Summary
Effective regenerative treatments for periodontal tissue defects have recently been demonstrated using mesenchymal stromal/stem cells (MSCs). Furthermore, current bioengineering techniques have enabled de novo fabrication of tooth-perio dental units in mice. These cutting-edge technologies are expected to address unmet needs within regenerative dentistry. However, to achieve efficient and stable treatment outcomes, preparation of an appropriate stem cell source is essential. Many researchers are investigating the use of adult stem cells for regenerative dentistry; bone marrow-derived MSCs (BM-MSCs) are particularly promising and presently used clinically. However, current BM-MSC isolation techniques result in a heterogeneous, non-reproducible cell population because of a lack of identified distinct BM-MSC surface markers. Recently, specific subsets of cell surface markers for BM-MSCs have been reported in mice (PDGFRα+ and Sca-1+) and humans (LNGFR+, THY-1+ and VCAM-1+), facilitating the isolation of unique enriched BM-MSCs (so-called “purified MSCs”). Notably, the enriched BM-MSC population contains neural crest-derived cells, which can differentiate into cells of neural crest- and mesenchymal lineages. In this review, characteristics of the enriched BM-MSCs are outlined with a focus on their potential application within future regenerative dentistry.
Keywords: Bone marrow-derived mesenchymal stem cell, Flow cytometric isolation, Neural crest cell, Enriched/purified mesenchymal stem cell, Regenerative dentistry
1. Introduction
Following tooth loss, damage to periodontal tissues or jaw bones caused by dental caries, periodontal disease, or tumor extraction, it is difficult for humans to self-regenerate the lost tissues [1]. Current dental therapies therefore use artificial materials and prostheses to restore lost dental structures. However, limitations of these therapies include incomplete recovery of functional tissues and esthetic issues [2]. Therefore, regenerative therapies are receiving increased attention within the dental field [3]. For example, patients with reduced alveolar bone height often undergo alveolar bone augmentation before a dental implant can be placed. Presently, autograft, allograft, xenograft, and bone grafting materials are used for alveolar bone augmentation; however, it is difficult to maintain the grafted bone height and volume for a long period after the augmentation treatment [4], even following autograft bone augmentation [5], [6], which is considered the gold-standard procedure.
Several studies have shown that fetal dental epithelium and dental mesenchyme can be suitable cell sources for bioengineered teeth [7], [8] that erupt at the transplant site of mouse alveolar bone defects [9]. A more recent technological approach for bioengineered teeth involves fabricating a tooth structure using periodontal tissues, including alveolar bone [10]. Therefore, stem cell-based therapy is expected to be a powerful tool in regenerative dentistry, with many researchers engaging in stem cell biology and tissue engineering research to establish cell-based approaches to regenerative dentistry [11].
There are basically two types of stem cells in bone marrow: hematopoietic stem cells (HSCs) [12], [13], [14], [15] and mesenchymal stromal/stem cells (MSCs) [16], [17]. Bone marrow-derived MSCs (BM-MSCs) are plastic-adherent and proliferative cells that are able to differentiate into osteoblasts, adipocytes and chondrocytes [17]. Human BM-MSCs (hBM-MSCs) are now clinically applied world-wide because of their proliferative ability and multi-potent differentiation potential.
One potential problem of using hBM-MSCs is that their therapeutic success rate varies among patients, partly because BM-MSCs as a population are heterogeneous and contain progenitors of osteoblasts, adipocytes, chondrocytes, and other mononuclear cells [18], [19]. According to the 2006 statement of the International Society for Cellular Therapy [20], hBM-MSCs are minimally defined by their (1) capacity to attach to tissue culture-treated plastic dishes, (2) specific surface antigen expression [CD105+, CD73+, CD90 (known as THY-1)+, CD45−, CD34−, CD14− or CD11b−, CD79a− or CD19−, and HLA-DR−], and (3) multipotent differentiation potential. However, these criteria are not definitive, and the statement mentioned that the criteria would require modification as new facts and data become available [20]. A lack of specific cell surface markers to define BM-MSCs has hampered progress in understanding the molecular basis of their clonal potential. In contrast, specific markers for HSCs and HSC-derived hematopoietic lineage cells are well-established [21], [22]; therefore these cells can be successfully isolated and analyzed using flow cytometry [12], [23]. Based on the resulting understanding of HSC characteristics, remarkable and reproducible progress in transplant therapy using HSCs has been achieved [24].
To provide standardized BM-MSC-based treatment outcomes, it is therefore important to pursue and define specific BM-MSC markers. Recently, combinations of cell surface markers for BM-MSCs, including PDGFRα+ and Sca-1+ (PαS) in mice [25] and LNGFR+, THY-1+, and VCAM-1+ (LTV) in humans [26], have been identified. These marker combinations enable the prospective isolation of highly enriched BM-MSC populations [25], [26], so-called “purified MSCs”, which show unique characteristics. Interestingly, recent studies have shown that the enriched BM-MSCs exhibit phenotypes of neural crest cells (NCCs) [25], [27], which are a multipotent migratory stem cell population. Indeed, a sub-population of BM-MSCs originates from NCCs [28]. Notably, during embryonic development, most cranial NCCs differentiate into cells of mesenchymal lineage, including osteoblasts, adipocytes, chondrocytes [29], and dental mesenchyme [30].
In this review, a specific combination of markers for identifying and isolating enriched BM-MSCs and their unique characteristics are introduced. The future prospects of using these enriched BM-MSCs for regenerative dentistry are also discussed.
2. Neural crest cells (NCCs)
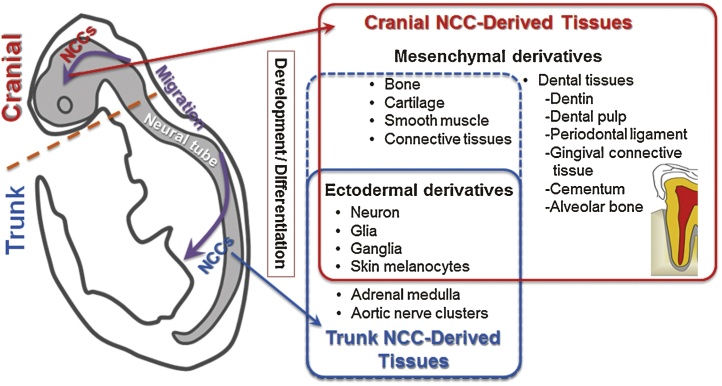
NCCs, known as the fourth germ layer, spread throughout the body following migration from the neural tube during fetal development [28], [29], [31]. In 1992, Anderson et al. [32] succeeded in isolating multi-potent and self-renewing NCCs (neural crest stem cells: NCSCs). Within the embryo, NCCs are detected at the border of the neural plate and the non-neural ectoderm after gastrulation. The borders of the neural plate (neural folds) converge at the dorsal midline to form the neural tube during neurulation. Subsequently, NCCs that have migrated from the neural tube undergo an epithelial-to-mesenchymal transition (EMT) [29]. After the EMT, NCCs leave the dorsal neural tube and migrate into other embryonic tissues in the head and trunk regions (Fig. 1). As a result, there are mainly two types of NCCs in the body, i.e., trunk and cranial NCCs [33], both of which can be identified by their Hox gene expression pattern [33], [34].
Figure 1.
Schematic diagram illustrating neural crest cell (NCC) location and progression. In the early embryo, the neural tube is formed and epithelial cells at the dorsal region of the neural tube undergo an epithelial-to-mesenchymal transition to become migratory NCCs. NCCs migrating from cranial and trunk regions of the neural tube differentiate into many diverse derivatives of mesenchymal- and ectodermal-lineage cells and tissues. In craniofacial development, cranial NCCs contribute to ectodermal tissues and mesenchymal tissues, including dental mesenchyme components, such as the dentin, dental pulp, periodontal ligament, gingival connective tissue, cementum, and alveolar bone. Trunk NCCs differentiate mainly into neurons and glial cells of the peripheral nervous system. A small mesenchymal contribution by trunk NCCs has also been reported.
2.1. Trunk NCCs
Most trunk NCCs differentiate into cells of ectodermal lineage, such as neurons, glia, and melanocytes [35], [36]. A very small mesenchymal contribution by trunk NCCs has been observed in endoneurial fibroblasts of the sciatic nerve [36], [37]. In addition, previous studies indicated that a certain population of mBM-MSCs from leg bones (the femur and tibia) originates from trunk NCCs [25], [28], [31]. It is now clear that trunk NCCs in amniotes have the capacity to give rise to diverse mesenchymal phenotypes, including skeletal cells, under specific conditions in vitro and in vivo. In appropriate culture conditions, trunk NCSCs differentiate into cells of mesenchymal lineage, such as osteoblasts [25], adipocytes [38], [39], chondrocytes [40], [41], and myofibroblasts [38].
2.2. Cranial NCCs
Cranial NCCs differentiate into a wider range of cell types than trunk NCCs during normal development [38]. During embryonic development, most cranial NCCs differentiate into mesenchymal lineage cells, including osteoblasts, adipocytes, chondrocytes [29], and dental mesenchyme [30]. After migration in the head regions of the embryo, cranial NCCs invade the surrounding cranial mesenchyme and ultimately cease migration, coalesce, and form the various cranial ganglia, craniofacial cartilage and bones [42], [43], and dental mesenchyme, such as dentin, dental pulp, cementum, and periodontal ligament [30], [44], [45], [46], [47], [48] (Fig. 1). In addition, some NCCs are preserved as NCSCs in adult craniofacial tissues, such as the nasal turbinates [49], submandibular glands [50], palate, gingiva, tongue, and buccal mucosa [51], [52].
Collectively, these data demonstrate that NCCs have multi-differentiation potential. In particular, NCCs in the craniofacial tissues possess the potential to undergo osteogenic differentiation [51], [53]. Therefore, multi-potent NCSCs have been isolated from adults [49] or produced from induced pluripotent stem (iPS) cells [54] with the goal of application to regenerative medicine, e.g., in craniofacial bone regeneration [55].
3. Bone marrow-derived mesenchymal stem cells (BM-MSCs)
In 1970, adherent fibroblastic cells were isolated from the bone marrow as colony-forming unit fibroblasts (CFU-F) [56]. In the early 1990s, these cells were recognized to differentiate into various mesenchymal tissues [57]. Subsequently, the proliferative ability of human bone marrow-derived cells partially attached to plastic dishes was reported [17]. These cells could directly differentiate into osteoblasts, chondrocytes, and adipocytes in vitro. However, these plastic-adherent cells are heterogeneous bulk BM-MSCs and contain unique subsets of BM-MSCs [58], [59], [60]. Therefore, inconsistent research outcomes cannot be completely avoided when using this traditional culture method, which has made it difficult to analyze the true phenotype and characteristics of BM-MSCs.
To date, the lack of unique markers has made it difficult to define BM-MSC origins, physiological roles, relationships with other stem cells, and in vivo localization. In 2002, the National Institutes of Health (NIH) described basic research on MSCs in the “Weekly NIH Funding Opportunities and Notices”, encouraging researchers to identify novel markers for MSC identification to enhance isolation techniques and facilitate the development of ex vivo stem cell expansion systems. To date, several MSC cell surface markers have been reported (Table 1), although hallmark, universally accepted cell surface markers for MSC detection still do not exist.
Table 1.
Previously reported surface markers of human and mouse multipotent mesenchymal stromal/stem cells.
| Cell surface marker | Human | Mouse | Distribution |
|---|---|---|---|
| CD11b/Integrin αM | − | − | Myeloid, macrophage, NK cells, T act, B subset |
| CD19/B4 | − | − | B cells, follicular dendritic cells |
| CD29/Integrin β1 | + | + | Leukocytes, fibroblasts, endothelium, epithelium |
| CD31/PECAM-1 | − | − | Platelets, gran, endothelium, dendritic cells, mono subset, T subset, B subset, lymphokine-activated killer cell |
| CD34/Mucocialin | − | − | Hematopoietic precursors, capillary endothelium, bone marrow stromal cells, mast cells |
| CD44 | + | + | Broad, memory T, fibroblast, epithelium, endothelium, cancer stem cells |
| CD45 | − | − | Leukocytes, not mature erythrocytes |
| CD49a/Integrin αI | + | ? | T act, endothelium |
| CD51/Integrin αV | + | + | Platelets, megakaryocytes, endothelium, osteoblasts, melanoma |
| CD56/NCAM-1 | + | + | Neural tissue, multiple isoforms |
| CD73/5′-Nucleotidase | + | + | T subset, B subset, follicular dendritic cells, endothelium, bone marrow stromal cells |
| CD90/THY1 | + | + | Thymocytes, T cells, hematopoietic subset, neurons |
| CD105/Endoglin | + | + | Endothelium, bone marrow cell subset, mac act |
| CD106/VCAM-1 | + | + | Endothelium act, follicular dendritic cells, bone marrow myeloid |
| CD117/c-Kit | + | ? | Hematopoietic stem and progenitors, neural crest-derived melanocytes, primordial germ cells, mast cells |
| CD140α/PDGFRα | + | + | Fibroblasts, smooth muscle, glial cells, chondrocytes |
| CD140β/PDGFRβ | + | + | Fibroblasts, smooth muscle, glial cells, chondrocytes |
| CD146/MCAM | + | ? | Embryonic tissue, mammary tumors |
| CD166/ALCAM | + | + | Neurons, T act, mono, epithelium, fibroblasts |
| CD271/LNGFR | + | ? | Neurons, mesenchymal stem cells |
| Nestin | + | + | Neural stem cells, glioma stem cells |
| Stro-1 | + | ? | Mesenchymal stem cells |
| Sca-1 | ? | + | Bone marrow hematopietic stem cells and precursors, bone marrow mesenchymal stem cells |
+: positive selection, −: negative selection, ?: unknown.
4. Enriched MSCs from limb bone marrow
In 2009, Morikawa et al. [25] first reported the prospective isolation of enriched mouse BM-MSCs (mBM-MSCs) using a combination of defined MSC surface markers and flow cytometry. This population of isolated mBM-MSCs was more homogenous than mBM-MSCs isolated using the traditional plastic-adherence technique, and the enriched mBM-MSCs could be analyzed and applied freshly without requiring cell culture after the initial flow cytometric cell sorting. Similar to traditionally isolated mBM-MSCs, the proliferative ability and differentiation capacity of the sorted cells can be evaluated by cell culture.
4.1. Enriched mBM-MSCs (PαS mBM-MSCs)
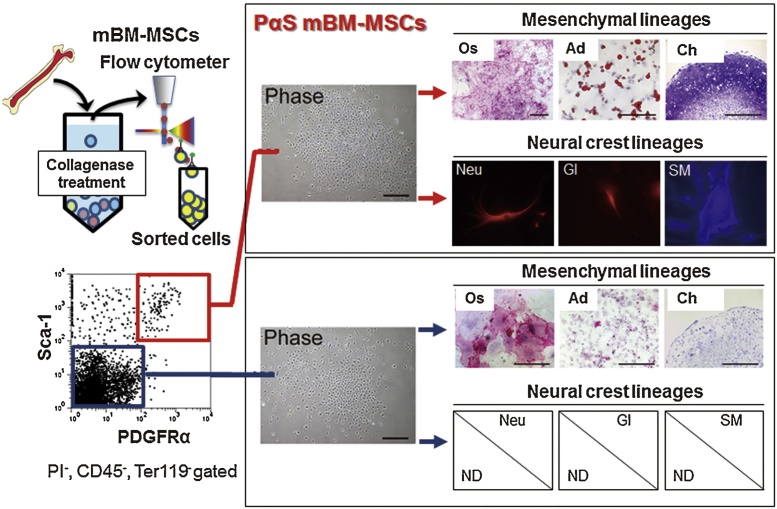
Highly enriched populations of mBM-MSCs can be isolated by flow cytometry as demonstrated for the PDGFRα+, Sca-1+, CD45−, Ter119− population [19], [25] (PαS mBM-MSCs: Fig. 2). PDGFRα is an NCC marker that is highly expressed particularly in cranial NCCs [38]. PαS mBM-MSCs (so-called “purified mouse MSCs”) have a CFU-F frequency approximately 120,000-fold higher than that of unfractionated bulk bone marrow mononuclear cells and show superior proliferative capacity in adherent cultures [19], [25]. Moreover, PαS mBM-MSCs maintain their differentiation capacity toward osteoblasts, adipocytes and chondrocytes [19], [25], [27] (Fig. 2). Additionally, PαS mBM-MSCs can differentiate into cells of neural crest lineage, including neurons, glia, and smooth muscle cells [25], [27].
Figure 2.
Schematic diagram illustrating mBM-MSC isolation by flow cytometry [27], [61]. Crushed bone fragments from adult mouse tibia are incubated in cell culture medium with collagenase to obtain a cell suspension. Cells are stained with monoclonal antibodies against CD45, Ter119, PDGFRα, and Sca-1. After staining, cells are sorted using a flow cytometer (see details in the protocol article [72]). Sorted Sca-1−/Ter119−/PDGFRα+/Sca-1+ cells (PαS mBM-MSCs: enriched mBM-MSCs) differentiate into cells of mesenchymal lineage (osteoblasts, adipocytes, and chondrocytes) and neural crest lineage (neurons, glia, and smooth muscle cells). Phase: phase contrast microscope image; Os: osteoblasts (alkaline phosphatase); Ad: adipocytes (Oil Red O); Ch: chondrocytes (toluidine blue); Neu: neurons (βIII tubulin); Gl: glia (glial fibrillary acidic protein: GFAP); SM: smooth muscle cells (α-smooth muscle actin); ND: not detected.
Furthermore, PαS mBM-MSCs innately express Klf4 and c-Myc at levels similar to those in embryonic stem (ES) cells [61]. Consequently, PαS mBM-MSCs have been demonstrated to efficiently produce iPS cells with introduction of three defined factors, i.e., Oct3, Klf4, and Sox2, without requiring c-Myc (3F-PαS-mBM-MSCs). Moreover, 3F-PαS-mBM-MSC-derived iPS cells can efficiently produce germline-competent chimeric mice [61]. These findings suggest that PαS mBM-MSCs can be maintained in an undifferentiated stem cell state while retaining multi-potency. These characteristics may facilitate analysis of the intrinsic characteristics and role of MSCs in bone marrow.
4.2. Origins of PαS mBM-MSCs: mesoderm and neural crest
The origins of BM-MSCs have been investigated using defined markers and transgenic mice. During development, the vertebrate limb bone marrow is developed from the lateral plate mesoderm [62], [63], [64]. Therefore, BM-MSCs have been considered to be derived from the mesoderm [62], [63], [64]. In contrast, Takashima et al. [28] have shown that Sox1+ neuroepithelium could give rise to mBM-MSCs, suggesting that NCCs supply the progenitors of BM-MSCs during embryogenesis and later are replaced by MSCs from other origins in postnatal development.
PαS mBM-MSCs contain both mesoderm- and neural crest-derived cells. A previous study using adult transgenic mice expressing Mesp1 (a mesodermal cell lineage marker in early development [65]) showed that PαS mBM-MSCs are partly derived from the mesoderm [27]. Interestingly, Mesp1+ PαS mBM-MSCs can differentiate into not only mesenchymal-lineage cells but also neural crest-lineage cells, even though they are derived from the mesoderm [27]. Morikawa et al. [25] also revealed that PαS mBM-MSCs originate partially from the neural crest using adult transgenic mice expressing neural crest-specific P0-Cre/Floxed-EGFP. These neural crest-derived PαS mBM-MSCs can also differentiate into mesenchymal- and neural crest-lineage cells [25].
4.3. Enriched human BM-MSCs (LTV hBM-MSCs)
Despite the widespread clinical use of hBM-MSCs, uncertainty exists with respect to many aspects of their biology because of the lack of unique markers, similar to mBM-MSCs. Recently, Mabuchi et al. [26] succeeded in prospectively isolating enriched hBM-MSCs by combining the LNGFR+, THY1+, and VCAM-1+ markers (LTV hBM-MSCs). Collectively, LTV hBM-MSCs demonstrate a CFU-F frequency ∼200,000 times higher than that observed for unfractionated bone marrow cells. Using a flow cytometric single-cell clonal assay, clonal LTV hBM-MSCs have been observed to differentiate into cells of mesenchymal lineage. In addition, LTV hBM-MSCs can differentiate into cells of neural crest lineage including neuronal and smooth muscle cells (Mabuchi et al. unpublished data).
This result implies that LTV hBM-MSCs contain neural crest-derived MSCs. More recently, enriched MSCs have also been isolated from MSCs in the human dental pulp using cell-surface markers LNGFR and THY1 [66]. Because craniofacial tissues originate from cranial NCCs, human LTV hBM-MSCs are expected to be an important cell source for achieving effective stem cell-based regenerative therapy of craniofacial tissues.
5. Potential of enriched BM-MSCs as a cell source for oral tissue/organ regeneration
New treatment methods are essential to repair large defects in periodontal tissues [1]. Stem cell transplantation strategies using the whole population of BM-MSCs have been successfully used in dentistry to regenerate these tissues, although further research is required to establish more reliable protocols [11]. It has been suggested that a cell source-dependent phenotype similar to that of the recipient site is associated with enhanced capacity of bone regeneration upon transplantation [67]. Embryologically, dental mesenchymal tissues are generated by cranial NCCs. Because enriched BM-MSCs also partly originate from NCCs, it would be expected that purified BM-MSCs with the NCC phenotype have an advantage in effective regeneration of craniofacial tissues/organs.
Tooth development requires an epithelial-mesenchymal interaction. Mouse dental epithelium in its embryonic early developmental stage stimulates mouse non-dental mesenchyme to differentiate into dental mesenchyme [68]. For tooth bioengineering, single-cell populations of fetal dental epithelium and mesenchyme can be used for generating bioengineered tooth germ, which can develop into fully functional teeth at transplanted sites in mouse mandibular bone [7], [9]. Advanced technology based on three-dimensional organ-germ culture using fetal dental cells enables the fabrication of a bioengineered tooth unit that regenerates not only the tooth but also the periodontal tissues [10]. These findings indicate that cells from embryonic oral epithelium and neural-crest-derived mesenchyme can be used to construct tooth and periodontal tissues. Although these technologies have opened the door to address unmet clinical needs in tooth regeneration therapy, the limited nature of cell sources for tooth bioengineering make them difficult apply clinically.
Recently, Jiang et al. [69] showed that postnatal dental epithelium and mesenchyme stem/progenitor cells in rat incisors can form tooth-like structures. Other research has shown that mouse embryonic dental mesenchyme cells, when reconstituted with adult human gingival epithelial cells [70] or cell sheets derived from iPS cells [8], form tooth-like structures. Additionally, adult mBM-MSCs were previously shown to successfully replace dental mesenchymal cells in forming a tooth primordium [71]. Because the dental mesenchyme originates in NCCs, enriched BM-MSCs, which possess neural crest phenotypes, would have more similar characteristics to dental mesenchyme compared to the general BM-MSC population. If the differentiation capacity of enriched BM-MSCs is comparable to that of dental mesenchyme, they could be a promising source for dental mesenchyme regeneration.
6. Future challenges for enriched BM-MSCs
Basic research into enriched BM-MSCs is in its infancy. These cells have been shown to possess some authentic BM-MSC characteristics; however, a complete BM-MSC marker set is yet to be defined. In addition, hBM-MSC markers are different from mBM-MSC markers. Therefore, simple comparisons of BM-MSCs across species are difficult. The identification of universal BM-MSC markers should greatly enhance the progress of basic research into BM-MSCs. Although attempts to regenerate tooth and periodontal tissues using enriched BM-MSCs have been initiated, one of the critical issues for clinical application of enriched BM-MSCs is their limited expansion potential in traditional adherent culture systems. Therefore, it is important to elucidate the mechanisms that regulate proliferation and differentiation of enriched BM-MSCs to develop suitable cell culture systems.
7. Concluding remarks
In this review, we have outlined two unique aspects of enriched BM-MSCs (PαS mBM-MSCs and LTV hBM-MSCs): (1) They partially contain NCSCs derived from the neural crest, and (2) their differentiation potential is similar to that of NCCs because they can also differentiate into neural crest lineage cells. The majority of the craniofacial mesenchyme is developed from the neural crest. Therefore, these unique characteristics of multi-potent enriched BM-MSCs with NCC phenotypes imply that they would be a suitable cell source for craniofacial mesenchymal tissue/organ regeneration, not only to form bioengineered tooth organs, but also for craniofacial bone regeneration or periodontal tissue regeneration (Fig. 3). Further biological research on enriched BM-MSCs is required to achieve efficient cell-based regenerative dentistry.
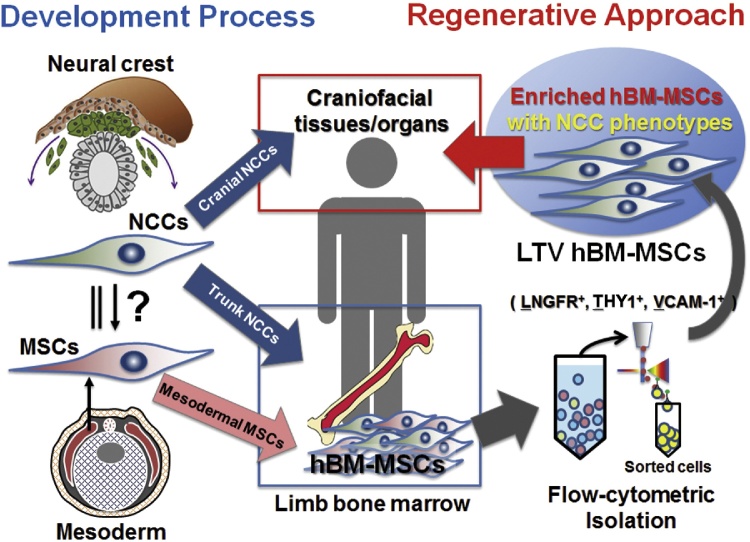
Figure 3.
Schematic diagram illustrating the prospects of using enriched hBM-MSCs from bone marrow for craniofacial tissue/organ regeneration. Craniofacial mesenchyme develops from migrating cranial NCCs. A certain portion of trunk NCCs migrates to limb bone marrow, where these cells reside as a part of adult BM-MSCs. Therefore, enriched hBM-MSCs, which are isolated from the BM-MSC population using a flow cytometer (LNGFR+, THY1+, and VCAM-1+ cells: LTV hBM-MSCs), show an NCC phenotype, and they are expected to be a useful cell source for craniofacial regenerative medicine.
Conflict of interest
The authors declare that no competing financial interests exist.
Acknowledgments
This work was supported by Grant-in-Aids for Young Scientists (B: 16K20480, K.N.) and Scientific Research (B: 16H05519, H.E. and K.N.) from the Japan Society for the Promotion of Science.
References
- 1.Egusa H., Sonoyama W., Nishimura M., Atsuta I., Akiyama K. Stem cells in dentistry – Part I: stem cell sources. J Prosthodont Res. 2012;56:151–165. doi: 10.1016/j.jpor.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 2.Masaki C., Nakamoto T., Mukaibo T., Kondo Y., Hosokawa R. Strategies for alveolar ridge reconstruction and preservation for implant therapy. J Prosthodont Res. 2015;59:220–228. doi: 10.1016/j.jpor.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 3.Kaku M., Akiba Y., Akiyama K., Akita D., Nishimura M. Cell-based bone regeneration for alveolar ridge augmentation – cell source, endogenous cell recruitment and immunomodulatory function. J Prosthodont Res. 2015;59:96–112. doi: 10.1016/j.jpor.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 4.Li P., Honda Y., Arima Y., Yasui K., Inami K., Nishiura A. Interferon-γ enhances the efficacy of autogenous bone grafts by inhibiting postoperative bone resorption in rat calvarial defects. J Prosthodont Res. 2016;60:167–176. doi: 10.1016/j.jpor.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 5.Verhoeven J.W., Ruijter J., Cune M.S., Terlou M., Zoon M. Onlay grafts in combination with endosseous implants in severe mandibular atrophy: one year results of a prospective, quantitative radiological study. Clin Oral Implants Res. 2000;11:583–594. doi: 10.1034/j.1600-0501.2000.011006583.x. [DOI] [PubMed] [Google Scholar]
- 6.Hatano N., Shimizu Y., Ooya K. A clinical long-term radiographic evaluation of graft height changes after maxillary sinus floor augmentation with a 2:1 autogenous bone/xenograft mixture and simultaneous placement of dental implants. Clin Oral Implants Res. 2004;15:339–345. doi: 10.1111/j.1600-0501.2004.00996.x. [DOI] [PubMed] [Google Scholar]
- 7.Nakao K., Morita R., Saji Y., Ishida K., Tomita Y., Ogawa M. The development of a bioengineered organ germ method. Nat Methods. 2007;4:227–230. doi: 10.1038/nmeth1012. [DOI] [PubMed] [Google Scholar]
- 8.Cai J., Zhang Y., Liu P., Chen S., Wu X., Sun Y. Generation of tooth-like structures from integration-free human urine induced pluripotent stem cells. Cell Regen. 2013;2:6. doi: 10.1186/2045-9769-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ikeda E., Morita R., Nakao K., Ishida K., Nakamura T., Takano-Yamamoto T. Fully functional bioengineered tooth replacement as an organ replacement therapy. Proc Natl Acad Sci U S A. 2009;106:13475–13480. doi: 10.1073/pnas.0902944106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oshima M., Mizuno M., Imamura A., Ogawa M., Yasukawa M., Yamazaki H. Functional tooth regeneration using a bioengineered tooth unit as a mature organ replacement regenerative therapy. PLoS ONE. 2011;6:e21531. doi: 10.1371/journal.pone.0021531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Egusa H., Sonoyama W., Nishimura M., Atsuta I., Akiyama K. Stem cells in dentistry – Part II: Clinical applications. J Prosthodont Res. 2012;56:229–248. doi: 10.1016/j.jpor.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 12.Spangrude G.J., Heimfeld S., Weissman I.L. Purification and characterization of mouse hematopoietic stem cells. Science. 1988;241:58–62. doi: 10.1126/science.2898810. [DOI] [PubMed] [Google Scholar]
- 13.Müller-Sieburg C.E., Cho R.H., Thoman M., Adkins B., Sieburg H.B. Deterministic regulation of hematopoietic stem cell self-renewal and differentiation. Blood. 2002;100:1302–1309. [PubMed] [Google Scholar]
- 14.Müller-Sieburg C.E., Cho R.H., Karlsson L., Huang J.F., Sieburg H.B. Myeloid-biased hematopoietic stem cells have extensive self-renewal capacity but generate diminished lymphoid progeny with impaired IL-7 responsiveness. Blood. 2004;103:4111–4118. doi: 10.1182/blood-2003-10-3448. [DOI] [PubMed] [Google Scholar]
- 15.Sieburg H.B., Cho R.H., Dykstra B., Uchida N., Eaves C.J., Muller-Sieburg C.E. The hematopoietic stem compartment consists of a limited number of discrete stem cell subsets. Blood. 2006;107:2311–2316. doi: 10.1182/blood-2005-07-2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prockop D.J. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- 17.Pittenger M.F., Mackay A.M., Beck S.C., Jaiswal R.K., Douglas R., Mosca J.D. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 18.Toyoda M., Takahashi H., Umezawa A. Ways for a mesenchymal stem cell to live on its own: maintaining an undifferentiated state ex vivo. Int J Hematol. 2007;86:1–4. doi: 10.1532/IJH97.07055. [DOI] [PubMed] [Google Scholar]
- 19.Morikawa S., Mabuchi Y., Kubota Y., Nagai Y., Niibe K., Hiratsu E. Prospective identification, isolation, and systemic transplantation of multipotent mesenchymal stem cells in murine bone marrow. J Exp Med. 2009;206:2483–2496. doi: 10.1084/jem.20091046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dominici M., Le Blanc K., Mueller I., Slaper-Cortenbach I., Marini F., Krause D. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 21.Morrison S.J., Uchida N., Weissman I.L. The biology of hematopoietic stem cells. Annu Rev Cell Dev Biol. 1995;11:35–71. doi: 10.1146/annurev.cb.11.110195.000343. [DOI] [PubMed] [Google Scholar]
- 22.Seita J., Weissman I.L. Hematopoietic stem cell: self-renewal versus differentiation. Wiley Interdiscip Rev Syst Biol Med. 2010;2:640–653. doi: 10.1002/wsbm.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baum C.M., Weissman I.L., Tsukamoto A.S., Buckle A.M., Peault B. Isolation of a candidate human hematopoietic stem-cell population. Proc Natl Acad Sci U S A. 1992;89:2804–2808. doi: 10.1073/pnas.89.7.2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Copelan E.A. Hematopoietic stem-cell transplantation. N Engl J Med. 2006;354:1813–1826. doi: 10.1056/NEJMra052638. [DOI] [PubMed] [Google Scholar]
- 25.Morikawa S., Mabuchi Y., Niibe K., Suzuki S., Nagoshi N., Sunabori T. Development of mesenchymal stem cells partially originate from the neural crest. Biochem Biophys Res Commun. 2009;379:1114–1119. doi: 10.1016/j.bbrc.2009.01.031. [DOI] [PubMed] [Google Scholar]
- 26.Mabuchi Y., Morikawa S., Harada S., Niibe K., Suzuki S., Renault-Mihara F. LNGFR(+)THY-1(+)VCAM-1(hi+) cells reveal functionally distinct subpopulations in mesenchymal stem cells. Stem Cell Rep. 2013;1:152–165. doi: 10.1016/j.stemcr.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Niibe K., Morikawa S., Mabuchi Y., Araki D., Nakagawa T., Okano H. Mesp1+ early paraxial mesodermal cells supply initial bone marrow mesenchymal stem cells capable of differentiating into neural crest lineage cells. Inflamm Regen. 2011;31:116–124. [Google Scholar]
- 28.Takashima Y., Era T., Nakao K., Kondo S., Kasuga M., Smith A.G. Neuroepithelial cells supply an initial transient wave of MSC differentiation. Cell. 2007;129:1377–1388. doi: 10.1016/j.cell.2007.04.028. [DOI] [PubMed] [Google Scholar]
- 29.Huang X., Saint-Jeannet J.P. Induction of the neural crest and the opportunities of life on the edge. Dev Biol. 2004;275:1–11. doi: 10.1016/j.ydbio.2004.07.033. [DOI] [PubMed] [Google Scholar]
- 30.Chai Y., Jiang X., Ito Y., Bringas P., Han J., Rowitch D.H. Fate of the mammalian cranial neural crest during tooth and mandibular morphogenesis. Development. 2000;127:1671–1679. doi: 10.1242/dev.127.8.1671. [DOI] [PubMed] [Google Scholar]
- 31.Nagoshi N., Shibata S., Kubota Y., Nakamura M., Nagai Y., Satoh E. Ontogeny and multipotency of neural crest-derived stem cells in mouse bone marrow, dorsal root ganglia, and whisker pad. Cell Stem Cell. 2008;2:392–403. doi: 10.1016/j.stem.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 32.Stemple D.L., Anderson D.J. Isolation of a stem cell for neurons and glia from the mammalian neural crest. Cell. 1992;71:973–985. doi: 10.1016/0092-8674(92)90393-q. [DOI] [PubMed] [Google Scholar]
- 33.Abzhanov A., Tzahor E., Lassar A.B., Tabin C.J. Dissimilar regulation of cell differentiation in mesencephalic (cranial) and sacral (trunk) neural crest cells in vitro. Development. 2003;130:4567–4579. doi: 10.1242/dev.00673. [DOI] [PubMed] [Google Scholar]
- 34.Couly G., Grapin-Botton A., Coltey P., Ruhin B., Le Douarin N.M. Determination of the identity of the derivatives of the cephalic neural crest: incompatibility between Hox gene expression and lower jaw development. Development. 1998;125:3445–3459. doi: 10.1242/dev.125.17.3445. [DOI] [PubMed] [Google Scholar]
- 35.Shah N.M., Groves A.K., Anderson D.J. Alternative neural crest cell fates are instructively promoted by TGFbeta superfamily members. Cell. 1996;85:331–343. doi: 10.1016/s0092-8674(00)81112-5. [DOI] [PubMed] [Google Scholar]
- 36.Dupin E., Sommer L. Neural crest progenitors and stem cells: from early development to adulthood. Dev Biol. 2012;366:83–95. doi: 10.1016/j.ydbio.2012.02.035. [DOI] [PubMed] [Google Scholar]
- 37.Joseph N.M., Mukouyama Y.S., Mosher J.T., Jaegle M., Crone S.A., Dormand E.L. Neural crest stem cells undergo multilineage differentiation in developing peripheral nerves to generate endoneurial fibroblasts in addition to Schwann cells. Development. 2004;131:5599–5612. doi: 10.1242/dev.01429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hagiwara K., Obayashi T., Sakayori N., Yamanishi E., Hayashi R., Osumi N. Molecular and cellular features of murine craniofacial and trunk neural crest cells as stem cell-like cells. PLOS ONE. 2014;9:e84072. doi: 10.1371/journal.pone.0084072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Billon N., Iannarelli P., Monteiro M.C., Glavieux-Pardanaud C., Richardson W.D., Kessaris N. The generation of adipocytes by the neural crest. Development. 2007;134:2283–2292. doi: 10.1242/dev.002642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ido A., Ito K. Expression of chondrogenic potential of mouse trunk neural crest cells by FGF2 treatment. Dev Dyn. 2006;235:361–367. doi: 10.1002/dvdy.20635. [DOI] [PubMed] [Google Scholar]
- 41.McGonnell I.M., Graham A. Trunk neural crest has skeletogenic potential. Curr Biol. 2002;12:767–771. doi: 10.1016/s0960-9822(02)00818-7. [DOI] [PubMed] [Google Scholar]
- 42.Taneyhill L.A. To adhere or not to adhere: the role of Cadherins in neural crest development. Cell Adh Migr. 2008;2:223–230. doi: 10.4161/cam.2.4.6835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chung I.H., Yamaza T., Zhao H., Choung P.H., Shi S., Chai Y. Stem cell property of postmigratory cranial neural crest cells and their utility in alveolar bone regeneration and tooth development. Stem Cells. 2009;27:866–877. doi: 10.1002/stem.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miletich I., Sharpe P.T. Neural crest contribution to mammalian tooth formation. Birth Defects Res C Embryo Today. 2004;72:200–212. doi: 10.1002/bdrc.20012. [DOI] [PubMed] [Google Scholar]
- 45.Kaukua N., Shahidi M.K., Konstantinidou C., Dyachuk V., Kaucka M., Furlan A. Glial origin of mesenchymal stem cells in a tooth model system. Nature. 2014;513:551–554. doi: 10.1038/nature13536. [DOI] [PubMed] [Google Scholar]
- 46.Duverger O., Zah A., Isaac J., Sun H.W., Bartels A.K., Lian J.B. Neural crest deletion of Dlx3 leads to major dentin defects through down-regulation of Dspp. J Biol Chem. 2012;287:12230–12240. doi: 10.1074/jbc.M111.326900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Janebodin K., Horst O.V., Ieronimakis N., Balasundaram G., Reesukumal K., Pratumvinit B. Isolation and characterization of neural crest-derived stem cells from dental pulp of neonatal mice. PLoS ONE. 2011;6:e27526. doi: 10.1371/journal.pone.0027526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ibarretxe G., Crende O., Aurrekoetxea M., Garcia-Murga V., Etxaniz J., Unda F. Neural crest stem cells from dental tissues: a new hope for dental and neural regeneration. Stem Cells Int. 2012;2012:103503. doi: 10.1155/2012/103503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hauser S., Widera D., Qunneis F., Muller J., Zander C., Greiner J. Isolation of novel multipotent neural crest-derived stem cells from adult human inferior turbinate. Stem Cells Dev. 2012;21:742–756. doi: 10.1089/scd.2011.0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Takahashi M., Suzawa T., Yamada A., Yamaguchi T., Mishima K., Osumi N. Identification of gene expression profile of neural crest-derived cells isolated from submandibular glands of adult mice. Biochem Biophys Res Commun. 2014;446:481–486. doi: 10.1016/j.bbrc.2014.02.130. [DOI] [PubMed] [Google Scholar]
- 51.Ono M., Suzawa T., Takami M., Yamamoto G., Hosono T., Yamada A. Localization and osteoblastic differentiation potential of neural crest-derived cells in oral tissues of adult mice. Biochem Biophys Res Commun. 2015 doi: 10.1016/j.bbrc.2015.07.106. [DOI] [PubMed] [Google Scholar]
- 52.Xu X., Chen C., Akiyama K., Chai Y., Le A.D., Wang Z. Gingivae contain neural-crest- and mesoderm-derived mesenchymal stem cells. J Dent Res. 2013;92:825–832. doi: 10.1177/0022034513497961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Greiner J.F., Grunwald L.M., Muller J., Sudhoff H., Widera D., Kaltschmidt C. Culture bag systems for clinical applications of adult human neural crest-derived stem cells. Stem Cell Res Ther. 2014;5:34. doi: 10.1186/scrt422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fukuta M., Nakai Y., Kirino K., Nakagawa M., Sekiguchi K., Nagata S. Derivation of mesenchymal stromal cells from pluripotent stem cells through a neural crest lineage using small molecule compounds with defined media. PLOS ONE. 2014;9:e112291. doi: 10.1371/journal.pone.0112291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Morikawa S., Ouchi T., Shibata S., Fujimura T., Kawana H., Okano H. Applications of mesenchymal stem cells and neural crest cells in craniofacial skeletal research. Stem Cells Int. 2016;2016:2849879. doi: 10.1155/2016/2849879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Friedenstein A.J., Chailakhjan R.K., Lalykina K.S. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970;3:393–403. doi: 10.1111/j.1365-2184.1970.tb00347.x. [DOI] [PubMed] [Google Scholar]
- 57.Caplan A.I. Mesenchymal stem cells. J Orthop Res. 1991;9:641–650. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- 58.Colter D.C., Class R., DiGirolamo C.M., Prockop D.J. Rapid expansion of recycling stem cells in cultures of plastic-adherent cells from human bone marrow. Proc Natl Acad Sci U S A. 2000;97:3213–3218. doi: 10.1073/pnas.070034097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jiang Y., Jahagirdar B.N., Reinhardt R.L., Schwartz R.E., Keene C.D., Ortiz-Gonzalez X.R. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–49. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- 60.Kuroda Y., Kitada M., Wakao S., Nishikawa K., Tanimura Y., Makinoshima H. Unique multipotent cells in adult human mesenchymal cell populations. Proc Natl Acad Sci U S A. 2010;107:8639–8643. doi: 10.1073/pnas.0911647107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Niibe K., Kawamura Y., Araki D., Morikawa S., Miura K., Suzuki S. Purified mesenchymal stem cells are an efficient source for iPS cell induction. PLoS ONE. 2011:6. doi: 10.1371/journal.pone.0017610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yusuf F., Brand-Saberi B. The eventful somite: patterning, fate determination and cell division in the somite. Anat Embryol. 2006;211(Suppl. 1):21–30. doi: 10.1007/s00429-006-0119-8. [DOI] [PubMed] [Google Scholar]
- 63.Onimaru K., Shoguchi E., Kuratani S., Tanaka M. Development and evolution of the lateral plate mesoderm: comparative analysis of amphioxus and lamprey with implications for the acquisition of paired fins. Dev Biol. 2011;359:124–136. doi: 10.1016/j.ydbio.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 64.Tanaka M. Molecular and evolutionary basis of limb field specification and limb initiation. Dev Growth Differ. 2013;55:149–163. doi: 10.1111/dgd.12017. [DOI] [PubMed] [Google Scholar]
- 65.Saga Y., Hata N., Kobayashi S., Magnuson T., Seldin M.F., Taketo M.M. MesP1: a novel basic helix-loop-helix protein expressed in the nascent mesodermal cells during mouse gastrulation. Development. 1996;122:2769–2778. doi: 10.1242/dev.122.9.2769. [DOI] [PubMed] [Google Scholar]
- 66.Yasui T., Mabuchi Y., Toriumi H., Ebine T., Niibe K., Houlihan D.D. Purified human dental pulp stem cells promote osteogenic regeneration. J Dent Res. 2016;95:206–214. doi: 10.1177/0022034515610748. [DOI] [PubMed] [Google Scholar]
- 67.Aghaloo T.L., Chaichanasakul T., Bezouglaia O., Kang B., Franco R., Dry S.M. Osteogenic potential of mandibular vs. long-bone marrow stromal cells. J Dent Res. 2010;89:1293–1298. doi: 10.1177/0022034510378427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mina M., Kollar E.J. The induction of odontogenesis in non-dental mesenchyme combined with early murine mandibular arch epithelium. Arch Oral Biol. 1987;32:123–127. doi: 10.1016/0003-9969(87)90055-0. [DOI] [PubMed] [Google Scholar]
- 69.Jiang N., Zhou J., Chen M., Schiff M.D., Lee C.H., Kong K. Postnatal epithelium and mesenchyme stem/progenitor cells in bioengineered amelogenesis and dentinogenesis. Biomaterials. 2014;35:2172–2180. doi: 10.1016/j.biomaterials.2013.11.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Volponi A., Kawasaki M., Sharpe P. Adult human gingival epithelial cells as a source for whole-tooth bioengineering. J Den Res. 2013;92:329–334. doi: 10.1177/0022034513481041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ohazama A., Modino S.A., Miletich I., Sharpe P.T. Stem-cell-based tissue engineering of murine teeth. J Dent Res. 2004;83:518–522. doi: 10.1177/154405910408300702. [DOI] [PubMed] [Google Scholar]
- 72.Houlihan D.D., Mabuchi Y., Morikawa S., Niibe K., Araki D., Suzuki S. Isolation of mouse mesenchymal stem cells on the basis of expression of Sca-1 and PDGFR-alpha. Nat Protoc. 2012;7:2103–2111. doi: 10.1038/nprot.2012.125. [DOI] [PubMed] [Google Scholar]