Abstract
Maternal vitamin A intake varies but its impact on offspring metabolic health is unknown. Here we found that maternal vitamin A or retinoic acid (RA) administration expanded PDGFRα+ adipose progenitor population in progeny, accompanied by increased blood vessel density and enhanced brown-like (beige) phenotype in adipose tissue, protecting offspring from obesity. Blockage of retinoic acid signaling by either BMS493 or negative RA receptor (RARαDN) over-expression abolished the increase in blood vessel density, adipose progenitor population, and beige adipogenesis stimulated by RA. Furthermore, RA-induced beige adipogenesis was blocked following vascular endothelial growth factor receptor (VEGFR) 2 knock out in PDGFRα+ cells, suggesting its mediatory role. Our data reveal an intrinsic link between maternal retinoid level and offspring health via promoting beige adipogenesis. Thus, enhancing maternal retinoids is an amiable therapeutic strategy to prevent obesity in offspring, especially for those born to obese mothers which account for one third of all pregnancies.
Keywords: Maternal care, Retinoic acid, Vitamin A, Adipose development, Beige adipocyte, Vasculature
Highlights
-
•
Maternal vitamin A supplementation increases blood vessel density and expands adipose progenitor population in progeny.
-
•
Maternal vitamin A supplementation enhances brown-like phenotype in adipose tissues.
-
•
Maternal vitamin A supplementation protects offspring from diet induced obesity.
Vitamin A and its metabolite, retinoic acid, play key roles in adipogenesis and energy expenditure of adipose tissues. In mice and humans, vitamin A intake is inversely correlated with adiposity. This study has uncovered a role for maternal retinoids in fetal adipose development. Maternal vitamin A supplementation or RA administration increases adipose progenitor population and promotes beige adipogenesis, which protects offspring from diet induced obesity in later life.
1. Introduction
With metabolic syndromes such as obesity yielding widespread contemporary concern, the race is on for discovering strategies which can ameliorate conditions detrimental to human health. Nutrigenomics is an emerging field of science aiming to address such conditions by studying the molecular interplay between a diet and the genome. Evidence shows that adipose development at the fetal stage have long term effects in later life. In mice, subcutaneous white fat (WAT) (Wang et al., 2013) and brown fat (BAT) (Billon and Dani, 2012) develop during the fetal stage. Maternal nutritional status during gestation epigenetically alters WAT differentiation (Borengasser et al., 2013). Zfp423, a gene encoding a transcription factor for preadipocyte commitment, is hypomethylated in fetuses born to obese mothers (Yang et al., 2013). Moreover, the population of adipose progenitor cells (Liang et al., 2016a) and the thermogenic function of BAT (Liang et al., 2016b) of offspring are altered due to maternal intake of high fat diet. Up to now, the relationship between early adipose development and obesity in later life remains poorly understood.
White adipocytes are healthy at normal sizes, but over-expansion of adipocyte sizes lead to hypoxia (Sun et al., 2011), inflammation, and interstitial fibrosis (Sun et al., 2013), which triggers adipose metabolic dysfunction (Sun et al., 2014b). Beige adipocytes, which distribute inside white adipose tissue, burn fatty acids to reduce adipocyte hypotrophy. The vascular system acts as an adipogenic niche by providing PDGFRα+ progenitors, which are able to differentiate into both beige and white adipocytes (Lee et al., 2012). Adipose tissue are highly vascularized (Cao, 2007), and adipogenesis is spatially and temporally associated with angiogenesis during fetal development (Cao, 2007), which provides PDGFRα+ progenitor cells (Crisan et al., 2008, Tran et al., 2012, Vishvanath et al., 2016). Thus, promoting beige adipogenesis of PDGFRα+ progenitors improves the metabolic health of adipose tissue.
Vitamin A and its metabolite, retinoic acid (RA), play key roles in fetal morphogenesis and organ development (Zile, 2001, Duester, 2008), and is a common supplement used during pregnancy. At pharmacological doses, RA increases energy consumption of white adipose tissue (Alvarez et al., 1995, Puigserver et al., 1996, Bonet et al., 2003, Mercader et al., 2006), suggesting that RA might promote beige adipogenesis in mature WAT. Vitamin A or RA supplementation is effective in preventing obesity in adult mice (Berry et al., 2012, Noy, 2013). In humans, overweight and obese individuals showed lower retinoid levels in serum (de Souza Valente da Silva et al., 2007, Pereira et al., 2012) and dietary vitamin A intake is inversely related with adiposity (Zulet et al., 2008). However, the effect of maternal vitamin A intake on beige adipogenesis during fetal development is unclear, which represents a critical knowledge gap because the fetal and neonatal stages are critical for adipogenesis. The long-term impact of maternal retinoid status on adipose metabolic health of offspring remains unclear. We hypothesized that maternal vitamin A supplementation promotes angiogenesis and beige adipogenesis during fetal development, which improve the metabolic health of offspring adipose tissue, protecting offspring from diet-induced obesity and metabolic dysfunction.
Currently, 190 million preschool-age children and 19.1 million pregnant women worldwide are at risk of vitamin A deficiency (WHO, 2009). This indicates that a large population of people do not have enough vitamin A intake, especially in the low-income countries. Thus, dietary vitamin A supplementation is expected to be beneficial. However, at very high supplementation levels, vitamin A may be harmful (Ritchie et al., 1998). In rodents, a dose of 75,000 IU/day/rat maternal vitamin A intake is teratogenic (Cohlan, 1954). In humans, a daily dose of 10,000 IU or a weekly dose of up to 25,000 IU during pregnancy are considered to be safe (WHO, 1998), which is a quite high threshold compared to the Recommended Dietary Allowance (RDA) for vitamin A at 700 μg/day or about 2333 IU/day during pregnancy (U.S. Department of Agriculture). Therefore, there is a knowledge gap on the effects of vitamin A at levels moderately above normal intake on fetal development. Our data demonstrate that maternal administration of vitamin A and RA during pregnancy and lactation at physiologically relevant levels enhances beige adipogenesis during early development, which protects offspring from diet-induced obesity and metabolic syndrome in later life.
2. Material and Methods
2.1. Antibodies and Chemicals
Antibodies against β-tubulin (#2146), cytochrome c (#4280) were purchased from Cell Signaling (Danvers, MA). Antibodies against UCP1 (Cat. No. PA1-24894) and PRDM16 (Cat. No. PA5-20872) were bought from TheromoFisher Scientific (Waltham, MA). Antibodies against PDGFRα (Cat. No. 1062-PR) and VEGFR2 (Cat. No. AF644) were bought from R&D. Alexa Fluor 488 anti-mouse CD309 (Cat. No. 136408), APC anti-mouse CD140a (Cat. No. 135908), PerCP/Cy5.5 anti-mouse Sca-1 (Cat. No. 108124), PE/Cy7 anti-mouse CD45 (Cat. No. 103114) were bought from Biolegend (San Diego, CA).
1,1′-Dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (42364), Tamoxifen (T5648), all-trans-Retinoic acid (R2625), insulin (I3536), dexamethasone (D4902), 3-isobutyl-1-methylxanthine (I5878), Triiodothyronine (T3) (IRMM469) and Oil-Red O (O0625) were purchased from Sigma (St Louis, MO, USA). BMS493 (Cat. No. 3509) were purchased from Tocris Bioscience (Ellisville, MO). Mouse recombinant VEGF165 (Cat. No. 583106) was purchased from Biolegend. Vitamin A (M4068, retinyl acetate, water soluble) was purchased from MP Biomedicals, LLC.
2.2. Mice
All animal studies were conducted in AAALAC-approved facilities and according to protocols approved by the Institutional Animal Care and Use Committee (IACUC). Wild-type (WT) C57BL/6 mice, Pdgfra-Cre-ER (stock number: 018280), Vegfr2e3loxP/e3loxP (stock number: 018977) and Gt(ROSA)26Sortm4(ACTB-tdTomato,-EGFP)Luo reporter (mT/mG) mice (stock number: 007676) were purchased from the Jackson Laboratory (Bar Harbor, ME). ROSA26-RARαDN mice were provided by Dr. Cathy Mendelsohn (Rosselot et al., 2010). Mice were mated overnight and the day of vaginal plug detection was marked as E0.5. Retinoic acid, BMS493 or vehicle (DMSO) dissolved in corn oil were injected to pregnant mice twice (10 mg/kg BW) at E10.5 and E13.5. Genetic recombination was induced by one injection of 20 mg/kg BW tamoxifen. For the maternal vitamin A supplementation, 6 litters were obtained for each treatment, and the pups in each litter were adjusted to 6 at birth. The pups were weighed at birth (male) and weaning (male). Twelve male pups in each group were euthanized at weaning for sample collection. Tissues from each litter (2 males) were pooled to get 6 samples for qRT-PCR or western blot analysis. Six male offspring in each group were further treated with HFD. For the RA/BMS493 administration during pregnancy, 6 litters were obtained for each treatment, and the pups in each litter were adjusted to 6 at birth. Six weanling male offspring in each group were euthanized for sample collection and further analysis. For the transgenic mouse lines, 6 weanling male mice in each group were euthanized for sample collection and further analysis.
A commercial diet (2018 Teklad Global) containing 15 IU/g Vitamin A (retinyl acetate which is water soluble) was used. In the current study, the pregnancy mice drank about 3 ml/day. The vitamin A provided by water (3000 IU/kg/day) is about twice of the vitamin A content in the diet. This dose is far below the lowest toxicity dose for rodent (~ 163,000 IU/kg/day) (Ritchie et al., 1998). To avoid oxidation, vitamin A solution was changed daily.
2.3. Tissue Processing and Histology
For adipose tissue structure and immunostaining, tissues were fixed in 4% paraformaldehyde (PFA) for 12 h at 4 °C then used for paraffin embedding, sectioning and H&E or immunostaining. For immunostaining, sections were heated in citrate buffer for 20 min, blocked with 5% goat serum in TBS containing 0.3% Triton X-100 for 2 h, then incubated sequentially with primary antibodies overnight and secondary antibodies for 1 h. Sections were then mounted in a mounting medium (Vector Lab, Burlingame, CA). H&E stained sections were used for measuring adipocyte size and number using Image J. Blood vessels were labeled according to a published protocol (Li et al., 2008). Briefly, mice were euthanized by CO2, then sequentially perfused with PBS, 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI) and 4% PFA into left ventricle (the right atrium was open). Tissues were then sectioned for further whole mount staining or imaging. For whole mount staining, tissues were blocked with 5% goat serum in TBS containing 1% Triton X-100 and 0.2% sodium azide for 2 h, then incubated sequentially with primary antibodies for 4 days and secondary antibodies for 2 days. The PDGFRα tracking mice were perfused with PBS and 4% PFA after euthanized by CO2. Tissues were then sectioned for direct examination and imaging under a Leica TCS SP8 confocal microscope, or tissues were used for further whole mount staining. The confocal microscope images were processed to create 3D Videos using Fiji Imaging Processing Package (Schindelin et al., 2012).
2.4. Isolation of Adipose-Derived Stromal Vascular Cells (SVCs)
Inguinal and epididymal fats were isolated, then washed with phosphate-buffered saline (PBS). Tissues were cut into small pieces and digested in digestion buffer containing 0.75 U/ml collagenase D (Roche, Pleasanton, CA) and 1.0 U/ml Dipase type II (Roche) for 30 min at 37 °C. The lysate was filtered sequentially through 100 μm cell strainers, then centrifuged for 5 min at 500g. The precipitated cells were then re-suspended and seeded in culture plates.
2.5. Oxygen Consumption Assay
Oxygen consumption was measured using a Thermo Scientific Orion 3-Star Dissolved Oxygen meter (Thermo Electron Corporation, Madison, WI). Fresh culture medium was added to the plates, and the dissolved oxygen concentration was measured at the start and after 30 min incubation.
2.6. Brown Adipogenesis Induction and Oil-red O Staining
Stromal vascular cells at 100% confluence were cultured in 1%FBS/DMEM medium for 2 days, and differentiation was then induced by 2 μg/ml insulin and 2 nM T3 for 6 days. For the P19 cells, hanging drops containing 103 P19 cells in 25 μl medium were maintained for 3 days on the lids of tissue culture dishes. The embryoid bodies (EB) formed were then transferred into bacteriological dishes in suspension in a medium supplemented with 1000 nM all-trans RA for 3 days (Dani et al., 1997). Embryoid bodies were then transferred to tissue culture plates in presence of differentiation medium supplemented with 2 μg/ml insulin and 2 nM T3. Differentiated Cells were fixed in 4% paraformaldehyde for 10 min at room temperature, rinsed 3 times with PBS. Fixed cells were stained with Oil-Red O solution (Sigma-Aldrich,) for 10 min then rinsed with PBS to remove excessive Oil-Red O dye. Following microscopic observation, Oil-Red O dye retained in cells was solubilized with isopropanol and the light absorbance measured at 510 nm.
2.7. Metabolic Chamber Analyses
The basal metabolic rate (BMR) (oxygen consumption (VO2), CO2 production (VCO2), respiratory exchange ratio (RER) and heat production) of mice during the day (quiescent phase) and the night (active phase) were measured using a CLAMS (Columbus Instruments, Columbus, OHIO) indirect open circuit calorimetry system. Mice were housed singly in CLAMS cages at 25 °C for 12 h and data were record every 30 s.
2.8. Thermal Image and Surface Temperature
Pups were taken away from the dams and put in a 6-wells-plates individually at 25 °C. The thermal images were taken by a FLIR E6 thermal imaging camera (FLIR System, Wilsonville, Oregon) and the surface temperature was quantified by the FLIR Tools. The core body temperature (oral cavity) was measured using a TH-5 Thermalert Monitoring Thermometer (Physitemp Instruments, Inc., Clifton, New Jersey).
2.9. Glucose Tolerance Test
Following 6 h-fasting, mice were administered 2 g/kg BW d-glucose. Blood samples were collected from the tail veil at 0, 15, 30, 60 and 90 min post injection and glucose concentration was measured using a glucose meter (Bayer Contour, Tarrytown, NY, USA).
2.10. High Performance Liquid Chromatography (HPLC) Analysis
Serum retinol (ROL), RA and retinaldehyde (RAL) were measured by HPLC using a reverse phase column (Luna 3 μm C18(2) 100 Å, LC Column 150 × 3 mm). The mobile phase, methanol/H2O (65/35) was pumped at 1.0 ml/min.
2.11. Flow Cytometry Analysis
Stromal vascular cells were isolated as previously described, then resuspended in PBS with 2.5% FBS, and stained with antibodies for 30 min at 4 °C. Stained cells were then sorted and analyzed by FACSAria Flow Cytometer (BD Biosciences, San Jose, CA).
2.12. Real-time Quantitative PCR (qRT-PCR)
Total mRNA was extracted from tissue or cells using TRIzol reagent (Sigma, St. Louis, MO) followed by DNase treatment, and reverse transcribed into cDNA using a kit (Qiagen, Valencia, CA). RT-PCR was performed using an iQ5 RT-PCR detection system (Bio-Rad Laboratories, Hercules, CA). A SYBR Green RT-PCR kit from Bio-Rad Laboratories (Hercules, CA) was used for PCR. Relative expression of mRNA was determined after normalization to 18S reference using ΔΔ − Ct method.
2.13. Immunoblotting Analysis
Immunoblotting were conducted as previously described using the Odyssey Infrared Imaging System (LI-COR Biosciences) (Yan et al., 2010), and beta-tubulin were used as the reference.
2.14. Statistical Analyses
Results were analyzed using two-tailed Student's t-test or one-way ANOVA (Bonferroni test was performed for differences between means) where appropriate, using SAS 9.0 (SAS Institute Inc., Cary, NC). All data were found to be normally distributed. Significance was accepted at p < 0.05. All data are reported as mean ± SEM.
3. Results
3.1. Maternal Vitamin A Supplementation Promotes Beige Adipogenesis of Offspring at Birth and Weaning
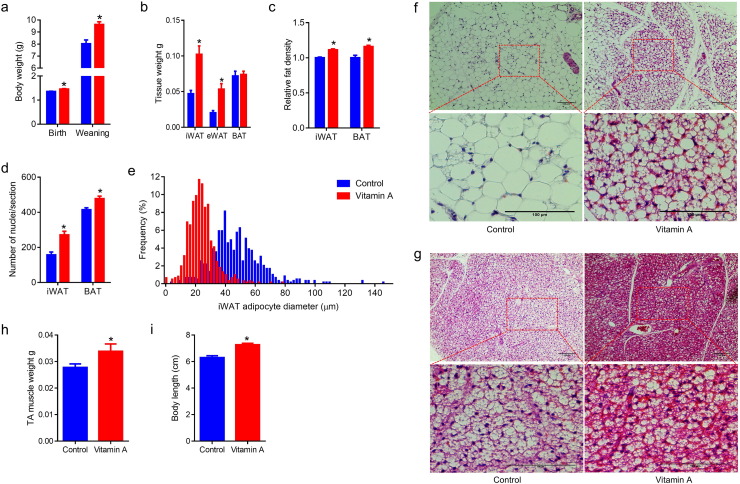
Adipogenesis is separated into adipogenic commitment and differentiation. Vitamin A and retinoic acid are well known to inhibit lipid accumulation (Jeyakumar et al., 2006) and adipogenic differentiation in preadipose cell lines (Castro-Munozledo et al., 1987, Kamei et al., 1994). At the same time, RA induces adipogenic commitment of embryonic stem cells (Dani et al., 1997). To determine the effects of retinoids on adipogenesis during the embryonic stage, pregnant C57BL/6 mice were supplemented with 0 or 30 IU/ml vitamin A through water. During the gestation and lactation stages, vitamin A supplementation didn't affect food intake of the dams (Fig. S1a). At birth and weaning, maternal vitamin A supplemented (MVA) offspring had higher body weight (Fig. 1a). At weaning, MVA offspring had larger inguinal adipose tissue (iWAT) and epididymal adipose tissue (eWAT) mass (Fig. 1b). When normalized to body weight, MVA offspring had a higher ratio of white adipose tissue (WAT) and a lower ratio of brown adipose tissue (BAT) (Fig. S1b). Interestingly, despite increased fat mass, MVA offspring had higher iWAT and BAT density, which was likely caused by lower lipid content (Fig. 1c). Furthermore, more adipocytes per unit area were observed in iWAT, BAT and eWAT of MVA offspring (Figs. 1d, f, g and S1c, d), which were likely due to adipocyte hyperplasia. Consistently, the adipocyte sizes in iWAT (Fig. 1e) and eWAT (Fig. S1e) of MVA offspring were much smaller than those of the control offspring. Consistent with the increased density, the BAT of MVA offspring was stained darker than the control (Fig. 1g). Furthermore, maternal vitamin A increased the Tibialis anterior (TA) muscle weight (Fig. 1h) and body length (tail included, Fig. 1i) of weanling offspring, suggesting the global effects of maternal vitamin A on the development of offspring. In summary, these data show that MVA induced adipocyte hyperplasia and reduced average adipocyte sizes in both white and brown adipose tissues.
Fig. 1.
Maternal vitamin A supplementation affects adipose tissue deposition and morphology. Adult C57BL/6 females during gestation and lactation were supplemented with 0 or 30 IU/ml vitamin A through water (designated as MVA experiment). (a) Body weight of offspring at birth and weaning. (b) Adipose tissue weight. (c) iWAT and BAT density. (d) Average number of nuclei per section. (e) Distribution of adipocytes size in iWAT. (f) Representative images of H&E stained iWAT. (g) Representative images of H&E stained BAT. (h) TA muscle weight. (i) Body length. Data presented are mean ± SEM, n = 6, unpaired two-tail t-test, *p < 0.05.
3.2. Maternal Vitamin A Supplementation Prevents Obesity of Adult Offspring Mice Challenged with a High Fat Diet
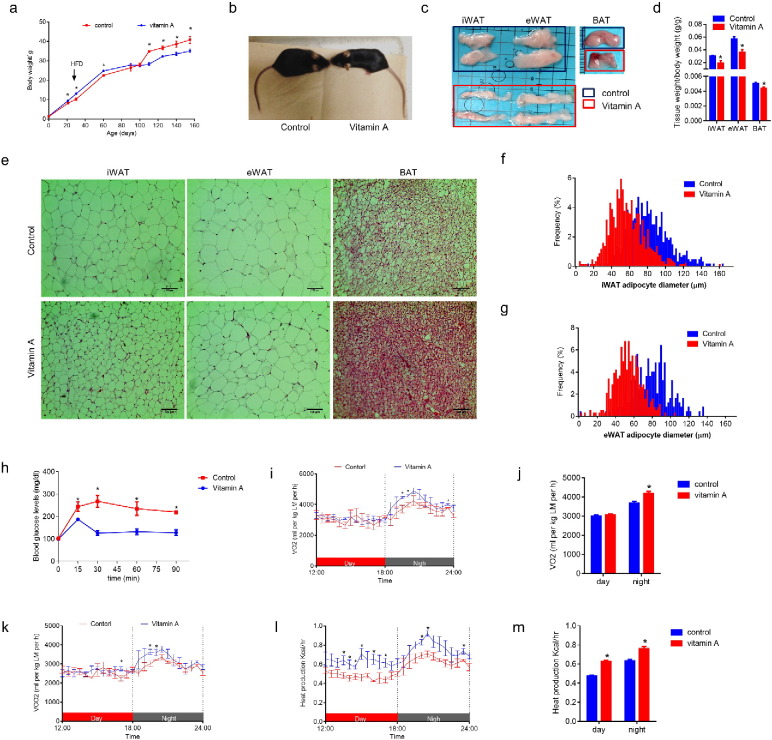
To further explore the long-term effects of MVA on offspring health, offspring of MVA and control mice were fed a high fat diet (HFD, fat 45 kcal%) for 5 months starting at 30 days of age. MVA treated mice had a higher body weight until 100 days of age, when the body weight of control mice exceeded (Fig. 2a). At necropsy, the MVA mice showed less accumulation of white and brown fat mass (Fig. 2c and d). The higher initial body weight was higher likely due to the global increase of tissue growth (fat, muscle and bone), but the later lower fat weight was due to reduced lipid accumulation in these mice. Indeed, the size of adipocytes was much smaller in MVA mice compared to their control counterparts (Fig. 2e–g). Before feeding the high fat diet, there was no difference in glucose intolerance (Fig. S2a). But after 5 months on a HFD, control mice became obese (Fig. 2a and b) and showed glucose intolerance, showing metabolic dysfunction, which was not observed in MVA mice (Fig. 2h). In addition, MVA mice showed higher O2 consumption during the night (Fig. 2i and j), higher CO2 production (Fig. 2k) and higher heat production during both day and night (Fig. 2l and m), which should account for a lower ratio of weight gain/food intake in MVA (Fig. S2d). In summary, these data show that maternal vitamin A supplementation enhances metabolic rate and prevents the development of obesity in offspring challenged with HFD.
Fig. 2.
Maternal vitamin A supplementation prevents offspring obesity induced by high fat diet. MVA offspring were fed a high fat diet (HFD, 45% energy from fat) from 30 days to 155 days old. (a) Growth curve of offspring fed with HFD. (b) Representative images of mice after HFD feeding. (c) Representative images of iWAT, eWAT and BAT tissues after HFD feeding. (d) Tissue weight/body weight ratio. (e) Representative images of fat tissue sections, stained by H&E. (f) Distribution of adipocyte sizes in iWAT. (g) Distribution of adipocyte sizes in eWAT. (h) Glucose tolerance test of offspring HFD feeding. (i–m) Oxygen consumption (i and j), CO2 production (k) and heat production (l and m) were measured in a metabolic chamber. Data presented are mean ± SEM, n = 6, unpaired two-tail t-test, *p < 0.05.
3.3. Maternal Vitamin A Supplementation Promotes Brown/Beige Adipogenesis in Both White and Brown Adipose Tissue
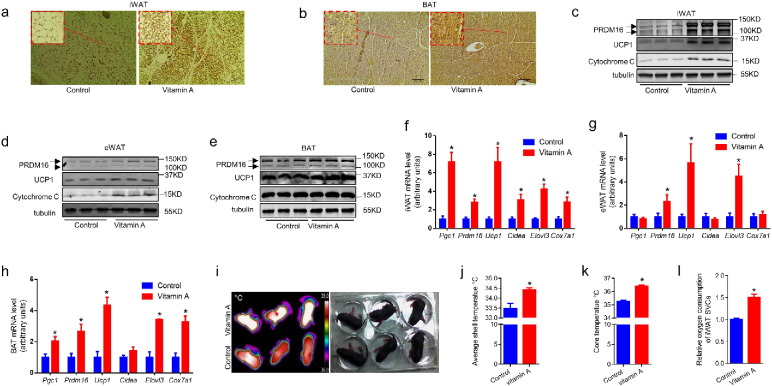
To explore the mechanisms that MVA prevented HFD induced obesity, the adipose tissue characteristics of offspring were further examined. At weaning, MVA increased the number of UCP-1 positive multilocular beige adipocytes in iWAT (Fig. 3a). MVA also increased UCP-1 expression in BAT (Fig. 3b). The contents of proteins enriched in BAT including PRDM16, UCP1 and Cytochrome C were higher in MVA mice (Fig. 3c–e). Consistently, the mRNA levels of brown adipose genes including Pgc1a, Prdm16, UCP1, Cidea, Elovl3 and Cox7a1 were higher in the adipose tissues of MVA offspring (Fig. 3f–h). Furthermore, the surface temperature (Fig. 3i, j) and core body temperature (Fig. 3k) of MVA offspring was higher than control mice, showing enhanced thermogenesis. Moreover, the SVCs isolated from iWAT of MVA offspring had higher oxygen consumption when compared to that of the control offspring (Fig. 3l). These data show that MVA enhanced brown and beige adipocyte function in offspring.
Fig. 3.
Maternal vitamin A supplementation promotes brown/beige adipogenesis in both white and brown adipose tissues. MVA offspring were fed a high fat diet (HFD, 45% energy from fat) from 30 days to 155 days old. (a–b) Immunohistochemistry (IHC) images of iWAT (a) and BAT (b) using anti-UCP1 antibody. (c–e) Brown adipose protein contents in iWAT (c), eWAT (d) and BAT (e) analyzed by western blot. (f–h) Brown adipose gene mRNA levels in iWAT (f, eWAT (g) and BAT (h) analyzed by qRT-PCR. (i–j) Thermal images of MVA treated offspring at 9 days old were captured by a thermal camera (i), the average surface temperature was analyzed (j). (k) Core body temperature. (l) Relative oxygen consumption of SVCs isolated from iWAT of control and MVA offspring. Panel f–h and k–l: n = 6, panel j: n = 3. Data presented are mean ± SEM, unpaired two-tail t-test, *p < 0.05.
Consistent with enhanced brown adipogenesis, MVA had elevated expression of pre-adipose genes (Pref1, Sox9 and Klf2) (Fig. S3a, b) and mature adipocyte markers including Pparg, Glut4, Cebpb and Fabp4 (Fig. S3c, d). These data align with a previous study showing that RA upregulates preadipose genes in mice (Berry et al., 2012). MVA also upregulated the expression of nuclear receptors Rara, Rarb and Rxra (Fig. S3e). MVA regulated the expression of Ehmt1 (Fig. S3e) which controls brown adipogenesis by stabilizing PRDM16 (Ohno et al., 2013). On the other hand, MVA downregulated the expression of Tle3 (Fig. S3e), which is a white adipogenesis-selective cofactor (Villanueva et al., 2013). Taken together, MVA promoted beige and brown adipogenesis in brown and white adipose tissues.
3.4. Retinoic Acid Signaling is Required for Fetal Adipose Tissue Development
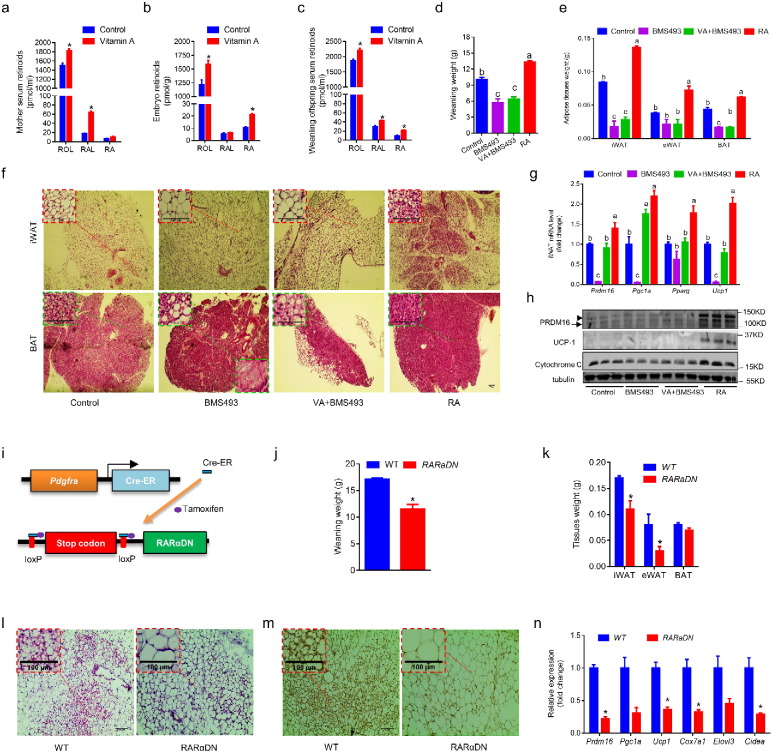
MVA increased retinoid levels in maternal mice (Fig. 4a), embryos (Fig. 4b) and the weanling offspring (Fig. 4c). Retinoic acid plays an important role in vertebrate organogenesis (Duester, 2008). To investigate the effects of RA signaling on adipose tissue development, pregnant mice were intraperitoneally injected with 10 mg/kg body weight RA, a pan-retinoic acid receptor inverse agonist BMS493 or vehicle (DMSO) at E10.5 and E13.5, or a combination of BMS493 injections and vitamin A supplementation (30 IU/ml) during gestation. BMS493 treated offspring had lower birth weight (Fig. S4a) and severely impaired postnatal growth (Fig. 4d and S4b), while RA treatment increased birth weight (Fig. S4a) and weanling weight of offspring (Fig. 4d and S4b). Consistent with the MVA data, RA increased both white and brown adipose mass (Fig. 4e and S4c), while BMS493 reduced (Fig. 4e and S4c). RA decreased lipid droplet size in both iWAT and BAT, and BMS493 had the opposite effects (Fig. 4f). Moreover, RA dramatically increased the mRNA levels of Prdm16, Pgc1a, Pparg and UCP1 (Fig. 4g) and the protein contents of PRDM16, UCP1 and Cytochrome C in iWAT (Fig. 4h). Notably, RA only slightly increased mRNA of Prdm16 (Fig. 4g) but dramatically increased PRDM16 protein (Fig. 4h) in iWAT, which was likely due to the enhanced expression of Ehmt1 stabilizing PRDM16 (Ohno et al., 2013). Consistent with data obtained from maternal vitamin A supplementation, RA administration during the fetal stage increased adipose tissue mass with enhanced brown phenotype.
Fig. 4.
Retinoic acid signaling is required for fetal adipose tissue development. (a) Retinoid levels in mother mice. (b) Retinoid levels in E18.5 fetuses (whole tissue lysate). (c) Retinoid levels in weanling offspring. (d–h) Pregnant mice were injected with BMS493 or RA at E10.5 and 13.5, one BMS493 treated group was supplemented with vitamin A during gestation. Samples were collected at weaning. Body weight (d) and adipose tissue weight (e) of offspring was measured at weaning. Adipose tissue sections were stained by H&E (f). Expression of brown adipose genes were analyzed by qRT-PCR (g) or western blot (h). (i–n) RARαDN expression was conditionally induced in PDGFRα+ cells at E10.5 by an injection of 20 mg/kg BW tamoxifen to the pregnant mothers (i). Offspring were euthanized at weaning, and body weight (j) and adipose tissue weight (k) were measured. iWAT and BAT tissue sections were stained by H&E (l) and IHC using anti-UCP1 antibody (m). Brown adipose genes expression were analyzed by qRT-PCR (n). Data presented are mean ± SEM, n = 6, unpaired two-tail t-test (a–c, j–k and n) *p < 0.05 or ANOVA (d–e and g), bars with different letters differ significantly.
To further confirm the role of RA signaling in mediating adipose tissue development, we used ROSA26-RARαDN (RARα403) and Pdgfra-cre/ER crossed mice. In these mice, when administered with tamoxifen, RARα403 is specifically expressed in PDGFRα+ progenitors, which blocked normal RARα function (Fig. 4i). Tamoxifen was administered at E10.5 to the mothers. The Pdgfra-Cre/ER+/−/RARαDN+/+ pups (expressing dominant negative RARα403 in PDGFRα + cells, which blocks RA signaling, RARαDN) were smaller than the Pdgfra-Cre/ER−/−/RARαDN+/+ ones (expressing normal RARα, WT) (Fig. 4j and S4d). RARαDN reduced the white and brown fat mass (Fig. 4k) and the population of beige adipocytes in iWAT (Fig. 4l). IHC showed less UCP1+ cells in iWAT of Pdgfra-Cre/ER+/−/RARαDN+/+ offspring (Fig. 4m). Furthermore, the expression of Pgc1a, Prdm16, UCP1, Cidea, Elovl3 and Cox7a1 was reduced (Fig. 4n). Collectively, these data show that RAR mediates the effect of maternal retinoids on beige adipogenesis of offspring.
3.5. Maternal Retinoic Acid Promotes Vasculature in Adipose Tissues
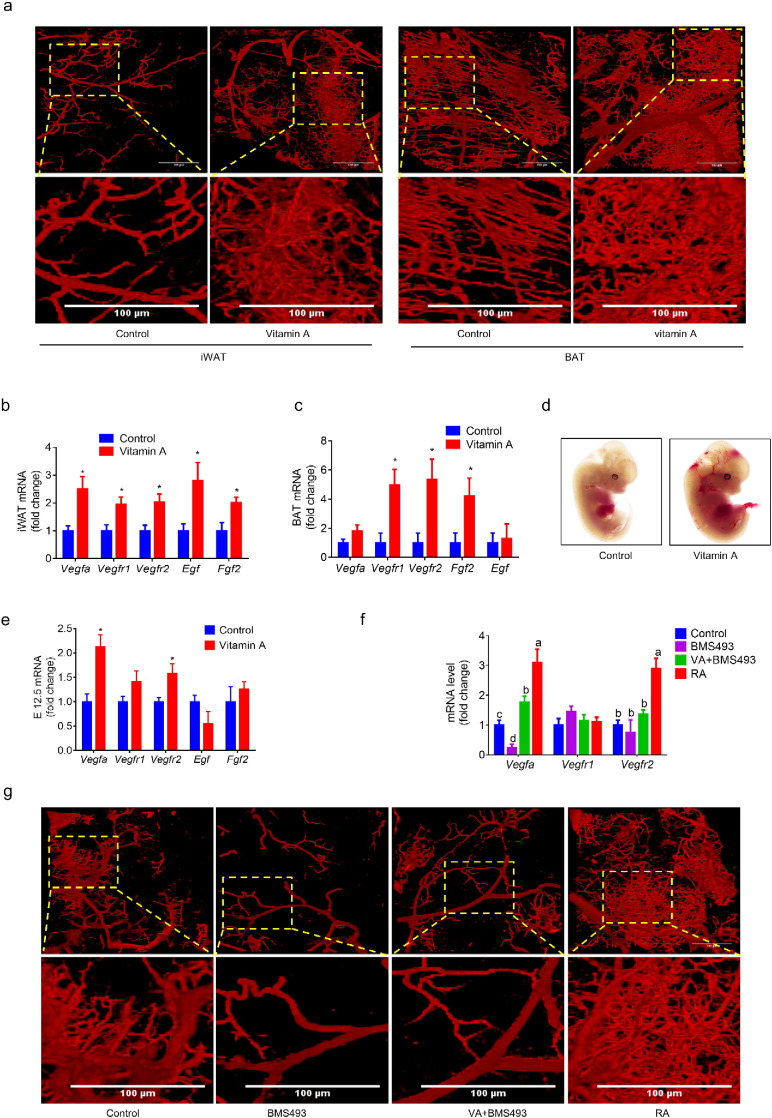
Adipogenesis is spatially and temporally associated with vascular development (Cao, 2007). Several types of endothelial related cells can differentiate into adipocytes (Tran et al., 2012, Min et al., 2016, Vishvanath et al., 2016). To explore the effects of maternal vitamin A on vascular development, we labeled blood vessels of weanling offspring using a lipophilic carbocyanine dye, 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI), which incorporates into endothelial cell membrane upon contact (Li et al., 2008). MVA dramatically increased blood vessel density in both white and brown adipose tissues of the offspring (Fig. 5a and S5a). Vitamin A supplementation also enhanced the expression of angiogenic genes including Vegfa, Vegfr1, Vegfr2, Fgf2 and epidermal growth factor (Egf) in adipose tissues (Fig. 5b, c and S5b). Furthermore, at E12.5, MVA fetuses had more visible blood vessels (Fig. 5d) and higher expression of angiogenic genes including Vegfa and Vegfr2 (Fig. 5e). No difference in iWAT Hif1a (Fig. S5c) was observed, indicating that MVA promotes angiogenesis in a hypoxia-independent manner. Consistently, RA administration at E10.5 increased blood vessel density (Fig. 5g) and expression of Vegfa and Vegfr2 (Fig. 5f) in weanling offspring while BMS493 had the opposite effects. Because higher blood vessel density was also observed in TA muscle of MVA mice (Fig. S5a), vitamin A or RA may have a widespread influence on angiogenesis of tissues. These data show that maternal vitamin A supplementation or RA administration promotes vascularization in adipose tissues of offspring.
Fig. 5.
Maternal retinoic acid promotes vasculature in adipose tissues. (a) Blood vessels in iWAT and BAT stained by DiI. (b) mRNA levels of angiogenic genes in iWAT. (c) mRNA levels of angiogenic genes in BAT. (d) Representative images of E12.5 embryos. (e) mRNA levels of angiogenic genes in E12.5 embryos. (f) mRNA levels of angiogenic genes in iWAT. (g) Blood vessels in iWAT and BAT of RA or BMS493 treated offspring at weaning. Data presented are mean ± SEM, n = 6, unpaired two-tail t-test (b, c and e) *p < 0.05 or ANOVA (f), bars with different letters differ significantly.
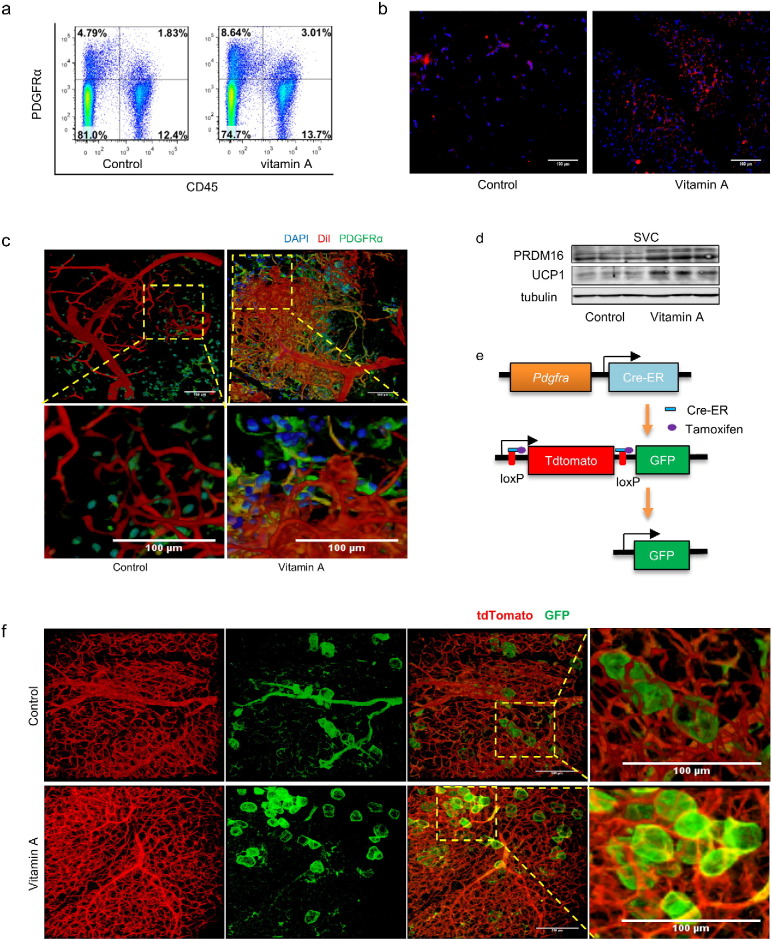
Consistent with enhanced vascularization, we found that MVA increased the PDGFRα+ CD45− population, a pool of adipogenic progenitors which are capable to differentiate into both white and brown adipocytes (Fig. 6a and S6a), as confirmed by immunohistochemical staining of both iWAT and eWAT tissue sections (Fig. 6b and S6b). The DiI labeled iWAT were further stained with an anti-PDGFRα antibody, which showed more abundant PDGFRα+ cells in iWAT of MVA mice located on or around the small blood vessels (Fig. 6c and S6c, Videos S1–4). To further confirm the effects of MVA on adipose progenitor populations, SVCs were isolated from iWAT and brown adipogenesis was induced. More adipocytes were formed in MVA cells than control cells (Fig. S6d), consistent with enhanced PRDM16 and UCP1 contents in MVA cells (Fig. 6d). To test the adipogenic potential in vivo, we generated a PDGFRα tracking mouse line, where, following tamoxifen treatment, cells derived from PDGFRα+ cells emanate green fluorescence (Fig. 6e). Two months old MVA or control offspring were put in 4 °C for 1 week to induce beige adipogenesis. More PDGFRα+ cells-derived beige adipocytes were found in iWAT of MVA offspring (Fig. 6f and Videos S5–6). In summary, maternal vitamin A promotes vascularization and increases beige adipocyte progenitor populations in offspring adipose tissues.
Fig. 6.
Maternal vitamin A increases PDGFRα+ population in adipose tissue. (a–d) For MVA weanling offspring. The population of PDGFRα+ cells in iWAT was analyzed by flow cytometry (a) (numbers in the quadrant indicate the percentages of cells) and IHC using anti-PDGFRα antibody (b). DiI labeled iWAT was further stained using anti-PDGFRα antibody (c). Brown adipose protein contents in iWAT-derived SVCs after 6 days of brown adipogenesis were analyzed (d). (e–f) PDGFRα+ cells in PDGFRα tracking mice were labeled by GFP in presence of tamoxifen (e). PDGFRα+ cells-derived adipocytes in iWAT after 1 week of cold stimulus were observed under confocal microscope (f).
3.6. RA Promotes Beige Adipogenesis by Enhancing VEGF Signaling
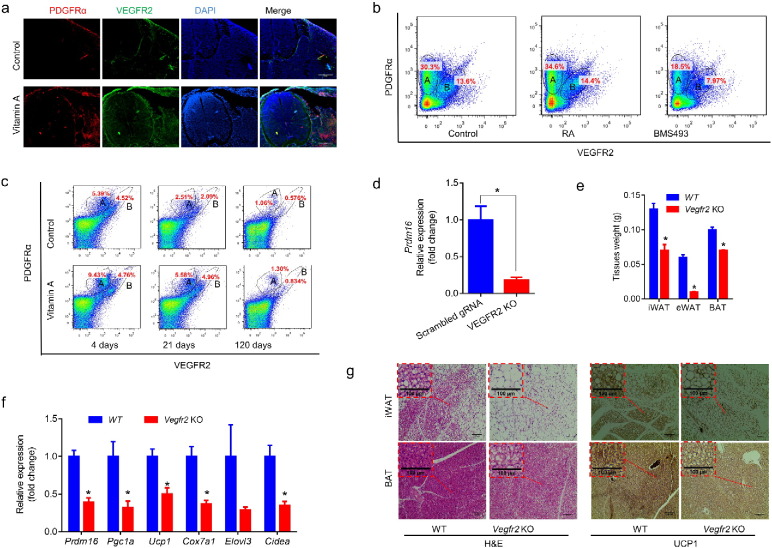
At E12.5 fetal stage, we found MVA increased both PDGFRα+ and VEGFR2+ population (Fig. 7a). Furthermore, two distinguished populations were observed by flow cytometry analysis of whole embryos (pre-sorted with CD45 antibody to exclude hematopoietic cells), one CD45−/PDGFRα+/VEGFR2− population (A) and another CD45−/PDGFRα+/VEGFR2+ population (B) (Fig. 7b). Two similar populations were detected in postnatal iWAT (Fig. 7c). From day 4 to day 120 after birth, the PDGFRα+-VEGFR2+ population in iWAT decreased (Fig. 7c and S7b–c). MVA increased these two populations both at prenatal and postnatal stages (Fig. 7b, c and S7a–c). These two populations might represent an uncommitted progenitor population associated with vasculature (both PDGFRα+ and VEGFR2+ positive), and an adipose lineage restricted population which was only PDGFRα+ positive.
Fig. 7.
VEGF signaling plays an important role in RA regulation of adipogenesis. (a) Representative PDGFRα and VEGFR2 immunohistochemical staining images of E12.5 embryo treated with MVA. (b) FACS analysis of PDGFRα +/VEGFR2+ cells (CD45+ cells were excluded) in iWAT of maternal RA or BMS493 treated (at E10.5) fetuses at E12.5 (numbers in the circles indicate the percentages of cells). (c) FACS analysis of PDGFRα+/VEGFR2+ cells in iWAT of weanling MVA offspring. (d) VEGFR2 was knocked out in P19 cells, then embryo bodies were formed and pretreated with RA for 3 days and, then, induced brown adipogenesis for 4 days. Prdm16 expression was analyzed by qRT-PCR (n = 3). (e–g) Vegfr2 were knocked out in PDGFRα+ cells at E10.5 by an injection of 20 mg/kg BW tamoxifen to the pregnant mothers. Adipose tissue weight was measured at weaning (e, n = 6). Brown adipose gene expression in iWAT was analyzed by qRT-PCR (f, n = 6). Adipose tissue sections were stained by H&E or IHC using anti-UCP1 antibody (g). Data presented are mean ± SEM, unpaired two-tail t-test, *p < 0.05.
To determine the role of VEGFR2 in brown adipogenesis during early embryonic development, Vegfr2 was knocked down in P19 cells using CRISPR/Cas9 system then cultured to form embryonic bodies with concomitant induction of brown adipogenesis. VEGFR2 deficiency reduced Prdm16 expression (Fig. 7d), suggesting that VEGFR2 has a critical role in brown adipogenesis.
To further explore the role of VEGF signaling in RA-stimulated brown adipogenesis, we administered 10 mg/kg BW SU5416, an inhibitor of VEGFR, to the mothers at E10.5, which stopped embryo development (Fig. S7d). We also conditionally knocked out Vegfr2 (Vegfr2e3loxP/e3loxP) in PDGFRα+ (Pdgfra-Cre-ER) cells (Fig. S7e) at E10.5 by tamoxifen, and the pregnant mice were fed with 30 IU/ml vitamin A during gestation. Vegfr2 null mice had lower body weight (Fig. S7f), smaller fat mass (Fig. 7e and S7g) and lower expression of brown adipose genes including Prdm16, Pgc1a, Ucp1, Cox7a1, Elovl3 and Cidea (Fig. 7f). Although there was no difference in Vegfa expression, Pdgfra-Cre-ER+/−/Vegfr2+/+ mice had lower expression of Vegfr2 in iWAT when compared with Pdgfra-Cre-ER−/−/Vegfr2+/+ mice (Fig. S7h). The lower expression of Vegfr2 was likely due to the fact that Pdgfra expressing cells accounted only a portion of cells. With Vegfr2 specifically knocked out in PDGFRα+ cells, less beige adipocytes were detected in iWAT (Fig. 7g) and eWAT (Fig. S7i) and the density of BAT (Fig. 7g) became lower, showing that VEGFR signaling promotes beige adipogenesis of PDGFRα+ progenitor cells. Taken together, these data show that VEGFA-VEGFR2 signaling mediates RA stimulated beige adipogenesis in PDGFRα+ progenitor cells.
4. Discussion
In this study, we found that maternal retinoid supplementation profoundly enhances brown/beige adipogenesis during fetal development, which has long-term effect on BAT and beige phenotype in offspring, and protects offspring from diet-induced obesity. This is an exciting discovery considering the easiness of vitamin A supplementation and the wide existence of vitamin A deficiency worldwide (WHO, 2009), particularly in low income countries. Low income correlates with obesity and metabolic diseases (Pan et al., 2013).
We identified that maternal retinoid status affects fetal and offspring brown/beige adipogenesis through promoting angiogenesis. Maternal vitamin A or RA supplementation enhances angiogenesis through upregulating Vegfa and Vegfr2 expression, which consequently increased the population of PDGFRα+ adipose progenitor cells in adipose tissue. Our data are consistent the enhanced angiogenesis in both white and brown adipose tissues of mice exposed to cold stimulus, where Vegfr2 blockage abolishes the cold-induced angiogenesis and impairs nonshivering thermogenesis capacity (Xue et al., 2009). Although it remains controversial whether beige adipocytes are generated by de novo adipogenesis or conversion of existing adipocytes (Rosenwald et al., 2013, Wang et al., 2013), progenitor cells on endothelial vessels are capable to differentiate into beige adipocytes (Tran et al., 2012, Min et al., 2016, Vishvanath et al., 2016). Obesity and diabetes impair the angiogenic potential of adipose tissue stem cells (Rennert et al., 2014, Togliatto et al., 2016), and stimulation of angiogenesis via Vegfa over-expression promotes adipose tissue thermogenesis and protects against diet-induced obesity (Wu et al., 2011, Sun et al., 2014a). Thus, enhancing angiogenesis is an effective strategy to promote beige adipogenesis in adipose tissue impaired due to obesity and diabetes.
Besides stimulating angiogenesis, our data also show that maternal vitamin A or RA supplementation up-regulates brown/beige adipogenesis of progenitor cells, which is associated with enhanced expression of Prdm16 and other brown adipocyte genes. Using mice with conditional Vegfr2 knockout specifically in PDGFRα+ progenitor cells, the promotion effect of RA on brown adipogenesis was severely reduced, demonstrating an angiogenesis independent effect of RA on beige adipogenesis. These observations are consistent with previous studies showing that the RA increases oxidation and energy consumption of white adipose tissue in mature animals (Alvarez et al., 1995, Puigserver et al., 1996, Bonet et al., 2003, Mercader et al., 2006). Thus, maternal RA stimulates both angiogenesis and beige adipogenesis during early development, which are mediated by RAR because its functional knockout blocks brown/beige adipogenesis.
Besides beige adipogenesis, increasing the progenitor pool in adipose tissues through angiogenesis has another advantage. It is known that PDGFRα+ progenitor cells are the source of both beige and white adipocytes (Lee et al., 2012, Lee et al., 2013, Lee et al., 2015). Thus, enhancing PDGFRα+ progenitor cell pool will not only increase beige but also white adipogenesis, as shown by the increased expression of white preadipose genes in MVA WAT of this study. Adipose tissue is the organ to store fat, and an insufficient number of adipocytes leads to adipocyte hypertrophy, hypoxia and inflammation, a key cause of metabolic dysfunction (Rosen and Spiegelman, 2014). Thus, adipocyte hyperplasia has protective effects on metabolic dysfunction induced by excessive energy intake. Consistently, there is one subgroup of people who are metabolically health despite being obese, while others exhibit severe metabolic syndromes (Denis and Obin, 2013). People who are called “metabolically healthy obese” (MHO) tend to have smaller adipocytes (Kloting et al., 2010) and higher mitochondrial transcription (Naukkarinen et al., 2014). These individuals have reduced visceral adiposity, reduced inflammation, improved glucose and lipid homeostasis when compared to other equally obese unhealthy subjects (Denis and Obin, 2013). Based on our discovery, maternal vitamin A or RA supplementation increases the progenitor pool in offspring, which reduces average adipocyte sizes and increases adipocyte hyperplasia, improving overall metabolic health of offspring.
In conclusion, offspring adipose tissue health is substantially improved due to maternal vitamin A or RA supplementation. Maternal vitamin A promotes vascular system development, which consequently increases the population of PDGFRα+ adipose progenitor cells. In addition, maternal vitamin A supplementation strongly upregulates beige adipogenesis of PDGFRα+ progenitor cells. In combination, maternal vitamin A treated offspring have increased beige adipogenesis and smaller adipocyte sizes, which protect offspring against diet-induced obesity and metabolic dysfunction.
The following are the supplementary data related to this article.
Supplementary material
Blood vessel and PDGFRα+ cells in iWAT of control offspring.
Blood vessel and PDGFRα+ cells in iWAT of MVA offspring.
Blood vessel and PDGFRα+ cells in eWAT of control offspring.
Blood vessel and PDGFRα+ cells in eWAT of MVA offspring.
Blood vessel and PDGFRα+ cells derived adipocytes in iWAT of control offspring after exposed to 4 °C for 1 week.
Blood vessel and PDGFRα+ cells derived adipocytes in iWAT of MVA offspring after exposed to 4 °C for 1 week.
Funding Sources
The work was funded by NIH R01HD067449 and R21AG049976, and the National Institute of Food and Agriculture (2015-67015-23219), U.S. Department of Agriculture, under award number 2015-67015-23219.
Conflicts of Interest
The authors declare no conflict of interest.
Author Contributions
BW and MD conceived the project, designed the experiments and wrote the manuscript. BW, XF, XWL, ZXW, QYY, TDZ, WN, JXZ and PFG performed the experiments. JMA assisted with the mouse experiments. JM and BDR assisted with the metabolic rate analyses. MJZ contributed to discussion and reviewed and edited the manuscript.
Acknowledgments
We thank Ann Norton in University of Idaho for assistance in FACS, and Dr. Cathy Mendelsohn for providing the RARαDN mice.
References
- Alvarez R., de Andres J., Yubero P., Vinas O., Mampel T., Iglesias R., Giralt M., Villarroya F. A novel regulatory pathway of brown fat thermogenesis. Retinoic acid is a transcriptional activator of the mitochondrial uncoupling protein gene. J. Biol. Chem. 1995;270(10):5666–5673. doi: 10.1074/jbc.270.10.5666. [DOI] [PubMed] [Google Scholar]
- Berry D.C., DeSantis D., Soltanian H., Croniger C.M., Noy N. Retinoic acid upregulates preadipocyte genes to block adipogenesis and suppress diet-induced obesity. Diabetes. 2012;61(5):1112–1121. doi: 10.2337/db11-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billon N., Dani C. Developmental origins of the adipocyte lineage: new insights from genetics and genomics studies. Stem Cell Rev. 2012;8(1):55–66. doi: 10.1007/s12015-011-9242-x. [DOI] [PubMed] [Google Scholar]
- Bonet M.L., Ribot J., Felipe F., Palou A. Vitamin A and the regulation of fat reserves. Cell. Mol. Life Sci. 2003;60(7):1311–1321. doi: 10.1007/s00018-003-2290-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borengasser S.J., Zhong Y., Kang P., Lindsey F., Ronis M.J., Badger T.M., Gomez-Acevedo H., Shankar K. Maternal obesity enhances white adipose tissue differentiation and alters genome-scale DNA methylation in male rat offspring. Endocrinology. 2013;154(11):4113–4125. doi: 10.1210/en.2012-2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y. Angiogenesis modulates adipogenesis and obesity. J. Clin. Invest. 2007;117(9):2362–2368. doi: 10.1172/JCI32239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Munozledo F., Marsch-Moreno M., Beltran-Langarica A., Kuri-Harcuch W. Commitment of adipocyte differentiation in 3T3 cells is inhibited by retinoic acid, and the expression of lipogenic enzymes is modulated through cytoskeleton stabilization. Differentiation. 1987;36(3):211–219. doi: 10.1111/j.1432-0436.1987.tb00195.x. [DOI] [PubMed] [Google Scholar]
- Cohlan S.Q. Congenital anomalies in the rat produced by excessive intake of vitamin A during pregnancy. Pediatrics. 1954;13(6):556–567. [PubMed] [Google Scholar]
- Crisan M., Yap S., Casteilla L., Chen C.W., Corselli M., Park T.S., Andriolo G., Sun B., Zheng B., Zhang L., Norotte C., Teng P.N., Traas J., Schugar R., Deasy B.M., Badylak S., Buhring H.J., Giacobino J.P., Lazzari L., Huard J., Peault B. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3(3):301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Dani C., Smith A.G., Dessolin S., Leroy P., Staccini L., Villageois P., Darimont C., Ailhaud G. Differentiation of embryonic stem cells into adipocytes in vitro. J. Cell Sci. 1997;110:1279–1285. doi: 10.1242/jcs.110.11.1279. [DOI] [PubMed] [Google Scholar]
- de Souza Valente da Silva L., Valeria da Veiga G., Ramalho R.A. Association of serum concentrations of retinol and carotenoids with overweight in children and adolescents. Nutrition. 2007;23(5):392–397. doi: 10.1016/j.nut.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Denis G.V., Obin M.S. ‘Metabolically healthy obesity’: origins and implications. Mol. Asp. Med. 2013;34(1):59–70. doi: 10.1016/j.mam.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duester G. Retinoic acid synthesis and signaling during early organogenesis. Cell. 2008;134(6):921–931. doi: 10.1016/j.cell.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeyakumar S.M., Vajreswari A., Giridharan N.V. Chronic dietary vitamin A supplementation regulates obesity in an obese mutant WNIN/Ob rat model. Obesity (Silver Spring) 2006;14(1):52–59. doi: 10.1038/oby.2006.7. [DOI] [PubMed] [Google Scholar]
- Kamei Y., Kawada T., Mizukami J., Sugimoto E. The prevention of adipose differentiation of 3T3-L1 cells caused by retinoic acid is elicited through retinoic acid receptor alpha. Life Sci. 1994;55(16):PL307–PL312. doi: 10.1016/0024-3205(94)90073-6. [DOI] [PubMed] [Google Scholar]
- Kloting N., Fasshauer M., Dietrich A., Kovacs P., Schon M.R., Kern M., Stumvoll M., Bluher M. Insulin-sensitive obesity. Am. J. Physiol. Endocrinol. Metab. 2010;299(3):E506–E515. doi: 10.1152/ajpendo.00586.2009. [DOI] [PubMed] [Google Scholar]
- Lee Y.H., Petkova A.P., Granneman J.G. Identification of an adipogenic niche for adipose tissue remodeling and restoration. Cell Metab. 2013;18(3):355–367. doi: 10.1016/j.cmet.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y.H., Petkova A.P., Konkar A.A., Granneman J.G. Cellular origins of cold-induced brown adipocytes in adult mice. FASEB J. 2015;29(1):286–299. doi: 10.1096/fj.14-263038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y.H., Petkova A.P., Mottillo E.P., Granneman J.G. In vivo identification of bipotential adipocyte progenitors recruited by beta3-adrenoceptor activation and high-fat feeding. Cell Metab. 2012;15(4):480–491. doi: 10.1016/j.cmet.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Song Y., Zhao L., Gaidosh G., Laties A.M., Wen R. Direct labeling and visualization of blood vessels with lipophilic carbocyanine dye DiI. Nat. Protoc. 2008;3(11):1703–1708. doi: 10.1038/nprot.2008.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X., Yang Q., Fu X., Rogers C.J., Wang B., Pan H., Zhu M.J., Nathanielsz P.W., Du M. Maternal obesity epigenetically alters visceral fat progenitor cell properties in male offspring mice. J. Physiol. 2016;594(15):4453–4466. doi: 10.1113/JP272123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X., Yang Q., Zhang L., Maricelli J.W., Rodgers B.D., Zhu M.J., Du M. Maternal high-fat diet during lactation impairs thermogenic function of brown adipose tissue in offspring mice. Sci. Rep. 2016;6:34345. doi: 10.1038/srep34345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercader J., Ribot J., Murano I., Felipe F., Cinti S., Bonet M.L., Palou A. Remodeling of white adipose tissue after retinoic acid administration in mice. Endocrinology. 2006;147(11):5325–5332. doi: 10.1210/en.2006-0760. [DOI] [PubMed] [Google Scholar]
- Min S.Y., Kady J., Nam M., Rojas-Rodriguez R., Berkenwald A., Kim J.H., Noh H.L., Kim J.K., Cooper M.P., Fitzgibbons T., Brehm M.A., Corvera S. Human 'brite/beige' adipocytes develop from capillary networks, and their implantation improves metabolic homeostasis in mice. Nat. Med. 2016;22(3):312–318. doi: 10.1038/nm.4031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naukkarinen J., Heinonen S., Hakkarainen A., Lundbom J., Vuolteenaho K., Saarinen L., Hautaniemi S., Rodriguez A., Fruhbeck G., Pajunen P., Hyotylainen T., Oresic M., Moilanen E., Suomalainen A., Lundbom N., Kaprio J., Rissanen A., Pietilainen K.H. Characterising metabolically healthy obesity in weight-discordant monozygotic twins. Diabetologia. 2014;57(1):167–176. doi: 10.1007/s00125-013-3066-y. [DOI] [PubMed] [Google Scholar]
- Noy N. The one-two punch: retinoic acid suppresses obesity both by promoting energy expenditure and by inhibiting adipogenesis. Adipocyte. 2013;2(3):184–187. doi: 10.4161/adip.23489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno H., Shinoda K., Ohyama K., Sharp L.Z., Kajimura S. EHMT1 controls brown adipose cell fate and thermogenesis through the PRDM16 complex. Nature. 2013;504(7478):163. doi: 10.1038/nature12652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan L., May A.L., Wethington H., Dalenius K., Grummer-Strawn L.M. Incidence of obesity among young US children living in low-income families, 2008–2011. Pediatrics. 2013:2013–2145. doi: 10.1542/peds.2013-2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira S.E., Saboya C.J., Saunders C., Ramalho A. Serum levels and liver store of retinol and their association with night blindness in individuals with class III obesity. Obes. Surg. 2012;22(4):602–608. doi: 10.1007/s11695-011-0522-y. [DOI] [PubMed] [Google Scholar]
- Puigserver P., Vazquez F., Bonet M.L., Pico C., Palou A. In vitro and in vivo induction of brown adipocyte uncoupling protein (thermogenin) by retinoic acid. Biochem. J. 1996;317(Pt 3):827–833. doi: 10.1042/bj3170827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennert R.C., Sorkin M., Januszyk M., Duscher D., Kosaraju R., Chung M.T., Lennon J., Radiya-Dixit A., Raghvendra S., Maan Z.N., Hu M.S., Rajadas J., Rodrigues M., Gurtner G.C. Diabetes impairs the angiogenic potential of adipose-derived stem cells by selectively depleting cellular subpopulations. Stem Cell. Res. Ther. 2014;5(3):79. doi: 10.1186/scrt468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie H.E., Webster W.S., Eckhoff C., Oakes D.J. Model predicting the teratogenic potential of retinyl palmitate, using a combined in vivo/in vitro approach. Teratology. 1998;58(3–4):113–123. doi: 10.1002/(SICI)1096-9926(199809/10)58:3/4<113::AID-TERA7>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Rosen E.D., Spiegelman B.M. What we talk about when we talk about fat. Cell. 2014;156(1–2):20–44. doi: 10.1016/j.cell.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenwald M., Perdikari A., Rulicke T., Wolfrum C. Bi-directional interconversion of brite and white adipocytes. Nat. Cell Biol. 2013;15(6):659–667. doi: 10.1038/ncb2740. [DOI] [PubMed] [Google Scholar]
- Rosselot C., Spraggon L., Chia I., Batourina E., Riccio P., Lu B., Niederreither K., Dolle P., Duester G., Chambon P., Costantini F., Gilbert T., Molotkov A., Mendelsohn C. Non-cell-autonomous retinoid signaling is crucial for renal development. Development. 2010;137(2):283–292. doi: 10.1242/dev.040287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindelin J., Arganda-Carreras I., Frise E., Kaynig V., Longair M., Pietzsch T., Preibisch S., Rueden C., Saalfeld S., Schmid B., Tinevez J.Y., White D.J., Hartenstein V., Eliceiri K., Tomancak P., Cardona A. Fiji: an open-source platform for biological-image analysis. Nat. Methods. 2012;9(7):676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun K., Kusminski C.M., Luby-Phelps K., Spurgin S.B., An Y.A., Wang Q.A., Holland W.L., Scherer P.E. Brown adipose tissue derived VEGF-A modulates cold tolerance and energy expenditure. Mol. Metab. 2014;3(4):474–483. doi: 10.1016/j.molmet.2014.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun K., Kusminski C.M., Scherer P.E. Adipose tissue remodeling and obesity. J. Clin. Invest. 2011;121(6):2094–2101. doi: 10.1172/JCI45887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun K., Park J., Gupta O.T., Holland W.L., Auerbach P., Zhang N., Goncalves Marangoni R., Nicoloro S.M., Czech M.P., Varga J., Ploug T., An Z., Scherer P.E. Endotrophin triggers adipose tissue fibrosis and metabolic dysfunction. Nat. Commun. 2014;5:3485. doi: 10.1038/ncomms4485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun K., Tordjman J., Clement K., Scherer P.E. Fibrosis and adipose tissue dysfunction. Cell Metab. 2013;18(4):470–477. doi: 10.1016/j.cmet.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Togliatto G., Dentelli P., Gili M., Gallo S., Deregibus C., Biglieri E., Iavello A., Santini E., Rossi C., Solini A., Camussi G., Brizzi M.F. Obesity reduces the pro-angiogenic potential of adipose tissue stem cell-derived extracellular vesicles (EVs) by impairing miR-126 content: impact on clinical applications. Int. J. Obes. (Lond.) 2016;40(1):102–111. doi: 10.1038/ijo.2015.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran K.V., Gealekman O., Frontini A., Zingaretti M.C., Morroni M., Giordano A., Smorlesi A., Perugini J., De Matteis R., Sbarbati A., Corvera S., Cinti S. The vascular endothelium of the adipose tissue gives rise to both white and brown fat cells. Cell Metab. 2012;15(2):222–229. doi: 10.1016/j.cmet.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Agriculture, A. R. S What We Eat in America. 2007–2008. https://www.ars.usda.gov/northeast-area/beltsville-md/beltsville-human-nutrition-research-center/food-surveys-research-group/docs/wweia-data-tables/
- Villanueva C.J., Vergnes L., Wang J., Drew B.G., Hong C., Tu Y., Hu Y., Peng X., Xu F., Saez E., Wroblewski K., Hevener A.L., Reue K., Fong L.G., Young S.G., Tontonoz P. Adipose subtype-selective recruitment of TLE3 or Prdm16 by PPARgamma specifies lipid storage versus thermogenic gene programs. Cell Metab. 2013;17(3):423–435. doi: 10.1016/j.cmet.2013.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vishvanath L., MacPherson K.A., Hepler C., Wang Q.A., Shao M., Spurgin S.B., Wang M.Y., Kusminski C.M., Morley T.S., Gupta R.K. Pdgfrbeta + mural Preadipocytes contribute to adipocyte hyperplasia induced by high-fat-diet feeding and prolonged cold exposure in adult mice. Cell Metab. 2016;23(2):350–359. doi: 10.1016/j.cmet.2015.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q.A., Tao C., Gupta R.K., Scherer P.E. Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nat. Med. 2013;19(10):1338–1344. doi: 10.1038/nm.3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . Safe Vitamin A Dosage during Pregnancy and Lactation: Recommendations and Report of a Consultation. World Health Organization; 1998. Safe vitamin A dosage during pregnancy and lactation: recommendations and report of a consultation. [Google Scholar]
- WHO . World Health Organization; 2009. Global Prevalence of Vitamin A Deficiency in Population at Risk: 1995-2005.http://www.who.int/vmnis/database/vitamina/x/en/ 09/25/2014. [Google Scholar]
- Wu J., Hansen J.M., Hao L., Taylor R.N., Sidell N. Retinoic acid stimulation of VEGF secretion from human endometrial stromal cells is mediated by production of reactive oxygen species. J. Physiol. 2011;589(Pt 4):863–875. doi: 10.1113/jphysiol.2010.200808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue Y., Petrovic N., Cao R., Larsson O., Lim S., Chen S., Feldmann H.M., Liang Z., Zhu Z., Nedergaard J., Cannon B., Cao Y. Hypoxia-independent angiogenesis in adipose tissues during cold acclimation. Cell Metab. 2009;9(1):99–109. doi: 10.1016/j.cmet.2008.11.009. [DOI] [PubMed] [Google Scholar]
- Yan X., Zhu M.J., Xu W., Tong J.F., Ford S.P., Nathanielsz P.W., Du M. Up-regulation of Toll-like receptor 4/nuclear factor-kappaB signaling is associated with enhanced adipogenesis and insulin resistance in fetal skeletal muscle of obese sheep at late gestation. Endocrinology. 2010;151(1):380–387. doi: 10.1210/en.2009-0849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q.Y., Liang J.F., Rogers C.J., Zhao J.X., Zhu M.J., Du M. Maternal obesity induces epigenetic modifications to facilitate Zfp423 expression and enhance adipogenic differentiation in fetal mice. Diabetes. 2013;62(11):3727–3735. doi: 10.2337/db13-0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zile M.H. Function of vitamin A in vertebrate embryonic development. J. Nutr. 2001;131(3):705–708. doi: 10.1093/jn/131.3.705. [DOI] [PubMed] [Google Scholar]
- Zulet M.A., Puchau B., Hermsdorff H.H.M., Navarro C., Martinez J.A. Vitamin A intake is inversely related with adiposity in healthy young adults. J. Nutr. Sci. Vitaminol. (Tokyo) 2008;54(5):347–352. doi: 10.3177/jnsv.54.347. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Blood vessel and PDGFRα+ cells in iWAT of control offspring.
Blood vessel and PDGFRα+ cells in iWAT of MVA offspring.
Blood vessel and PDGFRα+ cells in eWAT of control offspring.
Blood vessel and PDGFRα+ cells in eWAT of MVA offspring.
Blood vessel and PDGFRα+ cells derived adipocytes in iWAT of control offspring after exposed to 4 °C for 1 week.
Blood vessel and PDGFRα+ cells derived adipocytes in iWAT of MVA offspring after exposed to 4 °C for 1 week.