Abstract
High-dose chemotherapies to treat multiple myeloma (MM) can be life-threatening due to toxicities to normal cells and there is a need to target only tumor cells and/or lower standard drug dosage without losing efficacy. We show that pharmacologically-dosed ascorbic acid (PAA), in the presence of iron, leads to the formation of highly reactive oxygen species (ROS) resulting in cell death. PAA selectively kills CD138+ MM tumor cells derived from MM and smoldering MM (SMM) but not from monoclonal gammopathy undetermined significance (MGUS) patients. PAA alone or in combination with melphalan inhibits tumor formation in MM xenograft mice. This study shows PAA efficacy on primary cancer cells and cell lines in vitro and in vivo.
Keywords: Multiple myeloma, Pharmacologically-dosed ascorbic acid, Apoptosis-inducing factor 1
Abbreviation: MM, multiple myeloma; ROS, reactive oxygen species; SMM, smoldering MM; IMIDs, immunomodulatory drugs; ASCT, autologous stem cell transplantation; CRs, complete remissions; PAA, pharmacologically dosed ascorbic acid; LIP, labile iron pool; GEP, gene expression profiling; BM, bone marrow; IVIS, in vivo imaging system; WT, wild-type; DFO, deferoxamine; AIF1, apoptosis inducible factor 1
Highlights
-
•
Pharmacologically-dosed ascorbic acid kills Multiple Myeloma cells.
-
•
Pharmacologically-dosed ascorbic leads to apoptosis-inducing factor 1 cleavage.
-
•
Pharmacologically-dosed ascorbic lowers melphalan dosage.
Multiple myeloma (MM) remains a difficult to cure disease in the majority of cases. Several preclinical and clinical studies have shown that ascorbic acid in pharmacologic doses (PAA) selectively kills cancer cells, while sparing normal cells. This article reveals the biological mechanism by which PAA exerts its anti-cancer effects and should lead to the development of an innovative therapy in MM.
1. Introduction
Multiple myeloma (MM) is a plasma cell neoplasm. Four active classes of drugs including glucocorticoids, DNA alkylators (melphalan), proteasome inhibitors (bortezomib and carfilzomib) and immunomodulatory agents (thalidomide, lenalidomide, and pomalidomide), combined with or without autologous stem cell transplantation (ASCT) have led to complete remissions (CRs) in the large majority of newly diagnosed patients with MM (Alexanian et al., 2014, Fu et al., 2013, Terpos et al., 2014, Wang et al., 2014, Sonneveld et al., 2013, Gay et al., 2013, Liu et al., 2013, Bergsagel, 2014). These treatments have greatly improved patient progression-free and overall survival. However, there are at least three major problems limiting the administration of these agents: 1. All these drugs target both tumor and non-tumor cells; 2. Increased hematologic toxicity has been identifined by combining alkylators with either immunomodulatory drugs (IMIDs) (Bergsagel, 2014); and 3. High doses of the DNA alkalating agent, such as melphalan, have strong cytotoxicity on gut epithelial cells and hematopoietic stem cells (Shaw et al., 2014). One way to deal with non-selective toxicity of high dose melphalan is to combine it with another agent which very specifically targets tumor cells and therefore decreasing melphalan dosing without loss of efficacy.
In the 1970s, Cameron and Pauling reported that high doses of vitamin C increased survival of patients with cancer (Cameron and Pauling, 1976, Cameron and Pauling, 1978). Recently, reports have shown that pharmacologically dosed ascorbic acid (PAA) 50–100 g (Chen et al., 2008, Padayatty et al., 2004, Hoffer et al., 2008, Padayatty et al., 2006, Welsh et al., 2013), administered intravenously, has potent anti-cancer activity and its role as anti-cancer therapy is being studied at the University of Iowa and in other centers (Du et al., 2012, Ma et al., 2014). In the presence of catalytic metal ions like iron, PAA administered intravenously exerts pro-oxidant effects leading to the formation of highly reactive oxygen species (ROS), resulting in cell death (Yun et al., 2015, Ma et al., 2014, Du et al., 2012, Chen et al., 2007, Chen et al., 2005). In a previous study, we have reported that the labile iron pool (LIP) is significantly elevated in MM cells (Gu et al., 2015), suggesting that PAA treatment should target MM cells quite selectively. The higher LIP is the direct result of the low expression of the only known mammalian cellular iron exporter, Ferroportin 1 (Fpn1), in MM as demonstrated by our group (Gu et al., 2015). These findings led us to the hypothesis that PAA might specifically target MM cells with high iron content and may also act synergistically in combination with commonly used MM therapies.
2. Materials and Methods
2.1. Patients Samples
Peripheral-blood samples or bone marrow aspirates were obtained from patients with monoclonal gammopathy of undetermined significance (MGUS), smoldering multiple myeloma (SMM), and multiple myeloma (MM). Written informed consent was obtained from all participants. The de-identified clinical specimens in this study were approved by the institutional review board at the University of Iowa (HawkIRB protocol 201302833).
2.2. Gene Expression
Gene expression profiling (GEP) has been described previously (Zhan et al., 2006, Shaughnessy et al., 2007). The GEP access number of normal plasma cell (NPC), MGUS, and primary myeloma samples is GSE2658.
2.3. Viability Assay
Pharmacological ascorbic acid (PAA) was kindly provided by Dr. Garry R. Buettner (University of Iowa). Dr. Buettner prepares PAA as previously described (Du et al., 2010). Briefly, l-ascorbic acid was from MACRON Fine Chemicals/Avantor Performance Materia (Center Valley, PA, USA). A stock solution of 1.0 M ascorbate in de-ionized water (pH adjusted to 7.0 with NaOH) was made under argon and stored in a volumetric flask with a tight-fitting stopper at 4 °C. Ascorbate concentration was checked at 265 nm, ε = 14,500 M− 1 cm− 1(Buettner, 1998). The solution can be kept for several weeks without significant loss of ascorbate due to the lack of oxygen. CD138+ MM cells and CD138− non-MM cells were isolated from MGUS, SMM, and MM patient samples using anti-CD138 immunomagnetic beads (Miltenyl Biotec, Auburn, CA). Cells were cultured with or without PAA at the described concentration for 1 h. After incubation, the cells were washed and cultured up to 24 h. Cell counts and viable cell number were determined using Trypan Blue staining.
2.4. Xenograft Mouse Model
The animal study was performed according to the guidelines of the Institutional Animal Care and local veterinary office and ethics committee of the University of Iowa, USA under approved protocol (IACUC 5081482). NOD.Cγ-Rag1 mice 6–8 weeks old (Jackson Laboratory, Bar Harbor, Maine) were injected intravenously with ARP1 MM cells (1 × 106) expressing luciferase. After one-week injection of ARP1 cells, mice were treated with either PAA (4 mg/kg) injected intraperitoneal once a day, 5 days every week for 3 weeks. Melphalan (3 mg/kg) was injected intraperitoneal once a day, 2 days a week for 3 weeks (Sanchez et al., 2012). Carfilzomib (3 mg/kg) was injected by in vein once a day, 2 days every week for 3 weeks (Eda et al., 2014). Bortezomib (3 mg/kg) was injected intraperitoneal once a day, 2 days a week for 3 weeks. The mice were euthanized when a humane endpoint was reached.
2.5. In Vivo Imaging System
Xenogen IVIS-200, an in vivo imaging system (IVIS), was used to analyze tumor burden and was indicated by quantification of luciferase intensity of mice pre- and post-treatments.
2.6. Cell Culture
Human myeloma cell lines (ARP1, OCI-MY5 and their derivative cell lines) were cultured in RPMI 1640 (Invitrogen, Carlsbad, CA), supplemented with 10% heat-inactivated FBS (Invitrogen), penicillin (100 IU/mL), and streptomycin (100 μg/mL) in a humidified incubator at 37 °C and 5% CO2/95% air. To increase cellular iron concentration, ferric nitrilotriacatate (Fe-NTA) was added in the cell culture media.
2.7. Western Blotting
Cells were harvested and lysed with lysis buffer: 150 mM NaCl, 10 mM EDTA, 10 mM Tris, pH 7.4, 1% X-100 Triton. Cell lysates were subjected to SDS-PAGE, transferred onto a pure nitrocellulose membrane (BioRad) and blocked with 5% fat-free milk. Primary antibodies for immunoblotting included: anti-AIF1 (1:1000, Cell Signaling), anti-RIP (1:1000, Santa Cruz Biotechnology), anti-RIP3 (1:1000, Cell Signaling), anti-Caspase3 (1:1000, Cell Signaling), anti-Caspase 8 (1:1000, Cell Signaling), anti-Caspase 9 (1:1000, Cell Signaling), phosphorylated γ-H2AX (1:1000, Enzo Life Sciences), and β-actin (1:1000, Cell Signaling) as loading control. Membranes were incubated with horseradish peroxidase (HRP)-conjugated anti-mouse secondary antibody (1:10,000, Santa Cruz Biotechnology, cat#: sc-2005) or anti-rabbit secondary antibody (1: 10,000, AnaSpec Inc., cat#: AS-28177) for 1 h and chemi-luminescence signals were detected by HRP substrate (EMD Millipore). Pan-caspase inhibitor Q-VD-OPh (Sigma-Aldrich, MO) was at final concentration of 25 μM.
2.8. Statistical Analysis
GEP data were analyzed by one-way ANOVA test using log2 transformed Affymetrix Signals and presented by boxplot. The comparisons of tumor burden were analyzed either by student t-test (2 groups) or by one-way ANOVA test (> 2 groups). Kaplan-Meier test was performed for survival with the use of SPSS 16.0 software (SPSS, Chicago, IL). Two-tailed p value at an alpha level of 0.05 was considered to indicate statistical significance. Graphs were generated using Prism 6 software.
2.9. Electron Microscopy
Electron microscopy was performed by the Central Microscopy Research Facility personnel at the University of Iowa. Images were captured on JEOL JEM 1230.
3. Results
3.1. Pharmacological Ascorbic Acid Selectively Kills Primary Myeloma Cells
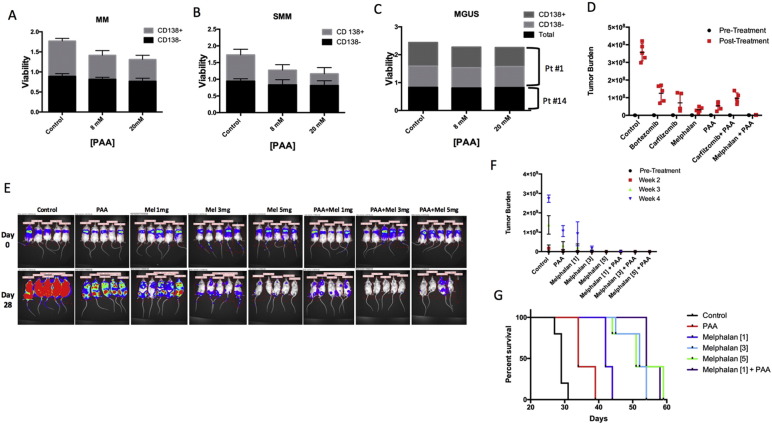
We analyzed the response to PAA of both CD138+ primary MM cells (high cytosolic iron) and CD138− non-MM bone marrow (BM) cells obtained from 13 patients. The 13 patients included 2 pre-cancer of monoclonal gammopathy of undetermined significance (MGUS), 2 smoldering MM (SMM) and 9 MM patients. Patient demographic, disease characteristics and therapy are listed in Supplementary Table 1 and Supplementary Fig. 1. The survival of CD138+ cells in vitro was significantly decreased following PAA treatment in all 9 MM (Fig. 1A, grey bars, p < 0.01). In contrast, no significant change of cell viability was observed in CD138− BM cells from the same patients (Fig. 1A, black bars). The same effect of PAA was also observed in the SMM patients (Fig. 1B). However, almost no response to PAA was detected in CD138+ cells from the 2 MGUS patients (Fig. 1C). We predicted that this would be the case because MGUS patients have much lower cytosolic iron compared to MM patients (Supplementary Fig. 2) as the consequence of lower expression of transferrin receptor 1 (TfR1), the cellular iron receptor-mediated importer (Supplementary Fig. 2A), and higher expression of Ferroportin 1 (Fpn1), the iron exporter (Supplementary Fig. 2B).
Fig. 1.
Pharmacologic ascorbic acid selectively kills tumor cells in MM and acts synergistically with melphalan in vivo. (A) Bar-view presents cell viability between CD138+ tumor cells and CD138− non-tumor cells treated with either PAA (8, 20 mM) or PBS (control) from MM patients (p < 0.01), SMM (B) and MGUS patients (C). (D) Xenografted NOD.Cγ-Rag1 mice were treated with PAA and in combination or not with melphalan, carfilzomib and bortezomib. After one-week injection of ARP1 cells, mice were treated with either PAA (4 mg/kg) injected intraperitoneal once a day, 5 days every week for 3 weeks. Melphalan (3 mg/kg) was injected intraperitoneal once a day, 2 days a week for 3 weeks. Carfilzomib (3 mg/kg) was injected by in vein once a day, 2 days every week for 3 weeks. Total flux indicates quantification of luciferase intensity (tumor burden) of mice pre- and post-PAA treatment at different time points. (E & F) Tumor burden was analyzed in ARP1 NOD.Cγ-Rag1 mice treated with PAA and with or without different doses of melphalan (1, 3, 5 mg/kg). (G) Treatment-related Kaplan-Meier survival curves of mice. The log-rank test was performed and indicated that mouse survivals among these groups are significantly different (p < 0.001) and that PAA, when combined with low dose of melphalan, extends MM mouse survival.
3.2. Pharmacological Ascorbic Acid Decreases Melphalan Doses in Myeloma Treatment
To investigate whether PAA may be effective in killing MM cells alone and when combined with currently used MM therapies. ARP1 MM cells expressing luciferase were injected intravenously into NOD.Cγ-Rag1 mice. Seven combinations (control, PAA, melphalan, carfilzomib, melphalan + PAA, carfilzomib + PAA and bortezomib) were tested in vivo (Sanchez et al., 2012, Eda et al., 2014). Tumor progression was monitored by an in vivo imaging system (IVIS). Compared to the control group, all treatments inhibited MM cell growth significantly (p < 0.05) (Fig. 1D). Within the single agent treatments, melphalan only showed a higher decrease in tumor burden when compared to PAA treatment and other single agents tested. Also, the combination of melphalan plus PAA showed greater tumor burden reduction than each drug alone, suggesting a synergistic activity between these two drugs (p < 0.05). Under the experimental conditions described in the Fig.1, no additive effect was found when PAA was combined with carfilzomib (p > 0.05). Bortezomib was not given in combination with PAA because ascorbic acid directly inactivates bortezomib by forming a tight and reversible complex through its vicinal diol group (Perrone et al., 2009, Harvey et al., 2009).
The above results showing that a synergistic effect was observed in MM treatment by combination of PAA with melphalan encouraged us to further determine if PAA addition would allow a decrease in melphalan dosing without losing efficacy. Therefore, mice were treated with 3 different doses of melphalan (1, 3, and 5 mg/kg) plus PAA. Tumor burden at three weeks of treatment showed that a single agent melphalan at the lowest dose was able to inhibit tumor growth better than PAA alone (Fig. 1E & F). Further, the presence of tumor at the highest dose of melphalan was detected only after four weeks confirming that the high dose of melphalan had greater anti-tumor effect. In contrast, no difference in outcome was observed when melphalan was combined with PAA even at the lowest dose. Reduction of mouse weight was not observed suggesting lack of toxicity (Supplementary Fig. 3). Tumor burden was almost undetectable in mice treated with any of the three combinational therapies (Fig. 1F). Survival curves confirmed that high doses of single agent melphalan (3 and 5 mg/kg) extended MM mouse survival (Fig. 1G) better than PAA alone. However, the combination of PAA with low-dose melphalan (1 mg/kg) extended MM mouse survival significantly compared with low-dose melphalan alone (Fig. 1G; p < 0.05). Importantly, no survival differences were observed between low and high doses of melphalan when given in combination with PAA (Fig.1G and Supplementary Fig. 4).
3.3. The Therapeutic Effect of Pharmacological Ascorbic Acid Depends on Cellular Iron and Reactive Oxygen Species
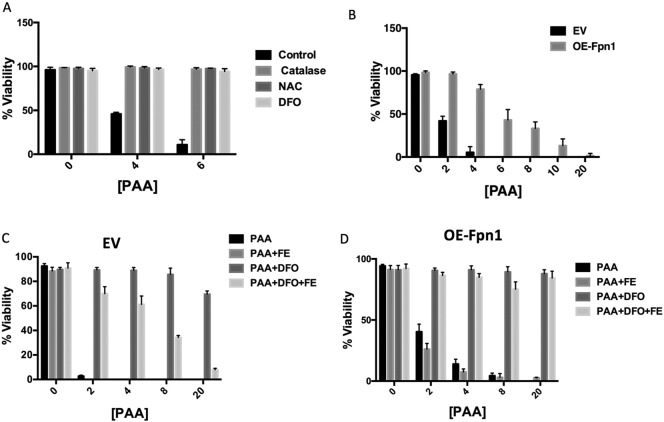
We subsequently asked whether PAA was selectively killing MM tumor cells by generating ROS, we treated OCI-MY5 MM wild-type (WT) cells with N-acetyl cysteine (NAC) or catalase. Both catalase and NAC protect cells from oxidative damage. OCI-MY5 cells pretreated with NAC and catalase became resistant to PAA even at high doses (Fig. 2A). Importantly, adding deferoxamine (DFO), an iron chelator, to OCI-MY5 cells before PAA treatment was also sufficient to prevent PAA-induced cellular death (Fig. 2A) but bathocuproinedisulfonic acid disodium salt (BCS), a selective copper chelator, was not able to block MM cell death (Supplementary Fig. 5A) suggesting that iron is essential for PAA to achieve its anti-cancer activity. DFO is a poorly membrane permeable iron chelator, however, it has been used to chelate intracellular iron in multiple studies including those ones that describe cells expressing Fpn1(Asano et al., 2011, Paradkar et al., 2008, Al-Qenaei et al., 2014). We reasoned that high cytosolic iron would catalyze PAA auto-oxidation leading to cell death. Because MM tumor cells have a higher labile iron pool (LIP) than non-tumor cells, we hypothesized that PAA's anti-cancer effect is dependent on LIP. We have previously shown that Fpn1, the only known mammalian iron exporter, is down-regulated in MM cells at the expression levels leading to higher LIP. We next sought to determine if higher Fpn1 levels in MM tumor cells could also block cell death mediated by PAA. We overexpressed and confirmed Fpn1 expression by qRT-PCR in OCI-MY5 cells (Supplementary Fig. 5B, OE-Fpn1). We noticed that 4 mM PAA was able to kill OCI-MY5 transfected with empty vector (EV) but not to overexpressing Fpn1 (OE-Fpn1) cells (Fig. 2B). Five-fold greater concentration of PAA (20 mM) was required to successfully kill OE-Fpn1 cells. Since the overexpression of Fpn1 in OCI-MY5 cells inhibits PAA anti-cancer activity, we next explored whether iron supplementation was able to restore sensitivity to PAA. Iron pre-treatment caused a rapid decrease in cell viability of OCI-MY5 EV (Fig. 2C) and the same effect was obtained in OE-Fpn1 OCI-MY5 cells (Fig. 2D). Consistent with our hypothesis, DFO, an iron chelator (Fig. 2C & D), abolished the ability of PAA to reduce cells viability in both EV and OE-Fpn1 OCI-MY5 cells pre-treated with iron. Interestingly, we noticed that EV OCI-MY5 cells in Fig. 2C treated with PAA alone showed a stronger sensitivity than those cells in Fig. 2B. We speculated that even if the PAA incubation is 1 h in both experiments the cells in Fig. 2C were kept in culture almost 24 h longer than cells in Fig. 2B for incubation with other reagents before PAA treatment. A possible explanation is that the longer incubation with culture media may slightly increase cellular iron (Goto et al., 1983). This was supported by the evidence that either addition of DFO (Fig. 2C) or overexpression of Fpn1 (Fig. 2D) rescued PAA-induced MM cell death even cell viability was different between control groups, because both DFO and overexpression of Fpn1 decrease cellular iron.
Fig. 2.
Pharmacologic ascorbic acid targets reactive oxidative species and labile iron pool. (A) OCI-MY5 WT cells were incubated with or without catalase (100 U/mL), NAC (15 mM) or DFO (200 μM) for 3 h following treatment with PAA. PAA was washed away after 1 h treatment and cell viability was determined 24 h later. (B) EV OCI-MY5 and OE-Fpn1 OCI-MY5 were treated with or without PAA (0–20 mM). PAA was washed away after 1 h and cell viability was measured 24 h later. EV OCI-MY5 (C) and OE-Fpn1 OCI-MY5 (d) were incubated with or without iron (Fe-NTA (FE), 100 μM). After 18 h cells were treated with or without DFO (200 μM) for 3 h followed by PAA treatment for 1 h and cell viability was measured as described in A.
3.4. Pharmacological Ascorbic Acid Induces Both Necrosis and Apoptosis in Myeloma Cells
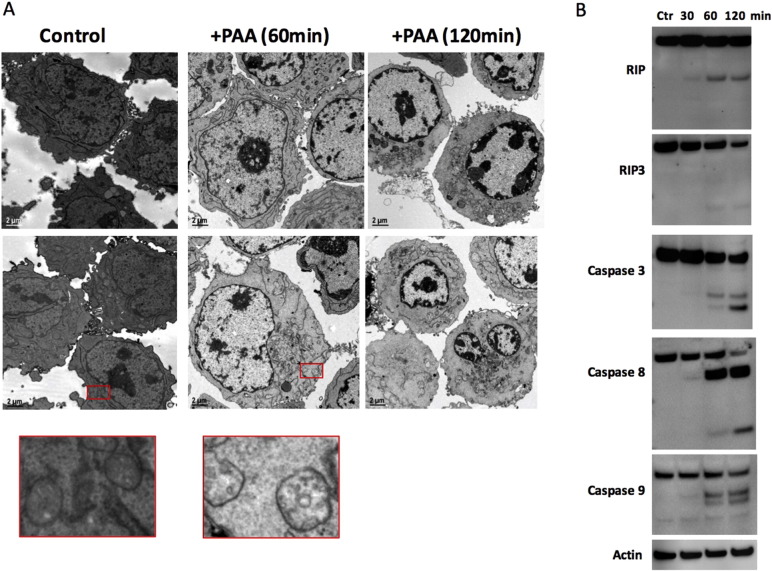
To determine the type of cell death induced by PAA, we performed transmission electron microscopy (TEM) experiments. Fig. 3A shows that in OCI-MY5 WT cells incubated with 4 mM PAA for 1 h and then left for another 2 h, PAA induced early necrosis (Fig. 3A, 60 min) followed by late apoptosis (Fig. 3A, 120 min). OCI-MY5 WT cells untreated appeared healthy and the mitochondria had visible cristae. However, once cells were treated with PAA, mitochondria started to swell and the cristae disappeared, but no remarkable chromatin condensation was identified (Fig. 3A, 60 min). In a later stage, chromatin condensation was seen in almost all cells, while mitochondrial membranes disappeared and most of cellular organelles were degraded (Fig. 3A, 120 min), consistent with apoptosis. Apoptosis can be induced by extrinsic stimuli through membrane death receptors or by intrinsic stimuli through mitochondrial signaling pathways (Hengartner, 2000, Kurokawa and Kornbluth, 2009). Our results further indicated that PAA induced mitochondria-mediated apoptosis with marked increase in caspases 3, 8, and 9 activity evidenced by increased cleavage of caspases (Fig. 3B). All three caspases were cleaved after 60 min post-PAA treatment. Consistently, PAA-induced MM cell death was partially blocked by adding a pan-caspase inhibitor Q-VD-OPh (p < 0.05; Supplementary Fig. 6). However, because necrosis was seen at earlier time points after PAA treatment we also hypothesized that extrinsic stimuli might be involved in PAA-mediated cell death and we tested the activation of receptor interacting protein kinase 1 and 3 (RIP1 and RIP3) (Nugues et al., 2014). Fig. 3B indicated that RIP1 and RIP3 were also cleaved.
Fig. 3.
Pharmacologic ascorbic acid induces mitochondria-mediated apoptosis in MM cells. (A) Transmission electron microscopy of OCI-MY5 WT cells treated with or without PAA (4 mM). After 1 h incubation, PAA was washed away and cells were fixed for TEM after 60 min and 120 min. Second row represents different field in the section. Red boxes represent zooming image of mitochondria in OCI-MY5 WT cells (left) and OCI-MY5 WT cells treated with PA (right). (B) OCI-MY5 WT cells were treated with or without PAA. After 1 h, PAA was washed away and cells were lysed at the specified times and RIP1, RIP3, Caspase 3, Caspase 8, Caspase 9, and β-actin levels were examined by western blots.
3.5. Apoptosis-inducing Factor 1 Plays a Critical Role in Pharmacological Ascorbic Acid-induced Myeloma Cell Death
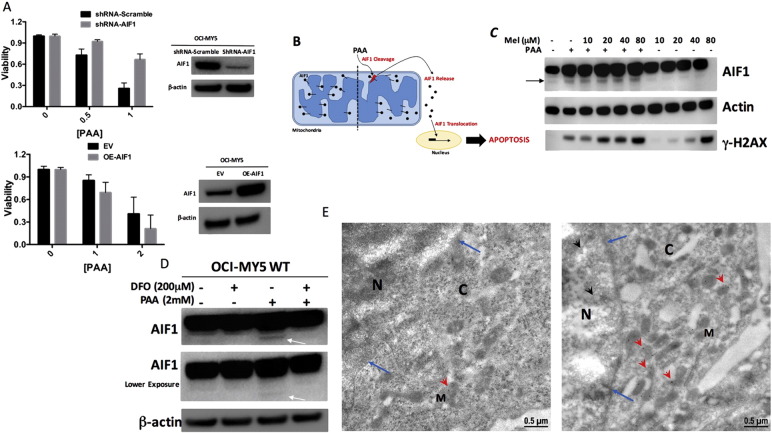
We subsequently tried to determine the molecular pathway by which PAA induced mitochondria-mediated apoptosis. Our hypothesis was that increased mitochondrial permeabilization was the trigger for the death signal transduction machinery. We focused our attention on apoptosis-inducing factor 1 (AIF1), because AIF1 induces cell death in caspase-dependent and caspase-independent manners (Nikoletopoulou et al., 2013). We evaluate if PAA induced MM cell death depends on AIF1 at least partially. We generated OCI-MY5 cells with AIF1 knockdown (shRNA-AIF1) or overexpression (OE-AIF1). The viability of AIF1-shRNA OCI-MY5 cells (Fig. 4A, top bar graph) was significantly higher than those cells expressing scrambled sequence after PAA treatment (Fig. 4A, top bar graph), while OE-AIF1 OCI-MY5 showed significantly less viability (Fig. 4A, bottom bar graph) than cells transfected with empty vector (EV) when treated with PAA (Fig. 4A, bottom bar graph). It is widely accepted that AIF1 must be cleaved and released from the mitochondria to the cytoplasm and then translocate to the nucleus to induce chromatolysis leading to cell death (Fig. 4B) (Sevrioukova, 2011). We thus examined if PAA induced AIF1 cleavage. OCI-MY5 cells treated with PAA showed an increase in the AIF1 cleaved form by western blotting (Fig. 4C). Melphalan was not able to induce AIF1 cleavage in OCI-MY5 cells (Fig. 4C) probably because melphalan is an alkylating agent and produces a number of DNA adducts with DNA interstrand crosslinks (ICLs) considered to be the critical cytotoxic lesion (Spanswick et al., 2002) in an AIF1 independent mechanism. We hypothesized that the AIF1 cleavage was mediated by PAA reacting with LIP to form ROS. Thus, we incubated OCI-MY5 cells with or without DFO followed by PAA treatment. AIF1 was not cleaved after PAA incubation in OCI-MY5 cells pretreated with DFO confirming the crucial role of LIP in this process (Fig. 4D, white arrow). Earlier studies have shown that DFO is able to deplete LIP (Al-Qenaei et al., 2014). We also tested the level of phosphorylated γ-H2AX, a biomarker for DNA double-stand breaks, after PAA and melphalan treatment, and determined that PAA and high dose of melphalan induced γ-H2AX. However, a lower dose of melphalan with PAA was also able to induce γ-H2AX (Fig. 4C). These data support our earlier in vivo data (Fig. 1F) that combination of PAA and melphalan at lower dose inhibits tumor formation at the same level or greater than melphalan alone. Cellular localization of AIF1 was examined by immunolabeling electron microscope with and without PAA treatment in OCI-MY5 cells. This staining revealed that AIF1 localizes not only in the mitochondria, as seen in untreated cells (Fig. 4E, left panel), but also in cytoplasm and nuclei in PAA-treated OCI-MY5 cells (Fig. 4E, right panel). These results indicate that PAA by reacting with LIP and generating ROS induces mitochondria-mediated apoptosis in which AIF1 cleavage is important for cell death.
Fig. 4.
Pharmacologic ascorbic acid induces AIF1 release from mitochondria. (A) Top bar graph represents OCI-MY5-Scramble and -shRNA-AIF1 cells incubated with doxycycline to knockdown AIF1 for 2 days. Bottom bar graph represents EV OCI-MY5 and OE-AIF1 OCI-MY5 cells. All cells were treated with or without PAA at the specified concentrations. After 1 h treatment, PAA was washed away and cell viability was measured after 24 h. Knockdown and overexpression of AIF1 was confirmed by western blots. (B) Schematic representation of PAA inducing AIF1 cleavage, release and nuclear translocation in MM tumor cells. (C) OCI-MY5 WT cells with or without PAA. After 1 h PAA was washed away and cells were incubated with melphalan (Mel, 0–80 μM) for 4 h then lysed. AIF1, β-actin and γ-H2AX levels were analyzed by western blots. (D) OCI-MY5 WT cells were incubated with or without DFO (200 μM) for 3 h followed PAA (2 mM) treatment. After 1 h PAA was washed away and cells were lysed. AIF1 and β-actin levels were analyzed by western blots. White arrow represent AIF1 cleaved form. (E) Electron microscopy shows AIF1 immunolabeling stain of OCI-MY5 WT cells treated without (left) or with (right) PAA (2 mM). N, M, C respectively represent nucleus, mitochondria and cytoplasm. Blue arrows indicate the nuclear membrane and red arrowheads indicate AIF1 gold beads in cytoplasm or mitochondria. Black arrowheads indicate AIF1 gold beads in nuclei.
4. Discussion
High-dose vitamin C has been studied in multiple cancers and has shown controversial clinical effects (Cameron and Pauling, 1978, Cameron and Pauling, 1976, Creagan et al., 1979, Moertel et al., 1985). The contradictory clinical results can be at least partially explained by different routes of vitamin C administration applied, i.e., either orally or intravenously. Recent reports indicate that a certain ROS concentration is required for high-dose vitamin C to induce cytotoxicity in cancer cells. The generation of ascorbyl- and H2O2 radicals by PAA increases ROS stress in cancer cells (Du et al., 2012). These studies including preclinical and clinical were performed in solid tumors, such as glioblastoma (Herst et al., 2012), pancreatic cancer (Du et al., 2015), ovarian cancer (Ma et al., 2014), prostate cancer (Chen et al., 2012, Pollard et al., 2010), hepatoma (Verrax and Calderon, 2009), colon cancer (Pires et al., 2016), mesothelioma (Ranzato et al., 2011), breast cancer (Yun et al., 2015), bladder cancer (Gilloteaux et al., 2010), and neuroblastoma (Deubzer et al., 2010). Reports are lacking to show that PAA can be used as a pro-oxidant drug in the treatment of “liquid” tumors, where tumor cells are surrounded by blood. This environmental difference between solid tumor and blood cancer has the potential to influence the PAA efficacy on cancer cell death even when given at high doses, because ascorbic acid generated ROS are much easier permeabilized in liquid tumor than in solid tumor. In this study, we report that PAA is efficacious in killing MM cells in vitro and in vivo models, which generated levels of 20–40 mM ascorbate and 500 nM ascorbyl radicals after intraperitoneal administration of 4 g ascorbate per kilogram of body weight (Chen et al., 2008), in xenograft MM mice. These data suggest that PAA may show a therapeutic advantage to blood cancers vs solid tumors because of the communication between tumor cells and blood plasma.
We have shown that Fpn1 regulates iron export in MM cells and LIP in vitro and in vivo (Gu et al., 2015). In addition, ferritin also regulates LIP by sequestering free iron in an oxidized form to prevent formation of free radicals (Pantopoulos et al., 2012). Our preliminary data show that overexpression of Fpn1 in MM cell line OCI-MY5 results in increased viability compared to wild type cells after PAA treatment. We hypothesize that Fpn1 expressing MM cells are less sensitive to PAA because their cytosolic iron content is reduced by Fpn1. To test if resistance to PAA is indeed due to low cytosolic iron content, we depleted cytosolic iron by pre-incubating cells with an iron chelator, deferoxamine (DFO). DFO is poorly membrane permeable. However, it has been used to chelate intracellular iron in multiple studies including cells expressing Fpn1 (Delaby et al., 2005, Knutson et al., 2003). ARP1 MM cells pre-treated with DFO (200 μM, 3 h) followed by PAA treatment showed a higher viability than cells not pre-treated with DFO. These results strongly suggest that the mechanism of PAA killing of MM cells is indeed iron-dependent. In addition, Fpn1 is significantly down-regulated in CD138+ primary MM cells, while the iron importer, transferrin receptor 1, is significantly upregulated in CD138+ MM cells compared to normal plasma cells, further supporting that MM cells have higher iron content than non-tumor cells. PAA showed increased killing of MM cells derived from almost all primary MM patients and smoldering MM, but not from MGUS patients. These results suggest that PAA administration in SMM may be able to prevent progression to symtomatic MM.
Though ROS and H2O2 are well known factors mediating PAA-induced cancer cell death (Espey et al., 2011, Levine et al., 2011), a single molecular mechanism cannot explain these observations because multiple pathways are involved in the downstream effects of ROS and H2O2 (Venturelli et al., 2015). Necorosis, caspase-dependent and caspase-independent apoptosis, and autophagy were reported in ascorbate-induced cell death in different types of cancer (Chen et al., 2015). A recent study by Yun and colleagues demonstrated that vitamin C selectively kills KRAS and BRAF mutant colorectal cancer cells by targeting GAPDH, but spares normal cells (Yun et al., 2015). Recently the deep-sequencing data have identified that RAS family genes show the most frequent mutations in MM. KRAS, NRAS and BRAF are mutated in 22%, 20% and 7% of MM samples respectively from the analysis of 733 patients, and these two RAS mutations occur exclusively in MM patients (Manier et al., 2017, Lohr et al., 2014). Although we did not specifically test if PAA was more sensitive to RAS-mutated MM samples, we found PAA was sensitive to all 9 MMs and 2 SMMs, in whom the RAS mutation is unknown, and 3 MM cell lines (without any RAS mutations) but insensitive to 2 MGUS samples, suggesting that the disease stage rather than the mutation of RAS and/or BRAF is the major predictive factor for PAA sensitivity in MM treatment. Other molecular mechanisms including ATP depletion and ATM-AMPK signaling have been reported to explain PAA-induced cell death (Cullen, 2010, Du et al., 2010, Chen et al., 2012, Ma et al., 2014). In this study, TEM data indicate that mitochondrial morphology and structure are significantly altered after PAA treatment. Furthermore, AIF1 was originally discovered as an intermembrane space (IMS) component of mitochondria and characterized as a pro-apoptotic gene (Susin et al., 1999, Joza et al., 2001). Therefore, we focused on AIF1 as one of the pathways by which PAA-induced MM cell death. The pro-apoptotic AIF1 or truncated AIF1 (tAIF) is cleaved from the full-length AIF1 by calpains and/or cathepsins after a caspase-independent cell death insult (Joza et al., 2009, Modjtahedi et al., 2006, Sevrioukova, 2011, Artus et al., 2010). tAIF moves from the mitochondria to the cytosol and nucleus, where it initiates chromatolysis and caspase-dependent and caspase-independent cell death (Nikoletopoulou et al., 2013, Artus et al., 2010). Our data show that PAA increases AIF1 cleavage and translocation from mitochondria to cytoplasm and nucleus. Overexpression of AIF1 in MM cells increases while knock-down of AIF1 prevents PAA-induced MM cell death, indicating that AIF1 plays a critical role in mediating PAA-induced MM cell death. Because the mitochondrial apoptogenic factors such as cytochrome c and Bcl-2 family proteins are also important for the activation of caspases, future work will have to determine if AIF1 is the major pathway related to PAA activity in cancer cells as well as the exact relationship with other mitochondrial apotogenetic factors. In addition, the necrosis and apoptosis markers, such as RIP1/3 and caspases 3/8/9, are cleaved after PAA administration. It is therefore possible that PAA activates caspase 8 resutling in RIP1 cleavage and necrosis (Rajput et al., 2011) evidenced by strong caspase 8 cleavage after a short-term treatment with PAA.
High oxidative stress and DNA damage activity are increased, while the anti-oxidant enzyme levels are decreased in MM patients (Mehdi et al., 2013). Several free radical drugs, such as As2O3 and ascorbic acid, have been used to treat MM, in which As2O3 generates ROS while ascorbic acid serves as an anti-oxidant agent. In MM preclinical and clinical studies, ascorbate was used as an adjunct drug and showed controversial results (Harvey et al., 2009, Perrone et al., 2009, Held et al., 2013, Sharma et al., 2012, Nakano et al., 2011, Takahashi, 2010, Sharma et al., 2009, Qazilbash et al., 2008). However, none of these tests used pharmacological doses of ascorbate and intravenous administration. It has been reported that ascorbate directly inactivates bortezomib activity by forming a tight but reversible complex through its vicinal diol group (Perrone et al., 2009, Harvey et al., 2009). This dose of ascorbate in the combination with bortezomib is at a physiological level which has anti-oxidant effect. We did not perform the combination of PAA with bortezomib due to a possible chemical reaction described above. However, our pilot study also suggested that PAA could overcome drug resistance to bortezomib in MM cells (data not shown).
Our findings complement reported studies and further address the mechanism of action using clinical samples in which we observed that PAA killed tumor cells with high iron content, suggesting that iron might be the initiator of PAA cytotoxicity. In addition, combination of PAA with standard therapeutic drugs, such as melphalan, may significantly reduce the dose of melphalan needed. This is beneficial because high doses of melphalan are very toxic not only to tumor cells but also to normal tissues, such as hematopoietic stem cell and epithelial cells in the gut (Shaw et al., 2014, Bayraktar et al., 2013). The efficacy of high-dose melphalan by itself is clearly dose-dependent. Combined treatment of reduced dose melphalan with PAA achieved a significantly longer progression-free survival than the same dose of melphalan alone. These data also suggest that the bone marrow suppression induced by high-dose melphalan can be ameliorated by the combination of PAA with lower dose of melphalan because of the lack of toxicity of PAA on normal cells with low iron content. It is important to consider for future clinical studies that renal insufficiency could be a contraindication for usage of PAA in MM patients. Renal impairment is a common complication of MM (50%) and up to 5% require dialysis (Yadav et al., 2016a, Yadav et al., 2016b). The clinical toxicity is probably from oxalate, an end-product of ascorbate metabolism. Therefore, if creatinine clearance is < 30 mL/min, high dose ascorbic acid should be not administrated.
Funding Sources
The data presented in this article were obtained at the Central Microscopy Research Facility, which is a Carver College of Medicine/Holden Comprehensive Cancer Center core research facility at the University of Iowa. The Facility is funded through user fees and the generous financial support of the Carver College of Medicine, Holden Comprehensive Cancer Center grant P30CA086862 and NIH grant 1S10RR018998-01. This work was also supported by NIH grants R01CA152105 (F.Z.), the Multiple Myeloma Research Foundation (F.Z.), the International Myeloma Foundation (F.Z.), the American Society of Hematology (ASH) Bridge (F.Z.), the UIHC-CCOM Research Investment Pilot Grants (F.Z.) and institutional start-up funds from the Department of Internal Medicine, Carver College of Medicine, University of Iowa (F.Z. and G.T.).
Conflict of Interest
Authors declare no conflict of interest.
Author Contribution
J.X., H.X. and X.Z. performed experiments and analyzed the data; C.A. performed, analyzed and reviewed the electron microscopy experiments; K.L.C. and G.T. collected patients' samples and clinical data and edited the manuscript; R.N. contributed in the electron microscopy experimental design; I.F. and F.Z. reviewed the data, wrote and edited the manuscript. All authors approved the manuscript.
Acknowledgements
The author thanks Dr. Garry R. Buettner (Free Radical and Radiation Biology Program, University of Iowa) for providing the ascorbic acid.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ebiom.2017.02.011.
Contributor Information
Guido Tricot, Email: guido-tricot@uiowa.edu.
Fenghuang Zhan, Email: fenghuang-zhan@uiowa.edu.
Appendix A. Supplementary Data
Supplementary material
References
- Al-Qenaei A., Yiakouvaki A., Reelfs O., Santambrogio P., Levi S., Hall N.D., Tyrrell R.M., Pourzand C. Role of intracellular labile iron, ferritin, and antioxidant defence in resistance of chronically adapted Jurkat T cells to hydrogen peroxide. Free Radic. Biol. Med. 2014;68:87–100. doi: 10.1016/j.freeradbiomed.2013.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexanian R., Wang M., Delasalle K., Wang S., Qazilbash M., Handy B., Weber D. Value of novel agents and intensive therapy for patients with multiple myeloma. Bone Marrow Transplant. 2014;49:422–425. doi: 10.1038/bmt.2013.189. [DOI] [PubMed] [Google Scholar]
- Artus C., Boujrad H., Bouharrour A., Brunelle M.N., Hoos S., Yuste V.J., Lenormand P., Rousselle J.C., Namane A., England P., Lorenzo H.K., Susin S.A. AIF promotes chromatinolysis and caspase-independent programmed necrosis by interacting with histone H2AX. EMBO J. 2010;29:1585–1599. doi: 10.1038/emboj.2010.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asano T., Komatsu M., Yamaguchi-Iwai Y., Ishikawa F., Mizushima N., Iwai K. Distinct mechanisms of ferritin delivery to lysosomes in iron-depleted and iron-replete cells. Mol. Cell. Biol. 2011;31:2040–2052. doi: 10.1128/MCB.01437-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayraktar U.D., Bashir Q., Qazilbash M., Champlin R.E., Ciurea S.O. Fifty years of melphalan use in hematopoietic stem cell transplantation. Biol. Blood Marrow Transplant. 2013;19:344–356. doi: 10.1016/j.bbmt.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergsagel P.L. Where we were, where we are, where we are going: progress in multiple myeloma. Am. Soc. Clin. Oncol. Educ. Book. 2014:199–203. doi: 10.14694/EdBook_AM.2014.34.199. [DOI] [PubMed] [Google Scholar]
- Buettner G.R. In the absence of catalytic metals ascorbate does not autoxidize at pH 7: ascorbate as a test for catalytic metals. J. Biochem. Biophys. Methods. 1998;16:27–40. doi: 10.1016/0165-022x(88)90100-5. [DOI] [PubMed] [Google Scholar]
- Cameron E., Pauling L. Supplemental ascorbate in the supportive treatment of cancer: prolongation of survival times in terminal human cancer. Proc. Natl. Acad. Sci. U. S. A. 1976;73:3685–3689. doi: 10.1073/pnas.73.10.3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron E., Pauling L. Supplemental ascorbate in the supportive treatment of cancer: reevaluation of prolongation of survival times in terminal human cancer. Proc. Natl. Acad. Sci. U. S. A. 1978;75:4538–4542. doi: 10.1073/pnas.75.9.4538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P., Yu J., Chalmers B., Drisko J., Yang J., Li B., Chen Q. Pharmacological ascorbate induces cytotoxicity in prostate cancer cells through ATP depletion and induction of autophagy. Anti-Cancer Drugs. 2012;23:437–444. doi: 10.1097/CAD.0b013e32834fd01f. [DOI] [PubMed] [Google Scholar]
- Chen Q., Espey M.G., Krishna M.C., Mitchell J.B., Corpe C.P., Buettner G.R., Shacter E., Levine M. Pharmacologic ascorbic acid concentrations selectively kill cancer cells: action as a pro-drug to deliver hydrogen peroxide to tissues. Proc. Natl. Acad. Sci. U. S. A. 2005;102:13604–13609. doi: 10.1073/pnas.0506390102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q., Espey M.G., Sun A.Y., Lee J.H., Krishna M.C., Shacter E., Choyke P.L., Pooput C., Kirk K.L., Buettner G.R., Levine M. Ascorbate in pharmacologic concentrations selectively generates ascorbate radical and hydrogen peroxide in extracellular fluid in vivo. Proc. Natl. Acad. Sci. U. S. A. 2007;104:8749–8754. doi: 10.1073/pnas.0702854104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q., Espey M.G., Sun A.Y., Pooput C., Kirk K.L., Krishna M.C., Khosh D.B., Drisko J., Levine M. Pharmacologic doses of ascorbate act as a prooxidant and decrease growth of aggressive tumor xenografts in mice. Proc. Natl. Acad. Sci. U. S. A. 2008;105:11105–11109. doi: 10.1073/pnas.0804226105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q., Polireddy K., Chen P., Dong R. The unpaved journey of vitamin C in cancer treatment. Can. J. Physiol. Pharmacol. 2015;93:1055–1063. doi: 10.1139/cjpp-2014-0509. [DOI] [PubMed] [Google Scholar]
- Creagan E.T., Moertel C.G., O'fallon J.R., Schutt A.J., O'connell M.J., Rubin J., Frytak S. Failure of high-dose vitamin C (ascorbic acid) therapy to benefit patients with advanced cancer. A controlled trial. N. Engl. J. Med. 1979;301:687–690. doi: 10.1056/NEJM197909273011303. [DOI] [PubMed] [Google Scholar]
- Cullen J.J. Ascorbate induces autophagy in pancreatic cancer. Autophagy. 2010;6:421–422. doi: 10.4161/auto.6.3.11527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaby C., Pilard N., Goncalves A.S., Beaumont C., Canonne-Hergaux F. Presence of the iron exporter ferroportin at the plasma membrane of macrophages is enhanced by iron loading and down-regulated by hepcidin. Blood. 2005;106:3979–3984. doi: 10.1182/blood-2005-06-2398. [DOI] [PubMed] [Google Scholar]
- Deubzer B., Mayer F., Kuci Z., Niewisch M., Merkel G., Handgretinger R., Bruchelt G. H(2)O(2)-mediated cytotoxicity of pharmacologic ascorbate concentrations to neuroblastoma cells: potential role of lactate and ferritin. Cell. Physiol. Biochem. 2010;25:767–774. doi: 10.1159/000315098. [DOI] [PubMed] [Google Scholar]
- Du J., Cieslak J.A., 3rd, Welsh J.L., Sibenaller Z.A., Allen B.G., Wagner B.A., Kalen A.L., Doskey C.M., Strother R.K., Button A.M., Mott S.L., Smith B., Tsai S., Mezhir J., Goswami P.C., Spitz D.R., Buettner G.R., Cullen J.J. Pharmacological ascorbate radiosensitizes pancreatic cancer. Cancer Res. 2015;75:3314–3326. doi: 10.1158/0008-5472.CAN-14-1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J., Cullen J.J., Buettner G.R. Ascorbic acid: chemistry, biology and the treatment of cancer. Biochim. Biophys. Acta. 2012;1826:443–457. doi: 10.1016/j.bbcan.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J., Martin S.M., Levine M., Wagner B.A., Buettner G.R., Wang S.H., Taghiyev A.F., Du C., Knudson C.M., Cullen J.J. Mechanisms of ascorbate-induced cytotoxicity in pancreatic cancer. Clin. Cancer Res. 2010;16:509–520. doi: 10.1158/1078-0432.CCR-09-1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eda H., Santo L., Cirstea D.D., Yee A.J., Scullen T.A., Nemani N., Mishima Y., Waterman P.R., Arastu-Kapur S., Evans E., Singh J., Kirk C.J., Westlin W.F., Raje N.S. A novel Bruton's tyrosine kinase inhibitor CC-292 in combination with the proteasome inhibitor carfilzomib impacts the bone microenvironment in a multiple myeloma model with resultant antimyeloma activity. Leukemia. 2014;28:1892–1901. doi: 10.1038/leu.2014.69. [DOI] [PubMed] [Google Scholar]
- Espey M.G., Chen P., Chalmers B., Drisko J., Sun A.Y., Levine M., Chen Q. Pharmacologic ascorbate synergizes with gemcitabine in preclinical models of pancreatic cancer. Free Radic. Biol. Med. 2011;50:1610–1619. doi: 10.1016/j.freeradbiomed.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu C., Wang J., Xin X., Liu H., Xue S., Ma X., Jin Z., Sun A., Qiu H., Wu D. Therapeutic effects of autologous hematopoietic stem cell transplantation in multiple myeloma patients. Exp. Ther. Med. 2013;6:977–982. doi: 10.3892/etm.2013.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay F., Magarotto V., Crippa C., Pescosta N., Guglielmelli T., Cavallo F., Pezzatti S., Ferrari S., Liberati A.M., Oliva S., Patriarca F., Offidani M., Omede P., Montefusco V., Petrucci M.T., Giuliani N., Passera R., Pietrantuono G., Boccadoro M., Corradini P., Palumbo A. Bortezomib induction, reduced-intensity transplantation, and lenalidomide consolidation-maintenance for myeloma: updated results. Blood. 2013;122:1376–1383. doi: 10.1182/blood-2013-02-483073. [DOI] [PubMed] [Google Scholar]
- Gilloteaux J., Jamison J.M., Neal D.R., Loukas M., Doberzstyn T., Summers J.L. Cell damage and death by autoschizis in human bladder (RT4) carcinoma cells resulting from treatment with ascorbate and menadione. Ultrastruct. Pathol. 2010;34:140–160. doi: 10.3109/01913121003662304. [DOI] [PubMed] [Google Scholar]
- Goto Y., Paterson M., Listowsky I. Iron uptake and regulation of ferritin synthesis by hepatoma cells in hormone-supplemented serum-free media. J. Biol. Chem. 1983;258:5248–5255. [PubMed] [Google Scholar]
- Gu Z., Wang H., Xia J., Yang Y., Jin Z., Xu H., Shi J., De Domenico I., Tricot G., Zhan F. Decreased ferroportin promotes myeloma cell growth and osteoclast differentiation. Cancer Res. 2015;75:2211–2221. doi: 10.1158/0008-5472.CAN-14-3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey R.D., Nettles J., Wang B., Sun S.Y., Lonial S. Commentary on Perrone et al.: 'vitamin C: not for breakfast anymore … if you have myeloma'. Leukemia. 2009;23:1939–1940. doi: 10.1038/leu.2009.128. [DOI] [PubMed] [Google Scholar]
- Held L.A., Rizzieri D., Long G.D., Gockerman J.P., Diehl L.F., De Castro C.M., Moore J.O., Horwitz M.E., Chao N.J., Gasparetto C. A Phase I study of arsenic trioxide (Trisenox), ascorbic acid, and bortezomib (Velcade) combination therapy in patients with relapsed/refractory multiple myeloma. Cancer Investig. 2013;31:172–176. doi: 10.3109/07357907.2012.756109. [DOI] [PubMed] [Google Scholar]
- Hengartner M.O. The biochemistry of apoptosis. Nature. 2000;407:770–776. doi: 10.1038/35037710. [DOI] [PubMed] [Google Scholar]
- Herst P.M., Broadley K.W., Harper J.L., Mcconnell M.J. Pharmacological concentrations of ascorbate radiosensitize glioblastoma multiforme primary cells by increasing oxidative DNA damage and inhibiting G2/M arrest. Free Radic. Biol. Med. 2012;52:1486–1493. doi: 10.1016/j.freeradbiomed.2012.01.021. [DOI] [PubMed] [Google Scholar]
- Padayatty S.J., Sun H., Wang Y., Riordan H.D., Hewitt S.M., Katz A., Wesley R.A., Levine M. Vitamin C pharmacokinetics: implications for oral and intravenous use. Ann. Intern. Med. 2004;140:533–537. doi: 10.7326/0003-4819-140-7-200404060-00010. [DOI] [PubMed] [Google Scholar]
- Hoffer L.J., Levine M., Assouline S., Melnychuk D., Padayatty S.J., Rosadiuk K., Rousseau C., Robitaille L., Miller W.H., Jr. Phase I clinical trial of i.v. ascorbic acid in advanced malignancy. Ann. Oncol. 2008;19:1969–1974. doi: 10.1093/annonc/mdn377. [DOI] [PubMed] [Google Scholar]
- Joza N., Pospisilik J.A., Hangen E., Hanada T., Modjtahedi N., Penninger J.M., Kroemer G. AIF: not just an apoptosis-inducing factor. Ann. N. Y. Acad. Sci. 2009;1171:2–11. doi: 10.1111/j.1749-6632.2009.04681.x. [DOI] [PubMed] [Google Scholar]
- Joza N., Susin S.A., Daugas E., Stanford W.L., Cho S.K., Li C.Y., Sasaki T., Elia A.J., Cheng H.Y., Ravagnan L., Ferri K.F., Zamzami N., Wakeham A., Hakem R., Yoshida H., Kong Y.Y., Mak T.W., Zuniga-Pflucker J.C., Kroemer G., Penninger J.M. Essential role of the mitochondrial apoptosis-inducing factor in programmed cell death. Nature. 2001;410:549–554. doi: 10.1038/35069004. [DOI] [PubMed] [Google Scholar]
- Knutson M.D., Vafa M.R., Haile D.J., Wessling-Resnick M. Iron loading and erythrophagocytosis increase ferroportin 1 (FPN1) expression in J774 macrophages. Blood. 2003;102:4191–4197. doi: 10.1182/blood-2003-04-1250. [DOI] [PubMed] [Google Scholar]
- Kurokawa M., Kornbluth S. Caspases and kinases in a death grip. Cell. 2009;138:838–854. doi: 10.1016/j.cell.2009.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M., Padayatty S.J., Espey M.G. Vitamin C: a concentration-function approach yields pharmacology and therapeutic discoveries. Adv. Nutr. 2011;2:78–88. doi: 10.3945/an.110.000109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Li J., Huang B., Zheng D., Chen M., Zhou Z., Xu D., Zou W. Determining the optimal time for bortezomib-based induction chemotherapy followed by autologous hematopoietic stem cell transplant in the treatment of multiple myeloma. Chin. J. Cancer Res. 2013;25:166–174. doi: 10.3978/j.issn.1000-9604.2013.02.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohr J.G., Stojanov P., Carter S.L., Cruz-Gordillo P., Lawrence M.S., Auclair D., Sougnez C., Knoechel B., Gould J., Saksena G., Cibulskis K., Mckenna A., Chapman M.A., Straussman R., Levy J., Perkins L.M., Keats J.J., Schumacher S.E., Rosenberg M., Multiple Myeloma Research Consortium, Getz G., Golub T.R. Widespread genetic heterogeneity in multiple myeloma: implications for targeted therapy. Cancer Cell. 2014;25:91–101. doi: 10.1016/j.ccr.2013.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y., Chapman J., Levine M., Polireddy K., Drisko J., Chen Q. High-dose parenteral ascorbate enhanced chemosensitivity of ovarian cancer and reduced toxicity of chemotherapy. Sci. Transl. Med. 2014;6:222ra18. doi: 10.1126/scitranslmed.3007154. [DOI] [PubMed] [Google Scholar]
- Manier S., Salem K.Z., Park J., Landau D.A., Getz G., Ghobrial I.M. Genomic complexity of multiple myeloma and its clinical implications. Nat. Rev. Clin. Oncol. 2017;14:100–113. doi: 10.1038/nrclinonc.2016.122. [DOI] [PubMed] [Google Scholar]
- Mehdi W.A., Zainulabdeen J.A., Mehde A.A. Investigation of the antioxidant status in multiple myeloma patients: effects of therapy. Asian Pac. J. Cancer Prev. 2013;14:3663–3667. doi: 10.7314/apjcp.2013.14.6.3663. [DOI] [PubMed] [Google Scholar]
- Modjtahedi N., Giordanetto F., Madeo F., Kroemer G. Apoptosis-inducing factor: vital and lethal. Trends Cell Biol. 2006;16:264–272. doi: 10.1016/j.tcb.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Moertel C.G., Fleming T.R., Creagan E.T., Rubin J., O'connell M.J., Ames M.M. High-dose vitamin C versus placebo in the treatment of patients with advanced cancer who have had no prior chemotherapy. A randomized double-blind comparison. N. Engl. J. Med. 1985;312:137–141. doi: 10.1056/NEJM198501173120301. [DOI] [PubMed] [Google Scholar]
- Nakano A., Abe M., Oda A., Amou H., Hiasa M., Nakamura S., Miki H., Harada T., Fujii S., Kagawa K., Takeuchi K., Watanabe T., Ozaki S., Matsumoto T. Delayed treatment with vitamin C and N-acetyl-L-cysteine protects Schwann cells without compromising the anti-myeloma activity of bortezomib. Int. J. Hematol. 2011;93:727–735. doi: 10.1007/s12185-011-0850-7. [DOI] [PubMed] [Google Scholar]
- Nikoletopoulou V., Markaki M., Palikaras K., Tavernarakis N. Crosstalk between apoptosis, necrosis and autophagy. Biochim. Biophys. Acta. 2013;1833:3448–3459. doi: 10.1016/j.bbamcr.2013.06.001. [DOI] [PubMed] [Google Scholar]
- Nugues A.L., El Bouazzati H., Hetuin D., Berthon C., Loyens A., Bertrand E., Jouy N., Idziorek T., Quesnel B. RIP3 is downregulated in human myeloid leukemia cells and modulates apoptosis and caspase-mediated p65/RelA cleavage. Cell Death Dis. 2014;5 doi: 10.1038/cddis.2014.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padayatty S.J., Riordan H.D., Hewitt S.M., Katz A., Hoffer L.J., Levine M. Intravenously administered vitamin C as cancer therapy: three cases. CMAJ. 2006;174:937–942. doi: 10.1503/cmaj.050346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantopoulos K., Porwal S.K., Tartakoff A., Devireddy L. Mechanisms of mammalian iron homeostasis. Biochemistry. 2012;51:5705–5724. doi: 10.1021/bi300752r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradkar P.N., De Domenico I., Durchfort N., Zohn I., Kaplan J., Ward D.M. Iron depletion limits intracellular bacterial growth in macrophages. Blood. 2008;112:866–874. doi: 10.1182/blood-2007-12-126854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrone G., Hideshima T., Ikeda H., Okawa Y., Calabrese E., Gorgun G., Santo L., Cirstea D., Raje N., Chauhan D., Baccarani M., Cavo M., Anderson K.C. Ascorbic acid inhibits antitumor activity of bortezomib in vivo. Leukemia. 2009;23:1679–1686. doi: 10.1038/leu.2009.83. [DOI] [PubMed] [Google Scholar]
- Pires A.S., Marques C.R., Encarnacao J.C., Abrantes A.M., Mamede A.C., Laranjo M., Goncalves A.C., Sarmento-Ribeiro A.B., Botelho M.F. Ascorbic acid and colon cancer: an oxidative stimulus to cell death depending on cell profile. Eur. J. Cell Biol. 2016;95:208–218. doi: 10.1016/j.ejcb.2016.04.001. [DOI] [PubMed] [Google Scholar]
- Pollard H.B., Levine M.A., Eidelman O., Pollard M. Pharmacological ascorbic acid suppresses syngeneic tumor growth and metastases in hormone-refractory prostate cancer. In Vivo. 2010;24:249–255. [PMC free article] [PubMed] [Google Scholar]
- Qazilbash M.H., Saliba R.M., Nieto Y., Parikh G., Pelosini M., Khan F.B., Jones R.B., Hosing C., Mendoza F., Weber D.M., Wang M., Popat U., Alousi A., Anderlini P., Champlin R.E., Giralt S. Arsenic trioxide with ascorbic acid and high-dose melphalan: results of a phase II randomized trial. Biol. Blood Marrow Transplant. 2008;14:1401–1407. doi: 10.1016/j.bbmt.2008.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajput A., Kovalenko A., Bogdanov K., Yang S.H., Kang T.B., Kim J.C., Du J., Wallach D. RIG-I RNA helicase activation of IRF3 transcription factor is negatively regulated by caspase-8-mediated cleavage of the RIP1 protein. Immunity. 2011;34:340–351. doi: 10.1016/j.immuni.2010.12.018. [DOI] [PubMed] [Google Scholar]
- Ranzato E., Biffo S., Burlando B. Selective ascorbate toxicity in malignant mesothelioma: a redox Trojan mechanism. Am. J. Respir. Cell Mol. Biol. 2011;44:108–117. doi: 10.1165/rcmb.2009-0340OC. [DOI] [PubMed] [Google Scholar]
- Sanchez E., Li M., Kitto A., Li J., Wang C.S., Kirk D.T., Yellin O., Nichols C.M., Dreyer M.P., Ahles C.P., Robinson A., Madden E., Waterman G.N., Swift R.A., Bonavida B., Boccia R., Vescio R.A., Crowley J., Chen H., Berenson J.R. Serum B-cell maturation antigen is elevated in multiple myeloma and correlates with disease status and survival. Br. J. Haematol. 2012;158:727–738. doi: 10.1111/j.1365-2141.2012.09241.x. [DOI] [PubMed] [Google Scholar]
- Sevrioukova I.F. Apoptosis-inducing factor: structure, function, and redox regulation. Antioxid. Redox Signal. 2011;14:2545–2579. doi: 10.1089/ars.2010.3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A., Tripathi M., Satyam A., Kumar L. Study of antioxidant levels in patients with multiple myeloma. Leuk. Lymphoma. 2009;50:809–815. doi: 10.1080/10428190902802323. [DOI] [PubMed] [Google Scholar]
- Sharma M., Khan H., Thall P.F., Orlowski R.Z., Bassett R.L., Jr., Shah N., Bashir Q., Parmar S., Wang M., Shah J.J., Hosing C.M., Popat U.R., Giralt S.A., Champlin R.E., Qazilbash M.H. A randomized phase 2 trial of a preparative regimen of bortezomib, high-dose melphalan, arsenic trioxide, and ascorbic acid. Cancer. 2012;118:2507–2515. doi: 10.1002/cncr.26517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaughnessy J.D., Jr., Zhan F., Burington B.E., Huang Y., Colla S., Hanamura I., Stewart J.P., Kordsmeier B., Randolph C., Williams D.R., Xiao Y., Xu H., Epstein J., Anaissie E., Krishna S.G., Cottler-Fox M., Hollmig K., Mohiuddin A., Pineda-Roman M., Tricot G., Van Rhee F., Sawyer J., Alsayed Y., Walker R., Zangari M., Crowley J., Barlogie B. A validated gene expression model of high-risk multiple myeloma is defined by deregulated expression of genes mapping to chromosome 1. Blood. 2007;109:2276–2284. doi: 10.1182/blood-2006-07-038430. [DOI] [PubMed] [Google Scholar]
- Shaw P.J., Nath C.E., Lazarus H.M. Not too little, not too much-just right! (Better ways to give high dose melphalan) Bone Marrow Transplant. 2014;49:1457–1465. doi: 10.1038/bmt.2014.186. [DOI] [PubMed] [Google Scholar]
- Sonneveld P., Goldschmidt H., Rosinol L., Blade J., Lahuerta J.J., Cavo M., Tacchetti P., Zamagni E., Attal M., Lokhorst H.M., Desai A., Cakana A., Liu K., Van De Velde H., Esseltine D.L., Moreau P. Bortezomib-based versus nonbortezomib-based induction treatment before autologous stem-cell transplantation in patients with previously untreated multiple myeloma: a meta-analysis of phase III randomized, controlled trials. J. Clin. Oncol. 2013;31:3279–3287. doi: 10.1200/JCO.2012.48.4626. [DOI] [PubMed] [Google Scholar]
- Spanswick V.J., Craddock C., Sekhar M., Mahendra P., Shankaranarayana P., Hughes R.G., Hochhauser D., Hartley J.A. Repair ofDNA interstrand crosslinks as a mechanism of clinical resistance to melphalan in multiple myeloma. Blood. 2002;100:224–229. doi: 10.1182/blood.v100.1.224. [DOI] [PubMed] [Google Scholar]
- Susin S.A., Lorenzo H.K., Zamzami N., Marzo I., Snow B.E., Brothers G.M., Mangion J., Jacotot E., Costantini P., Loeffler M., Larochette N., Goodlett D.R., Aebersold R., Siderovski D.P., Penninger J.M., Kroemer G. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature. 1999;397:441–446. doi: 10.1038/17135. [DOI] [PubMed] [Google Scholar]
- Takahashi S. Combination therapy with arsenic trioxide for hematological malignancies. Anti Cancer Agents Med. Chem. 2010;10:504–510. doi: 10.2174/1871520611009060504. [DOI] [PubMed] [Google Scholar]
- Terpos E., Christoulas D., Kastritis E., Roussou M., Migkou M., Eleutherakis-Papaiakovou E., Gavriatopoulou M., Gkotzamanidou M., Kanellias N., Manios E., Papadimitriou C., Dimopoulos M.A. VTD consolidation, without bisphosphonates, reduces bone resorption and is associated with a very low incidence of skeletal-related events in myeloma patients post ASCT. Leukemia. 2014;28:928–934. doi: 10.1038/leu.2013.267. [DOI] [PubMed] [Google Scholar]
- Venturelli S., Sinnberg T.W., Niessner H., Busch C. Molecular mechanisms of pharmacological doses of ascorbate on cancer cells. Wien. Med. Wochenschr. 2015;165:251–257. doi: 10.1007/s10354-015-0356-7. [DOI] [PubMed] [Google Scholar]
- Verrax J., Calderon P.B. Pharmacologic concentrations of ascorbate are achieved by parenteral administration and exhibit antitumoral effects. Free Radic. Biol. Med. 2009;47:32–40. doi: 10.1016/j.freeradbiomed.2009.02.016. [DOI] [PubMed] [Google Scholar]
- Wang L., Xu Y.L., Zhang X.Q. Bortezomib in combination with thalidomide or lenalidomide or doxorubicin regimens for the treatment of multiple myeloma: a meta-analysis of 14 randomized controlled trials. Leuk. Lymphoma. 2014;55:1479–1488. doi: 10.3109/10428194.2013.838232. [DOI] [PubMed] [Google Scholar]
- Welsh J.L., Wagner B.A., Van't Erve T.J., Zehr P.S., Berg D.J., Halfdanarson T.R., Yee N.S., Bodeker K.L., Du J., Roberts L.J., 2nd, Drisko J., Levine M., Buettner G.R., Cullen J.J. Pharmacological ascorbate with gemcitabine for the control of metastatic and node-positive pancreatic cancer (PACMAN): results from a phase I clinical trial. Cancer Chemother. Pharmacol. 2013;71:765–775. doi: 10.1007/s00280-013-2070-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav P., Cook M., Cockwell P. Current trends of renal impairment in multiple myeloma. Kidney Dis. (Basel) 2016;1:241–257. doi: 10.1159/000442511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav P., Hutchison C.A., Basnayake K., Stringer S., Jesky M., Fifer L., Snell K., Pinney J., Drayson M.T., Cook M., Cockwell P. Patients with multiple myeloma have excellent long-term outcomes after recovery from dialysis-dependent acute kidney injury. Eur. J. Haematol. 2016;96:610–617. doi: 10.1111/ejh.12644. [DOI] [PubMed] [Google Scholar]
- Yun J., Mullarky E., Lu C., Bosch K.N., Kavalier A., Rivera K., Roper J., Chio Ii, Giannopoulou E.G., Rago C., Muley A., Asara J.M., Paik J., Elemento O., Chen Z., Pappin D.J., Dow L.E., Papadopoulos N., Gross S.S., Cantley L.C. Vitamin C selectively kills KRAS and BRAF mutant colorectal cancer cells by targeting GAPDH. Science. 2015;350:1391–1396. doi: 10.1126/science.aaa5004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan F., Huang Y., Colla S., Stewart J.P., Hanamura I., Gupta S., Epstein J., Yaccoby S., Sawyer J., Burington B., Anaissie E., Hollmig K., Pineda-Roman M., Tricot G., Van Rhee F., Walker R., Zangari M., Crowley J., Barlogie B., Shaughnessy J.D., Jr. The molecular classification of multiple myeloma. Blood. 2006;108:2020–2028. doi: 10.1182/blood-2005-11-013458. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material