Abstract
In severe humoral immunodeficiency the indication for antibody replacement therapy (ART) is clear, and supported by several large studies. However, for milder forms of humoral immunodeficiency, the indication for ART is less clear. This is a retrospective cohort study of 87 adults with recurrent respiratory tract infections who received ART. The patients had severe or mild humoral immunodeficiency, and were followed up for a median of 62 months. Infection frequency, pharmacy-registered antibiotics use and hospital admissions significantly decreased under ART compared to the year prior to starting ART (median 5.50 (anamnestically)–0.82 (physician-confirmed) infections/year, p < 0.001; median 4.00–2.05 antibiotics courses/year, p < 0.001; mean 0.75–0.44 hospital admissions/year, p = 0.009). These beneficial effects of ART were seen in both severe and mild immunodeficiency. Bronchiectasis was present in 27 patients when ART was started, but was not associated with clinical outcomes. An increase in hospital admissions under ART, observed in some patients, was significantly associated with pulmonary emphysema and current smoking. In conclusion, this study shows that ART is a long-term effective therapy in adults with recurrent respiratory tract infections with severe as well as with milder forms of humoral immunodeficiency.
Keywords: Immunoglobulin, Gammaglobulin, Primary antibody deficiency, Immunodeficiency, Respiratory tract infections
Highlights
-
•
Long-time follow up of 87 adult primary antibody deficiency patients treated with antibody replacement therapy is reported.
-
•
Treatment leads to a significant decline in infection frequency, pharmacy-registered antibiotics use and hospital admissions.
-
•
This beneficial effect was seen in both patients with severe and milder forms of immune deficiency.
Patients with recurrent respiratory tract infections can have a primary antibody deficiency, characterized by reduced specificities of circulating antibodies. Antibody replacement therapy can prevent infections in these patients. The evidence supporting antibody replacement therapy is weaker for mild than for severe primary antibody deficiency. In this study of 87 patients with mild and severe primary antibody deficiency, antibody replacement therapy lead to a significant decrease in infections, antibiotics use and hospital admissions during a median follow up period of 62 months. Thus, this study supports the use of antibody replacement therapy, also in patients with mild primary antibody deficiency.
1. Introduction
In 1952 Colonel Ogden C. Bruton described the first case of agammaglobulinemia in a child with recurrent respiratory tract infections (RTI). This patient was successfully treated with antibody replacement therapy (ART) (Bruton, 1952). Agammaglobulinemia and hypogammaglobulinemia are states of absent or low levels of circulating antibodies. These conditions are the hallmark of primary antibody deficiencies (PADs). PADs vary in severity: from agammaglobulinemia to mild hypogammaglobulinemia to normogammaglobulinemia and impaired specific antibody production to vaccination. Various gene mutations have been found to cause PADs, but in most patients the precise cause remains unknown (Durandy et al., 2013). The estimated prevalence for PADs ranges from 1.3 to 2.9/100,000 persons in Europe (Edgar et al., 2014), although the actual prevalence is likely higher due to underdiagnosis of PADs and underrepresentation of mild PADs in these estimates (Edgar et al., 2014). The most notable clinical manifestations of PADs are recurrent RTI (Bonilla et al., 2015), and the treatment of choice is still ART, as introduced by Bruton 64 years ago. Prognosis is mainly determined by organ damage due to infections and the development of autoimmune or malignant disease.
The indication for ART is evident in case of hypo- or agammaglobulinemia (Bonilla et al., 2015, Orange et al., 2006). Despite the lack of randomized placebo-controlled trials, there is a broad consensus to provide ART to these patients. Non-randomized studies demonstrating the benefit of ART in hypo- or agammaglobulinemic patients have been published decades ago, as well as more recently (Nolte et al., 1979, Ammann et al., 1982, Cunningham-Rundles et al., 1984, Roifman et al., 1985, Quartier et al., 1999, Quinti et al., 2011, Lucas et al., 2010). The Primary Immunodeficiency Committee of the American Academy of Allergy, Asthma and Immunology performed a systematic literature review of the use of antibody replacement therapy (ART) in human disease in 2006 (Orange et al., 2006). Despite the absence of double-blind placebo-controlled studies the committee found the existing studies compelling enough to indicate ART in patients with severe antibody deficiency (agammaglobulinemia or hypogammaglobulinemia), such as common variable immunodeficiency (CVID) or X-lined agammaglobulinemia (XLA).
In milder forms of humoral immunodeficiencies e.g. IgG subclass deficiency (IgGSD) or specific antibody deficiency (SAD) there is less evidence supporting ART. There are few studies that thoroughly compare clinical and immunological characteristics before and during ART in these patients. The most extensive study is a retrospective study of 132 adult patients with IgGSD and ≥ 4 RTI per year (Olinder-Nielsen et al., 2007). Treatment with ART in the form of subcutaneous immunoglobulins (SCIG) resulted in a significant reduction of antibiotic-treated RTI in the most recent year of ART. Other studies have much smaller patient populations or were unable to obtain objective measures of infection frequency or baseline measurement with which to compare infection frequency under ART (Bjorkander et al., 1986, Abdou et al., 2009, Abrahamian et al., 2010). There have been no studies of ART with long-term follow up and robust outcome parameters in patients with mild immunodeficiency. As ART is an expensive therapy (20,000–30,000 euros per patient per year) it is important to establish clinical efficacy in patients with mild immunodeficiency.
The outpatient clinic of the Department of Pulmonology at the St. Antonius Hospital is a referral center for adult patients with recurrent RTI. Clinical and immunological characteristics of the patients are systematically evaluated, and then a multidisciplinary decision is made whether or not to initiate ART. This patient group comprises both severe adult-onset immunodeficiency such as CVID and mild adult-onset immunodeficiency such as SAD. Here we present the clinical and immunological data from adult patients with recurrent RTI who were treated with ART at our center. The objective of this study was to determine the efficacy of ART in this population. Clinical outcome parameters were compared between the period prior to ART and long-term follow-up.
2. Patients and Methods
In this retrospective cohort study we included 87 patients referred for analysis of recurrent RTI in the period 1992 until 2014. The clinical and immunological screening before starting ART comprised infection history and immune status investigation according to the immune status protocol of that time (the Dutch National Working Group for Immunodeficiencies (WID) and European Society for Immunodeficiencies (ESID) protocols) (Vossen and Zegers, 1988, de Vries et al., 2000, de Vries and Clinical Working Party of the European Society for Immunodeficiencies (ESID), 2006). Data were retrieved from patient records. For every patient, the history and laboratory results, including response to pneumococcal vaccination, at time of immunological screening were collected, as well as clinical and laboratory follow up data after that time. Data were collected up until July 2014, or until a patient was lost to follow up or died. Thus, there was no fixed length of the follow-up period. If patients gave written consent their home pharmacy was contacted with a request for providing the patient's antibiotics use. Antibiotics use was only available for patients who started with ART after 2002. The local medical ethics committee approved of the study and allowed contacting the pharmacies of patients that had passed away. The study was conducted in accordance with the principles of the Declaration of Helsinki (2013 version).
2.1. Antibody Replacement Therapy
ART was started in patients with a diagnosed immune deficiency and recurrent infections. Intravenous immunoglobulins (IVIG) were given monthly at the outpatient department of the hospital or at home, whereas SCIG was (self-)administered daily or weekly at home. The choice between these two administration routes was made on an individual basis. The preferred administration route for ART was IVIG in case of CVID. The starting dose depended on serum IgG levels. In patients with IgG levels < 5 g/l the dose was 400 mg/kg/every 4 weeks. In case of SAD or IgGSD the preferred route was SCIG with a starting dose of 2 ml (160 mg/ml) daily or 15 ml (160 mg/ml) every week by a subcutaneous infusion pump, depending on patient preference. Dose adjustments and changes in administration route were made based on IgG trough levels and infection frequency. The target IgG trough level was 7 g/l. ART with SCIG was usually discontinued after six months to evaluate infection frequency. ART was restarted if infection frequency and/or antibiotics use increased.
2.2. Immune Status Investigations
Standard laboratory investigations included blood leucocyte count with differentiation, complement status, serum immunoglobulins and IgG subclass-level measurement and pneumococcal antibody response after vaccination. All patients were vaccinated intramuscularly with a single dose of 23-valent pneumococcal polysaccharide vaccine (Pneumovax 23; Merck, Rahway, NJ, USA). Blood samples were routinely drawn before and 3–6 weeks after vaccination. Serum samples were stored at − 80 °C until use. Until 2008 total antibodies against capsular polysaccharides of two or three Streptococcus pneumoniae serotypes (3, 4 and 9) were measured by ELISA as previously described (van Kessel et al., 1999). After 2008 IgG antibodies against 8, 13 or 14 different pneumococcal polysaccharides were measured as described previously (Meerveld-Eggink et al., 2009, Elberse et al., 2010, van Kessel et al., 2014). For categorization of the antibody response to pneumococcal polysaccharide vaccination, the 2005 AAAAI/ACAAI classification criteria were used (Bonilla et al., 2005).
2.3. Diagnostic Classification of Immunodeficiencies
Patients were classified in different categories of primary immunodeficiency according to recent definitions. A patient was classified as CVID when fulfilling the following criteria: (1) IgG level < 7 g/l; (2) IgM level < 0.4 g/l and/or IgA level < 0.7 g/l; (3) onset of immunodeficiency at > 2 years of age; (4) absent isohemagglutinins and/or poor response to vaccinations; and (5) exclusion of other defined causes of hypogammaglobulinemia (Conley et al., 1999). When patients fulfilled criteria 1, 3 and 5, but not criteria 2 and/or 4, they were classified as Idiopathic Primary Hypogammaglobulinemia (IPH) (Driessen et al., 2013). Patients with IgA levels < 0.07 g/l and normal serum IgG and IgM levels in whom other causes of low IgA levels had been excluded, were categorized as IgA deficiency (Bonilla et al., 2015). When patients had normal levels of IgG, but IgG subclass levels below cut-off value they were classified as IgGSD (Bonilla et al., 2015). Patients with normal levels of immunoglobulins and IgG subclasses, but an impaired response to pneumococcal polysaccharide vaccination, were classified as SAD (Bonilla et al., 2015). Patients with a serum monoclonal protein were classified as MGUS when: (1) serum M-protein < 30 g/l; (2) bone marrow clonal plasma cells < 10%; (3) no evidence of other B-cell proliferative disorders; and (4) no related organ or tissue impairment (International Myeloma Working Group, 2003). For the purpose of this study, we refer to CVID and IPH as severe immunodeficiency, and to IgGSD and SAD as mild immunodeficiency.
2.4. Clinical Outcome Parameters
Recurrent RTI were defined as having three or more infectious periods per year. Infection frequency prior to starting ART was based on patient reporting. Infection frequency after ART was based on confirmation of infectious episodes by a physician. Infectious episodes were categorized as sinusitis, bronchitis or pneumonia. Data on antibiotics use was provided by patient's home pharmacies. In the Netherlands antibiotics are only available with a prescription from a physician and all antibiotics use is registered. Hospital admissions were scored in case of infection and/or exacerbation of underlying pulmonary disease.
2.5. Data Management and Statistics
Data collection and management were performed using Microsoft Office Excel 2011. Statistical analyses were performed using IBM SPSS Statistics version 24. Fisher's exact test was used for comparing dichotomous variables where appropriate. Mann-Whitney U test and Wilcoxon Signed Rank test were used for comparing continuous variables where appropriate. Graphs were created in IBM SPSS Statistics version 24.
3. Results
Eighty-seven patients were included in the study. Patient characteristics prior to ART are shown in Table 1. Fifty-five patients were female. The median age was 61 years at start of ART. In the majority of the patients comorbid conditions were present. Thirty-six patients were diagnosed with chronic obstructive pulmonary disease (COPD). COPD patients were significantly more likely to smoke or have smoked (32/36 COPD patients versus 20/51 non-COPD patients; p = 0.0001) to use corticosteroid maintenance therapy (11/36 COPD patients versus 6/51 non-COPD patients; p = 0.02), and to have been admitted to the hospital in the previous year (23/36 COPD patients versus 18/51 non-COPD patients; p = 0.001). HRCT-scan of the thorax showed bronchiectasis in 27 patients and pulmonary fibrosis in 3 patients. Infectious episodes prior to ART included sinusitis in 62 patients, bronchitis in 80 patients and pneumonia in 62 patients. Twenty-eight patients had undergone sinus surgery. Patients reported to have had infections for a median of 5 years (interquartile range 2–18.5 years) at time of immunological screening. The results of the immune status investigations are shown in Supplementary Table 1. The group of mild immunodeficiency consists of 37 patients with IgGSD (n = 27) or SAD (n = 10). The group of severe immunodeficiency consists of 44 patients with CVID (n = 15) or IPH (n = 28). The total group includes these two groups, as well as patients with MGUS (n = 5), IgA deficiency (n = 1) and no immunological defect (n = 1).
Table 1.
Clinical characteristics prior to the start of antibody replacement therapy.
| Demographic characteristics | |
| Total | 87 |
| Female | 55 |
| Median age at start of antibody replacement therapy (IQR) | 61 (50.5–67) |
| Comorbid conditions | |
| Allergic disease | 22 |
| Chronic obstructive pulmonary disease | 36 |
| Asthma | 21 |
| Auto-immune disease | 8 |
| Previous malignancy | 8 |
| Previous sinus surgery | 28 |
| Smoking status | |
| Never smoker | 35 |
| Ex-smoker | 28 |
| Current smoker | 24 |
| Lung functiona | |
| Normal | 25 |
| GOLD I | 10 |
| GOLD II | 20 |
| GOLD III | 11 |
| GOLD IV | 3 |
| Chest HRCT scanb | |
| Bronchopathy | 19 |
| Emphysema | 25 |
| Bronchiectasis | 27 |
| Pulmonary fibrosis | 3 |
| Medication | |
| Corticosteroid maintenance therapy | 17 |
| Other immunosuppressive maintenance therapy | 1 |
| Infectious episodes | |
| Sinusitis | 62 |
| Bronchitis | 80 |
| Pneumonia | 62 |
| Encapsulated bacteria in sputum culture | 62 |
| Median number of years since onset of infections (IQR)c | 5 (2–18.5) |
IQR: interquartile range.
Data available in 69 patients.
Data available in 75 patients.
Data available in 83 patients.
Seventeen patients were initially treated with IVIG and 70 patients with SCIG. In two patients with anti-IgA antibodies, pre-treatment with SCIG was given prior to starting IVIG. Twenty patients switched from SCIG to IVIG and one patient from IVIG to SCIG. Seventeen types of adverse effects were reported in 15 patients during IVIG. Adverse effects were reported in 23 patients during SCIG (Table 2). Adverse effects were mild except in one patient who developed a moderately severe systemic reaction after changing to another brand of IVIG. In 11 patients the therapy was stopped because of mild adverse reactions or because the therapy itself was perceived as too burdensome.
Table 2.
Adverse events during antibody replacement therapy.
| Adverse events | |
|---|---|
| IVIG | |
| Mild systemic reaction | |
|
1 |
|
3 |
|
1 |
|
1 |
|
10 |
| Moderately severe systemic reaction | |
|
1 |
| SCIG | |
| Mild local reaction | |
|
7 |
|
7 |
|
8 |
|
1 |
Each type of adverse event is scored only one time per patient.
The total study group comprised 543 patient-years including 383 patient-years under ART. The median follow-up time was 62 months (interquartile range 34.5–98.5). Thirty-seven patients were treated with IVIG during a median time of 23 months (interquartile range 6–63) and 71 patients with SCIG during a median of 11 months (interquartile range 7–50). The follow up of 10 patients, who were referred for immunological evaluation and initiation of ART, took place in the referring hospital. In 35 patients therapy with SCIG was discontinued after 4–8 months to evaluate the effect. In 19 of these patients it was necessary to restart ART after median of 12 months (interquartile range 2.5–15) due to increasing infection frequency. Details on comorbidities during the follow up, as well as details on mortality and the causes of death are shown in Supplementary Table 2. Mortality was associated with male gender (p = 0.005), higher age at start of ART (p = 0.007), COPD Gold 4 (p = 0.02), and development of malignancy during follow up (p = 0.0008).
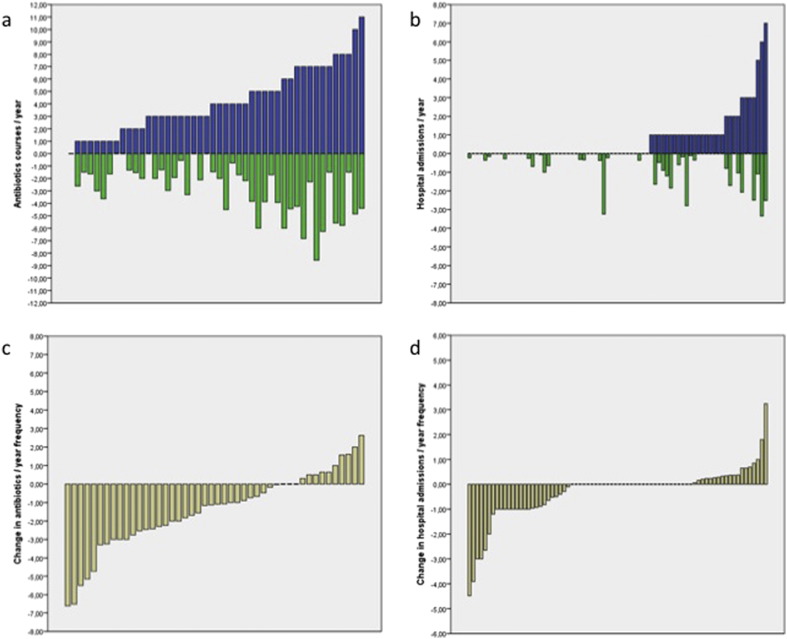
The outcome measures of efficacy of antibody replacement therapy are summarized in Table 3 and Fig. 1. Anamnestically patients had a median number of 5.50 infections per year (interquartile range 4.00–8.00; available in 50 patients). In the year prior to ART the median number of antibiotic courses was 4.00 (interquartile range 2.00–6.25; available in 46 patients). The mean number of hospital admissions was 0.75. Thirty of 76 patients were admitted at least one time in the year prior to ART.
Table 3.
Infectious outcome parameters under antibody replacement therapy.
| Infections | Mild immunodeficiency |
Severe immunodeficiency |
Total |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | Under ART | p-Value | Baseline | Under ART | p-Value | Baseline | Under ART | p-Value | |
| Data available in N patients | 21 | 25 | 50 | ||||||
| Median number of infections/year (IQR) | 6.00 (4.00–8.00) | 0.96 (0.00–1.66) | < 0.001 | 5.00 (4.00–7.50) | 0.73 (0.00–1.25) | < 0.001 | 5.50 (4.00–8.00) | 0.82 (0.00–1.38) | < 0.001 |
| Data available in N patients | 22 | 21 | 46 | ||||||
| Median courses of antibiotic therapy/year (IQR) | 4.50 (2.00–7.00) | 3.73 (1.67–5.63) | 0.02 | 3.00 (3.00–5.50) | 1.64 (0.28–2.62) | 0.002 | 4.00 (2.00–6.25) | 2.05 (1.49–4.28) | < 0.001 |
| Data available in N patients | 35 | 36 | 76 | ||||||
| Mean number of hospital admissions/year (SD) | 0.80 (1.32) | 0.51 (0.86) | 0.03 | 0.64 (1.29) | 0.39 (0.81) | 0.17 | 0.75 (1.36) | 0.44 (0.81) | 0.009 |
IQR: interquartile range. SD: standard deviation. The group of mild immunodeficiency consists of 37 patients with IgGSD (n = 27) or SAD (n = 10). The group of severe immunodeficiency consists of 44 patients with CVID (n = 15) or IPH (n = 28). The total group includes the above two groups, as well as patients with MGUS (n = 5), IgA deficiency (n = 1) and no immunological defect (n = 1). p-Values are calculated using only patients in whom data are available both before and under ART. The minimum period before start of ART is one year, the minimum period under ART is six months. In the case of hospital admissions, mean values are indicated in spite of non-normal distributions, because median values are all 0. Boldface indicates statistical significance (p-value < 0.05).
Fig. 1.
Infectious outcomes during antibody replacement therapy.
[a] Number of antibiotics courses per patient per year prior to antibody replacement therapy (blue bars) and under antibody replacement therapy (green bars). [b] Number of hospital admissions per patient per year prior to antibody replacement therapy (blue bars) and under antibody replacement therapy (green bars). [c] Net change in antibiotics use per patient per year, when comparing the year prior to antibody replacement therapy with antibiotics use under antibody replacement therapy. Negative values on the Y-axis represent a decrease in antibiotics use under antibody replacement therapy. [d] Net change in hospital admissions per patient per year, when comparing the year prior to antibody replacement therapy with hospital admissions under antibody replacement therapy. Negative values on the Y-axis represent a decrease in hospital admissions under antibody replacement therapy. N.B. patients were sorted on the X-axis for clarity and patient order is not the same in all graphs.
The yearly frequency of physician confirmed infections under ART was significantly lower than the anamnestically obtained infection frequency prior to therapy (p < 0.001). The yearly frequency of antibiotics use was significantly lower under ART compared to the year prior to ART (p < 0.001). The antibiotics use decreased under ART in 33 out of 46 patients and increased in 10 patients. Clinical and outcome parameters were not significantly different between the two groups. There were no significant differences in age, gender, time to diagnosis, type of PAD, or comorbid conditions.
The yearly number of hospital admissions was significantly lower under ART in the whole cohort compared to the year prior to ART (p = 0.009). The number of hospital admissions decreased in 26 out of 76 patients, increased in 19 patients and 31 patients had no admissions before or under ART. In the group with an increase in hospital admissions 15 out of 19 patients were not admitted to the hospital in the year before ART. In these 19 patients, there were more current smokers (p = 0.08) and significantly less ex-smokers (p = 0.02). Nine patients showed radiologically confirmed emphysema on thorax HRCT (p = 0.01 compared to patients with no increase in hospital admissions). Six of these 9 patients were current smokers. The combination of HRCT-confirmed emphysema and being a current smoker at the start of ART was significantly more present in patients with increased hospital admissions under ART (6/14 patients versus 2/57 patients; p = 0.0005). Five of 19 patients had a malignancy before ART (p = 0.02 compared to patients with no increase in hospital admissions). All 5 patients were considered disease free at the start of ART. There were no significant differences in age, gender, and time to diagnosis between the patients with an increase in hospital admissions compared to those with no increase in hospital admissions.
Subgroup analysis, with CVID and IPH patients combined in one group (n = 44) and SAD and IgGSD patients combined in another group (n = 37) showed a significant decrease under ART of infection frequency (p < 0.001 in both groups) and antibiotics use (p = 0.002 in CVID/IPH and p = 0.02 in SAD/IgGSD). The number of hospital admissions decreased in both groups as well, but not significantly in the severe immunodeficiency group (p = 0.17 in CVID/IPH and p = 0.03 in SAD/IgGSD). Before the start of ART, there were no significant differences in infection frequency, antibiotics use and number of hospital admissions.
Outcome measures for the different administration routes are shown in Supplementary Table 3. All three outcome parameters decreased under both IVIG and SCIG, but not always significantly. Immunoglobulin levels at baseline and mean immunoglobulin levels under IVIG and SCIG are shown in Table 4. IgG levels increased significantly under antibody replacement therapy (p = 0.001 for IVIG and p < 0.001 for SCIG respectively).
Table 4.
Serum immunoglobulin levels during antibody replacement therapy.
| IVIG |
SCIG |
|||||||
|---|---|---|---|---|---|---|---|---|
| N patients | Baseline level | Level under IVIG | p-Value | N patients | Baseline level | Level under SCIG | p-Value | |
| IgM median (IQR) | 25 | 0.60 (0.35–1.30) | 0.45 (0.30–1.23) | 0.08 | 33 | 0.70 (0.45–0.90) | 0.55 (0.41–0.96) | 0.009 |
| IgA median (IQR) | 21 | 1.00 (0.15–1.65) | 0.88 (0.18–1.65) | 0.73 | 33 | 1.10 (0.50–2.35) | 1.41 (0.48–2.34) | 0.92 |
| IgG median (IQR) | 29 | 5.40 (3.35–8.70) | 7.48 (6.44–8.55) | 0.001 | 45 | 6.70 (5.25–8.45) | 7.58 (6.15–9.57) | < 0.001 |
| IgG1 median (IQR) | 21 | 3.20 (2.10–5.75) | 5.45 (4.14–6.27) | < 0.001 | 59 | 4.60 (3.70–6.60) | 5.30 (4.20–6.65) | 0.04 |
| IgG2 median (IQR) | 21 | 0.90 (0.40–1.25) | 2.30 (1.75–2.79) | < 0.001 | 59 | 1.40 (1.00–2.20) | 1.88 (1.50–2.45) | < 0.001 |
| IgG3 median (IQR) | 21 | 0.30 (0.15–0.50) | 0.30 (0.16–0.43) | 0.22 | 59 | 0.40 (0.20–0.60) | 0.37 (0.20–0.56) | 0.03 |
| IgG4 median (IQR) | 19 | 0.10 (0.10–0.30) | 0.17 (0.06–0.38) | 0.28 | 59 | 0.20 (0.10–0.30) | 0.20 (0.10–0.37) | 0.02 |
IQR: interquartile range. p-Values were calculated by comparing baseline levels with patient-specific median levels under (a specific form of) antibody replacement therapy. Boldface indicates statistical significance (p-value < 0.05).
There were 27 patients with bronchiectasis. No significant correlation was found between bronchiectasis and the time since start of respiratory tract infections and start ART. Numbers of infections and hospital admissions prior to ART were not significantly different between patients with and without bronchiectasis. IgG and IgG1 levels prior to ART were significantly higher in the group of bronchiectasis patients (p = 0.04 and p = 0.02).
4. Discussion
This single center cohort study shows the long-term efficacy of ART severe but also in mild immunodeficiency. We report 383 patient-years under ART with a long follow up for most of the patients. In patients with severe immunodeficiency (CVID/IPH) and patients with mild immunodeficiency (IgGSD/SAD) treatment with ART resulted in a significant decrease of the use of antibiotics, hospital admissions and infection frequency. Only the decrease in hospital admissions in the severe immunodeficiency group was not significant. Our results confirm and extend earlier studies where the beneficial effect of ART was established mainly with IVIG. In contrast to other studies, our patient group is homogeneous in clinical symptomatology but heterogeneous in underlying humoral immunodeficiency. Analysis was carried out with the patients serving as their own controls using robust outcome parameters such as hospital admissions and pharmacy-registered antibiotics use. There is no over-the-counter availability of antibiotics in the Netherlands, which permitted us to register and analyze all antibiotics use of a patient. Treatment with ART was generally well tolerated. The therapy was discontinued in eleven patients because of mild adverse reactions or because it was considered too burdensome.
The number of hospital admissions increased under ART in 19 patients. This was significantly associated with smoking status and radiologically confirmed emphysema and even more strongly with both factors combined. Smoking and chronic respiratory diseases are both known risk factors for severe RTI (Torres et al., 2013). Obviously, ART will not overcome structural lung damage. This raises the question whether there is an indication for ART in patients with progressive underlying respiratory disease and immunodeficiency. Nevertheless, it is not known whether the number of hospital admissions would have been even higher without ART. ART might slow disease progression, as infectious episodes can cause worsening of underlying respiratory disease. Despite existing comorbidity, most patients in our study showed a significant response to ART.
In clinical practice there should be a high index of suspicion for an immunodeficiency in patients with lung disease and recurrent RTI not responding to conventional therapies. Immunological screening may reveal an immunodeficiency that contributes to the susceptibility to infections. ART can be given intravenously or subcutaneously depending on IgG levels and patient preference. In general, for patients with severe immunodeficiency the intravenous route is preferred. These patients need higher doses of immunoglobulins to achieve normal trough levels, and it is burdensome to give these quantities subcutaneously. All CVID patients in our cohort received immunoglobulins via the intravenous route. Therapy effect can be evaluated by monitoring infection frequency, use of antibiotics and hospital admissions in combination with IgG trough levels. Temporary discontinuation of therapy, followed by careful evaluation can also be helpful to select patients who benefit from ART.
In our cohort bronchiectasis was not associated with a higher mortality or a longer diagnostic delay. Bronchiectasis has been associated with a diagnostic delay in other studies (Brent et al., 2016) and is considered to be an important predictor of the prognosis of the disease. However an association with mortality has not always been found. We did not find an association with mortality or diagnostic delay possibly because we did not differentiate between mild and severe bronchiectasis. In our study 36% (27 of 75 patients) of the patients had bronchiectasis. The reported prevalence in other cohorts, consisting largely of CVID patients, varied from 11.2 to 47% (Quinti et al., 2011, Resnick et al., 2012, Brent et al., 2016).
Limitations of our study are mainly related to the retrospective character. Because of this not all data were complete. Infection frequency before ART was determined anamnestically, while during ART all infections were physician-confirmed. The latter is a stricter interpretation. The decrease of infection frequency during ART is very significant but should be interpreted with caution. In observational studies without control groups the phenomenon of regression to the mean can be inadvertently classified as a therapeutic effect. We find this unlikely in our study because the decision to start ART was based on an increase of infection frequency and spontaneous decrease of infection frequency in immunodeficient patients is not often seen. Another limitation is that the diagnoses of the patients receiving IVIG were different from the patients receiving SCIG. Patients receiving IVIG had more severe forms of immunodeficiency. However, in our study we did not intend to directly compare IVIG with SCIG, but rather to investigate the efficacy of ART as a whole. During the long follow up period there have inevitably been changes in diagnostic protocols and definition of antibody response. The IgG pneumococcal antibody response was categorized using the diagnostic criteria of 2005 (Bonilla et al., 2005), because these formed the basis for the decision to start ART in most patients. In 2015 the criteria were updated (Bonilla et al., 2015). The new criteria have a stricter definition of an adequate antibody response.
In conclusion, ART is an effective and well-tolerated therapy. Measurement of IgG levels and assessment of pneumococcal antibody response after pneumococcal polysaccharide vaccination leads to selection of patients that benefit from ART. In this study we confirm that adults with recurrent RTI and an underlying mild or severe humoral immunodeficiency show a decrease in infection frequency, antibiotics use and hospital admissions upon treatment with ART.
Funding Sources
None.
Conflicts of Interest
None reported.
Author Contributions
DvK conceived of the study; DvK, TH and HvVB collected patient data; DvK, TH and PZ performed statistical analyses; all authors interpreted the results; DvK and TH wrote the first draft of the manuscript; all authors critically reviewed and revised the manuscript; all authors approved the final manuscript.
Acknowledgements
We thank the St. Antonius Hospital Pharmacy, as well as the other pharmacies of the patients for their assistance in collecting patient antibiotics use.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ebiom.2017.03.025.
Appendix A. Supplementary Data
Supplementary tables
Dataset
References
- Abdou N.I., Greenwell C.A., Mehta R., Narra M., Hester J.D., Halsey J.F. Efficacy of intravenous gammaglobulin for immunoglobulin G subclass and/or antibody deficiency in adults. Int. Arch. Allergy Immunol. 2009;149(3):267–274. doi: 10.1159/000199723. [DOI] [PubMed] [Google Scholar]
- Abrahamian F., Agrawal S., Gupta S. Immunological and clinical profile of adult patients with selective immunoglobulin subclass deficiency: response to intravenous immunoglobulin therapy. Clin. Exp. Immunol. 2010;159(3):344–350. doi: 10.1111/j.1365-2249.2009.04062.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammann A.J., Ashman R.F., Buckley R.H., Hardie W.R., Krantmann H.J., Nelson J., Ochs H., Stiehm E.R., Tiller T., Wara D.W., Wedgwood R. Use of intravenous gamma-globulin in antibody immunodeficiency: results of a multicenter controlled trial. Clin. Immunol. Immunopathol. 1982;22(1):60–67. doi: 10.1016/0090-1229(82)90022-8. [DOI] [PubMed] [Google Scholar]
- Bjorkander J., Bengtsson U., Oxelius V.A., Hanson L.A. Symptoms in patients with lowered levels of IgG subclasses, with or without IgA deficiency, and effects of immunoglobulin prophylaxis. Monogr. Allergy. 1986;20:157–163. [PubMed] [Google Scholar]
- Bonilla F.A., Bernstein I.L., Khan D.A., Ballas Z.K., Chinen J., Frank M.M., Kobrynski L.J., Levinson A.I., Mazer B., Nelson R.P., Jr., Orange J.S., Routes J.M., Shearer W.T., Sorensen R.U., American Academy of Allergy, Asthma and Immunology, American College of Allergy, Asthma and Immunology and Joint Council of Allergy, Asthma and Immunology Practice parameter for the diagnosis and management of primary immunodeficiency. Ann. Allergy Asthma Immunol. 2005;94(5 Suppl. 1):S1–63. doi: 10.1016/s1081-1206(10)61142-8. [DOI] [PubMed] [Google Scholar]
- Bonilla F.A., Khan D.A., Ballas Z.K., Chinen J., Frank M.M., Hsu J.T., Keller M., Kobrynski L.J., Komarow H.D., Mazer B., Nelson R.P., Jr., Orange J.S., Routes J.M., Shearer W.T., Sorensen R.U., Verbsky J.W., Bernstein D.I., Blessing-Moore J., Lang D., Nicklas R.A., Oppenheimer J., Portnoy J.M., Randolph C.R., Schuller D., Spector S.L., Tilles S., Wallace D., Joint Task Force on Practice Parameters, representing the American Academy of Allergy, Asthma & Immunology, the American College of Allergy, Asthma & Immunology and the Joint Council of Allergy, Asthma & Immunology Practice parameter for the diagnosis and management of primary immunodeficiency. J. Allergy Clin. Immunol. 2015;136(5) doi: 10.1016/j.jaci.2015.04.049. 1186–205.e1–78. [DOI] [PubMed] [Google Scholar]
- Brent J., Guzman D., Bangs C., Grimbacher B., Fayolle C., Huissoon A., Bethune C., Thomas M., Patel S., Jolles S., Alachkar H., Kumaratne D., Baxendale H., Edgar J.D., Helbert M., Hambleton S., Arkwright P.D. Clinical and laboratory correlates of lung disease and cancer in adults with idiopathic hypogammaglobulinaemia. Clin. Exp. Immunol. 2016;184(1):73–82. doi: 10.1111/cei.12748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruton O.C. Agammaglobulinemia. Pediatrics. 1952;9(6):722–728. [PubMed] [Google Scholar]
- Conley M.E., Notarangelo L.D., Etzioni A. Diagnostic criteria for primary immunodeficiencies. Representing PAGID (Pan-American Group for Immunodeficiency) and ESID (European Society for Immunodeficiencies) Clin. Immunol. 1999;93(3):190–197. doi: 10.1006/clim.1999.4799. [DOI] [PubMed] [Google Scholar]
- Cunningham-Rundles C., Siegal F.P., Smithwick E.M., Lion-Boule A., Cunningham-Rundles S., O'Malley J., Barandun S., Good R.A. Efficacy of intravenous immunoglobulin in primary humoral immunodeficiency disease. Ann. Intern. Med. 1984;101(4):435–439. doi: 10.7326/0003-4819-101-4-435. [DOI] [PubMed] [Google Scholar]
- de Vries E., Clinical Working Party of the European Society for Immunodeficiencies (ESID) Patient-centred screening for primary immunodeficiency: a multi-stage diagnostic protocol designed for non-immunologists. Clin. Exp. Immunol. 2006;145(2):204–214. doi: 10.1111/j.1365-2249.2006.03138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries E., Kuijpers T.W., van Tol M.J., van der Meer J.W., Weemaes C.M., van Dongen J.J. Immunology in medical practice. XXXV. Screening of suspected immunodeficiency: diagnostic protocols for patients with opportunistic or recurrent severe infections, wasting and failure to thrive. Ned. Tijdschr. Geneeskd. 2000;144(46):2197–2203. [PubMed] [Google Scholar]
- Driessen G.J., Dalm V.A., van Hagen P.M., Grashoff H.A., Hartwig N.G., van Rossum A.M., Warris A., de Vries E., Barendregt B.H., Pico I., Posthumus S., van Zelm M.C., van Dongen J.J., van der Burg M. Common variable immunodeficiency and idiopathic primary hypogammaglobulinemia: two different conditions within the same disease spectrum. Haematologica. 2013;98(10):1617–1623. doi: 10.3324/haematol.2013.085076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durandy A., Kracker S., Fischer A. Primary antibody deficiencies. Nat. Rev. Immunol. 2013;13(7):519–533. doi: 10.1038/nri3466. [DOI] [PubMed] [Google Scholar]
- Edgar J.D., Buckland M., Guzman D., Conlon N.P., Knerr V., Bangs C., Reiser V., Panahloo Z., Workman S., Slatter M., Gennery A.R., Davies E.G., Allwood Z., Arkwright P.D., Helbert M., Longhurst H.J., Grigoriadou S., Devlin L.A., Huissoon A., Krishna M.T., Hackett S., Kumararatne D.S., Condliffe A.M., Baxendale H., Henderson K., Bethune C., Symons C., Wood P., Ford K., Patel S., Jain R., Jolles S., El-Shanawany T., Alachkar H., Herwadkar A., Sargur R., Shrimpton A., Hayman G., Abuzakouk M., Spickett G., Darroch C.J., Paulus S., Marshall S.E., Mcdermott E.M., Heath P.T., Herriot R., Noorani S., Turner M., Khan S., Grimbacher B. The United Kingdom Primary Immune Deficiency (UKPID) Registry: report of the first 4 years' activity 2008–2012. Clin. Exp. Immunol. 2014;175(1):68–78. doi: 10.1111/cei.12172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elberse K.E., Tcherniaeva I., Berbers G.A., Schouls L.M. Optimization and application of a multiplex bead-based assay to quantify serotype-specific IgG against Streptococcus pneumoniae polysaccharides: response to the booster vaccine after immunization with the pneumococcal 7-valent conjugate vaccine. Clin. Vaccine Immunol. 2010;17(4):674–682. doi: 10.1128/CVI.00408-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Myeloma Working Group Criteria for the classification of monoclonal gammopathies, multiple myeloma and related disorders: a report of the International Myeloma Working Group. Br. J. Haematol. 2003;121(5):749–757. [PubMed] [Google Scholar]
- Lucas M., Lee M., Lortan J., Lopez-Granados E., Misbah S., Chapel H. Infection outcomes in patients with common variable immunodeficiency disorders: relationship to immunoglobulin therapy over 22 years. J. Allergy Clin. Immunol. 2010;125(6) doi: 10.1016/j.jaci.2010.02.040. 1354–1360.e4. [DOI] [PubMed] [Google Scholar]
- Meerveld-Eggink A., van der Velden A.M., Ossenkoppele G.J., van de Loosdrecht A.A., Biesma D.H., Rijkers G.T. Antibody response to polysaccharide conjugate vaccines after nonmyeloablative allogeneic stem cell transplantation. Biol. Blood Marrow Transplant. 2009;15(12):1523–1530. doi: 10.1016/j.bbmt.2009.07.020. [DOI] [PubMed] [Google Scholar]
- Nolte M.T., Pirofsky B., Gerritz G.A., Golding B. Intravenous immunoglobulin therapy for antibody deficiency. Clin. Exp. Immunol. 1979;36(2):237–243. [PMC free article] [PubMed] [Google Scholar]
- Olinder-Nielsen A.M., Granert C., Forsberg P., Friman V., Vietorisz A., Bjorkander J. Immunoglobulin prophylaxis in 350 adults with IgG subclass deficiency and recurrent respiratory tract infections: a long-term follow-up. Scand. J. Infect. Dis. 2007;39(1):44–50. doi: 10.1080/00365540600951192. [DOI] [PubMed] [Google Scholar]
- Orange J.S., Hossny E.M., Weiler C.R., Ballow M., Berger M., Bonilla F.A., Buckley R., Chinen J., El-Gamal Y., Mazer B.D., Nelson R.P., Jr., Patel D.D., Secord E., Sorensen R.U., Wasserman R.L., Cunningham-Rundles C., Primary Immunodeficiency Committee of the American Academy of Allergy, Asthma and Immunology Use of intravenous immunoglobulin in human disease: a review of evidence by members of the Primary Immunodeficiency Committee of the American Academy of Allergy, Asthma and Immunology. J. Allergy Clin. Immunol. 2006;117(Suppl. 4):S525–S553. doi: 10.1016/j.jaci.2006.01.015. [DOI] [PubMed] [Google Scholar]
- Quartier P., Debre M., de Blic J., de Sauverzac R., Sayegh N., Jabado N., Haddad E., Blanche S., Casanova J.L., Smith C.I., Le Deist F., de Saint Basile G., Fischer A. Early and prolonged intravenous immunoglobulin replacement therapy in childhood agammaglobulinemia: a retrospective survey of 31 patients. J. Pediatr. 1999;134(5):589–596. doi: 10.1016/s0022-3476(99)70246-5. [DOI] [PubMed] [Google Scholar]
- Quinti I., Soresina A., Guerra A., Rondelli R., Spadaro G., Agostini C., Milito C., Trombetta A.C., Visentini M., Martini H., Plebani A., Fiorilli M., Ipinet Investigators Effectiveness of immunoglobulin replacement therapy on clinical outcome in patients with primary antibody deficiencies: results from a multicenter prospective cohort study. J. Clin. Immunol. 2011;31(3):315–322. doi: 10.1007/s10875-011-9511-0. [DOI] [PubMed] [Google Scholar]
- Resnick E.S., Moshier E.L., Godbold J.H., Cunningham-Rundles C. Morbidity and mortality in common variable immune deficiency over 4 decades. Blood. 2012;119(7):1650–1657. doi: 10.1182/blood-2011-09-377945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roifman C.M., Lederman H.M., Lavi S., Stein L.D., Levison H., Gelfand E.W. Benefit of intravenous IgG replacement in hypogammaglobulinemic patients with chronic sinopulmonary disease. Am. J. Med. 1985;79(2):171–174. doi: 10.1016/0002-9343(85)90006-3. [DOI] [PubMed] [Google Scholar]
- Torres A., Peetermans W.E., Viegi G., Blasi F. Risk factors for community-acquired pneumonia in adults in Europe: a literature review. Thorax. 2013;68(11):1057–1065. doi: 10.1136/thoraxjnl-2013-204282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kessel D.A., Hoffman T.W., van Velzen-Blad H., Zanen P., Rijkers G.T., Grutters J.C. Response to pneumococcal vaccination in mannose-binding lectin-deficient adults with recurrent respiratory tract infections. Clin. Exp. Immunol. 2014;177(1):272–279. doi: 10.1111/cei.12299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kessel D.A., Horikx P.E., van Houte A.J., de Graaff C.S., van Velzen-Blad H., Rijkers G.T. Clinical and immunological evaluation of patients with mild IgG1 deficiency. Clin. Exp. Immunol. 1999;118(1):102–107. doi: 10.1046/j.1365-2249.1999.01023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vossen J.M., Zegers B.J. Diagnostic studies in patients suspected of having an immunodeficiency disorder. Tijdschr. Kindergeneeskd. 1988;56(5):174–184. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary tables
Dataset