Abstract
Tenofovir disoproxil fumarate (TDF) genotypic resistance defined by K65R/N and/or K70E/Q/G occurs in 20% to 60% of individuals with virological failure (VF) on a WHO-recommended TDF-containing first-line regimen. However, the full spectrum of reverse transcriptase (RT) mutations selected in individuals with VF on such a regimen is not known. To identify TDF regimen-associated mutations (TRAMs), we compared the proportion of each RT mutation in 2873 individuals with VF on a WHO-recommended first-line TDF-containing regimen to its proportion in a cohort of 50,803 antiretroviral-naïve individuals. To identify TRAMs specifically associated with TDF-selection pressure, we compared the proportion of each TRAM to its proportion in a cohort of 5805 individuals with VF on a first-line thymidine analog-containing regimen. We identified 83 TRAMs including 33 NRTI-associated, 40 NNRTI-associated, and 10 uncommon mutations of uncertain provenance. Of the 33 NRTI-associated TRAMs, 12 – A62V, K65R/N, S68G/N/D, K70E/Q/T, L74I, V75L, and Y115F – were more common among individuals receiving a first-line TDF-containing compared to a first-line thymidine analog-containing regimen. These 12 TDF-selected TRAMs will be important for monitoring TDF-associated transmitted drug-resistance and for determining the extent of reduced TDF susceptibility in individuals with VF on a TDF-containing regimen.
Keywords: HIV-1, Antiretroviral therapy, Reverse transcriptase, Drug resistance, Tenofovir, WHO-recommended first-line
Highlights
-
•
We determined the spectrum of tenofovir-selected mutations associated with first-line tenofovir-containing regimens.
-
•
Several of these mutations should be added to the list of mutations used for drug-resistance surveillance.
-
•
Several of these mutations require further evaluation to determine their effects on tenofovir susceptibility.
Tenofovir disoproxil fumarate (TDF) plus a cytosine analog is the universally recommended NRTI backbone for first-line antiretroviral (ARV) therapy and for pre-exposure prophylaxis. TDF is also frequently recommended for second-line therapy provided it retains activity against viruses that emerge following virological failure (VF) on a first-line ARV treatment regimen. The expanded spectrum of TDF-selected mutations identified in this study will be important for monitoring TDF-associated drug-resistance. Their clinical significance requires phenotypic and clinical studies to characterize their effects on the continued activity of TDF and the newly developed prodrug tenofovir alafenamide in individuals with VF on a TDF-containing regimen.
1. Introduction
Tenofovir disoproxil fumarate (TDF) plus a cytosine analog is the universally recommended NRTI backbone for first-line antiretroviral (ARV) therapy and for pre-exposure prophylaxis. TDF is also frequently recommended for second-line therapy provided it retains activity against viruses that emerge following virological failure (VF) on a first-line ARV treatment regimen.
We recently performed a meta-analysis which showed that between 20% to 60% of individuals with VF on a TDF-containing regimen developed genotypic evidence of TDF resistance defined as the presence of either K65R/N or K70E/G/Q in the HIV-1 reverse transcriptase (RT) gene (TenoRes Study) (TenoRes Study, 2016). The risk of having TDF resistance at the time of VF was significantly higher in low and middle-income countries (LMICs) compared with upper-income countries (UICs). In addition, having a low baseline CD4 count, receiving lamivudine (3TC) rather than emtricitabine (FTC), and receiving nevirapine (NVP) rather than efavirenz (EFV) were also associated with a risk of TDF resistance.
It is not known, however, whether K65R/N and K70E/G/Q represent the full spectrum of NRTI-associated mutations in individuals receiving a WHO-recommended first-line TDF-containing regimen. Therefore, we analyzed the full set of publicly available RT sequences from studies of such individuals. To identify TDF regimen-associated mutations (TRAMs), we compared the proportion of each RT mutation in individuals with VF on a WHO-recommended first-line TDF-containing regimen with its proportion in a group of ARV-naïve individuals. To identify TRAMs specifically associated with TDF-selection pressure, we compared the proportion of each TRAM with its proportion in a group of individuals with VF on a first-line thymidine analog regimen. As resistance mutations are often accompanied by compensatory mutations, we also determined which combinations of TDF-selected mutations were likely to co-occur.
2. Materials and Methods
2.1. Individuals and Sequences
We analyzed HIV-1 RT sequences and clinical data from studies of individuals with VF on a WHO-recommended first-line regimen comprising TDF plus either 3TC or FTC plus either NVP or EFV. Study inclusion criteria required that individuals reported being ARV-naïve prior to receiving first-line therapy, that the year and country of virus sampling were known, and that RT sequences encompassing codons 40 to 240 were available.
Study individuals were from 51 datasets including 29 in the TenoRes study for which RT sequences were made publicly available (n = 1573 individuals) and 22 additional recently published studies (n = 1840 individuals) identified through GenBank HIV-1 sequence submissions (Supplementary Table 1 and Supplementary Data 1). VF was defined by local viral load thresholds or surveillance protocols, which was 1000 copies/ml for studies performed in LMICs. Viral load (VL) data, however, was not routinely made available.
Mutations were defined as amino acid differences at positions 1 to 240 between each sequence and the consensus subtype B amino acid reference sequence (Rhee et al., 2003). Because of the possibility that some individuals may have received a thymidine analog before receiving a TDF-containing regimen, as such switches may not have always reported (Gregson et al., 2016), we excluded from our analysis individuals with sequences containing one or more canonical thymidine analogue mutations (TAMs) – M41L, D67N, K70R, L210W, T215Y/F, and K219Q/E (Menendez-Arias, 2011).
Each sequence was subtyped using the Rega subtyping tool version 3 (Pineda-Pena et al., 2013). Sequence quality control measures were taken to identify sequences with APOBEC G-to-A hypermutation (Rhee et al., 2016). To exclude duplicate sequences, we examined the relatedness of sequences within each study and within the total study population by creating a neighbor-joining phylogenetic tree using the HKY85 substitution model with a gamma distribution for variable sites as implemented by PAUP (Supplementary Data 2).
2.2. Identification of TDF-regimen Associated Mutations (TRAMs)
We compared the proportion of each RT mutation with its proportion in a set of sequences from 50,803 ARV-naïve individuals described in a recent publication (Rhee et al., 2015). The proportion of each mutation was analyzed using Fisher's Exact Test and the p value was adjusted using the Holm's method to control the family-wise error rate for multiple hypothesis testing (Holm, 1979). Among mutations with an adjusted p < 0.05, those that occurred in ≥ 10 individuals receiving a TDF-containing regimen and ≥ 3 times more frequently than in the ARV-naïve population were classified as TRAMs. The rationale for requiring that a TRAM occur ≥ 3 times more commonly in the TDF recipients was to reduce the risk of false positive associations arising from differences in the distribution of subtype-dependent polymorphisms between the TDF recipients and ARV-naïve controls. The rationale for requiring that a TRAM appear in ≥ 10 individuals in the TDF group was to focus on the most commonly occurring TRAMs.
TRAMs were further classified as to whether they were previously reported to be at an NRTI-associated drug resistance mutation (DRM) position, an NNRTI-associated DRM position, an RTI-selected mutation position not clearly associated with either RTI class, or a position not previously associated with RTI selection pressure (Rhee et al., 2016). NRTI-associated DRM positions were referred to as established if they were on the IAS-USA DRM list (Wensing et al., 2015). NRTI-associated DRMs not among the exclusionary TAMs that had been reported to be selected by thymidine analogs were called non-canonical TAMs (Gonzales et al., 2003).
To determine whether the proportion of a TRAM was influenced by HIV-1 subtype, we performed a 2 × 5 Chi-square test to compare the proportion of each TRAM in the five most common subtypes in our dataset (A, B, C, G, CRF01_AE). The Holm's adjustment for multiple comparisons (adjusted p < 0.05) was used to identify which TRAMs occurred in significantly different proportions in different subtypes. For such TRAMs with a significantly large overall chi-square statistic, we identified the subtype that differed most markedly from the other subtypes using pairwise comparisons.
The proportion of each TRAM was also compared to its proportion in a published comparison group of 5805 individuals with VF on a first-line regimen containing a thymidine analog, zidovudine (AZT) or stavudine (d4T), plus 3TC plus NVP or EFV using Fisher's Exact Test with an adjusted p < 0.05 by Holm's adjustment.
2.3. Covariation of TDF-regimen Associated Mutations (TRAMs)
To identify patterns of covariation among the TRAMs, we calculated the binomial phi correlation coefficient for the simultaneous presence of each pair of TRAMs in the same virus sequence. A significant association between TRAMs was defined when two mutations occurred together in virus sequences from ten or more individuals and had a positive phi correlation coefficient with an adjusted p < 0.05 using the Holm's method.
To identify higher-level interactions among TRAMs, we used the R package igraph to create an undirected weighted network graph of mutations from the adjacency matrix of significantly positively correlated pairs. This network contains edges between all significantly correlated pairs of mutations with edge lengths inversely correlated to the pair's phi coefficient. Mutational cliques were defined as clusters of three or more mutations that were each significantly correlated with one another.
2.4. Mutational Predictors of Viral Load (VL)
To identify associations between TRAMs and the VL at the time of VF, we first performed univariate analyses for both mutational and non-mutational covariates. The mutational covariates included number of TRAMs, number of NRTI-associated TRAMs, number of NNRTI-associated TRAMs, M184V, K65R, K70E/Q/T/N, and K65R in combination with its mutational partners (defined as mutations with which K65R was significantly correlated in our covariation analysis). The non-mutational covariates included the region (UIC vs. LMIC), NNRTI (EFV vs. NVP), cytosine analog (3TC vs. FTC), and subtype. We then included in a multivariate linear regression analysis all mutational and non-mutational covariates significantly associated with VL in the univariate analysis. To ensure the multivariate analysis was robust to study heterogeneity, we included study as a random effect and performed a linear mixed model using the R package lme4.
3. Results
3.1. Study Population
HIV-1 RT sequences were available from 3413 individuals with VF on a WHO-recommended first-line TDF-containing regimen. Of these, 2873 (84.2%; 2873/3413) met study criteria. Five-hundred and forty (15.8%; 540/3413) were excluded because their sequences contained one or more TAMs (n = 523), were identical to another sequence in the same study (n = 12), or contained evidence of APOBEC-mediated G-to-A hypermutation (n = 5).
Seventy-nine percent (2262/2873) of study individuals received EFV and 21% (611/2873) received NVP. Sixty-four percent (1826/2873) received 3TC and 36% (1047/2873) received FTC. Seventy-two percent (2071/2873) were from LMICs. The most common subtypes were C (53%; 1524/2873), B (20%; 561/2873), CRF01_AE (13%; 365/2873), G (5%; 135/2873), and A (4%; 110/2873). The median sample year was 2012 (Interquartile Range, IQR: 2010–2013). The duration of therapy was available for 62% (1792/2873) of individuals. Among them, the median duration of therapy was 43.0 weeks (IQR: 21.0 to 83.0 weeks).
Table 1 summarizes the treatment regimens, location information, and subtype distributions of the TDF study group and the ARV-naïve and thymidine-analog control groups. Compared to the ARV-naïve control group, the TDF study group included a higher proportion of subtype C infected individuals. Compared to the thymidine-analog control group, the TDF study group was more recent, had a shorter duration of therapy, and a slightly lower proportion of individuals from LMICs.
Table 1.
Summary of individuals with virological failure (VF) on a first-line TDF-containing regimen and two comparison groups: (i) antiretroviral therapy (ARV)-naïve individuals and (ii) individuals with VF on a first-line thymidine analog-containing regimen according to treatment regimen, region and subtype.
| Characteristics | TDF-containing regimen (n = 2,873) | Comparison groups |
|
|---|---|---|---|
| ARV-naïve (n = 50,803) | Thymidine analog-containing regimen (n = 5,805) | ||
| NRTs | TDF + 3TC (64%), TDF + FTC (36%) | None | D4T + 3TC (64%), AZT + 3TC (36%) |
| NNRTIs | EFV (79%), NVP (21%) | None | EFV (53%), NVP (47%) |
| Regimens | TDF + 3TC + EFV (49%), TDF + FTC + EFV (30%), TDF + 3TC + NVP (15%), TDF + FTC + NVP (6%) | None | D4T + 3TC + EFV (35%), D4T + 3TC + NVP (30%), AZT + 3TC + EFV (18%), AZT + 3TC + NVP (17%) |
| Region | LMIC (72%) Sub-Saharan Africa (56%), South/Southeast Asia (15%), Latin America (1%) |
LMIC (49%) Sub-Saharan Africa (23%), South/Southeast Asia (13%), Latin America/Caribbean (11%), Former Soviet Union (3%) |
LMIC (88%) Sub-Saharan Africa (65%), South/Southeast Asia (19%), Latin America (2%), Former Soviet Union (2%) |
| UIC (28%) Europe (22%), North America (6%) |
UIC (51%) Europe (23%), North America (18%), Asia UICs (10%) |
UIC (12%) Europe (6%), North America (6%), Asia UICs (1%) |
|
| Subtype | C (53%), B (20%), CRF01_AE (13%), G (5%), A (4%), Other (6%) | B (53%), C (15%), CRF01_AG (11%), A (7%), CRF02_AG (5%), Other (10%) | C (52%), CRF01_AE (16%), B (10%), A (7%), CRF02_AG (5%), Other (11%) |
| Sample year, median (IQR) | 2012 (2010 − 2013) | 2006 (2003–2008) | 2008 (2006–2011) |
| Duration of therapy available, median (IQR) in weeks | 63%, 43 (21–85) | 39%, 97 (52–171) | |
Abbreviation: TDF – tenofovir disoproxil fumarate; FTC – emtricitabine; 3TC – lamivudine; AZT – zidovudine; D4T – stavudine; NVP – nevirapine; EFV – efavirenz; LMIC – low/middle-income country; UIC – upper-income country.
3.2. Identification and Classification of TDF-regimen Associated Mutations (TRAMs)
Overall 640 RT mutations occurred in sequences from one or more individuals. One hundred mutations (15.6%; 100/640) were significantly more common in the 2873 TDF recipients compared with the 50,803 ARV-naïve controls (adjusted p < 0.05; Fisher's Exact Test), and were at least three times more common in the TDF recipients compared with the ARV-naïve controls. Of these one hundred, 83 (13.0%; 83/640) occurred in ten or more individuals and met criteria for being a TRAM (Table 2). Supplementary Table 2 lists the 17 rare TRAMs that occurred in fewer than 10 individuals. Seventy-five percent (2155/2873) of sequences encompassed codons 1 to 240 and 90% (2586/2873) encompassed codons 30 to 240.
Table 2.
Prevalence of TDF-regimen associated mutations (TRAMs) in ARV-naïve and in individuals receiving a WHO-recommended first-line TDF-containing regimen.
| Pos | Cons | AA | Drug Class |
Overall (%Rx) n = 2,873 |
Overall (%ARV-Naive) n = 50,803 |
Fold | OR | Fisher's exact test (adjusted p) |
|---|---|---|---|---|---|---|---|---|
| 65 | K | R | NRTI | 39.5 | 0.1 | 489.1 | 803.6 | 0 |
| 106 | V | M | NNRTI | 19.8 | 0.0 | 559.9 | 707.8 | 0 |
| 115 | Y | F | NRTI | 11.9 | 0.0 | 303.2 | 344.1 | 0 |
| 184 | M | V | NRTI | 48.7 | 0.8 | 59.4 | 114.6 | 0 |
| 181 | Y | C | NNRTI | 23.0 | 0.5 | 44.9 | 58.0 | 0 |
| 190 | G | A | NNRTI | 13.3 | 0.4 | 35.3 | 40.5 | 0 |
| 103 | K | N | NNRTI | 38.5 | 1.8 | 21.3 | 34.0 | 0 |
| 184 | M | I | NRTI | 9.4 | 0.1 | 116.0 | 127.9 | 9.7E-295 |
| 225 | P | H | NNRTI | 8.7 | 0.1 | 71.6 | 78.1 | 5.1E-253 |
| 101 | K | E | NNRTI | 9.0 | 0.3 | 36.1 | 39.5 | 4.1E-230 |
| 100 | L | I | NNRTI | 7.2 | 0.1 | 114.3 | 123.0 | 4.1E-225 |
| 70 | K | E | NRTI | 6.6 | 0.0 | 241.2 | 256.6 | 1.6E-222 |
| 68 | S | N | NRTI | 8.1 | 0.3 | 30.7 | 33.4 | 7.6E-198 |
| 74 | L | I | NRTI | 5.7 | 0.1 | 78.8 | 83.7 | 7.1E-170 |
| 62 | A | V | NRTI | 10.4 | 1.0 | 10.8 | 11.9 | 1.7E-168 |
| 221 | H | Y | NNRTI | 6.6 | 0.2 | 34.6 | 37.0 | 2.9E-166 |
| 228 | L | R | NRTI | 5.5 | 0.1 | 45.7 | 48.4 | 9.0E-146 |
| 108 | V | I | NNRTI | 7.4 | 0.6 | 13.0 | 14.0 | 8.7E-132 |
| 227 | F | L | NNRTI | 4.6 | 0.1 | 58.3 | 61.0 | 1.6E-128 |
| 188 | Y | L | NNRTI | 4.7 | 0.1 | 39.5 | 41.3 | 7.2E-122 |
| 190 | G | S | NNRTI | 3.6 | 0.0 | 80.0 | 83.0 | 2.1E-106 |
| 98 | A | G | NNRTI | 5.2 | 0.3 | 17.3 | 18.2 | 3.8E-103 |
| 230 | M | L | NNRTI | 3.3 | 0.0 | 88.2 | 91.2 | 2.7E-96 |
| 68 | S | G | NRTI | 13.3 | 4.1 | 3.2 | 3.6 | 1.1E-77 |
| 70 | K | T | NRTI | 2.6 | 0.0 | 64.0 | 65.7 | 1.8E-74 |
| 90 | V | I | NNRTI | 8.6 | 2.0 | 4.2 | 4.5 | 3.8E-68 |
| 188 | Y | C | NNRTI | 2.4 | 0.0 | 63.3 | 64.8 | 2.5E-66 |
| 74 | L | V | NRTI | 2.6 | 0.1 | 29.9 | 30.6 | 1.9E-62 |
| 190 | G | E | NNRTI | 2.0 | 0.0 | 53.1 | 54.1 | 1.7E-53 |
| 67 | D | G | NRTI | 2.5 | 0.1 | 22.4 | 23.0 | 3.6E-53 |
| 203 | E | K | NRTI | 3.0 | 0.3 | 11.4 | 11.8 | 2.0E-48 |
| 46 | K | Q | UNK | 2.5 | 0.2 | 16.1 | 16.5 | 9.5E-47 |
| 75 | V | M | NRTI | 2.0 | 0.1 | 24.4 | 24.9 | 1.9E-44 |
| 70 | K | Q | NRTI | 1.6 | 0.0 | 66.3 | 67.3 | 9.9E-44 |
| 179 | V | D | NNRTI | 6.5 | 1.8 | 3.6 | 3.8 | 1.0E-42 |
| 68 | S | D | NRTI | 1.3 | 0.0 | 163.6 | 165.6 | 3.6E-40 |
| 190 | G | Q | NNRTI | 1.0 | 0.0 | 495.1 | Inf | 1.3E-33 |
| 101 | K | Q | NNRTI | 3.3 | 0.6 | 5.4 | 5.6 | 4.2E-32 |
| 232 | Y | H | NNRTI | 1.2 | 0.0 | 56.0 | 56.6 | 2.2E-30 |
| 101 | K | P | NNRTI | 1.1 | 0.0 | 48.6 | 49.1 | 8.3E-30 |
| 219 | K | R | NRTI | 1.5 | 0.1 | 16.5 | 16.8 | 8.6E-29 |
| 75 | V | I | NRTI | 1.5 | 0.1 | 15.6 | 15.8 | 4.7E-28 |
| 103 | K | S | NNRTI | 1.5 | 0.1 | 15.3 | 15.5 | 8.3E-28 |
| 106 | V | A | NNRTI | 1.2 | 0.0 | 31.6 | 32.0 | 1.3E-27 |
| 4 | P | S | UNK | 5.0 | 1.3 | 3.9 | 4.0 | 1.6E-25 |
| 101 | K | N | NNRTI | 1.0 | 0.0 | 39.5 | 39.8 | 8.8E-25 |
| 138 | E | G | NNRTI | 1.8 | 0.2 | 7.6 | 7.7 | 2.7E-22 |
| 69 | T | del | NRTI | 0.6 | 0.0 | 300.8 | Inf | 1.3E-19 |
| 181 | Y | V | NNRTI | 0.7 | 0.0 | 88.4 | 89.1 | 1.8E-19 |
| 188 | Y | H | NNRTI | 0.9 | 0.0 | 23.0 | 23.2 | 8.8E-19 |
| 88 | W | C | NRTI | 2.6 | 0.6 | 4.0 | 4.1 | 4.9E-18 |
| 31 | I | L | RTI | 1.5 | 0.2 | 7.8 | 7.9 | 1.3E-16 |
| 28 | E | K | UNK | 3.4 | 1.0 | 3.3 | 3.3 | 6.2E-16 |
| 109 | L | I | RTI | 0.8 | 0.0 | 17.7 | 17.8 | 1.5E-15 |
| 179 | V | E | NNRTI | 2.4 | 0.7 | 3.6 | 3.7 | 1.6E-14 |
| 228 | L | Q | NRTI | 1.0 | 0.1 | 9.5 | 9.6 | 2.8E-14 |
| 163 | S | T | UNK | 1.7 | 0.4 | 4.8 | 4.8 | 2.9E-14 |
| 101 | K | H | NNRTI | 0.6 | 0.0 | 56.6 | 56.9 | 3.9E-14 |
| 219 | K | N | NRTI | 0.9 | 0.1 | 11.3 | 11.4 | 4.5E-13 |
| 65 | K | N | NRTI | 0.7 | 0.0 | 21.0 | 21.1 | 6.6E-13 |
| 138 | E | Q | NNRTI | 0.8 | 0.1 | 14.4 | 14.5 | 6.9E-13 |
| 58 | T | S | RTI | 0.5 | 0.0 | 29.5 | 29.6 | 3.8E-11 |
| 139 | T | K | NNRTI | 1.5 | 0.3 | 4.3 | 4.4 | 1.2E-10 |
| 88 | W | S | NRTI | 1.0 | 0.2 | 6.4 | 6.5 | 5.3E-10 |
| 102 | K | N | NNRTI | 0.7 | 0.1 | 11.2 | 11.3 | 1.5E-09 |
| 181 | Y | I | NNRTI | 0.5 | 0.0 | 20.6 | 20.7 | 4.5E-09 |
| 234 | L | I | NNRTI | 0.5 | 0.0 | 18.3 | 18.4 | 1.4E-08 |
| 70 | K | N | NRTI | 0.5 | 0.0 | 14.0 | 14.0 | 3.2E-08 |
| 75 | V | L | NRTI | 1.1 | 0.2 | 4.5 | 4.6 | 6.2E-08 |
| 188 | Y | F | NNRTI | 0.5 | 0.0 | 14.6 | 14.6 | 9.3E-08 |
| 238 | K | T | NNRTI | 0.8 | 0.1 | 6.4 | 6.4 | 1.8E-07 |
| 223 | K | E | RTI | 0.5 | 0.0 | 11.3 | 11.3 | 2.8E-07 |
| 132 | I | L | NNRTI | 0.6 | 0.1 | 8.6 | 8.6 | 4.6E-07 |
| 228 | L | H | NRTI | 1.2 | 0.3 | 3.6 | 3.7 | 1.3E-06 |
| 69 | T | I | NRTI | 0.6 | 0.1 | 6.8 | 6.8 | 2.9E-06 |
| 138 | E | K | NNRTI | 0.7 | 0.1 | 5.5 | 5.6 | 3.1E-06 |
| 165 | T | L | NRTI | 0.4 | 0.0 | 11.5 | 11.5 | 4.9E-05 |
| 4 | P | T | UNK | 1.4 | 0.5 | 3.1 | 3.1 | 1.8E-04 |
| 165 | T | V | NRTI | 0.4 | 0.1 | 7.3 | 7.3 | 5.2E-04 |
| 31 | I | R | RTI | 0.4 | 0.1 | 7.3 | 7.3 | 1.5E-03 |
| 218 | D | E | NRTI | 0.6 | 0.1 | 4.4 | 4.4 | 2.8E-03 |
| 68 | S | R | NRTI | 0.4 | 0.1 | 5.7 | 5.7 | 9.8E-03 |
| 139 | T | R | NNRTI | 0.5 | 0.1 | 4.2 | 4.2 | 2.9E-02 |
83 TRAMs were listed in the order of the significance estimated by Fisher's exact test. Estimated odds ratios (OR) by Fisher's exact test and their p-values adjusted by Holm's method are shown. Each TRAM was classified to NRTI-associated, NNRTI-associated or undifferentiated (in the DrugClass; see Methods).
Abbreviation: TDF – tenofovir disoproxil fumarate; ARV- antiretroviral; Cons - consensus amino acid (defined in the Methods); RTI - reverse transcriptase inhibitor; NRTI - nucleos(t)ide RTI; NNRTI - non-NRTI; UNK - undifferentiated.
Of the 83 TRAMs, 16 (21%; 16/83) were established NRTI-associated resistance mutations including A62V, K65R/N, T69deletion, K70E/Q/T/N, L74V/I, V75M/I/L, Y115F, and M184V/I and 40 (48%; 40/83) were established NNRTI-associated resistance mutations. The remaining 26 mutations included 12 mutations at five positions previously associated with NRTI therapy including S68D/G/N/R, T69I, W88S/C, T165L/V, L228H/Q/R; five non-canonical TAMs including D67G, E203K, D218E, and K219N/R; five uncommon previously undifferentiated RTI-associated mutations (I31L/R, T58S, L109I, and K223E) and five previously unreported mutations P4S/T, E28K, K46Q, and S163T.
The proportions of 53 TRAMs were not uniform among the five most common subtypes. For 13 TRAMs, the mutation proportion was highest with subtype C (Supplementary Table 3). For four, two, and two TRAMs, the mutation proportion was highest for subtypes CRF01_AE, A and G, respectively. For 28 and four TRAMs, the mutation proportion was lowest for subtype B and CRF01_AE, respectively. The most notable findings among the NRTI-associated TRAMs were an increased proportion of K65R, S68N, and A62V in subtype C viruses, an increased proportion of S68G in CRF01_AE viruses, and an increased proportion of K70E in subtype G viruses.
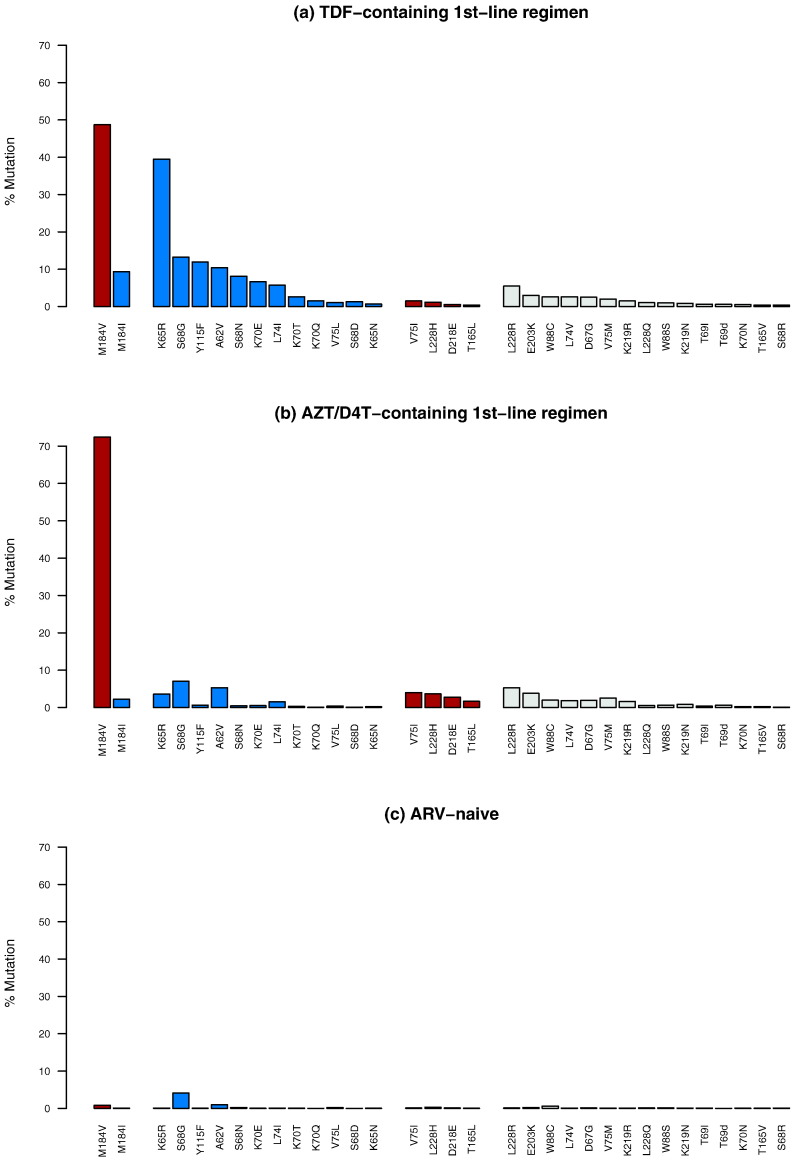
Table 3 compares the proportions of the TRAMs in this study compared with their proportions in 5805 individuals who receiving a first-line thymidine analog-containing regimen. Twelve NRTI-associated TRAMs – A62V, K65R/N, S68D/G/N, K70E/Q/T, L74I, V75L and Y115F, and the established cytosine analog resistance mutation M184I, occurred significantly more commonly in the TDF group (Fig. 1). Five NRTI-associated TRAMs occurred significantly more commonly in the thymidine analog group including V75I, T165L, M184V, D218E and L228H. M184V occurred in 72.5% (4202/5799) of individuals in the thymidine analog group and in 48.7% (1400/2873) of individuals in the TDF group (p < 0.001). M184I occurred in 9.4% (264/2873) of individuals in the TDF group and in 2.2% (128/5799) of individuals in the thymidine analog group (p < 0.001). Because M184V/I are established cytosine analog resistance mutations known to increase TDF susceptibility, we excluded M184I from the list of TDF-selected TRAMs.
Table 3.
Comparison of proportion of each TDF-regimen associated mutations (TRAMs) between in individuals receiving a TDF-containing first-line and individuals receiving an AZT/D4T-containing first-line regimen.
| Pos | Cons | AA | DrugClass | TDF (%) n = 2,837 |
AZT/D4T (%) n = 5,805 |
Fold | OR | Fisher's exact test (adjusted p) |
|---|---|---|---|---|---|---|---|---|
| 65 | K | R | NRTI | 39.5 | 3.6 | 11.1 | 17.6 | 0 |
| 115 | Y | F | NRTI | 11.9 | 0.6 | 19.2 | 21.7 | 7.5E-125 |
| 184 | M | V | NRTI | 48.7 | 72.5 | 0.7 | 0.4 | 5.6E-101 |
| 68 | S | N | NRTI | 8.1 | 0.4 | 18.8 | 20.4 | 5.8E-83 |
| 70 | K | E | NRTI | 6.6 | 0.5 | 13.3 | 14.2 | 5.5E-61 |
| 184 | M | I | NRTI | 9.4 | 2.2 | 4.2 | 4.6 | 2.4E-45 |
| 100 | L | I | NNRTI | 7.2 | 1.5 | 4.7 | 5.0 | 6.0E-38 |
| 74 | L | I | NRTI | 5.7 | 1.5 | 3.8 | 4.0 | 1.7E-24 |
| 70 | K | T | NRTI | 2.6 | 0.3 | 10.2 | 10.5 | 2.1E-21 |
| 46 | K | Q | UNK | 2.5 | 0.3 | 9.5 | 9.7 | 2.5E-19 |
| 68 | S | G | NRTI | 13.3 | 7.0 | 1.9 | 2.0 | 1.2E-18 |
| 70 | K | Q | NRTI | 1.6 | 0.1 | 30.3 | 30.7 | 7.9E-17 |
| 62 | A | V | NRTI | 10.4 | 5.3 | 2.0 | 2.1 | 2.1E-15 |
| 106 | V | M | NNRTI | 19.8 | 13.4 | 1.5 | 1.6 | 1.5E-12 |
| 68 | S | D | NRTI | 1.3 | 0.1 | 18.7 | 18.9 | 2.2E-12 |
| 218 | D | E | NRTI | 0.6 | 2.8 | 0.2 | 0.2 | 4.1E-12 |
| 190 | G | E | NNRTI | 2.0 | 0.4 | 5.5 | 5.6 | 3.5E-11 |
| 228 | L | H | NRTI | 1.2 | 3.6 | 0.3 | 0.3 | 2.6E-10 |
| 75 | V | I | NRTI | 1.5 | 4.0 | 0.4 | 0.4 | 6.8E-09 |
| 238 | K | T | NNRTI | 0.8 | 2.8 | 0.3 | 0.3 | 1.0E-08 |
| 179 | V | D | NNRTI | 6.5 | 3.7 | 1.8 | 1.8 | 6.4E-07 |
| 132 | I | L | NNRTI | 0.6 | 2.1 | 0.3 | 0.3 | 7.0E-07 |
| 165 | T | L | NRTI | 0.4 | 1.7 | 0.2 | 0.2 | 3.9E-06 |
| 139 | T | R | NNRTI | 0.5 | 1.6 | 0.3 | 0.3 | 6.7E-05 |
| 181 | Y | C | NNRTI | 23.0 | 18.7 | 1.2 | 1.3 | 1.6E-04 |
| 31 | I | L | RTI | 1.5 | 3.3 | 0.5 | 0.5 | 2.5E-04 |
| 190 | G | Q | NNRTI | 1.0 | 0.2 | 4.0 | 4.1 | 5.5E-04 |
| 190 | G | A | NNRTI | 13.3 | 16.9 | 0.8 | 0.8 | 6.2E-04 |
| 101 | K | H | NNRTI | 0.6 | 1.6 | 0.4 | 0.3 | 1.1E-03 |
| 138 | E | Q | NNRTI | 0.8 | 1.9 | 0.4 | 0.4 | 1.3E-03 |
| 221 | H | Y | NNRTI | 6.6 | 9.3 | 0.7 | 0.7 | 1.4E-03 |
| 138 | E | G | NNRTI | 1.8 | 0.8 | 2.3 | 2.3 | 2.7E-03 |
| 98 | A | G | NNRTI | 5.2 | 7.4 | 0.7 | 0.7 | 4.2E-03 |
| 190 | G | S | NNRTI | 3.6 | 2.2 | 1.7 | 1.7 | 5.6E-03 |
| 75 | V | L | NRTI | 1.1 | 0.4 | 2.8 | 2.9 | 8.9E-03 |
| 108 | V | I | NNRTI | 7.4 | 9.9 | 0.8 | 0.7 | 9.0E-03 |
| 28 | E | K | UNK | 3.4 | 5.3 | 0.6 | 0.6 | 9.6E-03 |
| 179 | V | E | NNRTI | 2.4 | 1.3 | 1.8 | 1.9 | 1.5E-02 |
| 65 | K | N | NRTI | 0.7 | 0.2 | 3.5 | 3.5 | 3.4E-02 |
| 188 | Y | C | NNRTI | 2.4 | 1.4 | 1.7 | 1.7 | 4.8E-02 |
Proportion of each of the TRAMs was compared with those receiving AZT/D4T containing first-line regimens. The comparisons were performed using Fisher's exact test. Estimated odds ratios (OR) by Fisher's exact test and their p-values adjusted by Holm's method are shown. TRAMs were shown in the order of their p values. Abbreviation: TDF – tenofovir disoproxil fumarate; AZT – zidovudine; D4T – stavudine; RTI - reverse transcriptase inhibitor; NRTI - nucleos(t)ide RTI; NNRTI - non-NRTI; UNK - undifferentiated.
Fig. 1.
Prevalence of nucleoside RT inhibitor (NRTI)-associated tenofovir disoproxil fumarate (TDF)-regimen associated mutations (TRAMs) in 2,873 individuals with virological failure (VF) on a TDF-containing 1st-line regimen (a); in 5,805 individuals with VF on a thymidine analog-containing 1st-line regimen (b); and in 50,803 antiretroviral (ARV)-naive individuals (c). NRTI TRAMs that were significantly more common in individuals receiving a TDF-containing regimen compared to in individuals receiving a thymidine analog-containing regimen are shown in blue. NRTI TRAMs that were significantly more common in individuals receiving a thymidine analog-containing regimen compared to in individuals receiving a TDF-containing regimen are shown in brown.
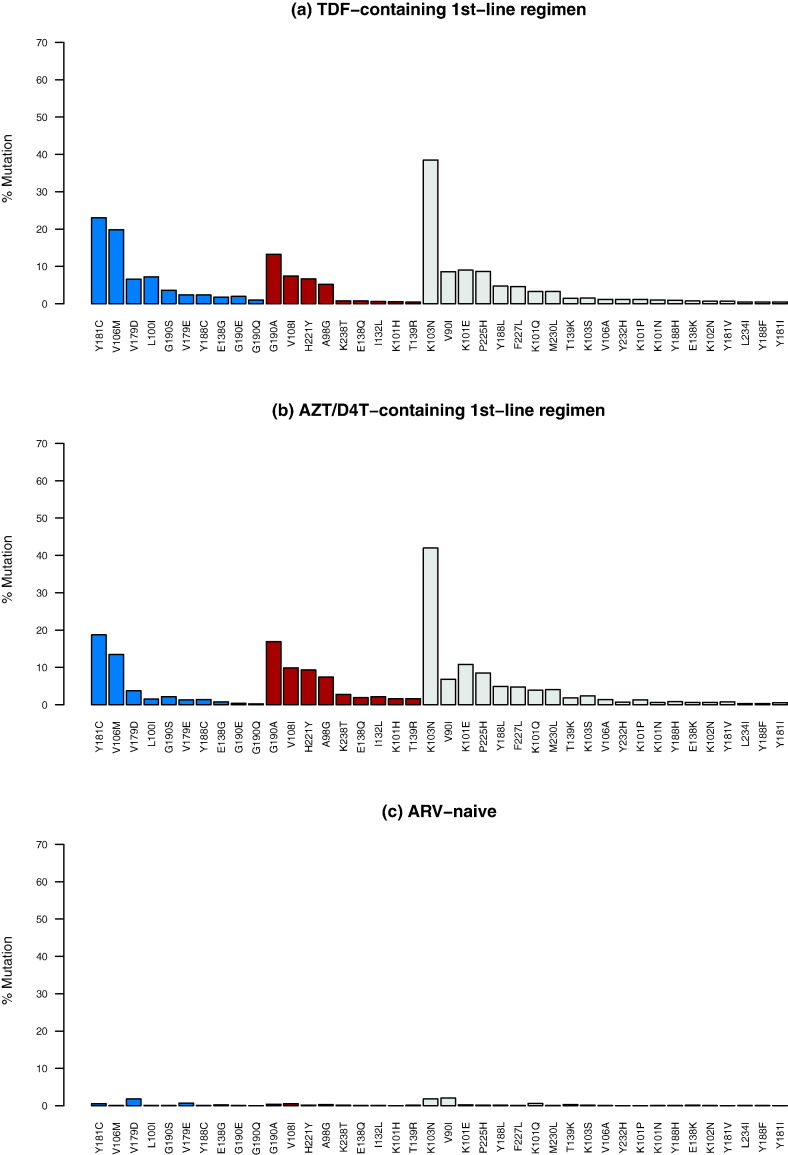
Although ten NNRTI-associated TRAMs occurred significantly more commonly in the TDF group and nine occurred significantly more commonly in the thymidine analog group, the differences in the proportions of these TRAMs were often not large (Fig. 2). Among the unclassified TRAMs, K46Q and E28K occurred more commonly in the TDF group and in the thymidine analog group, respectively (Supplementary Fig. 1).
Fig. 2.
Prevalence of non-nucleoside RT inhibitor (NRTI)-associated tenofovir disoproxil fumarate (TDF)-regimen associated mutations (TRAMs) in 2,873 individuals with virological failure (VF) on a TDF-containing 1st-line regimen (a); in 5,805 individuals with VF on a thymidine analog-containing 1st-line regimen (b); and in 50,803 antiretroviral (ARV)-naive individuals (c). NNRTI TRAMs that were significantly more common in individuals receiving a TDF-containing regimen compared to in individuals receiving a thymidine analog-containing regimen are shown in blue. NNRTI TRAMs that were significantly more common in individuals receiving a thymidine analog-containing regimen compared to in individuals receiving a TDF-containing regimen are shown in brown.
3.3. Covariation
Overall, 149 pairs of TRAMs co-occurred in ten or more individuals and had significant positive phi correlation coefficients (adjusted p < 0.05). Thirty-five (23.5%; 35/149) pairs comprised two NRTI-associated TRAMs, 30 (20.1%; 30/149) comprised two NNRTI-associated TRAMs, 65 (43.6%; 65/149) comprised one NRTI- and one NNRTI-associated TRAMs, and 19 (12.8%; 19/149) comprised at least one TRAM that had not previously been associated with a specific RTI class.
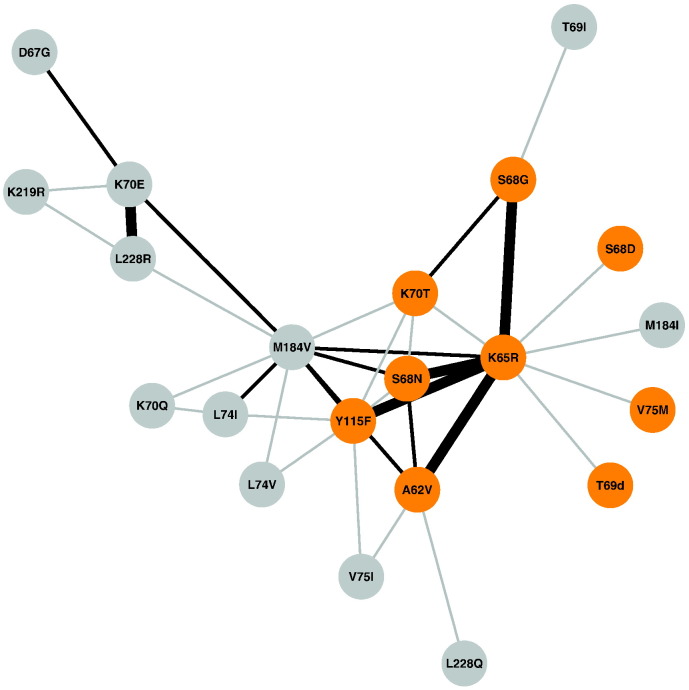
The 35 pairs of significantly correlated NRTI-associated TRAMs included 21 of the 33 NRTI-associated TRAMs (Table 4). The NRTI-associated TRAMs most commonly correlated with other NRTI-associated TRAMs were K65R (n = 10 pairs), M184V (n = 10 pairs), Y115F (n = 7 pairs), A62V (n = 5 pairs), S68N (n = 5 pairs), K70T (n = 5 pairs), K70E (n = 4 pairs), S68G (n = 3 pairs), L74I (n = 3 pairs), and L228R (n = 3 pairs). The most strongly correlated pairs (phi > 0.3) were K65R + A62V, K65R + S68N, K65R + S68G, K65R + Y115F, and K70E + L228R (Fig. 3). The largest clique included the following five TRAMs, which were significantly correlated with one another: M184V, K65R, S68N, K70T and Y115F.
Table 4.
Significantly correlated NRTI-associated TDF-regimen associated mutations (TRAMs) pairs.
| Mut1 | Mut2 | Mut1% | Mut2% | Phi | Mut1 + Mut2 | Mut1 alone | Mut2 alone | Neither | Adjusted p |
|---|---|---|---|---|---|---|---|---|---|
| K70E | L228R | 6.6 | 5.5 | 0.38 | 72 | 86 | 119 | 2596 | 0 |
| A62V | K65R | 10.4 | 39.5 | 0.35 | 268 | 866 | 30 | 1709 | 0 |
| K65R | S68N | 39.5 | 8.1 | 0.35 | 225 | 8 | 909 | 1731 | 0 |
| K65R | S68G | 39.5 | 13.3 | 0.32 | 301 | 80 | 833 | 1659 | 0 |
| K65R | Y115F | 39.5 | 11.9 | 0.31 | 277 | 66 | 857 | 1673 | 0 |
| K70T | S68G | 2.6 | 13.3 | 0.29 | 56 | 325 | 20 | 2472 | 0 |
| M184V | K65R | 48.7 | 39.5 | 0.27 | 740 | 394 | 660 | 1079 | 0 |
| A62V | S68N | 10.4 | 8.1 | 0.25 | 84 | 149 | 214 | 2426 | 0 |
| K70E | D67G | 6.7 | 2.5 | 0.25 | 32 | 39 | 159 | 2642 | 0 |
| M184V | K70E | 48.7 | 6.6 | 0.24 | 178 | 13 | 1222 | 1460 | 0 |
| M184V | L74I | 48.7 | 5.7 | 0.24 | 159 | 6 | 1241 | 1467 | 0 |
| M184V | A62V | 48.7 | 10.4 | 0.22 | 243 | 55 | 1157 | 1418 | 0 |
| M184V | S68N | 48.7 | 8.1 | 0.2 | 193 | 40 | 1207 | 1433 | 0 |
| M184V | Y115F | 48.7 | 11.9 | 0.2 | 258 | 85 | 1142 | 1388 | 0 |
| K65R | K70T | 39.5 | 2.6 | 0.19 | 73 | 3 | 1061 | 1736 | 0 |
| S68G | T69I | 13.3 | 0.6 | 0.19 | 17 | 1 | 364 | 2489 | 0 |
| K70T | S68N | 2.6 | 8.1 | 0.16 | 26 | 207 | 50 | 2590 | 0 |
| L74V | Y115F | 2.6 | 11.9 | 0.15 | 32 | 311 | 44 | 2486 | 2.9E-12 |
| M184V | L228R | 48.7 | 5.5 | 0.15 | 127 | 31 | 1273 | 1442 | 2.9E-12 |
| K65R | S68D | 39.5 | 1.3 | 0.13 | 35 | 2 | 1099 | 1737 | 1.0E-08 |
| K70E | K219R | 6.6 | 1.5 | 0.13 | 14 | 30 | 177 | 2652 | 1.0E-08 |
| K70T | Y115F | 2.6 | 11.9 | 0.12 | 27 | 316 | 49 | 2481 | 4.1E-07 |
| L74I | Y115F | 5.7 | 11.9 | 0.12 | 45 | 298 | 120 | 2410 | 4.1E-07 |
| Y115F | S68N | 11.9 | 8.1 | 0.12 | 59 | 174 | 284 | 2356 | 4.1E-07 |
| M184I | K65R | 9.4 | 39.5 | 0.12 | 157 | 977 | 112 | 1627 | 4.1E-07 |
| M184V | K70Q | 48.7 | 1.6 | 0.11 | 41 | 4 | 1359 | 1469 | 1.2E-05 |
| A62V | V75I | 10.4 | 1.5 | 0.1 | 15 | 29 | 283 | 2546 | 2.7E-04 |
| K70Q | L74I | 1.6 | 5.7 | 0.1 | 11 | 154 | 34 | 2674 | 2.7E-04 |
| M184V | L74V | 48.7 | 2.6 | 0.1 | 60 | 16 | 1340 | 1457 | 2.7E-04 |
| K65R | T69del | 39.5 | 0.6 | 0.1 | 17 | 0 | 1116 | 1738 | 2.7E-04 |
| A62V | L228Q | 10.4 | 1.0 | 0.09 | 11 | 19 | 287 | 2556 | 4.5E-03 |
| K65R | V75M | 39.5 | 2.0 | 0.09 | 40 | 18 | 1094 | 1721 | 4.5E-03 |
| V75I | Y115F | 1.5 | 11.9 | 0.09 | 15 | 328 | 29 | 2501 | 4.5E-03 |
| M184V | K70T | 48.7 | 2.6 | 0.09 | 57 | 19 | 1343 | 1454 | 4.5E-03 |
| K219R | L228R | 1.5 | 5.5 | 0.09 | 10 | 148 | 34 | 2681 | 4.5E-03 |
Pairs of NRTI TRAMs co-occurring in ten or more sequences with a significant Phi correlation coefficient are listed in the order of their p values (an adjusted p < 0.05 by Holm's method). Mut1 + Mut2 contains number of sequences containing Mut1 and Mut2. Mut1 Alone contains number of sequences containing Mut1 but not Mut2. Mut2 Alone contains number of sequences containing Mut2 but not Mut1. Neither contains number of sequences containing neither of Mut1 or Mut2.
Abbreviation: TDF – tenofovir disoproxil fumarate; NRTI - nucleos(t)ide reverse transcriptase inhibitor.
Fig. 3.
Covariation patterns of nucleoside RT inhibitor (NRTI)-associated tenofovir disoproxil fumarate (TDF)-regimen associated mutations (TRAMs) in a network created from the adjacency matrix of phi correlation coefficients for each pair of NRTI TRAMs. Each edge in the network represents a strongly significant correlation (phi ≥ 0.1 and an adjusted p < 0.05) and the thickness of the edge is proportional to the strength of the correlation (phi) of a pair. Shown in orange are the NRTI TRAMs that were strongly associated with K65R: A62V, S68G/N/D, T69deletion, K70T, V75M and Y115F.
K65R was negatively correlated with both K70Q and K70E. There were just two virus sequences with both K65R and K70Q but in each sequence both K65R and K70Q were present as electrophoretic mixtures. There were 21 viruses with both K65R and K70E but in 16 both K65R and K70E were present as electrophoretic mixtures and in five either K65R or K70E was present as an electrophoretic mixture.
The 30 pairs of significantly correlated NNRTI-associated TRAMs and the 84 pairs comprising significant correlations between NRTI- and NNRTI-associated TRAMs are shown in the Supplementary Table 4, and Supplementary Figs. 2 and 3.
3.4. Association of TDF-regimen Associated Mutations (TRAMs) With Viral Load (VL)
VL at the time of VF was available in 32 of 51 studies including 50% (1445/2873) of the TDF study population. The strongest predictor of VL was being from an LMIC: VLs were significantly higher in individuals from LMICs (median 4.8 log10 copies/mL) compared with individuals from UICs (median 3.9 log10 copies/mL; p < 0.001) (Supplementary Table 5). Additional non-genotypic factors significantly associated with an increased VL at VF included receiving 3TC (compared with FTC) and being infected with a non-subtype B virus. Significant genotypic factors associated with an increased VL included the number of TRAMs, the number of NRTI-associated TRAMs, the number of NNRTI-associated TRAMs, M184V/I, K70 mutations, and K65R with and without one or more K65R partners (A62V, S68G/N/D, T69deletion, K70T, V75M and Y115F). However, in our multivariate analysis only region (p < 0.004) and number of NNRTI-associated TRAMs (p < 0.0001) were significant predictors of an increased VL.
4. Discussion
The earliest clinical trials of TDF-containing first-line regimens were performed in UICs (Gallant et al., 2006, 2004). In these trials, few individuals with VF developed RT mutations other than NNRTI-resistance mutations and the cytosine analog resistance mutations M184V/I. K65R was the most commonly occurring TDF-selected mutation, however, it rarely emerged in individuals undergoing frequent virological monitoring in whom therapy was changed when VF was first detected.
As TDF-containing regimens have become preferred for first-line therapy in LMICs (World Health Organization HIV/AIDS Programme, 2013), there have been an increasing number of studies of VF in individuals receiving such a regimen who have undergone less frequent monitoring than performed in clinical trials. We assembled sequences from nearly 3000 individuals in these studies to characterize the spectrum of RT mutations selected in individuals with VF on a WHO recommended TDF-containing first-line regimen. By comparing these sequences to those from a historical control population of ARV-naïve individuals, we identified 83 TRAMs. By comparing the proportion of TRAMs in TDF recipients to their proportions in a historical control population of thymidine analog-treated individuals, we identified 12 TDF-selected TRAMs.
The 12 TDF-selected TRAMs included A62V, K65R/N, S68G/D/N, K70E/Q/T, L74I, V75L, and Y115F. The most commonly occurring of these were K65R (39.5%; 1134/2873), S68G/N (21.4%; 614/2873), Y115F (11.9%; 343/2873), K70E/Q/T (10.9%; 312/2873), A62V (10.4%; 298/2873), and L74I (6.4%; 165/2873).
Because most of the additional TDF-selected TRAMs usually occurred in combination with K65R, the overall prevalence of one or more of the 12 TDF-selected TRAMs in this study was only slightly higher than the overall prevalence of K65R/N and/or K70E/G/Q in individuals with VF on a TDF-containing first-line WHO recommended regimen (54% vs. 47%; 1551/2873 vs. 1350/2873). However, previously published data outlined below suggest that these additional mutations may confer greater reductions in TDF susceptibility than K65R alone.
Several of the 12 TDF-selected TRAMs have been previously associated with K65R, TDF selection pressure, or reduced in vitro TDF susceptibility. Specifically, A62V and S68G have been reported to improve the replication of viruses with K65R (Svarovskaia et al., 2008). S68N has been selected in vitro by TDF and shown to further reduce TDF susceptibility when present with K65R (Margot et al., 2006). Y115F, although it is primarily an abacavir (ABC)-resistance mutation, has been selected in vitro by TDF and shown to reduce TDF susceptibility (Margot et al., 2006, Melikian et al., 2012, Stone et al., 2004). L74I, which is also primarily an ABC-resistance mutation, has been reported to be selected by TDF (Wirden et al., 2009).
K70E/G/Q/N/T have previously been reported to occur rarely in individuals receiving d4T, ABC, and TDF-containing regimens and to be associated with slightly reduced susceptibility to these NRTIs (Tang et al., 2013, Delaugerre et al., 2008, Sluis-Cremer et al., 2007, Hachiya et al., 2011). In this study, we observed a significant association with a TDF-containing regimen for each of these mutations. Of these, K70G occurred in just four individuals (Supplementary Table 2). K70E has been reported to rarely occur in combination with K65R because of the reduced replication fitness when both mutations occur in the same virus (Kagan et al., 2007). We confirmed this observation and demonstrated that the same antagonistic interaction exists between K65R and K70Q. Their negative association and the high proportion of mixed bases in cases where they did co-occur suggest they rarely occur in the same virus genomes. The same antagonistic interaction was not observed between K65R and K70T/N.
Of the cytosine analog resistance mutations, M184V occurred more commonly in the thymidine analog group whereas M184I occurred more commonly in the TDF group. M184I has been reported to often precede the development of M184V among individuals receiving a cytosine analog (Back et al., 1996). In these individuals, M184V eventually replaces M184I because its replication capacity is less attenuated than that of M184I. We speculate that M184I occurred more commonly in the TDF group compared with the thymidine analog group because the duration of therapy was shorter in the TDF group – possibly because of more frequent virological monitoring in this somewhat more recent cohort. Consistent with this is the fact that the thymidine analog cohort contained a higher proportion of individuals from LMICs (88% vs. 72%; 5082/5805 vs. 2071/2873) and preceded the TDF cohort by a median of four years (2008 vs. 2012) (Table 1).
The association between subtypes and TRAMs was most notable for an increased proportion of several TRAMs in subtype C and less commonly CRF01_AE, and G and a decreased proportion of many TRAMs in subtype B viruses. However, this analysis is likely to be confounded by the fact that fewer number of viruses was available for subtype G, A and CRF01_AE and that subtype B was more common in the UICs where virological monitoring has been frequent and the time on a regimen following VF was likely to have been shorter.
The established TDF-resistance mutations K65R and K70E have been shown to attenuate virus replication in vitro and in animal models (Miller et al., 2002, Van Rompay et al., 2007, White et al., 2002, Sluis-Cremer et al., 2007). To determine whether these and other TDF-selected TRAMs are associated with reduced VL and a potentially reduced risk of TDF resistance transmission, we attempted to correlate specific TDF-selected TRAMs with VL at the time of VF. However, this analysis was limited by the availability of VL data in only half of study individuals and the likely differences in the frequency of viral load monitoring in different study populations. Although LMIC region and the number of NNRTI-associated TRAMs appeared to be independently associated with an increased VL at VF in our multivariate analysis, the limited availability of VL data prevents us from drawing any conclusions about the potential transmissibility of viruses containing TDF-selected TRAMs.
The widely used list of DRMs for the surveillance of transmitted drug-resistance (SDRMs) was developed at a time when the number of available sequences from individuals with VF on a first-line TDF-containing regimen was low (Bennett et al., 2009). Of the 12 TDF-selected TRAMs, the SDRM list includes the established TDF-resistance mutations K65R and K70E, and the established ABC-resistance mutations L74I and Y115F. The remaining eight TDF-selected TRAMs are not on the SDRM list. Two of these TRAMs are polymorphic and are therefore not SDRM candidates for monitoring transmitted drug-resistance: S68G occurs in 1.6% to 6.6% of ARV-naïve individuals depending on subtype and A62V is the consensus residue in subtype A viruses in the Former Soviet Union countries (Rhee et al., 2003). The remaining six TDF-selected TRAMs, K65N, S68N/D, K70Q/T, and V75L are nonpolymorphic and should be considered for possible addition to the SDRM list.
In conclusion, this study shows that the spectrum of TDF-selected mutations extends beyond K65R/N and K70E/G/Q. Several of the additional TDF-selected mutations are nonpolymorphic mutations that are currently not considered surveillance DRMs yet may be important for monitoring TDF-associated transmitted drug-resistance (Bennett et al., 2009). Additionally, the clinical significance of several mutations that occurred commonly in combination with K65R including A62V, S68G/N/D, L74I, V75L and Y115F requires further phenotypic and clinical studies to better understand their effects on both TDF and the newly developed prodrug tenofovir alafenamide (TAF) (Margot et al., 2016).
Funding Sources
This study was funded in part by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Number NIAID AI068581. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflicts of Interest
GH reports receipt of grants from the Swiss National Science Foundation, Swiss HIV Cohort Study, University of Zurich, Yvonne Jacob Foundation, and Gilead Sciences outside of the submitted work; fees for data and safety monitoring board membership from Merck outside of the submitted work; consulting/advisory board membership fees from Gilead Sciences outside of the submitted work; and travel reimbursement from Gilead, Bristol-Myers Squibb, and Janssen outside of the submitted work. JMS has received research support, honorarium or consulting fees from Abbvie, Merck, Gilead Sciences, GlaxoSmithKline, Tibotec-Janssen, BMS, Teva, Virology Education and ViiV Healthcare outside of the submitted work; and travel support and stipends for advisory work for the World Health Organization outside of the submitted work. MAE became a Merck employee in December of 2016. RKG has received honoraria for non-promotional lectures from Gilead outside of the submitted work. RWS has received research support and consulting fees from Gilead Sciences, Bristol-Myers Squibb, Merck, and ViiV outside of the submitted work. VCM has received funding from Gilead, Bayer and ViiV outside of the submitted work.
Author Contributions
RKG and RWS conceived the study; RKG, RWS, and SYR designed the study; RWS, SPH, and SYR analyzed data; AJ, BK, CC, CJH, CR, CY, DAC, DG, DS, FG, GH, GUvanZ, HFG, HS, JdAN, JMS, KCES, KS, LS, LS, MAB, MAE, MMS, MT, NGHN, NN, PG, PK, PK, RK, RKG, RLH, RWS, SC, SN, SS, SS, SYR, TdeO, VCM, and VV generated data; RKG, RWS and SYR wrote the manuscript; and all authors interpreted data, and approved the final report.
Acknowledgments
We acknowledge University of North Carolina at Chapel Hill (UNC) Center for AIDS Research (CFAR) and ACTG (U01AI068636) team for making data available. We also acknowledge Dr. Ricardo Camacho for providing Portugal cohort data for this study.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ebiom.2017.03.024.
Appendix A. Supplementary Data
Supplementary material
References
- Back N.K., Nijhuis M., Keulen W., Boucher C.A., Oude Essink B.O., Van Kuilenburg A.B., Van Gennip A.H., Berkhout B. Reduced replication of 3TC-resistant HIV-1 variants in primary cells due to a processivity defect of the reverse transcriptase enzyme. EMBO J. 1996;15:4040–4049. [PMC free article] [PubMed] [Google Scholar]
- Bennett D.E., Camacho R.J., Otelea D., Kuritzkes D.R., Fleury H., Kiuchi M., Heneine W., Kantor R., Jordan M.R., Schapiro J.M., Vandamme A.M., Sandstrom P., Boucher C.A., Van De Vijver D., Rhee S.Y., Liu T.F., Pillay D., Shafer R.W. Drug resistance mutations for surveillance of transmitted HIV-1 drug-resistance: 2009 update. PLoS One. 2009;4:e4724. doi: 10.1371/journal.pone.0004724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaugerre C., Flandre P., Marcelin A.G., Descamps D., Tamalet C., Cottalorda J., Schneider V., Yerly S., Legoff J., Morand-Joubert L., Chaix M.L., Costagliola D., Calvez V. National survey of the prevalence and conditions of selection of HIV-1 reverse transcriptase K70E mutation. J. Med. Virol. 2008;80:762–765. doi: 10.1002/jmv.21158. [DOI] [PubMed] [Google Scholar]
- Gallant J.E., Dejesus E., Arribas J.R., Pozniak A.L., Gazzard B., Campo R.E., Lu B., Mccoll D., Chuck S., Enejosa J., Toole J.J., Cheng A.K., Study G. Tenofovir DF, emtricitabine, and efavirenz vs. zidovudine, lamivudine, and efavirenz for HIV. N. Engl. J. Med. 2006;354:251–260. doi: 10.1056/NEJMoa051871. [DOI] [PubMed] [Google Scholar]
- Gallant J.E., Staszewski S., Pozniak A.L., Dejesus E., Suleiman J.M., Miller M.D., Coakley D.F., Lu B., Toole J.J., Cheng A.K. Efficacy and safety of tenofovir DF vs stavudine in combination therapy in antiretroviral-naive patients: a 3-year randomized trial. JAMA. 2004;292:191–201. doi: 10.1001/jama.292.2.191. [DOI] [PubMed] [Google Scholar]
- Gonzales M.J., Wu T.D., Taylor J., Belitskaya I., Kantor R., Israelski D., Chou S., Zolopa A.R., Fessel W.J., Shafer R.W. Extended spectrum of HIV-1 reverse transcriptase mutations in patients receiving multiple nucleoside analog inhibitors. AIDS. 2003;17:791–799. doi: 10.1097/01.aids.0000050860.71999.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregson J., Kaleebu P., Marconi V.C., Van Vuuren C., Ndembi N., Hamers R.L., Kanki P., Hoffmann C.J., Lockman S., Pillay D., De Oliveira T., Clumeck N., Hunt G., Kerschberger B., Shafer R.W., Yang C., Raizes E., Kantor R., Gupta R.K. Occult HIV-1 drug resistance to thymidine analogues following failure of first-line tenofovir combined with a cytosine analogue and nevirapine or efavirenz in sub Saharan Africa: a retrospective multi-centre cohort study. Lancet Infect. Dis. 2016;17:296–304. doi: 10.1016/S1473-3099(16)30469-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hachiya A., Kodama E.N., Schuckmann M.M., Kirby K.A., Michailidis E., Sakagami Y., Oka S., Singh K., Sarafianos S.G. K70Q adds high-level tenofovir resistance to "Q151M complex" HIV reverse transcriptase through the enhanced discrimination mechanism. PLoS One. 2011;6:e16242. doi: 10.1371/journal.pone.0016242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm S. A simple sequentially rejective multiple test procedure. Scand. J. Stat. 1979;6:65–70. [Google Scholar]
- Kagan R.M., Lee T.S., Ross L., Lloyd R.M., Jr., Lewinski M.A., Potts S.J. Molecular basis of antagonism between K70E and K65R tenofovir-associated mutations in HIV-1 reverse transcriptase. Antivir. Res. 2007;75:210–218. doi: 10.1016/j.antiviral.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Margot N.A., Liu Y., Miller M.D., Callebaut C. High resistance barrier to tenofovir alafenamide is driven by higher loading of tenofovir diphosphate into target cells compared to tenofovir disoproxil fumarate. Antivir. Res. 2016;132:50–58. doi: 10.1016/j.antiviral.2016.05.012. [DOI] [PubMed] [Google Scholar]
- Margot N.A., Waters J.M., Miller M.D. In vitro human immunodeficiency virus type 1 resistance selections with combinations of tenofovir and emtricitabine or abacavir and lamivudine. Antimicrob. Agents Chemother. 2006;50:4087–4095. doi: 10.1128/AAC.00816-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melikian G.L., Rhee S.Y., Taylor J., Fessel W.J., Kaufman D., Towner W., Troia-Cancio P.V., Zolopa A., Robbins G.K., Kagan R., Israelski D., Shafer R.W. Standardized comparison of the relative impacts of HIV-1 reverse transcriptase (RT) mutations on nucleoside RT inhibitor susceptibility. Antimicrob. Agents Chemother. 2012;56:2305–2313. doi: 10.1128/AAC.05487-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menendez-Arias L. A structural frame for understanding the role of thymidine analogue resistance mutations in resistance to zidovudine and other nucleoside analogues. Antivir. Ther. 2011;16:943–946. doi: 10.3851/IMP1889. [DOI] [PubMed] [Google Scholar]
- Miller V., Stark T., Loeliger A.E., Lange J.M. The impact of the M184V substitution in HIV-1 reverse transcriptase on treatment response. HIV Med. 2002;3:135–145. doi: 10.1046/j.1468-1293.2002.00101.x. [DOI] [PubMed] [Google Scholar]
- Pineda-Pena A.C., Faria N.R., Imbrechts S., Libin P., Abecasis A.B., Deforche K., Gomez-Lopez A., Camacho R.J., De Oliveira T., Vandamme A.M. Automated subtyping of HIV-1 genetic sequences for clinical and surveillance purposes: performance evaluation of the new REGA version 3 and seven other tools. Infect. Genet. Evol. 2013;19:337–348. doi: 10.1016/j.meegid.2013.04.032. [DOI] [PubMed] [Google Scholar]
- Rhee S.Y., Blanco J.L., Jordan M.R., Taylor J., Lemey P., Varghese V., Hamers R.L., Bertagnolio S., Rinke de Wit T.F., Aghokeng A.F., Albert J., Avi R., Avila-Rios S., Bessong P.O., Brooks J.I., Boucher C.A., Brumme Z.L., Busch M.P., Bussmann H., Chaix M.L., Chin B.S., D'aquin T.T., De Gascun C.F., Derache A., Descamps D., Deshpande A.K., Djoko C.F., Eshleman S.H., Fleury H., Frange P., Fujisaki S., Harrigan P.R., Hattori J., Holguin A., Hunt G.M., Ichimura H., Kaleebu P., Katzenstein D., Kiertiburanakul S., Kim J.H., Kim S.S., Li Y., Lutsar I., Morris L., Ndembi N., Ng K.P., Paranjape R.S., Peeters M., Poljak M., Price M.A., Ragonnet-Cronin M.L., Reyes-Teran G., Rolland M., Sirivichayakul S., Smith D.M., Soares M.A., Soriano V.V., Ssemwanga D., Stanojevic M., Stefani M.A., Sugiura W., Sungkanuparph S., Tanuri A., Tee K.K., Truong H.M., Van De Vijver D.A., Vidal N., Yang C., Yang R., Yebra G., Ioannidis J.P., Vandamme A.M., Shafer R.W. Geographic and temporal trends in the molecular epidemiology and genetic mechanisms of transmitted HIV-1 drug resistance: an individual-patient- and sequence-level meta-analysis. PLoS Med. 2015;12 doi: 10.1371/journal.pmed.1001810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee S.Y., Gonzales M.J., Kantor R., Betts B.J., Ravela J., Shafer R.W. Human immunodeficiency virus reverse transcriptase and protease sequence database. Nucleic Acids Res. 2003;31:298–303. doi: 10.1093/nar/gkg100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee S.Y., Sankaran K., Varghese V., Winters M.A., Hurt C.B., Eron J.J., Parkin N., Holmes S.P., Holodniy M., Shafer R.W. HIV-1 protease, reverse transcriptase, and integrase variation. J. Virol. 2016;90:6058–6070. doi: 10.1128/JVI.00495-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluis-Cremer N., Sheen C.W., Zelina S., Torres P.S., Parikh U.M., Mellors J.W. Molecular mechanism by which the K70E mutation in human immunodeficiency virus type 1 reverse transcriptase confers resistance to nucleoside reverse transcriptase inhibitors. Antimicrob. Agents Chemother. 2007;51:48–53. doi: 10.1128/AAC.00683-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone C., Ait-Khaled M., Craig C., Griffin P., Tisdale M. Human immunodeficiency virus type 1 reverse transcriptase mutation selection during in vitro exposure to tenofovir alone or combined with abacavir or lamivudine. Antimicrob. Agents Chemother. 2004;48:1413–1415. doi: 10.1128/AAC.48.4.1413-1415.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svarovskaia E.S., Feng J.Y., Margot N.A., Myrick F., Goodman D., Ly J.K., White K.L., Kutty N., Wang R., Borroto-Esoda K., Miller M.D. The A62V and S68G mutations in HIV-1 reverse transcriptase partially restore the replication defect associated with the K65R mutation. J. Acquir. Immune Defic. Syndr. 2008;48:428–436. doi: 10.1097/QAI.0b013e31817bbe93. [DOI] [PubMed] [Google Scholar]
- Tang M.W., Rhee S.Y., Bertagnolio S., Ford N., Holmes S., Sigaloff K.C., Hamers R.L., De Wit T.F., Fleury H.J., Kanki P.J., Ruxrungtham K., Hawkins C.A., Wallis C.L., Stevens W., Van Zyl G.U., Manosuthi W., Hosseinipour M.C., Ngo-Giang-Huong N., Belec L., Peeters M., Aghokeng A., Bunupuradah T., Burda S., Cane P., Cappelli G., Charpentier C., Dagnra A.Y., Deshpande A.K., El-Katib Z., Eshleman S.H., Fokam J., Gody J.C., Katzenstein D., Koyalta D.D., Kumwenda J.J., Lallemant M., Lynen L., Marconi V.C., Margot N.A., Moussa S., Ndung'u T., Nyambi P.N., Orrell C., Schapiro J.M., Schuurman R., Sirivichayakul S., Smith D., Zolfo M., Jordan M.R., Shafer R.W. Nucleoside reverse transcriptase inhibitor resistance mutations associated with first-line stavudine-containing antiretroviral therapy: programmatic implications for countries phasing out stavudine. J. Infect. Dis. 2013;207(Suppl. 2):S70–S77. doi: 10.1093/infdis/jit114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenores Study G. Global epidemiology of drug resistance after failure of WHO recommended first-line regimens for adult HIV-1 infection: a multicentre retrospective cohort study. Lancet Infect. Dis. 2016;16:565–575. doi: 10.1016/S1473-3099(15)00536-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Rompay K.K., Johnson J.A., Blackwood E.J., Singh R.P., Lipscomb J., Matthews T.B., Marthas M.L., Pedersen N.C., Bischofberger N., Heneine W., North T.W. Sequential emergence and clinical implications of viral mutants with K70E and K65R mutation in reverse transcriptase during prolonged tenofovir monotherapy in rhesus macaques with chronic RT-SHIV infection. Retrovirology. 2007;4:25. doi: 10.1186/1742-4690-4-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wensing A.M., Calvez V., Gunthard H.F., Johnson V.A., Paredes R., Pillay D., Shafer R.W., Richman D.D. 2015 update of the drug resistance mutations in HIV-1. Top Antivir. Med. 2015;23:132–141. [PMC free article] [PubMed] [Google Scholar]
- White K.L., Margot N.A., Wrin T., Petropoulos C.J., Miller M.D., Naeger L.K. Molecular mechanisms of resistance to human immunodeficiency virus type 1 with reverse transcriptase mutations K65R and K65R + M184V and their effects on enzyme function and viral replication capacity. Antimicrob. Agents Chemother. 2002;46:3437–3446. doi: 10.1128/AAC.46.11.3437-3446.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirden M., Lambert-Niclot S., Marcelin A.G., Schneider L., Ait-Mohand H., Brunet C., Angleraud F., Amard S., Katlama C., Calvez V. Antiretroviral combinations implicated in emergence of the L74I and L74V resistance mutations in HIV-1-infected patients. AIDS. 2009;23:95–99. doi: 10.1097/QAD.0b013e328319bc91. [DOI] [PubMed] [Google Scholar]
- World Health Organization Hiv/Aids Programme . 2013. Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material