Abstract
Soil salinity is a serious threat to agriculture sustainability worldwide. Salt tolerance at the seedling stage is crucial for plant establishment and high yield in saline soils; however, little information is available on rapeseed (Brassica napus L.) salt tolerance. We evaluated salt tolerance in different rapeseed accessions and conducted a genome-wide association study (GWAS) to identify salt tolerance-related quantitative trait loci (QTL). A natural population comprising 368 B. napus cultivars and inbred lines was genotyped with a Brassica 60K Illumina Infinium SNP array. The results revealed that 75 single-nucleotide polymorphisms (SNPs) distributed across 14 chromosomes were associated with four salt tolerance-related traits. These SNPs integrated into 25 QTLs that explained 4.21–9.23% of the phenotypic variation in the cultivars. Additionally, 38 possible candidate genes were identified in genomic regions associated with salt tolerance indices. These genes fell into several functional groups that are associated with plant salt tolerance, including transcription factors, aquaporins, transporters, and enzymes. Thus, salt tolerance in rapeseed involves complex molecular mechanisms. Our results provide valuable information for studying the genetic control of salt tolerance in B. napus seedlings and may facilitate marker-based breeding for rapeseed salt tolerance.
Keywords: rapeseed (Brassica napus L.), genome-wide association study (GWAS), salt tolerance, quantitative trait loci (QTL), single-nucleotide polymorphisms (SNP), candidate genes
Introduction
Soil salinity is a major abiotic stress that threatens agricultural production all over the world (Zhu, 2001). Approximately 280 million hectares of agricultural land are affected by salt stress (Ruan et al., 2010). Due to traditional and unscientific irrigation management, soil salinity will become progressively more severe over time, and salt stress is predicted to affect over 50% of all agricultural land by 2050 (Flowers, 2004; Demiral and Türkan, 2006).
Plant response to salt stress is a complex genetic and physiological trait controlled by several quantitative trait loci (QTL) (Flowers, 2004). The response can be subdivided into two stages: early-occurring osmotic stress and accumulation of toxic ions (e.g., Na+ and Cl− if in excess) (Munns and Tester, 2008). In the first stage, salt stress reduces soil water potential, thus decreasing root water-uptake ability. This water deficit leads to over-production of reactive oxygen species (ROS), including super oxides, hydrogen peroxide, and hydroxyl radicals (Mittler, 2002). Elevated ROS content disturbs normal cellular activity through oxidative damage of membrane lipids and proteins (Imlay, 2003). During the second stage, roots absorb Na+ at high concentrations; these ions are then transported to shoots later. Excess Na+ negatively affect plant growth rate and development, compromising metabolic processes and decreasing photosynthetic efficiency (Munns, 2005; Munns and Tester, 2008). Specifically, excess Na+ impedes K+ absorption, thus disrupting intracellular Na+ and K+ homeostasis, which is critical to plant growth and development (Schachtman and Liu, 1999; Shabala and Cuin, 2008; Ahmadi et al., 2011).
Saline land has traditionally been reclaimed through engineering technologies that repair salt-affected soil (Epstein et al., 1980). With recent biotechnological developments, plant scientists have increasingly favored breeding and screening salt-tolerant crop varieties through genetic modification as a cost-saving, convenient, and efficient alternative. Mapping the QTL of salt-related traits and cloning salt-tolerant genes have become effective approaches for coping with soil salinization in agricultural land. Conventionally, QTL mapping uses bi-parental populations to dissect the genetic mechanisms of salt stress-related traits (Kumar et al., 2015), resulting in the discovery of salt tolerance-linked QTLs from several crops. In rice, for example, Lin et al. (2004) mapped QTL for root and shoot Na+/K+ concentration and transportation to five chromosomes, while also cloning SKC1, a key gene that controls shoot K+ content. In soybean, a major QTL controlling salt tolerance was identified, accounting for 41, 60, and 79% of the total genetic variation for salt tolerance in field, greenhouse, and combined environments, respectively (Lee et al., 2004). Finally, in maize, Cui et al. (2015) detected 20 additive and nine epistatic QTLs for salt tolerance, based on SNP markers expressed at the seedling stage. With the recent introduction of genome-wide association studies (GWAS; also called association mapping) to crop genetic research, the utility of QTL has been further enhanced (Mackay and Powell, 2007; Cockram et al., 2010). Compared with traditional screening methods, genotyping, and sequencing technologies are more efficient (Zhu et al., 2008), making association mapping a widely used technique for dissecting salt-related traits in crops such as rice (Kumar et al., 2015), barley (Long et al., 2013), and soybean (Kan et al., 2015).
Rapeseed (Brassica napus L., genome AACC, 2n = 38) is one of the most important oil crops in the world. Annually, rapeseed oil production reaches 26 million tons, accounting for approximately 15% of global vegetable oil production (USDA ERS, 2014). Therefore, understanding the genetic architecture for a complex trait such as salt tolerance is crucial in the development of new, robust rapeseed cultivars. Recently, with the development of the Brassica 60K Infinium SNP array, association mapping has been successful in detecting QTL for complex traits in rapeseed. Recently, Li et al. (2014, 2016) identified 23 QTLs for erucic acid content, glucosinolate content, oil content, seed weight, plant height, and primary branch. Additionally, Xu et al. (2016) identified 35 QTLs for flowering time found near Bn-scaff_16362_1-p380982, approximately 13 kb from BnaC09g41990D, which is an orthologous to A. thaliana CONSTANS (CO), an important gene in the photoperiod flowering pathway. Liu et al. (2016) identified 50 QTLs for seed oil content that could explain approximately 80% of the total phenotypic variance in rapeseed, while Luo et al. (2015) identified nine SNPs for harvest index and seed yield per plant that explained 3.42% of the phenotypic variance in the former trait. Moreover, Sun et al. (2016a,b) identified 68 loci associated with plant height and 53 loci associated with branch angle in rapeseed. Yet despite the recent increase in rapeseed association mapping, limited QTLs for rapeseed salt tolerance have been reported.
In the present study, we examined a panel of 368 rapeseed accessions using 19,167 genomic SNPs from the Illumina BrassicaSNP60 Bead Chip. Seedling growth-related traits from the panel were investigated in three environments. The objectives of this study were as follows: (i) obtain a better understanding the effect of salt stress on rapeseed seedlings, (ii) examine the relationships between salt stress-related traits in seedlings, and (iii) perform GWAS to identify salt-tolerance-related SNPs and possible candidate genes underlying salt tolerance at the seedling stage.
Materials and methods
Plant materials
The association panel used in this study consisted of 368 diverse rapeseed accessions derived from recently published studies (Liu et al., 2016; Xu et al., 2016). These germplasms originated from 10 countries on four continents (Table S1); most came from China and included winter oilseed rape (OSR) [26], semi-winter OSR [307], and spring OSR [35].
Experimental design and trait measurement
To minimize environmental impact, rapeseed salt-tolerance traits were evaluated at the seedling stage using a previously described hydroponic system (Tocquin et al., 2003), with slight modifications to accommodate larger hydroponic containers (60× 40× 10 cm). Thirty healthy seeds from all 368 rapeseed lines were germinated for 7 d in a salver (60× 40× 10 cm) containing double-distilled water, separated into 96 plots with nylon rope, and covered with one sheet of medical gauze on the bottom. At the beginning of the 8th day, 10 similar seedlings per plot were selected and individually transferred to the hydroponic system for another 4 weeks of growth. According to the use of the nutrient solution method for rapeseed seedling growth in our laboratory, different concentrations of Hoagland solution (10 L) were used as the hydroponic growing medium: 0.25× for the first week, 0.5× for the second week, and 1× for the third to fourth weeks. We designed eight concentration gradients (0, 110, 140, 170, 200, 230, 260, 290 mM) NaCl to carry out salt stress treatment on rapeseed seedlings, and the results shown that the 230 mM NaCl was suitable to evaluated salt tolerance of rapeseed seedlings (Unpublished). Thus, at the beginning of the fifth week, 134 g NaCl was mixed with 10 L 1× Hoagland solution to generate a 230 mM NaCl solution, while 1× Hoagland solution without NaCl was used for the control condition and the NaCl and without NaCl solution were used to plant rapeseed seedling for another 2 weeks. Experimental and control 1× Hoagland solutions were replaced every 7 d.
The seedlings were harvested for measuring shoot length (SL), taproot length (RL), and shoot fresh weight (SFW). Harvested material from salt-stressed seedlings were washed with distilled water and oven-dried at 105°C for 30 min, then dried further at 80°C for 48 h. To determine shoot Na+ content in each rapeseed line, dried shoots from five replicates per line were pooled, placed in a 50-mL centrifuge tube containing two 6-mm steel balls, and pulverized for 5 min with a high-speed oscillator (model SK450, F&FM, Australia). Pulverized samples were then sieved (100 mesh). A 100 mg sample from each rapeseed line was placed in test tubes with 2 mL nitric acid, incubated in a 100°C water bath for 1 h, and then distilled water was added until a final volume of 10 mL was attained. A flame photometer (model FP640) was used for Na+ estimation (μg/mg dry weight), calculated with the following equation (Ammar et al., 2009):
where C is the flame photometer reading, d is the dilution factor, V is extraction volume (mL), and dwt is the dry weight (mg).
The salt tolerance index was calculated following previous publications, as the ratio of experimental (saline) values to control values (Munns and James, 2003; Long et al., 2013). The hydroponics system used for testing consisted of 10 units and involved 10 plants grown separately in containers (five per condition), with 77 possible plant positions. Each plant represented one experimental unit. Thus, there were 10 replicates per genotype: five for the salt treatment and five for the control. Each experiment comprised 10 randomized units allocated to control (0 mM NaCl) and salt (230 mM NaCl) hydroponic treatment groups. The experiment was repeated three times, once in a natural outdoor environment during October 2014 and twice in a greenhouse (20°C ± 2) during November 2013 and September 2014.
Phenotypic data analysis
Statistical analysis of all phenotypic data was performed in SPSS version 18.0 (IBM Corp, Armonk, NY, USA). Descriptive statistics and Pearson's correlations between traits were performed using the mean values of all phenotypic data from 368 rapeseed accessions. The frequency distribution of each trait was calculated in R (R Core Team, Vienna, Austria). The broad-sense heritability for the panel was calculated with the R package lme4 (Merk et al., 2012), using the equation h2 = o,2g/(o,2g + o,2ge/n + o,2e/nr), where o,2g is the genetic variance, o,2ge is the genotype × environment interaction variance, o,2e is the error variance, n is the number of environments, and r is the number of replications.
Population structure and relative kinship analysis
The genotypes of the 368 rapeseed accessions in this study have been previously published (Liu et al., 2016; Xu et al., 2016) and were evaluated here using the Brassica 60 K Illumina® Infinium SNP array. Following previously described methods (Sun et al., 2016b), 19,167 high-quality SNPs (call rate > 0.7; SNP call frequency > 0.75; minor frequency > 0.05; AA, BB frequency > 0.03; and GenTrain score > 0.5) was used for population structure analysis and relative kinship analysis. The population structure was estimated in STRUCTURE 2.3.4 (Falush et al., 2003) with the Bayesian Markov Chain Monte Carlo (MCMC) model. The K-value representing the number of subgroups was set from 1 to 10, and five independent runs for each K were performed, with burn-in length and iterations both set to 100,000 under the admixture and correlated allele frequencies models. The optimal K-value was determined using the log likelihood of the date [LnP(D)] and ΔK based on the rate of change in LnP(D) between successive K-values, as previously described (Evanno et al., 2005). The Q matrix was obtained in CLUMPP (Jakobsson and Rosenberg, 2007), through integrating replicate runs from STRUCTURE. The relative kinship matrix (K matrix) was obtained using SPAGeDi (Hardy and Vekemans, 2002), and negative values between two rapeseed accessions were set to 0 (Yu et al., 2006).
Genome-wide association analysis
Association mapping was conducted in TASSEL 4.0 (Bradbury et al., 2007) with four models: GLM without considering Q and K, GLM considering Q (Q), MLM considering K (K), and MLM considering Q and K (Q + K). The SNP markers that were significantly associated with the three salt tolerance indices (ST-RL, ST-SL, ST-SFW) and shoot Na+ content were identified according to a probability level of P ≤ 0.0001 or −logP ≥ 4.00, following existing published thresholds (Yong et al., 2015).
Results
Phenotypic variation among accessions for growth-related traits and salt tolerance index traits
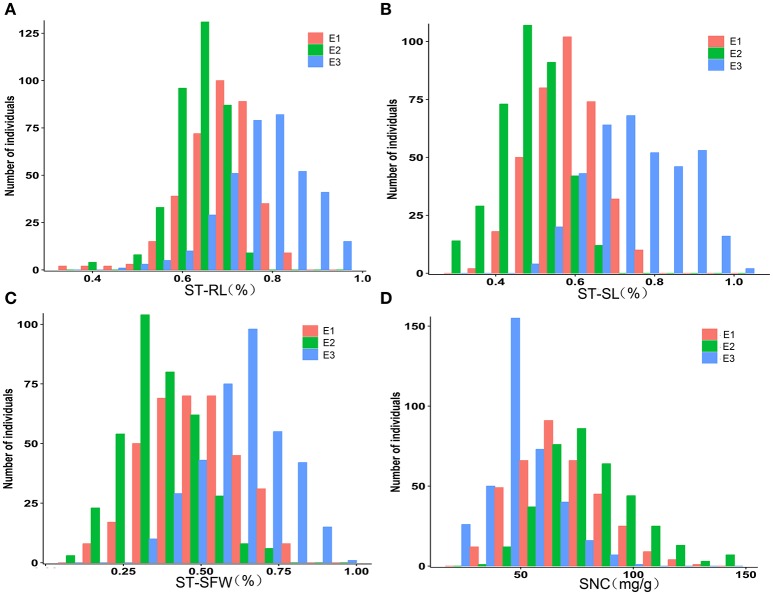
The 368 rapeseed accessions were planted in three environments (E1: greenhouse 2013, E2: greenhouse 2014, E3: natural environment 2014) and extensive phenotypic variation was observed for RL, SL, and SFW in salt stress and control conditions (Table 1, Figure S1, Data S1). Furthermore, salt stress significantly reduced RL, SL, and SFW compared with the control. To control for background differences, salt tolerance indices (ST) of RL, SL, and SFW were used in our evaluation of rapeseed salt stress response. Across the three environments, ST-RL, ST-SL, and ST-SFW varied between 0.370 and 0.960 (averages: 0.636 ± 0.058–0.776 ± 0.091), 0.310 and 0.930 (0.532 ± 0.080–0.715 ± 0.094), and 0.087 and 0.940 (0.374 ± 0.121–0.617 ± 0.132) (Figures 1A–C). Control growth traits had higher mean values and less variation than those measured under salt stress. In addition, salt-stressed shoot Na+ content (SNC) ranged 20.2–143.5 mg g−1 with averages of 47.7 ± 13.7–80.3 ± 21.3 mg g−1 in the three growth environments (Figure 1D). For the salt tolerance indices, the STs in the greenhouse were lower than that in outdoor, suggesting that the environment would affect the salt tolerance of rapeseed at seedling stage (Table 1). Heritability (h2) estimates of all traits ranged from 79.60% (control SFW) to 93.46% (ST-SL), suggesting their stable inheritance (Table 1).
Table 1.
Phenotypic variation in growth−related traits and salt tolerance index traits.
| Trait | Treat | Env | Minimum | Maximum | Mean | SD | CV (%) | Skewness | Kurtosis | h2 (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| RL | C | E1 | 19.2 | 36 | 27.1 | 2.28 | 8.41 | 0.43 | 1.22 | 82.27 |
| E2 | 20 | 35 | 25.8 | 1.96 | 7.60 | 0.46 | 1.00 | |||
| E3 | 16.6 | 33 | 23.6 | 2.57 | 10.87 | 0.53 | 0.32 | |||
| S | E1 | 10.2 | 26.5 | 18.7 | 2.30 | 12.31 | −0.41 | 1.28 | 80.78 | |
| E2 | 9.7 | 21.1 | 16.4 | 1.76 | 10.73 | −0.26 | 0.70 | |||
| E3 | 9.6 | 26 | 18.3 | 2.78 | 15.15 | 0.12 | 0.18 | |||
| ST | E1 | 0.37 | 0.85 | 0.69 | 0.08 | 11.18 | −0.95 | 2.07 | 87.37 | |
| E2 | 0.38 | 0.76 | 0.64 | 0.06 | 9.06 | −1.00 | 2.41 | |||
| E3 | 0.47 | 0.96 | 0.78 | 0.09 | 11.79 | −0.43 | 0.19 | |||
| SL | C | E1 | 19.1 | 39.2 | 27.9 | 3.46 | 12.40 | 0.18 | −0.52 | 81.40 |
| E2 | 18.18 | 38.9 | 26.4 | 4.02 | 15.24 | 0.98 | 0.63 | |||
| E3 | 16.4 | 34.1 | 24.9 | 3.78 | 15.19 | 0.02 | −1.01 | |||
| S | E1 | 9.9 | 24.2 | 16.3 | 2.16 | 13.29 | 0.08 | 0.52 | 86.40 | |
| E2 | 7.2 | 20 | 12.7 | 2.13 | 16.76 | 0.09 | 0.30 | |||
| E3 | 10.9 | 28.1 | 18.4 | 3.44 | 18.66 | 0.29 | −0.33 | |||
| ST | E1 | 0.37 | 0.8 | 0.59 | 0.09 | 14.52 | −0.04 | −0.31 | 93.46 | |
| E2 | 0.27 | 0.65 | 0.49 | 0.08 | 16.33 | −0.15 | −0.27 | |||
| E3 | 0.47 | 1 | 0.75 | 0.12 | 15.74 | 0.05 | −0.90 | |||
| SFW | C | E1 | 5.05 | 18.59 | 9.66 | 2.61 | 27.01 | 0.67 | 0.00 | 79.60 |
| E2 | 4.46 | 19.62 | 10.71 | 2.67 | 24.92 | 0.26 | −0.02 | |||
| E3 | 2.85 | 12.32 | 7.26 | 1.86 | 25.60 | 0.16 | −0.23 | |||
| S | E1 | 0.85 | 11.04 | 4.63 | 1.68 | 36.36 | 0.60 | 0.79 | 90.09 | |
| E2 | 0.79 | 9.43 | 3.97 | 1.52 | 38.28 | 0.51 | 0.40 | |||
| E3 | 1.35 | 9.92 | 4.45 | 1.41 | 31.80 | 0.46 | 0.34 | |||
| ST | E1 | 0.14 | 0.83 | 0.49 | 0.14 | 29.70 | 0.00 | −0.50 | 92.31 | |
| E2 | 0.09 | 0.75 | 0.37 | 0.12 | 32.41 | 0.36 | −0.01 | |||
| E3 | 0.29 | 0.95 | 0.62 | 0.13 | 21.35 | −0.15 | −0.38 | |||
| SNC | E1 | 29.6 | 133 | 68.72 | 18.71 | 27.23 | 0.45 | 0.07 | 91.02 | |
| E2 | 35.6 | 145.3 | 81.28 | 20.26 | 24.93 | 0.60 | 0.36 | |||
| E3 | 20 | 94.1 | 47.69 | 13.68 | 28.68 | 0.55 | 0.44 |
Trait: RL, root length; SL, shoot length; SFW, shoot fresh weight; ST, salt tolerance index; SNC, shoot Na+ content.
Treatment: C, no salt treatment; S, salt treatment; ST, salt tolerance index.
Environment: E1, greenhouse in 2013; E2, greenhouse in 2014; E3, natural environment in 2014.
SD, standard deviation; CV (%), coefficient of variation; h2, heritability of means.
Figure 1.
Distribution of the three salt indices and SNC in the association panel of 368 accessions grown in three environments. (A) ST-RL. (B) ST-SL. (C) ST-SFW. (D) SNC. E1, greenhouse in 2013; E2, greenhouse in 2014; E3, natural environment condition in 2014. ST, salt tolerance index; RL, root length; SL, shoot length; SFW, shoot fresh weight.
Salt tolerance index and its correlation with other traits in rapeseed
Pearson's correlations between the three growth-related traits (under control or salt stress conditions) and various STs were calculated from 2013 greenhouse (Table 2). In the control, RL was not significantly correlated with SL or SFW; however, SL and SFW were positively correlated (P < 0.01). Under salt stress, SL was positively correlated with RL (P < 0.05) and SFW (P < 0.01). Further, ST-RL was negatively correlated with RL in control conditions (P < 0.01) and positively correlated with RL in salt stress conditions (P < 0.01). The same patterns were observed for ST-SFW and ST-SL, but not for ST-SL and SL, which were uncorrelated in the control. Furthermore, SNC under salt stress was not significantly correlated with any traits, indicating that rapeseed salt tolerance is not linked to Na+ content in shoots. Results were consistent across all environments (Table S2). As shown in Table S3, the correlations between phenotypic data in different environments were generally high, and majority reached to 0.8, indicating our screenings were reliable.
Table 2.
Phenotypic correlations between all of the salt tolerance traits based on the trait means of plants grown in the greenhouse in 2013.
| Trait | CRL | TRL | ST-RL | CSL | TSL | ST-SL | CSFW | TSFW | ST-SFW | SNC |
|---|---|---|---|---|---|---|---|---|---|---|
| CRL | 1 | 0.451** | −0.249** | 0.082 | 0.065 | −0.001 | 0.086 | 0.089 | 0.029 | 0.066 |
| TRL | 1 | 0.748** | 0.1 | 0.094 | 0.01 | 0.033 | 0.055 | 0.042 | 0.091 | |
| ST-RL | 1 | 0.051 | 0.056 | 0.011 | −0.027 | −0.004 | 0.026 | 0.047 | ||
| CSL | 1 | 0.346** | −0.529** | 0.654** | 0.357** | −0.131* | 0.037 | |||
| TSL | 1 | 0.605** | 0.335** | 0.605** | 0.437** | −0.037 | ||||
| ST-SL | 1 | −0.247** | 0.239** | 0.504** | −0.063 | |||||
| CSFW | 1 | 0.561** | −0.186** | 0.01 | ||||||
| TSFW | 1 | 0.681** | −0.062 | |||||||
| ST-SFW | 1 | −0.086 | ||||||||
| SNC | 1 |
Trait: CRL, root length under control treatment; CSL, shoot length under control treatment; CSFW, shoot fresh weight under control treatment; TRL, root length under salt treatment; TSL, shoot length under salt treatment; TSFW, shoot fresh weight under salt treatment; ST-RL, ST-SL, and ST-SFW, salt tolerance index of RL, SL, and SFW, respectively.
P < 0.05,
P < 0.01.
Population structure and genetic relatedness in rapeseed cultivars
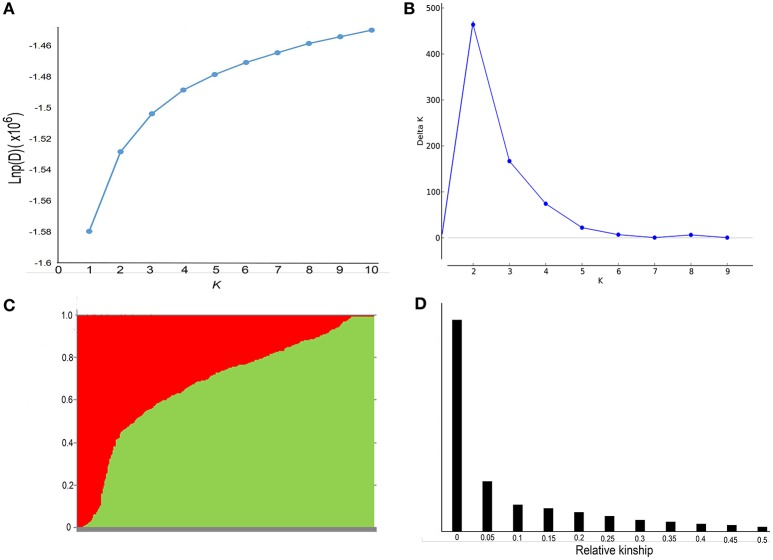
Population structure and genetic relatedness was calculated from 19,167 SNPs in the rapeseed accessions. As K increased from 1 to 10, LnP(D) increased continuously, and the most significant changes appeared when K shifted from 1 to 2 (Figure 2A). A sharp peak in ΔK was also observed at K = 2 (Figure 2B). Thus, the association panel was divided into two sub-groups, designated P1 and P2 (Figure 2C). Rapeseed accessions with Q matrix values < 0.6 or ≥0.6 were assigned to the P1 or P2 sub-groups, respectively, resulting in 105 lines for the former and 263 lines for the latter (Table S1, Data S2). The analysis of relative kinship showed that 56.8% of between-line kinship coefficients equaled 0, and 95.5% kinship coefficients ranged from 0 to 0.2 (Figure 2D, Data S2). Thus, most lines in the panel were very weakly related.
Figure 2.
Analysis of the population structure and relative kinships of the 368 rapeseed accessions. (A) Estimated LnP(D) of possible clusters (K) from 1 to 10. (B) ΔK was based on the rate of change of LnP(D) between successive K-values. (C) Population structure based on K = 2. Red represents Subgroup Q1; green, Subgroup Q2. (D) Distribution of pairwise relative kinship estimated in the entire population P (368 rapeseed accessions). Only kinship values ranging from 0 to 0.5 are shown.
Association mapping for salt tolerance indices and SNC
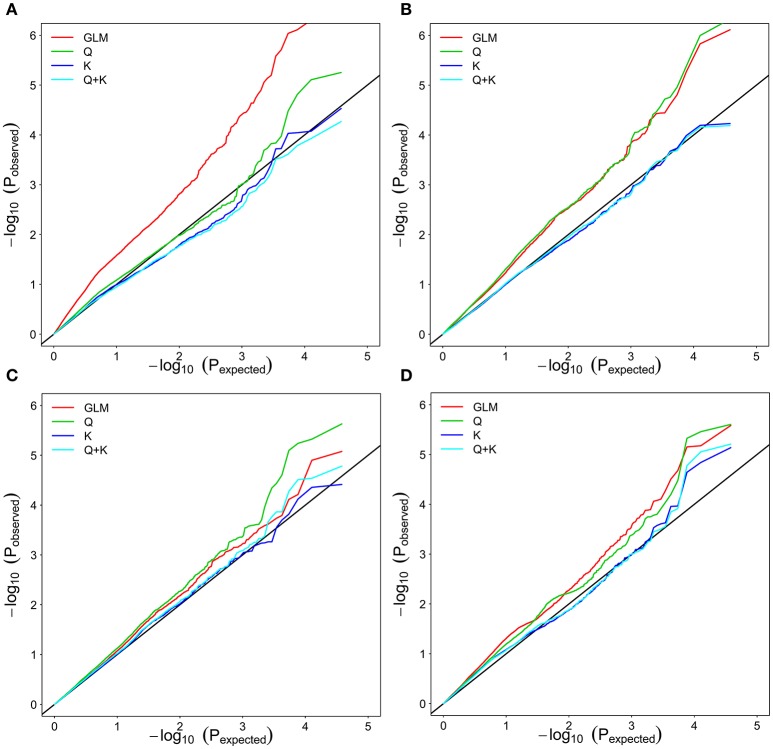
To determine the most appropriate model (see Genome-Wide Association Analysis) for a GWAS, model fit was tested with salt tolerance traits (ST-RL, ST-SL, ST-SFW, and SNC) (Figure 3). Based on quantile–quantile (Q-Q) plots, the best-fit model varied depending on rapeseed traits. For example, the Q, K, and Q + K models were a better fit for the expected P-values of ST-RL (Figure 3A). The K and Q + K models were a better fit for the expected P-values of ST-SL (Figure 3B). All four models were good fits for expected P-values of ST-SFW (Figure 3C). The Q, K, and Q + K models were a better fit for the expected P-values of SNC (Figure 3D). To make the most efficient use of genotypic and phenotypic data in this study, the Q and Q + K models were applied for the association analysis.
Figure 3.
Quantile–quantile plots of estimated−log10(P) from association analysis of associated traits in rapeseed cultivars. (A) ST-RL. (B) ST-SL. (C) ST-SFW. (D) SNC. GLM considering no Q and K; Q: GLM considering Q; K: MLM considering K; Q + K: MLM considering Q and K; ST, salt tolerance index; RL, root length; SL, shoot length; SFW, shoot fresh weight.
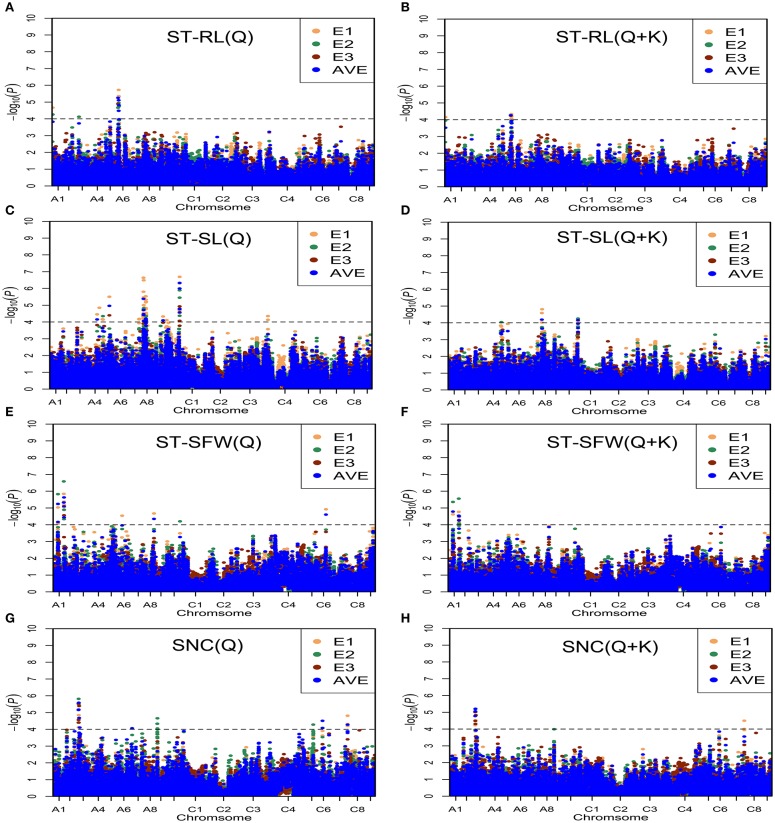
With these two models, we identified 75 significantly associated (P < 0.0001 or −Log(P) > 4.0) SNPs for the salt-related traits ST-RL, ST-SL, ST-SFW, and SNC (Figure 4, Table S4). Among the 75 associated SNPs, 58 SNPs were identified in greenhouse in 2013 (E1); 29 SNPs were identified in greenhouse in 2014 (E2); 10 SNPs were identified in natural environment in 2014 (E3); 34 SNPs were identified using average values (AVE) across three environments (Table S4). In addition, 32% (24/75) of the SNPs were identified in more than one growth environment, indicating their high reliability. Additionally, six, 47, 10, and 12 SNPs were distributed on chromosomes A1, A3, A4, A5, A6, A7, A8, A9, A10, C1, C4, C5, C6, and C7.
Figure 4.
Manhattan plots generated from genome-wide association analysis results for salt tolerance related-traits using Q and Q + K models. (A) ST-RL (Q). (B) ST-RL (Q + K). (C) ST-SL (Q). (D) ST-SL (Q + K). (E) ST-SFW (Q). (F) ST-SFW (Q + K). (G) SNC (Q). (H) SNC (Q + K). E1, greenhouse in 2013; E2, greenhouse in 2014; E3, natural environment condition in 2014; AVE, average values across three environments. Gray horizontal line depicts significance threshold (P < 0.0001).
To integrate putative QTLs, we modified the method as previously described (Mohammadi et al., 2015; Liu et al., 2016). If the lead SNP and following SNPs were within 1 Mb, or the LD statistic pairwise r2 between lead SNP and the following SNPs was > 0.35, these SNPs were regarded as identifiers for the same QTL. Finally, these 75 significant SNPs constituted 25 QTLs and explained 4.21–9.23% of the phenotypic variation among the rapeseed accessions (Table 3, Table S5).
Table 3.
SNPs significantly associated with salt tolerance indices and SNC in the three growth environments.
| Trait | SNPsa | Chr | Site (b) | Allele | MAF | Q | Q + K | Envc | ||
|---|---|---|---|---|---|---|---|---|---|---|
| −Log10P | R2(%)b | −Log10P | R2(%)b | |||||||
| ST-RL | Bn-A01-p1372430 | A01 | 973485 | A/G | 0.135 | 4.67 | 5.96 | 4.15 | 5.67 | E1 |
| 4.26 | 5.37 | E2 | ||||||||
| Bn-A03-p9873327 | A03 | 9088303 | T/C | 0.41 | 4.12 | 5.59 | E1 | |||
| 4.12 | 5.54 | E2 | ||||||||
| Bn-A06-p3066372 | A06 | 2959211 | A/G | 0.16 | 5.73 | 7.44 | 4.33 | 6.69 | E1 | |
| 4.95 | 6.27 | E2 | ||||||||
| 5.11 | 6.5 | AVE | ||||||||
| ST-SL | Bn-A04-p11164883 | A04 | 12315774 | A/G | 0.305 | 4.45 | 5.65 | E1 | ||
| 4.15 | 5.24 | AVE | ||||||||
| Bn-A04-p14778146 | A04 | 15229011 | A/G | 0.395 | 4.85 | 6.17 | E1 | |||
| Bn-A05-p1744534 | A05 | 1887184 | A/C | 0.465 | 4.16 | 4.27 | E1 | |||
| Bn-A05-p2246038 | A05 | 2366787 | A/C | 0.304 | 4.14 | 4.24 | E1 | |||
| 4.35 | 4.46 | 4.04 | 4.3 | E2 | ||||||
| Bn-A05-p17420613 | A05 | 14469584 | T/C | 0.419 | 5.5 | 7.69 | E1 | |||
| 4.39 | 6.02 | E3 | ||||||||
| 4.96 | 6.88 | AVE | ||||||||
| Bn-A07-p15589001 | A07 | 17485098 | T/C | 0.15 | 4.18 | 4.78 | E1 | |||
| Bn-A09-p33179406 | A09 | 30892924 | T/C | 0.17 | 4.32 | 5.56 | E1 | |||
| Bn-A08-p3269074 | A08 | 2746242 | T/C | 0.117 | 6.48 | 8.04 | 4.81 | 6.24 | E1 | |
| 4.81 | 5.8 | E2 | ||||||||
| 4.72 | 5.71 | AVE | ||||||||
| Bn-Scaffold000211-p65635 | A10 | 4230491 | T/G | 0.467 | 4.11 | 4.21 | E1 | |||
| Bn-scaff_15838_1-p1804241 | C01 | 2159899 | T/C | 0.424 | 6.69 | 9.23 | 4.09 | 6.06 | E1 | |
| 5.89 | 8.03 | 4.25 | 6.23 | E2 | ||||||
| 4.93 | 6.68 | E3 | ||||||||
| 6.33 | 8.71 | 4.16 | 6.19 | AVE | ||||||
| Bn-scaff_15908_1-p535407 | C04 | 5694092 | A/G | 0.259 | 4.35 | 5.19 | E1 | |||
| ST-SFW | Bn-A01-p7574355 | A01 | 6916594 | T/C | 0.276 | 5.03 | 6.2 | 4.61 | 6.02 | E1 |
| 5.82 | 7.26 | 5.36 | 7.05 | E2 | ||||||
| 5.24 | 6.48 | 4.78 | 6.31 | AVE | ||||||
| Bn-A01-p21855770 | A01 | 18441121 | T/C | 0.212 | 5.84 | 7.53 | 4.77 | 6.43 | E1 | |
| 6.58 | 8.49 | 5.55 | 7.75 | E2 | ||||||
| 5.32 | 6.81 | 4.54 | 6.21 | AVE | ||||||
| Bn-A06-p7703242 | A06 | 7333685 | T/G | 0.23 | 4.54 | 4.71 | E1 | |||
| Bn-A08-p20091818 | A08 | 17365577 | T/C | 0.173 | 4.67 | 5.51 | E1 | |||
| 4.35 | 5.09 | AVE | ||||||||
| Bn-A10-p15741819 | A10 | 15808392 | A/G | 0.477 | 4.2 | 5.59 | E2 | |||
| Bn-scaff_16116_1-p406330 | C06 | 28299662 | T/G | 0.191 | 4.92 | 5.15 | E1 | |||
| 4.6 | 4.78 | AVE | ||||||||
| SNC | Bn-A03-p8032849 | A03 | 7321761 | A/G | 0.266 | 4.12 | 4.85 | E1 | ||
| 5.81 | 7.04 | 5.19 | 6.37 | E2 | ||||||
| 4.56 | 5.45 | 4.48 | 5.29 | E3 | ||||||
| 5.46 | 6.6 | 5.05 | 6.11 | AVE | ||||||
| Bn-A09-p13614414 | A09 | 10135644 | T/G | 0.411 | 4.65 | 4.81 | E2 | |||
| Bn-scaff_16268_1-p640088 | C05 | 12621782 | A/G | 0.493 | 4.28 | 5.52 | E2 | |||
| Bn-scaff_18206_1-p435713 | C06 | 18701952 | A/G | 0.362 | 4.07 | 5.39 | E2 | |||
| 4.2 | 5.59 | AVE | ||||||||
| Bn-scaff_16110_1-p3586046 | C07 | 41328003 | T/C | 0.213 | 4.81 | 5.74 | 4.49 | 5.42 | E1 | |
Only the lead SNPs at a defined locus are shown.
Percentage of phenotypic variance explained by the lead SNP.
E1 greenhouse in 2013, E2 greenhouse in 2014, E3 natural environment in 2014, AVE, average values across three environments.
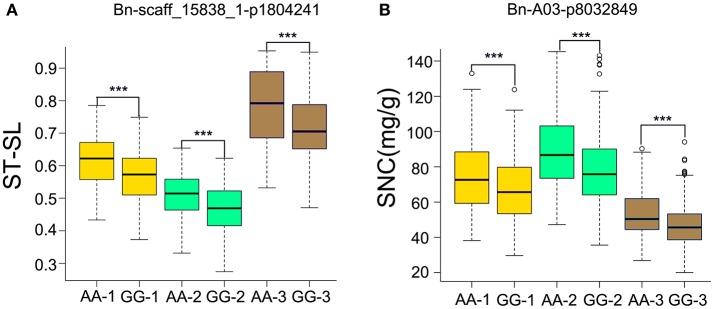
For ST-RL, six significantly associated SNPs, constituting three QTLs, were identified on chromosomes A1, A3, and A6 and explained 5.37–7.44% of the phenotypic variation (Table 3, Table S4). Among these six SNPs, five were validated in more than one growth environment. For ST-SL, 47 significantly associated SNPs (constituting 11 QTLs) were distributed on the A4, A5, A7, A9, A10, C1, and C4 chromosomes and explained 4.21–9.23% of phenotypic variation. Among them, 11 SNPs were validated in more than one individual environment (Table 3, Table S4). For ST-SFW, 10 significantly associated SNPs (constituting six QTLs) were identified on A1, A6, A8, A10, and C6 chromosomes and explained 4.39–8.49% of the phenotypic variation observed (Table 3, Table S4). Among them, five were validated in more than one individual environment. For SNC, 12 significantly associated SNPs (five QTLs) were identified on A3, A9, C5, C6, and C7 chromosomes and explained 4.69–7.04% of the phenotypic variation (Table 3, Table S4). Among these, three SNPs were validated in all tested environments. The SNP Bn-scaff_15838_1-p1804241 (ST-SL) and Bn-A03-p8032849 (SNC) were identified both in three environments and using average values across three environments (Table 3). In order to know the impact of these stable QTLs on phenotype. We found that ST-SL of accessions carrying the AA allele of Bn-scaff_15838_1-p1804241 was significantly higher (P < 0.001) than those with the GG allele in all three environments (Figure 5A). The SNC of accessions carrying the AA allele for Bn-A03-p8032849 was significantly higher (P < 0.001) than those with the GG allele in all three environments (Figure 5B).
Figure 5.
Phenotypic differences between lines with different alleles of the SNPs associated with SNC and ST-SL in three growth environments. (A) Bn-A03-p8032849 associated with ST-SL. (B) Bn-scaff_15838_1-p1804241 associated with SNC. The numbers in brackets behind AA or GG refer to the three growth environments. ***P < 0.001.
Predicting salt-tolerant genes in rapeseed cultivars
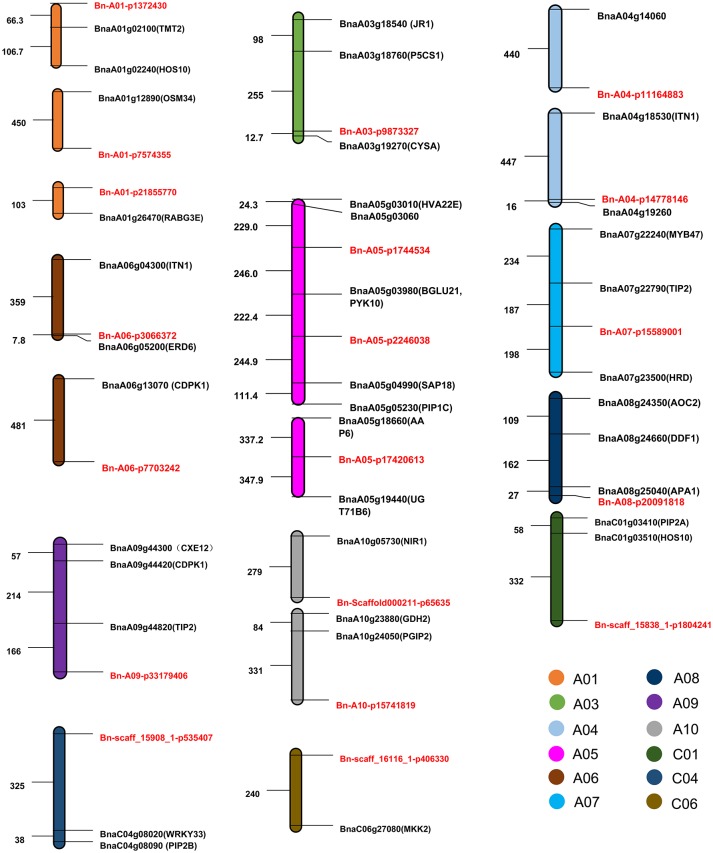
To predict candidate genes related to ST-RL, ST-SL, ST-SFW, and SNC, we identified B. napus genes which were the orthologous to A. thaliana salt stress-related genes located within the 500 kb of peak SNPs. Based on the B. napus reference genome (Chalhoub et al., 2014), we obtained 38 candidate salt stress-related genes (Figure 6, Table S6). The physical distance between these candidate genes and the significant SNPs varied from 8 to 490 kb (Figure 6). Among these genes, BnaA01g02240, BnaA01g02100, BnaA03g18540, BnaA03g18760, BnaA03g19270, BnaA06g04300, and BnaA06g05200 were identified for ST-RL; they are genes which were the orthologous to the Arabidopsis genes HOS10, TMT2, JR1, P5CS1, CYSA, ITN1, and ERD6, and located within 500 kb of peak SNPs Bn-A01-p1372430, Bn-A03-p9873327, and Bn-A06-p3066372. For ST-SL, 22 genes (including BnaA04g18530, BnaA05g03980, BnaA05g05230, BnaA05g04990, BnaA07g22240, BnaA07g22790, BnaC01g03510, and BnaC04g08090) were identified within 500 kb of peak SNPs (e.g., Bn-A04-p11164883, Bn-A04-p14778146, Bn-A05-p2246038, Bn-A07-p15589001, Bn-A09-p33179406, Bn-scaff_158381-p1804241, and Bn-scaff_159081-p535407); they are genes which were the orthologous to Arabidopsis ITN1, PYK10, PIP1C, SAP18, MYB47, TIP2, HOS10, and PIP2B. For ST-SFW, nine genes (BnaA01g12890, BnaA01g26470, BnaA06g13070, BnaA08g24350, BnaA08g24660, BnaA08g25040, BnaA10g23880, BnaA10g24050, and BnaC06g27080) were identified within 500 kb of the peak SNPs (Bn-A01-p7574355, Bn-A01-p21855770, Bn-A06-p7703242, Bn-A08-p20091818, Bn-A10-p15741819, and Bn-scaff_161161-p406330); they are genes which were the orthologous to the Arabidopsis genes OSM34, RABG3E, CDPK1, AOC2, DDF1, APA1, GDH2, PGIP2, and MAPKK2. Unfortunately, we did not find candidate genes related to SNC.
Figure 6.
The distribution pattern of candidate genes and their corresponding SNPs associated with salt tolerance (ST). The abbreviations of orthologous genes in Arabidopsis thaliana are shown in brackets after the candidate genes. SNPs are marked in red. Numbers represent the relative distances in the genome, 1 = 1 kb.
Discussion
Population structure and model comparison
Association mapping has become a critical tool for identifying alleles and loci responsible for agronomically important traits. Its success, however, depends largely on species, target traits, marker density, and study population (Zhu et al., 2008). Spurious associations may result because genotypes of association populations from the same area may be more closely related than those from different areas (Zhao et al., 2007). Thus, the critical first step before initiating association mapping is to collect a wide range of germplasm resources with different genetic backgrounds. In the current study, we chose from a worldwide rapeseed collection to map loci responsible for salt-stress response and related growth, with the ultimate goal of discovering useful candidate genes for improving salt tolerance in plants. Our collection consisted of 368 rapeseed genotypes originating from 10 countries on four continents, indicative of a sample population with sufficient genetic variety for a feasible GWAS. Population structure (Q) and kinship (K) are major factors leading to false positive results in association analyses. To reduce the risk of false positives, we used a corrected MLM that considered both Q and K matrices, which was more effective than the Q or K alone (Stich et al., 2008). However, in some cases, the MLM (Q + K) model was too strict, and important SNPs could not be detected. Thus, other better models should be developed and used in future research.
QTL for salt tolerance indices and shoot Na+ content
The salt tolerance index is considered a reliable measure for evaluating salt tolerance that controls for background effects among different genotypes (Long et al., 2013; Kan et al., 2015; Yong et al., 2015). We evaluated the salt tolerance indices of rapeseed seedlings for RL, SL, and SFW. Our results suggested that root and shoot salt-tolerance mechanisms may be different; ST-SL and ST-SFW were significantly and positively correlated with each other (P < 0.01), but ST-RL was not significantly correlated with either ST-SL or ST-SFW (Table 2).
Unraveling the genetic factors controlling salt tolerance in plants is a challenge due to the complexity of adaptive mechanisms that involve various pathways and molecular components (Long et al., 2013). To date, although some studies exist that focus on salt tolerance in B. napus (Farhoudi and Saeedipour, 2011; Jian et al., 2016; Zhao et al., 2016), but the studie on salt tolerance-related QTL are limited. To our knowledge, only one study like ours had been performed previously: association mapping of 85 inbred rapeseed lines identified 62 QTL for salt tolerance, shoot biomass, and ion homeostasis-related traits (Yong et al., 2015). In the present study, we identified 20 QTL associated with salt tolerance indices and five QTL associated with SNC in different regions of the B. napus genome (Table 3). Among these 25 QTL, 40% (10/25) were detected in more than one environment, and 60% (15/25) were detected in a single environment. These data indicate that significant interactions exist between QTL and growth environments. No co-associated QTL, however, was observed between any two salt tolerance indices, suggesting that the salt tolerance genetic mechanisms are complex and tissue-specific (Wang et al., 2011). Furthermore, QTLs distribution revealed that salt tolerance in B. napus is exclusively attributable to the A (19 QTLs) and C (6 QTLs) chromosomes.
The most important function of plant roots is to absorb water and nutrients from the soil. Thus, roots are the primary site for sensing abiotic stress from soil, including salinity and drought. In many circumstances, root sensitivity to stress limits the productivity of the entire plant (Ruan et al., 2010). Thus, understanding salt tolerance mechanisms in roots is essential to improve plant salt resistance. In our study, the three QTLs, Bn-A01-p1372430, Bn-A03-p9873327, and Bn-A06-p3066372 root-related were identified on A1, A3, and A6 chromosomes, respectively, and were detected in more than one environment. Furthermore, 11 QTL associated with ST-SL were identified and four QTLs Bn-scaff_15838_1-p1804241, Bn-A05-p2246038, Bn-A05-p17420613, and Bn-A08-p3269074 were validated in more than one individual environment. Remarkably, Bn-scaff_15838_1-p1804241 was detected in all three environments, indicating that it is a stable QTL not easily affected by environmental change. No QTL associated with ST-RL and ST-SL was reported previously, thus all above QTLs identified in our study should be the novel QTLs. Additionally, six QTLs associated with ST-SFW were identified. In contrast to a previous study (Yong et al., 2015), we found that Bn-A06-p7703242 was 2.39 Mb away from the salt tolerance-related 3075557d, suggesting that association signals detected in our study are valid. And Bn-A01-p7574355, Bn-A01-p21855770, Bn-A08-p20091818, Bn-A10-p15741819, Bn-scaff_16116_1-p406330 should be the novel QTLs associated with ST-SFW.
Although shoot Na+ content was not correlated with salt tolerance in rapeseed seedlings, the mechanism for Na+ uptake and exclusion still appeared to have some possible effects on rapeseed salt tolerance. We identified five QTLs associated with SNC on A3, A9, C5, C6, and C7 chromosomes. Notably, Bn-A03-p8032849 was detected in all three environments and was only 244 kb away from the SNC-related SNP 3099118d identified previously (Yong et al., 2015), suggesting that Bn-A03-p8032849 could be a reliable site affecting shoot Na+ content. Most identified QTLs have not been reported in previous studies and can be considered novel. They thus provide new references for the study of salt tolerance mechanisms and the breeding of salt-tolerant B. napus cultivars. Compared with the research published by Yong et al. (2015), the number of rapeseed accessions in our study was larger (368 to 85) and the NaCl concentration was higher (230–100 mM). Therefore, our results can provide a reference for the genetic mechanism of rapeseed salt tolerance under a relatively high salt concentration. Besides, another important point for the current study is that the experiments were conducted both at the natural condition and greenhouse but the experiment of Yong et al. (2015) was only conducted in greenhouse.
Salt tolerance in B. napus L. is not linked to Na+ content in shoots
In many crop species such as durum wheat (Munns et al., 2012), barley (Nguyen et al., 2013), and rice (Lin et al., 2004), salt tolerance is significantly and negatively correlated with Na+ content in shoots. These plants have developed diverse strategies to resist salt stress, such as restricting Na+ uptake in root, activating shoot Na+ exclusion or cellular compartmentalization of excessive in root and shoot (Zhu, 2000; Shi et al., 2002), and K+ retention in root (Chen et al., 2005) and mesophyll (Wu et al., 2013). Nevertheless, our results indicate that the salt tolerance indices in rapeseed were not linked to shoot Na+ content, suggesting that the salt tolerance mechanism in rapeseed is more complicated and possibly influenced by multiple factors. Our results are consistent with previous work showing no significant correlation between salt tolerance index and leaf Na+ content (Yong et al., 2015). Plants evolved a variety of mechanisms in response to salt stress, including tissue tolerance to toxic ions, tolerance to osmotic stress, ROS scavenging, and beneficial ion homeostasis (e.g., K+, Ca2+) (Leshem et al., 2006; Shabala et al., 2006; Munns and Tester, 2008), and these different mechanisms probably combine to affect plant salt tolerance. Future comprehensive studies of rapeseed salt tolerance should introduce additional indicators of salt stress responses, such as malonaldehyde (MAD), chlorophyll content, and photosynthetic rate (Moradi and Ismail, 2007; Ellouzi et al., 2011). Nonetheless, our results provide an important first step to the study of salt tolerance mechanisms in rapeseed.
Salt stress-related candidate genes
Currently, little is known about the genes involved in B. napus salt tolerance. However, many reports have dissected the molecular mechanisms of salt tolerance in Arabidopsis (Qiu et al., 2002), rice (Lin et al., 2004), and other crop species (Misra and Dwivedi, 2004). The candidate genes identified in our study can be split into several functional groups, including transcription factors, aquaporin, transporters, and enzymes. Most of these candidate genes have known orthologs in Arabidopsis and multiple crops.
Transcription factors include HOS10, encoding a nuclear localized R2R3-type MYB transcription factor; MYB47, a member of the R2R3 factor gene family; HRD and DDF1, both part of the DREB subfamily within A-4 of ERF/AP2 transcription factors; and WRKY33, a WRKY transcription factor. R2R3-type MYB transcription factor is involved in stress responses to cold, osmotic imbalance, and salt. The hos10-1 mutant plants are extremely sensitive to NaCl and freezing temperatures (Zhu et al., 2005). Similarly, the link we found between MYB genes and salt tolerance in rapeseed is corroborated in previous research. OsMYB2-overexpressing rice is more tolerant to salt, cold, and dehydration stresses, but more sensitive to abscisic acid than wild-type rice. In addition, overexpressing wheat TaMYB30-B in Arabidopsis improves drought stress tolerance during the germination and seedling stages (Zhang et al., 2012). AtWRKY33 (WRKY DNA-binding protein 33) is a transcription factors that participate in various stress responses, including against salt and drought (Mare et al., 2004; Pandey and Somssich, 2009). Loss-of-function analysis of wrky33 mutants showed a moderate increase in NaCl sensitivity, and WRKY33 overexpression increased Arabidopsis NaCl tolerance (Jiang and Deyholos, 2009).
PIP1C, PIP2A, PIP2B, and TIP2 are aquaporins (AQPs) that act as water channels and respond to water deprivation and salt stress, which has two subfamilies—the plasma membrane intrinsic proteins (PIP) and tonoplast intrinsic proteins (TIP) (Alexandersson et al., 2005). PIP1C is a member of PIP subfamily PIP1, while PIP2A and PIP2B are members of PIP2. TIP2 is a member of the tonoplast intrinsic protein (TIP) family. Overexpressing rice PIP genes in wild-type Arabidopsis enhanced tolerance to salt and drought (Guo et al., 2006).
Next, transporter proteins include TMT2, a tonoplast monosaccharide transporter 2; ERD6, a putative sucrose transporter; and AAP6, a high affinity amino acid transporter. The expression of ERD6, which encoded a putative sugar transporter of Arabidopsis, was up-regulated by dehydration and cold treatment (Kiyosue et al., 1998).
Additionally, P5CS1, CXE12, CDPK1, RABG3E, AOC2, GDH2, MAPKK2, and PGIP2 belong to the enzyme functional group. Previous studies have shown that proline accumulation frequently occurs in plants under salt stress and can improve the salt tolerance of plants (Nanjo et al., 1999; Jimenez-Bremont et al., 2006). P5CS1 encodes a delta-1-pyrroline-5-carboxylate synthase that catalysis the rate-limiting step in proline biosynthesis. P5CS1-knockout lines exhibit lowered proline synthesis, resulting in salt hypersensitivity and AtP5CS1 expression was induced under salt stress in B. napus (Xue et al., 2009). CXE12 and CDPK1 encode a protein carboxylesterase and a calcium-dependent protein kinase, respectively. Plant CDPKs may function as calcium sensors and are important to the regulation of plant growth and development, as well as to biotic and abiotic stress responses (Zou et al., 2010). RABG3E encodes a small GTPase involved in membrane trafficking. Hydrogen peroxide induces AtRADG3E expression, and transgenic plants expressing this gene exhibited increased tolerance to salt and osmotic stresses, as well as reduced ROS accumulation during salt stress (Mazel et al., 2004). AOC2 encodes allene-oxide cyclase. GDH2 encodes the beta-subunit of the glutamate dehydrogenase. MKK2 (MAP kinase kinase 2) is a regulator of MPK6 and MPK4 in response to cold and salt stresses. Arabidopsis plants overexpressing MKK2 exhibited constitutive MPK4 and MPK6 activity, as well as heightened expression of stress-induced marker genes, all of which increased freezing and salt tolerance. In contrast, mkk2 null plants are hypersensitive to salt and cold stress (Teige et al., 2004). Cold and salinity can induce MKK2 expression in oilseed rape, suggesting that MKK2 is important for the regulation of plant response to those stresses. In addition, OsMKK6 overexpressing lines exhibit higher salt stress tolerance in rice (Kumar and Sinha, 2013). PGIP2 (polygalacturonase inhibiting protein 2), a gene that encodes a polygalacturonase inhibiting protein involved in plant defense response. Abiotic stress (e.g., cold, NaCl, dehydration) upregulates BrPGIP2 in Chinese cabbage leaves (Hwang et al., 2010).
In addition to the above mentioned genes, some other genes were identified related to salt tolerance. At HVA22E, which is up-regulated to varying degrees in response to cold, salt, ABA treatment, or dehydration (Chen et al., 2002). HARDY is a part of the stress-related AP2/ERF gene family. Overexpression of AtHARDY improved drought and salt tolerance in rice (Karaba et al., 2007) and Trifolium alexandrinum L (Abogadallah et al., 2011).
Interestingly, most of the salt tolerance-related genes we identified were related to osmotic tolerance, whereas candidate genes related to ion stress were not found. Previous studies have reported that plants can experience NaCl stress gradually or in one step, leading to either salt stress or salt shock, respectively (Shavrukov, 2013). Salt shock is an extreme salt stress, wherein plants are exposed suddenly to high salinity levels. Gene expression patterns differ in response to salt stress and salt shock. Salt stress initiates relatively fewer expression changes in osmotic stress tolerance-related genes and more in ionic stress tolerance-related genes, whereas salt shock leads to the opposite outcome. Thus, our study provides a valuable reference for the study of both salt tolerance and osmotic tolerance mechanisms in B. napus. Future studies should examine these candidate genes to determine their functional characteristics in relation to rapeseed salt tolerance.
Author contributions
JS, TF, and JW conceived and designed the study. BY, CM, and JT provided advice on the experimental design. HW, LC, JG, and QL performed phenotype measurements. HW and LC analyzed all data. HW wrote the manuscript. All authors reviewed and edited the manuscript.
Funding
This research was financially supported by the National Key Research and Development Program of China (grant number 2016YFD0101300) and the Program for Modern Agricultural Industrial Technology System (grant number CARS-13).
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The reviewer MZ and handling Editor declared their shared affiliation, and the handling Editor states that the process nevertheless met the standards of a fair and objective review.
Acknowledgments
We appreciate the instructive suggestions from Chengming Sun, and Zhikun Wu, and the technical support from Dr. Laiqiang Song.
Supplementary material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fpls.2017.00593/full#supplementary-material
References
- Abogadallah G. M., Nada R. M., Malinowski R., Quick P. (2011). Overexpression of HARDY, an AP2/ERF gene from Arabidopsis, improves drought and salt tolerance by reducing transpiration and sodium uptake in transgenic Trifolium alexandrinum L. Planta 233, 1265–1276. 10.1007/s00425-011-1382-3 [DOI] [PubMed] [Google Scholar]
- Ahmadi N., Negrao S., Katsantonis D., Frouin J., Ploux J., Letourmy P., et al. (2011). Targeted association analysis identified japonica rice varieties achieving Na+/K+ homeostasis without the allelic make-up of the salt tolerant indica variety Nona Bokra. Theor. Appl. Genet. 123, 881–895. 10.1007/s00122-011-1634-4 [DOI] [PubMed] [Google Scholar]
- Alexandersson E., Fraysse L., Sjovall-Larsen S., Gustavsson S., Fellert M., Karlsson M., et al. (2005). Whole gene family expression and drought stress regulation of aquaporins. Plant Mol. Biol. 59, 469–484. 10.1007/s11103-005-0352-1 [DOI] [PubMed] [Google Scholar]
- Ammar M. H. M., Pandit A., Singh R. K., Sameena S., Chauhan M. S., Singh A. K., et al. (2009). Mapping of QTLs controlling Na+, K+ and Cl− ion concentrations in salt tolerant indica rice variety CSR27. J. Plant Biochem. Biotechnol. 18, 139–150. 10.1007/BF03263312 [DOI] [Google Scholar]
- Bradbury P. J., Zhang Z., Kroon D. E., Casstevens T. M., Ramdoss Y., Buckler E. S. (2007). TASSEL: software for association mapping of complex traits in diverse samples. Bioinformatics 23, 2633–2635. 10.1093/bioinformatics/btm308 [DOI] [PubMed] [Google Scholar]
- Chalhoub B., Denoeud F., Liu S. Y., Parkin I. A., Tang H. B., Wang X. Y., et al. (2014). Early allopolyploid evolution in the post-Neolithic Brassica napus oilseed genome. Science 345, 950–953. 10.1126/science.1253435 [DOI] [PubMed] [Google Scholar]
- Chen C.-N., Chu C.-C., Zentella R., Pan S.-M., Ho T.-H. D. (2002). AtHVA22 gene family in Arabidopsis: phylogenetic relationship, ABA and stress regulation, and tissue-specific expression. Plant Mol. Biol. 49, 633–644. 10.1023/A:1015593715144 [DOI] [PubMed] [Google Scholar]
- Chen Z., Newman I., Zhou M., Mendham N., Zhang G., Shabala S. (2005). Screening plants for salt tolerance by measuring K+ flux: a case study for barley. Plant Cell Environ. 28, 1230–1246. 10.1111/j.1365-3040.2005.01364.x [DOI] [Google Scholar]
- Cockram J., White J., Zuluaga D. L., Smith D., Comadran J., Macaulay M., et al. (2010). Genome-wide association mapping to candidate polymorphism resolution in the unsequenced barley genome. Proc. Natl. Acad. Sci. U.S.A. 107, 21611–21616. 10.1073/pnas.1010179107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui D. Z., Wu D. D., Somarathna Y., Xu C. Y., Li S., Li P., et al. (2015). QTL mapping for salt tolerance based on snp markers at the seedling stage in maize (Zea mays L.). Euphytica 203, 273–283. 10.1007/s10681-014-1250-x [DOI] [Google Scholar]
- Demiral T., Türkan I. (2006). Exogenous glycinebetaine affects growth and proline accumulation and retards senescence in two rice cultivars under NaCl stress. Environ. Exp. Bot. 56, 72–79. 10.1016/j.envexpbot.2005.01.005 [DOI] [Google Scholar]
- Ellouzi H., Hamed K. B., Cela J., Munne-Bosch S., Abdelly C. (2011). Early effects of salt stress on the physiological and oxidative status of Cakile maritima (halophyte) and Arabidopsis thaliana (glycophyte). Physiol. Plant. 142, 128–143. 10.1111/j.1399-3054.2011.01450.x [DOI] [PubMed] [Google Scholar]
- Epstein E., Norlyn J. D., Rush D. W., Kingsbury R. W., Kelley D. B., Cunningham G. A., et al. (1980). Saline culture of crops: a genetic approach. Science 210, 399–404. 10.1126/science.210.4468.399 [DOI] [PubMed] [Google Scholar]
- Evanno G., Regnaut S., Goudet J. (2005). Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study. Mol. Ecol. 14, 2611–2620. 10.1111/j.1365-294X.2005.02553.x [DOI] [PubMed] [Google Scholar]
- Falush D., Stephens M., Pritchard J. K. (2003). Inference of population structure using multilocus genotype data: linked loci and correlated allele frequencies. Genetics 164, 1567–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farhoudi R., Saeedipour S. (2011). Effect of exogenous abscisic acid on antioxidant activity and salt tolerance in rapeseed (Brassica napus) cultivars. Res. Crop. 12, 122–130. [Google Scholar]
- Flowers T. J. (2004). Improving crop salt tolerance. J. Exp. Bot. 55, 307–319. 10.1093/jxb/erh003 [DOI] [PubMed] [Google Scholar]
- Guo L., Wang Z. Y., Lin H., Cui W. E., Chen J., Liu M. H., et al. (2006). Expression and functional analysis of the rice plasma-membrane intrinsic protein gene family. Cell Res. 16, 277–286. 10.1038/sj.cr.7310035 [DOI] [PubMed] [Google Scholar]
- Hardy O. J., Vekemans X. (2002). SPAGEDi: a versatile computer program to analyse spatial genetic structure at the individual or population levels. Mol. Ecol. 2, 618–620. 10.1046/j.1471-8286.2002.00305.x [DOI] [Google Scholar]
- Hwang B. H., Bae H., Lim H. S., Kim K. B., Kim S. J., Im M. H., et al. (2010). Overexpression of polygalacturonase-inhibiting protein 2 (PGIP2) of Chinese cabbage (Brassica rapa ssp. pekinensis) increased resistance to the bacterial pathogen Pectobacterium carotovorum ssp. carotovorum. Plant Cell Tissue Org. 103, 293–305. 10.1007/s11240-010-9779-4 [DOI] [Google Scholar]
- Imlay J. A. (2003). Pathways of oxidative damage. Annu. Rev. Microbiol. 57, 395–418. 10.1146/annurev.micro.57.030502.090938 [DOI] [PubMed] [Google Scholar]
- Jakobsson M., Rosenberg N. A. (2007). CLUMPP: a cluster matching and permutation program for dealing with label switching and multimodality in analysis of population structure. Bioinformatics 23, 1801–1806. 10.1093/bioinformatics/btm233 [DOI] [PubMed] [Google Scholar]
- Jian H., Wang J., Wang T., Wei L., Li J., Liu L. (2016). Identification of rapeseed microRNAs involved in early stage seed germination under salt and drought stresses. Front. Plant Sci. 7:658. 10.3389/fpls.2016.00658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y. Q., Deyholos M. K. (2009). Functional characterization of Arabidopsis NaCl-inducible WRKY25 and WRKY33 transcription factors in abiotic stresses. Plant Mol. Biol. 69, 91–105. 10.1007/s11103-008-9408-3 [DOI] [PubMed] [Google Scholar]
- Jimenez-Bremont J. F., Becerra-Flora A., Hernandez-Lucero E., Rodriguez-Kessler M., Acosta-Gallegos J. A., Ramirez-Pimentel J. G. (2006). Proline accumulation in two bean cultivars under salt stress and the effect of polyamines and ornithine. Biol. Plant. 50, 763–766. 10.1007/s10535-006-0126-x [DOI] [Google Scholar]
- Kan G. Z., Zhang W., Yang W. M., Ma D. Y., Zhang D., Hao D. R., et al. (2015). Association mapping of soybean seed germination under salt stress. Mol. Genet. Genomics 290, 2147–2162. 10.1007/s00438-015-1066-y [DOI] [PubMed] [Google Scholar]
- Karaba A., Dixit S., Greco R., Aharoni A., Trijatmiko K. R., Marsch-Martinez N., et al. (2007). Improvement of water use efficiency in rice by expression of HARDY, an Arabidopsis drought and salt tolerance gene. Proc. Natl. Acad. Sci. U.S.A. 104, 15270–15275. 10.1073/pnas.0707294104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyosue T., Abe H., Yamaguchi-Shinozaki K., Shinozaki K. (1998). ERD6, a cDNA clone for an early dehydration-induced gene of Arabidopsis, encodes a putative sugar transporter. Biochim. Biophys. Acta 187–191. 10.1016/S0005-2736(98)00007-8 [DOI] [PubMed] [Google Scholar]
- Kumar K., Sinha A. K. (2013). Overexpression of constitutively active mitogen activated protein kinase kinase 6 enhances tolerance to salt stress in rice. Rice 6:25. 10.1186/1939-8433-6-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V., Singh A., Mithra S. V. A., Krishnamurthy S. L., Parida S. K., Jain S., et al. (2015). Genome-wide association mapping of salinity tolerance in rice (Oryza sativa). DNA Res. 22, 133–145. 10.1093/dnares/dsu046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee G. J., Boerma H. R., Villagarcia M. R., Zhou X., Carter T. E., Li Z., et al. (2004). A major QTL conditioning salt tolerance in S-100 soybean and descendent cultivars. Theor. Appl. Genet. 109, 1610–1619. 10.1007/s00122-004-1783-9 [DOI] [PubMed] [Google Scholar]
- Leshem Y., Melamed-Book N., Cagnac O., Ronen G., Nishri Y., Solomon M., et al. (2006). Suppression of Arabidopsis vesicle-SNARE expression inhibited fusion of H2O2-containing vesicles with tonoplast and increased salt tolerance. Proc. Natl. Acad. Sci. U.S.A. 103, 18008–18013. 10.1073/pnas.0604421103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F., Chen B., Xu K., Gao G., Yan G., Qiao J., et al. (2016). A genome-wide association study of plant height and primary branch number in Rapeseed (Brassica napus). Plant Sci. 242, 169–177. 10.1016/j.plantsci.2015.05.012 [DOI] [PubMed] [Google Scholar]
- Li F., Chen B., Xu K., Wu J., Song W., Bancroft I., et al. (2014). Genome-wide association study dissects the genetic architecture of seed weight and seed quality in rapeseed (Brassica napus L.). DNA Res. 21, 355–367. 10.1093/dnares/dsu002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H. X., Zhu M. Z., Yano M., Gao J. P., Liang Z. W., Su W. A., et al. (2004). QTLs for Na+ and K+ uptake of the shoots and roots controlling rice salt tolerance. Theor. Appl. Genet. 108, 253–260. 10.1007/s00122-003-1421-y [DOI] [PubMed] [Google Scholar]
- Liu S., Fan C., Li J., Cai G., Yang Q., Wu J., et al. (2016). A genome-wide association study reveals novel elite allelic variations in seed oil content of Brassica napus. Theor. Appl. Genet. 129, 1203–1215. 10.1007/s00122-016-2697-z [DOI] [PubMed] [Google Scholar]
- Long N. V., Dolstra O., Malosetti M., Kilian B., Graner A., Visser R. G. F., et al. (2013). Association mapping of salt tolerance in barley (Hordeum vulgare L.). Theor. Appl. Genet. 126, 2335–2351. 10.1007/s00122-013-2139-0 [DOI] [PubMed] [Google Scholar]
- Luo X., Ma C., Yue Y., Hu K., Li Y., Duan Z., et al. (2015). Unravelling the complex trait of harvest index in rapeseed (Brassica napus L.) with association mapping. BMC Genomics 16, 379. 10.1186/s12864-015-1607-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay I., Powell W. (2007). Methods for linkage disequilibrium mapping in crops. Trends Plant Sci. 12, 57–63. 10.1016/j.tplants.2006.12.001 [DOI] [PubMed] [Google Scholar]
- Mare C., Mazzucotelli E., Crosatti C., Francia E., Stanca A. M., Cattivelli L. (2004). Hv-WRKY38: a new transcription factor involved in cold- and drought-response in barley. Plant Mol. Biol. 55, 399–416. 10.1007/s11103-004-0906-7 [DOI] [PubMed] [Google Scholar]
- Mazel A., Leshem Y., Tiwari B. S., Levine A. (2004). Induction of salt and osmotic stress tolerance by overexpression of an intracellular vesicle trafficking protein AtRab7 (AtRabG3e). Plant Physiol. 134, 118–128. 10.1104/pp.103.025379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merk H. L., Yarnes S. C., Van Deynze A., Tong N. K., Menda N., Mueller L. A., et al. (2012). Trait diversity and potential for selection indices based on variation among regionally adapted processing tomato germplasm. J. Am. Soc. Hortic. Sci. 137, 427–437. [Google Scholar]
- Misra N., Dwivedi U. N. (2004). Genotypic difference in salinity tolerance of green gram cultivars. Plant Sci. 166, 1135–1142. 10.1016/j.plantsci.2003.11.028 [DOI] [Google Scholar]
- Mittler R. (2002). Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 7, 405–410. 10.1016/S1360-1385(02)02312-9 [DOI] [PubMed] [Google Scholar]
- Mohammadi M., Blake T. K., Budde A. D., Chao S. M., Hayes P. M., Horsley R. D., et al. (2015). A genome-wide association study of malting quality across eight US barley breeding programs. Theor. Appl. Genet. 128, 705–721. 10.1007/s00122-015-2465-5 [DOI] [PubMed] [Google Scholar]
- Moradi F., Ismail A. M. (2007). Responses of photosynthesis, chlorophyll fluorescence and ROS-scavenging systems to salt stress during seedling and reproductive stages in rice. Ann. Bot. 99, 1161–1173. 10.1093/aob/mcm052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munns R. (2005). Genes and salt tolerance: bringing them together. New Phytol. 167, 645–663. 10.1111/j.1469-8137.2005.01487.x [DOI] [PubMed] [Google Scholar]
- Munns R., James R. A. (2003). Screening methods for salinity tolerance: a case study with tetraploid wheat. Plant Soil. 253, 201–218. 10.1023/A:1024553303144 [DOI] [Google Scholar]
- Munns R., James R. A., Xu B., Athman A., Conn S. J., Jordans C., et al. (2012). Wheat grain yield on saline soils is improved by an ancestral Na+ transporter gene. Nat. Biotechnol. 30, 360–364. 10.1038/nbt.2120 [DOI] [PubMed] [Google Scholar]
- Munns R., Tester M. (2008). Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 59, 651–681. 10.1146/annurev.arplant.59.032607.092911 [DOI] [PubMed] [Google Scholar]
- Nanjo T., Kobayashi M., Yoshiba Y., Kakubari Y., Yamaguchi-Shinozaki K., Shinozaki K. (1999). Antisense suppression of proline degradation improves tolerance to freezing and salinity in Arabidopsis thaliana. FEBS Lett. 461, 205–210. 10.1016/S0014-5793(99)01451-9 [DOI] [PubMed] [Google Scholar]
- Nguyen V. L., Ribot S. A., Dolstra O., Niks R. E., Visser R. G. F., Van Der Linden C. G. (2013). Identification of quantitative trait loci for ion homeostasis and salt tolerance in barley (Hordeum vulgare L.). Mol. Breed. 31, 137–152. 10.1007/s11032-012-9777-9 [DOI] [Google Scholar]
- Pandey S. P., Somssich I. E. (2009). The role of WRKY transcription factors in plant immunity. Plant Physiol. 150, 1648–1655. 10.1104/pp.109.138990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Q. S., Guo Y., Dietrich M. A., Schumaker K. S., Zhu J. K. (2002). Regulation of SOS1, a plasma membrane Na+/H+ exchanger in Arabidopsis thaliana, by SOS2 and SOS3. Proc. Natl. Acad. Sci. U.S.A. 99, 8436–8441. 10.1073/pnas.122224699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan C. J., Da Silva J. A. T., Mopper S., Qin P., Lutts S. (2010). Halophyte improvement for a salinized world. Crit. Rev. Plant Sci. 29, 329–359. 10.1080/07352689.2010.524517 [DOI] [Google Scholar]
- Schachtman D., Liu W. (1999). Molecular pieces to the puzzle of the interaction between potassium and sodium uptake in plants. Trends Plant Sci. 4, 281–287. 10.1016/S1360-1385(99)01428-4 [DOI] [PubMed] [Google Scholar]
- Shabala S., Cuin T. A. (2008). Potassium transport and plant salt tolerance. Physiol. Plant. 133, 651–669. 10.1111/j.1399-3054.2007.01008.x [DOI] [PubMed] [Google Scholar]
- Shabala S., Demidchik V., Shabala L., Cuin T. A., Smith S. J., Miller A. J., et al. (2006). Extracellular Ca2+ ameliorates NaCl-induced K+ loss from Arabidopsis root and leaf cells by controlling plasma membrane K+-permeable channels. Plant Physiol. 141, 1653–1665. 10.1104/pp.106.082388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shavrukov Y. (2013). Salt stress or salt shock: which genes are we studying? J. Exp. Bot. 64, 119–127. 10.1093/jxb/ers316 [DOI] [PubMed] [Google Scholar]
- Shi H., Quintero F. J., Pardo J. M., Zhu J. K. (2002). The putative plasma membrane Na+/H+ antiporter SOS1 controls long-distance Na+ transport in plants. Plant Cell 14, 465–477. 10.1105/tpc.010371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stich B., Mohring J., Piepho H. P., Heckenberger M., Buckler E. S., Melchinger A. E. (2008). Comparison of mixed-model approaches for association mapping. Genetics 178, 1745–1754. 10.1534/genetics.107.079707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C., Wang B., Wang X., Hu K., Li K., Li Z., et al. (2016a). Genome-wide association study dissecting the genetic architecture underlying the branch angle trait in rapeseed (Brassica napus L.). Sci. Rep. 6:33673. 10.1038/srep33673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C. M., Wang B. Q., Yan L., Hu K. N., Liu S., Zhou Y. M., et al. (2016b). Genome-wide association study provides insight into the genetic control of plant height in rapeseed (Brassica napus L.). Front. Plant Sci. 7:1102. 10.3389/fpls.2016.01102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teige M., Scheikl E., Eulgem T., Doczi F., Ichimura K., Shinozaki K., et al. (2004). The MKK2 pathway mediates cold and salt stress signaling in Arabidopsis. Mol. Cell 15, 141–152. 10.1016/j.molcel.2004.06.023 [DOI] [PubMed] [Google Scholar]
- Tocquin P., Corbesier L., Havelange A., Pieltain A., Kurtem E., Bernier G., et al. (2003). A novel high efficiency, low maintenance, hydroponic system for synchronous growth and flowering of Arabidopsis thaliana. BMC Plant Biol. 3:2. 10.1186/1471-2229-3-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- USDA ERS (2014). Oil Crops Yearbook. Available online at: http://www.ers.usda.gov/data-products/oil-crops-yearbook.aspx
- Wang Z. F., Wang J. F., Bao Y. M., Wu Y. Y., Zhang H. S. (2011). Quantitative trait loci controlling rice seed germination under salt stress. Euphytica 178, 297–307. 10.1007/s10681-010-0287-8 [DOI] [Google Scholar]
- Wu H. H., Shabala L., Barry K., Zhou M. X., Shabala S. (2013). Ability of leaf mesophyll to retain potassium correlates with salinity tolerance in wheat and barley. Physiol. Plant. 149, 515–527. 10.1111/ppl.12056 [DOI] [PubMed] [Google Scholar]
- Xu L., Hu K., Zhang Z., Guan C., Chen S., Hua W., et al. (2016). Genome-wide association study reveals the genetic architecture of flowering time in rapeseed (Brassica napus L.). DNA Res. 23, 43–52. 10.1093/dnares/dsv035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue X. N., Liu A. H., Hua X. J. (2009). Proline accumulation and transcriptional regulation of proline biothesynthesis and degradation in Brassica napus. BMB Rep. 42, 28–34. 10.5483/BMBRep.2009.42.1.028 [DOI] [PubMed] [Google Scholar]
- Yong H. Y., Wang C. L., Bancroft I., Li F., Wu X. M., Kitashiba H., et al. (2015). Identification of a gene controlling variation in the salt tolerance of rapeseed (Brassica napus L.). Planta 242, 313–326. 10.1007/s00425-015-2310-8 [DOI] [PubMed] [Google Scholar]
- Yu J. M., Pressoir G., Briggs W. H., Bi I. V., Yamasaki M., Doebley J. F., et al. (2006). A unified mixed-model method for association mapping that accounts for multiple levels of relatedness. Nat. Genet. 38, 203–208. 10.1038/ng1702 [DOI] [PubMed] [Google Scholar]
- Zhang L. C., Zhao G. Y., Xia C., Jia J. Z., Liu X., Kong X. Y. (2012). A wheat R2R3-MYB gene, TaMYB30-B, improves drought stress tolerance in transgenic Arabidopsis. J. Exp. Bot. 63, 5873–5885. 10.1093/jxb/ers237 [DOI] [PubMed] [Google Scholar]
- Zhao B.-Y., Hu Y.-F., Li J.-J., Yao X., Liu K.-d. (2016). BnaABF2, a bZIP transcription factor from rapeseed (Brassica napus L.), enhances drought and salt tolerance in transgenic Arabidopsis. Bot. Stud. 57:12 10.1186/s40529-016-0127-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J., Paulo M. J., Jamar D., Lou P., Van Eeuwijk F., Bonnema G., et al. (2007). Association mapping of leaf traits, flowering time, and phytate content in Brassica rapa. Genome 50, 963–973. 10.1139/G07-078 [DOI] [PubMed] [Google Scholar]
- Zhu C. S., Gore M., Buckler E. S., Yu J. M. (2008). Status and prospects of association mapping in plants. Plant Genome 1, 5–20. 10.3835/plantgenome2008.02.0089 [DOI] [Google Scholar]
- Zhu J. K. (2000). Genetic analysis of plant salt tolerance using Arabidopsis. Plant Physiol. 124, 941–948. 10.1104/pp.124.3.941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J.-K. (2001). Plant salt tolerance. Trends Plant Sci. 6, 66–71. 10.1016/S1360-1385(00)01838-0 [DOI] [PubMed] [Google Scholar]
- Zhu J., Verslues P. E., Zheng X., Lee B. H., Zhan X., Manabe Y., et al. (2005). HOS10 encodes an R2R3-type MYB transcription factor essential for cold acclimation in plants. Proc. Natl. Acad. Sci. U.S.A. 102, 9966–9971. 10.1073/pnas.0503960102 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Zou J. J., Wei F. J., Wang C., Wu J. J., Ratnasekera D., Liu W. X., et al. (2010). Arabidopsis Calcium-Dependent Protein Kinase CPK10 functions in abscisic acid- and Ca2+-mediated stomatal regulation in response to drought stress. Plant Physiol. 154, 1232–1243. 10.1104/pp.110.157545 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.