Abstract
Purpose
T-cell lymphomas are a molecularly heterogeneous group of non-Hodgkin lymphomas (NHL) that account for a disproportionate number of NHL disease-related deaths due to their inherent and acquired resistance to standard multiagent chemotherapy regimens. Despite their molecular heterogeneity and frequent loss of various T-cell specific receptors, the T-cell antigen receptor is retained in the majority of these lymphomas. As T-cell receptor (TCR) engagement activates a number of signaling pathways and transcription factors that regulate T-cell growth and survival, we examined the TCR’s role in mediating resistance to chemotherapy.
Experimental Design
Genetic and pharmacologic strategies were utilized to determine the contribution of tyrosine kinases and transcription factors activated in conventional T cells following T-cell receptor (TCR) engagement in acquired chemotherapy resistance in primary T-cell lymphoma cells and patient-derived cell lines.
Results
Here we report that TCR signaling activates a signaling axis that includes ITK, NF-κB, and GATA-3, and promotes chemotherapy resistance.
Conclusions
These observations have significant therapeutic implications, as pharmacologic inhibition of ITK prevented activation of this signaling axis and overcame chemotherapy resistance.
Keywords: Peripheral T-cell lymphoma, T-cell receptor, ITK, GATA-3
Introduction
T-cell lymphoproliferative disorders are derived from mature (post-thymic) T cells and account for ≈10% of non-Hodgkin lymphomas (NHL). In contrast to many aggressive B-cell derived NHL that are highly curable with modern multiagent chemotherapy regimens, acquired chemotherapy resistance is frequently observed in the majority of similarly treated T-cell lymphoma (TCL) patients(1, 2). Despite initial chemosensitivity, primary refractory disease and cross-resistance to alternative agents is frequently observed, thus explaining the high mortality rates observed with these aggressive NHL(3, 4). Therefore, improved understanding of chemotherapy resistance mechanisms and the development of novel therapeutic strategies to overcome them are needed.
Despite their proliferative capacity, resistance to chemotherapy, and survival in vivo, the establishment of long-term TCL cell lines has been challenging, as malignant T cells undergo spontaneous apoptosis during in vitro culture(5, 6). Therefore, chemotherapy resistance is unlikely explained by intrinsic resistance mechanisms alone, but is likely explained by the provision of extrinsic growth and survival signals by non-neoplastic cells within the tumor microenvironment (TME). Every milestone in the lifecycle of a conventional T cell is regulated by professional antigen-presenting cells (APC). Professional APC provide ligands for antigen, costimulatory and cytokine receptors, the provision of which may similarly promote T-cell lymphomagenesis and resistance to chemotherapy, as engagement of these receptors activates signaling pathways and transcription factors, including NF-κB, that promote cell growth and survival, and have been previously implicated in TCL pathogenesis and chemotherapy resistance(7–10). This hypothesis is further supported by the observation that professional APC, including lymphoma-associated macrophages and dendritic cells, directly promote the growth and survival of malignant T cells and are abundant constituents of the TME in most TCL(5, 6, 11).
Collectively, these observations suggest that TCL are regulated by lineage-specific pathways, like the antigen-receptor, that are engaged by professional APC. Therefore, we sought to investigate the extent to which TCR engagement regulates TCL growth, survival, and particularly chemotherapy resistance. In doing so, we observed that engagement of the TCR on primary malignant T cells activates an ITK/NF-kB/GATA-3 axis that promotes chemotherapy resistance, but is abrogated by the BTK/ITK inhibitor ibrutinib.
Materials and Methods
Patient samples and cell lines
Normal donors and TCL patients at the University of Michigan Comprehensive Cancer Center, Ann Arbor, MI were considered for study participation. Study approval was granted by the Institutional Review Board of the University of Michigan, in accordance with U.S. federal regulations and the Declaration of Helsinki. TCL patients [with Sezary syndrome, n=6; or systemic peripheral T-cell lymphoma, not otherwise specified (PTCL, NOS), n=1] with extensive leukemic involvement, representing >80% of peripheral blood lymphocytes by flow cytometry, as determined by clinical flow cytometry and reported in the electronic medical record, were preferentially selected for isolation and in vitro studies, after provided informed consent. Normal T-cells and T-cell lymphoma cells were isolated from peripheral blood mononuclear cells by density centrifugation (Ficoll-Hypaque, GE Healthcare) and subjected to positive selection using CD4 magnetic beads (Miltenyi Biotec). Isolation and purity of T-cell lymphoma cells was examined by multicolor flow cytometry. Malignant T cells were recognized by their expression of T-cell markers (CD3, CD4 and/or CD8) and the aberrant loss of other T-cell associated antigens (most commonly CD7), as per EORTC and ISCL guidelines(12). Purity was assessed by flow cytometry, and was ≥93% (Supplementary Figure 1). Lymphoma cells were used immediately following isolation for the experiments described. Monocytes were isolated in a similar fashion using CD14 magnetic beads. Coculture experiments were performed, as previously described(5), with the addition of functional grade anti-HLA-DR/DP/DQ (clone Tu39, BD Biosciences) or an isotype control. Cells were cocultured for 7–10 days prior to assessing Ki67 expression on CD4+CD7− malignant T cells. The T-cell lymphoma cell lines (T8ML-1, H9, MyLa, SU-DHL-1) used in this study were mycoplasma free and independently validated, as previously described(11, 13). T8ML-1 cells were derived from a patient with refractory PTCL, NOS. These cells are CD3+CD8+, and are immunophenotypically identical to the patient’s primary tumor. Clonality was confirmed by multiplex PCR, and expression of Vα12.2/J6/Cα and Vβ15.1/J2.1/Cβ2 TCR chains confirmed, as previously reported. Primary cells and cell lines were grown at 37°C in 5% CO2 in RPMI 1640 (Life Technologies) supplemented with 10% fetal bovine serum (FBS; ATLANTA biologicals), 10 mM HEPES, 1 mM L-glutamine, 50 I.U./ml penicillin and 50 µg/ml streptomycin. For T8ML1 and its derivative cell lines, 100 IU/ml recombinant human IL-2 was also added. To engage T-cell receptor signaling, anti-CD3/CD28 Dynabeads (Life Technologies) were cocultured with TCL cells for various times (5 minutes for phosphorylation events, examined by western blot; 1 hour for NF-κB nuclear localization, by ELISA and western blot; 24 hours for NF-κB target gene mRNA quantitation, cytokine measurement by ELISA, or assessment of T-cell activation and GATA-3 expression by flow cytometry). Chemotherapeutic agents were obtained from Selleckchem or the University of Michigan Pharmacy.
Microarray gene expression profiling and statistical analysis
Microarray profiling was used to determine gene expression changes following T-cell receptor engagement. T8ML-1 and 3 TCL patient (2 Sezary syndrome, 1 PTCL, NOS) primary cells were used as study subjects. Briefly, at various time points (4, 12, or 24 hours) following stimulation, total RNA was extracted and submitted to the University of Michigan DNA Sequencing core for processing, QC, and analysis of the data. The RNA samples were processed using the NuGen WT-pico kit and were hybridized to the Human Gene ST 2.1 arrays and were scanned on the Gene atlas system. Probe intensity values were converted to expression values using the Robust Multi-Array Average (RMA) Method for each experiment independently. This was implemented in the R statistical environment using the “oligo” package of Bioconductor(14, 15). This technique includes a quantile normalization step effectively making the distribution of probe intensities the same across the chips. For this reason density plots were used to confirm that no sample had a different distribution in intensities. RMA log transformed expression values were used for downstream analysis. The affyplm package of Bioconductor was also used to fit probe level models and plot boxplots of the normalized unscaled standard errors. These boxplots showed very little variation. Lastly we used PCA plots to confirm there were no major outliers. All plots together suggested this data was of very good quality. We fit linear models to the data using the limma package of Bioconductor(16, 17). We limited the analysis to ‘main’ probesets as defined by Affymetrix and we filtered out probeset with a variance less than .05. A gene-by-gene update algorithm was used to help down-weight samples deemed less reproducible(18). P-values were adjusted for multiplicity using the Benjamini and Hochberg FDR(19). We used Venn diagrams to compare gene lists that were shared among groups, or unique to a given sample. Functional/pathway enrichment analysis was performed using the Database for Annotation, Visualization, and Integrated Discovery (DAVID; david.ncifcrf.gov).
Additional details and methods are provided in the supplementary Materials and Methods.
Results
The TCR regulates gene expression in primary malignant T cells
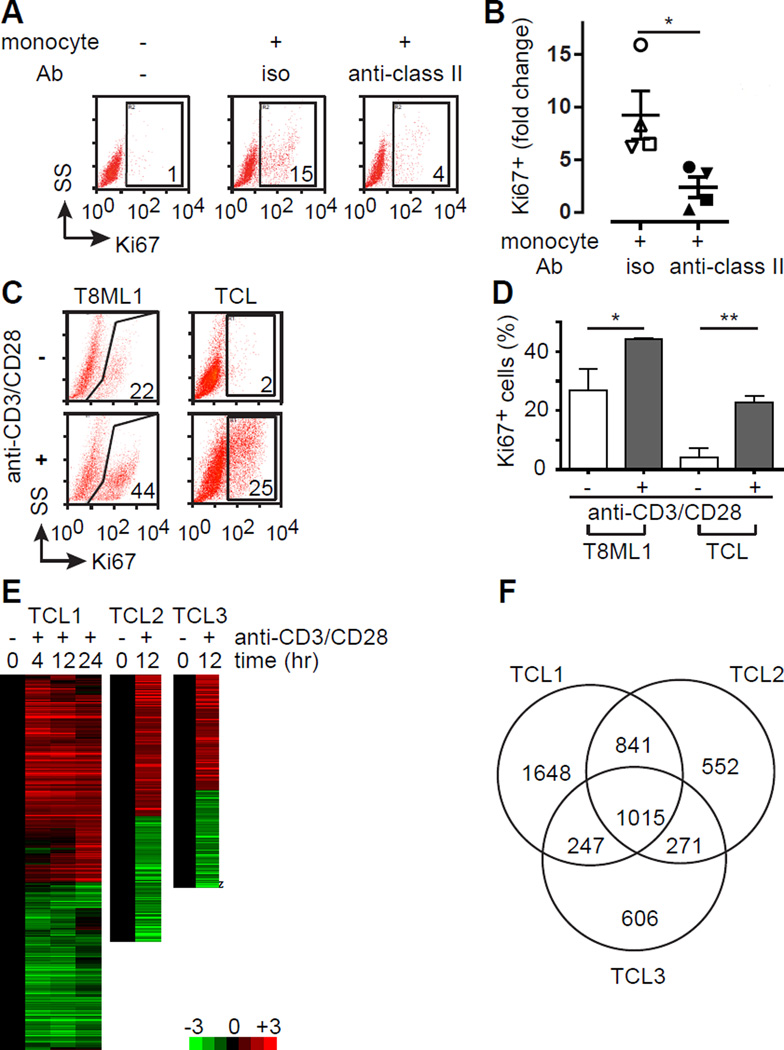
Monocytes and their progeny promote the expansion and long-term survival of malignant T cells during in vitro culture(5, 6). Therefore, this experimental system was initially employed to interrogate the role of the TCR in stimulating the proliferation of malignant T cells. To do so, malignant CD4+ T cells and autologous monocytes were isolated from TCL patient PBMC. T cells were either cultured alone or cocultured with autologous monocytes in the presence of an antagonistic MHC class II monoclonal antibody (or isotype control) and T-cell proliferation determined by Ki67 expression. Consistent with previous studies, TCL cells cultured in isolation did not expand (data not shown) and a significant population of Ki67+ T cells was not observed (Figure 1, A and B). In contrast, a marked increase in Ki67 expression was observed in the presence of autologous monocytes, but was abrogated upon MHC class II neutralization (Figure 1, A and B), thus implicating the TCR in TCL proliferation. To more directly interrogate the role of the TCR, primary malignant T cells were positively selected from peripheral blood (purity ≥93%, Supplemental Figure 1). We have previously shown that APC’s expressing costimulatory ligands are abundant constituents of the TME in TCL(5, 11, 20). Therefore, we sought to recapitulate the context in which TCR engagement is likely to occur in situ using anti-CD3/CD28 coated microbeads. A significant increase in proliferating Ki67+ cells was observed upon activation of either a patient-derived cell line (T8ML-1 cells(13)) or purified primary malignant T cells (Figure 1, C and D). Consistent with these findings, an ≈50% increase in T8ML-1 viable cell mass (data not shown) and an >100% increase in primary TCL viable cell mass was observed following stimulation (Supplementary Figure 6A). Gene expression profiling was subsequently performed using Affymetrix microarrays following bead stimulation of primary TCL cells (Sezary syndrome, n=2; PTCL, NOS, n=1) and unsupervised hierarchical clustering performed (Figure 1, E and F). A significant (log2 transformed fold-change >1 with FDR <0.05) change in expression was observed in 1,015 transcripts that were shared among the 3 biologic replicates examined (Figure 1F). Observed changes in gene expression were concordant (uniformly up- or down-regulated among all samples) for 98.7% (n=1,002) of transcripts. Pathway enrichment analysis was performed using these shared genes (Supplemental Table 1; Supplemental Figure 2), and pathways involved in T-cell proliferation and metabolism, including those that are TCR-dependent, identified. Similar changes in gene expression were observed following stimulation of T8ML-1 cells (Supplemental Figure 3), as 19% of differentially expressed genes identified in T8ML-1 were shared among all 3 primary samples, while 59% of genes identified were shared with at least one primary sample. Therefore, we concluded that TCR engagement on malignant T cells has a profound impact on gene expression and conceivably activates relevant pathways involved in conferring resistance to chemotherapy. We next sought to confirm these findings in independent primary TCL specimens. To do so, cytokine production and the inducible expression of T-cell activation markers were examined. We preferentially selected gene products that were identified in the gene expression profiling dataset, including those that are inducibly expressed following TCR activation of conventional T cells, and examined their expression following TCR engagement in T8ML-1 (Supplemental Figure 4) and primary TCL cells (Supplemental Figure 5). As anticipated, TCR engagement in T8ML-1 and primary TCL cells led to the phosphorylation of proximal tyrosine kinases, including Zap-70 and ITK (Supplemental Figure 5, A and B), culminating in a significant increase in IL-2, IL-13, IL-10 and IFN-γ production (Supplemental Figure 5B), and the upregulation of T-cell activation markers (Supplemental Figure 5, C and D).
Figure 1.
The TCR stimulates proliferation and activates gene expression in T-cell lymphomas. (A) Primary TCL cells were cultured for 7 days in the presence or absence of autologous monocytes with an isotype control (iso) or blocking MHC class II antibody (anti-class II), as indicated. TCL proliferation was determined by Ki67 expression, and the percentage of CD4+CD7− TCL cells that were Ki67+ is indicated in the gates shown in a representative FACS analysis (SS, side scatter). (B) TCL proliferation in the presence of autologous monocytes was independently determined (n=4) and was expressed as the fold increase in Ki67 expression compared with TCL cells cultured alone (mean ± s.e.m.). (C) The primary TCL cell line T8ML-1 and primary TCL cells were cultured in the presence (1:1 ratio) or absence of anti-CD3/CD28 beads and proliferation determined by Ki67 expression, as indicated in the representative examples shown. (D) The percentage of Ki67+ TCL cells (mean ± s.e.m.) cultured alone (open histogram) or with beads (gray histogram) is shown for T8ML-1 (in technical replicates) and for primary TCL cells (n=3). (E) Unsupervised hierarchical clustering highlighting changes (log2 transformed fold-change) in gene expression over time upon bead stimulation is shown for 3 TCL patients (Sezary syndrome=2, PTCL, NOS=1). (F) Venn diagram showing substantial overlap in differentially expressed genes among the 3 TCL patients examined. (*p<0.05, **p<0.01, ***p<0.001)
TCR engagement activates NF-κB and decreases responsiveness to chemotherapy in T-cell lymphomas
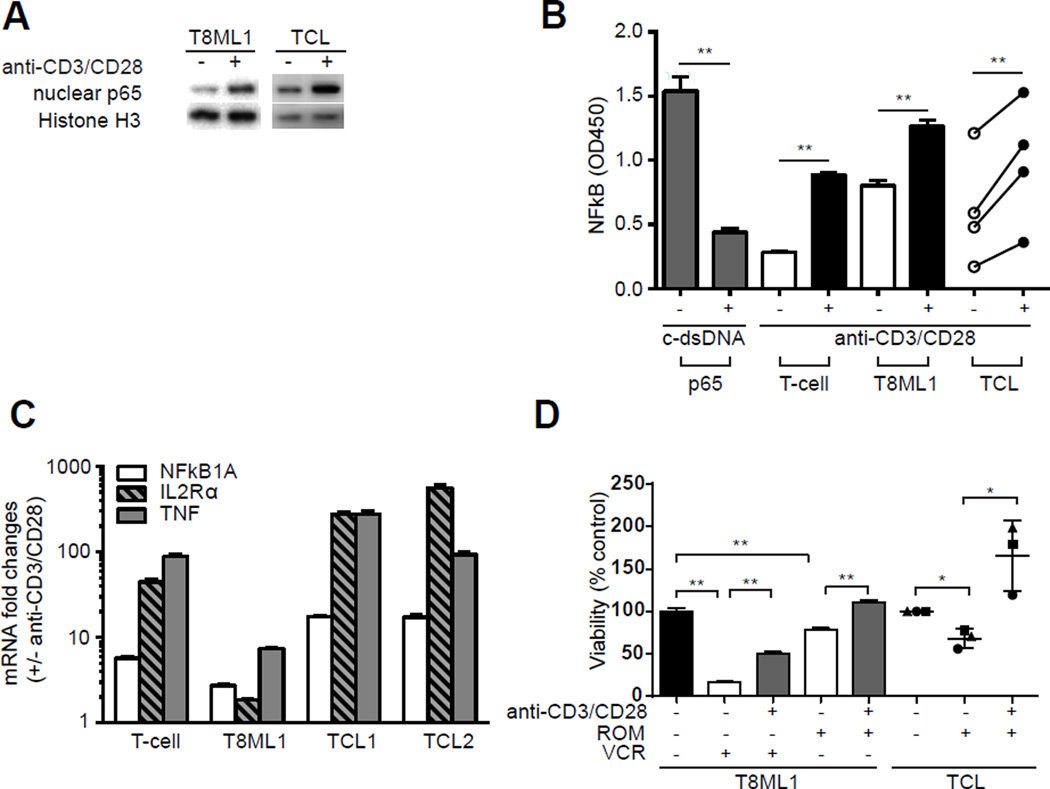
TCR engagement culminates in the activation of signaling cascades and transcription factors, including NF-κB, that are required for the homeostatic survival of conventional T cells. Similarly, TCR engagement led to the activation of downstream kinases, including IL-2 inducible T-cell kinase (ITK) and PLC-γ (Supplemental Figure 5A), in TCL. Examination of NF-κB (p65) nuclear localization (Figure 2A, Supplementary Figure 5F), DNA binding (Figure 2B), and expression of NF-κB target genes (Figure 2C) demonstrated that NF-κB is activated upon TCR engagement in TCL cells. As NF-κB regulates the expression of many genes involved in T-cell growth and survival, TCL cell growth and viability were examined following TCR engagement. TCR engagement significantly enhanced the growth and viability of cultured TCL cells, conferring decreased susceptibility to chemotherapy (Figure 2D). T8ML-1 cells were treated with either a microtubule-targeting agent (vincristine) or with a histone deacetylase inhibitor (romidepsin) at concentrations approximating the IC50. A significant increase in TCL cell viability was observed in bead-stimulated T8ML-1 cells. Primary TCL cells failed to proliferate in culture and were consequently not susceptible to the cell-cycle dependent agent vincristine (data not shown). Therefore, primary TCL cells were treated with romidepsin alone, whereby TCR stimulation was observed to provide a similar degree of protection (Figure 2D). Sotrastaurin, a PKC-Θ inhibitor, was utilized to block NF-κB activation following TCR engagement, and was found to significantly inhibit TCR-mediated chemotherapy resistance in T8ML-1 and in most primary TCL cells (Supplemental Figure 6). Collectively, these findings demonstrate that TCR activation, as may be provided by MHC-expressing APC within the TME, supports the growth and survival of malignant T cells. Improved understanding of the mechanisms involved in TCR-mediated resistance to chemotherapy may unveil novel therapeutic strategies that abrogate resistance.
Figure 2.
TCR engagement activates NF-κB and confers resistance to chemotherapy in T-cell lymphomas. (A) NF-κB (p65) was examined in nuclear extracts following bead stimulation in T8ML-1 and primary TCL cells. Representative western blots are shown. (B) Nuclear NF-κB (p65) DNA binding was determined following bead stimulation by ELISA in conventional T-cells (“T-cell”), in T8ML-1 and in primary TCL cells (n=4). (C) Expression of selected NF-κB target genes (NFκB1A, IL2Rα, TNF) was examined by qRT-PCR following bead stimulation. The relative increase (compared with unstimulated cells) in mRNA for normal T cells, T8ML-1, and primary TCL cells (n=2) is shown. (D) T8ML-1 cells and primary TCL specimens (n=3) were cultured with romidepsin (2 nM) or vincristine (6 nM) in the presence or absence of CD3/CD28 beads, as indicated, and cell viability determined 48 hours later in a standard MTT assay and reported as relative to control cells (neither treated with CD3/CD28 beads nor chemotherapy). (*p<0.05, **p<0.01, ***p<0.001)
TCR-mediated activation of NF-kB and chemotherapy resistance are ITK dependent
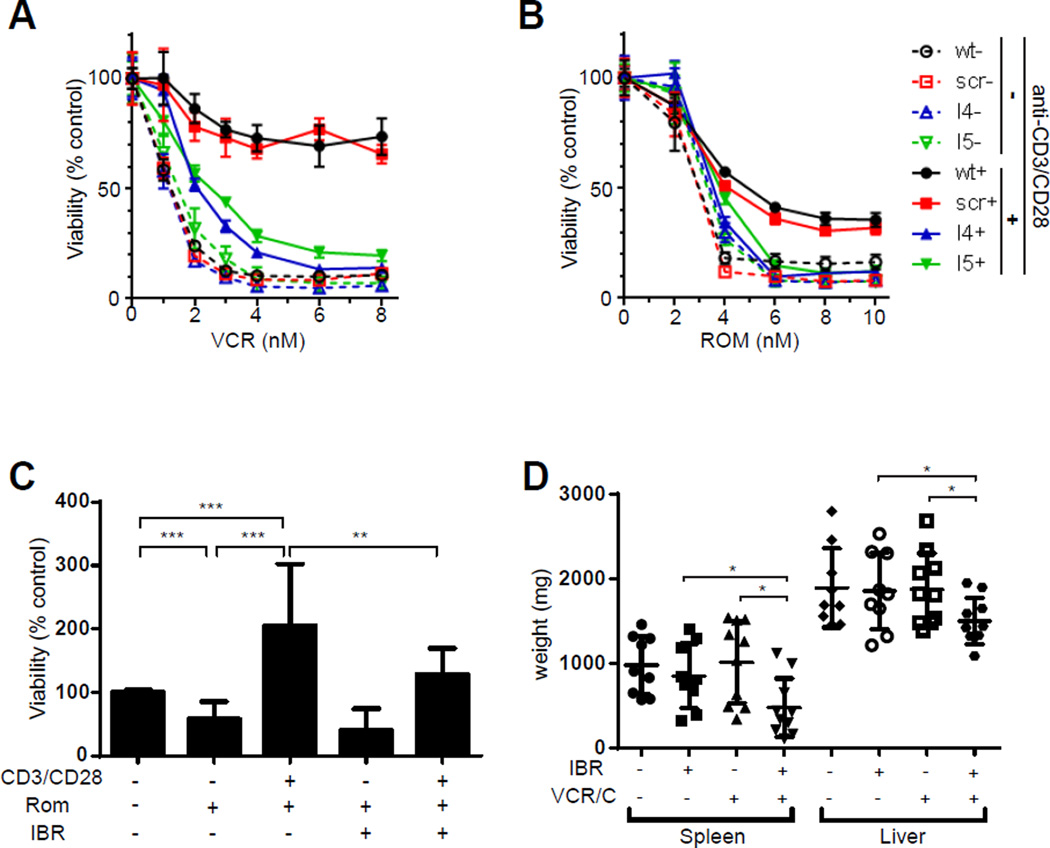
The IL-2 inducible T-cell kinase (ITK), by regulating the spatiotemporal localization of the TCR signalosome(21), is a critically important mediator of TCR signaling, and is commonly expressed(22), if not overexpressed(23), in T-cell lymphomas. Therefore, its localization to the cell membrane following CD3 cross-linking in primary TCL cells may not be surprising (Supplemental Figure 7). In order to examine its role in TCL cells, a shRNA-mediated gene targeting approach was adopted in T8ML-1 cells, and ITK knockdown confirmed (Supplemental Figure 8). Upon ITK knockdown, TCR-dependent cytokine production (Supplemental Figure 9A), upregulation of activation-dependent cell surface receptors (Supplemental Figure 9B), and NF-κB activation (Supplemental Figure 9C) were significantly impaired. Therefore, the extent to which ITK loss abrogated TCR-mediated chemotherapy resistance was examined. In its absence, resistance to vincristine (Figure 3A) and romidepsin (Figure 3B) following TCR engagement was significantly impaired in T8ML-1 cells. Ibrutinib, an irreversible inhibitor of ITK(24), and its homologue BTK, was used to pharmacologically inhibit ITK in primary TCL cells. As expected, ibrutinib significantly impaired TCR-dependent signaling (Supplemental Figure 10A), T-cell activation (Supplemental Figure 10, B and C), and NF-kB activation (Supplemental Figure 11, A, B, and C) in primary TCL cells. Consistent with these results, ibrutinib significantly impaired TCR-mediated resistance to romidepsin in primary TCL cells (Figure 3C). As ibrutinib inhibits murine ITK, we next examined the extent to which ibrutinib abrogates chemotherapy resistance in vivo using T8–28 cells, a spontaneously generated murine TCL expressing an intact TCR(25), In vitro studies were challenging, as ≈90% of T8–28 cells undergo apoptosis within 48 hours of in vitro culture and must be maintained by serial passage in vivo. Nonetheless, TCR engagement inhibited spontaneous apoptosis of T8–28 cells during in vitro culture, and was abrogated by ibrutinib (Supplemental Figure 12). In this model, lymphoma-bearing mice develop massive hepatosplenomegaly secondary to lymphomatous involvement (Supplemental Figure 13)(25). Consistent with results obtained in primary human TCL cells, ibrutinib treatment alone had no significant impact on T8–28 progression. In contrast, ibrutinib administration significantly improved the responses observed with conventional chemotherapeutic agents (vincristine, cyclophosphamide), further implicating ITK in mediating resistance to chemotherapy (Figure 3D, Supplemental Figure 14).
Figure 3.
Chemotherapy resistance following TCR engagement is ITK dependent. (A, B) T8ML-1 cells (wt) were lentivirally transduced with either nontargeting (scr) or ITK specific shRNA (I4, I5). Cells were cultured in the presence (solid line) or absence (dashed line) of beads in the presence of vincristine (A) or romidepsin (B) and viability determined 48 hours later (mean ± s.e.m.). (C) Primary TCL cells were cultured with beads, romidepsin (2 nM), and/or ibrutinib (1 µM), as indicated, and viability determined 48 hours later. Pooled data from 5 independent patients is shown. (D) T8–28 cells were adoptively transferred into Balb/c recipient mice and lymphoma-bearing mice treated with cyclophosphamide, vincristine, and ibrutinib or vehicle control, as indicated, and spleen and liver weights determined. Pooled data from two independent experiments is shown. (*p<0.05, **p<0.01, ***p<0.001)
GATA-3 expression in T-cell lymphomas is regulated by the TCR
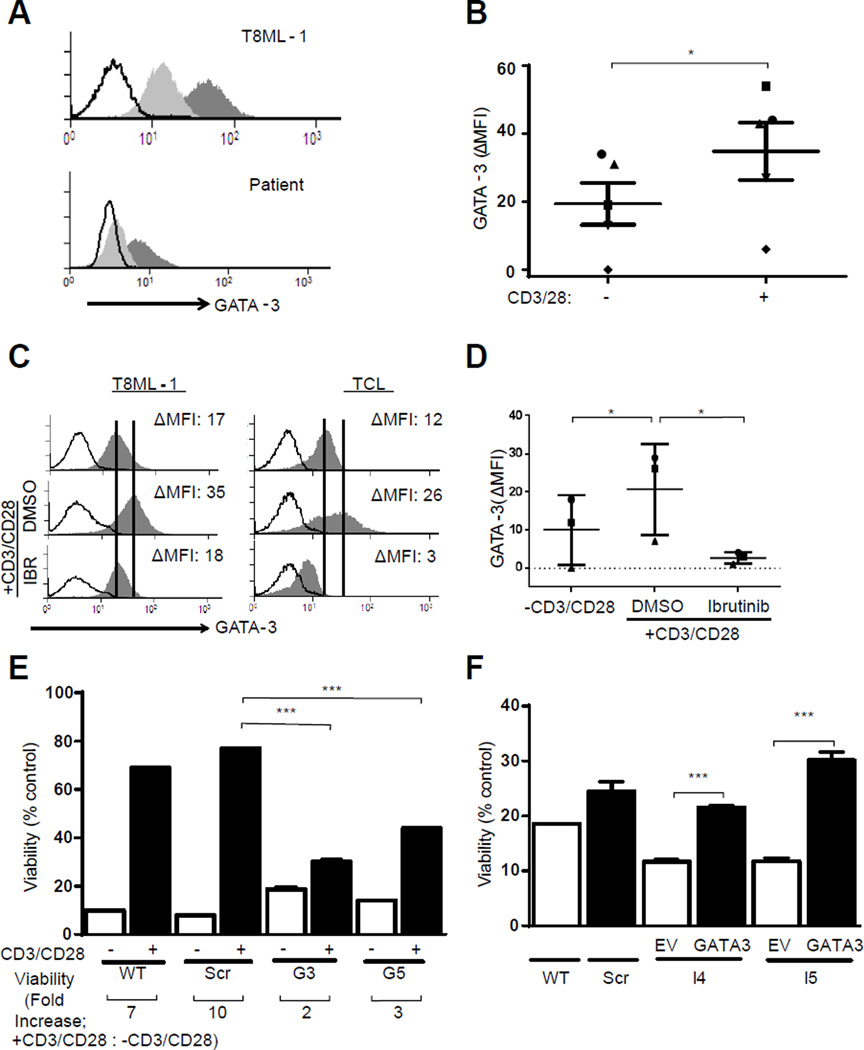
The transcription factor GATA-3 promotes the homeostatic survival of conventional T cells in a TCR- and NF-κB-dependent manner(26). We have previously shown that GATA-3 is expressed in various TCL subtypes, including the most common TCL in North America, that is, peripheral T-cell lymphoma, not otherwise specified (PTCL, NOS)(10, 11). PTCL, NOS are clinically and molecularly heterogeneous, but expression of GATA-3 and its gene targets identifies a distinct subset of these aggressive T-cell lymphomas. While recognizing that the TCR and NF-κB regulate the expression of many pro-growth and survival genes, these previous observations suggested that GATA-3 may have a significant role in mediating chemotherapy resistance following TCR engagement in TCL. Therefore, GATA-3 expression was examined following TCR stimulation in T8ML-1 and primary TCL cells. Following TCR engagement, a significant increase in GATA-3 expression was observed (Figure 4, A and B). GATA-3 upregulation following TCR engagement was significantly inhibited by ibrutinib, thus implicating ITK in TCR-mediated GATA-3 upregulation (Figure 4, C and D). The observed increase in GATA-3 transcripts following TCR engagement of T8ML-1 cells (Supplemental Figure 15A), and inhibition by sotrastaurin (Supplemental Figure 15B), collectively implicate an NF-κB-driven increase in GATA-3 transcription as a mechanism for TCR-mediated GATA-3 upregulation(26). However, a similar increase in GATA-3 transcription was not uniformly observed in primary TCL cells (Supplemental Figure 15C), implicating an additional post-transcriptional mechanism of GATA-3 regulation. Significant differences in the stability of GATA-3 mRNA (Supplemental Figure 15D) or protein (Supplemental Figure 15E) were not observed following TCR engagement, suggesting that GATA-3 upregulation following TCR stimulation may be attributed, at least in part(27), to the global increase in mRNA translation that was observed (Supplemental Figure 15F). In order to examine the role of GATA-3 in TCR-mediated chemotherapy resistance, shRNA-mediated loss-of-function studies were performed. Cells transduced with GATA-3-targeting shRNA failed to upregulate GATA-3 following TCR engagement (Supplemental Figure 16A), and chemotherapy resistance was curtailed (Figure 4E). Conversely, chemotherapy resistance in ITK-deficient T8ML-1, which also fail to upregulate GATA-3 following TCR engagement (Supplemental Figure 16B), was partially restored upon TCR-independent GATA-3 overexpression (Supplemental Figure 16C, Figure 4F).
Figure 4.
TCR-mediated chemotherapy resistance is GATA-3 dependent. (A, B) T8ML-1 and primary TCL cells were activated with beads for 24 hours and GATA-3 expression in unstimulated (light gray histogram) and bead stimulated (dark gray histogram) cells determined by FACS analysis (isotype control, open histogram). Representative examples are shown in (A), and data obtained from 5 TCL patients summarized in (B). (C, D) TCL cells were activated in the presence of ibrutinib (1 µM) or vehicle control (DMSO) and GATA-3 expression determined in resting and bead stimulated cells. Representative examples are shown in (C) and data from 3 TCL patients summarized in (D). (E) T8ML-1 cells (wt) were transduced with either nontargeting (scr) or GATA-3 specific shRNA (G3, G5). Cells were cultured with or without beads, as indicated, in the presence of vincristine (6 nM). Cell viability was determined 48 hours later, and is reported relative to cells cultured without vincristine. The fold increase in viability between unstimulated and bead stimulated cells is shown below the x-axis. (F) GATA-3 was overexpressed in T8ML-1 transduced with ITK-targeting shRNA (I4, I5) following transduction with a vector containing full-length GATA-3 (GATA-3) or an empty vector (EV) control. Cells were cultured alone, or in the presence of vincristine (6 nM), and viability determined 48 hours later. Viability is reported relative to cells cultured without vincristine. (*p<0.05, **p<0.01, ***p<0.001 in unpaired two-sided Student t-test)
GATA-3 confers chemotherapy resistance
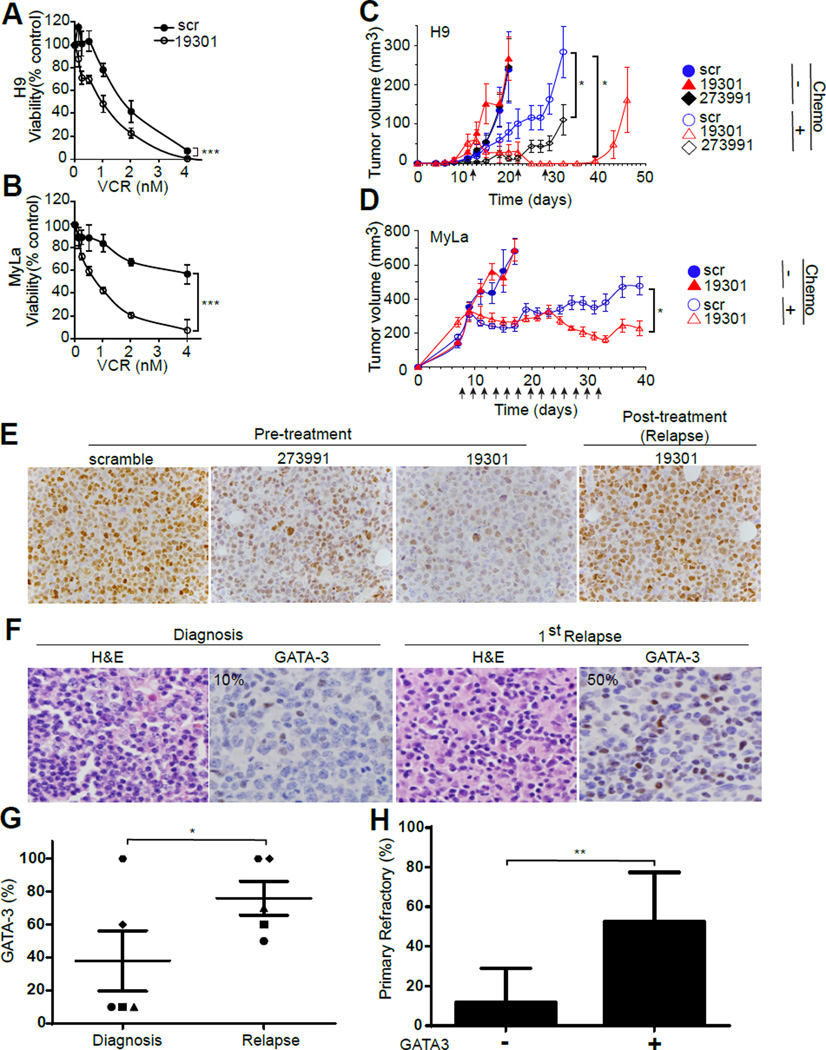
Given these findings, and to determine the extent to which they may be generalizable, we sought to examine the potential role of GATA-3 in mediating chemotherapy resistance in cutaneous T-cell lymphomas (CTCL), as these lymphomas classically express GATA-3 and its gene targets(28, 29), including Th2-associated cytokines(11, 30, 31), and are clinically resistant to conventional chemotherapeutic agents(32). Therefore, cell growth and viability was examined using CTCL cell lines (i.e. H9 and MyLa) stably transduced with non-targeting or GATA-3-targeting shRNA, as previously published(11). Given their molecular heterogeneity, including genetic alterations (e.g. activating NF-κB mutation) that are downstream from the TCR and ITK, these particular CTCL cells were selected for these studies(7, 8, 33–37). GATA-3 loss was associated with a significant decrease in chemotherapy resistance, both in vitro (Figure 5, A and B; Supplemental Figure 17) and in vivo (Figure 5, C and D). Interestingly, following chemotherapy administration and complete regression of tumors in mice injected with H9 cells expressing GATA-3-targeting shRNA, tumors recurred following cessation of chemotherapy administration. GATA-3 expression was examined by immunohistochemistry in these recurrent tumors. As previously published, GATA-3 knockdown is observed in tumor xenografts obtained from untreated mice(11), but the tumors that reemerge following chemotherapy administration highly express GATA-3 (Figure 5E). These findings suggest that chemotherapy imposes a positive selection pressure for TCL subclones that highly express GATA-3, and provides further evidence for its role in mediating resistance to chemotherapy. In order to explore the extent to which this may be observed in relapsed/refractory PTCL, we searched our clinical database and tissue archive for serial PTCL specimens obtained from the time of initial diagnosis and at the time of relapse (following multiagent chemotherapy). Five cases (PTCL, NOS, n=3; AITL, n=2) were identified and had tissue available for immunohistochemical examination of GATA-3 expression. An approximately two-fold increase in GATA-3 expression was appreciated in biopsies obtained at the time of relapse or progression when compared to paired biopsy specimens obtained at diagnosis and prior to treatment (Figure 5, F and G; Supplemental Figure 18). Using a previously described cohort of PTCL, NOS patients(11), the relationship between GATA-3 expression and primary refractory disease following treatment with anthracycline-based multiagent chemotherapy (i.e. CHOP/CHOEP) was examined (Figure 5H). Primary refractory disease was observed in 11.8% of GATA-3 negative PTCL, NOS (n=17), in contrast to 52.6% of GATA-3 positive PTCL, NOS (n=19). Collectively, these results strongly suggest that GATA-3 promotes chemotherapy resistance in TCL, and that mediators of TCR-dependent signaling, including ITK, promote its expression and are thus attractive therapeutic targets.
Figure 5.
GATA-3 confers resistance to chemotherapy in T-cell lymphomas. (A, B) The CTCL cell lines H9 and MyLa were transduced with nontargeting (scr) or GATA-3-targeting shRNA (19301). H9 (in A) and MyLa (in B) were cultured with or without vincristine (VCR) and cell viability (reported as a percent of untreated cells) determined 48 hours later. (C) H9 transduced with nontargeting (scr, ○/●) or GATA-3-targeting shRNA’s 273991 (◊/♦) or 19301 (△/▲), were injected subcutaneously into the shoulders and flanks of NSG mice. Following tumor engraftment, mice were either untreated (closed symbols) or treated with weekly cyclophosphamide and vincristine (as indicated by the arrows) and tumor volumes determined (n=12). (D) In a similar fashion, NSG mice were engrafted with MyLa cells transduced with nontargeting (scr, ○/●) or GATA-3-targeting shRNA 19301 (△/▲) and either untreated (closed symbols) or treated with single-agent vincristine (as indicated by the arrows) and tumor volumes determined. (E) H9 19301 tumors previously treated with cyclophosphamide/vincristine subsequently relapsed (shown in C) and were analyzed for GATA-3 expression by immunohistochemistry (“post-treatment”). For comparison, GATA-3 expression in established H9 scramble, 273991, and 19301 tumors (“pre-treatment”) is shown at left. (F, G) The extent of GATA-3 expression was determined by immunohistochemistry in serial patient biopsies obtained at the time of diagnosis and at the time of relapse after treatment (representative example shown in F). GATA-3 expression, as a percentage of total TCL cells, in paired samples (n=5) is shown (G). (H) PTCL, NOS patients treated with CHOP or CHOEP were stratified by GATA-3 expression, and the rate of primary refractory disease reported (mean ± 95% CI is shown). (*p<0.05, **p<0.01, ***p<0.001)
Discussion
The widespread expression of both the TCR and its associated kinases and adaptor proteins (23, 38, 39), in conjunction with the biased TCR Vβ usage that is observed in selected T-cell lymphoproliferative disorders(40–42), suggests that malignant T cells exploit the growth and survival signals provided by the TCR. This hypothesis is further supported by the growing appreciation that tyrosine kinases classically associated with TCR signaling in conventional T cells are recurrently mutated in a minority of T-cell lymphomas [reviewed in(43)]. However, for the majority of TCL lacking such activating mutations, signaling may require engagement by antigenic peptide/MHC complexes provided by constituents of the tumor microenvironment (TME), particularly professional antigen-presenting cells, which are particularly abundant constituents of the TME in most T-cell lymphomas(5, 6, 11). Our findings are consistent with this hypothesis, as are previous observations demonstrating that survival of malignant T cells when cocultured with immature dendritic cells or monocytes/macrophages is abrogated by either clonotypic TCR- or MHC-blocking antibodies(6, 44). Nonetheless, the hypothesis that TCL exploit TCR-dependent signals upon recognition of peptide/MHC in vivo, and the extent to which ITK, NF-κB, and GATA-3 are involved, will require future studies in mouse models. Positively selected T cells emerging from the thymus have significant, albeit low, affinity for self-peptide/MHC, and their continued homeostatic survival in the periphery requires continuous, low-level (or “tonic”) engagement of the TCR(45). Therefore, T-cell lymphomas could be similarly regulated by tonic TCR engagement(46). In the current study, antibody-mediated cross-linking of the TCR was utilized, and in contrast to “tonic” signaling, more closely resembles TCR activation by high-affinity antigenic peptides leading to “active” TCR signaling. Recurrent genomic alterations in the TCL, including the loss of negative feedback mechanisms or gain of positive regulators, may lower the threshold for TCR activation, and thus potentiate low affinity interactions with peptide/MHC normally associated with “tonic” signaling(47–52). While previous observational studies demonstrating biased usage of specific TCR-Vβ chains implicate the TCR in disease pathogenesis(40, 41), most TCL are not associated with T-cell mediated autoimmunity or chronic infections. With the possible exception of bacterial superantigen-mediated engagement of the TCR in Sezary syndrome(42, 53), potential antigenic drivers remain largely speculative(54).
ITK is a critically important component of the proximal TCR signalosome(21). Therefore, we exploited the BTK/ITK inhibitor ibrutinib as a means to pharmacologically inhibit TCR signaling and NF-κB activation in primary TCL(24). Ibrutinib significantly abrogated both TCR-dependent activation of NF-κB and chemotherapy resistance. While the findings reported here implicate TCR- and ITK-dependent GATA-3 expression in chemotherapy resistance, GATA-3 is not uniformly expressed in all TCL subtypes, including those that may be driven by the antigen-receptor. Therefore, it seems quite likely that alternative GATA-3-independent pathways may also contribute to chemotherapy resistance following TCR engagement. The observation that genetic or pharmacologic approaches to inhibit ITK uniformly impaired TCR-mediated GATA-3 upregulation has significant clinical implications. Whether similar strategies targeting ITK, or other mediators of TCR signaling, will be effective strategies to inhibit GATA-3 expression and overcome acquired chemotherapy resistance warrant further study in well-designed clinical trials. Interestingly, single-agent ibrutinib did not significantly impair TCL growth in our studies (e.g. Figure 3D). This may be explained by the bidirectional relationship between ITK and GATA-3 in T-cell activation and differentiation. Studies performed in ITK deficient mice demonstrate that ITK is required for optimal Th2-biased immunity, as ITK qualitatively regulates TCR signaling and negatively regulates the expression of T-bet(55). Conversely, established Th2 cells are increasingly dependent upon ITK, as GATA-3 promotes the selective downregulation of RLK, a TEC family kinase that is, at least partially, redundant with ITK and is not sensitive to ibrutinib. Therefore, it is noteworthy that ITK inhibition was associated with decreased GATA-3 expression in primary T-cell lymphomas (Figure 4C, D) and in T8ML-1 cells (Supplementary Figure 16B). These observations may imply significant heterogeneity among PTCL subtypes in their dependence upon ITK and susceptibility to ibrutinib. Impaired GATA-3 expression alone does not inhibit T-cell lymphoma growth (e.g. Figure 5C, D), despite diminishing chemotherapy resistance. The mechanism(s) underlying these apparently discordant, but not unprecedented, observations are poorly understood. In contrast, the selection pressure imposed by chemotherapy favors the outgrowth of “GATA-3 high” cells (Figure 5E, G), but the emergence of these resistant clones may be subverted upon ITK inhibition. Whether the emergence of “GATA-3 high” cells is associated with an increased ITK/RLK ratio and increasing sensitivity to ibrutinib, while biologically plausible, was not examined. We cannot exclude the possibility that ibrutinib’s effects are, at least partially, indirect and due to increased host anti-tumor immunity in the T8–28 model.
Activation of NF-κB is commonly observed in TCL and, not surprisingly, promotes resistance to chemotherapy(7, 8). A myriad of NF-κB target genes regulate T-cell growth and survival and likely collaborate in conferring chemotherapy resistance to their malignant counterparts. Nonetheless, the data we present clearly implicates GATA-3 in chemotherapy resistance, but the GATA-3 target genes responsible are unknown. Gene expression profiling studies performed following GATA-3 knockdown failed to clearly identify a single candidate gene, including those involved in multidrug resistance (data not shown). The number of GATA-3 binding sites identified in the human genome (≈7000 in Th2 cells) rivals that observed for c-myc in Burkitt’s lymphoma(56). Therefore, it may be naïve to imagine that GATA-3 regulates chemotherapy resistance by regulating a single gene target, or that its role in chemotherapy resistance isn’t influenced by the context, including the cell-of-origin, in which it’s expressed. In addition, GATA-3 may regulate chemotherapy resistance in a non-cell-autonomous, as its targets genes may functionally polarize constituents of the tumor microenvironment, particularly lymphoma-associated macrophages(11).
We initially hypothesized that ITK, by activating NF-κB, transcriptionally regulates GATA-3(26). While ITK-dependent NF-κB activation was demonstrated, a significant increase in GATA-3 transcription was not uniformly observed following TCR activation of primary TCL cells, implicating an additional post-transcriptional mechanism of regulation. The collective data implies that inducible GATA-3 expression following TCR engagement may be translationally regulated. The GATA-3 5’-UTR is GC rich and is likely amenable to “cap-dependent”, and mTOR-regulated, translational control (data not shown). This is pertinent, as TCR activation leads to mTOR activation, which has recently been implicated in regulating GATA-3 expression and Th2 differentiation(27, 57). More importantly, mTOR-dependent genes were enriched in GATA-3 positive, but not GATA-3 negative, TCL.(10) In contrast to TCR-inducible GATA-3 expression, basal expression of GATA-3, as in conventional T cells, appeared to be NF-κB dependent, as its inhibition with the protein kinase C-Θ inhibitor sotrastaurin reproducibly impaired basal GATA-3 expression in TCL (data not shown)(58).
In summary, activation of the TCR in TCL culminates in ITK-dependent NF-κB activation, GATA-3 upregulation, and chemotherapy resistance. As GATA-3 regulates chemotherapy resistance in these lymphomas, ITK inhibition, or similar therapeutic strategies that inhibit TCR-dependent signaling and GATA-3 expression, is a novel and promising therapeutic strategy that may overcome the challenge of chemotherapy resistance in these aggressive lymphomas.
Supplementary Material
Translational Relevance.
The acquisition of chemotherapy resistant disease, observed in ≈25% of peripheral T-cell lymphoma patients treated with standard regimens, remains a clinical challenge, as the mechanism(s) driving the emergence of resistant clones is poorly understood. We demonstrate that engagement of the T-cell receptor on malignant T cells culminates in NF-κB activation and the upregulation of GATA-3 expression, both of which regulate the growth and survival of conventional T cells, and were shown to promote chemotherapy resistance in malignant T cells. ITK regulates the formation of the TCR signalosome, thus its pharmacologic inhibition significantly impaired TCR signaling and chemotherapy resistance in T-cell lymphoma cells. Therefore, ITK inhibition, or similar therapeutic strategies that inhibit TCR-dependent signaling, is a novel and promising therapeutic strategy that may overcome the challenge of chemotherapy resistance in the T-cell lymphomas.
Acknowledgments
This work was supported in part by grants from the Leukemia & Lymphoma Society (6270-13 and 6503-16), the V Foundation for Cancer Research, and the NIH-NCI (K08CA172215). The authors wish to thank the University of Michigan lymphoma group for helpful discussions and the DNA microarray and sequencing core for technical assistance.
Footnotes
Author Contributions
T.W. performed research, analyzed results, and assisted in manuscript preparation; Y.L., A.P., P.C., and C.M.Z. provided technical assistance, performed research, and assisted with data analysis; H.F., K.S., N.B. provided reagents and assisted with data analysis; A.C.H., M.S.L., and N.G.B. performed research and assisted with data analysis; R.A.W. conceived, designed, and performed research, analyzed results, and wrote the manuscript. All authors approved the final manuscript.
Conflict-of-interest disclosure: The authors declare no conflict of interest.
References
- 1.Vose J, Armitage J, Weisenburger D. International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol. 2008;26:4124–4130. doi: 10.1200/JCO.2008.16.4558. [DOI] [PubMed] [Google Scholar]
- 2.Briski R, Feldman AL, Bailey NG, Lim MS, Ristow K, Habermann TM, et al. The role of front-line anthracycline-containing chemotherapy regimens in peripheral T-cell lymphomas. Blood Cancer J. 2014;4:e214. doi: 10.1038/bcj.2014.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Briski R, Feldman AL, Bailey NG, Lim MS, Ristow K, Habermann TM, et al. Survival in Patients with Limited-stage Peripheral T-cell Lymphomas. Leuk Lymphoma. 2014:1–17. doi: 10.3109/10428194.2014.963078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mak V, Hamm J, Chhanabhai M, Shenkier T, Klasa R, Sehn LH, et al. Survival of patients with peripheral T-cell lymphoma after first relapse or progression: spectrum of disease and rare long-term survivors. J Clin Oncol. 2013;31:1970–1976. doi: 10.1200/JCO.2012.44.7524. [DOI] [PubMed] [Google Scholar]
- 5.Wilcox RA, Wada DA, Ziesmer SC, Elsawa SF, Comfere NI, Dietz AB, et al. Monocytes promote tumor cell survival in T-cell lymphoproliferative disorders and are impaired in their ability to differentiate into mature dendritic cells. Blood. 2009;114:2936–2944. doi: 10.1182/blood-2009-05-220111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berger CL, Hanlon D, Kanada D, Dhodapkar M, Lombillo V, Wang N, et al. The growth of cutaneous T-cell lymphoma is stimulated by immature dendritic cells. Blood. 2002;99:2929–2939. [PubMed] [Google Scholar]
- 7.Sors A, Jean-Louis F, Begue E, Parmentier L, Dubertret L, Dreano M, et al. Inhibition of IkappaB kinase subunit 2 in cutaneous T-cell lymphoma down-regulates nuclear factor-kappaB constitutive activation, induces cell death, and potentiates the apoptotic response to antineoplastic chemotherapeutic agents. Clin Cancer Res. 2008;14:901–911. doi: 10.1158/1078-0432.CCR-07-1419. [DOI] [PubMed] [Google Scholar]
- 8.Sors A, Jean-Louis F, Pellet C, Laroche L, Dubertret L, Courtois G, et al. Down-regulating constitutive activation of the NF-kappaB canonical pathway overcomes the resistance of cutaneous T-cell lymphoma to apoptosis. Blood. 2006;107:2354–2363. doi: 10.1182/blood-2005-06-2536. [DOI] [PubMed] [Google Scholar]
- 9.Iqbal J, Wilcox R, Naushad H, Rohr J, Heavican TB, Wang C, et al. Genomic signatures in T-cell lymphoma: How can these improve precision in diagnosis and inform prognosis? Blood reviews. 2015 doi: 10.1016/j.blre.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 10.Iqbal J, Wright G, Wang C, Rosenwald A, Gascoyne RD, Weisenburger DD, et al. Gene expression signatures delineate biological and prognostic subgroups in peripheral T-cell lymphoma. Blood. 2014;123:2915–2923. doi: 10.1182/blood-2013-11-536359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang T, Feldman AL, Wada DA, Lu Y, Polk A, Briski R, et al. GATA-3 expression identifies a high-risk subset of PTCL, NOS with distinct molecular and clinical features. Blood. 2014;123:3007–3015. doi: 10.1182/blood-2013-12-544809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Olsen E, Vonderheid E, Pimpinelli N, Willemze R, Kim Y, Knobler R, et al. Revisions to the staging and classification of mycosis fungoides and Sezary syndrome: a proposal of the International Society for Cutaneous Lymphomas (ISCL) and the cutaneous lymphoma task force of the European Organization of Research and Treatment of Cancer (EORTC) Blood. 2007;110:1713–1722. doi: 10.1182/blood-2007-03-055749. [DOI] [PubMed] [Google Scholar]
- 13.An J, Fujiwara H, Suemori K, Niiya T, Azuma T, Tanimoto K, et al. Activation of T-cell receptor signaling in peripheral T-cell lymphoma cells plays an important role in the development of lymphoma-associated hemophagocytosis. Int J Hematol. 2011;93:176–185. doi: 10.1007/s12185-010-0758-7. [DOI] [PubMed] [Google Scholar]
- 14.Irizarry RA, Bolstad BM, Collin F, Cope LM, Hobbs B, Speed TP. Summaries of Affymetrix GeneChip probe level data. Nucleic acids research. 2003;31:e15. doi: 10.1093/nar/gng015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Irizarry RA, Hobbs B, Collin F, Beazer-Barclay YD, Antonellis KJ, Scherf U, et al. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics. 2003;4:249–264. doi: 10.1093/biostatistics/4.2.249. [DOI] [PubMed] [Google Scholar]
- 16.Gentleman RC, Carey VJ, Bates DM, Bolstad B, Dettling M, Dudoit S, et al. Bioconductor: open software development for computational biology and bioinformatics. Genome biology. 2004;5:R80. doi: 10.1186/gb-2004-5-10-r80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wettenhall JM, Smyth GK. limmaGUI: a graphical user interface for linear modeling of microarray data. Bioinformatics. 2004;20:3705–3706. doi: 10.1093/bioinformatics/bth449. [DOI] [PubMed] [Google Scholar]
- 18.Ritchie ME, Diyagama D, Neilson J, van Laar R, Dobrovic A, Holloway A, et al. Empirical array quality weights in the analysis of microarray data. BMC bioinformatics. 2006;7:261. doi: 10.1186/1471-2105-7-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hochberg Y, Benjamini Y. More powerful procedures for multiple significance testing. Statistics in medicine. 1990;9:811–818. doi: 10.1002/sim.4780090710. [DOI] [PubMed] [Google Scholar]
- 20.Wilcox RA, Feldman AL, Wada DA, Yang ZZ, Comfere NI, Dong H, et al. B7-H1 (PD-L1, CD274) suppresses host immunity in T-cell lymphoproliferative disorders. Blood. 2009;114:2149–2158. doi: 10.1182/blood-2009-04-216671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singleton KL, Gosh M, Dandekar RD, Au-Yeung BB, Ksionda O, Tybulewicz VL, et al. Itk controls the spatiotemporal organization of T cell activation. Sci Signal. 2011;4:ra66. doi: 10.1126/scisignal.2001821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Agostinelli C, Rizvi H, Paterson J, Shende V, Akarca AU, Agostini E, et al. Intracellular TCR-signaling pathway: novel markers for lymphoma diagnosis and potential therapeutic targets. Am J Surg Pathol. 2014;38:1349–1359. doi: 10.1097/PAS.0000000000000309. [DOI] [PubMed] [Google Scholar]
- 23.Liang PI, Chang ST, Lin MY, Hsieh YC, Chu PY, Chen CJ, et al. Angioimmunoblastic T-cell lymphoma in Taiwan shows a frequent gain of ITK gene. International journal of clinical and experimental pathology. 2014;7:6097–6107. [PMC free article] [PubMed] [Google Scholar]
- 24.Dubovsky JA, Beckwith KA, Natarajan G, Woyach JA, Jaglowski S, Zhong Y, et al. Ibrutinib is an irreversible molecular inhibitor of ITK driving a Th1-selective pressure in T lymphocytes. Blood. 2013;122:2539–2549. doi: 10.1182/blood-2013-06-507947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beyersdorf N, Werner S, Wolf N, Herrmann T, Kerkau T. Characterization of a new mouse model for peripheral T cell lymphoma in humans. PLoS One. 2011;6:e28546. doi: 10.1371/journal.pone.0028546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Y, Misumi I, Gu AD, Curtis TA, Su L, Whitmire JK, et al. GATA-3 controls the maintenance and proliferation of T cells downstream of TCR and cytokine signaling. Nat Immunol. 2013 doi: 10.1038/ni.2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cook KD, Miller J. TCR-dependent translational control of GATA-3 enhances Th2 differentiation. J Immunol. 2010;185:3209–3216. doi: 10.4049/jimmunol.0902544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hsi AC, Lee SJ, Rosman IS, Carson KR, Kelley A, Viele V, et al. Expression of helper T cell master regulators in inflammatory dermatoses and primary cutaneous T-cell lymphomas: diagnostic implications. Journal of the American Academy of Dermatology. 2015;72:159–167. doi: 10.1016/j.jaad.2014.09.022. [DOI] [PubMed] [Google Scholar]
- 29.Kari L, Loboda A, Nebozhyn M, Rook AH, Vonderheid EC, Nichols C, et al. Classification and prediction of survival in patients with the leukemic phase of cutaneous T cell lymphoma. J Exp Med. 2003;197:1477–1488. doi: 10.1084/jem.20021726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilcox RA. Cutaneous T-cell lymphoma: 2011 update on diagnosis, risk-stratification, and management. Am J Hematol. 2011;86:928–948. doi: 10.1002/ajh.22139. [DOI] [PubMed] [Google Scholar]
- 31.Vowels BR, Lessin SR, Cassin M, Jaworsky C, Benoit B, Wolfe JT, et al. Th2 cytokine mRNA expression in skin in cutaneous T-cell lymphoma. J Invest Dermatol. 1994;103:669–673. doi: 10.1111/1523-1747.ep12398454. [DOI] [PubMed] [Google Scholar]
- 32.Hughes CF, Khot A, McCormack C, Lade S, Westerman DA, Twigger R, et al. Lack of durable disease control with chemotherapy for mycosis fungoides and Sezary syndrome: a comparative study of systemic therapy. Blood. 2015;125:71–81. doi: 10.1182/blood-2014-07-588236. [DOI] [PubMed] [Google Scholar]
- 33.Kiessling MK, Klemke CD, Kaminski MM, Galani IE, Krammer PH, Gulow K. Inhibition of constitutively activated nuclear factor-kappaB induces reactive oxygen species- and iron-dependent cell death in cutaneous T-cell lymphoma. Cancer Res. 2009;69:2365–2374. doi: 10.1158/0008-5472.CAN-08-3221. [DOI] [PubMed] [Google Scholar]
- 34.Juvekar A, Manna S, Ramaswami S, Chang TP, Vu HY, Ghosh CC, et al. Bortezomib induces nuclear translocation of IkappaBalpha resulting in gene-specific suppression of NF-kappaB--dependent transcription and induction of apoptosis in CTCL. Mol Cancer Res. 2011;9:183–194. doi: 10.1158/1541-7786.MCR-10-0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang J, Chang CC, Lombardi L, Dalla-Favera R. Rearranged NFKB2 gene in the HUT78 T-lymphoma cell line codes for a constitutively nuclear factor lacking transcriptional repressor functions. Oncogene. 1994;9:1931–1937. [PubMed] [Google Scholar]
- 36.Lamprecht B, Kreher S, Mobs M, Sterry W, Dorken B, Janz M, et al. The tumour suppressor p53 is frequently nonfunctional in Sezary syndrome. The British journal of dermatology. 2012;167:240–246. doi: 10.1111/j.1365-2133.2012.10918.x. [DOI] [PubMed] [Google Scholar]
- 37.Velusamy T, Kiel MJ, Sahasrabuddhe AA, Rolland D, Dixon CA, Bailey NG, et al. A novel recurrent NPM1-TYK2 gene fusion in cutaneous CD30-positive lymphoproliferative disorders. Blood. 2014;124:3768–3771. doi: 10.1182/blood-2014-07-588434. [DOI] [PubMed] [Google Scholar]
- 38.Geissinger E, Sadler P, Roth S, Grieb T, Puppe B, Muller N, et al. Disturbed expression of the T-cell receptor/CD3 complex and associated signaling molecules in CD30+ T-cell lymphoproliferations. Haematologica. 2010;95:1697–1704. doi: 10.3324/haematol.2009.021428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rodriguez-Pinilla SM, Sanchez ME, Rodriguez J, Garcia JF, Sanchez-Espiridion B, Lamana LF, et al. Loss of TCR-beta F1 and/or EZRIN expression is associated with unfavorable prognosis in nodal peripheral T-cell lymphomas. Blood Cancer J. 2013;3:e111. doi: 10.1038/bcj.2013.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Clemente MJ, Przychodzen B, Jerez A, Dienes BE, Afable MG, Husseinzadeh H, et al. Deep sequencing of the T-cell receptor repertoire in CD8+ T-large granular lymphocyte leukemia identifies signature landscapes. Blood. 2013;122:4077–4085. doi: 10.1182/blood-2013-05-506386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vonderheid EC, Boselli CM, Conroy M, Casaus L, Espinoza LC, Venkataramani P, et al. Evidence for restricted Vbeta usage in the leukemic phase of cutaneous T cell lymphoma. J Invest Dermatol. 2005;124:651–661. doi: 10.1111/j.0022-202X.2004.23586.x. [DOI] [PubMed] [Google Scholar]
- 42.Wang L, Ni X, Covington KR, Yang BY, Shiu J, Zhang X, et al. Genomic profiling of Sezary syndrome identifies alterations of key T cell signaling and differentiation genes. Nature genetics. 2015;47:1426–1434. doi: 10.1038/ng.3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wilcox RA. A three-signal model of T-cell lymphoma pathogenesis. Am J Hematol. 2016;91:113–122. doi: 10.1002/ajh.24203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen J, Petrus M, Bryant BR, Nguyen VP, Goldman CK, Bamford R, et al. Autocrine/paracrine cytokine stimulation of leukemic cell proliferation in smoldering and chronic adult T-cell leukemia. Blood. 2010;116:5948–5956. doi: 10.1182/blood-2010-04-277418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sprent J, Surh CD. Normal T cell homeostasis: the conversion of naive cells into memory-phenotype cells. Nat Immunol. 2011;12:478–484. doi: 10.1038/ni.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang X, Werneck MB, Wilson BG, Kim HJ, Kluk MJ, Thom CS, et al. TCR-dependent transformation of mature memory phenotype T cells in mice. J Clin Invest. 2011;121:3834–3845. doi: 10.1172/JCI37210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Feldman AL, Dogan A, Smith DI, Law ME, Ansell SM, Johnson SH, et al. Discovery of recurrent t(6;7)(p25.3;q32.3) translocations in ALK-negative anaplastic large cell lymphomas by massively parallel genomic sequencing. Blood. 2011;117:915–919. doi: 10.1182/blood-2010-08-303305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li JP, Yang CY, Chuang HC, Lan JL, Chen DY, Chen YM, et al. The phosphatase JKAP/DUSP22 inhibits T-cell receptor signalling and autoimmunity by inactivating Lck. Nature communications. 2014;5:3618. doi: 10.1038/ncomms4618. [DOI] [PubMed] [Google Scholar]
- 49.Alonso A, Merlo JJ, Na S, Kholod N, Jaroszewski L, Kharitonenkov A, et al. Inhibition of T cell antigen receptor signaling by VHR-related MKPX (VHX), a new dual specificity phosphatase related to VH1 related (VHR) J Biol Chem. 2002;277:5524–5528. doi: 10.1074/jbc.M107653200. [DOI] [PubMed] [Google Scholar]
- 50.Streubel B, Vinatzer U, Willheim M, Raderer M, Chott A. Novel t(5;9)(q33;q22) fuses ITK to SYK in unspecified peripheral T-cell lymphoma.[see comment] Leukemia. 2006;20:313–318. doi: 10.1038/sj.leu.2404045. [DOI] [PubMed] [Google Scholar]
- 51.Hussain A, Mohammad DK, Gustafsson MO, Uslu M, Hamasy A, Nore BF, et al. Signaling of the ITK (interleukin 2-inducible T cell kinase)-SYK (spleen tyrosine kinase) fusion kinase is dependent on adapter SLP-76 and on the adapter function of the kinases SYK and ZAP70. J Biol Chem. 2013;288:7338–7350. doi: 10.1074/jbc.M112.374967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pechloff K, Holch J, Ferch U, Schweneker M, Brunner K, Kremer M, et al. The fusion kinase ITK-SYK mimics a T cell receptor signal and drives oncogenesis in conditional mouse models of peripheral T cell lymphoma. J Exp Med. 2010;207:1031–1044. doi: 10.1084/jem.20092042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jackow CM, Cather JC, Hearne V, Asano AT, Musser JM, Duvic M. Association of erythrodermic cutaneous T-cell lymphoma, superantigen-positive Staphylococcus aureus, and oligoclonal T-cell receptor V beta gene expansion. Blood. 1997;89:32–40. [PubMed] [Google Scholar]
- 54.Jahan-Tigh RR, Huen AO, Lee GL, Pozadzides JV, Liu P, Duvic M. Hydrochlorothiazide and cutaneous T cell lymphoma: prospective analysis and case series. Cancer. 2013;119:825–831. doi: 10.1002/cncr.27740. [DOI] [PubMed] [Google Scholar]
- 55.Miller AT, Wilcox HM, Lai Z, Berg LJ. Signaling through Itk promotes T helper 2 differentiation via negative regulation of T-bet. Immunity. 2004;21:67–80. doi: 10.1016/j.immuni.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 56.Seitz V, Butzhammer P, Hirsch B, Hecht J, Gutgemann I, Ehlers A, et al. Deep sequencing of MYC DNA-binding sites in Burkitt lymphoma. PLoS One. 2011;6:e26837. doi: 10.1371/journal.pone.0026837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee K, Gudapati P, Dragovic S, Spencer C, Joyce S, Killeen N, et al. Mammalian target of rapamycin protein complex 2 regulates differentiation of Th1 and Th2 cell subsets via distinct signaling pathways. Immunity. 2010;32:743–753. doi: 10.1016/j.immuni.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Evenou JP, Wagner J, Zenke G, Brinkmann V, Wagner K, Kovarik J, et al. The potent protein kinase C-selective inhibitor AEB071 (sotrastaurin) represents a new class of immunosuppressive agents affecting early T-cell activation. The Journal of pharmacology and experimental therapeutics. 2009;330:792–801. doi: 10.1124/jpet.109.153205. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.