cannabinoids); 3) the lack of information regarding optimal routes of administration and appropriate dosing; and 4) the absence of risk benefit analyses in different patient populations to guide prescribing practices and use (differential impact on, for example, terminally ill patients with no other options, children with severe intractable seizure disorders, or young adults with minor aches and pains).
The Food and Drug Administration’s (FDA’s) drug review and approval process is designed to ensure that new medicines, including those derived from botanicals, are appropriately evaluated for safety and effectiveness, are cultivated and manufactured under safe conditions for human consumption, and are consistent from batch to batch (U.S. Food and Drug Administration, 2014). In the current environment, many patients are using cannabis-derived products or extracts that have not undergone rigorous clinical trials, are not regulated for consistency or quality, and are indicated for medical conditions with an insufficient (or no) evidence base supporting their effectiveness.
Research is ongoing using the entire cannabis plant or its extracts, and most of it has focused on two cannabinoids: THC and CBD or their combination. As stated previously, the euphoric psychoactive effects of cannabis are caused by THC’s actions on CB1 receptors. CBD has a very low affinity for the CB receptors (100-fold less than THC), and does not appear to produce euphoria or intoxication. In fact, CBD’s actions may attenuate some of the effects of THC, although there is some disagreement about this in the literature. CBD’s (Vann et al., 2008) mechanism of action is not well understood, however, multiple signaling systems (e.g., the serotonin 5-HT1a receptor, orphan G-protein-coupled receptor GPR55, the α3 and α1 glycine receptors, and others) have been implicated (Devinsky et al., 2014) (Detyniecki & Hirsch, 2015). Preclinical studies have provided some evidence that CBD may have neuroprotective and anti-inflammatory effects (Hampson, Grimaldi, Axelrod, & Wink, 1998). CBD has also sparked a lot of attention for its potential therapeutic effects in severe forms of pediatric epilepsy, as well as in psychiatric conditions, including anxiety, psychosis, and addiction (Devinsky, Marsh, et al., 2015) (Paolino, Ferretti, Papetti, Villa, & Parisi, 2016) (Leweke et al., 2012) (Zuardi et al., 2012) (Mechoulam, Parker, & Gallily, 2002) (Blessing, Steenkamp, Manzanares, & Marmar, 2015) (Prud’homme, Cata, & Jutras-Aswad, 2015).
To date, the FDA has approved three cannabinoid-based medications, all of which are synthetic formulations identical or similar to THC: dronabinol (Marinol), an oral dronabinol solution (Syndros), and nabilone (Cesamet). These compounds have been approved for the treatment of severe nausea and vomiting in patients undergoing cancer chemotherapy, or wasting in patients with AIDS. A number of other countries have approved an oromucosal spray (nabiximols, trade name Sativex) that contains a purified cannabis extract with approximately equal parts THC and CBD for the treatment of spasms and pain associated with multiple sclerosis (Chaplin & Dobson, 2010) (Sastre-Garriga, Vila, Clissold, & Montalban, 2011). In addition, Phase 3 clinical trials are ongoing for the use of another GW Pharma product containing only CBD extracted from the cannabis plant (trade name Epidiolex) for the treatment of Dravet and Lennox-Gastaut Syndromes (severe forms of epilepsy) (Devinsky, Thiele, et al., 2015).
Recent scientific reviews in JAMA (Whiting et al., 2015) and by the American Academy of Neurology (Koppel et al., 2014) have concluded that there is strong evidence for the use of phytocannabinoids (nabiximols) to treat spasms and pain associated with multiple sclerosis, and moderate evidence for the treatment of neuropathic pain (using THC and nabiximols), although these indications are not yet approved by the FDA. A number of other conditions or symptoms are also being investigated, including Tourette’s syndrome, insomnia, obesity, cancer, anxiety, irritable bowel disease, autoimmune disorders, and more; however, the clinical trials data are not yet sufficient to show efficacy, and some of these indications are based solely on preclinical (animal or cell culture) data, which, more often than not, fail to translate into effective therapeutics (Zettl, Rommer, Hipp, & Patejdl, 2016) (Tsang & Giudice, 2016) (Curtis, Clarke, & Rickards, 2009) (Ben Amar, 2006) (Tanasescu & Constantinescu, 2010) (Grotenhermen & Müller-Vahl, 2012).
Adverse Consequences of Cannabis Use: Acute and Chronic Effects
While certain aspects and attributes associated with the chronic use of cannabis remain contested, the acute effects of cannabis intoxication i.e., relaxation, appetite stimulation, heightened sensation, impairment of balance and motor coordination, increased heart rate, impairment of short-term memory and learning, interference with executive function, including judgement and decision-making, and possible mental health disturbances, including psychosis and paranoia (especially with high doses or oral administration) have been well established (Volkow, Baler, Compton, & Weiss, 2014) (Volkow et al., 2016). However, our understanding of the sequelae of chronic cannabis use is less developed, particularly with regard to causality and permanence of effects. There are a number of reasons for this in particular, the preclusion of randomized controlled exposures (for ethical reasons) that could rule out pre-existing differences, and the common use of multiple substances (e.g., alcohol and tobacco) especially in adolescents. Nevertheless, there are some outcomes that are clearly linked to chronic cannabis use while animal studies can help determine causality.
Cannabis Use Disorder (CUD; Addiction)
There is overwhelming evidence that a subset of cannabis users will develop cannabis use disorders (CUD--formerly referred to as abuse, dependence, or addiction), evinced by trouble controlling intake, use in risky situations, social impairment, tolerance and withdrawal symptoms (Elkashef et al., 2008) (Budney & Hughes, 2006) (Stinson, Ruan, Pickering, & Grant, 2006). Prior research on the development of cannabis dependence suggests that approximately 9% of individuals that report ever using cannabis ultimately develop dependence (addiction) (Anthony, Warner, & Kessler, 1994) (Lopez-Quintero et al., 2011). Data collected from cannabis users in 2004–2005 indicated that the probability of transitioning to dependence was 8.9% with transition occurring more rapidly in cannabis users as compared to nicotine or alcohol users (Lopez-Quintero et al., 2011). Reports of global prevalence suggest that over 13 million individuals met the criteria for dependence in 2010, with higher population prevalence rates in males (0.23 percent [0.20–0.27 percent]) than in females (0.14 percent [0.12–0.16 percent]) (Degenhardt et al., 2013).
Trends in the prevalence of cannabis dependence or CUD are unclear, with two national surveys reporting discrepant results. NSDUH, which is conducted annually, reports no change or decreasing rates of dependence (among 12–17 year olds) between 2002 and 2014, but NESARC found a doubling in the rates of cannabis use and disorders in two waves of data collection covering approximately the same time frame (2002–2003–2012–2013) (Grucza, Agrawal, Krauss, Cavazos-Rehg, & Bierut, 2016) (Center for Behavioral Health Statistics and Quality, 2015) (Hasin, Saha, et al., 2015). Further analyses are ongoing to clarify the reasons for this difference.
Psychosis
Adverse mental health outcomes, including anxiety, depression, and suicidal ideation have all been linked to chronic cannabis use; however, the data supporting these associations have not been consistent (Volkow et al., 2016) (Minozzi et al., 2010) (McLaren, Silins, Hutchinson, Mattick, & Hall, 2010). In contrast, the link to psychosis (or schizophrenia) has been well replicated even though most cannabis users do not develop schizophrenia.
It is well established that ingestion of high doses of cannabis can produce an acute psychotic reaction, which typically resolves once the intoxication wears off and cannabis is cleared from the user’s system (Radhakrishnan, Wilkinson, & D’Souza, 2014). However, multiple lines of research have also suggested that cannabis use can be a risk (or component causal) factor for the development of chronic psychosis or schizophrenia in individuals with a genetic vulnerability. In addition, cannabis use is linked to an earlier onset of disease, and a more severe course of illness (Moore et al., 2007) (McLaren et al., 2010) (Volkow et al., 2016) (Arseneault et al., 2002). Cannabis is also frequently used by people with schizophrenia, in spite of the fact that it may worsen positive symptoms (hallucinations, delusions, confused thinking).
The link between cannabis use and psychosis was first described in a longitudinal study of Swedish conscripts by Andreasson et al, where 45,570 individuals were followed for 15 years, beginning in 1969/1970. Conscripts who reported cannabis use onset before or at age 18 were 2.4 times more likely to develop schizophrenia and those reporting more than 50 lifetime uses of cannabis were 6 times more likely (Andreasson, Engstrom, Allebeck, & Rydberg, 1987). These findings have been confirmed in other cohorts studies, which have also reported that individuals with cannabis use onset before or at age 18 are at an increased risk of developing psychosis (Arseneault et al., 2002) (Large, Sharma, Compton, Slade, & Nielssen, 2011) (Di Forti et al., 2014). Among the factors that may contribute to this enhanced vulnerability are genetic predisposition (e.g., polymorphisms of catechol-O-methyltransferase (COMT), AKT1, and DAT1, DRD2genes), family history, early initiation and regular use, and daily use of high THC potency products (Caspi et al., 2005) (Proal, Fleming, Galvez-Buccollini, & DeLisi, 2014) (Giordano, Ohlsson, Sundquist, Sundquist, & Kendler, 2015) (Volkow et al., 2016).
Neuroimaging results support the linkage, since there are common morphological changes in CUD and schizophrenia, which may contribute to an exacerbation of psychotic symptoms in chronic cannabis users (Smith et al., 2014) (Yucel et al., 2008) (Van Erp et al., 2016); and research showing altered cannabinoid receptors in patients with schizophrenia suggest a role for the ECS in vulnerability to psychosis (Volk, Eggan, Horti, Wong, & Lewis, 2014) (Wong et al., 2010). These findings, and the typical age of onset of schizophrenia (late adolescence to early adulthood) suggest that cannabis may disrupt brain development during a vulnerable period, in those with a family history/genetic predisposition to psychosis. Additional research is needed to delineate the exact nature of the association and the potential mechanisms by which cannabis use can affect psychosis risk and onset; as well as determining risk and protective factors that influence outcome.
Effects on the Developing Brain
The ECS is a critical signaling system that helps guide neuronal and glial cell proliferation, differentiation, and migration, beginning as early as embryogenesis (Maccarrone, Guzmán, Mackie, Doherty, & Harkany, 2014). From this early period of fetal development until the approximate age of 26 the brain continues to mature, mainly through experience-dependent synaptic pruning and increases in connectivity between cortical and subcortical regions, which become particularly extensive during the adolescent and early adult years (Gogtay et al., 2004). Since recent data suggest that prenatal use of cannabis during pregnancy is common globally (approximately 2–13 percent of women worldwide report cannabis use during pregnancy), and cannabis use often begins in early adolescence, understanding the impact of cannabis use on the developing brain is of critical importance (Marroun et al., 2010).
While there is a paucity of research evaluating the morphological effects of prenatal cannabis exposure on the human brain, a recent prospective study from the Generation R cohort in the Netherlands suggests that children with in utero exposure to cannabis develop thicker frontal cortices compared to children not exposed (Marroun et al., 2015). In addition, results from two older prospective longitudinal studies [Ottawa Prenatal Prospective Study (1978) and the Maternal Health Practices and Child Development Study (1982)], suggest deleterious behavioral and cognitive impacts, including problems with visual memory, language, attention and executive function in early childhood and adolescence, as well increased substance use (Fried, 1995) (Day et al., 1994) (Richardson, Ryan, Willford, Day, & Goldschmidt, 2002). Several caveats should be mentioned here, including the fact that it is often difficult to attribute findings to specific drug use, as there may be other variables that distinguish pregnant cannabis users from non-users, including other drug use (especially tobacco and alcohol), prenatal care, nutrition, etc. In addition, the early cohort studies, described above, occurred when cannabis’ potency was much lower than it is today; thus, effects of cannabis exposure may be underestimated compared to more recent cohorts, including Generation R, which began with women who were pregnant in 2002.
Taken together with the preclinical findings demonstrating a crucial role of the ECS in neural development, it would be reasonable to expect that cannabis use during pregnancy could interfere with normal neurodevelopmental maturation. More research is needed on the impact of timing, patterns, and quantities of exposures to cannabis, and its combined use with tobacco or other nicotine products. This is particularly urgent if use by pregnant women rises because of its anti-nausea properties and a failure to recognize its potential harms.
Adolescence
Adolescence is the stage in life when most drug use starts, and it is also a time of dramatic brain development synaptic pruning, axon myelination, and strengthening connections between cortical and subcortical regions (Casey, Jones, & Hare, 2008) (Spear, 2013)There is a growing literature suggesting that individuals who report frequent cannabis use during adolescence and into early adulthood suffer significantly worse outcomes in a variety of domains, and these effects are frequently “dose related”, with daily users being the most strongly impacted (Hurd, Michaelides, Miller, & Jutras-Aswad, 2014). These include academic achievement, income, life satisfaction, mental health, and other substance use or substance use disorders (Silins et al., 2014) (H. G. Pope et al., 2003) (Fergusson & Boden, 2008) (M. T. Lynskey, Coffey, Degenhardt, Carlin, & Patton, 2003). Alterations in brain structure and function may underlie some of these outcomes, along with disruptions in academic and social maturational experiences.
Cognitive function has been studied intensively after both acute and chronic cannabis exposure, and following various periods of abstinence (Broyd, van Hell, Beale, Yucel, & Solowij, 2016). While it is clear that cannabis use impairs short-term memory and other cognitive functions during intoxication, and for hours to days afterwards in chronic users, the persistence and accumulation of cognitive deficits over the long run is less well established (Volkow et al., 2014) (Volkow et al., 2016). Multiple studies have documented worse performance on neurocognitive tests in long-term heavy cannabis users compared to non-users, often correlated with duration and frequency of use, and earlier age of initiation (Volkow et al., 2014) (Volkow et al., 2016). Some, but not all, studies suggest that these cognitive deficiencies can be reversed following a month or more of abstinence (Bolla, Brown, Eldreth, Tate, & Cadet, 2002) (H. G. J. Pope, Gruber, Hudson, Huestis, & Yurgelun-Todd, 2002). Even so, regular cannabis use has been consistently associated with higher rates of school dropout, which may reflect learning, memory and attention problems leading to poorer school performance (M. Lynskey & Hall, 2000) (Bray, Zarkin, Ringwalt, & Qi, 2000) (M. T. Lynskey, Coffey, et al., 2003) (Solowij, 2002). Motivational factors may also contribute, including reduced dopamine synthesis capacity an amotivational syndrome linked to chronic cannabis use has been known for years; however, its course and underlying neurobiology is not well characterized (Bloomfield et al., 2014) (Volkow et al., 2016). Significant and possibly permanent declines in intelligence quotient (IQ) were reported in a longitudinal study (Dunedin cohort) among cannabis users who started as adolescents and developed cannabis dependence, with losses corresponding to severity or duration of dependence (Meier et al., 2012). A recent twin study questioned the causality implied by these findings; however, differences in methods make a direct comparison difficult (Jackson et al., 2016).
A growing body of neuroimaging research also suggests that early cannabis use negatively impacts the structure and function of developing brain circuits (Jacobus & Tapert, 2014). The adolescent brain, because it is undergoing dramatic growth, may be particularly vulnerable. Structural alterations have been reported in cortical (orbitofrontal, medial) and subcortical (amygdala, hippocampus, cerebellum) regions (Filbey et al., 2014). However, one important exception was a study comparing the size and shape of various subcortical structures in adolescent cannabis users vs. controls, which found no differences (Weiland et al., 2015). The subjects in this study were carefully matched for alcohol consumption, and this appeared to account for any regional differences in their findings. This highlights one of the important limitations of these studies, since many adolescents that are heavy cannabis users also use other substances, it can be difficult to parse out the various influences. Researchers try to account for these differences statistically, but that may not always be adequate, especially if there are interactions between the effects of various substances.
Research also suggests that cannabis users have impaired neural connectivity. A study of adults who began to use cannabis regularly as adolescents found marked decreases in the connectivity of the fimbria of the hippocampus which is important for memory formation, and the precuneus region, which is a major hub for many brain circuits (Zalesky et al., 2012). Functional and resting state neuroimaging studies have also shown altered neural activity or connectivity, which in some cases, correlated with poorer performance on neurocognitive tests (Batalla et al., 2013) (Houck, Bryan, & Feldstein Ewing, 2013).
Gateway effects
The age-old concept of cannabis as the paradigmatic “gateway drug” has fueled a particularly contentious area of research regarding causality linkages (D. Kandel, 1975) (D. B. Kandel, Kessler, & Margulies, 1978) (Morral, McCaffrey, & Paddock, 2002). In other words, does exposure to cannabis, particularly during critical periods such as prenatal or in adolescence, lead to other drug use or drug use disorders? There are a number of relevant issues to take into account as well as competing explanations: 1) temporal sequencing: cannabis usually precedes other substance use, except for nicotine and tobacco (which may be confounded by cannabis’ wide availability); 2) access: users of an illicit substance are more likely to have access to dealers or peers who can provide other illicit substances; 3) common liability: the same factors (genetic or environmental) that make an individual likely to use cannabis also increase their chances of using other substances; and 4) neurobiological alterations: exposure to cannabis changes a person’s neurobiology in a way that makes them more likely to use other substances. While all of these factors (and others) are likely to play a role, it remains true that among users of other substances, cannabis usually comes first; and also that most cannabis users do not go on to use other drugs. However, two lines of research are of interest in relation to gateway causal effects. One involves a twin cohort design comparing outcomes from monozygotic (identical) twins, raised together, who are discordant for early cannabis use. This controls for genetic and environmental influences as much as possible in a human study. Several groups have found that early cannabis users are more likely than their twins to use other drugs and develop a substance use disorder later in life (cannabis or other) (M. T. Lynskey, Heath, et al., 2003) (M. T. Lynskey, Vink, & Boomsma, 2006) (Grant et al., 2010). Animal research has also provided intriguing findings, indicative of not just a gateway effect, but one that may be transmitted across generations. Research by Yasmin Hurd and her colleagues has demonstrated that exposing mice to THC prenatally, or during the rodent equivalent of adolescence, leads to greater intake of heroin when these animals grow up and are given access to it (Spano, Ellgren, Wang, & Hurd, 2007). Hurd has further documented changes in the sensitivity of the reward system that are long-lasting and may mediate this effect (Ellgren, Spano, & Hurd, 2007). Animals first exposed to THC as adults were not similarly affected. More recently, Dr. Hurd found that rats whose parents were exposed to THC as adolescents (prior to the female rat becoming pregnant) inherited a vulnerability to heroin when given access that is, they worked harder than rats whose parents were never exposed to THC to obtain then heroin (Szutorisz et al., 2014). Whether these effects will translate into human predispositions remains an open question, but they are intriguing nevertheless.
Research Gaps
Teasing apart the many factors that contribute to worse outcomes in chronic cannabis users, particularly those who start early, remains a difficult undertaking. Data are rarely available for the same individuals before they started using substances, and polysubstance use is the norm, rather than the exception. Also, findings are not always consistent because it is hard to identify all of the relevant variables that can affect outcomes. In spite of challenges, the literature has been converging on several findings, especially the one pertaining to the association between cannabis use during vulnerable periods (i.e., adolescence) and greater risks of persistent adverse consequences. A more focused basic research effort will be paramount to help us understand why this may be the case. In particular, large scale, longitudinal studies using diverse populations are needed to disentangle the multiple interacting variables (confounding factors) associated with cannabis and other drug use, that influence brain development and functional outcomes. NIH has launched the Adolescent Brain Cognitive Development (ABCD) Study in order to address some of these questions in a large and diverse cohort (~10,000 participants) of 9–10 year olds who will be followed into early adulthood using brain imaging, behavioral assessment tools, including mobile technologies, and more(Collaborative Research on Addiction at the National Institutes of Health, 2016). Additional research will be needed beginning as early as pregnancy to improve our understanding of normal brain development and how exactly it can be affected by genetic factors and environmental exposures, including substance use.
Conclusions and Recommendations
Here, we highlight the state of scientific knowledge around cannabis’ therapeutic potential and adverse effects. This is not an exhaustive review, and it is clear that there are areas where the data are not yet sufficient to inform policy decisions. However, in addition to the basic science being conducted in various laboratories, there are many ongoing natural experiments that could prove very useful in the long-term. In the US, the legal landscape for cannabis is changing rapidly, with multiple such “experiments” taking place in different States. There are also many different decriminalization/legalization models implemented around the world that have been in effect for various periods of time and with very different track records (Greenwald, 2009) (Walsh & Ramsey, 2016) (MacCoun, 2011) (Rocky Mountains High Intensity Drug Trafficking Area, 2015). It is critical that we learn from all these approaches as much as possible, so that we can take steps to minimize harm, especially to those most vulnerable who are often the most adversely impacted by ill-advised, even if well-intentioned, policies. We also need to recognize our biases and measure outcomes rationally and thoughtfully, including consideration of both benefits and harms.
We know full well that policy decisions are complex, and guided by multiple competing interests. Moreover, it is unlikely that smart solutions will become universal recipes that will be effectively applicable, without modifications or adjustments, to very different national circumstances and cultural identities. As mentioned earlier, it is abundantly clear that we do not have all the answers we need. However, it may be useful to highlight some of what we consider to be key scientific questions for policy researchers and decision makers to focus our efforts as we explore the best way forward, to wit:
On the general issue of legalized cannabis:
How do specific cannabis laws influence incidence and prevalence of use? Should strain, potency, and routes of administration be regulated, and if so, how will that be monitored?
How will legalization affect academic achievement, vehicular and work related accidents, other drug use, including tobacco, alcohol, and prescription opioids? How will these be monitored and what preventive measures can be taken to counter negative effects?
Will changes in availability have differential populational impacts on prevalence and further widen existing social, academic, or economic gaps?
How will any unforeseen yet potentially significant public benefits derived from cannabis legalization be identified, monitored, and exploited?
What are the implications of government vs. private vs. non-profit models of commercialization?
What is the actual economic impact related to new revenues, healthcare costs (or savings), criminal justice repercussions, and workplace productivity?
Should marketing/targeted advertising be permitted? How will it impact youth and other vulnerable populations?
What percentage of revenue should be earmarked for research, treatment, or for education/prevention campaigns intended to reduce demand, and how can revenue diversion be prevented?
On the issue of “medical cannabis”:
What policies can be implemented to counteract the dissemination of unsubstantiated information?
What policies should be pursued to speed up the research needed to fully exploit the therapeutic potential of cannabis-derived medications, either as rigorously validated and standardized botanical products or in the form of pure or synthetic compounds?
Once cannabis derived (botanical) formulations are approved, how do we develop and enforce Good Manufacturing Practices (GMP) to protect the health of the public?
Will approved complex cannabis formulations offer additional benefits, or be more affordable than isolated active ingredients for specific clinical indications?
We hope that this list of questions will help to partially circumscribe the universe of potential research avenues and spur international collaborations to advance the goals of what is shaping up to become an important new sub-field in policy research.
Far from being prescriptive, the goal of this review is to inform policy researchers, policymakers, and other stakeholders about the current state of the science and the research gaps and needs vis á vis cannabis. We hope this information will prove useful to their efforts to craft, promote, and implement smart evidence-based cannabis policy.
Highlights.
Cannabis is the most commonly used illicit substance in the world.
More research is needed to better understand cannabis’ adverse effects.
Rapidly changing cannabis policies should be informed by science.
Researchers should study the consequences of current approaches to cannabis regulation.
More research is needed to explore the ECS and cannabinoid’s therapeutic potential.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alger BE. Endocannabinoids at the synapse a decade after the dies mirabilis (29 March 2001): what we still do not know. The Journal of Physiology. 2012;590(10):2203–2212. doi: 10.1113/jphysiol.2011.220855. http://doi.org/10.1113/jphysiol.2011.220855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasson S, Engstrom A, Allebeck P, Rydberg U. Cannabis and schizophrenia. A longitudinal study of Swedish conscripts. Lancet. 1987;330(8574):1483–1486. doi: 10.1016/s0140-6736(87)92620-1. http://doi.org/10.1016/S0140-6736(87)92620-1. [DOI] [PubMed] [Google Scholar]
- Anthony JC, Warner La, Kessler RC. Comparative epidemiology of dependence on tobacco, alcohol, controlled substances, and inhalants: Basic findings from the National Comorbidity Survey. Experimental and Clinical Psychopharmacology. 1994;2(3):244–268. http://doi.org/10.1037/1064-1297.2.3.244. [Google Scholar]
- Arseneault L, Cannon M, Poulton R, Murray R, Caspi A, Moffitt TE. Cannabis use in adolescence and risk for adult psychosis: longitudinal prospective study. BMJ (Clinical Research Ed) 2002;325:1212–1213. doi: 10.1136/bmj.325.7374.1212. http://doi.org/10.1136/bmj.325.7374.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baillie GL, Horswill JG, Anavi-Goffer S, Reggio PH, Bolognini D, Abood ME, … Ross Ra. CB(1) receptor allosteric modulators display both agonist and signaling pathway specificity. Molecular Pharmacology. 2013;83(2):322–338. doi: 10.1124/mol.112.080879. http://doi.org/10.1124/mol.112.080879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batalla A, Bhattacharyya S, Yucel M, Fusar-Poli P, Crippa JA, Nogue S, … Martin-Santos R. Structural and functional imaging studies in chronic cannabis users: a systematic review of adolescent and adult findings. PLoS One. 2013;8(2):e55821. doi: 10.1371/journal.pone.0055821. http://doi.org/10.1371/journal.pone.0055821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Amar M. Cannabinoids in medicine: A review of their therapeutic potential. Journal of Ethnopharmacology. 2006 doi: 10.1016/j.jep.2006.02.001. http://doi.org/10.1016/j.jep.2006.02.001. [DOI] [PubMed]
- Blankman JL, Cravatt BF. Chemical probes of endocannabinoid metabolism. Pharmacological Reviews. 2013;65(2):849–871. doi: 10.1124/pr.112.006387. http://doi.org/10.1124/pr.112.006387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blessing EM, Steenkamp MM, Manzanares J, Marmar CR. Cannabidiol as a Potential Treatment for Anxiety Disorders. Neurotherapeutics. 2015 doi: 10.1007/s13311-015-0387-1. http://doi.org/10.1007/s13311-015-0387-1. [DOI] [PMC free article] [PubMed]
- Bloomfield MAP, Morgan CJA, Egerton A, Kapur S, Curran HV, Howes OD. Dopaminergic function in cannabis users and its relationship to cannabis-induced psychotic symptoms. Biological Psychiatry. 2014;75(6):470–478. doi: 10.1016/j.biopsych.2013.05.027. http://doi.org/10.1016/j.biopsych.2013.05.027. [DOI] [PubMed] [Google Scholar]
- Bolla KI, Brown K, Eldreth D, Tate K, Cadet JL. Dose-related neurocognitive effects of marijuana use. Neurology. 2002;59:1337–1343. doi: 10.1212/01.wnl.0000031422.66442.49. http://doi.org/10.1212/01.WNL.0000031422.66442.49. [DOI] [PubMed] [Google Scholar]
- Bray JW, Zarkin GA, Ringwalt C, Qi J. The relationship between marijuana initiation and dropping out of high school. Health Economics. 2000;9(1):9–18. doi: 10.1002/(sici)1099-1050(200001)9:1<9::aid-hec471>3.0.co;2-z. http://doi.org/10.1002/(SICI)1099-1050(200001)9:1<9::AID-HEC471>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Broyd SJ, van Hell HH, Beale C, Yucel M, Solowij N. Acute and Chronic Effects of Cannabinoids on Human Cognition-A Systematic Review. Biological Psychiatry. 2016;79(7):557–567. doi: 10.1016/j.biopsych.2015.12.002. http://doi.org/10.1016/j.biopsych.2015.12.002. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Hughes JR. The cannabis withdrawal syndrome. Current Opinion in Psychiatry. 2006;19(3):233–238. doi: 10.1097/01.yco.0000218592.00689.e5. http://doi.org/10.1097/01.yco.0000218592.00689.e5. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Jones RM, Hare TA. The adolescent brain. Annals of the New York Academy of Sciences. 2008 doi: 10.1196/annals.1440.010. http://doi.org/10.1196/annals.1440.010. [DOI] [PMC free article] [PubMed]
- Caspi A, Moffitt TE, Cannon M, McClay J, Murray R, Harrington H, … Craig IW. Moderation of the effect of adolescent-onset cannabis use on adult psychosis by a functional polymorphism in the catechol-O-methyltransferase gene: longitudinal evidence of a gene X environment interaction. Biological Psychiatry. 2005;57(10):1117–1127. doi: 10.1016/j.biopsych.2005.01.026. [DOI] [PubMed] [Google Scholar]
- Ceccarini J, Kuepper R, Kemels D, Van Os J, Henquet C, Van Laere K. [18F]MK-9470 PET measurement of cannabinoid CB1 receptor availability in chronic cannabis users. Addiction Biology. 2015;20(2):357–367. doi: 10.1111/adb.12116. http://doi.org/10.1111/adb.12116. [DOI] [PubMed] [Google Scholar]
- Center for Behavioral Health Statistics and Quality. Behavioral health trends in the United States: Results from the 2014 National Survey on Drug Use and Health. 2015 HHS Pulication No. SMA 15-4927, NSDUH Series H-50. Retrieved from http://www.samhsa.gov/data/sites/default/files/NSDUH-FRR1-2014/NSDUH-FRR1-2014.pdf\nhttp://www.samhsa.gov/data/
- Chaplin S, Dobson R. Sativex: oromucosal cannabinoid spray for spasticity in MS. Prescriber. 2010;21(20):34–37. http://doi.org/10.1002/psb.684. [Google Scholar]
- Collaborative Research on Addiction at the National Institutes of Health. Adolescent Brain Cognitive Development Study. 2016 Retrieved from https://addictionresearch.nih.gov/abcd-study.
- Console-Bram L, Marcu J, Abood ME. Cannabinoid receptors: Nomenclature and pharmacological principles. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2012 doi: 10.1016/j.pnpbp.2012.02.009. http://doi.org/10.1016/j.pnpbp.2012.02.009. [DOI] [PMC free article] [PubMed]
- Curtis A, Clarke CE, Rickards H. Cochrane Database of Systematic Reviews. John Wiley & Sons, Ltd; 2009. Cannabinoids in Tourette’s syndrome. http://doi.org/10.1002/14651858.CD006565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day NL, Richardson GA, Goldschmidt L, Robles N, Taylor PM, Stoffer DS, … Geva D. Effect of prenatal marijuana exposure on the cognitive development of offspring at age three. Neurotoxicology and Teratology. 1994;16(2):169–175. doi: 10.1016/0892-0362(94)90114-7. http://doi.org/10.1016/0892-0362(94)90114-7. [DOI] [PubMed] [Google Scholar]
- Degenhardt L, Ferrari AJ, Calabria B, Hall WD, Norman RE, McGrath J, … Vos T. The Global Epidemiology and Contribution of Cannabis Use and Dependence to the Global Burden of Disease: Results from the GBD 2010 Study. PLoS ONE. 2013;8(10):e76635. doi: 10.1371/journal.pone.0076635. http://doi.org/10.1371/journal.pone.0076635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detyniecki K, Hirsch L. Marijuana Use in Epilepsy: The Myth and the Reality. Current Neurology and Neuroscience Reports. 2015 doi: 10.1007/s11910-015-0586-5. http://doi.org/10.1007/s11910-015-0586-5. [DOI] [PubMed]
- Devane WA, Dysarz FA, III, Johnson MR, Melvin LS, Howlett AC. Determination and characterization of a cannabinoid receptor in rat brain. Molecular Pharmacology. 1988;34(5):605–613. [PubMed] [Google Scholar]
- Devinsky O, Cilio MR, Cross H, Fernandez-Ruiz J, French J, Hill C, … Friedman D. Cannabidiol: Pharmacology and potential therapeutic role in epilepsy and other neuropsychiatric disorders. Epilepsia. 2014;55(6):791–802. doi: 10.1111/epi.12631. http://doi.org/10.1111/epi.12631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devinsky O, Marsh E, Friedman D, Thiele E, Laux L, Sullivan J, … Cilio MR. Cannabidiol in patients with treatment-resistant epilepsy: An open-label interventional trial. The Lancet Neurology. 2015;15(3):270–278. doi: 10.1016/S1474-4422(15)00379-8. http://doi.org/10.1016/S1474-4422(15)00379-8. [DOI] [PubMed] [Google Scholar]
- Devinsky O, Thiele E, Laux L, Friedman D, Patel A, Bluvstein J, … Marsh E. Efficacy and safety of epidiolex (cannabidiol) in children and young adults with treatment-resistant epilepsy: Initial data from an expanded access program. American Epilepsy Society: Annual Meeting. 2015 (p. Abstract 3.397) Retrieved from http://ovidsp.ovid.com/ovidweb.cgi?T=JS&CSC=Y&NEWS=N&PAGE=fulltext&D=emed13&AN=71845857\nhttp://sfx.ucl.ac.uk/sfx_local?sid=OVID:embase&id=pmid:&id=doi:&issn=153-5-7597&isbn=&volume=15&issue=&spage=532&pages=532&date=2015&title=Epilepsy+Currents&atitle=Eff.
- Di Forti M, Sallis H, Allegri F, Trotta A, Ferraro L, Stilo SA, … Murray RM. Daily use, especially of high-potency cannabis, drives the earlier onset of psychosis in cannabis users. Schizophrenia Bulletin. 2014;40(6):1509–1517. doi: 10.1093/schbul/sbt181. http://doi.org/10.1093/schbul/sbt181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drucker E. Drug Prohibition and Public Health: 25 Years of Evidence. Public Health Reports. 1999;114(1):14–29. doi: 10.1093/phr/114.1.14. http://doi.org/10.1093/phr/114.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkashef A, Vocci F, Huestis M, Haney M, Budney A, Gruber A, El-Guebaly N. Marijuana neurobiology and treatment. Substance Abuse. 2008;29(3):17–29. doi: 10.1080/08897070802218166. http://doi.org/10.1080/08897070802218166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellgren M, Spano SM, Hurd YL. Adolescent cannabis exposure alters opiate intake and opioid limbic neuronal populations in adult rats. Neuropsychopharmacology. 2007;32:607–615. doi: 10.1038/sj.npp.1301127. http://doi.org/10.1038/sj.npp.1301127. [DOI] [PubMed] [Google Scholar]
- ElSohly MA, Mehmedic Z, Foster S, Gon C, Chandra S, Church JC. Changes in cannabis potency over the last 2 decades (1995–2014): Analysis of current data in the United States. Biological Psychiatry. 2016;79(7):613–619. doi: 10.1016/j.biopsych.2016.01.004. http://doi.org/10.1016/j.biopsych.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairman BJ. Cannabis problem experiences among users of the tobacco-cannabis combination known as blunts. Drug and Alcohol Dependence. 2015;150:77–84. doi: 10.1016/j.drugalcdep.2015.02.014. http://doi.org/10.1016/j.drugalcdep.2015.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fergusson DM, Boden JM. Cannabis use and later life outcomes. Addiction. 2008;103(6):969–976. doi: 10.1111/j.1360-0443.2008.02221.x. http://doi.org/10.1111/j.1360-0443.2008.02221.x. [DOI] [PubMed] [Google Scholar]
- Filbey FM, Aslan S, Calhoun VD, Spence JS, Damaraju E, Caprihan A, Segall J. Long-term effects of marijuana use on the brain. Proceedings of the National Academy of Sciences. 2014;111(47):16913–16918. doi: 10.1073/pnas.1415297111. http://doi.org/10.1073/pnas.1415297111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fride E, Mechoulam R. Pharmacological activity of the cannabinoid receptor agonist, anandamide, a brain constituent. European Journal of Pharmacology. 1993;231(2):313–314. doi: 10.1016/0014-2999(93)90468-w. http://doi.org/10.1016/0014-2999(93)90468-W. [DOI] [PubMed] [Google Scholar]
- Fried PA. The Ottawa Prenatal Prospective Study (OPPS): methodological issues and findings--it’s easy to throw the baby out with the bath water. Life Sciences. 1995;56(23–24):2159–2168. doi: 10.1016/0024-3205(95)00203-i. http://doi.org/10.1016/0024-3205(95)00203-I. [DOI] [PubMed] [Google Scholar]
- Gaoni Y, Mechoulam R. Isolation, structure and partial synthesis of an active constituent of hashish. Journal of the American Chemical Society. 1964;86(8):1646–1647. http://doi.org/10.1039/ps9640000073. [Google Scholar]
- Giordano GN, Ohlsson H, Sundquist K, Sundquist J, Kendler K. The association between cannabis abuse and subsequent schizophrenia: a Swedish national co-relative control study. Psychological Medicine. 2015;45(2):407–414. doi: 10.1017/S0033291714001524. http://doi.org/10.1017/S0033291714001524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis aC, … Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(21):8174–9179. doi: 10.1073/pnas.0402680101. http://doi.org/10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman DM, Huber CJ., Jr Do medical cannabis laws encourage cannabis use? International Journal of Drug Policy. 2007;18(3):160–167. doi: 10.1016/j.drugpo.2006.10.001. http://doi.org/10.1016/j.drugpo.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Grant JD, Lynskey MT, Scherrer JF, Agrawal A, Heath AC, Bucholz KK. A cotwin-control analysis of drug use and abuse/dependence risk associated with early-onset cannabis use. Addictive Behaviors. 2010;35(1):35–41. doi: 10.1016/j.addbeh.2009.08.006. http://doi.org/10.1016/j.addbeh.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwald G. Portugal: Lessons For Creating Fair and Successful Drug Policies. CATO INSTITUTE; 2009. Drug Decriminalization. http://doi.org/10.1126/science.246.4934.1104-a. [Google Scholar]
- Grotenhermen F, Muller-Vahl K. The therapeutic potential of cannabis and cannabinoids. Deutsches A rzteblatt International. 2012;109(29–30):495–501. doi: 10.3238/arztebl.2012.0495. http://doi.org/10.3238/arztebl.2012.0495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grucza RA, Abbacchi AM, Przybeck TR, Gfroerer JC. Discrepancies in estimates of prevalence and correlates of substance use and disorders between two national surveys. Addiction. 2007;102(4):623–629. doi: 10.1111/j.1360-0443.2007.01745.x. http://doi.org/10.1111/j.1360-0443.2007.01745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grucza RA, Agrawal A, Krauss MJ, Cavazos-Rehg PA, Bierut LJ. Recent Trends in the Prevalence of Marijuana Use and Associated Disorders in the United States. JAMA Psychiatry. 2016;73(3):300–301. doi: 10.1001/jamapsychiatry.2015.3111. http://doi.org/10.1001/jamapsychiatry.2015.3111.Author. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunduz-Cinar O, Hill MN, McEwen BS, Holmes A. Amygdala FAAH and anandamide: Mediating protection and recovery from stress. Trends in Pharmacological Sciences. 2013 doi: 10.1016/j.tips.2013.08.008. http://doi.org/10.1016/j.tips.2013.08.008. [DOI] [PMC free article] [PubMed]
- Hall W. What has research over the past two decades revealed about the adverse health effects of recreational cannabis use? Addiction. 2015;110(1):19–35. doi: 10.1111/add.12703. http://doi.org/10.1111/add.12703. [DOI] [PubMed] [Google Scholar]
- Hall W, Renstrom M, Poznyak V, editors. The health and social effects of nonmedical cannabis use. 2016 Retrieved from http://www.who.int/substance_abuse/publications/msb_cannabis_report.pdf?ua=1.
- Hampson AJ, Grimaldi M, Axelrod J, Wink DA. Cannabidiol and (−)Delta9-tetrahydrocannabinol are neuroprotective antioxidants. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(14):8268–8273. doi: 10.1073/pnas.95.14.8268. http://doi.org/10.1073/pnas.95.14.8268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin DS, Saha TD, Kerridge BT, Goldstein RB, Chou SP, Zhang H, … Grant BF. Prevalence of marijuana use disorders in the United States between 2001–2002 and 2012–2013. JAMA Psychiatry. 2015;72(12):1235–1242. doi: 10.1001/jamapsychiatry.2015.1858. http://doi.org/10.1001/jamapsychiatry.2015.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin DS, Wall M, Keyes KM, Cerda M, Schulenberg J, O’Malley PM, … Feng T. Medical marijuana laws and adolescent marijuana use in the USA from 1991 to 2014: results from annual, repeated cross-sectional surveys. The Lancet Psychiatry. 2015;2(7):601–608. doi: 10.1016/S2215-0366(15)00217-5. http://doi.org/10.1016/S2215-0366(15)00217-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Little MD, Johnson MR, Melvin LS, de Costa BR, Rice KC. Cannabinoid receptor localization in brain. Proceedings of the National Academy of Sciences of the United States of America. 1990;87(5):1932–1936. doi: 10.1073/pnas.87.5.1932. http://doi.org/10.1073/pnas.87.5.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirvonen J, Goodwin RS, Li CT, Terry GE, Zoghbi SS, Morse CL, … Innis RB. Reversible and regionally selective downregulation of brain cannabinoid CB1 receptors in chronic daily cannabis smokers. Molecular Psychiatry. 2012;17(6):642–649. doi: 10.1038/mp.2011.82. http://doi.org/10.1038/mp.2011.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman AF, Lupica CR. Synaptic targets of Delta9-tetrahydrocannabinol in the central nervous system. Cold Spring Harbor Perspectives in Medicine. 2013;3(8) doi: 10.1101/cshperspect.a012237. http://doi.org/10.1101/cshperspect.a012237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houck JM, Bryan AD, Feldstein Ewing SW. Functional connectivity and cannabis use in high-risk adolescents. The American Journal of Drug and Alcohol Abuse. 2013;39(6):414–423. doi: 10.3109/00952990.2013.837914. http://doi.org/10.3109/00952990.2013.837914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurd YL, Michaelides M, Miller ML, Jutras-Aswad D. Trajectory of adolescent cannabis use on addiction vulnerability. Neuropharmacology. 2014;76(Part B):416–424. doi: 10.1016/j.neuropharm.2013.07.028. http://doi.org/10.1126/scisignal.2001449.Engineering. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilan AB, Gevins A, Coleman M, ElSohly MA, de Wit H. Neurophysiological and subjective profile of marijuana with varying concentrations of cannabinoids. Behavioural Pharmacology. 2005;16(5–6):487–496. doi: 10.1097/00008877-200509000-00023. http://doi.org/10.1097/00008877-200509000-00023. [DOI] [PubMed] [Google Scholar]
- Jackson NJ, Isen JD, Khoddam R, Irons D, Tuvblad C, Iacono WG, … Baker LA. Impact of adolescent marijuana use on intelligence: Results from two longitudinal twin studies. Proceedings of the National Academy of Sciences of the United States of America. 2016;113(5):E500–E508. doi: 10.1073/pnas.1516648113. http://doi.org/10.1073/pnas.1516648113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobus J, Tapert SF. Effects of cannabis on the adolescent brain. Current Pharmaceutical Design. 2014;20(13):2186–2193. doi: 10.2174/13816128113199990426. http://doi.org/10.2174/13816128113199990426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston LD, Malley PMO, Miech RA, Bachman JG, Schulenberg JE. Monitoring the Future national survey results on drug use, 1975–2015: Overview, key findings on adolescent drug use. Ann Arbor 2016 [Google Scholar]
- Kandel D. Stages in adolescent involvement in drug use. Science. 1975;190(4217):912–914. doi: 10.1126/science.1188374. http://doi.org/10.1126/science.1188374. [DOI] [PubMed] [Google Scholar]
- Kandel DB, Kessler RC, Margulies RZ. Antecedents of adolescent initiation into stages of drug use: A developmental analysis. Journal of Youth and Adolescence. 1978;7(1):13–40. doi: 10.1007/BF01538684. http://doi.org/10.1007/BF01538684. [DOI] [PubMed] [Google Scholar]
- Keyes KM, Wall M, Cerda M, Schulenberg J, O’Malley PM, Galea S, … Hasin DS. How does state marijuana policy affect U.S. youth? Medical marijuana laws, marijuana use and perceived harmfulness: 1991–2014. Addiction. 2016 doi: 10.1111/add.13523. http://doi.org/DOI:10.1111/add.13523. [DOI] [PMC free article] [PubMed]
- Koppel BS, Brust JCM, Fife T, Bronstein J, Youssof S, Gronseth G, Gloss D. Systematic review: Efficacy and safety of medical marijuana in selected neurologic disorders: Report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2014;82(17):1556–1563. doi: 10.1212/WNL.0000000000000363. http://doi.org/10.1212/WNL.0000000000000363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Large M, Sharma S, Compton MT, Slade T, Nielssen O. Cannabis use and earlier onset of psychosis: a systematic meta-analysis. Archives of General Psychiatry. 2011;68(6):555–561. doi: 10.1001/archgenpsychiatry.2011.5. http://doi.org/10.1016/j.ypsy.2011.09.029. [DOI] [PubMed] [Google Scholar]
- Leweke FM, Piomelli D, Pahlisch F, Muhl D, Gerth CW, Hoyer C, … Koethe D. Cannabidiol Enhances Anandamide Signaling and Alleviates Psychotic Symptoms of Schizophrenia. Translational Psychiatry. 2012;2(3):e94. doi: 10.1038/tp.2012.15. http://doi.org/10.1038/tp.2012.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Quintero C, de los Cobos JP, Hasin DS, Okuda M, Wang S, Grant BF, Blanco C. Probability and predictors of transition from first use to dependence on nicotine, alcohol, cannabis, and cocaine: Results of the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC) Drug and Alcohol Dependence. 2011;115(1–2):120–130. doi: 10.1016/j.drugalcdep.2010.11.004. http://doi.org/10.1016/j.drugalcdep.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynne-Landsman SD, Livingston MD, Wagenaar AC. Effects of state medical marijuana laws on adolescent marijuana use. American Journal of Public Health. 2013;103(8):1500–1506. doi: 10.2105/AJPH.2012.301117. http://doi.org/10.2105/AJPH.2012.301117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynskey M, Hall W. The effects of adolescent cannabis use on educational attainment: a review. Addiction. 2000;95(11):1621–1630. doi: 10.1046/j.1360-0443.2000.951116213.x. http://doi.org/10.1046/j.1360-0443.2000.951116213.x. [DOI] [PubMed] [Google Scholar]
- Lynskey MT, Coffey C, Degenhardt L, Carlin JB, Patton G. A longitudinal study of the effects of adolescent cannabis use on high school completion. Addiction. 2003;98(5):685–692. doi: 10.1046/j.1360-0443.2003.00356.x. http://doi.org/10.1046/j.1360-0443.2003.00356.x. [DOI] [PubMed] [Google Scholar]
- Lynskey MT, Heath AC, Bucholz KK, Slutske WS, Madden PAF, Nelson EC, … Martin NG. Escalation of drug use in early-onset cannabis users vs co-twin controls. JAMA. 2003;289(4):427–433. doi: 10.1001/jama.289.4.427. http://doi.org/10.1001/jama.289.4.427. [DOI] [PubMed] [Google Scholar]
- Lynskey MT, Vink JM, Boomsma DI. Early onset cannabis use and progression to other drug use in a sample of dutch twins. Behavior Genetics. 2006;36(2):195–200. doi: 10.1007/s10519-005-9023-x. http://doi.org/10.1007/s10519-005-9023-x. [DOI] [PubMed] [Google Scholar]
- Maccarrone M, Guzman M, Mackie K, Doherty P, Harkany T. Programming of neural cells by (endo)cannabinoids: from physiological rules to emerging therapies. Nature Reviews Neuroscience. 2014;15(12):786–801. doi: 10.1038/nrn3846. http://doi.org/10.1038/nrn3846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacCoun RJ. What can we learn from the Dutch cannabis coffeeshop system? Addiction. 2011;106(11):1899–1910. doi: 10.1111/j.1360-0443.2011.03572.x. http://doi.org/10.1111/j.1360-0443.2011.03572.x. [DOI] [PubMed] [Google Scholar]
- Mackie K. Cannabinoid receptors: Where they are and what they do. Journal of Neuroendocrinology. 2008;20:10–14. doi: 10.1111/j.1365-2826.2008.01671.x. http://doi.org/10.1111/j.1365-2826.2008.01671.x. [DOI] [PubMed] [Google Scholar]
- Madras BK. Update of Cannabis and its medical use. 2015 Retrieved from http://www.who.int/medicines/access/controlled-substances/6_2_cannabis_update.pdf.
- Malfitano AM, Basu S, Maresz K, Bifulco M, Dittel BN. What we know and do not know about the cannabinoid receptor 2 (CB2) Seminars in Immunology. 2014 doi: 10.1016/j.smim.2014.04.002. http://doi.org/10.1016/j.smim.2014.04.002. [DOI] [PMC free article] [PubMed]
- Marco EM, Rapino C, Caprioli A, Borsini F, Laviola G, Maccarrone M, Lodola A. Potential therapeutic value of a novel FAAH inhibitor for the treatment of anxiety. PLoS ONE. 2015;10(9) doi: 10.1371/journal.pone.0137034. http://doi.org/10.1371/journal.pone.0137034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Marroun H, Tiemeier H, Franken IHA, Jaddoe VWV, Van Der Lugt A, Verhulst FC, … White T. Prenatal Cannabis and Tobacco Exposure in Relation to Brain Morphology: A Prospective Neuroimaging Study in Young Children. Biological Psychiatry. 2015;79(12):1–9. doi: 10.1016/j.biopsych.2015.08.024. http://doi.org/10.1016/j.biopsych.2015.08.024. [DOI] [PubMed] [Google Scholar]
- El Marroun H, Tiemeier H, Jaddoe VWV, Hofman A, Verhulst FC, Van Den Brink W, Huizink AC. Agreement between maternal cannabis use during pregnancy according to self-report and urinalysis in a population-based cohort: The generation R study. European Addiction Research. 2010;17(1):37–43. doi: 10.1159/000320550. http://doi.org/10.1159/000320550. [DOI] [PubMed] [Google Scholar]
- McCaffrey DF, Pacula RL, Han B, Ellickson P. Marijuana Use and High School Dropout: The Influence of Unobservables. Health Economics. 2010;19(11):1281–1299. doi: 10.1002/hec.1561. http://doi.org/10.1002/hec.1561.Marijuana. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaren JA, Silins E, Hutchinson D, Mattick RP, Hall W. Assessing evidence for a causal link between cannabis and psychosis: A review of cohort studies. International Journal of Drug Policy. 2010 doi: 10.1016/j.drugpo.2009.09.001. http://doi.org/10.1016/j.drugpo.2009.09.001. [DOI] [PubMed]
- Mechoulam R, Hanus L. A historical overview of chemical research on cannabinoids. Chemistry and Physics of Lipids. 2000;108(1–2):1–13. doi: 10.1016/s0009-3084(00)00184-5. http://doi.org/10.1016/S0009-3084(00)00184-5. [DOI] [PubMed] [Google Scholar]
- Mechoulam R, Parker La, Gallily R. Cannabidiol: an overview of some pharmacological aspects. Journal of Clinical Pharmacology. 2002;42:11S–19S. doi: 10.1002/j.1552-4604.2002.tb05998.x. http://doi.org/10.1177/0091270002238789. [DOI] [PubMed] [Google Scholar]
- Meier MH, Caspi A, Ambler A, Harrington H, Houts R, Keefe RSE, … Moffitt TE. Persistent cannabis users show neuropsychological decline from childhood to midlife. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(40):E2657–E2664. doi: 10.1073/pnas.1206820109. http://doi.org/10.1073/pnas.1206820109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minozzi S, Davoli M, Bargagli AM, Amato L, Vecchi S, Perucci CA. An overview of systematic reviews on cannabis and psychosis: Discussing apparently conflicting results. Drug and Alcohol Review. 2010 doi: 10.1111/j.1465-3362.2009.00132.x. http://doi.org/10.1111/j.1465-3362.2009.00132.x. [DOI] [PubMed]
- Moore TH, Zammit S, Lingford-Hughes A, Barnes TR, Jones PB, Burke M, Lewis G. Cannabis use and risk of psychotic or affective mental health outcomes: a systematic review. Lancet. 2007;370(9584):319–328. doi: 10.1016/S0140-6736(07)61162-3. http://doi.org/10.1016/s0140-6736(07)61162-3. [DOI] [PubMed] [Google Scholar]
- Morales P, Goya P, Jagerovic N, Hernandez-Folgado L. Allosteric Modulators of the CB1 Cannabinoid Receptor: A Structural Update Review. Cannabis and Cannabinoid Research. 2016;1:22–30. doi: 10.1089/can.2015.0005. http://doi.org/10.1089/can.2015.0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morral AR, McCaffrey DF, Paddock SM. Reassessing the marijuana gateway effect. Addiction. 2002 doi: 10.1046/j.1360-0443.2002.00280.x. http://doi.org/10.1046/j.1360-0443.2002.00280.x. [DOI] [PubMed]
- Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365(6441):61–65. doi: 10.1038/365061a0. http://doi.org/10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- National Conference of State Legislatures. State Medical Marijuana Laws. 2016 Retrieved July 27, 2016, from http://www.ncsl.org/research/health/state-medical-marijuana-laws.aspx.
- Pacher P, Kunos G. Modulating the endocannabinoid system in human health and disease - Successes and failures. FEBS Journal. 2013 doi: 10.1111/febs.12260. http://doi.org/10.1111/febs.12260. [DOI] [PMC free article] [PubMed]
- Pacula RL, Powell D, Heaton P, Sevigny EL. Assessing the effects of medical marijuana laws on marijuana use: the devil is in the details. Journal of Policy Analysis and Management. 2014;34(1):7–31. doi: 10.1002/pam.21804. http://doi.org/10.1002/jhm.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolino MC, Ferretti A, Papetti L, Villa MP, Parisi P. Cannabidiol as potential treatment in refractory pediatric epilepsy. Expert Review of Neurotherapeutics. 2016;16(1):17–21. doi: 10.1586/14737175.2016.1121098. http://doi.org/10.1586/14737175.2016.1121098. [DOI] [PubMed] [Google Scholar]
- Pertwee RG. Ligands that target cannabinoid receptors in the brain: From THC to anandamide and beyond. Addiction Biology. 2008 doi: 10.1111/j.1369-1600.2008.00108.x. http://doi.org/10.1111/j.1369-1600.2008.00108.x. [DOI] [PubMed]
- Pertwee RG. Targeting the endocannabinoid system with cannabinoid receptor agonists: pharmacological strategies and therapeutic possibilities. Philosophical Transactions of the Royal Society of London Series B, Biological Sciences. 2012;367(1607):3353–3363. doi: 10.1098/rstb.2011.0381. http://doi.org/10.1098/rstb.2011.0381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piomelli D. The molecular logic of endocannabinoid signalling. Nature Reviews Neuroscience. 2003;4(11):873–884. doi: 10.1038/nrn1247. http://doi.org/10.1038/nrn1247. [DOI] [PubMed] [Google Scholar]
- Piomelli D, Tarzia G, Duranti A, Tontini A, Mor M, Compton TR, … Putman D. Pharmacological profile of the selective FAAH inhibitor KDS-4103 (URB597) CNS Drug Reviews. 2006 doi: 10.1111/j.1527-3458.2006.00021.x. http://doi.org/10.1111/j.1527-3458.2006.00021.x. [DOI] [PMC free article] [PubMed]
- Pope HG, Gruber AJ, Hudson JI, Cohane G, Huestis MA, Yurgelun-Todd D. Early-onset cannabis use and cognitive deficits: What is the nature of the association? Drug and Alcohol Dependence. 2003;69(3):303–310. doi: 10.1016/s0376-8716(02)00334-4. http://doi.org/10.1016/S0376-8716(02)00334-4. [DOI] [PubMed] [Google Scholar]
- Pope HGJ, Gruber AJ, Hudson JI, Huestis MA, Yurgelun-Todd D. Cognitive measures in long-term cannabis users. Journal of Clinical Pharmacology. 2002;42(11 Suppl):41S–47S. doi: 10.1002/j.1552-4604.2002.tb06002.x. http://doi.org/10.1212/01.WNL.0000031422.66442.49. [DOI] [PubMed] [Google Scholar]
- Proal AC, Fleming J, Galvez-Buccollini JA, DeLisi LE. A controlled family study of cannabis users with and without psychosis. Schizophrenia Research. 2014;152(1):283–288. doi: 10.1016/j.schres.2013.11.014. http://doi.org/10.1016/j.schres.2013.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prud’homme M, Cata R, Jutras-Aswad D. Cannabidiol as an Intervention for Addictive Behaviors: A Systematic Review of the Evidence. Substance Abuse: Research and Treatment. 2015;9:33–38. doi: 10.4137/SART.S25081. http://doi.org/10.4137/SART.S25081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radhakrishnan R, Wilkinson ST, D’Souza DC. Gone to Pot - A Review of the Association between Cannabis and Psychosis. Frontiers in Psychiatry. 2014;5:54. doi: 10.3389/fpsyt.2014.00054. http://doi.org/10.3389/fpsyt.2014.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahn EJ, Thakur GA, Wood JAT, Zvonok AM, Makriyannis A, Hohmann AG. Pharmacological characterization of AM1710, a putative cannabinoid CB 2 agonist from the cannabilactone class: Antinociception without central nervous system side-effects. Pharmacology Biochemistry and Behavior. 2011;98(4):493–502. doi: 10.1016/j.pbb.2011.02.024. http://doi.org/10.1016/j.pbb.2011.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson GA, Ryan C, Willford J, Day NL, Goldschmidt L. Prenatal alcohol and marijuana exposure: Effects on neuropsychological outcomes at 10 years. Neurotoxicology and Teratology. 2002;24(3):309–320. doi: 10.1016/s0892-0362(02)00193-9. http://doi.org/10.1016/S0892-0362(02)00193-9. [DOI] [PubMed] [Google Scholar]
- Roche M, Finn DP. Brain CB2 receptors: Implications for neuropsychiatric disorders. Pharmaceuticals. 2010 doi: 10.3390/ph3082517. http://doi.org/10.3390/ph3082517. [DOI] [PMC free article] [PubMed]
- Rocky Mountains High Intensity Drug Trafficking Area. Legalization of Marijuana in Colorado - The Impact. Annual Reports on the Impact of Legalized Marijuana in Colorado. 2015;3 http://doi.org/10.1017/CBO9781107415324.004. [Google Scholar]
- Rosenbaum CD, Carreiro SP, Babu KM. Here Today, Gone Tomorrow. and Back Again? A Review of Herbal Marijuana Alternatives (K2, Spice), Synthetic Cathinones (Bath Salts), Kratom, Salvia divinorum, Methoxetamine, and Piperazines. Journal of Medical Toxicology. 2012;8(1):15–32. doi: 10.1007/s13181-011-0202-2. http://doi.org/10.1007/s13181-011-0202-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomonsen-Sautel S, Sakai JT, Thurstone C, Corley R, Hopfer C. Medical marijuana use among adolescents in substance abuse treatment. Journal of the American Academy of Child and Adolescent Psychiatry. 2012;51(7):694–702. doi: 10.1016/j.jaac.2012.04.004. http://doi.org/10.1016/j.jaac.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sastre-Garriga J, Vila C, Clissold S, Montalban X. THC and CBD oromucosal spray (SativexR) in the management of spasticity associated with multiple sclerosis. Expert Review of Neurotherapeutics. 2011;11(5):627–637. doi: 10.1586/ern.11.47. http://doi.org/10.1586/ern.11.47. [DOI] [PubMed] [Google Scholar]
- Scherma M, Medalie J, Fratta W, Vadivel SK, Makriyannis A, Piomelli D, … Goldberg SR. The endogenous cannabinoid anandamide has effects on motivation and anxiety that are revealed by fatty acid amide hydrolase (FAAH) inhibition. Neuropharmacology. 2008;54(1):129–140. doi: 10.1016/j.neuropharm.2007.08.011. http://doi.org/10.1016/j.neuropharm.2007.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scigliano JA. THC Therapeutic Research by Independent and State-Sponsored Investigators: A Historical Review. Journal of Clinical Pharmacology. 1981;21(8–9 Suppl):113S–121S. doi: 10.1002/j.1552-4604.1981.tb02584.x. [DOI] [PubMed] [Google Scholar]
- Silins E, Horwood LJ, Patton GC, Fergusson DM, Olsson CA, Hutchinson DM, … Slade T. Young adult sequelae of adolescent cannabis use: An integrative analysis. Lancet Psychiatry. 2014;1(4):286–293. doi: 10.1016/S2215-0366(14)70307-4. http://doi.org/10.1016/S2215-0366(14)70307-4. [DOI] [PubMed] [Google Scholar]
- Smith MJ, Cobia DJ, Wang L, Alpert KI, Cronenwett WJ, Goldman MB, … Csernansky JG. Cannabis-related working memory deficits and associated subcortical morphological differences in healthy individuals and schizophrenia subjects. Schizophrenia Bulletin. 2014;40(2):287–299. doi: 10.1093/schbul/sbt176. http://doi.org/10.1093/schbul/sbt176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solowij N. Cognitive Functioning of Long-term Heavy Cannabis Users Seeking Treatment. JAMA. 2002;287(9):1123–1131. doi: 10.1001/jama.287.9.1123. http://doi.org/10.1001/jama.287.9.1123. [DOI] [PubMed] [Google Scholar]
- Solowij N, Lorenzetti V, Yucel M. Effects of Cannabis Use on Human Behavior A Call for Standardization of Cannabis Use Metrics. JAMA Psychiatry. 2016:E1. doi: 10.1001/jamapsychiatry.2016.1329. http://doi.org/10.1001/jamapsychiatry.2016.1329. [DOI] [PubMed]
- Spano MS, Ellgren M, Wang X, Hurd YL. Prenatal Cannabis Exposure Increases Heroin Seeking with Allostatic Changes in Limbic Enkephalin Systems in Adulthood. Biological Psychiatry. 2007;61(4):554–563. doi: 10.1016/j.biopsych.2006.03.073. http://doi.org/10.1016/j.biopsych.2006.03.073. [DOI] [PubMed] [Google Scholar]
- Spear LP. Adolescent neurodevelopment. Journal of Adolescent Health. 2013 doi: 10.1016/j.jadohealth.2012.05.006. http://doi.org/10.1016/j.jadohealth.2012.05.006. [DOI] [PMC free article] [PubMed]
- Stinson FS, Ruan WJ, Pickering R, Grant BF. Cannabis use disorders in the USA: prevalence, correlates and co-morbidity. Psychological Medicine. 2006;36(10):1447–1460. doi: 10.1017/S0033291706008361. http://doi.org/10.1017/S0033291706008361. [DOI] [PubMed] [Google Scholar]
- Stolzenberg L, D’Alessio SJ, Dariano D. The effect of medical cannabis laws on juvenile cannabis use. International Journal of Drug Policy. 2016;27:82–88. doi: 10.1016/j.drugpo.2015.05.018. http://doi.org/10.1016/j.drugpo.2015.05.018. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Results from the 2013 National Survey on Drug Use and Health: Summary of National Findings. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2014. NSDUH Series H-48, HHS Publication No. (SMA) 14-4863. http://doi.org/NSDUH Series H-41, HHS Publication No. (SMA) 11-4658. [Google Scholar]
- Sugiura T, Kondo S, Sukagawa A, Nakane S, Shinoda A, Itoh K, … Waku K. 2-Arachidonoylgylcerol: A Possible Endogenous Cannabinoid Receptor Ligand in Brain. Biochemical and Biophysical Research Communications. 1995;215(1):89–97. doi: 10.1006/bbrc.1995.2437. http://doi.org/10.1006/bbrc.1995.2437. [DOI] [PubMed] [Google Scholar]
- Szutorisz H, DiNieri J, Sweet E, Gabor Egervari G, Michaelides M, Carter J, … Hurd Y. Parental THC Exposure Leads to Compulsive Heroin-Seeking and Altered Striatal Synaptic Plasticity in the Subsequent Generation. Neuropsychopharmacology. 2014;39:1315–1323. doi: 10.1038/npp.2013.352. http://doi.org/10.1038/npp.2013.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanasescu R, Constantinescu CS. Cannabinoids and the immune system: An overview. Immunobiology. 2010;215(8):588–597. doi: 10.1016/j.imbio.2009.12.005. http://doi.org/10.1016/j.imbio.2009.12.005. [DOI] [PubMed] [Google Scholar]
- Tsang CC, Giudice MG. Nabilone for the Management of Pain. Pharmacotherapy. 2016 doi: 10.1002/phar.1709. http://doi.org/10.1002/phar.1709. [DOI] [PubMed]
- U.S. Food and Drug Administration. The FDA’s Drug Review Process: Ensuring Drugs Are Safe and Effective. 2014 Retrieved from http://www.fda.gov/drugs/resourcesforyou/consumers/ucm143534.htm.
- Van Erp TGM, Hibar DP, Rasmussen JM, Glahn DC, Pearlson GD, Andreassen OA, … Turner JA. Subcortical brain volume abnormalities in 2028 individuals with schizophrenia and 2540 healthy controls via the ENIGMA consortium. Molecular Psychiatry. 2016;21:1–7. doi: 10.1038/mp.2015.118. http://doi.org/10.1038/mp.2015.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vann RE, Gamage TF, Warner JA, Marshall EM, Taylor NL, Martin BR, Wiley JL. Divergent effects of cannabidiol on the discriminative stimulus and place conditioning effects of Δ9-tetrahydrocannabinol. Drug and Alcohol Dependence. 2008;94(1–3):191–198. doi: 10.1016/j.drugalcdep.2007.11.017. http://doi.org/10.1016/j.drugalcdep.2007.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk DW, Eggan SM, Horti AG, Wong DF, Lewis DA. Reciprocal alterations in cortical cannabinoid receptor 1 binding relative to protein immunoreactivity and transcript levels in schizophrenia. Schizophrenia Research. 2014;159(1):124–129. doi: 10.1016/j.schres.2014.07.017. http://doi.org/10.1016/j.schres.2014.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Baler RD, Compton WM, Weiss SRB. Adverse health effects of marijuana use. The New England Journal of Medicine. 2014;370:2219–2227. doi: 10.1056/NEJMra1402309. http://doi.org/10.1056/NEJMra1402309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Swanson JM, Evins AE, DeLisi LE, Meier MH, Gonzalez R, … Baler R. Effects of Cannabis Use on Human Behavior, Including Cognition, Motivation, and Psychosis: A Review. JAMA Psychiatry. 2016;73(3):292–297. doi: 10.1001/jamapsychiatry.2015.3278. http://doi.org/10.1001/jamapsychiatry.2015.3278. [DOI] [PubMed] [Google Scholar]
- Walsh J, Ramsey G. Uruguay’s drug policy: major innovations, major challenges. Improving Global Drug Policy: Comparative Perspectives and UNGASS. 2016 Retrieved from https://www.brookings.edu/wp-content/uploads/2016/07/Walsh-Uruguay-final.pdf.
- Weiland BJ, Thayer RE, Depue BE, Sabbineni A, Bryan AD, Hutchison KE. Daily Marijuana Use Is Not Associated with Brain Morphometric Measures in Adolescents or Adults. The Journal of Neuroscience. 2015;35(4):1505–1512. doi: 10.1523/JNEUROSCI.2946-14.2015. http://doi.org/10.1523/JNEUROSCI.2946-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiting PF, Wolff RF, Deshpande S, Di Nisio M, Duffy S, Hernandez AV, … Kleijnen J. Cannabinoids for Medical Use: A Systematic Review and Meta-analysis. JAMA. 2015;313(24):2456–2473. doi: 10.1001/jama.2015.6358. http://doi.org/10.1001/jama.2015.6358. [DOI] [PubMed] [Google Scholar]
- Wong DF, Kuwabara H, Horti AG, Raymont V, Brasic J, Guevara M, … Cascella N. Quantification of cerebral cannabinoid receptors subtype 1 (CB1) in healthy subjects and schizophrenia by the novel PET radioligand [11C]OMAR. NeuroImage. 2010;52(4):1505–1513. doi: 10.1016/j.neuroimage.2010.04.034. http://doi.org/10.1016/j.neuroimage.2010.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi Z, Peng X, Li X, Song R, Zhang H, Liu QR, … Gardner EL. Brain Cannabinoid CB2 Receptors Modulate Cocaine’s Actions in Mice. Nature Neuroscience. 2011;14(9):1160–1166. doi: 10.1038/nn.2874. http://doi.org/10.1038/nn.2874.Brain. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yücel M, Solowij N, Respondek C, Whittle S, Fornito A, Pantelis C, Lubman DI. Regional brain abnormalities associated with long-term heavy cannabis use. Archives of General Psychiatry. 2008;65(6):694–701. doi: 10.1001/archpsyc.65.6.694. http://doi.org/10.1001/archpsyc.65.6.694. [DOI] [PubMed] [Google Scholar]
- Zalesky A, Solowij N, Y??cel M, Lubman DI, Takagi M, Harding IH, … Seal M. Effect of long-term cannabis use on axonal fibre connectivity. Brain. 2012;135(7):2245–2255. doi: 10.1093/brain/aws136. http://doi.org/10.1093/brain/aws136. [DOI] [PubMed] [Google Scholar]
- Zettl UK, Rommer P, Hipp P, Patejdl R. Evidence for the efficacy and effectiveness of THC-CBD oromucosal spray in symptom management of patients with spasticity due to multiple sclerosis. Therapeutic Advances in Neurological Disorders. 2016;9(1):9–30. doi: 10.1177/1756285615612659. http://doi.org/10.1177/1756285615612659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuardi AW, Crippa JAS, Hallak JEC, Bhattacharyya S, Atakan Z, Martin-Santos R, … Guimaraes FS. A Critical Review of the Antipsychotic Effects of Cannabidiol: 30 Years of a Translational Investigation. Current Pharmaceutical Design. 2012;18(32):5131–5140. doi: 10.2174/138161212802884681. http://doi.org/10.2174/138161212802884681. [DOI] [PubMed] [Google Scholar]