Abstract
We previously reported that overexpression of DHX32 contributes to the growth and metastasis of colorectal cancer (CRC). However, the underlying mechanism is not largely characterized. Herein, we reported that DHX32 in CRC cells upregulated expression of vascular endothelial growth factor A (VEGFA) at the transcription level through interacting with and stabilizing β-catenin. This promoted the recruitment of host endothelial cells to the tumor, and therefore, formation of microvessel in the tumor. Xenograft model revealed that depletion of DHX32 in CRC cells significantly reduced the microvessel density in the grafts and suppressed the growth of grafts. Furthermore, the expression level of DHX32 was positively associated with microvessel density in human CRC and poor outcome of CRC patients. Therefore, the report demonstrates that DHX32 is a pro-angiogenic factor, that inhibition of DHX32-β-catenin pathway can provide a strategy for CRC treatment, and that the expression level of DHX32 has the potential to serve as a biomarker for CRC diagnosis and prognosis.
Keywords: Colorectal cancer, DHX32, β-catenin, VEGFA, Angiogenesis
Highlights
-
•
DHX32 upregulates VEGFA expression through interacting with and stabilizing β-catenin.
-
•
DHX32 promotes colorectal cancer cells to recruit endothelial cells and induces angiogenesis.
-
•
DHX32 is associated with tumor angiogenesis and poor prognosis of colorectal cancer patients.
Tumor angiogenesis is required for cancer growth and metastasis. Understanding the molecular mechanism by which cancer cells promote angiogenesis is required to develop effective cancer treatment. In this study, we reported that DHX32 is a pro-angiogenic factor in colorectal cancer. Aberrantly expressed DHX32 promoted tumor angiogenesis by stabilizing β-catenin and increasing the expression of vascular endothelial growth factor. The results suggested that suppression of DHX32 can be of therapeutic value for colorectal cancer and that expression level of DHX32 has the potential to serve as a biomarker for colorectal cancer diagnosis and prognosis.
1. Introduction
Colorectal cancer (CRC) is the third most common cancer worldwide and accounts for 14% of all new cancer diagnoses(Fitzmaurice et al., 2015). Tumor angiogenesis that is required for cancer growth and metastasis by nourishing cancer cells and helping spread of metastatic cells to distant tissues has been considered as a potential target for CRC treatment (Hanahan and Weinberg, 2011). Understanding the molecular mechanism by which CRC cells promote angiogenesis is required to develop effective antiangiogenesis treatment for CRC.
Angiogenesis is a tightly regulated multistep process that includes endothelial cells breaking through the basement membrane, migrating toward angiogenic stimuli released from tumor cells, proliferating to provide sufficient cells for making a new vessel, and forming tubular structures (Bergers and Benjamin, 2003). The angiogenic switch depends on the balance of pro- and anti-angiogenic factors. Vascular endothelial growth factor (VEGF) is one of the key pro-angiogenic stimuli produced within the tumor microenvironment in pathological states (Carmeliet and Jain, 2011, Conway et al., 2001, Yancopoulos et al., 2000). In CRC, the level of VEGF is elevated and correlate with a poor clinical outcome (Takahashi et al., 1995, Ellis et al., 2000). VEGF has, therefore, been a major focus for the development of anti-cancer drug and is considered a negative prognostic indicator for CRC. Many signaling pathways are interconnected to activate VEGF expression during tumorigenesis and progression (Des Guetz et al., 2006, Kerbel, 2008, Grothey and Galanis, 2009). Transcription of VEGF is regulated by multiple external factors. The VEGFA promoter contains binding sites for numerous transcription factors, including SP1, AP2, c-JUN, EGR-1, HIF-1, and TCF (Pages and Pouyssegur, 2005, Liu et al., 2016). Recent work reveals that activated β-catenin translocates to the nucleus and complexes with TCF to promote transcription of VEGFA (Easwaran et al., 2003, Clifford et al., 2008), indicating that Wnt/β-catenin signaling regulates vessel formation. Therefore, targeting Wnt/β-catenin mediated VEGFA expression can be a new strategy for inhibiting angiogenesis.
RNA helicases are members of the DEAD/H-box family, which are characterized by the presence of a helicase domain and are involved in RNA posttranscriptional procession. In addition to their roles in RNA procession, multiple members of RNA helicases are also implicated in transcription regulations. Aberrant expression of these proteins have been reported in various solid and hematologic malignancies (Wilson and Giguere, 2007, Causevic et al., 2001, Schlegel et al., 2003, Abdelhaleem, 2004a). We reported previously that DHX32, a novel member of the DEAH family, is up-regulated in CRC and contributes to CRC proliferation, apoptosis, migration, and invasion. Array analyses revealed that depleting DHX32 in CRC cells suppressed expression of VEGFA, indicating that DHX32 is involved in tumor angiogenesis (Huang et al., 2009, Lin et al., 2015). However, the mechanism by which DHX32 upregulates expression of VEGFA remains unknown.
In this study, we showed that DHX32 promoted expression of VEGFA through augmenting β-catenin signaling. Ablation of DHX32 in CRC cells compromised their tumorigenicity and angiogenesis when they were grafted in mice. Furthermore, overexpression of DHX32 was associated with angiogenesis in CRC and poor outcomes of human CRC patients. These results reveal a mechanism by which DHX32 enhances angiogenesis and growth of CRC. It also uncovers the potential of DHX32 as a target for CRC treatments and as a biomarker for CRC diagnosis and prognosis.
2. Materials and Methods
2.1. Cell Culture and Reagents
Human CRC SW480, HCT-8, SW620, and HCT116 cells were grown in the RPMI-1640 medium (Gibco), human CRC HT-29 cells were grown in the Myco5A medium (Gibco), and HUVEC cells were grown in the DMEM medium (Gibco), with supplements of 10% (v/v) fetal bovine serum (Gibco) and 100 units/ml streptomycin and penicillin (Millipore), at 37 °C. Stable strains of SW480 cell with DHX32-overexpression or depletion were generated, characterized, and cultured as described in our previous publication (Lin et al., 2015). The conditioned medium was collected from confluent SW480 cells 24 h after changing the medium to the 0.2% FBS-DMEM medium. The medium was filtered with 0.22 μm filters prior to being used.
The anti-DHX32 antibody, anti-VEGFA antibody and anti-CD31 antibody were purchased from Abcam; anti-β-catenin antibody, anti-phospho-β-catenin (Ser33/37/Thr41) antibody, anti-β-TrCP antibody and anti-ubiquitin antibody from Cell Signaling; anti-β-Actin antibody and anti-HA antibody from Santa Cruz; anti-Flag antibody from Sigma.
2.2. DNA constructs and transfection
Expression vectors carrying the Flag- and His-tagged DHX32 cDNAs were constructed by sub-cloning the PCR product of human DHX32 cDNA into pCMV5 vector and were confirmed by sequencing. Human β-catenin cDNA, β-catenin truncated mutants, pTOPFLASH, and pFOPFLASH were generous gifts from Prof. B. Li. (Xiamen University, China). The renilla luciferase pRL-TK reporter was kindly provided by Prof. H-R Wang. (Xiamen University, China). Τhe β-catenin cDNA was subcloned into pGEX4T-1 vector for bacterial expression. siRNA targeting β-catenin and the control siRNA were purchased from Santa Cruz. siRNA targeting DHX32 and the control siRNA were purchased from QIAGEN.
Transfections of siRNA and plasmid were performed using the HiPerFect Transfection Reagent (QIAGEN) and ViaFect Transfection Reagent (Promega) according to the manufacturer's protocol, respectively.
2.3. Quantitative real-time RT-PCR
Total RNA was extracted from cells using Trizol (TIANGEN) according to the manufacturer's protocol and the cDNA was synthesized using the RevertAid First Strand cDNA Synthesis Kits (Fermentas). Quantitative real-time RT-PCR was performed using gene-specific primers as described in our previous study (Huang et al., 2009). The expression of target transcripts was normalized to the β-actin internal control, and relative changes of gene expression were determined using the 2− ΔΔCt method. The primers for DHX32 are 5′-GTCTTTCCATCCACTACCAGCAC-3′(forward) and 5′-ATGATGACCCCATAGCTACCCAA-3′(reverse), β-actin are 5′-TCACCCACACTGTGCCCATCTACGA-3′(forward) and 5′-CAGCGGAACCGCTCATTGCCAATGG-3′(reverse), VEGFA are 5′-AGGGCAGAATCATCACGAAGT-3′(forward) and 5′-AGGGTCTCGATTGGATGGCA-3′(reverse), and β-catenin are 5′-CATCTACACAGTTTGATGCTGCT-3′ (forward) and 5′-GCAGTTTTGTCAGTTCAGGGA-3′(reverse).
2.4. Matrigel Invasion Assay
Cells were seeded in 24-well plates at a density of 2.5 × 104 per well. After attaching to the surface, the cells were washed with PBS and the culture media were replaced with 700 μl 0.2% FBS-DMEM Medium. After being cultured for 48 h, each well was then inserted a Matrigel invasion chamber with an 8.0 μm pore size membrane (BD) containing 0.75 × 105 serum-starved HUVECs in 0.5 ml 0.2% FBS-DMEM medium. After co-cultured for 24 h, non-migrating cells were removed from the upper chamber with a cotton swab and cells migrated through the membrane were fixed with 4% formaldehyde and stained with crystal violet staining solutions. Cell numbers in three randomly selected fields were photographed and counted under a microscope (Leica) with 200 × magnification.
2.5. Scrap-wound Healing Assay
Cells were seeded in 6-well plates at a density of 105 cells per well and incubated for 24 h. Then a 20 μl pipette tip was used to scratch a linear wound in the cell monolayer. Photographs were taken at 0 and 24 h after scrapping.
2.6. Cell Proliferation Assays
The Cell Counting Kit-8 (CCK-8) (Dojindo) was used to measure cell density. 3 × 103 cells per well were seeded in 96-well plates and incubated for 48 h. The cells were then incubated with the CCK-8 reagent (10 μl per well) for 2 h prior to measure the absorbance of 450 nm with an ELISA plate reader (Thermo).
2.7. In vitro Angiogenesis Assay
HUVECs (2 × 104) were seeded in 24-well plates containing 0.5 ml solidified Matrigel (10 mg/ml) and cultured in the conditioned medium for 12 h. Images were acquired with a phase-contrast microscope. Average numbers of microtubes were counted in three individual wells and presented as mean ± s.d.
2.8. Affinity Purification of DHX32 Binding Proteins
DHX32 transiently expressed in SW480 cells was purified with anti-DHX32 antibody and protein A/G beads (Santa Cruz) in lysis buffer TNPE 0.2% (0.2% NP-40, 20 mM Tris-HCl, pH 7.5, 150 mM NaCl and 1 mM EDTA). The bead-binding proteins were eluted by boiling in the loading buffer and then subjected to SDS-PAGE. The proteins were detected with silver staining, and specific bands were excised and analyzed by mass spectrometry.
2.9. Immunoprecipitation, Immunoblotting, and Ubiquitination Assays
Immunoprecipitation (IP), immunoblotting (IB), and ubiquitination assays were performed as previously described (Lin et al., 2014). Briefly, cell lysates prepared using ice-cold lysis buffer TNTE 0.5% (50 mM Tris-HCl [pH 7.5], 150 mM NaCl, 1 mM EDTA, and 0.5% Triton X-100, containing 10 mg/ml pepstatin A, 10 mg/ml leupeptin, and 1 mM PMSF) were applied to IP or immunoblotting assays with appropriate antibodies. For ubiquitination assay, cell lysates were subjected to anti-β-catenin IP and the ubiquitin-conjugated proteins were detected by immunoblotting and Chemiluminescent HRP substrates (Millipore).
2.10. GST Pull-down
GST fusion proteins were expressed in the BL21 strain of E. coli. To purify the GST fusion proteins, the cells were lysed by sonication in TNTE 0.5% and the resulting lysates were incubated for 3 h at 4 °C with glutathione-sepharose beads. The beads were pelleted by centrifugation and washed with dialysis buffer for subsequent experiments. His-fusion protein lysates from BL21 were then incubated with the resin-bound proteins by rotating at 4 °C for 2 h. The samples were analyzed by Western blotting using the appropriate antibodies.
2.11. Immunofluorescence Assay
SW480 cells were washed in PBS, fixed with 4% paraformaldehyde, and permeabilized with 0.25% Triton X-100. DHX32 proteins were detected using rabbit anti-DHX32 antibody followed by Alexa Fluor 555-conjugated donkey anti-rabbit secondary antibody (Life Technologies). β-catenin was detected using mouse anti-β-catenin antibody followed by Alexa Fluor 488-conjugated donkey antibody to mouse immunoglobulin G (Life Technologies). The cells were counterstained with DAPI and imaged using a confocal laser-scanning microscope (Zeiss LSM 780).
2.12. Luciferase Assay
Luciferase reporter plasmids were transiently transfected into SW480 cells. Thirty-six hours after transfection, the cells were lysed with the Luc-lysis buffer. The lysates were harvested for measuring the firefly and renilla luciferase activities using Dual-Glo Luciferase Assay System (Promega). The firefly luciferase activity was normalized for transfection efficiency using the corresponding renilla luciferase activity.
2.13. ELISA Assay
The concentration of VEGF in culture medium supernatants was determined by enzyme-linked immunosorbent assay (ELISA). The human VEGF ELISA kits were purchased from BOSTER. The assay was performed according to the manufacturer's protocol.
2.14. Chromatin Immunoprecipitation (ChIP)
After cross-linking with 1% formaldehyde, SW480 stable cells were lysed and subjected to ChIP analyses with the ChIP assay kit from Millipore according to the manufacturer's protocols. Cells were collected and resuspended in SDS lysis buffer and sonicated. After centrifugation, the supernatant was diluted and incubated with the appropriate antibodies overnight at 4 °C. Protein A/G sepharose beads were then added, and the mixture was incubated for an additional 1 h at 4 °C. The sepharose beads were washed once in low-salt wash buffer and once in high-salt wash buffer, followed by washes with LiCl buffer and a final two washes with TE buffer. The beads were then extracted twice with elution buffer (1% SDS, 0.1 M NaHCO3). 5 M NaCl was then added, and the mixture was incubated at 65 °C overnight to reverse cross-linking. The DNA fragments were purified and subjected to PCR using specific primers. Nonimmunoprecipitated chromatin was used as an “input” control, and an IgG antibody control was performed on all occasions. The VEGF primers yielded a 161-bp pair product corresponding to − 262 to − 101 of the VEGF gene promoter and were as follows: 5′-GCGTGTCTCTGGACAGAGTTT-3′(forward) and 5′-AGCCTCAGCCCTTCCACA-3′(reverse).
2.15. Tumorigenicity Assay in Nude Mice
SW480 stable cells were used for the tumorigenicity assays. 5 × 106 of control and experimental cells suspended in 100 μl PBS were injected subcutaneously into the flank of BALB/c mice. Tumor volume was determined by external measurement according to the formula volume (mm3) = width2 × length/2 and the growth of tumors were monitored for 40 days. All mice were then sacrificed and xenografts were obtained, weighed, and fixed with 10% formaldehyde. All mice were kept under specific pathogen-free conditions at Xiamen University Laboratory Animal Center (Xiamen University, China) in accordance with institutional guidelines. All experimental protocols were approved by the Ethics Committee for Animal Experimentation of Xiamen University.
2.16. Patient Samples
Patient samples including 139 primary human colorectal cancer and 93 corresponding adjacent non-cancerous tissues collected between 2009 and 2013 were obtained from Xiamen University Affiliated Zhongshan Hospital. The samples are collected with a patient consent and are approved by the Institutional Review Board of Zhongshan Hospital. These samples were subsequently de-identified to ensure patient confidentiality. All samples taken after the surgery were fixed, dehydrated, and embedded in paraffin for further assays.
2.17. Immunohistochemistry Assay
Tissues were fixed, dehydrated, embedded, and sectioned according to standard procedures. Antigens were retrieved by boiling in the citrate buffer (10 mM) for 3 min, and then incubated in 3% hydrogen peroxide for 20 min to block endogenous peroxidase. All sections were incubated with primary antibodies at 4 °C overnight. The UltraSensitive SP kit (Maxim) was then used to detect the specifically bound primary antibodies. The stain intensity was scored from 0 to 3 as following: no staining (score 0), weak staining (score 1), moderate staining (score 2), or strong staining (score 3). The percentage of stained cells was scored from 0 to 3 as following: < 10% (score 0), 11%–50% (score 1), 51%–75% (score 2), 76%–100% (score 3).
2.18. Statistical Analysis
The data were expressed as mean ± SD of triplicate samples. Comparison of non-normal data was analyzed using Mann-Whitney U test, and analysis of normally data was performed using the Student's t-test between two groups. Univariate analysis and multivariate logistic regression were performed to analysis the association between patient characteristics and DHX32 expression. OS and MFS were estimated by Kaplan-Meier method. All P values were based on two-sided testing and statistical analysis was performed using SPSS 20.0 statistical software. P < 0.05 was considered a statistically significant difference.
3. Results
3.1. DHX32 Increases the Stability of β-catenin Through Direct Interaction With it
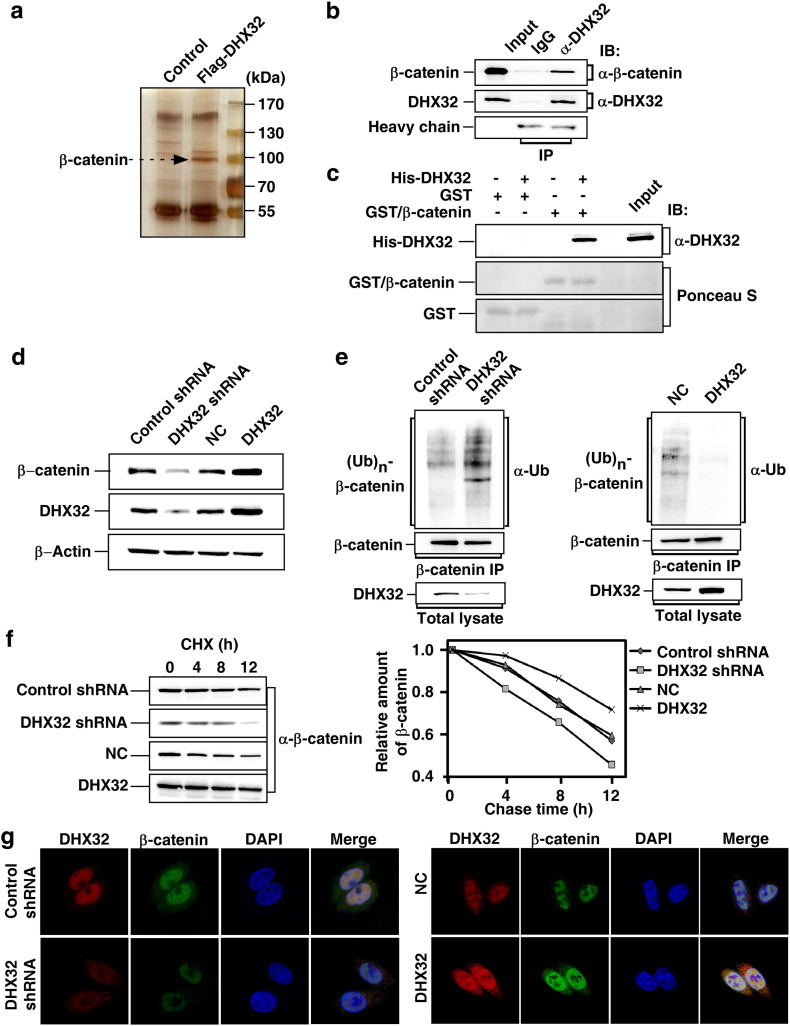
We reported previously that DHX32 is aberrantly expressed in CRC and promotes CRC progression and metastasis (Huang et al., 2009, Lin et al., 2015). To determine the downstream target of DHX32 that contributes to CRC progression, immunoprecipitation of FLAG-tagged DHX32 followed by mass spectrometry analyses was carried out to identify the proteins binding to DHX32. Among the detected proteins, β-catenin was identified (Fig. 1a). β-catenin has been reported to affect the transcription of multiple genes and its expression is dysregulated in CRC (Korinek et al., 1997). To verify the interaction between β-catenin and DHX32, Western blot was carried out to analyze the immunocomplex of DHX32. The results clearly demonstrated that β-catenin was indeed pulled down by endogenous DHX32 in SW480 cells (Fig. 1b). Moreover, we confirmed that DHX32 could be detected in β-catenin immunocomplexes (Supplementary Fig. S1). Next, we performed in vitro GST-pull down assay to examine whether DHX32 and β-catenin directly interacted. Using purified proteins produced in bacterial, we demonstrated that DHX32 was pulled down by GST/β-catenin fusion protein, but not by GST only, indicating a direct interaction between DHX32 and β-catenin (Fig. 1c). Furthermore, we mapped the interaction domain of β-catenin for DHX32 using β-catenin truncations and demonstrated the direct binding of DHX32 with β-catenin at the N terminus and armadillo repeat region (Supplementary Fig. S2).
Fig. 1.
DHX32 interacts with β-catenin and protects β-catenin from degradation. (a) Flag-tagged DHX32 transiently expressed in SW480 cells was purified and applied to SDS-PAGE. The proteins were visualized by silver staining, and indicated spots were analyzed by mass spectrometry. (b) Interaction of endogenous DHX32 and β-catenin proteins. Cell lysates from SW480 cells were subjected to IP with antibody against DHX32 followed by immunoblotting (IB) to detect endogenous β-catenin. (c) In vitro interaction between DHX32 and β-catenin. Bacterial-expressed and purified His-tagged DHX32 and GST-tagged β-catenin were subjected to GST pull-down assays as indicated. DHX32 proteins were detected with anti-DHX32 antibody. GST and GST/β-catenin were determined by Ponceau S staining. (d) Endogenous β-catenin protein levels in SW480 cells with DHX32 depletion or overexpression were detected by immunoblotting. (e) DHX32 decreases ubiquitination of β-catenin. After overnight treatment of MG-132 (10 μM), SW480 stable cells were subjected to anti-β-catenin IP. The ubiquitin-conjugated β-catenin ((Ub)n-β-catenin) was detected with anti-Ub antibody. (f) DHX32 regulates turnover rates of β-catenin. The levels of β-catenin at different time points after cycloheximide (CHX) treatment with SW480 stable cells were determined by immunoblotting the total cell lysates and quantification using Image Lab software (Bio-Rad) with β-Actin as a loading control. Results plotted are the amounts of β-catenin at each time point relative to the level at time 0. (g) DHX32 colocalizes with β-catenin proteins at nucleus. Localization of DHX32 (red) and β-catenin (green) in SW480 cells with DHX32 overexpression or depletion were detected by immunofluorescence. The nuclei were stained with DAPI (blue).
We next investigated whether the activity of β-catenin was regulated by DHX32. Since the activity of β-catenin is mainly regulated through post-translational modifications (Kikuchi et al., 2006), we first examined whether the protein level of β-catenin is affected by DHX32. SW480 human CRC cells with DHX32-depletion or overexpression were generated by stable transfection (Lin et al., 2015). Western blot showed that the abundance of β-catenin was reduced in DHX32-depleted, and increased in DHX32 overexpressing SW480 cells (Fig. 1d). Of note, neither depletion nor overexpression of DHX32 affected the expression of β-catenin at the mRNA level (Supplementary Fig. S3), suggesting that DHX32 regulated the abundance of β-catenin at the protein level. Ubiquitination-mediated degradation is the main mechanism for downregulating β-catenin (Aberle et al., 1997). Therefore, we next assessed the ubiquitination of β-catenin in SW480 cells with or without depletion or overexpression of DHX32 to determine whether DHX32 regulated ubiquitination of β-catenin. It was clear that depletion of DHX32 markedly enhanced ubiquitination of β-catenin, while overexpression of DHX32 decreased the levels of ubiquitin-conjugated β-catenin (Fig. 1e). Accordingly, depletion of DHX32 significantly decreased the half-lives of β-catenin proteins, whereas overexpression of DHX32 prolonged the half-lives of β-catenin proteins (Fig. 1f).
It has been reported that DHX32 is located in the nucleus and mitochondrial in myeloid leukemia cells HL-60(Alli et al., 2006). In SW480 cells, β-catenin is constitutively accumulated in nucleus due to the loss of wild-type adenomatous polyposis coli (APC) (Korinek et al., 1997). Consistently, immunofluorescent microscopy also demonstrated that DHX32 and β-catenin were predominantly colocalized in the nucleus. Furthermore, depletion of DHX32 reduced nuclear β-catenin whereas overexpression of DHX32 increased nuclear β-catenin (Fig. 1g).
3.2. DHX32 Stimulates VEGFA Expression in a β-catenin/TCF-Dependent Manner
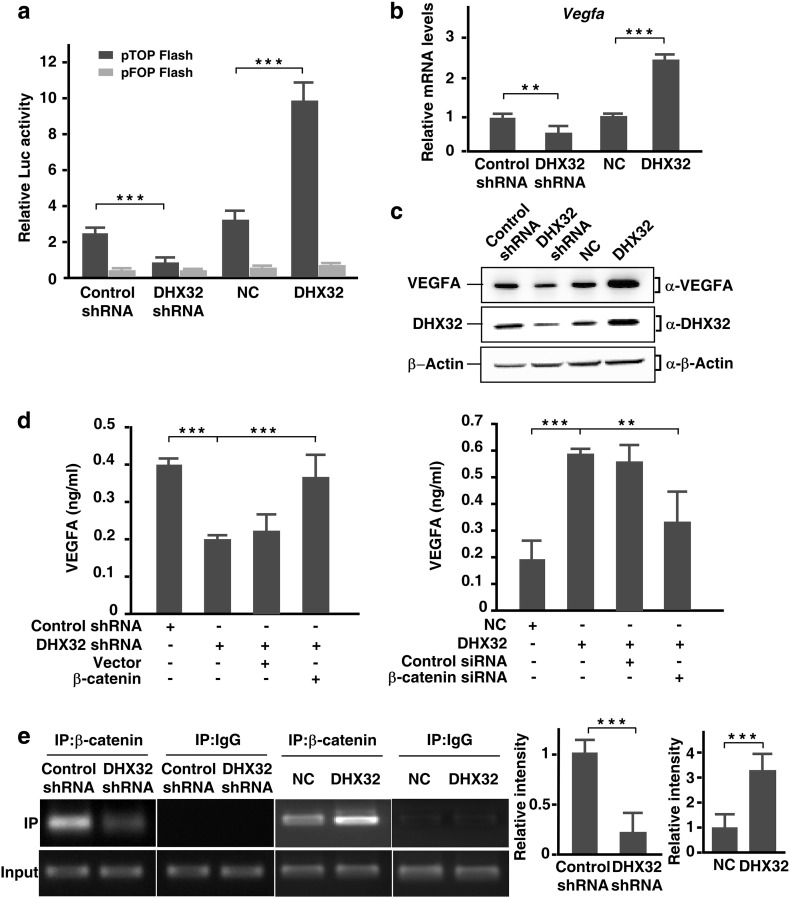
The data that the expression level of DHX32 affected nuclear expression of β-catenin prompted us to determine whether the transcription activity of β-catenin was regulated by DHX32. Therefore, we first employed the β-catenin/TCF luciferase reporter pTOPFLASH (Bai et al., 2015) to determine whether DHX32 upregulated the expression of the reporter. As shown in Fig. 2a, depletion of DHX32 suppressed the expression of pTOPFLASH and overexpression of DHX32 enhanced the expression of the reporter. The expression of the control vector pFOPFLASH that did not have an active β-catenin/TCF responsive elements was not affected by DHX32 (Fig. 2a). The result demonstrates that activity of β-catenin is upregulated by DHX32. It has been recently reported that VEGFA, a key regulator of angiogenesis, is a downstream target of β-catenin/TCF signaling in colon cancer cells (Easwaran et al., 2003). In addition, we reported previously that depletion of DHX32 in colon cancer cells attenuates expression of VEGFA at the mRNA level (Lin et al., 2015). Therefore, we hypothesized that DHX32 controlled VEGFA expression via the β-catenin/TCF signaling pathway. To test this hypothesis, we first employed quantitative RT-PCR analyses to assess VEGFA expression in the DHX32-depleted or overexpressing SW480 cells. In comparison with controls cells, the expression of VEGFA in DHX32 depleted cells was reduced, while it was increased in DHX32 overexpressing cells (Fig. 2b). Western blot analyses further demonstrated that expression of VEGFA in SW480 cells was upregulated by DHX32 at the protein level (Fig. 2c). Furthermore, we confirmed the effects of DHX32 knockdown on VEGFA expression in different types of colorectal cancer cells, including HCT-8, HT-29, SW620, and HCT116 (Supplementary Fig. S4).
Fig. 2.
DHX32 promotes transcription of VEGFA in CRC cells. (a) SW480 stable cells were transfected with β-catenin/TCF luciferase reporter gene pTOPFLASH or mutated pFOPFLASH, pRL-TK as internal control. The mean ± SD of a representative result of three independent experiments is shown. (b) Real-time RT-PCR analyses of VEGFA expression in SW480 cells with DHX32 depletion or overexpression. (c) Endogenous VEGFA protein levels in SW480 cells with DHX32 overexpression or depletion were detected by immunoblotting. (d) SW480 stable cells with depletion DHX32 were transfected with β-catenin and DHX32-overexpressed stable cells were transfected with control siRNA or siRNA against β-catenin. VEGFA protein levels in condition medium were quantified by ELISA analysis. (e) Representative PCR gel of ChIP assays showing binding of β-catenin to the VEGF promoter over the IgG control. Immunoprecipitate was carried out using an antibody to β-catenin. Nonimmunoprecipitated chromatin was used as an “input” control, and an IgG antibody control was performed on all occasions. The PCR primers were amplified in the − 262 to − 101 region of the VEGF promoter. **P < 0.01, ***P < 0.001.
To investigate whether β-catenin was required for upregulation of VEGFA expression by DHX32 in SW480 cells, β-catenin was overexpressed in SW480 cells with DHX32 depletion or depleted in SW480 cells with DHX32 overexpression. Enzyme-linked immunosorbent assay (ELISA) was carried out to determine the VEGFA level in the mediums conditioned by these SW480 cells. The data showed that overexpression of β-catenin compensated the loss of VEGFA expression due to depletion of DHX32. On the other hand, depletion of β-catenin attenuated the increase of VEGFA expression caused by DHX32 overexpression (Fig. 2d). Together, the results demonstrate that β-catenin is required for DHX32 to upregulate the expression of VEGFA in SW480 cells.
Furthermore, chromatin immunoprecipitation (ChIP) assays revealed that the capture of the VEGFA promoter region by β-catenin was reduced in DHX32 depleted SW480 cells. Consistently, the VEGFA promoter region pulled down by β-catenin was increased in SW480 cells with DHX32 overexpression (Fig. 2e).
3.3. Ablation of DHX32 in CRC Cells Compromises the Activity of the Cells to Interact With Endothelial Cells
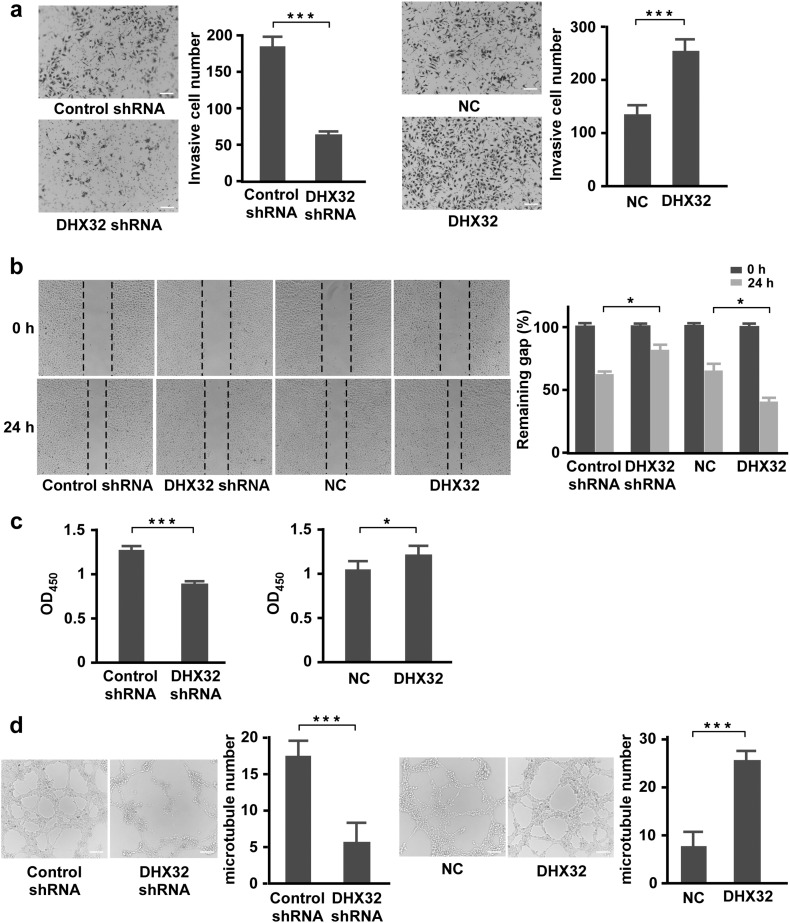
We then carried out the Transwell coculture with SW480 in the bottom chamber and HUVECs in the upper chamber to determine whether the manipulation of DHX32 expression affected the activity of SW480 cells to recruit endothelial cells. Relative to the control, depletion of DHX32 decreased, whereas overexpression of DHX32 increased, the activity of SW480 to induce endothelial cells to migrate across the membrane (Fig. 3a). Moreover, the necessity of DHX32 for HUVEC cells migration was confirmed in different types of cancer cells, including HCT-8, HT-29, SW620, and HCT116 (Supplementary Fig. S5). Furthermore, the medium conditioned by DHX32 depleted cells had a lower activity to stimulate migration in wound healing assays (Fig. 3b), proliferation (Fig. 3c), and microtube formation in Matrigel (Fig. 3d) of HUVECs than the medium conditioned by control SW480 cells. Consistently, forced expression of DHX32 in SW480 cells increased their activity to promote migration in wound healing assay, proliferation, and microtubes formation of HUVECs. Together, the results indicate that DHX32 promotes CRC cells to recruits endothelial cells and induces angiogenesis.
Fig. 3.
DHX32 promotes CRC cells to recruits endothelial cells and induces angiogenesis. (a) Transwell Matrigel invasion assays with HUVECs in the upper chamber and SW480 in the lower chamber. The average numbers of migrated cells were quantitated and expressed as mean ± SD. (b) Scratch wound assays for HUVECs followed by addition of medium containing indicated SW480 cell-conditioned medium. Images were taken before and after culture for 24 h with the conditioned medium. The average sizes of the gaps were measured at the indicated times and expressed as mean ± SD of triplicated samples. (c) HUVECs were cultured for 48 h with indicated SW480 cell-conditioned medium. The cell density was then determined with the CCK-8 Assay Kit as described in Methods. (d) HUVECs were seeded in Matrigel with medium conditioned by SW480 cells with depleted DHX32 for 12 h or with medium conditioned by SW480 cells with DHX32 overexpression for 8 h. The average number of tubes formed from HUVECs were calculated and presented as mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001. The scale bars indicate 10 μm.
3.4. Ablation of DHX32 Inhibits the Tumorigenicity of CRC Cells
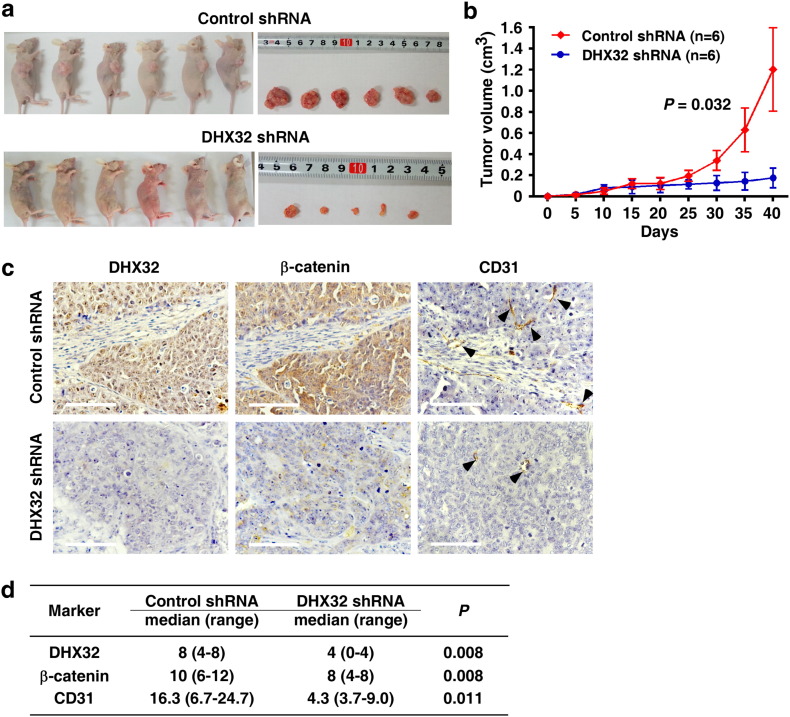
Angiogenesis is essential for tumor growth and invasion (Bergers and Benjamin, 2003). To further investigate whether DHX32 contributed to angiogenesis and tumor progression in vivo, SW480 cells with or without depletion of DHX32 were grafted to the flank of nude mice by injecting the cells subcutaneously. The sizes of the tumors were measured with a caliper every 5 days and the tumors were excised for analyses 40 days after the implantation. Strikingly, tumors derived from the cells with DHX32 depletion were smaller than those derived from control cells (Fig. 4a) and had a reduced growth rate compared with the control (Fig. 4b).
Fig. 4.
Ablation of DHX32 inhibits the tumorigenicity of CRC cells. (a & b) SW480 cells with or without depletion of DHX32 were grafted to the flank of nude mice by injecting the cells subcutaneously to observe tumor development, and volumes of tumor were determined and plotted as mean ± SD of six independent experiments. (c) Expression levels of DHX32, β-catenin and CD31 in transplanted tumor tissues were determined by immunohistochemistry analysis. The scale bars indicate 100 μm. (d) Statistical analyses of expression of DHX32, β-catenin and CD31 in DHX32 depletion and control tumors. *P < 0.05.
To determine whether ablation of DHX32 compromised angiogenesis in the tumors, the abundance of CD31+ endothelial cells in tumor tissues was assessed by immunohistochemical staining as described(Hasan et al., 2002) (Fig. 4c). Statistical analyses of expression of DHX32, β-catenin, and CD31 were shown in Fig. 4d. Tumors with depletion of DHX32 had less CD31+ endothelial cells than control groups, indicating a reduction in vasculature in DHX32 deficient tumors. In addition, expression of β-catenin was also lower in DHX32 deficient tumors than that in control tumors. Consistently, these data indicated that ablation of DHX32 decreased the expression of β-catenin and reduced angiogenesis in the tumors.
3.5. Overexpression of DHX32 in hUman CRC is Associated With Tumor Angiogenesis and Poor Prognosis of CRC Patients
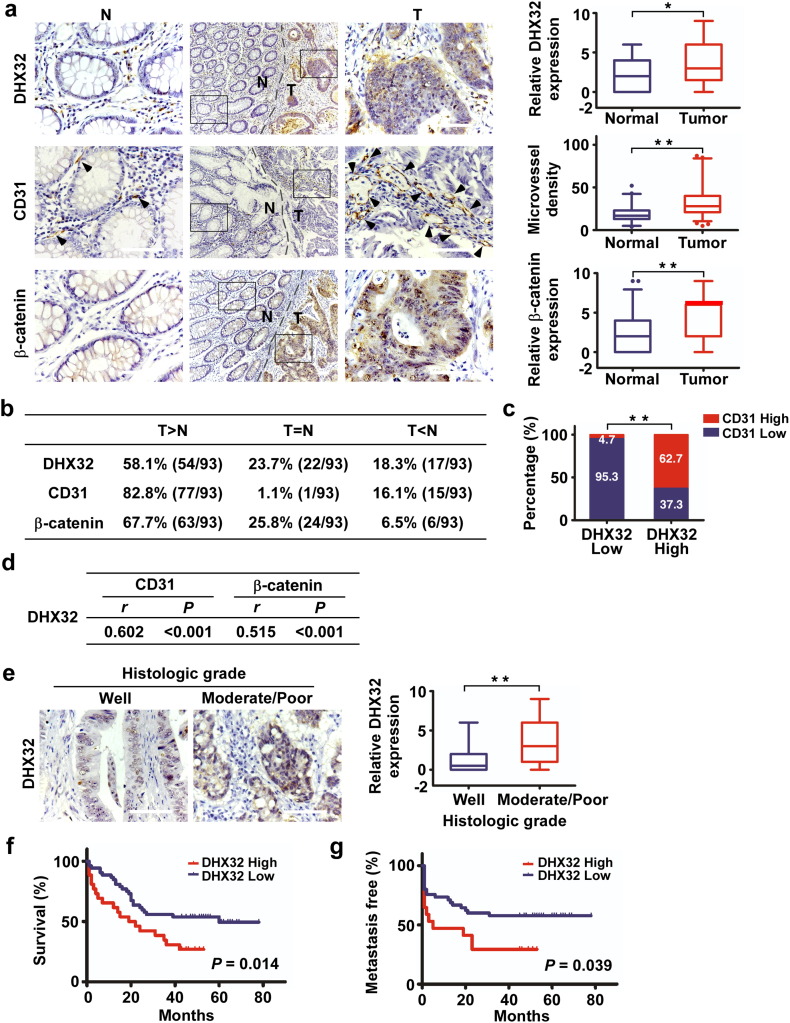
DHX32 is upregulated in human CRC compared to its adjacent normal tissues (Huang et al., 2009). However, whether the expression of DHX32 is associated with and contributes to tumor angiogenesis in human CRC has not been established. To determine the clinical relevance of DHX32 expression and its correlation with the microvessel density in human CRC, immunohistochemical staining with anti-DHX32, anti-CD31, and anti-β-catenin antibodies was carried out to assessed the expression of DHX32, CD31, and β-catenin in a total of 139 CRC tissues and 93 adjacent non-cancerous tissues. Detailed quantitative analyses revealed that there were significant differences in relative DHX32 protein levels (P = 0.028), microvessel density (P < 0.001) and β-catenin expression levels (P < 0.001) between paired CRC and non-tumor tissues (Fig. 5a, Supplementary Fig. S6a). Compared with paired non-cancerous tissues, 58.1% (54/93) of the CRC samples showed a higher expression of DHX32 than did the non-cancerous samples, 82.8% (77/93) showed elevated microvessel density, and 67.7% (63/93) showed increased expression of β-catenin (Fig. 5b). The median expression value of all 139 cases was chosen as the cutoff value for separating the dataset into DHX32-low group (score ≤ 4) and DHX32-high group (score > 4). As shown in Fig. 5c, 62.7% (47/75) of the DHX32-high group (75 out of 139) also had high abundance of CD31+ cells (CD31+ endothelial cells ≥ 34), while the DHX32-low group (64 out of 139) only 4.7% (3/64) had high abundance of CD31+ cells. We than carried out statistical analyses for the correlation between DHX32 expression and microvessel density as well as that between the expression of DHX32 and β-catenin (Fig. 5d). The results demonstrated a positive correlation between DHX32 expression and microvessel density determined by CD31 staining (Pearson contingency coefficient: r = 0.602, P < 0.001) and between the expression of DHX32 and β-catenin (Pearson contingency coefficient: r = 0.515, P < 0.001).
Fig. 5.
Overexpression of DHX32 is associated with tumor angiogenesis and poor prognosis of CRC patients. (a) Representative images and statistical analyses of DHX32, CD31 and β-catenin expressions in CRC tissues and adjacent non-tumor tissues. (b) Summary of the differences in the expression of DHX32 protein, CD31 and β-catenin protein between paired CRC and adjacent non-tumor tissues. (c) Correlation between DHX32 protein levels and CD31 in CRC tissues. (d) Statistical analyses of the association between DHX32 and CD31 expressions as well as DHX32 and β-catenin. (e) Representative images and statistical analyses of DHX32 expressions in different histologic grade samples. (f & g) Statistical analyses of the association of DHX32 expression with overall survival time (f) and metastasis free survival time (g) of CRC patients. N, Adjacent non-tumor control; T, Tumor; r, Pearson correlation coefficient; *P < 0.05, **P < 0.01. The scale bars indicate 100 μm.
To further understand the significance of DHX32 in CRC, the correlation between DHX32 expression and clinicopathological characteristics was analyzed. As shown in Table 1 and Fig. 5e, high DHX32 expression levels were significantly associated with poor pathologic grades of CRC. In addition, to determine whether DHX32 expression correlated with the prognosis of CRC patients, the association of DHX32 expression and the CRC overall survival (OS) and metastasis-free survival (MFS) times of the 139 patients were analyzed (Fig. 5f & g, Supplementary Fig. S6b). Kaplan-Meier analysis revealed a reverse association between the expression level of DHX32 and the overall survival time of the patients (Hazard ratio [HR] = 2.33; 95% CI: 1.19–4.58, P = 0.014), as well as with the metastasis-free survival time (Hazard ratio [HR] = 2.55; 95% CI: 1.05–6.17, P = 0.039). Together, our findings demonstrate that overexpressed DHX32 promotes CRC angiogenesis and progression of the clinical course of human CRC. The level of DHX32 expression in CRC tissues has a potential to serve as a biomarker for predicting outcomes of CRC patients.
Table 1.
Univariate and multivariate analysis for associations between DHX32 expression and patient features of colorectal cancer.
| Feature | n | Univariate analysis |
Multivariate analysis |
||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95%CI | P | OR | 95%CI | P | ||||
| Gender | |||||||||
| Male | 78 | 1 | |||||||
| Female | 61 | 0.99 | 0.45 | 2.15 | 0.975 | ||||
| Missing | 0 | ||||||||
| Age | |||||||||
| ≤ 50 | 37 | 1 | |||||||
| > 50 | 102 | 0.51 | 0.19 | 1.36 | 0.173 | ||||
| Missing | 0 | ||||||||
| T stage | |||||||||
| 1 + 2 + 3 | 30 | 1 | |||||||
| 4 | 94 | 1.06 | 0.4 | 2.81 | 0.91 | ||||
| Missing | 15 | ||||||||
| Histologic grade | |||||||||
| Well | 22 | 1 | 1 | ||||||
| Poor/moderate | 110 | 4.24 | 1.62 | 11.09 | 0.002 | 3.79 | 1.32 | 10.85 | 0.013* |
| Missing | 7 | ||||||||
| Lymphatic invasion | |||||||||
| Absent | 73 | 1 | 1 | ||||||
| Present | 51 | 3.24 | 1.21 | 8.69 | 0.016 | 2.38 | 0.84 | 6.7 | 0.102 |
| Missing | 15 | ||||||||
| Distant metastasis | |||||||||
| Absent | 98 | 1 | 1 | ||||||
| Present | 25 | 4.15 | 0.92 | 18.85 | 0.049 | 2.24 | 0.46 | 10.91 | 0.317 |
| Missing | 16 | ||||||||
| Perineural invasion | |||||||||
| Absent | 44 | 1 | |||||||
| Present | 90 | 1.8 | 0.78 | 4.15 | 0.165 | ||||
| Missing | 5 | ||||||||
| Vascular invasion | |||||||||
| Absent | 60 | 1 | |||||||
| Present | 76 | 1.9 | 0.85 | 4.23 | 0.114 | ||||
| Missing | 3 | ||||||||
n: sample number; OR: odd ratio; CI: confidence interval; P: P-value
Bold values indicate significance at P < 0.05.
Significant association between DHX32 expression and pathologic grades of CRC.
4. Discussion
With a few exceptions, the activities and roles of the RNA helicase family, including DHX32, are generally not well understood. Our previous work showed that overexpression of DHX32 contributes to the growth and metastasis of CRC. In this report, we further demonstrated that aberrantly expressed DHX32 promoted tumor angiogenesis by binding to and stabilizing β-catenin, and therefore, increasing the expression of VEGFA. Ablation of DHX32 in SW480 human CRC cells significantly impaired the growth of tumors derived from the cells. Furthermore, immunostaining of 139 CRC and 93 adjacent non-tumor tissues revealed that the expression level of DHX32 was associated with high microvessel density of the tumor and poor prognosis of CRC patients. Therefore, the results, for the first time, demonstrate that DHX32 is a potent tumor angiogenesis promoter and that the abundance of DHX32 has the potential to serve as a biomarker for CRC diagnosis and prognosis.
As a pro-oncogenic factor, β-catenin is one of the most important regulators that trigger the onset of tumor formation. In normal cells, signals of β-catenin are mainly activated by the canonical Wnt signaling pathway. Activation of the Wnt pathway leads to the dissociation of β-catenin from the APC/axin/GSK-3β complex. The released β-catenin then translocates to the nucleus and activates gene expressions. About 80% of CRC has loss of function mutations in APC. The mutations compromise the degradation of β-catenin, resulting in translocation of β-catenin to the nucleus where it dimerizes with the TCF/LEF factor, activates gene transcriptions, and contributes to angiogenesis in CRC (Clevers and Nusse, 2012, Pendas-Franco et al., 2008, Kiewisz et al., 2015). Several studies demonstrate that targeting the oncogenic activity of β-catenin has potent anti-tumor effects (Bienz and Clevers, 2000). In this study, we identified that DHX32 directly interacted with β-catenin in CRC cells. Binding of DHX32 to β-catenin prevented β-catenin from ubiquitination and degradation, and therefore, enhanced the activity of β-catenin. Previous studies showed that phosphorylation at N-terminal of β-catenin result in β-TrCP-mediated degradation (Yost et al., 1996). To address how DHX32 is stabilizing β-catenin, we tested whether DHX32 influenced N-terminal phosphorylation of β-catenin and whether it regulates β-TRCP protein level. However, neither over-expression nor depletion of DHX32 has significant effect on phosphorylation level of β-catenin and protein level of β-TrCP (Supplementary Fig. S7), indicating that DHX32-dependent regulation of β-catenin is not through inhibiting N-terminal phosphorylation of β-catenin or expression of β-TrCP. The mechanism by which DHX32 stabilizes β-catenin remains unknown, and is the next challenge for us to understand the function of DHX32.
It has been known that RNA helicases are aberrantly expressed and play a key role in various solid and hematologic malignancies. In addition to their roles in RNA procession, multiple members of the DEXD/H-proteins, including DDX1, DDX3, DHX5, DHX9 and DHX17, are also implicated in transcription regulations (Abdelhaleem, 2004b, Fuller-Pace, 2006, Ishaq et al., 2009). Our previous work shows that DHX32 is up-regulated in CRC compared to its adjacent normal tissues and contributes to the growth and metastasis of CRC (Huang et al., 2009, Lin et al., 2015). However, how DHX32 regulates gene expression is largely unknown. Our study here revealed a mechanism by which DHX32 regulates the signaling intensity of the Wnt/β-catenin pathway and controls the expression of VEGFA. We demonstrated that the multifunctional RNA helicase DHX32 also regulates gene expression at the transcription level.
Tumor angiogenesis is critical for tumor growth, maintenance, and metastasis. Many studies have demonstrated that angiogenesis inhibitors have a significant therapeutic value (Zhao et al., 2011, Faivre et al., 2007). In this regard, DHX32 can be a potential druggable target for cancer treatment. Our result that ablation of DHX32 reduces microvessel density and inhibits tumor growth suggests that inhibition of DHX32 has the potential for CRC treatments. Furthermore, our result shows that high expression of DHX32 correlates with disease aggressiveness and poor outcomes of CRC patients, including short metastatic-free and overall survival times.
In conclusion, herein we report that DHX32 is an upstream regulator for β-catenin, which promotes the activity of β-catenin to upregulate expression of VEGFA in CRC cells. Depletion of DHX32 expression suppressed CRC tumor angiogenesis and tumor growth. Furthermore, overexpression of DHX32 is associated with high microvessel density and pathological features of human CRC. The results suggest that suppression of DHX32 can be of therapeutic value for CRC and that expression level of DHX32 has the potential to serve as a biomarker for CRC diagnosis and prognosis.
Funding Sources
This work was supported by the National Natural Science Foundation of China (Grant No 81072016, 81311120473, 81401971), Postdoctoral Science Foundation of China (Grant No 2014M561865, 2015T80682) and Scientific Research Project of Xiamen (Grant No 3502Z20150052).
Author Contributions
Z.Z. and H.L. conceived experiments. F.W. and H.L. wrote the manuscript. C.P., H.L., Z.F., Y.S. and P.L. prepared figures. Y.S., P.L. and J.W. performed experiments. C.P., C.Y., H.L., Q.H., Y.F., Q.L., and Z.L. provided technical support. All authors discussed the results and commented on the manuscript.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgements
We are grateful to Prof. B. Li. and Prof. H-R Wang for providing DNA constructs.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ebiom.2017.03.012.
Contributor Information
Chao Pan, Email: panchao123a@126.com.
Fen Wang, Email: fwang@ibt.tmc.edu.
Zhong-Ying Zhang, Email: zhangzy1121@xmu.edu.cn.
Appendix A. Supplementary Data
Supplementary figures
References
- Abdelhaleem M. Do human RNA helicases have a role in cancer? Biochim. Biophys. Acta. 2004;1704:37–46. doi: 10.1016/j.bbcan.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Abdelhaleem M. Over-expression of RNA helicases in cancer. Anticancer Res. 2004;24:3951–3953. [PubMed] [Google Scholar]
- Aberle H., Bauer A., Stappert J., Kispert A., Kemler R. Beta-catenin is a target for the ubiquitin-proteasome pathway. EMBO J. 1997;16:3797–3804. doi: 10.1093/emboj/16.13.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alli Z., Ackerley C., Chen Y., Al-Saud B., Abdelhaleem M. Nuclear and mitochondrial localization of the putative RNA helicase DHX32. Exp. Mol. Pathol. 2006;81:245–248. doi: 10.1016/j.yexmp.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Bai X.L., Zhang Q., Ye L.Y., Liang F., Sun X., Chen Y., Hu Q.D., Fu Q.H., Su W., Chen Z., Zhuang Z.P., Liang T.B. Myocyte enhancer factor 2C regulation of hepatocellular carcinoma via vascular endothelial growth factor and Wnt/beta-catenin signaling. Oncogene. 2015;34:4089–4097. doi: 10.1038/onc.2014.337. [DOI] [PubMed] [Google Scholar]
- Bergers G., Benjamin L.E. Tumorigenesis and the angiogenic switch. Nat. Rev. Cancer. 2003;3:401–410. doi: 10.1038/nrc1093. [DOI] [PubMed] [Google Scholar]
- Bienz M., Clevers H. Linking colorectal cancer to Wnt signaling. Cell. 2000;103:311–320. doi: 10.1016/s0092-8674(00)00122-7. [DOI] [PubMed] [Google Scholar]
- Carmeliet P., Jain R.K. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473:298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Causevic M., Hislop R.G., Kernohan N.M., Carey F.A., Kay R.A., Steele R.J., Fuller-Pace F.V. Overexpression and poly-ubiquitylation of the DEAD-box RNA helicase p68 in colorectal tumours. Oncogene. 2001;20:7734–7743. doi: 10.1038/sj.onc.1204976. [DOI] [PubMed] [Google Scholar]
- Clevers H., Nusse R. Wnt/beta-catenin signaling and disease. Cell. 2012;149:1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- Clifford R.L., Deacon K., Knox A.J. Novel regulation of vascular endothelial growth factor-A (VEGF-A) by transforming growth factor (beta)1: requirement for Smads, (beta)-CATENIN, AND GSK3(beta) J. Biol. Chem. 2008;283:35337–35353. doi: 10.1074/jbc.M803342200. [DOI] [PubMed] [Google Scholar]
- Conway E.M., Collen D., Carmeliet P. Molecular mechanisms of blood vessel growth. Cardiovasc. Res. 2001;49:507–521. doi: 10.1016/s0008-6363(00)00281-9. [DOI] [PubMed] [Google Scholar]
- Des Guetz G., Uzzan B., Nicolas P., Cucherat M., Morere J.F., Benamouzig R., Breau J.L., Perret G.Y. Microvessel density and VEGF expression are prognostic factors in colorectal cancer. Meta-analysis of the literature. Br. J. Cancer. 2006;94:1823–1832. doi: 10.1038/sj.bjc.6603176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easwaran V., Lee S.H., Inge L., Guo L., Goldbeck C., Garrett E., Wiesmann M., Garcia P.D., Fuller J.H., Chan V., Randazzo F., Gundel R., Warren R.S., Escobedo J., Aukerman S.L., Taylor R.N., Fantl W.J. Beta-catenin regulates vascular endothelial growth factor expression in colon cancer. Cancer Res. 2003;63:3145–3153. [PubMed] [Google Scholar]
- Ellis L.M., Takahashi Y., Liu W., Shaheen R.M. Vascular endothelial growth factor in human colon cancer: biology and therapeutic implications. Oncologist. 2000;5(Suppl. 1):11–15. doi: 10.1634/theoncologist.5-suppl_1-11. [DOI] [PubMed] [Google Scholar]
- Faivre S., Demetri G., Sargent W., Raymond E. Molecular basis for sunitinib efficacy and future clinical development. Nat. Rev. Drug Discov. 2007;6:734–745. doi: 10.1038/nrd2380. [DOI] [PubMed] [Google Scholar]
- Fuller-Pace F.V. DExD/H box RNA helicases: multifunctional proteins with important roles in transcriptional regulation. Nucleic Acids Res. 2006;34:4206–4215. doi: 10.1093/nar/gkl460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzmaurice C., Dicker D., Pain A., Hamavid H., Moradi-Lakeh M., Macintyre M.F., Allen C., Hansen G., Woodbrook R., Wolfe C., Hamadeh R.R., Moore A., Werdecker A., Gessner B.D., Te Ao B., Mcmahon B., Karimkhani C., Yu C., Cooke G.S., Schwebel D.C., Carpenter D.O., Pereira D.M., Nash D., Kazi D.S., de Leo D., Plass D., Ukwaja K.N., Thurston G.D., Yun Jin K., Simard E.P., Mills E., Park E.K., Catala-Lopez F., Deveber G., Gotay C., Khan G., Hosgood H.D., 3rd, Santos I.S., Leasher J.L., Singh J., Leigh J., Jonas J.B., Sanabria J., Beardsley J., Jacobsen K.H., Takahashi K., Franklin R.C., Ronfani L., Montico M., Naldi L., Tonelli M., Geleijnse J., Petzold M., Shrime M.G., Younis M., Yonemoto N., Breitborde N., Yip P., Pourmalek F., Lotufo P.A., Esteghamati A., Hankey G.J., Ali R., Lunevicius R., Malekzadeh R., Dellavalle R., Weintraub R., Lucas R., Hay R., Rojas-Rueda D., Westerman R., Sepanlou S.G., Nolte S., Patten S., Weichenthal S., Abera S.F., Fereshtehnejad S.M., Shiue I., Driscoll T., Vasankari T., Alsharif U., Rahimi-Movaghar V., Vlassov V.V., Marcenes W.S., Mekonnen W., Melaku Y.A., Yano Y., Artaman A., Campos I., Maclachlan J., Mueller U., Kim D., Trillini M., Eshrati B., Williams H.C., Shibuya K., Dandona R., Murthy K., Cowie B. The Global Burden of Cancer 2013. JAMA Oncol. 2015;1:505–527. doi: 10.1001/jamaoncol.2015.0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grothey A., Galanis E. Targeting angiogenesis: progress with anti-VEGF treatment with large molecules. Nat. Rev. Clin. Oncol. 2009;6:507–518. doi: 10.1038/nrclinonc.2009.110. [DOI] [PubMed] [Google Scholar]
- Hanahan D., Weinberg R.A. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Hasan J., Byers R., Jayson G.C. Intra-tumoural microvessel density in human solid tumours. Br. J. Cancer. 2002;86:1566–1577. doi: 10.1038/sj.bjc.6600315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Liang X., Huang R., Zhang Z. Up-regulation and clinical relevance of novel helicase homologue DHX32 in colorectal cancer. J. Exp. Clin. Cancer Res. 2009;28:11. doi: 10.1186/1756-9966-28-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishaq M., Ma L., Wu X., Mu Y., Pan J., Hu J., Hu T., Fu Q., Guo D. The DEAD-box RNA helicase DDX1 interacts with RelA and enhances nuclear factor kappaB-mediated transcription. J. Cell. Biochem. 2009;106:296–305. doi: 10.1002/jcb.22004. [DOI] [PubMed] [Google Scholar]
- Kerbel R.S. Tumor angiogenesis. N. Engl. J. Med. 2008;358:2039–2049. doi: 10.1056/NEJMra0706596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiewisz J., Wasniewski T., Kmiec Z. Participation of WNT and beta-catenin in physiological and pathological endometrial changes: association with angiogenesis. Biomed. Res. Int. 2015;2015:854056. doi: 10.1155/2015/854056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi A., Kishida S., Yamamoto H. Regulation of Wnt signaling by protein-protein interaction and post-translational modifications. Exp. Mol. Med. 2006;38:1–10. doi: 10.1038/emm.2006.1. [DOI] [PubMed] [Google Scholar]
- Korinek V., Barker N., Morin P.J., Van Wichen D., De Weger R., Kinzler K.W., Vogelstein B., Clevers H. Constitutive transcriptional activation by a beta-catenin-Tcf complex in APC −/− colon carcinoma. Science. 1997;275:1784–1787. doi: 10.1126/science.275.5307.1784. [DOI] [PubMed] [Google Scholar]
- Lin H., Lin Q., Liu M., Lin Y., Wang X., Chen H., Xia Z., Lu B., Ding F., Wu Q., Wang H.R. PKA/Smurf1 signaling-mediated stabilization of Nur77 is required for anticancer drug cisplatin-induced apoptosis. Oncogene. 2014;33:1629–1639. doi: 10.1038/onc.2013.116. [DOI] [PubMed] [Google Scholar]
- Lin H., Liu W., Fang Z., Liang X., Li J., Bai Y., Lin L., You H., Pei Y., Wang F., Zhang Z.Y. Overexpression of DHX32 contributes to the growth and metastasis of colorectal cancer. Sci. Report. 2015;5:9247. doi: 10.1038/srep09247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., You P., Chen G., Fu X., Zeng X., Wang C., Huang Y., An L., Wan X., Navone N., Wu C.L., Mckeehan W.L., Zhang Z., Zhong W., Wang F. Hyperactivated FRS2alpha-mediated signaling in prostate cancer cells promotes tumor angiogenesis and predicts poor clinical outcome of patients. Oncogene. 2016;35:1750–1759. doi: 10.1038/onc.2015.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pages G., Pouyssegur J. Transcriptional regulation of the vascular endothelial growth factor gene--a concert of activating factors. Cardiovasc. Res. 2005;65:564–573. doi: 10.1016/j.cardiores.2004.09.032. [DOI] [PubMed] [Google Scholar]
- Pendas-Franco N., Garcia J.M., Pena C., Valle N., Palmer H.G., Heinaniemi M., Carlberg C., Jimenez B., Bonilla F., Munoz A., Gonzalez-Sancho J.M. DICKKOPF-4 is induced by TCF/beta-catenin and upregulated in human colon cancer, promotes tumour cell invasion and angiogenesis and is repressed by 1alpha,25-dihydroxyvitamin D3. Oncogene. 2008;27:4467–4477. doi: 10.1038/onc.2008.88. [DOI] [PubMed] [Google Scholar]
- Schlegel B.P., Starita L.M., Parvin J.D. Overexpression of a protein fragment of RNA helicase A causes inhibition of endogenous BRCA1 function and defects in ploidy and cytokinesis in mammary epithelial cells. Oncogene. 2003;22:983–991. doi: 10.1038/sj.onc.1206195. [DOI] [PubMed] [Google Scholar]
- Takahashi Y., Kitadai Y., Bucana C.D., Cleary K.R., Ellis L.M. Expression of vascular endothelial growth factor and its receptor, KDR, correlates with vascularity, metastasis, and proliferation of human colon cancer. Cancer Res. 1995;55:3964–3968. [PubMed] [Google Scholar]
- Wilson B.J., Giguere V. Identification of novel pathway partners of p68 and p72 RNA helicases through Oncomine meta-analysis. BMC Genomics. 2007;8:419. doi: 10.1186/1471-2164-8-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yancopoulos G.D., Davis S., Gale N.W., Rudge J.S., Wiegand S.J., Holash J. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407:242–248. doi: 10.1038/35025215. [DOI] [PubMed] [Google Scholar]
- Yost C., Torres M., Miller J.R., Huang E., Kimelman D., Moon R.T. The axis-inducing activity, stability, and subcellular distribution of beta-catenin is regulated in Xenopus embryos by glycogen synthase kinase 3. Genes Dev. 1996;10:1443–1454. doi: 10.1101/gad.10.12.1443. [DOI] [PubMed] [Google Scholar]
- Zhao Y., Bao Q., Renner A., Camaj P., Eichhorn M., Ischenko I., Angele M., Kleespies A., Jauch K.W., Bruns C. Cancer stem cells and angiogenesis. Int. J. Dev. Biol. 2011;55:477–482. doi: 10.1387/ijdb.103225yz. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figures