Abstract
Background
Neurophysiological and behavioral processes regulated by hypocretin (orexin) are severely affected in depression. However, alterations in hypocretin have so far not been studied in the human brain. We explored the hypocretin system changes in the hypothalamus and cortex in depression from male and female subjects.
Methods
We quantified the differences between depression patients and well-matched controls, in terms of hypothalamic hypocretin-1 immunoreactivity (ir) and hypocretin receptors (Hcrtr-receptors)-mRNA in the anterior cingulate cortex (ACC) and dorsolateral prefrontal cortex. In addition, we determined the alterations in the hypocretin system in a frequently used model for depression, the chronic unpredictable mild stress (CUMS) rat.
Results
i) Compared to control subjects, the amount of hypocretin-immunoreactivity (ir) was significantly increased in female but not in male depression patients; ii) hypothalamic hypocretin-ir showed a clear diurnal fluctuation, which was absent in depression; iii) male depressive patients who had committed suicide showed significantly increased ACC Hcrt-receptor-2-mRNA expression compared to male controls; and iv) female but not male CUMS rats showed a highly significant positive correlation between the mRNA levels of corticotropin-releasing hormone and prepro-hypocretin in the hypothalamus, and a significantly increased Hcrt-receptor-1-mRNA expression in the frontal cortex compared to female control rats.
Conclusions
The clear sex-related change found in the hypothalamic hypocretin-1-ir in depression should be taken into account in the development of hypocretin-targeted therapeutic strategies.
Keywords: Depression, Hypocretin, Hypocretin receptors, Hypothalamus, Sex difference
Highlights
-
•
Hypocretin (orexin) changes were studied in human postmortem brain in depression.
-
•
A clear sex-related change was found in the hypothalamic hypocretin-1-immunoreactivity in depression.
-
•
A rat depression model did not reflect the changes in the hypocretin system in the human brain in depression.
The stress systems of depressed patients are put into a higher gear by genetic and developmental factors. Over-reaction of these systems to stressful environmental situations makes people vulnerable to depression and suicide. This is the first postmortem study on changes in a relatively novel stress system in depression, consisting of the hypothalamic hypocretin neurons and hypocretin receptors in the prefrontal cortex. A clear sex-related change was found in the hypothalamic hypocretin-1-immunoreactivity in depression. Evaluation of the hypocretin system in a frequently used depression animal model, i.e. chronic unpredictable mild stress rats, did not replicate changes found in the hypocretin systems in the human brain in depression.
1. Introduction
In individuals vulnerable to depression and suicide an exaggerated reaction to environmental stressors, such as life events, is found in the stress-regulating systems of the brain (Turecki et al., 2012). This hyper-reactivity stems from the interaction of genetic, early developmental and environmental factors (Sun et al., 2013). The hypothalamo-pituitary-adrenal (HPA) axis holds a prominent position in the network mediated by stress- and reward-related neurotransmitters and neuromodulators, and is significantly affected in depression (Bao et al., 2012) and suicide (Turecki et al., 2012).In depression, the hypothalamic paraventricular nucleus (PVN), which is the regulating center of the HPA-axis, shows not only an increased production of corticotropin-releasing hormone (CRH) (Raadsheer et al., 1995, Bao et al., 2005), but also changes in the expression of certain CRH-activity-related receptors that render the PVN more sensitive to stress (Wang et al., 2008). Both increased CRH and increased corticosteroid levels may induce depressive-like behavioral changes (Holsboer, 2001).
Hypocretin-producing neurons are localized in the hypothalamus (Hunt et al., 2015) and act via their two G-protein-coupled receptors, hypocretin receptor-1 and -2 (Hcrt-receptor-1 and Hcrt-receptor-2) (Sakurai et al., 1998). Hypocretin projections and Hcrt-receptors are found in many brain areas, including the prefrontal cortex (PFC) (Peyron et al., 1998). It is of interest to note that the neurophysiological and behavioral processes regulated by hypocretin, such as the sleep-wake cycle, food intake, sexual behavior, and stress response, are severely affected in depression (Nollet and Leman, 2013). Several studies have indicated the possible involvement of the hypocretin system in depression with suicide. For example, reduced CSF hypocretin levels were observed in suicidal patients with major depressive disorder (MDD) compared with patients suffering from adjustment disorder or dysthymia with suicide attempts (Brundin et al., 2007a). In addition, significant negative correlations were found between CSF-hypocretin levels and the symptoms of lassitude (difficulty to initiate activities) and slowness of movement, as well as the ratings of global illness (Brundin et al., 2007b). Hypocretin has close functional interactions with the HPA-axis. In rat, CRH directly stimulates the release of hypocretin during acute stress (Winsky-Sommerer et al., 2004). Hcrt-receptor antagonists were found to attenuate anxiety and panic-like behaviors associated with stress or hyperarousal states in rat (Johnson et al., 2015, Bonaventure et al., 2015). Moreover, the changes in the transcripts for the Hcrt-receptor-1 and Hcrt-receptor-2 were found to be divergent, i.e. Hcrt-receptor-1 increased, while Hcrt-receptor-2 decreased in the basolateral amygdala in chronically stressed c57bl/6 mice (Arendt et al., 2014). A clinical study of single-nucleotide polymorphisms suggested that the hcrt-receptor-1 gene, or a linked locus, may modulate the risk for mood disorders (Rainero et al., 2011). This possibility was confirmed in hcrt-receptor-1 knockout mice, which showed increased anxiety-like behavior and altered depression-like behaviors (Abbas et al., 2015). These data indicate that the hypocretin system may have a bidirectional regulatory capacity in terms of the stress response.
In light of the data mentioned above, we hypothesized that the hypocretin/orexin system may play a role in the pathogenesis of depression and suicide, possibly by interaction with the HPA-axis. To test this possibility, we measured hypocretin-1 expression in its production area, the postmortem hypothalamus. Second, Hcrt-receptors-mRNA content was determined in the PFC of depressive patients, some of whom had committed suicide. Finally, we determined prepro-hypocretin-mRNA and CRH-mRNA in the hypothalamus and Hcrt-receptors-mRNA levels in the frontal cortex in a frequently used animal model for depression, i.e. chronic unpredictable mild stress (CUMS) rats. Because of the clear sex differences in depression and suicide, special attention was given to the possible sex differences, both in the human and in the animal studies (Bao and Swaab, 2011).
2. Materials and Methods
2.1. Part I: Post-mortem Brain Material Study
In total, 120 human post-mortem samples were studied: 32 hypothalami and 52 cortex samples from the Netherlands Brain Bank (NBB), and 36 cortex samples from the Stanley Medical Research Institute (SMRI). Informed consent for a brain autopsy and for the use of the brain material and medical records for research purposes was given by the donor or their next of kin.
The chronic mood disorder patients and their controls were well-matched for confounding factors, including age, sex, postmortem delay, fixation time, clock time of death, month of death, CSF-pH (a measure of agonal state), brain weight and Braak stages of Alzheimer's pathology (Braak and Braak, 1991). Clinico-pathological details and p-values of matching are given in Table 1 and Supplementary Table 1, Supplementary Table 2. The diagnosis of MDD or bipolar disorder (BD) was confirmed according to the Diagnostic and Statistical Manual of Mental Disorders IV by qualified psychiatrists using the extensive medical records of the NBB, which also contained well-documented diagnoses and onset of depression from psychiatric clinics. Exclusion criteria for control subjects were in the first place the use of corticosteroids, as they inhibit the CRH cells in the human hypothalamus (Watts, 2005), which may subsequently influence the hypocretin system (Brunton and Russell, 2003). In addition, primary neurological or psychiatric diseases were exclusion criteria, unless stated otherwise. The absence of pathology was verified in all subjects by a systematic neuropathological analysis (Fronczek et al., 2007, Gao et al., 2013).
Table 1.
Clinico-pathological information of subjects for hypothalamus study.
| NBB | Group | Sex | Age at death/age of onset (y) | PMD (hr:min) | FT (d) | CTD | MOD | CSF pH | BW (g) | Braak stage | Medication in the past | Medication in the last 3 months | Suicide thought | Suicide attempt | Cause of death |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 92-003 | MDD | F | 55/2 | 4:54 | 52 | 07:45 | 11 | 6.40 | 1320 | ND | BZD, SSRI, TeCA | SSRI, BZD, bromocriptine | Yes | Yes | Suspected urosepsis, heart failure |
| 94-032 | MDD | M | 71/2 | 16:15 | 38 | 16:15 | 2 | ND | 975 | ND | ZUC, BZD, MAOI, digoxine | None | Yes | Yes | Probably broncho-pnemonia, next to cerebral ischaemia |
| 94-017 | MDD | F | 72/20 | 22:00 | 39 | 19:00 | 1 | ND | 1287 | ND | TeCA, BZD, prednison | None | NO | No | Bronchopneumonia, mesothelioma |
| 12-097 | MDD | F | 73/9 | 5:45 | 61 | 15:30 | 9 | 6.70 | 1205 | ND | TeCA | TCA, TeCA, BZD, pancuronium, Ba | No | No | Heart failure, legal euthanasia |
| 95-036 | MDD | M | 74/0.5 | 62 | 35 | 17:05 | 3 | ND | 1444 | ND | SSRI, BZD, cisordinol | SSRI, BZD, cisordinol | Yes | Yes | Suicide by hanging |
| 02-051 | MDD | M | 81/34 | 15:30 | 34 | 15:30 | 6 | 6.50 | 1345 | 3 | None | Hal | No | No | Renal insufficiency |
| 11-058a | MDD | M | 83/24 | 10:40 | 57 | 05:00 | 7 | 6.50 | 1200 | 2 | TCA, TeCA, Hal, Pipamperon, SSRI | BZD, gabapentin, clonazepam, Mo | No | No | PTSS with depression, acute heart failure |
| 08-076 | MDD | F | 91/36 | 5:20 | 36 | 09:05 | 8 | 6.53 | 1163 | 3 | Hal, SSRI, BZD, levothyroxine | SSRI, BZD, levothyroxine | No | No | Cachexia and dehydration by pneumonia and renal insufficiency |
| 08-031 | MDD | F | 93/63 | 4:20 | 51 | 04:55 | 3 | 6.80 | 1023 | 2 | SSRI, BZD | Mo, BZD, SSRI | Yes | Yes | Pneumonia |
| 02-014 | BD | M | 68/26 | 12:00 | 30 | 00:00 | 2 | 6.64 | 1424 | 1 | Li, Hal, ZUC, MAOI | MAOI | No | No | Subdural hematoma |
| 99–118 | BD | M | 68/30 | 5:55 | 33 | 23:15 | 10 | 6.82 | 1204 | 1 | Li, SSRI | Li | Yes | No | Cardiac ischemia |
| 00–111 | BD | M | 70/35 | 4:50 | 43 | 02:45 | 10 | 6.26 | 1490 | 1 | Li, ZUC, valproate, BZD | Li, ECT | No | No | Cardiac arrest, ileus due to intestinal haemorrhage |
| 00-088b | BD | M | 73/45 | 5:15 | 36 | 09:30 | 7 | 6.38 | 1260 | 2 | Li, BZD, SSRI, Hal, ECT, MAOI, methylphenidate | Li, SSRI, Hal, methylphenidate | Yes | No | Cachexia, dehydration |
| 98-010 | BD | F | 75/20 | 4:00 | 38 | 20:45 | 1 | ND | 1123 | 1 | TeCA, TCA | BZD, TeCA, SSRI, Hal, Mo | No | No | Acute abdomen secondary to a perforation of stomach/intestines due to NSAIDs |
| 12-048 | BD | M | 81/15 | 6:40 | 60 | 20:00 | 5 | 6.70 | 1283 | 1 | Li, prednisolon | Li, BZD, amitriptyline, valproate | No | No | Legal euthanasia |
| 12-110 | BD | M | 87/10 | 3:15 | 53 | 23:00 | 10 | 6.39 | 1285 | 3 | BZD, valproate | BZD, Mo, valproate | No | No | CVA, pneumonia |
| Median | – | – | 76/20 | 5:50 | 44 | 15:30 | 6 | 6.51 | 1271 | – | – | – | – | – | – |
| 99-033 | CTR | F | 61 | 17:45 | 119 | 23:15 | 12 | ND | 1296 | 0 | None | None | – | – | Acute heart failure, gastric blood loss |
| 97-042 | CTR | F | 65 | 12:50 | 28 | 02:00 | 4 | 6.94 | 910 | 1 | None | Adrenalin | – | – | Cardiac arrest, pneumonia, pulmonary oedema |
| 99-101 | CTR | M | 69 | 19:15 | 41 | 03:30 | 8 | 6.40 | 1352 | 1 | None | βB, Hal, BZD | – | – | Pneumonia |
| 92-049 | CTR | M | 71 | 5:40 | 32 | 12:00 | 4 | 7.40 | 1250 | ND | None | None | – | – | Found death |
| 08-032 | CTR | M | 71 | 8:55 | 70 | 03:15 | 3 | 6.64 | 1520 | 2 | Mo | Mo | – | – | Pancreas carcinoma, rectum carcinoma with hepatic metastases |
| 98-104 | CTR | F | 74 | 7:25 | 31 | 09:50 | 7 | 6.95 | 1167 | 2 | BZD | BZD, βB | – | – | Necrosis of the intestines secondary to thrombosis |
| 95-106 | CTR | M | 74 | 8:00 | 60 | 13:00 | 11 | 6.75 | 1317 | 0 | None | Dopamine | – | – | Myocardial infarction |
| 06-028 | CTR | M | 76 | 19:35 | 27 | 20:00 | 4 | 6.50 | 1514 | 3 | None | None | – | – | Prostate carcinoma, cardiac arrest |
| 94-039 | CTR | M | 78 | 53 | 88 | 12:00 | 1 | ND | 1354 | ND | None | Nitroglycerine | – | – | Myocardial infarction |
| 99-116 | CTR | M | 78 | 4:20 | 43 | 16:15 | 9 | ND | 1310 | 0 | None | Mo, BZD | – | – | Pancreatic cancer |
| 95-006 | CTR | M | 81 | 8:25 | 28 | 04:45 | 1 | 6.70 | 1274 | 1 | None | ND | – | – | Cardiogenic shock |
| 00-022 | CTR | F | 83 | 7:45 | 34 | 21:00 | 2 | 6.52 | 1102 | 2 | Digoxin, methimazole | Digoxin, methimazole | – | – | Acute myocardial infarction |
| 09-075 | CTR | M | 88 | 7:00 | 44 | 02:25 | 10 | 6.76 | 1230 | 3 | Salbutamol, prednisolone, | Mo, Hal, goserelin | – | – | Cachexia and dehydration by rectum carcinoma and prostate carcinoma |
| 09-001 | CTR | M | 88 | 4:43 | 51 | 19:47 | 1 | 6.17 | 1418 | 2 | None | None | – | – | Gastro-intestinal bleeding |
| 08-105 | CTR | F | 89 | 3:52 | 58 | 12:10 | 12 | 7.30 | 1258 | 3 | prednisolone | Hal, digoxine, Mo | – | – | Pneumonia |
| 08-054 | CTR | F | 92 | 7:00 | 67 | 09:45 | 6 | 6.55 | 1230 | 1 | Digoxine, prednison | prednison | – | – | Acute death, probably pulmonary embolism |
| Median | – | – | 77 | 7:52 | 43 | 12:00 | 5 | 6.7 | 1285 | – | – | – | – | – | – |
| p-Value | – | – | 0.616 | 0.361 | 0.780 | 0.711 | 0.93 | 0.137 | 0.515 | – | – | – | – | – | – |
Note: βB, beta-blocker; Ba, barbiturate; BD, bipolar disorder; Braak stage, progression of pathological changes for Alzheimer's disease according to Braak and Braak, 1991; BW, brain weight; BZD, benzodiazepine; CSF, cerebrospinal fluid; CTD, clock time at death; CTR, control; CVA, cerebrovascular accident; ECT, electroshock treatment; F, female; FT(d), fixation time in days; F, female; Hal, haloperidol; Li, lithium; M, male; MAOI, monoamine oxidase inhibitor; MDD, major depressive disorder; Mo, morphine; MOD, month of death; NBB, Netherlands Brain Bank; ND, no data; None, no medication; NSAIDs, nonsteroidal anti-inflammatory drugs; PMD, postmortem delay; PTSS, posttraumatische stressstoornis; SSRI, selective serotonin reuptake inhibitor; TCA, tricyclic antidepressant; TeCA, tetracyclic antidepressants; ZUC, zuclopenthixol. a: Patient also be diagnosis with post traumatic stress syndrome. b: Patient also be diagnosis with old cerebrovascular accident mildly demanded.
2.1.1. Immunocytochemistry and Quantification
The hypothalami were fixed in 10% PBS (pH 7.4) formalin at room temperature and were paraffin-embedded and serially-sectioned at 6 μm in rostro-caudal direction. Hypothalami of 16 mood disorder patients, i.e. 9 MDD and 7 BD patients, and 16 control subjects were used. Every 100th section of 6 μm thickness in the expected hypocretin cell area was stained using a hypocretin-1 antibody (catalog no. H-003-30, Phoenix Pharmaceuticals, Inc., Belmont, CA, USA) at 1:20,000 dilution. The specificity of the antibody had been confirmed in our previous study (Fronczek et al., 2007). In addition, we stained the hypothalamus of a narcoleptic patient as a negative control whose hypothalamus was indeed virtually devoid of hypocretin-1-immunoreactive (ir) neurons.
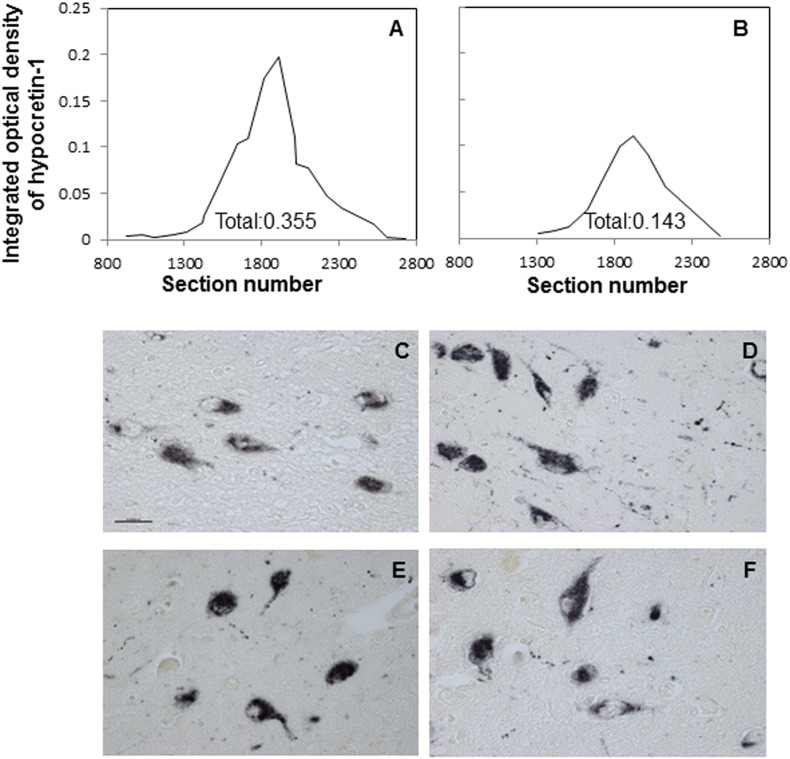
Hypocretin-1-ir was quantified by the image analysis procedure described in our previous studies (Gao et al., 2013). In brief, the set-up consisted of an image analysis system (Image Proversion 6.3, Media Cybernetics, Rockville, USA) connected to a black and white camera (SONY XC-77E) mounted on a microscope (Zeis Axios-kop with Plan-NEOFLUAR Zeiss objectives, Carl Zeiss GmbH, Jena, Germany). The hypothalamus area covered by hypocretin-stained neurons was outlined manually at a 20 × objective. The threshold for the positive signal was set at twice the optical density (OD) of the background. The computer determined the OD of the pixels and percentage surface area covered by the signal (area mask). The integrated optical density (IOD) was calculated by multiplying the OD by the masked area corrected for background. For each subject, the total IOD was calculated as the final parameter for the total amount of Hypocretin-1-ir, by a conversion program based upon multiplication of the separate IOD by sample frequency of the sections, as described previously (Goldstone et al., 2002). Completeness of the hypocretin-1-ir in the hypothalamus was confirmed by graphically presenting the IODs measured in every section from rostral to caudal, with a line drawn by the excel trend-line option ‘Moving Average’. The value under each curve showed the total IOD of the subject (see Fig. 1A–B). The total IOD of hypothalamic hypocretin-1-ir as determined by computer-assisted morphometry is not only an objective method but also less time-consuming than neuron-counting. In addition, the IOD shows a good positive correlation with neuron-counting (for 35 sections of 2 patients and 2 controls, rho = 0.781, p < 0.001), performed in the way we described previously (Fronczek et al., 2007).
Fig. 1.
Immunocytochemistry of hypocretin-1. A–B: representative distribution patterns of the integrated optical density (IOD) of hypocretin-1 immunoreactivity from rostral to caudal along the hypothalamus. (A) A female depression patient, (B) a male depression patient. C–F: representative hypocretin-1 staining in the hypothalamus of a female control subject. (C) A female control subject. (D) A female depression patient. (E) A male control subject and (F) a male depression patient. Bar = 0·025 mm.
2.1.2. Quantitative PCR (qPCR) for mRNA Expression of Hcrt-receptors
In our present study 50 μm cryostat sections of anterior cingulate cortex (ACC) or dorsal lateral prefrontal cortex (DLPFC) were cut from the left side of the cortex and the grey matter containing all six layers was isolated as described before (Gao et al., 2013). Levels of Hcrt-receptor-1- and Hcrt-receptor-2-mRNA were determined by qPCR. Information regarding gene selection and primers are shown in Supplementary Table 3. RNA isolation, cDNA synthesis and qPCR were performed as described before (Wang et al., 2008).
In addition, a normalization strategy was used to remove sampling-related differences in RNA quantity (Wang et al., 2008). The expression of target genes was normalized using the sets of stable reference genes, including actin-beta (ACTb), glyceraldehyde-3-phosphate dehydrogenase (GAPDH), hypoxanthine phosphoribosyltransferase 1 (HPRT1), and ubiquitin-C for the postmortem human brain study, while ACTb, GAPDH, HPRT1, and elongation factor-1 alpha (EF1α) for the animal study (see below).
2.2. Part II: Animal Study
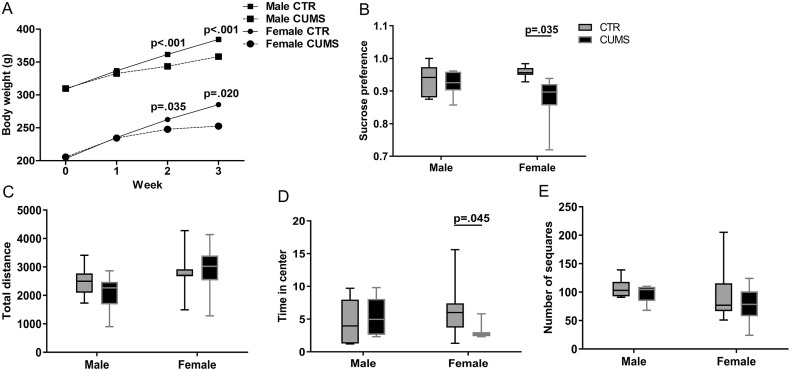
Adult Sprague Dawley male (n = 12, 280–300 g, ~ 8 weeks of age) and female (n = 24, 230–250 g, ~ 8 weeks of age) rats were randomly divided into a control and a CUMS group. CUMS was applied according to our previous study (Lu et al., 2015). Briefly: this involved a three-week daily exposure to alternating stressors along with occasional overnight stressors. The following stressors were given in random order: damp bedding (300 ml of water spilled in the bedding), 40°-cage-tilt along the vertical axis, paired housing, exposure to an empty water bottle for 1 h immediately following a period of acute water deprivation (25 h from 0900 h until 1000 h the next day), stroboscopic illumination (300 flashes/min), and white noise. Body weight was assessed weekly during the CUMS procedure, and an open field test for anxiety-behavior (Chen et al., 2009) and sucrose preference test for depression-like behavior such as anhedonia (Cai et al., 2015) were performed following the CUMS paradigm. In the open field test we measured the distance the rat travelled in the field, the time spent in the central squares, together with the number of grid lines crossed. In the 5% sucrose preference test sucrose preference was determined by the ratio of the weight of sucrose solution versus water consumed during the 24 h.
2.2.1. Tissue Collection
Rats were sacrificed by decapitation between 0900 h and 1000 h upon conclusion of the behavioral tests. Female rats, both of the control and CUMS groups, were sacrificed, in equal numbers, in proestrus or diestrus. Trunk blood, hypothalamus and frontal cortex were collected. The hypothalamus and frontal lobe were immediately dissected, frozen in liquid nitrogen and stored at − 80 °C until assayed.
All animal experimental procedures were approved by the local animal care committee of Zhejiang University in accordance with the relevant regulations and laws.
2.2.2. Sample Measurement
Plasma corticosterone (CORT) levels were assayed by ELISA (RE52211, IBL Corporation, USA). Hypothalamic mRNA levels of prepro-hypocretin, Hcrt-receptor-1, Hcrt-receptor-2 and CRH, and frontal cortex mRNA levels of Hcrt-receptor-1 and Hcrt-receptor-2 were determined by qPCR. The primers were designed by Primer Premier (version 5.0) to amplify specific amplicons. Sequences and size of the primer pairs are shown in Supplementary Table 3. The RNA isolation and qPCR were performed as described before by our group (Wang et al., 2008).
2.3. Statistics
As the data were not always normally distributed, non-parametric tests were applied. Differences between 2 groups were tested using the Mann-Whitney U test. Differences among the 3 groups were first tested with Kruskal-Wallis (K-W), and if a significant difference was identified, the difference between 2 groups was checked with the Mann-Whitney U test. Generalized linear model (GLM) was applied to analyze the contribution of certain factors to the changes observed in Hypocretin-1-ir (see Section 3). Correlations were examined with the Spearman's test. Differences in clock time and month of death were analyzed with the Mardia-Watson-Wheeler test. All tests were 2-tailed, p-values ≤ 0.05 were considered to be significant, while p-value < 0.1 and > 0.05 was considered to be a trend.
3. Results
3.1. Postmortem Human Brain Material
3.1.1. Hypocretin-1 Immunocytochemistry
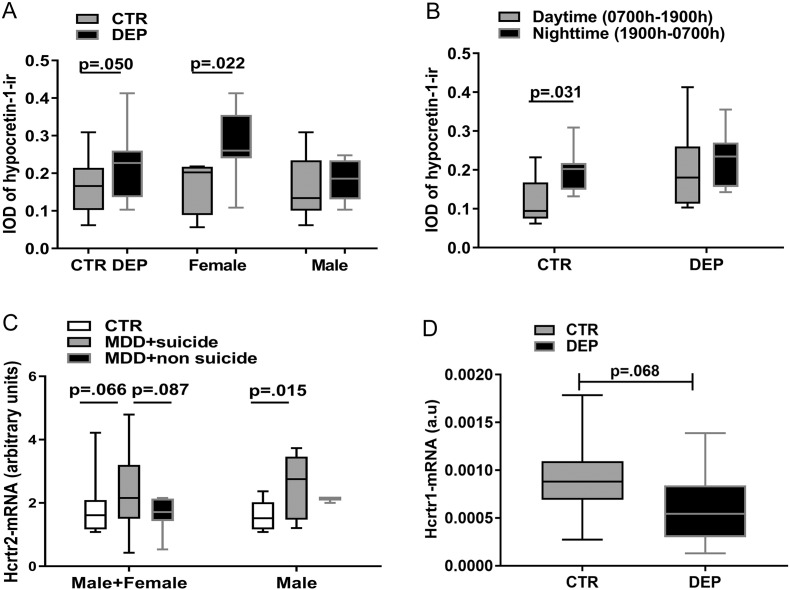
Hypocretin-1-ir neurons showed the same distribution pattern as described before (Fronczek et al., 2007), and hypocretin-1-ir was present in the cytoplasm and fibers of hypothalamic neurons, both in controls and depressive patients (Fig. 1C–F). Data are presented as median (25th–75th percentile). The IOD of hypocretin-1-ir showed a significantly higher level in the depression group compared with the control group (depression: 0.23 (0.14–0.26); controls: 0·17 (0.10–0.22), p = 0.050), which appeared to be due to the female depressive patients, who showed significantly (40%) higher IOD values (0.27 (0.22–0.37)) than the matched female controls (0.19 (0.09–0.21); p = 0.022), while the IOD of hypocretin-1-ir of male depressive patients (0.17 (0.13–0.23)) showed no significant differences from the control male subjects (0.15 (0.11–0.22); p = 0.406) (Fig. 2A). GLM was applied with depression and sex as the main factors to investigate the effect of these two factors on hypocretin-1-ir and the interaction between them. GLM revealed that the interaction between depression and sex was significant (p = 0.041). The main effect of depression on hypocretin-1-ir remained significant (p = 0.003), as well as the effect of sex (p = 0.004).
Fig. 2.
Changes of hypocretin-1 system in depression. A: Comparison of the amount of hypocretin-1-immunoreactivity (ir) between the entire control (CTR, n = 16) and the entire depression group (DEP, n = 16), the female CTR (n = 6) and female DEP group (n = 6), and the male CTR (n = 9) and male DEP group (n = 9). B: The amount of hypocretin-1-ir is on the left side for the CTR group (between nighttime (1900 h–0700 h, n = 9) and daytime (0700 h–1900 h, n = 7), and on the right side (n = 8 vs. n = 8) for the DEP group. The time segmentation was based upon a study on the diurnal rhythm of cerebrospinal fluid hypocretin-1 levels (Chen et al., 2009).
Note the absence of a day-night fluctuation in DEP patients. C: The hypocretin (Hcrt) receptor-2-mRNA levels in the anterior cingulated cortex (ACC) of the entire group comparison, i.e. CTR (n = 12), major depressive disorder patients who committed suicide (MDD + suicide, n = 17) and MDD patients who died of causes other than suicide (MDD + non-suicide, n = 7) on the left side, and for male groups comparison on the right side.
Note that male MDD + suicide patients (n = 10) had significantly higher Hcrt receptor-2-mRNA levels compared with male controls (n = 8). D: The Hcrt-receptor-1-mRNA levels in the ACC of these non-suicide depressive patients (n = 12) showed a trend for decrease compared with the controls (n = 12).
There was no difference in hypocretin-1-ir IOD between the MDD and BD subgroups (p = 0.874), either between female and male subjects within the control group (p = 0.652) or between female and male subjects within the control group (p = 0.875). Furthermore, there were no significant correlations between the IOD of hypocretin-1-ir with age or with the duration of depression (p ≥ 0.15).
3.1.2. Hypocretin-1-ir Day-Night Fluctuation
In the control group, the IOD of hypocretin-1-ir was significantly (40%) higher for those with a clock time of death at night (0.20 (0.15–0.22), 1900 h–0700 h) than for those with a clock time of death during the daytime (0.09 (0.07–0.17); p = 0.031), whereas such a difference was not present in the depression subjects (nighttime: median = 0.23; daytime: median = 0.18; p = 0.382, Fig. 2B). The time segmentation was based upon a previous study on the diurnal rhythm of CSF hypocretin-1 levels (Salomon et al., 2003). The daytime and nighttime control subgroups did not differ significantly in terms of possible confounders, i.e. age, sex, PMD, FT, or CSF-pH (p ≥ 0.137).
3.1.3. The mRNA Expression of Hcrt Receptors in the ACC and DL PFC
In the cortex samples of the relatively younger patients of the SMRI series (depression: 42 years (24–63 years); controls 46 years (24–63 years), Supplementary Table 2), both Hcrt-receptor-1- and Hcrt-receptor-2-mRNA expression were detectable in the ACC and the DLPFC. No significant differences in ACC Hcrt-receptor-1-mRNA expression were seen among the 3 groups, i.e. depressive patients who had committed suicide, depressive patients who had died of causes other than suicide, and control subjects (K-W: Hcrt-receptor-1, p = 0.102), but there was a trend for changes in Hcrt-receptor-2-mRNA in these 3 groups (K-W: p = 0.085). We observed a significant difference in Hcrt-receptor-2-mRNA expression among these 3 groups in male subjects (K-W: p = 0.035), which was subsequently found to be due to the significantly (38%) higher Hcrt-receptor-2-mRNA levels in male suicide MDD patients (n = 10) compared with male controls (n = 8, p = 0.015, Fig. 2C). Please note that although we did not observe similar significant changes in female subjects or in male subjects who died of causes other than suicide (K-W: Hcrt-receptors, p ≥ 0.222), the number of these subgroups was too small to draw a firm conclusion in relation to sex difference and/or suicide. In the DLPFC, Hcrt-receptor-1-mRNA expression showed a significant negative correlation with age in the control group (rho = − 0.887, p = 0.001). No significant changes in Hcrt-receptors-mRNA expression in the DLPFC were observed, either among the 3 entire (female + male) groups, i.e. depressive patients who had committed suicide, depressive patients who had died of causes other than suicide, and control subjects, or among 3 different female or male subgroups (K-W: Hcrt-receptors, p ≥ 0.345).
In cortex samples of the relatively older patients (depression: 78 years (45–96 years); controls 73 years (45–90 years), Supplementary Table 1), which contained only non-suicide depressive patients, Hcrt-receptor-1-mRNA expression was only detectable in the ACC, while Hcrt-receptor-2-mRNA expression was only detectable in the DLPFC, both in the control and in the depression group. In the ACC of these non-suicide depressive patients there was a trend (p = 0.068, Fig. 2D) towards lower Hcrt-receptor-1-mRNA expression compared with the controls. In the DLPFC, no significant differences in Hcrt-receptor-2-mRNA expression were found between the non-suicide depressive patients and the control group (p = 0.894). There were no sex differences in HCRTs either in the ACC or in the DLPFC (p ≥ 0.209). There was no significant correlation between the duration of depression and the Hcrt-receptors-mRNA levels in the ACC or DLPFC (p ≥ 0.401).
3.1.4. The CUMS Animals
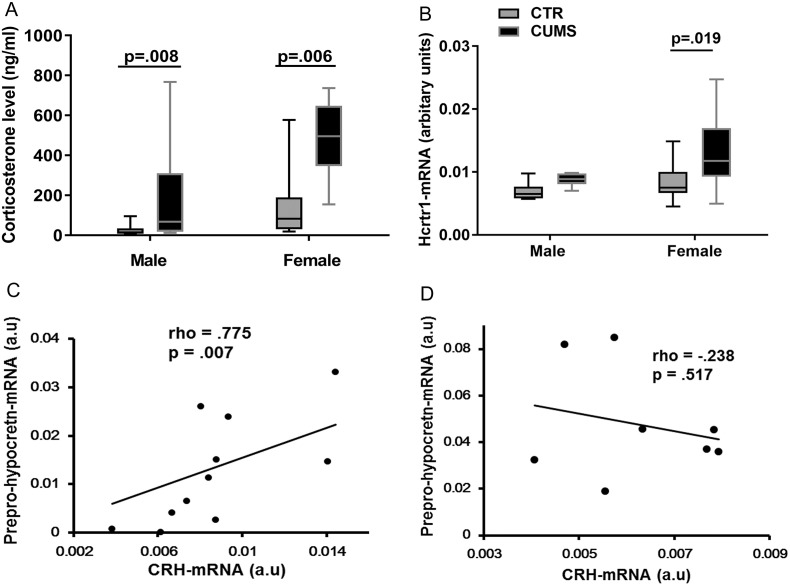
Although the body weight of both female and male rats showed, as expected, a diminished increase during and after the CUMS procedure (Supplementary Fig. 1A), only the female CUMS rats showed significant depression-like (sucrose preference tests, Supplementary Fig. 1B) and anxiety-like (open field test) behaviors (Supplementary Fig. 1C–E). Plasma CORT levels were significantly increased both in female and male (p ≤ 0.008) CUMS rats (Fig. 3A). It should be noted that we pooled these female rats for the correlation analysis, since we did not observe a significant difference in the plasma CORT levels, hypothalamic mRNA levels of prepro-hypocretin, Hcrt-receptor-1, Hcrt-receptor-2 and CRH, and frontal cortex mRNA levels of Hcrt-receptor-1 and Hcrt-receptor-2 between female rats sacrificed in proestrus and in diestrus, we pooled these female rats for the correlation analysis. The Hcrt-receptor-1-mRNA expression was significantly higher in the frontal cortex of female CUMS rats (0.012 (0.009–0.017)), compared with the female control rats (0.008 (0.007–0.010); p = 0.019, Fig. 3B), while male rats did not show significant alterations in these molecules after CUMS (p ≥ 0.328). There were no significant differences in the mRNA expression of prepro-hypocretin, Hcrt-receptor-1, Hcrt-receptor-2 or CRH in the hypothalamus between the CUMS and the control rats in either sex (p ≥ 0.234). However, female CUMS rats showed a significant positive correlation between the mRNA levels of CRH and prepro-hypocretin (p = 0.007, rho = 0.775, Fig. 3C), which was absent in male CUMS rats (p = 0.517, rho = − 0.238, Fig. 3D).
Supplementary Fig. 1.
Changes in bodyweight and behaviours in rats expososed to chronic unpredictable mild stress.
Fig. 3.
A: Plasma corticosterone levels of control (male CTR, n = 6; female CTR, n = 12) rats and chronic unpredictable mild stress (CUMS) rats (male CUMS, n = 6; female CUMS, n = 12). B: Hypocretin (Hcrt) receptor-1-mRNA expression in frontal cortex of CTR and CUMS rats. Please note that female CUMS rats (n = 12) had significantly higher Hcrt receptor-1-mRNA levels compared with female controls (n = 12). C: There was a significant positive correlation between prepro-hypocretin-mRNA and corticotropin-releasing hormone (CRH)-mRNA in the hypothalamus of female CUMS rats (rho = 0·775, p = 0·007, n = 12). D: There was no significant correlation between prepro-hypocretin-mRNA and CRH-mRNA in the hypothalamus of male CUMS rats (rho = − 0·238, p = 0·517, n = 6).
4. Discussion
Our study shows, for the first time in postmortem human brain, that hypothalamic hypocretin/orexin is increased in female - but not in male - depressive patients. In addition, there was a diurnal fluctuation in hypothalamic hypocretin-1-ir in the control subjects, which was absent in depression. Moreover, we observed that Hcrt-receptor-mRNA expression showed differences in the ACC and DLPFC depending on age. Male depressive patients who had committed suicide had significantly increased ACC Hcrt-receptor-2-mRNA expression. Our data thus indicate sex-, brain area-, age-, and potentially suicide-related changes in the hypocretin/orexin system in depression. Finally, a significant positive correlation between hypothalamic CRH-mRNA and prepro-hypocretin-mRNA and a significant increase in Hcrt-receptor-1-mRNA expression in the frontal cortex in female - but not male - CUMS rats strengthen the presence of sexually dimorphic hypocretin/orexin system changes in mood disorder.
4.1. Hypocretin-ir in the Hypothalamus in Depression
It should be noted that the increased IOD of hypocretin-ir may indicate an increase in either hypocretin-expressing neuron number (related to the area stained) and/or staining intensity (measured as OD). An increase of either of these parameters indicates an increased expression of hypocretin protein levels. The significantly increased hypocretin-ir in female depressive patients indicates that hypocretin may play a key role in the etiology of depression, which is more prevalent in females than in males (Piccinelli and Wilkinson, 2000). As we have indicated in the Introduction section, studies have found that Hcrt-receptor-1 gene, or a linked locus, may modulate the risk for mood disorders (Rainero et al., 2011) and Hcrt-receptor-1 gene knockout mice showed increased anxiety-like behavior and altered depression-like behaviors (Abbas et al., 2015). One may thus speculate that the higher levels of hypocretin-1-ir in female patients may enhance depressive symptoms. It should be noted that since all the female subjects studied in our study were in their postmenopausal stage, one would not expect to see the hot flash-related hypocretin changes reported earlier (Federici et al., 2016).
The sex difference in the alterations of hypocretin, which happens in the framework of the stress-hypothesis, was further supported by our animal study, showing a significant positive correlation between hypothalamic prepro-hypocretin-mRNA and CRH-mRNA only in female CUMS rats. In view of this, it is of interest to note that the dual Hcrt-receptor antagonist almorexant was found to prevent HPA axis dysregulation caused by CUMS and offers evidence for the possibility that pharmacological blockade of the hypocretin system induces a robust antidepressant-like effect as well as the restoration of the stress-related HPA axis defect (Nollet et al., 2012). It should be noted, however, that other animal models of depression showed different results in terms of changes in the hypocretin system, such as the genetically depressed Wistar-Kyoto male rats, which showed a lower number and size of hypocretin-1 neurons than its control Wistar male rats (Allard et al., 2004). In another genetically depressed rat model, i.e. the Flinders Sensitive Line (FSL), the number of hypothalamic hypocretin-positive neurons was higher in female FSL rats than in the female control rats, i.e. Flinders Resistant Line (FRL) although this publication by Mikrouli et al. offers no data on male rats (Mikrouli et al., 2011). It is a frequent phenomenon that animal models tend to mimic “just a few symptoms rather than a complete psychiatric disorder”. This is a reason to validate the data obtained in animal models on human postmortem material.
We found a clear day-night fluctuation in hypothalamic hypocretin-1-ir in the control subjects, with higher levels at night, which is similar to the pattern reported for lumbar puncture CSF hypocretin-1 levels obtained by continuous in vivo sampling (Salomon et al., 2003). These findings are in agreement with the concept that hypocretin neurons may play a key role in sleep-wake regulation (Saper, 2013), and the absence of the day-night hypocretin-1-ir fluctuation in depression may thus relate to the frequently occurring symptoms of sleep disorders in this condition. It should be noted that in our earlier research we demonstrated a clear diurnal rhythm in the biological clock, the suprachiasmatic nucleus (SCN), for its main neuropeptide, vasopressin, both on the protein level (Hofman et al., 1993) and on the mRNA level (Zhou et al., 2001) in postmortem material, when the patients were grouped according to time of death. Interestingly, a direct projection from the SCN onto the hypocretin neurons was observed in the brains of rat and human (Abrahamson et al., 2001), which indicates that the SCN may directly regulate the function of hypocretin-immunoreactive neurons. Our finding of the absence of a diurnal hypocretin rhythm in depression agrees with our earlier observation of a diminished SCN function in depression (Zhou et al., 2001). Our data (Zhou et al., 2001) and those of others (Li et al., 2013) thus also show that postmortem studies can indeed reflect the day-night fluctuations during life.
It is of interest to note that in rats the maximal activity of the hypocretin system takes place in their active period, i.e. at night (Mileykovskiy et al., 2005, Yoshida et al., 2001) Surprisingly, we found the highest hypocretin-1-ir levels in the human hypothalamus at night. However, this observation agrees with two human studies that reported the lowest CSF hypocretin levels during the daytime (Salomon et al., 2003, Grady et al., 2006). This means that the nocturnal elevation of hypocretin-1-ir in the hypothalamus is not simply due to a lack of transport or secretion. A similar phenomenon was observed for melatonin, which is also involved in sleep-wake control: in rats, melatonin levels increase during the dark period (their active phase) and decrease during the light period (rest phase) (Gutjahr et al., 2004), while in humans its levels increase during the dark period (rest phase) and decrease during the light period (active phase) (Zeitzer et al., 2007). The possibility that the diurnal regulation systems act in a different way in a diurnal and a nocturnal species (such as rodents) warrants further investigation.
A recent study showed an age-related decline in the number of hypothalamic hypocretin neurons in the range from 0 to 60 years of age in control subjects (Hunt et al., 2015). We did not find such a correlation, but it should be noted that, unlike in the study by others, the control subjects we studied did not contain very young ages.
4.2. PFC Hcrt-receptors in Depression and in Relation to Suicide
Earlier, our group found, with NBB cortex samples (depression patients without suicide), that the ACC seems to be more vulnerable than the DLPFC to alterations in depression-related molecules, such as nitric oxide synthase, gamma-aminobutyric acid and glutamate (Gao et al., 2013, Zhao et al., 2012). In our study, with NBB cortex samples, again we observed a trend for lower Hcrt-receptor-1-mRNA expression in the ACC in depression but no changes in Hcrt-receptors in the DLPFC. The novel finding with SMRI cortex samples (containing depressive patients who committed suicide or died of causes other than suicide) that there was a significantly increased Hcrt-receptor-2-mRNA expression in the ACC, but not in the DLPFC, in male MDD patients who had committed suicide is in agreement with previous findings that the ACC is more vulnerable to suicide than the DLPFC (Zhao et al., 2012, Drevets et al., 2008) and that there is a sex difference in the prevalence of suicide (Maguen et al., 2015). Our data concerning increased ACC Hcrt-receptor-2-mRNA expression in male suicide patients are thus interesting, although too limited for a final conclusion. They do represent, however, a strong rationale for further studies on this topic. Finally, the decreased Hcrt-receptor-1-mRNA expression with aging we observed in the DLPFC may at least partly explain the findings that in SMRI cortex samples (younger) both Hcrt-receptors were detectable in ACC and DLPFC, while in the NBB series (older) Hcrt-receptor-1-mRNA expression was only detectable in ACC and Hcrt-receptor-2-mRNA expression was only detectable in the DLPFC.
Some concerns of the present postmortem brain material study should be mentioned. We did not find significant differences in the hypothalamic hypocretin-1-ir expression between MDD and BD patients, which is in accordance with our previous findings for CRH, AVP and OXT and for receptors in the hypothalamus (Bao et al., 2005, Wang et al., 2008), although a final conclusion on this phenomenon should be based upon a larger sample size. Secondly, one of the inherent potential confounding factors in a postmortem study is medication use. However, we do not think that our main conclusions are cofounded by antidepressants, since increased hypothalamic hypocretin-1-ir was only observed in female depressive patients and increased expression of Hcrt-receptor-2-mRNA in the ACC was only observed in male depressive suicides, although all the depressive groups had been on antidepressants. In addition, animal studies showed that benzodiazepines (Panhelainen and Korpi, 2012), haloperidol (Dalal et al., 2003) and fluoxetine (Nollet et al., 2011), taken by the depressive patients in the present study, may inhibit hypocretin neurons and/or decrease hypocretin levels. Therefore, had antidepressants interfered with our measurements, this would have led to an underestimation of the increased hypocretin-1 levels observed in female depressive patients. It is noted that Calegare et al. found that sub-chronic treatment of adult male rats with fluoxetine increased the levels of prepro-hypocretin mRNA in the hypothalamus without affecting the hypocretin-1 CSF levels (Calegare et al., 2016). In our study, there were 2 out of 10 male depression patients who had taken fluoxetine, while their hypothalamic hypocretin-1-ir levels (IOD: 0.128 and 0.134) were fully within the range of the other male depression patients (IOD range from 0.103 to 0.248, median value 0.166). Finally, it should also be noted that the Hcrt-receptor data are based upon mRNA measurements and have yet to be confirmed on the protein level.
5. Conclusions
A clear sex-related change was found in the hypothalamic hypocretin-1-ir in depression. The CUMS rat depression model did not replicate changes found in the hypocretin systems in the human brain in depression. Since sex-related changes in hypothalamic hypocretin-1-ir expression were observed in depression, this factor should be taken into account in the development of hypocretin-targeted therapeutic strategies.
The following are the supplementary data related to this article.
Clinico-pathological information of subjects for prefrontal cortex study (material obtained from the Netherlands Brain Bank).
Clinico-pathological information of subjects for prefrontal cortex study (material obtained from the Stanley Medical Research Institue).
Sequence of the primers, gene bank accession numbers, the size of amplified product for the target genes and reference genes.
Funding Sources
AB is funded by the National Key Research and Development Program of China (2016YFC1306701), and the National Natural Science Foundation of China (91332102), DFS is funded through the Programme of Introducing Talents of Discipline Universities of China (B13026). JL was funded by the China Scholarship Council [grant number (201206320053)].
Conflicts of Interests
All authors declare no competing interests.
Authors Contributions
JL, AB and DFS had full access to all the data in the study and take full responsibility for the integrity of the data and the accuracy of the data analysis. AB and DFS financed and designed the study and supervised the data collection and analysis, JL analyzed the data and wrote the first draft with AB and DFS. All other authors provided data, reviewed the results, and contributed to the final draft of the report.
Acknowledgements
The authors are grateful to the Netherlands Brain Bank (Director Dr. Inge Huitinga) for providing human brain material and clinical details, and to Ms. W.T.P. Verweij for her secretarial assistance.
References
- Abbas M.G., Shoji H., Soya S., Hondo M., Miyakawa T., Sakurai T. Comprehensive behavioral analysis of male Ox1r (−/−) mice showed implication of orexin receptor-1 in mood, anxiety, and social behavior. Front. Behav. Neurosci. 2015;9:324. doi: 10.3389/fnbeh.2015.00324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrahamson E.E., Leak R.K., Moore R.Y. The suprachiasmatic nucleus projects to posterior hypothalamic arousal systems. Neuroreport. 2001;12(2):435–440. doi: 10.1097/00001756-200102120-00048. [DOI] [PubMed] [Google Scholar]
- Allard J.S., Tizabi Y., Shaffery J.P., Trouth C.O., Manaye K. Stereological analysis of the hypothalamic hypocretin/orexin neurons in an animal model of depression. Neuropeptides. 2004;38(5):311–315. doi: 10.1016/j.npep.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Arendt D.H., Hassell J., Li H., Achua J.K., Guarnieri D.J., Dileone R.J. Anxiolytic function of the orexin 2/hypocretin A receptor in the basolateral amygdala. Psychoneuroendocrinology. 2014;40:17–26. doi: 10.1016/j.psyneuen.2013.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao A.M., Swaab D.F. Sexual differentiation of the human brain: relation to gender identity, sexual orientation and neuropsychiatric disorders. Front. Neuroendocrinol. 2011;32(2):214–226. doi: 10.1016/j.yfrne.2011.02.007. [DOI] [PubMed] [Google Scholar]
- Bao A.M., Hestiantoro A., Van Someren E.J., Swaab D.F., Zhou J.N. Colocalization of corticotropin-releasing hormone and oestrogen receptor-alpha in the paraventricular nucleus of the hypothalamus in mood disorders. Brain. 2005;128(Pt 6):1301–1313. doi: 10.1093/brain/awh448. [DOI] [PubMed] [Google Scholar]
- Bao A.M., Ruhe H.G., Gao S.F., Swaab D.F. Neurotransmitters and neuropeptides in depression. Handb. Clin. Neurol. 2012;106:107–136. doi: 10.1016/B978-0-444-52002-9.00008-5. [DOI] [PubMed] [Google Scholar]
- Bonaventure P., Yun S., Johnson P.L., Shekhar A., Fitz S.D., Shireman B.T. A selective orexin-1 receptor antagonist attenuates stress-induced hyperarousal without hypnotic effects. J. Pharmacol. Exp. Ther. 2015;352(3):590–601. doi: 10.1124/jpet.114.220392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H., Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82(4):239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Brundin L., Bjorkqvist M., Petersen A., Traskman-Bendz L. Reduced orexin levels in the cerebrospinal fluid of suicidal patients with major depressive disorder. Eur. Neuropsychopharmacol. 2007;17(9):573–579. doi: 10.1016/j.euroneuro.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Brundin L., Petersen A., Bjorkqvist M., Traskman-Bendz L. Orexin and psychiatric symptoms in suicide attempters. J. Affect. Disord. 2007;100(1–3):259–263. doi: 10.1016/j.jad.2006.10.019. [DOI] [PubMed] [Google Scholar]
- Brunton P.J., Russell J.A. Hypothalamic-pituitary-adrenal responses to centrally administered orexin-A are suppressed in pregnant rats. J. Neuroendocrinol. 2003;15(7):633–637. doi: 10.1046/j.1365-2826.2003.01045.x. [DOI] [PubMed] [Google Scholar]
- Cai L., Li R., Tang W.J., Meng G., Hu X.Y., Wu T.N. Antidepressant-like effect of geniposide on chronic unpredictable mild stress-induced depressive rats by regulating the hypothalamus-pituitary-adrenal axis. Eur. Neuropsychopharmacol. 2015;25(8):1332–1341. doi: 10.1016/j.euroneuro.2015.04.009. [DOI] [PubMed] [Google Scholar]
- Calegare B.F., Costa A., Fernandes L., Dias A.L., Torterolo P., Almeida V.D. Subchronical treatment with Fluoxetine modifies the activity of the MCHergic and hypocretinergic systems. Evidences from peptide CSF concentration and gene expression. Sleep Sci. 2016;9(2):89–93. doi: 10.1016/j.slsci.2016.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X.N., Meng Q.Y., Bao A.M., Swaab D.F., Wang G.H., Zhou J.N. The involvement of retinoic acid receptor-alpha in corticotropin-releasing hormone gene expression and affective disorders. Biol. Psychiatry. 2009;66(9):832–839. doi: 10.1016/j.biopsych.2009.05.031. [DOI] [PubMed] [Google Scholar]
- Dalal M.A., Schuld A., Pollmacher T. Lower CSF orexin A (hypocretin-1) levels in patients with schizophrenia treated with haloperidol compared to unmedicated subjects. Mol. Psychiatry. 2003;8(10):836–837. doi: 10.1038/sj.mp.4001363. [DOI] [PubMed] [Google Scholar]
- Drevets W.C., Savitz J., Trimble M. The subgenual anterior cingulate cortex in mood disorders. CNS Spectr. 2008;13(8):663–681. doi: 10.1017/s1092852900013754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federici L.M., Caliman I.F., Molosh A.I., Fitz S.D., Truitt W.A., Bonaventure P., Carpenter J.S., Shekhar A., Johnson P.L. Hypothalamic orexin's role in exacerbated cutaneous vasodilation responses to an anxiogenic stimulus in a surgical menopause model. Psychoneuroendocrinology. 2016;65:127–137. doi: 10.1016/j.psyneuen.2015.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fronczek R., Overeem S., Lee S.Y., Hegeman I.M., van Pelt J., van Duinen S.G. Hypocretin (orexin) loss in Parkinson's disease. Brain. 2007;130(Pt 6):1577–1585. doi: 10.1093/brain/awm090. [DOI] [PubMed] [Google Scholar]
- Gao S.F., Qi X.R., Zhao J., Balesar R., Bao A.M., Swaab D.F. Decreased NOS1 expression in the anterior cingulate cortex in depression. Cereb. Cortex. 2013;23(12):2956–2964. doi: 10.1093/cercor/bhs285. [DOI] [PubMed] [Google Scholar]
- Goldstone A.P., Unmehopa U.A., Bloom S.R., Swaab D.F. Hypothalamic NPY and agouti-related protein are increased in human illness but not in Prader-Willi syndrome and other obese subjects. J. Clin. Endocrinol. Metab. 2002;87(2):927–937. doi: 10.1210/jcem.87.2.8230. [DOI] [PubMed] [Google Scholar]
- Grady S.P., Nishino S., Czeisler C.A., Hepner D., Scammell T.E. Diurnal variation in CSF orexin-A in healthy male subjects. Sleep. 2006;29(3):295–297. doi: 10.1093/sleep/29.3.295. [DOI] [PubMed] [Google Scholar]
- Gutjahr G.H., van Rensburg L.J., Malpaux B., Richter T.A., Bennett N.C. The endogenous rhythm of plasma melatonin and its regulation by light in the highveld mole-rat (Cryptomys hottentotus pretoriae): a microphthalmic, seasonally breeding rodent. J. Pineal Res. 2004;37(3):185–192. doi: 10.1111/j.1600-079X.2004.00151.x. [DOI] [PubMed] [Google Scholar]
- Hofman M.A., Purba J.S., Swaab D.F. Annual variations in the vasopressin neuron population of the human suprachiasmatic nucleus. Neuroscience. 1993;53(4):1103–1112. doi: 10.1016/0306-4522(93)90493-y. [DOI] [PubMed] [Google Scholar]
- Holsboer F. Stress, hypercortisolism and corticosteroid receptors in depression: implications for therapy. J. Affect. Disord. 2001;62(1–2):77–91. doi: 10.1016/s0165-0327(00)00352-9. [DOI] [PubMed] [Google Scholar]
- Hunt N.J., Rodriguez M.L., Waters K.A., Machaalani R. Changes in orexin (hypocretin) neuronal expression with normal aging in the human hypothalamus. Neurobiol. Aging. 2015;36(1):292–300. doi: 10.1016/j.neurobiolaging.2014.08.010. [DOI] [PubMed] [Google Scholar]
- Johnson P.L., Federici L.M., Fitz S.D., Renger J.J., Shireman B., Winrow C.J. Orexin 1 and 2 receptor involvement in Co2-induced panic-associated behavior and autonomic responses. Depress. Anxiety. 2015;32(9):671–683. doi: 10.1002/da.22403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J.Z., Bunney B.G., Meng F., Hagenauer M.H., Walsh D.M., Vawter M.P. Circadian patterns of gene expression in the human brain and disruption in major depressive disorder. Proc. Natl. Acad. Sci. U. S. A. 2013;110(24):9950–9955. doi: 10.1073/pnas.1305814110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J., Wu X.Y., Zhu Q.B., Li J., Shi L.G., Wu J.L. Sex differences in the stress response in SD rats. Behav. Brain Res. 2015;284:231–237. doi: 10.1016/j.bbr.2015.02.009. [DOI] [PubMed] [Google Scholar]
- Maguen S., Skopp N.A., Zhang Y., Smolenski D.J. Gender differences in suicide and suicide attempts among US Army soldiers. Psychiatry Res. 2015;225(3):545–549. doi: 10.1016/j.psychres.2014.11.050. [DOI] [PubMed] [Google Scholar]
- Mikrouli E., Wortwein G., Soylu R., Mathe A.A., Petersen A. Increased numbers of orexin/hypocretin neurons in a genetic rat depression model. Neuropeptides. 2011;45(6):401–406. doi: 10.1016/j.npep.2011.07.010. [DOI] [PubMed] [Google Scholar]
- Mileykovskiy B.Y., Kiyashchenko L.I., Siegel J.M. Behavioral correlates of activity in identified hypocretin/orexin neurons. Neuron. 2005;46(5):787–798. doi: 10.1016/j.neuron.2005.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nollet M., Leman S. Role of orexin in the pathophysiology of depression: potential for pharmacological intervention. CNS Drugs. 2013;27(6):411–422. doi: 10.1007/s40263-013-0064-z. [DOI] [PubMed] [Google Scholar]
- Nollet M., Gaillard P., Minier F., Tanti A., Belzung C., Leman S. Activation of orexin neurons in dorsomedial/perifornical hypothalamus and antidepressant reversal in a rodent model of depression. Neuropharmacology. 2011;61(1–2):336–346. doi: 10.1016/j.neuropharm.2011.04.022. [DOI] [PubMed] [Google Scholar]
- Nollet M., Gaillard P., Tanti A., Girault V., Belzung C., Leman S. Neurogenesis-independent antidepressant-like effects on behavior and stress axis response of a dual orexin receptor antagonist in a rodent model of depression. Neuropsychopharmacology. 2012;37(10):2210–2221. doi: 10.1038/npp.2012.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panhelainen A.E., Korpi E.R. Evidence for a role of inhibition of orexinergic neurons in the anxiolytic and sedative effects of diazepam: a c-Fos study. Pharmacol. Biochem. Behav. 2012;101(1):115–124. doi: 10.1016/j.pbb.2011.12.011. [DOI] [PubMed] [Google Scholar]
- Peyron C., Tighe D.K., van den Pol A.N., de Lecea L., Heller H.C., Sutcliffe J.G. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J. Neurosci. 1998;18(23):9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccinelli M., Wilkinson G. Gender differences in depression. Critical review. Br. J. Psychiatry. 2000;177:486–492. doi: 10.1192/bjp.177.6.486. [DOI] [PubMed] [Google Scholar]
- Raadsheer F.C., van Heerikhuize J.J., Lucassen P.J., Hoogendijk W.J., Tilders F.J., Swaab D.F. Corticotropin-releasing hormone mRNA levels in the paraventricular nucleus of patients with Alzheimer's disease and depression. Am. J. Psychiatry. 1995;152(9):1372–1376. doi: 10.1176/ajp.152.9.1372. [DOI] [PubMed] [Google Scholar]
- Rainero I., Ostacoli L., Rubino E., Gallone S., Picci L.R., Fenoglio P., Negro E., Rosso C., De Martino P., De Marchi M., Furlan P.M., Pinessi L. Association between major mood disorders and the hypocretin receptor 1 gene. J. Affect. Disord. 2011;130(3):487–491. doi: 10.1016/j.jad.2010.10.033. [DOI] [PubMed] [Google Scholar]
- Sakurai T., Amemiya A., Ishii M., Matsuzaki I., Chemelli R.M., Tanaka H. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92(5) doi: 10.1016/s0092-8674(02)09256-5. (1 page following 696) [DOI] [PubMed] [Google Scholar]
- Salomon R.M., Ripley B., Kennedy J.S., Johnson B., Schmidt D., Zeitzer J.M. Diurnal variation of cerebrospinal fluid hypocretin-1 (Orexin-A) levels in control and depressed subjects. Biol. Psychiatry. 2003;54(2):96–104. doi: 10.1016/s0006-3223(02)01740-7. [DOI] [PubMed] [Google Scholar]
- Saper C.B. The neurobiology of sleep. Continuum. 2013;19:19–31. doi: 10.1212/01.CON.0000427215.07715.73. (1 sleep disorders) [DOI] [PubMed] [Google Scholar]
- Sun H., Kennedy P.J., Nestler E.J. Epigenetics of the depressed brain: role of histone acetylation and methylation. Neuropsychopharmacology. 2013;38(1):124–137. doi: 10.1038/npp.2012.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turecki G., Ernst C., Jollant F., Labonte B., Mechawar N. The neurodevelopmental origins of suicidal behavior. Trends Neurosci. 2012;35(1):14–23. doi: 10.1016/j.tins.2011.11.008. [DOI] [PubMed] [Google Scholar]
- Wang S.S., Kamphuis W., Huitinga I., Zhou J.N., Swaab D.F. Gene expression analysis in the human hypothalamus in depression by laser microdissection and real-time PCR: the presence of multiple receptor imbalances. Mol. Psychiatry. 2008;13(8):786–799. doi: 10.1038/mp.2008.38. (41) [DOI] [PubMed] [Google Scholar]
- Watts A.G. Glucocorticoid regulation of peptide genes in neuroendocrine CRH neurons: a complexity beyond negative feedback. Front. Neuroendocrinol. 2005;26(3–4):109–130. doi: 10.1016/j.yfrne.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Winsky-Sommerer R., Yamanaka A., Diano S., Borok E., Roberts A.J., Sakurai T. Interaction between the corticotropin-releasing factor system and hypocretins (orexins): a novel circuit mediating stress response. J. Neurosci. 2004;24(50):11439–11448. doi: 10.1523/JNEUROSCI.3459-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida Y., Fujiki N., Nakajima T., Ripley B., Matsumura H., Yoneda H. Fluctuation of extracellular hypocretin-1 (orexin A) levels in the rat in relation to the light-dark cycle and sleep-wake activities. Eur. J. Neurosci. 2001;14(7):1075–1081. doi: 10.1046/j.0953-816x.2001.01725.x. [DOI] [PubMed] [Google Scholar]
- Zeitzer J.M., Duffy J.F., Lockley S.W., Dijk D.J., Czeisler C.A. Plasma melatonin rhythms in young and older humans during sleep, sleep deprivation, and wake. Sleep. 2007;30(11):1437–1443. doi: 10.1093/sleep/30.11.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J., Bao A.M., Qi X.R. Gene expression of GABA and glutamate pathway markers in the prefrontal cortex of non-suicidal elderly depressed patients. J. Affect. Disord. 2012;138(3):494–502. doi: 10.1016/j.jad.2012.01.013. [DOI] [PubMed] [Google Scholar]
- Zhou J.N., Riemersma R.F., Unmehopa U.A., Hoogendijk W.J., van Heerikhuize J.J., Hofman M.A. Alterations in arginine vasopressin neurons in the suprachiasmatic nucleus in depression. Arch. Gen. Psychiatry. 2001;58(7):655–662. doi: 10.1001/archpsyc.58.7.655. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Clinico-pathological information of subjects for prefrontal cortex study (material obtained from the Netherlands Brain Bank).
Clinico-pathological information of subjects for prefrontal cortex study (material obtained from the Stanley Medical Research Institue).
Sequence of the primers, gene bank accession numbers, the size of amplified product for the target genes and reference genes.