Abstract
HIV-1 superinfection, in which an infected individual acquires a second HIV-1 infection from a different partner, is one of the only settings in which HIV acquisition occurs in the context of a pre-existing immune response to natural HIV infection. There is evidence that initial infection provides some protection from superinfection, particularly after 6 months of initial infection, when development of broad immunity occurs. Comparison of the immune response of superinfected individuals at the time of superinfection acquisition to that of individuals who remain singly infected despite continued exposure can shed light on immune correlates of HIV acquisition to inform prophylactic vaccine design. We evaluated a panel of humoral immune responses in the largest published group of superinfected individuals (n = 21), compared to a set of 3:1 matched singly infected controls from the same cohort. The immune functions studied included plasma neutralization, plasma and cervical antibody-dependent cellular cytotoxicity, and plasma IgG and IgA binding to a panel of 18 envelope antigens, including correlates of HIV acquisition in the RV144 vaccine trial, IgG binding to V1V2 and IgA binding to gp140. Association between each immune function and HIV superinfection was evaluated using conditional logistic regression. No significant associations were detected between any of the immune functions and superinfection acquisition. This study constitutes the most comprehensive and detailed characterization of multiple immune correlates of superinfection to date. The results suggest that immune responses not commonly measured in current HIV studies may be important in protection from HIV infection, and these or a more robust humoral response than that seen in naturally infected women may be needed for a protective vaccine.
Keywords: HIV, Superinfection, Antibody, Neutralization, Binding, Immune correlate
Highlights
-
•
We conducted a case-control study of the association between humoral immune functions and HIV superinfection (SI).
-
•
Neutralization, antibody-dependent cellular cytotoxicity, and IgG and IgA binding to Env antigens were interrogated.
-
•
We found no significant associations between SI acquisition and neutralizing or non-neutralizing antibody activity.
HIV superinfection (repeat infection from a second partner) is a unique situation in which infection occurs in the presence of a pre-existing HIV-specific immune response. Identification of immune deficits in superinfected individuals prior to superinfection can shed light on immune functions associated with HIV acquisition, and help inform prophylactic vaccine development. We compared various antibody measures in superinfected women vs. women who remained singly infected. We found no evidence that deficits in any of the measures analyzed were associated with superinfection risk. This suggests a prophylactic vaccine may need to elicit stronger or different immune responses than those investigated here.
1. Introduction
In 2015, 2.1 million people were newly infected with HIV-1 globally (UNAIDS, 2016). While growing numbers have access to treatment and significant advances have been made in prevention (UNAIDS, 2016), a protective vaccine is essential to ending the HIV pandemic. However, development of an efficacious prophylactic HIV vaccine has been enormously challenging. An important barrier to HIV vaccine development has been the field's limited understanding of the nature of protective HIV-specific immunity. Recent analysis of participants in the RV144 HIV vaccine trial, which showed modest (~ 31%) efficacy, identified several humoral correlates of acquisition among vaccine recipients (Haynes et al., 2012). HIV acquisition was inversely correlated with plasma IgG binding to the V1V2 portion of the HIV envelope protein (Env), and directly correlated with IgA binding to Env. No significant effects of neutralizing antibody responses were detected. The results of this hypothesis-generating study have prompted validation of these correlates of acquisition risk in other settings.
Apart from vaccine studies, superinfection (SI), in which an HIV-infected individual acquires a second HIV infection, is one of the only settings in which HIV exposure occurs in the presence of a pre-existing HIV-specific immune response. SI has been documented in a number of settings (Altfeld et al., 2002, Smith et al., 2004, Chohan et al., 2005, Piantadosi et al., 2007, Redd et al., 2012, Ronen et al., 2013). Early epidemiologic studies in several high-risk cohorts suggested the incidence of SI was similar to that of initial infection (Smith et al., 2004, Kraft et al., 2010, Redd et al., 2013). However, analysis of a larger cohort of SI cases (n = 21) from the Mombasa Cohort, a cohort of high-risk women in Mombasa, Kenya, suggests that HIV-infected individuals are partially protected from SI: the incidence of SI was roughly half that of initial infection in the cohort after adjustment for differences in risk behavior, with evidence of greater protection after 6 months of infection, suggesting a potential immune mechanism (Ronen et al., 2013). Comparison of immune responses immediately before SI acquisition with responses among individuals exposed to HIV who do not acquire SI is therefore a promising strategy to identify immune correlates of HIV acquisition. Importantly, these correlates may differ from correlates of virologic control after establishment of chronic infection.
To date, no studies have examined the immune correlates identified in the RV144 trial for their possible role in protection against SI. Moreover, studies that have investigated other humoral correlates of SI, which have had conflicting findings, have generally been limited to examining one response at a time and small numbers of cases. Two case-controls studies in the Mombasa Cohort found no difference between cases and singly-infected controls: one included 6 SI cases and investigated neutralizing antibody breadth and potency (Blish et al., 2008, UNAIDS, 2016) and the other included 12 SI cases and investigated antibody-dependent cellular viral inhibition (ADCVI) (Haynes et al., 2012, Forthal et al., 2013). However, studies in other cohorts, each comparing 3 SI cases to singly-infected controls, have suggested lower autologous neutralizing antibody (Altfeld et al., 2002, Smith et al., 2004, Chohan et al., 2005, Smith et al., 2006, Piantadosi et al., 2007, Basu et al., 2012, Redd et al., 2012, Ronen et al., 2013) and antibody-dependent cellular cytotoxicity (ADCC) (Basu et al., 2014) in SI cases than controls. Given these conflicting findings, larger studies are needed. Additionally, because synergistic or antagonistic relationships likely exist between different immune functions, any correlate of protection may in fact consist of a combination of responses, which would be missed in studies of a single immune measure. Indeed, interaction analysis in the RV144 study showed that in the presence of low levels of Env-specific IgA, ADCC activity and infection risk were inversely associated, while in the presence of high Env-specific IgA, no such association was present (Haynes et al., 2012). This finding has been hypothesized to be due to competition between binding antibodies (Tomaras et al., 2013). Evaluating multiple immune correlates in aggregate may provide a more nuanced understanding of HIV acquisition risk.
Here, we present a comprehensive analysis of an array of humoral immune responses in 21 SI cases from the Mombasa Cohort compared to individually-matched singly infected controls from the same cohort. This study constitutes the most detailed characterization of multiple immune correlates of SI in the largest group of SI cases analyzed to date.
2. Materials and Methods
2.1. Study Population and Sample Selection
Subjects were drawn from the Mombasa Cohort, an ongoing prospective cohort of initially HIV-negative high-risk women in Mombasa, Kenya, as previously described (Martin et al., 1998). All participants provided written informed consent. Ethical approval was provided by the ethical review boards of the University of Nairobi, University of Washington and Fred Hutchinson Cancer Research Center. Twenty-one SI cases were identified by subjecting samples collected from 146 HIV-1 infected women with well-defined initial infection dates to screening by 454 pyrosequencing or Sanger sequencing, as previously described (Chohan et al., 2005, Piantadosi et al., 2007, Piantadosi et al., 2008, Ronen et al., 2013). SI was detected based on evidence of a phylogenetically distinct viral variant at a timepoint after initial infection. Timing of SI was estimated as the midpoint between the first timepoint in which the SI variant was detected and the last singly infected timepoint. All participants were antiretroviral naïve at the time of sampling. Antiretroviral therapy became available in 2004, after which it was offered to eligible patients in accordance with World Health Organization and Kenyan Ministry of Health guidelines. Participant followup included quarterly sampling of plasma and cervical swabs. Cervical swabs were stored in Fetal Bovine Serum with 10% DMSO. All samples were frozen at − 80 °C within 24 h of collection.
The present study employed a case-control design, and examined immune functions in both plasma and cervical swab supernatants to assess systemic responses and responses at the likely mucosal site of HIV exposure in this sex worker cohort. From the 21 identified SI cases, 20 plasma specimens and 17 cervical swab supernatants were available at the last available singly infected timepoint. For each case sample, 3 singly-infected control samples of the same specimen type were selected, matched to each SI case based on initial infection viral subtype in the env gene and on time since initial infection (± 30 days). All controls were HIV-infected women who seroconverted after enrollment in the Mombasa Cohort and were screened for and had no evidence of SI.
2.2. Plasma and Cervical IgG Titer
IgG ELISA was performed as described in (Williams et al., 2015). In brief, Immunolon 2-HB plates were coated with 2500 ng of goat anti-human antibody (Sigma) diluted in 0.1 M sodium bicarbonate coating buffer (pH 7.4) overnight at 4 °C. Plates were washed with PBS-0.05% Tween and blocked with 10% non-fat dry milk (NFDM) diluted in PBS-0.05% Tween. After at least 1 h, the milk block was removed, and 100 μL of plasma or cervical samples diluted in NFDM added for 1 h at 37 °C. Samples were serially 10-fold diluted to identify the end-point titer: plasma dilutions spanned 1 in 104 to 1 in 106; cervical sample dilutions spanned 1 in 102 to 1 in 105. Plates were washed after sample incubation, and 100 μL anti-human-IgG-HRP (Sigma), diluted in 1:2500 in NFDM added and incubated for 1 h at room temperature. Plates were and 50 μL Ultra-TMB (Thermo Scientific) substrate was added for 10 min. The reaction was stopped by adding an equal volume of 0.1 M H2SO4 and the absorbance read within 30 min using 450 nM optical density. The endpoint titer was defined as the average Ab concentration with binding greater than double the binding by NFDM only.
2.3. Neutralization Assays
To score neutralizing antibody activity, we used a previously published 4-pseudovirus panel (Cortez et al., 2015). This panel was constructed to include diverse envelope variants isolated early in infection whose neutralization profiles could provide a range of breadth and potency scores (scoring described below). We first identified variants whose IC50 values varied across individuals and were predictive of NAb breadth scores generated using larger pseudovirus panels in previous studies in the Mombasa Cohort (Blish et al., 2008, Piantadosi et al., 2009, Cortez et al., 2012). A subset of 4 viruses from these panels was found to be predictive of findings based on the larger panels: Q461.d1 (Tier 1b, subtype A) (Long et al., 2002), QD435.100M.a4 (Tier 2, subtype D) (Blish et al., 2009), Q842.d16 (Tier 2, subtype A) (Long et al., 2002), Du156.12 (Tier 2, subtype C) (Li et al., 2006). For example, in a prior study of breadth at 5 years post initial infection among singly and superinfected individuals (Cortez et al., 2012), superinfected individuals had on average 1.68 (95% CI 1.24–2.26) times greater breadth scores than singly infected controls when using an 8-virus panel. The smaller 4-virus panel estimated 1.65 (95% CI 1.08–2.50) times greater breadth scores in superinfected than singly infected controls.
Pseudoviruses were produced in HEK 293T cells by cotransfecting equimolar concentrations of the cloned env gene and Q23Δenv (Long et al., 2002), a subtype A full-length proviral clone with a partial deletion in env, using Fugene-6. Forty-eight hours post-transfection, supernatants were harvested, filtered through a 0.22 μm filter and tittered on TZM-bl cells.
Case-control sets were assessed for neutralizing antibody activity if specimens were dated at least two months post initial infection, allowing for development of a detectable neutralizing antibody response (n = 13 cases, 39 controls). We confirmed there was limited measurable neutralization activity before this cut-off by assessing neutralization activity of 4 representative early cases (< 2 months post-infection) and their 12 match controls against a neutralization sensitive virus (Q461.d1). Of the 16 early samples tested, 15 had undetectable neutralization activity (IC50 < 100) and one control sample showed modest neutralization (IC50 241.2) (data not shown). Plasma samples were assessed for their ability to neutralize the pseudovirus panel using a TZM-bl neutralization assay as previously described (Blish et al., 2007). Briefly, five two-fold dilutions of heat-inactivated plasma (from 1:100 to 1:1600) were incubated in duplicate with 500 pseudovirus infectious particles for one hour before the addition of 10,000 TZM-bl reporter cells in the presence of 10 μg/mL DEAE-dextran. Forty-eight hours post-infection, infectivity was assessed by β-galactosidase activity using Gal-Screen (Life Technologies). IC50s (reciprocal plasma dilution at which 50% of viruses are neutralized) were calculated based on linear interpolation of the percent neutralization curve. The assay was performed using a Tecan Freedom Evo 150 liquid handling robot, with cases and control always being run on the same plate. The assay was run twice; if the two IC50 values were not within 3-fold of one another for a given sample, the assay was repeated a third time for that case-control set. The final IC50 value was calculated as the geometric mean of all available replicates. Pooled HIV-positive plasma and plasma from an HIV-negative individual were used as positive and negative controls respectively; both were tested against all viruses in each experiment. To assess background neutralization, plasma samples were also tested for neutralization of a pseudovirus bearing SIVMneCL8 Env (Pineda et al., 2007).
Plasma neutralization breadth and potency were scored based on TZM-bl neutralization assays against the panel of pseudoviruses as previously described (Cortez et al., 2015). Briefly, breadth scores were calculated by adding one point for each virus that a plasma sample neutralized at an IC50 greater than the median IC50 value for that virus across all plasma samples. Potency was scored as the sum of the ratio of the plasma sample's IC50 to the cohort median IC50 for each virus. For score calculation purposes, if a plasma sample did not display > 50% neutralization at the lowest dilution tested, it was given an IC50 value of 50, midway between zero and the lower limit of detection (100). If the IC50 value was greater than the highest dilution tested, it was given a value of 1600, the upper limit of our assay. In the small number of cases in which background neutralization of SIVMneCL8 pseudovirus was observed (IC50 > 100, occurring in 7 of 176 plasma-SIVMneCL8 assays), we assigned that IC50 value as the lower limit of detection for that plasma sample in that experiment. We accordingly adjusted the IC50 value assigned to that plasma sample for any virus that had an IC50 value below this new limit of detection to halfway between this limit and zero. In addition to calculating breadth and potency scores, geometric mean IC50 was calculated across the pseudovirus panel for confirmatory analysis.
2.4. Antibody-dependent Cellular Cytotoxicity (ADCC) Assay
The Rapid Fluorometric ADCC (RF-ADCC) assay was performed as described in (Gómez-Román et al., 2006, Mabuka et al., 2012). Briefly, CEM.NKr cells (AIDS Research and Reference Reagent Program, NIAID, NIH) were double labeled with PKH26-cell membrane dye (Sigma Aldrich) and cytoplasmic-staining CFSE dye (Vybrant CFDA SE Cell Tracer Kit, Life Technologies) and coated with BL035.W6M.Env.C1 gp120 protein (Immune Tech) for 1 h at room temperature at a ratio of 1.5 μg of protein (1 μg/μL) per 100,000 double-stained target cells. Coated target cells were washed once with complete RPMI media (Gibco) supplemented with 10% FBS (Gibco), 4.0 mM Glutamax (Gibco) and 1% antibiotic-antimycotic (Life Technologies). Samples were diluted in complete RPMI media and mixed with 5 × 103 target cells for 10 min at room temperature. Peripheral blood mononuclear cells (PBMCs) from an HIV-negative donor were added at a ratio of 50 effector cells: 1 target cell. Coated target cells, antibody dilutions and effector cells were co-cultured for 4 h at 37 °C before being fixed in 150 μL 1% paraformaldehyde (Affymetrix) and acquired by flow cytometry (LSR II, BD). ADCC activity was defined as the percent of PE-positive, FITC-negative cells, analyzed using FlowJo software (Treestar). Plasma samples were run at a 1:10,000 dilution. Pooled IgG from HIV-positive individuals, HIVIg (NIH AIDS Reagent Program), was used as a positive control and target cells coated with SIV gp120 were used as a negative control. Cervical samples were run at a 1:3 or 1:20 dilution, whichever achieved an IgG concentration of 5000–50,000 ng/mL (the RFADCC assay dynamic range) based on IgG ELISA data. Positive and negative control samples in assays of cervical samples contained 10% DMSO to match the cervical samples. Samples were run in duplicate in each assay. Two biological replicates of each assay were performed, using PBMCs from two different donors. Within each assay, mean ADCC activity against SIV gp120-coated cells was subtracted from experimental values as background. Percent ADCC activity was normalized to HIVIg-positive control activity as described previously (Milligan et al., 2015) and the mean of the two biological replicates calculated.
2.5. Antibody Binding Assays
The Binding Antibody Multiplex Assay (BAMA) was used to measure IgG and IgA binding to a panel of antigens, as previously described (Tomaras et al., 2008, Haynes et al., 2012, Fouda et al., 2015). Briefly, carboxylated fluorescent beads (Luminex) were covalently coupled to antigens. Coupled beads were incubated with samples diluted in 5% goat serum (Sigma), 1% NFDM, 0.05% Tween. After a 30-min incubation at room temperature, the beads were washed in PBS pH 7.4 containing 1% BSA and 0.05% Tween. Biotin-conjugated mouse anti-human-IgG (Southern Biotec) or goat anti-human-IgA (Jackson Immunoresearch) were used at 4 μg/mL for detection. After a 30-minute incubation at room temperature, the beads were washed and incubated with 1:100 Streptavidin-PE (BD Pharmingen) for 30 min at room temperature. Beads were washed and acquired using a Bio-Plex 200 instrument (Bio-Rad Laboratories). Results were expressed as mean fluorescence intensity (MFI).
The antigen panel used consisted of: gp140 consensus protein for clade A (Duke Human Vaccine Institute Protein Production Facility); gp120 protein from BG505.W6M.ENV.C1, BL035.W6M.Env.C1, C2-94UG114, ZM109F.PB4, SF162 (Immune Tech); v1v2 scaffolds MuLVgp70-caseA2_v1v2 (Immune Tech), 2J9C-ZM53_v1v2, 1FD6-Fc-ZM109_v1v2 (Jiang et al., 2016); v3 consensus peptides for clades A1 (CTRPNNNTRKSIRIGPGQAFYATGDIIGDIRQAHC), B (CTRPNNNTRKSIHIGPGRAFYTTGEIIGDIRQAHC), C (CTRPNNNTRKSIRIGPGQTFYATGDIIGDIRQAHC), D (CTRPYNNTRQRTPIGPGQALYTTRIKGDIRQAHC) (Genscript); scaffold proteins MuLV gp70 (Duke Human Vaccine Institute Protein Production Facility), 2J9C and 1FD6-Fc (Jiang et al., 2016); SOSIP Env trimer BG505 (courtesy of Marit van Gils, Rogier Sanders and John Moore (Sanders et al., 2013)); gp41 protein from MN (AIDS Reagent Program), gp41 ectodomain from ZA.1197/MB; C1 consensus peptide for clade A1 (MHTDIISLWDQSLKPCVKLTPLCV) (JPT Peptide Technologies); resurfaced Env core protein (RSC3) and CD4-binding site defective mutant (RSC3 Δ371I) (construct obtained from AIDS Reagent Program and produced as described in (Cortez et al., 2015)); SIV gp120 (Immune Tech). Gp120 proteins were purified prior to conjugation to beads by gel filtration using a HiLoad™ 16/60 Superdex 200 column (GE Healthcare) equilibrated with phosphate-buffered saline (1 × PBS, pH 7.4, 1 mM EDTA, 0.02% NaN3). Fractions containing monomeric gp120 were pooled.
Sample dilutions used in the assay were empirically determined for each antigen based on experiments with plasma and cervical samples from 6 randomly selected participants in other Kenya-based cohorts. For each antigen and sample type, the dilution that yielded an MFI in the linear range of the assay (100–50,000 units) was selected. Samples for detection of IgG binding were whole plasma. For detection of IgA binding, plasma was depleted of IgG by processing through a protein G spin plate (Pierce). Most IgA binding was assayed using plasma that had been depleted by passing the sample over the column twice. One replicate of the samples used to measure binding to V1V2 scaffolds, unconjugated scaffolds, gp140 and C1 used samples depleted by passing the sample over the column three times. IgA and IgG ELISAs on a subset of samples suggested depleting twice or three times yielded similar levels of IgG depletion and negligible loss of IgA (data not shown). Samples were run at 1:200 dilution for detection of IgG binding to consensus A gp140, V1V2 scaffolds, scaffold proteins, gp120 BG505.W6M.ENV.C1, gp120 BL035.W6M.Env.C1, gp120 ZM109F.PB4, gp120 SF162, Env trimer, and consensus D V3. Samples were run at 1:800 dilution for detection of IgG binding to RSC3, RSC3 Δ371I, consensus A1 V3, B V3 and D V3, and gp120 C2-94UG114. Samples were run at 1:10,000 for detection of IgG binding to gp41 antigens. Samples were run at 1:10 for detection of IgA binding to all antigens. SIV gp120 coated beads were run as negative controls at each sample dilution. Strong correlation was observed between sample binding to SIV gp120 and mock-conjugated (blank) beads (data not shown). Samples were run in duplicate on each plate and two biological replicates of each assay were performed.
To control for non-specific background binding to beads, a similar approach was used to that by Haynes et al. (Haynes et al., 2012). For each antigen, a negative control was designated: for v1v2 scaffolds MuLVgp70-caseA2_v1v2, 2J9C-ZM53_v1v2 and 1FD6-Fc-ZM109_v1v2 this was matched scaffold proteins MuLV gp70, 2J9C and 1FD6-Fc respectively; for RSC3, RSC3 Δ371I; for all other antigens, SIV gp120. MFIs < 100 units above the appropriate negative control MFI were set to zero. The negative control MFI was subtracted from the remaining MFIs within each biological replicate. The arithmetic mean of control-subtracted MFI of the two biological duplicates was calculated. Mean control-subtracted MFIs were log transformed for statistical analysis.
2.6. Statistical Analysis
Conditional logistic regression was used to evaluate differences in the immune correlates studied in SI cases compared with matched singly infected controls. Continuous predictors were tested in the model unless the proportion of data points below the lower limit of detection exceeded 40%, in which case data were dichotomized as detectable or undetectable. Contemporaneous viral load was identified a priori as a potential confounder based on its reported association with the development of humoral immunity (Piantadosi et al., 2009, Gray et al., 2011, Mabuka et al., 2012) and SI (Ronen et al., 2014). This variable was included as an adjustment in conditional logistic regression models. Statistical analyses were performed using Stata 12 and R. Two-sided Wald tests were used to evaluate statistical significance, at an α level of 0.05.
A statistical analysis plan was specified a priori. Our primary predictors of interest were neutralizing antibody breadth, neutralizing antibody potency, geometric mean neutralizing IC50, ADCC activity, IgG binding to a panel of V1V2 antigens (individually and the arithmetic mean binding to the panel), and IgA binding to gp140. We additionally performed hypothesis-generating analyses exploring the association between SI acquisition and secondary predictors including binding to gp120, V3, C1, gp41, and Env trimer. Only crude models were performed for these exploratory analyses. These results were viewed as exploratory; p-values for these analyses were not adjusted for multiple comparisons.
3. Results
3.1. Characteristics of SI Cases and Singly Infected Controls
Table 1 summarizes the characteristics of the 21 SI case samples and their corresponding 63 singly infected controls, matched to cases based on time since initial infection and initial viral subtype. They are representative of the three main viral subtypes circulating in Kenya (subtypes A, D and C), and include both inter- and intra-subtype superinfections (Table S1). The median time from initial infection to SI acquisition was 391 days (inter-quartile range [IQR] 112–878), where the timing of SI was defined as the midpoint between the last singly infected and first dually infected timepoints. The median timing of the last singly infected sample available was 255 days after initial infection (IQR 58–714). The number of cases included in the different analyses differed based on sample availability. Of the 21 cases, plasma samples immediately pre-SI were available for 20 cases, and cervical swab samples were available for 17 cases. Samples used for neutralization assays were restricted to the 13 whose samples were at least 2 months post-infection, due to the delay in development of detectable neutralization activity. Three matched controls were selected for each case. With the exception of the samples used for neutralization, which were later in infection by design, the subset of cases with available samples had similar timing, contemporaneous VL and sexual behavior characteristics to the full set of 21 cases. Cases and controls had similar sexual behavior characteristics.
Table 1.
Characteristics of cases and controls.
| Cases |
Controls |
|||
|---|---|---|---|---|
| Median | IQR | Median | IQR | |
| Full set | n = 21 | |||
| Estimated timing of SI (dpi) | 391 | 112–878 | ||
| Last singly infected timepoint | 255 | 58–714 | ||
| Contemporaneous plasma VL (log copies/mL) | 4.51 | 3.97–5.21 | ||
| Sex partners in the last week | 1 | 0–1 | ||
| Unprotected sex acts in the last week | 0 | 0–0 | ||
| Plasma assayed for binding & ADCC | n = 20 | n = 60 | ||
| Estimated timing of SI (dpi) | 420 | 110–881 | ||
| Assayed timepoint (dpi) | 260 | 57–723 | 251 | 56–724 |
| Contemporaneous plasma VL (log copies/mL) | 4.52 | 4.07–5.21 | 4.91 | 4.36–5.51 |
| Sex partners in the last week | 1 | 0–1 | 1 | 0–1 |
| Unprotected sex acts in the last week | 0 | 0–0 | 0 | 0.0–0.5 |
| Plasma assayed for neutralization | n = 13 | n = 39 | ||
| Estimated timing of SI (dpi) | 861 | 391–1041 | ||
| Assayed timepoint (dpi) | 341 | 264–996 | 350 | 254–997 |
| Contemporaneous plasma VL (log copies/mL) | 4.30 | 4.07–4.94 | 4.76 | 4.30–5.35 |
| Sex partners in the last week | 1 | 0–1 | 0.67 | 0.33–1.00 |
| Unprotected sex acts in the last week | 0 | 0–0 | 0 | 0.00–0.33 |
| Cervical swabs assayed for ADCC | n = 17 | n = 51 | ||
| Estimated timing of SI (dpi) | 448 | 293–890 | ||
| Assayed timepoint (dpi) | 275 | 155–749 | 271 | 152–750 |
| Contemporaneous plasma VL (log copies/mL) | 4.40 | 3.97–5.16 | 4.76 | 4.29–5.29 |
| Sex partners in the last week | 1 | 0–1 | 0.5 | 0.0–1.0 |
| Unprotected sex acts in the last week | 0 | 0–0 | 0 | 0–0 |
Controls matched to cases on time since HIV infection and initial viral subtype.
ADCC = antibody-dependent cell-mediated cytotoxicity, Dpi = days post-infection, IQR = interquartile range, SI = superinfection, VL = viral load.
3.2. No Association Between SI Acquisition and Plasma or Cervical Total IgG Titer
Fig. S1 summarizes total IgG titer in plasma and cervical samples from SI cases and controls. In both compartments, no association was found between total IgG titer and SI acquisition by conditional logistic regression: the odds ratio (OR) was 0.98 (95% confidence interval [CI] 0.92–1.03, p = 0.38) in plasma and 0.64 (95% CI 0.05–7.81, p = 0.72) in cervical supernatant.
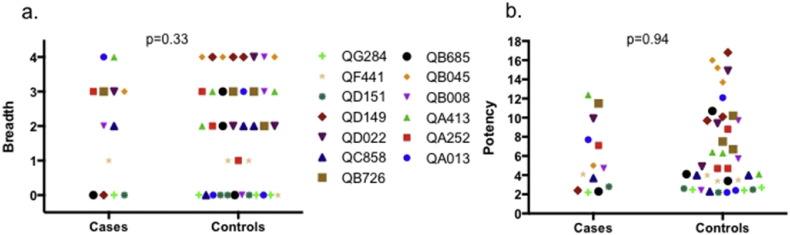
3.3. No Association Between SI Acquisition and Neutralizing Antibody Breadth and Potency
Fig. 1 summarizes neutralizing antibody breadth and potency in SI cases and controls, calculated based on plasma neutralization against a panel of 4 viruses selected to be representative of results of larger virus panels based on prior studies (as described in Materials and Methods). In conditional logistic regression, no association was found between either breadth or potency and SI acquisition. For antibody breadth a crude OR of 1.00 was estimated (95% CI 0.52–1.93, p = 1.00). Adjustment for contemporaneous VL did not alter the point estimate appreciably (adjusted OR [aOR] 1.35, 95% CI 0.73–2.53, p = 0.33). Similarly, for neutralizing potency, a crude OR of 0.93 was estimated (95% CI 0.75–1.15, p = 0.51), with a VL-adjusted OR of 0.99 (95% CI 0.74–1.32, p = 0.94). Findings were similar when using the geometric mean IC50 value across the virus panel rather than breadth or potency scores (OR 1.00 [95% CI 0.99–1.01, p = 0.62] and VL-adjusted OR of 1.00 [95% CI 0.99–1.02, p = 0.92]), and when using alternative methods to score neutralization activity, such as that of Simek et al. (2009) (OR 1.20 [95% CI 0.04–36.71, p = 0.92] and VL-adjusted OR of 1.35 [95% CI 0.73–2.53, p = 0.33]) or factor analysis (OR 0.71 [95% CI 0.28–1.80, p = 0.47] and VL-adjusted OR of 0.99 [95% CI 0.74–1.32, p = 0.94]).
Fig. 1.
Neutralizing antibody breadth (a) and potency (b) in superinfection cases and controls.
Colors and symbols represent superinfection cases (study identification numbers displayed) and their matched singly infected controls. Breadth and potency scores were calculated as described in the Materials and Methods. P-values are from conditional logistic regression.
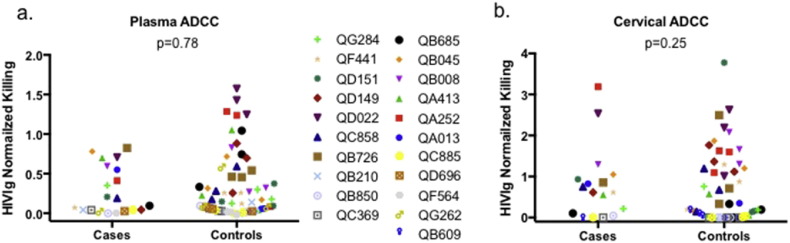
3.4. No Association Between SI Acquisition and Plasma and Cervical ADCC Activity
Fig. 2 and Table 2 summarize plasma and cervical ADCC activity in SI cases and controls, normalized to positive control (HIVIg) values. Seventy-six of 80 plasma samples (95%) and 70 of 83 cervical samples tested (84%) had detectable ADCC activity. In conditional logistic regression, no association was found between plasma ADCC activity and SI acquisition (OR 0.44, 95% CI 0.06–3.10, p = 0.41). Adjustment for contemporaneous viral load did not substantially alter the estimate (aOR 0.74, 95% CI 0.09–6.00, p = 0.78). Similarly, no association was found between cervical RFADCC activity and SI acquisition in either crude (OR 1.16, 95% CI 0.51–2.63, p = 0.73) or adjusted models (aOR 1.18, 95% CI 0.52–2.67, p = 0.25).
Fig. 2.
Plasma (a) and cervical (b) antibody-dependent cellular cytotoxicity in superinfection cases and controls.
Colors and symbols represent superinfection cases (study identification numbers displayed) and their matched singly infected controls. Results are normalized to a positive control (HIVIg). P-values are from conditional logistic regression.
Table 2.
Associations between primary predictors and SI status.
| Correlate | N (case, control) | OR | 95% CI | p-Value | aORb | 95% CI | p-Value |
|---|---|---|---|---|---|---|---|
| Plasma neutralization breadth | 13, 39 | 1.00 | 0.52–1.93 | 1.00 | 1.35 | 0.73–2.53 | 0.33 |
| Plasma neutralization potency | 13, 39 | 0.93 | 0.75–1.15 | 0.51 | 0.99 | 0.74–1.32 | 0.94 |
| Plasma ADCC activity | 20, 60 | 0.44 | 0.06–3.10 | 0.41 | 0.74 | 0.09–6.00 | 0.78 |
| Cervical ADCC activity | 17, 51 | 1.16 | 0.51–2.63 | 0.73 | 0.18 | 0.52–2.67 | 0.70 |
| Plasma V1V2 IgG bindinga | |||||||
| ZM109 | 20, 60 | 1.00 | 0.31–3.18 | 1.00 | 0.96 | 0.30–3.04 | 0.94 |
| ZM53 | 20, 60 | 0.89 | 0.24–3.33 | 0.89 | 0.97 | 0.26–3.63 | 0.96 |
| Case A2 | 20, 60 | 1.00 | 0.27–3.69 | 1.00 | 1.05 | 0.27–4.08 | 0.94 |
| Average | 20, 60 | 0.79 | 0.48–1.29 | 0.34 | 0.64 | 0.20–2.06 | 0.46 |
| Plasma gp140 IgA binding | 20, 60 | 0.93 | 0.58–1.49 | 0.78 | 0.85 | 0.51–1.41 | 0.52 |
ADCC = antibody-dependent cell-mediated cytotoxicity, aOR = adjusted odds ratio, CI = confidence interval, OR = odds ratio, SI = superinfection, VL = viral load.
Dichotomized detectable vs. undetectable.
Adjusted for VL.
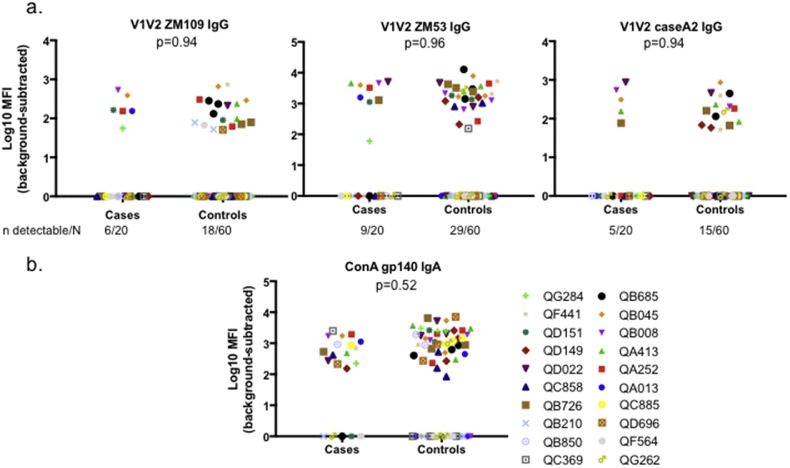
3.5. No Association Between SI Acquisition and Plasma IgG or IgA Binding to a Panel of Antigens
Fig. 3 and Table 2 summarize key pre-specified outcome measures in a screen of binding antibodies: IgG binding to each of three V1V2 scaffold antigens and IgA binding to gp140. Limited IgG binding to any of the V1V2 scaffold antigens was detected (Fig. 3a and Table 2). Of the 80 samples tested, detectable binding to 1FD6-Fc-ZM109_v1v2, 2J9C-ZM53_v1v2 and MuLVgp70-caseA2_v1v2 was observed in 24 (30%), 38 (48%) and 20 (25%) samples respectively (Fig. 3). When binding was averaged over the three variants, 42 (53%) of samples had detectable binding. In conditional logistic regression of dichotomized data (detectable vs. undetectable), no association was detected between SI acquisition and binding to any single V1V2 variant or mean binding (Table 2): aOR for 1FD6-Fc-ZM109_v1v2 0.96, 95% CI 0.30–3.04, p = 0.94; aOR for 2J9C-ZM53_v1v2 0.97, 95% CI 0.26–3.63, p = 0.96; aOR for MuLVgp70-caseA2_v1v2 1.05, 95% CI 0.27–4.08, p = 0.94; aOR for average binding 0.64, 95% CI 0.20–2.06, p = 0.46. Crude and VL-adjusted estimates of the association were similar (Table 2).
Fig. 3.
Plasma binding antibody in superinfection cases and controls a. IgG binding to V1V2 scaffold proteins. b. IgA binding to gp140. Antigenic variants are named above each graph.
Colors and symbols represent superinfection cases (study identification numbers displayed) and their matched singly infected controls. Results are background-subtracted and log-transformed. P-values are from conditional logistic regression.
Fig. 3b and Table 2 summarize plasma IgA binding to clade A consensus gp140. Fifty-eight samples (73%) had detectable IgA binding to gp140. In conditional logistic regression, no association was detected between gp140 binding and SI acquisition, in both crude and VL-adjusted models (OR 0.93, 95% CI 0.58–1.49, p = 0.78; aOR 0.85, 95% CI 0.51–1.41, p = 0.52).
In addition to IgG binding to V1V2 and IgA binding to gp140, IgG and IgA binding to a panel of additional exploratory antigens was evaluated (18 total antigens), including 5 gp120 variants, Env trimer, 4 V3 peptide variants (a consensus for each subtype), consensus A C1 peptide, 2 gp41 variants and the CD4 binding site core. No associations were found between any of the antigens examined and SI acquisition (Table 3).
Table 3.
Associations between secondary predictors and SI status.
| Binding correlate | OR | 95% CI | p | |
|---|---|---|---|---|
| bg505 gp120 | IgG | 1.17 | 0.61–2.23 | 0.64 |
| c294 gp120 | IgG | 0.80 | 0.42–1.53 | 0.50 |
| bl035 gp120 | IgG | 1.00 | 0.62–1.64 | 0.99 |
| zm109 gp120 | IgG | 0.98 | 0.56–1.72 | 0.94 |
| sf162 gp120 | IgG | 0.99 | 0.49–2.01 | 0.98 |
| conA gp140 | IgG | 2.74 | 0.72–10.43 | 0.14 |
| bg505 trimer | IgG | 0.93 | 0.48–1.84 | 0.84 |
| conA1 v3 | IgG | 1.59 | 0.47–5.43 | 0.46 |
| conB v3 | IgG | 1.00 | 0.57–1.78 | 1.00 |
| conC v3 | IgG | 0.47 | 0.14–1.56 | 0.22 |
| conD v3 | IgG | 1.00 | 0.68–1.47 | 0.99 |
| conA C1 | IgG | 1.44 | 0.80–2.60 | 0.22 |
| mn gp41 | IgG | 1.47 | 0.74–2.95 | 0.28 |
| za1197 gp41 | IgG | 1.51 | 0.65–3.61 | 0.34 |
| rsc3a, b | IgG | – | – | – |
| zm109 v1v2 | IgA | 2.79 | 0.81–9.68 | 0.11 |
| caseA2 v1v2 | IgA | 1.69 | 0.47–6.13 | 0.42 |
| zm53 v1v2 | IgA | 1.24 | 0.86–1.79 | 0.26 |
| bg505 gp120 | IgA | 1.08 | 0.66–1.78 | 0.76 |
| c294 gp120 | IgA | 0.88 | 0.58–1.35 | 0.56 |
| bl035 gp120 | IgA | 0.75 | 0.49–1.15 | 0.19 |
| zm109 gp120 | IgA | 1.00 | 0.69–1.44 | 0.99 |
| sf162 gp120 | IgA | 0.97 | 0.62–1.53 | 0.90 |
| bg505 trimer | IgA | 0.72 | 0.41–1.26 | 0.24 |
| conA1 v3 | IgA | 1.59 | 0.47–5.43 | 0.46 |
| conB v3 | IgA | 2.31 | 0.57–9.46 | 0.24 |
| conC v3 | IgA | 1.45 | 0.42–4.99 | 0.56 |
| conD v3 | IgA | 2.06 | 0.89–4.80 | 0.09 |
| conA C1 | IgA | 1.47 | 0.60–3.58 | 0.40 |
| mn gp41 | IgA | 1.24 | 0.50–3.06 | 0.64 |
| za1197 gp41 | IgA | 2.48 | 0.72–8.56 | 0.15 |
| rsc3 | IgA | 1.36 | 0.33–5.62 | 0.68 |
N = 20 cases, 60 controls for all analyses.
CI = confidence interval, OR = odds ratio, SI = superinfection.
Dichotomized detectable vs. undetectable.
Model failed to converge due to too many undetectable observations (78 of 80).
3.6. No Interaction Between Humoral Responses
Interaction analysis evaluated whether the relationships between SI acquisition and either IgG-mediated ADCC activity or V1V2 IgG binding were modified by the level of gp140 IgA binding, as was observed in the RV144 analysis (Haynes et al., 2012). Conditional logistic regression did not demonstrate evidence of an interaction between either IgG-mediated immune function and gp140 IgA binding (Supplementary Table S2).
3.7. Sensitivity Analyses
We conducted two sensitivity analyses. The first included only samples ≥ 180 days after initial infection, at a time when immune responses would have had more time to mature, and at which greater evidence of protection from SI was observed in a prior study (Ronen et al., 2013). Twelve case-control sets were included this analysis. No associations were detected with any of the immune correlates assayed when restricting to samples ≥ 180 days after initial infection (Supplementary Table S3). The second sensitivity analysis included only intra-subtype SI cases, based on the env subtype, to enable comparison with studies of intra-subtype SI in other cohorts (Smith et al., 2006, Basu et al., 2012, Basu et al., 2014). Ten to twelve case-control sets were included in this analysis, based on sample availability of intra-subtype SI cases. No associations were detected with any of the immune correlates assayed (Supplementary Table S4).
4. Discussion
Elucidation of the immune responses that provide protection from HIV infection is an important step in designing protective vaccines. We have previously reported that the incidence of SI is two-fold lower than that of initial infection, after adjustment for differences in sexual risk behavior (Ronen et al., 2013), suggesting the immune response to initial infection may provide some protection against subsequent infection. In the present study, we employed a matched case-control study design to characterize differences between the immune response of individuals who acquired SI and that of individuals who remained singly infected despite ongoing HIV exposure. Association between SI status and a panel of measures of humoral responses against HIV was evaluated, including plasma IgG binding to V1V2, plasma IgA binding to gp140, plasma neutralizing antibody breadth and potency, and plasma and genital ADCC. We found no statistically significant association between SI acquisition and any of the antibody functions assayed. Interaction analysis did not find evidence of modification of the effect of IgG-mediated binding or killing by IgA binding. Sensitivity analyses restricted to individuals who had been HIV-infected for at least 6 months, and to intra-subtype SI cases, did not change these results. Exploratory analysis of 32 additional binding antibody activities did not suggest any additional associations with SI acquisition.
Our study hypotheses were motivated by the findings of several prior studies. Analysis of recipients of the RV144 vaccine pointed to IgA binding to gp140 and IgG binding to V1V2 as positive and negative correlates of HIV acquisition respectively, with evidence of antagonism between the two functions (Haynes et al., 2012). Additionally, several antibody effector functions have been implicated as correlates of natural HIV infection. In the context of mother-to-child transmission, breast milk IgA binding to gp140 (Pollara et al., 2015) and ADCC activity (Mabuka et al., 2012) were associated with reduced breast milk transmission, and maternal plasma IgG binding to V3 was associated with reduced perinatal transmission (Permar et al., 2015, Martinez et al., 2017). While several small studies have previously investigated the humoral immune correlates of SI, the results were conflicting. Comparing 3 early SI cases and 11 matched controls in the first year of infection from a cohort of subtype B infected men who have sex with men, Smith et al. reported lower neutralizing antibody activity in cases (Smith et al., 2006). Similarly, Basu et al. compared 3 SI cases and 10 controls in the first year of infection from a cohort of subtype C infected heterosexual men and women and reported lower neutralizing antibody activity, lower ADCC activity and a non-significant trend for lower V1V2 IgG binding and higher gp120 IgA binding in cases (Basu et al., 2012, Basu et al., 2014). However, while Basu et al. found the 3 SI cases to have delayed autologous neutralizing antibody kinetics compared to controls, they reported no difference in heterologous neutralizing antibody breadth between cases and controls (Basu et al., 2012), similar to the findings reported here. Previous analysis of 12 of the Mombasa Cohort SI cases included in this study to 36 matched controls detected no significant difference in neutralization breadth and potency or antibody-dependent cell-mediated viral inhibition (ADCVI, which captures ADCC and other immune-mediated viral inhibition) between cases and controls (Blish et al., 2008, Forthal et al., 2013).
Our findings for neutralizing and ADCC responses are consistent with those of the previous studies of superinfection in the Mombasa Cohort, but not the other studies based on 3 cases of superinfection. These differences may simply be due to unstable estimates in the small samples studied. In this study we included 20 cases assayed for plasma binding and ADCC activity, 13 cases assayed for neutralization and 17 samples assayed for cervical ADCC, with 3 controls per sample. This larger sample size improves the reliability of our findings, and allowed us to employ regression techniques with adjustment for confounding by viral load. The different findings in this and prior studies may also be due to differences in study design. Previous studies used frequency-matching of cases to controls based on time since infection, whereas we individually matched, potentially allowing for more complete adjustment for confounding by time since infection. This may be critical given that timing of sampling relative to initial infection and SI acquisition is an important determinant of the strength of the immune response. Indeed, we specifically excluded cases of SI that occurred in the first two months after initial infection because small differences between a case and its controls in the accuracy of infection timing could lead to spurious differences in neutralization activity when assaying early on in infection. Later in infection, neutralization activity would be expected to be less influenced by small differences in timing.
It is of interest to note that the Mombasa Cohort differs from the other SI cohorts studied in its inclusion of intra-subtype and inter-subtype SI cases. It is possible that certain immune correlates identified in previous studies are protective against antigenically similar viruses. Sensitivity analysis of our data restricted to 12 intra-subtype SI cases did not indicate associations with any of the immune functions assayed. Sample size limitations in this and previous studies make it impossible to reliably address this aspect; additional studies of comparable size to the current study in other cohorts or pooling of samples and data are needed to clarify these immune correlates in intra-subtype infections. If there is some immune protection within clades but limited protection between clades, this would be highly relevant for vaccine design and implementation.
Our observation of no statistically significant associations between any of the immune responses assayed and acquisition of SI suggests that the protection from superinfection observed in our epidemiologic study of the Mombasa Cohort (Ronen et al., 2013) was not mediated by the humoral immune functions ascertained in this study. This finding has a number of explanations.
First, it is possible that the assays we performed did not adequately capture the immune deficit of individuals who acquire SI. Ours is the most comprehensive analysis of immune correlates of SI published to date, evaluating 5 primary humoral immune responses in plasma and genital samples, and over 30 additional exploratory binding antibody activities using the same samples. Several of the activities assayed have not previously been characterized in studies of superinfection, including ADCC activity in genital samples or binding to gp140, V3, Env trimer, CD4 binding site or gp41. Nonetheless, our assays were limited. Use of a 4-virus panel for neutralization assays, though validated against a larger panel, may have reduced the assay's sensitivity. Moreover, non-humoral mechanisms of protection such as T-cell or NK-cell responses, or immune activation prior to SI, which can lead to increased target cells for HIV, were not assessed in this study and could contribute to SI acquisition risk, as suggested by a small prior study from the Mombasa Cohort (Blish et al., 2012). These results, coupled with the observed temporal association with SI acquisition we have previously reported (Ronen et al., 2013), prompt greater focus on these mechanisms. Second, unlike participants in the RV144 vaccine trial, in which all vaccinees were exposed to the same immune stimulus, each participant in the Mombasa Cohort was exposed to a different initial virus. These different antigens may have stimulated heterogeneous immune responses, which could make it more challenging to detect any single correlate. Third, ongoing HIV exposure could not be directly evaluated in singly infected controls. Unknown HIV exposure status in the singly infected controls, some of whom may not have been exposed, would be expected to yield an attenuated estimate of the true association between immune response and SI acquisition. Similarly, our study has no information on viral or host factors in the source partners that could contribute to infection outcome. While there is little biological support for immune responses in the source partner driving transmission outcomes in the setting of sexual HIV-1 transmission, this has been observed in the context of mother-to-child transmission, where ADCC activity in maternal breastmilk was associated with transmission to the infant (Mabuka et al., 2012). Fourth, while this is the largest set of SI cases published to date all available samples were included and individual 3:1 matching was employed to increase sensitivity, sample sizes were still relatively small (n = 13–20 SI cases per analysis). It remains possible that an association exists in our data that we were underpowered to detect. Pooled analysis of SI cases and controls from multiple cohorts could increase power to detect immune deficits. Finally, it is possible that the protection from SI acquisition previously observed in the cohort is not explained by immune protection, but by residual confounding by behavioral risk.
In conclusion, these findings do not provide evidence for a strong protective effect of humoral immune responses in the setting of natural exposure to diverse circulating HIV variants. This suggests that a protective HIV vaccine may need to elicit cellular immune mechanisms such as NK or T-cell responses, or a stronger humoral response than that seen in the naturally infected women included in our study.
Funding Sources
This work was supported by the National Institutes of Health, grant numbers R37 AI038518 to J.O. and T32 CA080416 to K.R. and A.S.D. The funders had no role in study design, data collection, analysis, decision to publish, or preparation of the manuscript.
Conflicts of Interest
The authors have no conflicts of interest to declare.
Author Contributions
Designed the study: KR, ASD, SMG, RSM, JO. Performed the experiments: KR, ASD. Analyzed data: KR, ASD, SMG, RSM. Contributed reagents and materials: WJ, KM. Wrote the paper: KR, ASD, JO, with input from all authors.
Acknowledgements
We gratefully acknowledge to the participants of the Mombasa Cohort and staff at the study clinic. We thank Xiangpeng Kong and Xunqing Jiang for providing 2J9C and 1FD6-Fc V1V2 scaffold proteins.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ebiom.2017.04.005.
Appendix A. Supplementary data
Supplementary material
References
- Altfeld M., Allen T.M., Yu X.G., Johnston M.N., Agrawal D., Korber B.T., Montefiori D.C., O'Connor D.H., Davis B.T., Lee P.K., Maier E.L., Harlow J., Goulder P.J.R., Brander C., Rosenberg E.S., Walker B.D. HIV-1 superinfection despite broad CD8 + T-cell responses containing replication of the primary virus. Nature. 2002;420(6914):434–439. doi: 10.1038/nature01200. [DOI] [PubMed] [Google Scholar]
- Basu D., Kraft C.S., Murphy M.K., Campbell P.J., Yu T., Hraber P.T., Irene C., Pinter A., Chomba E., Mulenga J., Kilembe W., Allen S.A., Derdeyn C.A., Hunter E. HIV-1 subtype C superinfected individuals mount low autologous neutralizing antibody responses prior to intrasubtype superinfection. Retrovirology. 2012;9:76. doi: 10.1186/1742-4690-9-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu D., Xiao P., Ende Z., Bere A., Britt W.J., Mulenga J., Kilembe W., Allen S.A., Derdeyn C.A., Hunter E. Low antibody-dependent cellular cytotoxicity responses in Zambians prior to HIV-1 intrasubtype C superinfection. Virology. 2014;462-463:295–298. doi: 10.1016/j.virol.2014.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blish C.A., Dogan O.C., Derby N.R., Nguyen M.-A., Chohan B., Richardson B.A., Overbaugh J. Human immunodeficiency virus type 1 superinfection occurs despite relatively robust neutralizing antibody responses. J. Virol. 2008;82(24):12094–12103. doi: 10.1128/JVI.01730-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blish C.A., Dogan O.C., Jaoko W., McClelland R.S., Mandaliya K., Odem-Davis K.S., Richardsonb B.A., Overbaugh J. Cellular immune responses and susceptibility to HIV-1 superinfection: a case-control study. AIDS. 2012;26(5):643–646. doi: 10.1097/QAD.0b013e3283509a0b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blish C.A., Jalalian-Lechak Z., Rainwater S., Nguyen M.-A., Dogan O.C., Overbaugh J. Cross-subtype neutralization sensitivity despite monoclonal antibody resistance among early subtype A, C, and D envelope variants of human immunodeficiency virus type 1. J. Virol. 2009;83(15):7783–7788. doi: 10.1128/JVI.00673-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blish C.A., Nedellec R., Mandaliya K., Mosier D.E., Overbaugh J. HIV-1 subtype A envelope variants from early in infection have variable sensitivity to neutralization and to inhibitors of viral entry. AIDS. 2007;21(6):693–702. doi: 10.1097/QAD.0b013e32805e8727. [DOI] [PubMed] [Google Scholar]
- Chohan B., Lavreys L., Rainwater S.M.J., Overbaugh J. Evidence for frequent reinfection with human immunodeficiency virus type 1 of a different subtype. J. Virol. 2005;79(16):10701–10708. doi: 10.1128/JVI.79.16.10701-10708.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortez V., Odem-Davis K., McClelland R.S., Jaoko W., Overbaugh J. HIV-1 superinfection in women broadens and strengthens the neutralizing antibody response. PLOS Pathog. 2012;8(3):e1002611. doi: 10.1371/journal.ppat.1002611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortez V., Wang B., Dingens A., Chen M.M., Ronen K., Georgiev I.S., McClelland R.S., Overbaugh J. The broad neutralizing antibody responses after HIV-1 superinfection are not dominated by antibodies directed to epitopes common in single infection. PLOS Pathog. 2015;11(7):e1004973. doi: 10.1371/journal.ppat.1004973. http://dx.doi.org/10.1371/journal.ppat.1004973.s008 Edited by R. C. Desrosiers. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forthal D.N., Landucci G., Chohan B., Richardson B.A., McClelland R.S., Jaoko W., Blish C., Overbaugh J. Antibody-dependent cell-mediated virus inhibition antibody activity does not correlate with risk of HIV-1 superinfection. J. Acquir. Immune Defic. Syndr. 2013;63(1):31–33. doi: 10.1097/QAI.0b013e3182874d41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouda G.G., Cunningham C.K., McFarland E.J., Borkowsky W., Muresan P., Pollara J., Song L.Y., Liebl B.E., Whitaker K., Shen X., Vandergrift N.A., Overman R.G., Yates N.L., Moody M.A., Fry C., Kim J.H., Michael N.L., Robb M., Pitisuttithum P., Kaewkungwal J., Nitayaphan S., Rerks-Ngarm S., Liao H.-X., Haynes B.F., Montefiori D.C., Ferrari G., Tomaras G.D., Permar S.R. Infant HIV type 1 gp120 vaccination elicits robust and durable anti-V1V2 immunoglobulin G responses and only rare envelope-specific immunoglobulin A responses. J. Infect. Dis. 2015;211(4):508–517. doi: 10.1093/infdis/jiu444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Román V.R., Florese R.H., Patterson L.J., Peng B., Venzon D., Aldrich K., Robert-Guroff M. A simplified method for the rapid fluorometric assessment of antibody-dependent cell-mediated cytotoxicity. J. Immunol. Methods. 2006;308(1–2):53–67. doi: 10.1016/j.jim.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Gray E.S., Madiga M.C., Hermanus T., Moore P.L., Wibmer C.K., Tumba N.L., Werner L., Mlisana K., Sibeko S., Williamson C., Abdool Karim S.S., Morris L., CAPRISA002 Study Team The neutralization breadth of HIV-1 develops incrementally over four years and is associated with CD4 + T cell decline and high viral load during acute infection. J. Virol. 2011;85(10):4828–4840. doi: 10.1128/JVI.00198-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes B.F., Gilbert P.B., Mcelrath M.J., Zolla-Pazner S., Tomaras G.D., Alam S.M., Evans D.T., Montefiori D.C., Karnasuta C., Sutthent R., Liao H.-X., DeVico A.L., Lewis G.K., Williams C., Pinter A., Fong Y., Janes H., DeCamp A., Huang Y., Rao M., Billings E., Karasavvas N., Robb M.L., Ngauy V., de Souza M.S., Paris R., Ferrari G., Bailer R.T., Soderberg K.A., Andrews C., Berman P.W., Frahm N., De Rosa S.C., Alpert M.D., Yates N.L., Shen X., Koup R.A., Pitisuttithum P., Kaewkungwal J., Nitayaphan S., Rerks-Ngarm S., Michael N.L., Kim J.H. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N. Engl. J. Med. 2012;366(14):1275–1286. doi: 10.1056/NEJMoa1113425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X., Totrov M., Li W., Sampson J.M., Williams C., Lu H., Wu X., Lu S., Wang S., Zolla-Pazner S., Kong X.-P. Rationally designed immunogens targeting HIV-1 gp120 V1V2 induce distinct conformation-specific antibody responses in rabbits. J. Virol. 2016;90(24):11007–11019. doi: 10.1128/JVI.01409-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraft C.S., Hunter E., Manigart O. Identification of HIV superinfection requires longitudinal samples and sequence analysis. J. Infect. Dis. 2010:1–32. [Google Scholar]
- Li M., Salazar-Gonzalez J.F., Derdeyn C.A., Morris L., Williamson C., Robinson J.E., Decker J.M., Li Y., Salazar M.G., Polonis V.R., Mlisana K., Karim S.A., Hong K., Greene K.M., Bilska M., Zhou J., Allen S., Chomba E., Mulenga J., Vwalika C., Gao F., Zhang M., Korber B.T.M., Hunter E., Hahn B.H., Montefiori D.C. Genetic and neutralization properties of subtype C human immunodeficiency virus type 1 molecular env clones from acute and early heterosexually acquired infections in Southern Africa. J. Virol. 2006;80(23):11776–11790. doi: 10.1128/JVI.01730-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long E.M., Rainwater S.M.J., Lavreys L., Mandaliya K., Overbaugh J. HIV type 1 variants transmitted to women in Kenya require the CCR5 coreceptor for entry, regardless of the genetic complexity of the infecting virus. AIDS Res. Hum. Retrovir. 2002;18(8):567–576. doi: 10.1089/088922202753747914. [DOI] [PubMed] [Google Scholar]
- Mabuka J., Nduati R., Odem-Davis K., Peterson D., Overbaugh J. HIV-specific antibodies capable of ADCC are common in breastmilk and are associated with reduced risk of transmission in women with high viral loads. PLOS Pathogens. 2012;8(6):e1002739. doi: 10.1371/journal.ppat.1002739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin H.L., Nyange P.M., Richardson B.A., Lavreys L., Mandaliya K., Jackson D.J., Ndinya-Achola J.O., Kreiss J. Hormonal contraception, sexually transmitted diseases, and risk of heterosexual transmission of human immunodeficiency virus type 1. J. Infect. Dis. 1998;178(4):1053–1059. doi: 10.1086/515654. [DOI] [PubMed] [Google Scholar]
- Martinez D.R., Vandergrift N., Douglas A.O., McGuire E., Bainbridge J., Nicely N.I., Montefiori D.C., Tomaras G.D., Fouda G.G., Permar S.R. Maternal binding and neutralizing IgG responses targeting the C terminal region of the V3 loop are predictive of reduced peripartum HIV-1 transmission risk. J. Virol. 2017 doi: 10.1128/JVI.02422-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan C., Richardson B.A., John-Stewart G., Nduati R., Overbaugh J. Passively acquired antibody-dependent cellular cytotoxicity (ADCC) activity in HIV-infected infants is associated with reduced mortality. Cell Host Microbe. 2015;17(4):500–506. doi: 10.1016/j.chom.2015.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Permar S.R., Fong Y., Vandergrift N., Fouda G.G., Gilbert P., Parks R., Jaeger F.H., Pollara J., Martelli A., Liebl B.E., Lloyd K., Yates N.L., Overman R.G., Shen X., Whitaker K., Chen H., Pritchett J., Solomon E., Friberg E., Marshall D.J., Whitesides J.F., Gurley T.C., Holle Von T., Martinez D.R., Cai F., Kumar A., Xia S.-M., Lu X., Louzao R., Wilkes S., Datta S., Sarzotti-Kelsoe M., Liao H.-X., Ferrari G., Alam S.M., Montefiori D.C., Denny T.N., Moody M.A., Tomaras G.D., Gao F., Haynes B.F. Maternal HIV-1 envelope–specific antibody responses and reduced risk of perinatal transmission. J. Clin. Investig. 2015;125(7):2702–2706. doi: 10.1172/JCI81593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piantadosi A., Chohan B., Chohan V., McClelland R.S., Overbaugh J. Chronic HIV-1 infection frequently fails to protect against superinfection. PLOS Pathog. 2007;3(11):e177. doi: 10.1371/journal.ppat.0030177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piantadosi A., Ngayo M.O., Chohan B., Overbaugh J. Examination of a second region of the HIV type 1 genome reveals additional cases of superinfection. AIDS Res. Hum. Retrovir. 2008;24(9):1221–1224. doi: 10.1089/aid.2008.0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piantadosi A., Panteleeff D., Blish C.A., Baeten J.M., Jaoko W., McClelland R.S., Overbaugh J. Breadth of neutralizing antibody response to human immunodeficiency virus type 1 is affected by factors early in infection but does not influence disease progression. J. Virol. 2009;83(19):10269–10274. doi: 10.1128/JVI.01149-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineda M.J., Orton B.R., Overbaugh J. A TRIM5alpha-independent post-entry restriction to HIV-1 infection of macaque cells that is dependent on the path of entry. Virology. 2007;363(2):310–318. doi: 10.1016/j.virol.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollara J., McGuire E., Fouda G.G., Rountree W., Eudailey J., Overman R.G., Seaton K.E., Deal A., Edwards R.W., Tegha G., Kamwendo D., Kumwenda J., Nelson J.A.E., Liao H.-X., Brinkley C., Denny T.N., Ochsenbauer C., Ellington S., King C.C., Jamieson D.J., van der Horst C., Kourtis A.P., Tomaras G.D., Ferrari G., Permar S.R. Association of HIV-1 Envelope-Specific Breast Milk IgA Responses with Reduced Risk of Postnatal Mother-to-Child Transmission of HIV-1. J. Virol. 2015;89(19):9952–9961. doi: 10.1128/JVI.01560-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redd A.D., Mullis C.E., Serwadda D., Kong X., Martens C., Ricklefs S.M., Tobian A.A.R., Xiao C., Grabowski M.K., Nalugoda F., Kigozi G., Laeyendecker O., Kagaayi J., Sewankambo N., Gray R.H., Porcella S.F., Wawer M.J., Quinn T.C. The rates of HIV superinfection and primary HIV incidence in a general population in Rakai, Uganda. J. Infect. Dis. 2012;206(2):267–274. doi: 10.1093/infdis/jis325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redd A.D., Quinn T.C., Tobian A.A. Frequency and implications of HIV superinfection. Lancet Infect. Dis. 2013;13(7):622–628. doi: 10.1016/S1473-3099(13)70066-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronen K., McCoy C.O., Matsen F.A., Boyd D.F., Emery S., Odem-Davis K., Jaoko W., Mandaliya K., McClelland R.S., Richardson B.A., Overbaugh J. HIV-1 superinfection occurs less frequently than initial infection in a cohort of high-risk Kenyan women. PLOS Pathog. 2013;9(8):e1003593. doi: 10.1371/journal.ppat.1003593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronen K., Richardson B.A., Graham S.M., Jaoko W., Mandaliya K., McClelland R.S., Overbaugh J. HIV-1 superinfection is associated with an accelerated viral load increase but has a limited impact on disease progression. AIDS. 2014;28(15):2281–2286. doi: 10.1097/QAD.0000000000000422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders R.W., Derking R., Cupo A., Julien J.-P., Yasmeen A., de Val N., Kim H.J., Blattner C., la Peña, de, A. T., Korzun, J., Golabek, M., de los Reyes, K., Ketas, T. J., van Gils, M. J., King, C. R., Wilson, I. A., Ward, A. B., Klasse, P. J. and Moore, J. P. A Next-Generation Cleaved, Soluble HIV-1 Env Trimer, BG505 SOSIP.664 gp140, Expresses Multiple Epitopes for Broadly Neutralizing but Not Non-Neutralizing Antibodies. PLOS Pathog. 2013;9(9):e1003618. doi: 10.1371/journal.ppat.1003618. http://dx.doi.org/10.1371/journal.ppat.1003618.s004 Edited by A. Trkola. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simek M.D., Rida W., Priddy F.H., Pung P., Carrow E., Laufer D.S., Lehrman J.K., Boaz M., Tarragona-Fiol T., Miiro G., Birungi J., Pozniak A., McPhee D.A., Manigart O., Karita E., Inwoley A., Jaoko W., Dehovitz J., Bekker L.-G., Pitisuttithum P., Paris R., Walker L.M., Poignard P., Wrin T., Fast P.E., Burton D.R., Koff W.C. Human immunodeficiency virus type 1 elite neutralizers: individuals with broad and potent neutralizing activity identified by using a high-throughput neutralization assay together with an analytical selection algorithm. J. Virol. 2009;83(14):7337–7348. doi: 10.1128/JVI.00110-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D.M., Strain M.C., Frost S.D.W., Pillai S.K., Wong J.K., Wrin T., Liu Y., Petropolous C.J., Daar E.S., Little S.J., Richman D.D. Lack of neutralizing antibody response to HIV-1 predisposes to superinfection. Virology. 2006;355(1):1–5. doi: 10.1016/j.virol.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Smith D.M., Wong J.K., Hightower G.K., Ignacio C.C., Koelsch K.K., Daar E.S., Richman D.D., Little S.J. Incidence of HIV superinfection following primary infection. JAMA. 2004;292(10):1177–1178. doi: 10.1001/jama.292.10.1177. [DOI] [PubMed] [Google Scholar]
- Tomaras G.D., Ferrari G., Shen X., Alam S.M., Liao H.-X., Pollara J., Bonsignori M., Moody M.A., Fong Y., Chen X., Poling B., Nicholson C.O., Zhang R., Lu X., Parks R., Kaewkungwal J., Nitayaphan S., Pitisuttithum P., Rerks-Ngarm S., Gilbert P.B., Kim J.H., Michael N.L., Montefiori D.C., Haynes B.F. Vaccine-induced plasma IgA specific for the C1 region of the HIV-1 envelope blocks binding and effector function of IgG. Proceedings of the National Academy of Sciences. 2013;110(22):9019–9024. doi: 10.1073/pnas.1301456110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomaras G.D., Yates N.L., Liu P., Qin L., Fouda G.G., Chavez L.L., Decamp A.C., Parks R.J., Ashley V.C., Lucas J.T., Cohen M., Eron J., Hicks C.B., Liao H.-X., Self S.G., Landucci G., Forthal D.N., Weinhold K.J., Keele B.F., Hahn B.H., Greenberg M.L., Morris L., Karim S.S.A., Blattner W.A., Montefiori D.C., Shaw G.M., Perelson A.S., Haynes B.F. Initial B-cell responses to transmitted human immunodeficiency virus type 1: virion-binding immunoglobulin M (IgM) and IgG antibodies followed by plasma anti-gp41 antibodies with ineffective control of initial viremia. J. Virol. 2008;82(24):12449–12463. doi: 10.1128/JVI.01708-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNAIDS . 2016. Fact Sheet 2016; www.unaids.orgpp. 1–8.http://www.unaids.org/sites/default/files/media_asset/UNAIDS_FactSheet_en.pdf 18 July. Available at: Accessed: 21 October 2016. [Google Scholar]
- Williams K.L., Cortez V., Dingens A.S., Gach J.S., Rainwater S., Weis J.F., Chen X., Spearman P., Forthal D.N., Overbaugh J. HIV-specific CD4-induced antibodies mediate broad and potent antibody-dependent cellular cytotoxicity activity and are commonly detected in plasma from HIV-infected humans. EBioMedicine. 2015;2(10):1464–1477. doi: 10.1016/j.ebiom.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material