Abstract
Despite the recognized role of the ATP-binding Cassette Transporter A1 (ABCA1) in high-density lipoprotein (HDL) metabolism, our understanding of ABCA1 deficiency in human hepatocytes is limited. To define the functional effects of human hepatocyte ABCA1 deficiency, we generated induced pluripotent stem cell (iPSC)-derived hepatocyte-like cells (HLCs) from Tangier disease (TD) and matched control subjects. Control HLCs exhibited robust cholesterol efflux to apolipoprotein A-I (apoA-I) and formed nascent HDL particles. ABCA1-deficient HLCs failed to mediate lipid efflux or nascent HDL formation, but had elevated triglyceride (TG) secretion. Global transcriptome analysis revealed significantly increased ANGPTL3 expression in ABCA1-deficient HLCs. Angiopoietin-related protein 3 (ANGPTL3) was enriched in plasma of TD relative to control subjects. These results highlight the required role of ABCA1 in cholesterol efflux and nascent HDL formation by hepatocytes. Furthermore, our results suggest that hepatic ABCA1 deficiency results in increased hepatic TG and ANGPTL3 secretion, potentially underlying the elevated plasma TG levels in TD patients.
Keywords: Tangier disease, Induced pluripotent stem cells, iPSC-derived hepatocytes, HDL biogenesis, Triglyceride secretion
Highlights
-
•
ABCA1 deficiency in human hepatocytes abolishes nascent HDL formation, but elevates triglyceride secretion
-
•
ABCA1 deficiency increases hepatic ANGPTL3 expression and secretion
-
•
Tangier disease patients display higher plasma ANGPTL3 levels relative to normal HDL control subjects
ATP-Binding Cassette Transporter A1 (ABCA1) is a key regulator of high-density lipoprotein metabolism, but the intrinsic functional impact of human hepatocyte ABCA1 deficiency is yet to be defined. We generated hepatocyte-like cells (HLCs) from induced pluripotent stem cell (iPSC) of patients with Tangier disease (TD), a rare genetic disorder caused by mutations in ABCA1. ABCA1 deficiency in HLCs abrogates lipid efflux and nascent HDL formation but increases triglyceride secretion. ANGPTL3 has also been uncovered as a potential mediator of hypertriglyceridemia in TD. This study thus highlights the utility of iPSC-derived cells in disease modeling.
1. Introduction
Tangier disease (TD), a rare autosomal recessive disorder, is characterized by the near absence of plasma high-density lipoprotein (HDL), elevated triglyceride (TG) levels and sterol deposition in tissue macrophages (Clifton-Bligh et al., 1972). TD is caused by mutations in the ABCA1 gene (Oram and Heinecke, 2005), which encodes the integral membrane protein ATP-binding cassette transporter A1 (ABCA1). ABCA1 facilitates transport of cellular free cholesterol (FC) and phospholipids (PL) to lipid-poor apolipoprotein A-I (apoA-I). Studies with murine models have demonstrated the pivotal role of hepatic ABCA1 in promoting nascent HDL formation and maintaining normal plasma HDL levels (Bi et al., 2013, Timmins et al., 2005). However, primary hepatocytes from TD patients have never been investigated and thus the intrinsic functional effects of the loss of human hepatocyte ABCA1 have yet to be established.
Reprogramming differentiated somatic cells to induced pluripotent stem cells (iPSCs) enables re-differentiation to a wide array of cell types (Yamanaka, 2012) and is of considerable value for disease modeling, functional genomics, drug discovery, and regenerative medicine. The Next Generation Genetic Association Studies consortium was developed to exploit the power of iPSC-derived cells for gaining insight into the functional implications of human genetic variation, and has generated iPSCs from thousands of patients with defined genetic variations (http://www.wicell.org/home/stem-cell-lines/collections/collections.cmsx). The liver plays a crucial role in many physiological processes, including lipid and lipoprotein metabolism and the differentiation of human iPSCs to hepatocyte-like cells (HLCs) provides a model system to study hepatocyte-specific functions of human disease and gain mechanistic insights.
In the current study, iPSCs from TD and matched control subjects were generated and differentiated into HLCs. The TD HLCs were shown to have severely impaired cholesterol efflux and nascent HDL formation, as well as increased TG secretion. Gene expression analysis of TD and control HLCs revealed an increase in ANGPTL3 expression, confirmed by assay of this protein in the media and plasma. These results illustrate the utility of iPSC-derived HLCs in disease modeling, highlight the importance of human hepatic ABCA1 in both HDL and TG metabolism, and show that ABCA1 deficiency results in upregulation of ANGPTL3, which is known to influence HDL and TG metabolism.
2. Materials and Methods
All human protocols for the study were approved by the University of Pennsylvania Human Subjects Research Institutional Review Board. The participants provided their informed written consent for the study. The study was conducted according to standards indicated by the Declaration of Helsinki.
2.1. Flow Cytometry Analysis of Pluripotency Markers
iPSCs were detached and dissociated into single cells with Accutase Enzyme Cell Detachment Medium (Innovative Cell Technologies) and gentle pipetting. Cells were then stained with Alexa Fluor 647-SSEA4 (Biolegend 330408), PE-Tra-1-60 (BD Bioscience 560193) or corresponding isotype controls (Biolegend 401321 and BD Bioscience 555584) for cell surface expression analysis by Flow Cytometry using BD FACSCalibur (BD Biosciences, San Jose, CA).
2.2. Karyotypic Analysis of iPSCs
iPSCs were cultured in T25 flasks and live cultures were sent to WiCell Cytogenetics Lab (Madison, WI) for cytogenetic analysis using G-banded Karyotyping. An average of 20 cells per cell line was analyzed for chromosome integrity.
2.3. Generation of Subject-Specific iPSCs
Subject-specific PBMC-derived iPSCs were generated using Sendai viral vectors by the iPSC Core Facility at the University of Pennsylvania as previously described (Yang et al., 2012, Zhang et al., 2015). These cells lines have been deposited at WiCell Research Institute.
2.4. Differentiation of iPSCs to Hepatocytes
HLCs were generated from iPSCs using standard protocols as reported (Mallanna and Duncan, 2013, Si-Tayeb et al., 2010).
2.5. Lipid Efflux
Lipid efflux assays were performed as previously described (Lyssenko et al., 2013). Twenty days after differentiation, HLCs were radiolabeled, with 0.12 μCi/ml 14C-cholesterol and 1.3 μCi/ml 3H-choline or with 0.12 μCi/ml 14C-cholesterol only (PerkinElmer, Waltham, MA, USA) in the presence of T0901317 (10 μM, Sigma-Aldrich T2320) for 24 h. Cells were then washed twice and incubated in fresh medium with or without exogenous human apoA-I (20 μg/ml) for 6 h. At the end of incubation, cell medium was collected and filtered through a 96-well filter plate (EMD Millipore) to remove cell debris. ApoB-containing lipoprotein was precipitated using phosphotungstate. Remaining HDL-containing medium underwent lipid extraction by Bligh-Dyer method. Cellular lipids were extracted with hexane-isopropanol (3:2, v/v) and the solvent was then evaporated for scintillation counting. The percentage of cholesterol export was calculated by dividing the 14C counts in the medium by the sum of counts in the medium and cells and multiplying by 100.
2.6. Nascent HDL formation
Nascent HDL formation and gel-filtration chromatography analysis of nascent HDL were performed as previously described (Lyssenko et al., 2013). Briefly, HLCs were dual radiolabeled with 1.3 μCi/ml 3H-choline and 0.12 μCi/ml 14C-cholesterol (PerkinElmer, Waltham, MA, USA) in the presence of 10 μM T0901317 (Sigma-Aldrich T2320) for 24 h. After two washes, efflux medium with or without human apoAI (20 μg/ml) were added to cells for a 6-hour incubation. Cell medium was collected, filtered through a 0.45 μm PVDF membrane filter unit (EMD Millipore), and concentrated using Amicon Ultracel-10 K centrifugal filters (EMD Millipore). A 1-ml aliquot of the concentrated cell medium was resolved into 1-ml fractions on a calibrated HiLoad 16/60 Superdex 200 gel-filtration column (GE Healthcare, Mickleton, NJ, USA). 3H and 14C counts in each fraction were determined by scintillation counting. Results were presented as counts per fraction for regions containing HDLs (fraction 45–90).
2.7. TG Secretion
HLCs were preloaded with oleic acid (1.5 mM) in the presence of 3H-glycerol (5 μCi/ml) for 6 h. Culture media samples were collected for low speed spin to remove cell debris. Supernatant from the spin was harvested. An aliquot of media was used for lipid extraction by Bligh-Dyer method while another portion was used for albumin quantification using ELISA (BETHYL Laboratories E88–129). Cellular lipids were extracted with isopropanol, after which 0.1 N NaOH was added to collect cell lysates. Lipid extracts from medium and cells were used for thin layer chromatography and scintillation counting. Lowry protein assay was performed to measure cellular protein contents. 3H-TG counts in cells and medium were normalized to medium albumin mass to determine TG secretion (Chung et al., 2010a).
2.8. Microarray Data Analysis
Microarray was performed using Affymetrix Human Gene 2.0 ST Array. Genes with a false discovery rate (FDR) of < 5% and a fold change (FC) > 1.5 were considered differentially expressed. Heat maps illustrating expression patterns of genes were generated using pheatmap package in R. Results have been deposited at GEO (GSE95482).
2.9. ELISA
Albumin (BETHYL Laboratories, Inc.) in the cell culture medium, ANGPTL3 (R&D DANL30) concentrations in the cell culture medium and plasma were determined using commercial kits.
2.10. Statistical Analysis
Values are shown as mean ± standard error of the mean. Data were analyzed using two-tailed unpaired Student's t-test (Graphpad Prism 7). A p value < 0.05 was considered statistically significant.
3. Results
3.1. Generation of Subject-Specific iPSCs and Differentiation into HLCs
Two TD patients, one homozygous for the E1005X nonsense mutation (TD-1) and one compound heterozygote at S2046R/K531N (TD-2), together with ethnicity, gender, and age-matched control healthy subjects (control-1 and control-2 respectively) were recruited for the study (Zhang et al., 2015). The extremely low plasma HDL cholesterol of patients is characteristic of TD. E1005X homozygote (TD-1) also exhibits hypertriglyceridemia (Table S1). The mutations of ABCA1 cause synthesis of proteins that are unable to mediate cholesterol export from macrophages (Zhang et al., 2015). Peripheral blood mononuclear cells (PBMC) from the TD and control subjects were used to generate iPSCs following a standard protocol (Yang et al., 2012, Zhang et al., 2015), with multiple clones established for each condition (Table S2). These iPSCs expressed typical pluripotency surface markers, such as Tra-1-60 and SSEA-4 (Fig. S1A), and all clones maintained a normal karyotype (Fig. S1B).
To investigate the effects of ABCA1 deficiency on hepatic lipid metabolism, TD and control iPSCs were differentiated into HLCs using a standard procedure (Mallanna and Duncan, 2013, Si-Tayeb et al., 2010). TD and control HLCs stained positively for the hepatocyte markers α-fetoprotein (AFP) and HNF4α after twenty days of differentiation (Fig. S2A). Similar expression of hepatocyte markers including AFP, ALB, TF and ASGR1 was also confirmed at the transcript level by real time-PCR analysis (Fig. S2B). In addition, albumin secretion from TD and control HLCs was comparable (Fig. S2C). Together, these results show efficient differentiation of both control and TD iPSCs to hepatocytes.
3.2. Impaired Cholesterol Efflux in TD and Control HLCs
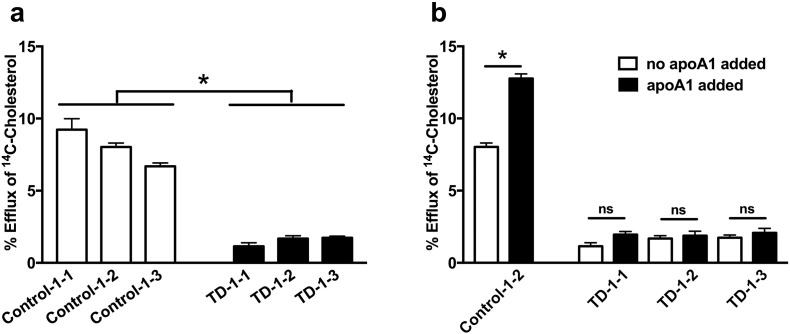
The essential role of ABCA1 in mediating FC and PL efflux to apoA-I has been well-documented in a variety of in vitro and ex vivo cell culture systems (Clee et al., 2000, Oram and Heinecke, 2005, Timmins et al., 2005, van Dam et al., 2002). However, owing to inaccessibility of human TD liver tissue, the impact of ABCA1 deficiency on lipid efflux from human primary hepatocytes has not been described. To remedy this, we first studied cholesterol efflux from TD-1 and control-1 HLCs (3 clones each). TD-1 and control-1 HLCs secreted comparable amounts of apoA-I (~ 1 μg/well, data not shown), the main acceptor of ABCA1-mediated lipid efflux and a major component of the HDL particle (Oram and Heinecke, 2005). Control, but not TD, HLCs exhibited robust cholesterol efflux, likely to endogenous apoAI (Fig. 1A). Addition of exogenous human apoA-I (20 μg/ml) stimulated cholesterol efflux from control HLCs, increasing it by ~ 60% over the endogenous level. The response of TD HLCs to exogenous apoA-I was negligible (Fig. 1B). Similarly, cholesterol and phospholipid efflux to exogenous apoA-I from TD-2 HLCs was severely impaired, compared to control-2 HLCs (Fig. S3). These results demonstrate significantly impaired lipid efflux from human hepatocytes deficient in ABCA1.
Fig. 1.
Impaired cholesterol efflux in TD HLCs.
(A) Cholesterol efflux in the absence of exogenous apoA-I was compared between control-1 and TD-1 HLCs. All samples from the 3 clones of each group were compared. n = 9, * denotes p < 0.05. (B) Cholesterol efflux without (white bar) or with (black bar) addition of exogenous apoA-I (20 μg/ml) was compared for each group. n = 3, * denotes p < 0.05. Values are shown as mean ± SEM.
3.3. Lack of Nascent HDL Formation in TD HLCs
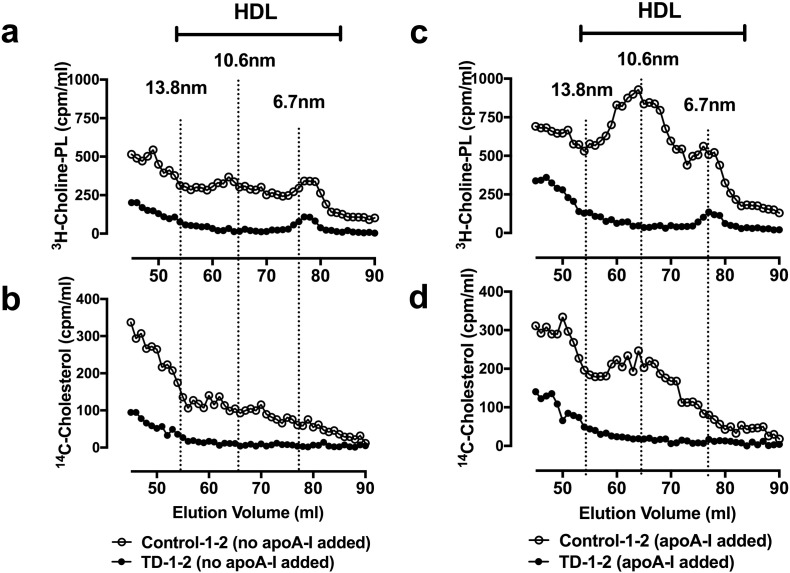
To further assess nascent HDL formation, TD and control HLCs were radiolabeled with 14C-cholesterol and 3H-choline and incubated with apoA-I-free or apoA-I-containing medium. Cell medium was collected and resolved on a gel filtration column by size. Control HLCs formed cholesterol and choline-phospholipid particles that eluted in two size species: the larger-sized (10.6 nm) species predominated over the smaller-sized species (6.7 nm) in terms of lipid mass (Fig. 2A–B). Exogenous apoA-I increased formation of both species, but had no effect on the species sizes or relative amounts (Fig. 2C–D). This two-species elution pattern is typical of nascent HDL formed by human hepatoma cells HepG2 and other ABCA1-expressing cells (Lyssenko et al., 2013), indicating that control HLCs formed bona fide HDL. TD HLCs failed to form nascent HDL particles regardless of whether or not exogenous apoAI was added (Fig. 2A–D). These observations show complete loss of nascent HDL formation as a result of human hepatocyte ABCA1 deficiency.
Fig. 2.
Lack of nascent HDL formation in TD HLCs.
(A and B) Gel-filtration chromatography profiles of nascent HDL formed in the absence of exogenous apoA-I based on 3H-choline-PL (A) and 14C-cholesterol (B) counts. (C and D) Gel-filtration chromatography profiles of nascent HDL formed in the presence of 20 μg/ml exogenous apoA-I based on 3H-choline-PL (C) and 14C-cholesterol (D) counts across fractions.
3.4. Increased TG Secretion from TD HLCs
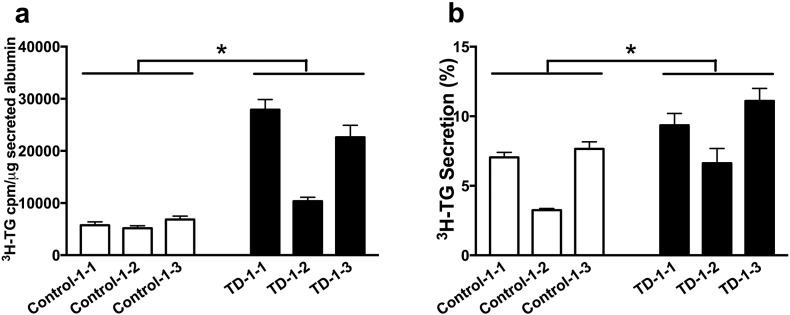
Beyond HDL deficiency, TD subjects often exhibit significantly elevated fasting or postprandial TG levels (Kolovou et al., 2003). Previous in vitro studies and in vivo work with hepatocyte-specific ABCA1 knockout mice have suggested that increased secretion of very low density lipoprotein (VLDL)-TG may be responsible for the elevated TG levels in TD (Chung et al., 2010a, Chung et al., 2010b). We used HLCs to test whether human hepatic ABCA1 affects TG secretion. Cells were concurrently incubated for 6 h with oleic acid to stimulate neutral lipid secretion and 3H-glycerol to radiolabel newly synthesized TG. When medium 3H-TG counts were normalized to albumin mass, TD HLCs secreted significantly more TG than control HLCs (Fig. 3A). TD HLCs also secreted more 3H-TG as percent of the total synthesized 3H-TG (Fig. 3B). The results did not change when 3H-TG counts were normalized to total cellular protein (data not shown). These observations suggest enhanced hepatic TG secretion in the absence of HDL formation.
Fig. 3.
Elevated TG secretion in TD vs. control HLCs.
(A) Newly synthesized TG that was secreted into medium. Medium 3H-TG counts were normalized to media albumin mass. Data is presented as cpm 3H-TG/μg secreted albumin. (B) Percent of newly synthesized TG secretion. The percentage of TG secretion was calculated by dividing 3H-TG in the medium by the sum of counts in the medium and cells and multiplying by 100. All samples from the 3 clones of each group were compared. Values are shown as mean ± SEM. n = 8–9, * denotes p < 0.05.
3.5. Transcriptomic Profiling of HLCs and Validation of ANGPTL3
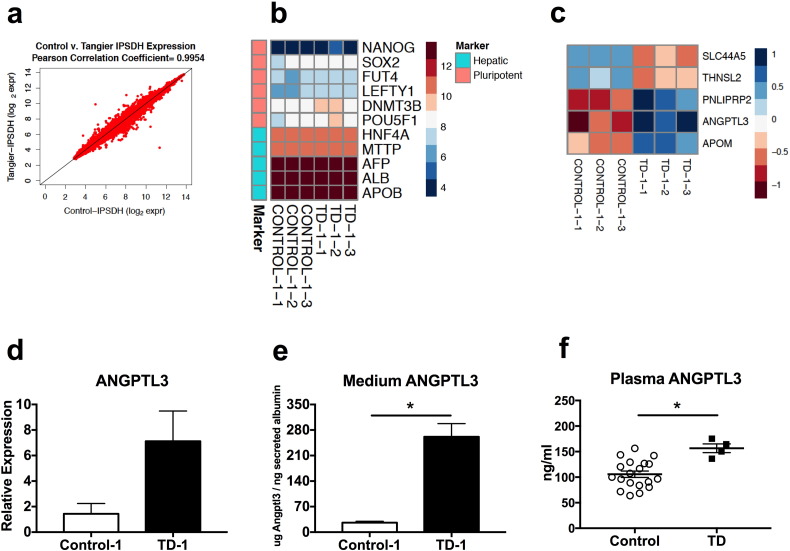
To explore transcriptional effects of human hepatic ABCA1 deficiency, we performed microarray analysis to compare transcriptome profiles of TD-1 and control-1 HLCs. There was significant overlap in gene expression between TD and control HLCs (n = 3 for each; Pearson r = 0.9954; Fig. 4A). A total of 80 genes were expressed differentially (false discovery rate < 5%, fold change > 1.5). Among the differentially expressed genes, 37 were expressed at higher levels in TD HLCs, whereas 43 had higher expression in control HLCs. It is important to note that key hepatocyte markers such as AFP, ALB, HNF4A and APOB were not among the differentially expressed genes. Hepatocyte markers were strongly expressed in both control and TD HLCs. In contrast to highly expressed hepatic markers, expression of pluripotency genes, including SOX2 and NANOG, was markedly low, suggesting efficient transition of iPSCs to HLCs (Fig. 4B). The 80 differentially expressed genes were compared to genes in multiple Gene Ontology (GO) lipid terms, such as lipoprotein metabolic process and high-density lipoprotein particle, to identify lipid-related genes. A small set of 5 genes was significantly different between TD and control HLCs (Fig. 4C). Expression of ANGPTL3, APOM and PNLIPRP2 was highly increased, while SLC44A5 and THNSL2 expression was significantly decreased in TD relative to control HLCs (Fig. 4C). ANGPTL3 is predominantly produced in the liver and is a potent regulator of lipoprotein lipase (LPL) activity and plasma triglyceride clearance (Shan et al., 2009). Gene expression analysis confirmed pronouncedly induced ANGPTL3 expression in TD HLCs (Fig. 4D), which translated to significantly increased secretion of angiopoietin-like protein 3 (ANGPTL3) (Fig. 4E). To determine the in vivo effect of ABCA1 deficiency on ANGPTL3 secretion, plasma samples from a TD cohort and a matched (normal HDL-C) male control subject group were tested for ANGPTL3 by ELISA. Interestingly, a significant 48% increase (156.6 ± 8.525 ng/ml in TD vs. 105.7 ± 6.209 ng/ml in controls) in plasma ANGPTL3 level was observed in TD relative to control subjects (Fig. 4F). Thus, loss of hepatic ABCA1 affects not only TG secretion, but also potentially TG clearance by way of elevated ANGPTL3 expression and secretion.
Fig. 4.
Transcriptome profiles of HLCs and validation of ANGPTL3.
(A) The overlap of genes expressed in TD-1 and control-1 HLCs was evaluated by Pearson Correlation Coefficient. (B) Heat map of pluripotency and hepatic markers between groups. (C) Heat map of differentially expressed lipid-related genes according to Gene Ontology lipid terms. (D) Expression of ANGPTL3 measured by RT-PCR. (E) Culture medium ANGPTL3 protein concentrations determined by ELISA. (F) Plasma ANGPTL3 levels were measured by ELISA. Values are shown as mean ± SEM. n = 9 for RT-PCR analysis. n = 3 for culture medium ELISA. n = 4–19 for plasma ANGPTL3 ELISA. * denotes p < 0.05.
4. Discussion
Our understanding of human hepatocyte ABCA1 deficiency is limited because of unavailability of primary cells for cellular and molecular studies. Subject-specific iPSCs can serve as a renewable source of human primary cells to study molecular processes underlying the pathogenesis of complex diseases. In the present work, we generated iPSC-derived HLCs from Tangier disease patients and matched control subjects to investigate the functional effects of human hepatic ABCA1 deficiency. IPSC-derived macrophages from TD patients exhibited the hallmark defect in cholesterol efflux (Zhang et al., 2015). TD HLCs failed to mediate cholesterol and PL efflux to lipid-free apoA-I or produce nascent HDL particles. Interestingly, TD HLCs displayed increased TG secretion together with increased ANGPTL3 expression and secretion, consistent with the increased plasma ANGPTL3 and elevated plasma TG levels seen in TD patients.
The critical role of hepatic ABCA1 in maintaining plasma HDL has been established in the hepatocyte-specific ABCA1 knockout (HSKO) mice (Timmins et al., 2005). However, primary hepatocytes from patients with rare genetic disorders are typically not available. As a result, the functional significance of human hepatic ABCA1 deficiency has remained unexplored. iPSC-derived HLCs allowed us to directly test the canonical function of human hepatocyte ABCA1. Control HLCs in the presence or absence of exogenous apoA-I produced HDL particles that eluted in the typical two-size species pattern. TD HLCs, however, were not able to mediate lipid efflux for nascent HDL formation. These observations emphasize the essential role of hepatic ABCA1 in human HDL biogenesis.
Besides severe HDL deficiency, most TD patients have increased fasting or postprandial TG at variable levels (Kolovou et al., 2003, Liu et al., 2012). The inverse association between plasma TG and HDL also exists in ABCA1 heterozygous subjects (Clee et al., 2000). Increased hepatic VLDL-TG secretion and delayed clearance of TG owing to decreased LPL activity have been suggested as two mechanisms responsible for elevated TG in TD subjects. Support for the first mechanism has come from a series of studies in McA cells and HSKO mice that have demonstrated increased VLDL-TG secretion in a PI3 kinase-dependent manner when ABCA1 was silenced or deleted (Chung et al., 2010a, Chung et al., 2010b). The findings of the present study provide evidence for a similar mechanism linking ABCA1 expression and plasma TG levels in human hepatocytes. Secretion of newly synthesized TG from TD HLCs was significantly increased relative to control cells. Increased TG secretion in TD HLCs may be secondary to the loss of nascent HDL formation, and may be mediated by PI3 kinase, as proposed by Liu et al. (Liu et al., 2012).
LPL is the rate-limiting enzyme in hydrolysis of TG in TG-rich lipoproteins, and LPL activity is inversely correlated with plasma TG levels (Shimizugawa et al., 2002). Support for the second mechanism linking ABCA1 and plasma TG rests on the observations that TD subjects exhibit decreased LPL activity (Alaupovic et al., 1991, Clifton-Bligh et al., 1972, Greten et al., 1974, Wang et al., 1987) and delayed clearance of postprandial lipid (Kolovou et al., 2003). Post-heparin LPL activity is also reduced in HSKO compared to WT mice, resulting in delayed clearance of postprandial TG (Chung et al., 2010b). The observations in TD patients and HSKO mice regarding LPL activity raise the question as to how the loss of ABCA1 in liver leads to a decrease in LPL activity. Here, we identified ANGPTL3 as a potential mediating factor between ABCA1 and TG levels. TD HLCs displayed elevated ANGPTL3 expression and a ~ 10 fold increase in ANGPTL3 protein secretion. More importantly, male TD patients, including the two recruited to the current study, exhibited significantly increased plasma ANGPTL3 (Fig. 4F) and TG levels (M. Cuchel, unpublished observation). ANGPTL3 is a hepatokine that inhibits LPL activity. In agreement with its known TG regulatory effect, circulating ANGPTL3 positively correlates with TG levels in a larger cohort of TD patients and matched controls (M. Cuchel, unpublished observation). Inactivation of ANGPTL3 in mice has been recently shown to reduce VLDL-TG secretion (Tikka et al., 2014, Wang et al., 2015), which is thought to be a result of reduced free fatty acid supply to the liver. Therefore, the increased ANGPTL3 expression in TD hepatocytes may result in efficient secretion of ANGPTL3, confer enhanced LPL inhibition and delay TG clearance in vivo, contributing to hypertriglyceridemia in TD patients. Overall, our data are compatible with a model of increased secretion, reduced lipolysis and delayed clearance of TG as the underlying mechanisms of hypertriglyceridemia in TD patients.
The increased ANGPTL3 expression and secretion with ABCA1 deficiency is intriguing. ANGPTL3 is a target gene of liver X receptors (LXR) (Tikka and Jauhiainen, 2016). HSKO mice fed with chow diet do not have altered liver lipids or evidence of LXR activation, but have phenotypes of early insulin resistance, including defective PI3 kinase activation (Chung et al., 2010b). Insulin signaling has been implicated as a negative regulator of ANGPTL3 mRNA in HepG2 cells, immortalized human hepatocytes and in vivo in human livers (Nidhina Haridas et al., 2015, Shimamura et al., 2004). The possibility of attenuated hepatic insulin signaling under conditions of ABCA1 deficiency in ANGPTL3 regulation will need to be explored in future studies. Regardless of the mechanism, ANGPTL3 as a potential mediator of altered TG metabolism in TD is an exciting finding that also has significant clinical implications.
The global transcriptional profile of ABCA1-deficient human hepatocytes has never been examined. In this study, we performed microarray analysis with TD and control HLCs. As exemplified by ANGPTL3, we gained insights into transcriptional changes in human hepatic ABCA1 deficiency. Of note, some genes of immune response pathways were differentially expressed, including HLA-A and HLA-C, members of the human leukocyte antigen family. The HLA gene complex encodes the human proteins of the major histocompatibility complex (MHC), which is important for presenting processed peptide antigens (Choo, 2007, Neefjes et al., 2011). It is of interest to know whether these changes represent another link between ABCA1 and immune responses, considering the increasingly appreciated relationship between ABCA1 and inflammation, lipid-regulatory effects dependent or not (Bi et al., 2015, Bochem et al., 2015, Tall and Yvan-Charvet, 2015). The HLCs system enables future explorations using unbiased strategies to enhance our understanding of the functions of human hepatic ABCA1.
In conclusion, we have shown that human HLCs deficient in ABCA1 lack the ability to form nascent HDL, secrete elevated levels of VLDL-TG and express and secrete increased amounts of ANGPTL3, an inhibitor of LPL activity. These findings illuminate the mechanisms of the inverse association between HDL cholesterol and TG in plasma. Our work also illustrates the power of HLCs to answer intractable questions regarding lipid metabolism in a manner that is immediately relevant to human physiology.
Funding Sources
This work was supported by NIH grant U01HG006398. The Clinical Translational Research Center at PENN was supported by NIH grant CTSA UL1TR0000003. E.E.P. also received support from NIH training grant T32HL007954. X.B. is supported by AHA Postdoctoral Fellowship 15POST25160019 and N.N.L. is supported by AHA Scientist Development Grant 14SDG20230024.
Conflicts of Interest
The authors have no conflict of interest in this study.
Author Contributions
Conceptualization, D.J.R., E.E.M. and S.A.D.; Methodology, W.Y., M.C.P. and S.A.D.; Formal Analysis, X.B., E.E.P. and C.Y.; Investigation, E.E.P., N.N.L., M.H., A.P., J.M., X.B., M.C., Y.L. and R.Y.; Data Curation, X.B., E.E.P., S.L.D. and C.Y.; Writing – Original Draft, X.B.; Writing – Review and Editing, D.J.R., S.A.D, M.C.P., N.N.L. and M.C.; Visualization, X.B. and E.E.P. and C.Y.; Supervision, D.J.R. and W.Y.; Funding Acquisition, D.J.R., E.E.M. and S.A.D.
Acknowledgements
We thank the participants of the study and the staff at the University of Pennsylvania Clinical Translational Research Center. We also thank the Induced Pluripotent Stem Cell Core Facility of the Institute for Regenerative Medicine at the University of Pennsylvania (PENN) for iPSC generation, and the Molecular Profiling Facility of the Dept. of Genetics at PENN for microarray analysis. The authors also thank Dawn Marchadier for project management.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ebiom.2017.03.018.
Appendix A. Supplementary data
Supplementary material
References
- Alaupovic P., Knight-Gibson C., Wang C.S., Downs D., Koren E., Brewer H.B., Jr., Gregg R.E. Isolation and characterization of an apoA-II-containing lipoprotein (LP-A-II:B complex) from plasma very low density lipoproteins of patients with Tangier disease and type V hyperlipoproteinemia. J. Lipid Res. 1991;32:9–19. [PubMed] [Google Scholar]
- Bi X., Zhu X., Duong M., Boudyguina E.Y., Wilson M.D., Gebre A.K., Parks J.S. Liver ABCA1 deletion in LDLrKO mice does not impair macrophage reverse cholesterol transport or exacerbate atherogenesis. Arterioscler. Thromb. Vasc. Biol. 2013;33:2288–2296. doi: 10.1161/ATVBAHA.112.301110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi X., Vitali C., Cuchel M. ABCA1 and inflammation: from animal models to humans. Arterioscler. Thromb. Vasc. Biol. 2015;35:1551–1553. doi: 10.1161/ATVBAHA.115.305547. [DOI] [PubMed] [Google Scholar]
- Bochem A.E., van der Valk F.M., Tolani S., Stroes E.S., Westerterp M., Tall A.R. Increased systemic and plaque inflammation in ABCA1 mutation carriers with attenuation by statins. Arterioscler. Thromb. Vasc. Biol. 2015;35:1663–1669. doi: 10.1161/ATVBAHA.114.304959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choo S.Y. The HLA system: genetics, immunology, clinical testing, and clinical implications. Yonsei Med. J. 2007;48:11–23. doi: 10.3349/ymj.2007.48.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung S., Gebre A.K., Seo J., Shelness G.S., Parks J.S. A novel role for ABCA1-generated large pre-beta migrating nascent HDL in the regulation of hepatic VLDL triglyceride secretion. J. Lipid Res. 2010;51:729–742. doi: 10.1194/jlr.M900083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung S., Timmins J.M., Duong M., Degirolamo C., Rong S., Sawyer J.K., Singaraja R.R., Hayden M.R., Maeda N., Rudel L.L. Targeted deletion of hepatocyte ABCA1 leads to very low density lipoprotein triglyceride overproduction and low density lipoprotein hypercatabolism. J. Biol. Chem. 2010;285:12197–12209. doi: 10.1074/jbc.M109.096933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clee S.M., Kastelein J.J., van Dam M., Marcil M., Roomp K., Zwarts K.Y., Collins J.A., Roelants R., Tamasawa N., Stulc T. Age and residual cholesterol efflux affect HDL cholesterol levels and coronary artery disease in ABCA1 heterozygotes. J. Clin. Invest. 2000;106:1263–1270. doi: 10.1172/JCI10727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifton-Bligh P., Nestel P.J., Whyte H.M. Tangier disease. Report of a case and studies of lipid metabolism. N.Engl. J. Med. 1972;286:567–571. doi: 10.1056/NEJM197203162861103. [DOI] [PubMed] [Google Scholar]
- van Dam M.J., de Groot E., Clee S.M., Hovingh G.K., Roelants R., Brooks-Wilson A., Zwinderman A.H., Smit A.J., Smelt A.H., Groen A.K. Association between increased arterial-wall thickness and impairment in ABCA1-driven cholesterol efflux: an observational study. Lancet. 2002;359:37–42. doi: 10.1016/S0140-6736(02)07277-X. [DOI] [PubMed] [Google Scholar]
- Greten H., Hannemann T., Gusek W., Vivell O. Lipoproteins and lipolytic plasma enzymes in a case of tangier disease. N. Engl. J. Med. 1974;291:548–552. doi: 10.1056/NEJM197409122911103. [DOI] [PubMed] [Google Scholar]
- Kolovou G., Daskalova D., Anagnostopoulou K., Hoursalas I., Voudris V., Mikhailidis D.P., Cokkinos D.V. Postprandial hypertriglyceridaemia in patients with Tangier disease. J. Clin. Pathol. 2003;56:937–941. doi: 10.1136/jcp.56.12.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M., Chung S., Shelness G.S., Parks J.S. Hepatic ABCA1 and VLDL triglyceride production. Biochim. Biophys. Acta. 2012;1821:770–777. doi: 10.1016/j.bbalip.2011.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyssenko N.N., Nickel M., Tang C., Phillips M.C. Factors controlling nascent high-density lipoprotein particle heterogeneity: ATP-binding cassette transporter A1 activity and cell lipid and apolipoprotein AI availability. FASEB J. 2013;27:2880–2892. doi: 10.1096/fj.12-216564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallanna S.K., Duncan S.A. vol. 26. 2013. Differentiation of hepatocytes from pluripotent stem cells. (Current Protocols in Stem Cell Biology). (Unit 1G 4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neefjes J., Jongsma M.L., Paul P., Bakke O. Towards a systems understanding of MHC class I and MHC class II antigen presentation. Nat. Rev. Immunol. 2011;11:823–836. doi: 10.1038/nri3084. [DOI] [PubMed] [Google Scholar]
- Nidhina Haridas P.A., Soronen J., Sadevirta S., Mysore R., Quagliarini F., Pasternack A., Metso J., Perttila J., Leivonen M., Smas C.M. Regulation of Angiopoietin-Like Proteins (ANGPTLs) 3 and 8 by insulin. J. Clin. Endocrinol. Metab. 2015;100:E1299–E1307. doi: 10.1210/jc.2015-1254. [DOI] [PubMed] [Google Scholar]
- Oram J.F., Heinecke J.W. ATP-binding cassette transporter A1: a cell cholesterol exporter that protects against cardiovascular disease. Physiol. Rev. 2005;85:1343–1372. doi: 10.1152/physrev.00005.2005. [DOI] [PubMed] [Google Scholar]
- Shan L., Yu X.C., Liu Z., Hu Y., Sturgis L.T., Miranda M.L., Liu Q. The angiopoietin-like proteins ANGPTL3 and ANGPTL4 inhibit lipoprotein lipase activity through distinct mechanisms. J. Biol. Chem. 2009;284:1419–1424. doi: 10.1074/jbc.M808477200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimamura M., Matsuda M., Ando Y., Koishi R., Yasumo H., Furukawa H., Shimomura I. Leptin and insulin down-regulate angiopoietin-like protein 3, a plasma triglyceride-increasing factor. Biochem. Biophys. Res. Commun. 2004;322:1080–1085. doi: 10.1016/j.bbrc.2004.08.024. [DOI] [PubMed] [Google Scholar]
- Shimizugawa T., Ono M., Shimamura M., Yoshida K., Ando Y., Koishi R., Ueda K., Inaba T., Minekura H., Kohama T. ANGPTL3 decreases very low density lipoprotein triglyceride clearance by inhibition of lipoprotein lipase. J. Biol. Chem. 2002;277:33742–33748. doi: 10.1074/jbc.M203215200. [DOI] [PubMed] [Google Scholar]
- Si-Tayeb K., Noto F.K., Nagaoka M., Li J., Battle M.A., Duris C., North P.E., Dalton S., Duncan S.A. Highly efficient generation of human hepatocyte-like cells from induced pluripotent stem cells. Hepatology. 2010;51:297–305. doi: 10.1002/hep.23354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tall A.R., Yvan-Charvet L. Cholesterol, inflammation and innate immunity. Nat. Rev. Immunol. 2015;15:104–116. doi: 10.1038/nri3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tikka A., Jauhiainen M. The role of ANGPTL3 in controlling lipoprotein metabolism. Endocrine. 2016;52:187–193. doi: 10.1007/s12020-015-0838-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tikka A., Soronen J., Laurila P.P., Metso J., Ehnholm C., Jauhiainen M. Silencing of ANGPTL 3 (angiopoietin-like protein 3) in human hepatocytes results in decreased expression of gluconeogenic genes and reduced triacylglycerol-rich VLDL secretion upon insulin stimulation. Biosci. Rep. 2014;34 doi: 10.1042/BSR20140115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmins J.M., Lee J.Y., Boudyguina E., Kluckman K.D., Brunham L.R., Mulya A., Gebre A.K., Coutinho J.M., Colvin P.L., Smith T.L. Targeted inactivation of hepatic Abca1 causes profound hypoalphalipoproteinemia and kidney hypercatabolism of apoA-I. J. Clin. Invest. 2005;115:1333–1342. doi: 10.1172/JCI23915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C.S., Alaupovic P., Gregg R.E., Brewer H.B., Jr. Studies on the mechanism of hypertriglyceridemia in Tangier disease. Determination of plasma lipolytic activities, k1 values and apolipoprotein composition of the major lipoprotein density classes. Biochim. Biophys. Acta. 1987;920:9–19. doi: 10.1016/0005-2760(87)90305-5. [DOI] [PubMed] [Google Scholar]
- Wang Y., Gusarova V., Banfi S., Gromada J., Cohen J.C., Hobbs H.H. Inactivation of ANGPTL3 reduces hepatic VLDL-triglyceride secretion. J. Lipid Res. 2015;56:1296–1307. doi: 10.1194/jlr.M054882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka S. Induced pluripotent stem cells: past, present, and future. Cell Stem Cell. 2012;10:678–684. doi: 10.1016/j.stem.2012.05.005. [DOI] [PubMed] [Google Scholar]
- Yang W., Mills J.A., Sullivan S., Liu Y., French D.L., Gadue P. StemBook. Cambridge; MA: 2012. iPSC reprogramming from human peripheral blood using sendai virus mediated gene transfer. [PubMed] [Google Scholar]
- Zhang H., Xue C., Shah R., Bermingham K., Hinkle C.C., Li W., Rodrigues A., Tabita-Martinez J., Millar J.S., Cuchel M. Functional analysis and transcriptomic profiling of iPSC-derived macrophages and their application in modeling Mendelian disease. Circ. Res. 2015;117:17–28. doi: 10.1161/CIRCRESAHA.117.305860. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material