ABSTRACT
Health care-onset health care facility-associated Clostridium difficile infection (HO-CDI) is overdiagnosed for several reasons, including the high prevalence of C. difficile colonization and the inability of hospitals to limit testing to patients with clinically significant diarrhea. We conducted a quasiexperimental study from 22 June 2015 to 30 June 2016 on consecutive inpatients with C. difficile test orders at an academic hospital. Real-time electronic patient data tracking was used by the laboratory to enforce testing criteria (defined as the presence of diarrhea [≥3 unformed stools in 24 h] and absence of laxative intake in the prior 48 h). Outcome measures included C. difficile test utilization, HO-CDI incidence, oral vancomycin utilization, and clinical complications. During the intervention, 7.1% (164) and 9.1% (211) of 2,321 C. difficile test orders were canceled due to absence of diarrhea and receipt of laxative therapy, respectively. C. difficile test utilization decreased upon implementation from an average of 208.8 tests to 143.0 tests per 10,000 patient-days (P < 0.001). HO-CDI incidence rate decreased from an average of 13.0 cases to 9.7 cases per 10,000 patient-days (P = 0.008). Oral vancomycin days of therapy decreased from an average of 13.8 days to 9.4 days per 1,000 patient-days (P = 0.009). Clinical complication rates were not significantly different in patients with 375 canceled orders compared with 869 episodes with diarrhea but negative C. difficile results. Real-time electronic clinical data tracking is an effective tool for verification of C. difficile clinical testing criteria and safe reduction of inflated HO-CDI rates.
KEYWORDS: Clostridium difficile, data tracking, evidence-based medicine, verification
INTRODUCTION
Clostridium difficile is the most common identifiable cause of antibiotic-associated diarrhea (1). Clostridium difficile infection (CDI) is estimated to be the most expensive health care-associated infection, costing the U.S. health system $5.4 billion annually (2). A major challenge with accurate diagnosis of CDI is the high prevalence of C. difficile colonization. Studies indicate that 4.4% to 21% of hospitalized patients are asymptomatically colonized with toxigenic C. difficile (3–10, 30). In the absence of disease-specific diagnostic biomarkers to accurately distinguish between colonization and disease, American guidelines define a CDI case as the presence of diarrhea (i.e., 3 or more episodes of unformed stools over 24 or fewer consecutive hours) and laboratory evidence of toxigenic C. difficile in stool (11). European guidelines recommend multistep testing algorithms with an emphasis on detection of free fecal toxins in symptomatic patients to confirm CDI and to avoid the requirement for three unformed stools (12). Inclusion of diarrhea in the diagnostic algorithm has been shown to improve the specificity of C. difficile diagnostics (13). Most clinical laboratories have recommendations and clinical criteria for appropriate testing. However, current laboratory rejection criteria are inadequate to stop inappropriate testing and distinguish patients with clinically significant diarrhea from those with transient loose bowel movements. In routine practice, providers often test for C. difficile despite the absence of diarrhea. In North American studies, diarrhea has been reported to be absent in up to 39.0% of patients tested for C. difficile and up to 66.5% of patients diagnosed with CDI (13–17). Laxative therapy was also documented in up to 50% of patients undergoing testing or diagnosed with CDI in some studies (13, 14, 16). Furthermore, in some studies, 100% of patients with positive C. difficile results were treated for CDI whether diarrhea was present or not (16, 18). Combined with the fact that most cases of nosocomial diarrhea have a noninfectious etiology (19), current testing practices lead to inappropriate C. difficile diagnosis and overtreatment. Given that provider education is often insufficient to improve ordering practice (16), novel interventions are needed to improve appropriate C. difficile testing.
In an era of hospital information systems with real-time patient data tracking capabilities, it is possible to utilize clinical data to verify the presence of clinical testing criteria in patients undergoing C. difficile testing. The aims of this study were to implement and evaluate the impact of real-time electronic patient data tracking for verification of C. difficile testing criteria in hospitalized patients based on the presence of diarrhea and the absence of laxative intake.
RESULTS
Compliance with the intervention testing criteria.
Of the 2,321 C. difficile test orders received for 1,457 hospitalized patients during the postintervention period, overall, 16.2% (375 patients) were canceled by the clinical microbiology laboratory for not meeting the intervention testing criteria, and 7.1% (164) and 9.1% (211) were canceled due to the absence of diarrhea and to administration of laxative therapy, respectively (Table 1). Real-time data tracking enabled the laboratory to cancel 84.3% (375) of 445 C. difficile orders not meeting the intervention testing criteria. Laboratory personnel noncompliance with the intervention testing criteria decreased during the first three 4-week periods, reaching and remaining at <10%, with the exception of period 7, which coincided with the Christmas holiday (Fig. 1). In contrast, provider (i.e., physician and nurse) noncompliance with the intervention criteria (i.e., order placed and sample collected despite the patient not meeting the criteria) persisted at 18% on average and did not improve in trend over the course of the study (Fig. 1).
TABLE 1.
C. difficile orders received and accepted for testing during the postintervention period
| Subject parameter | Value(s) |
|
|---|---|---|
| Received orders | Accepted orders | |
| Median age (yrs) (IQ range)a | 60.8 (47.0–70.9) | 60.8 (46.4–71.0) |
| Sex (% female) | 47.3 | 48.3 |
| No. (%) with unformed stool | 2,018 | 1,631 (80.8) |
| ≥3 in 24 h | 1,000 | 990 (99.0) |
| <3 in 24 h | 224 | 48b (21.4), 12c (5.4) |
| ≥3 in 24 h and laxative in 48 h | 288 | 22b (7.6), 55c (19.1) |
| No. (%) hospitalized <24 h | 192 | 192 (100) |
| No. (%) with rectal tube/ostomy | 314 | 312 (99.4) |
| No. (%) with formed stool | 129 | 0 (0) |
| No. (%) with repeat in 7 days | 174 | 15 (8.6) |
| Total | 2,321 | 1,646 (70.9) |
IQ, interquartile.
Incorrectly accepted by the laboratory.
Requested by physician versus laboratory error.
FIG 1.
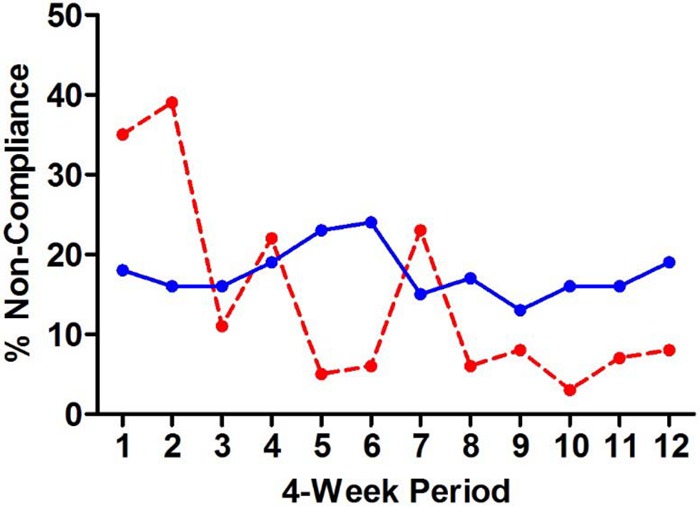
Provider and laboratory noncompliance with C. difficile testing criteria during the postintervention period. The solid blue line shows the fraction of provider test orders not meeting the intervention testing criteria. The dashed red line shows the fraction of C. difficile test orders not meeting the intervention testing criteria accepted by the laboratory for testing.
Impact of intervention.
Cancellation of 375 C. difficile test orders not meeting the intervention testing criteria reduced the accepted test volume by 18.6%. C. difficile test utilization volume in hospitalized patients decreased between the 14 preintervention and 4 postintervention periods from an average of 208.8 tests per 10,000 patient-days (95% confidence interval [CI], 203.9 to 213.7) to 143.0 tests per 10,000 patient-days (CI, 140.0 to 146.1; P < 0.001) (Fig. 2A). The HO-CDI rate decreased from an average of 13.0 cases per 10,000 patient-days (CI, 11.7 to 14.3) during the preintervention period to 9.7 cases per 10,000 patient-days (CI, 7.3 to 12.0; P = 0.008) after the intervention (Fig. 2B). Oral vancomycin days of therapy in hospitalized patients decreased from 13.8 per 1,000 patient-days (CI, 12.2 to 15.4) during the preintervention period to 9.4 per 1,000 patient-days (CI, 6.4 to 12.5; P = 0.009) during the postintervention period (Fig. 2C).
FIG 2.
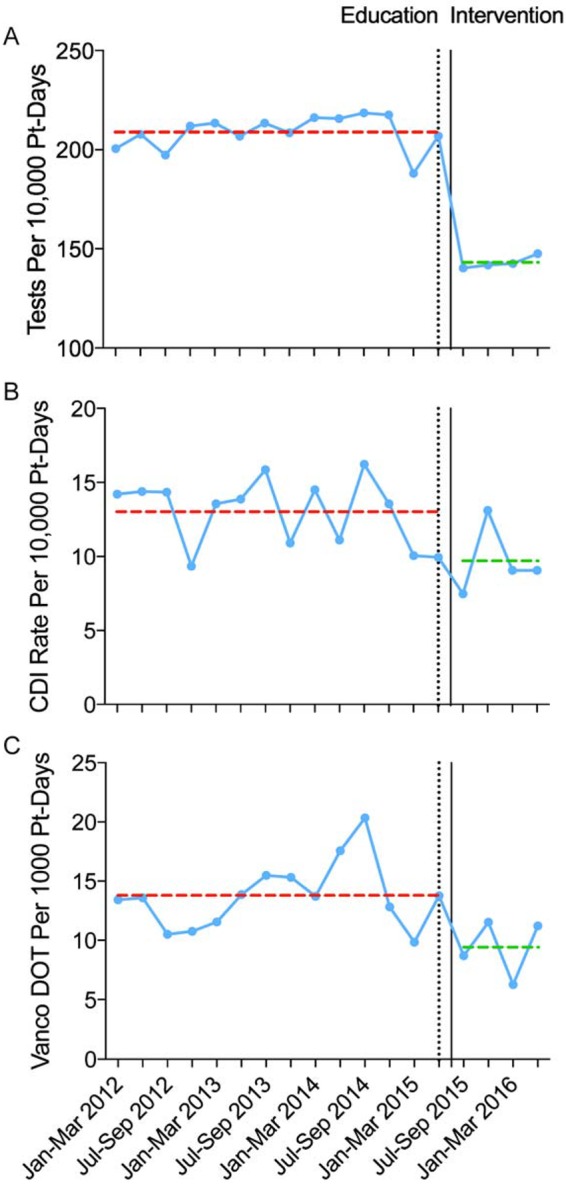
Impact of the intervention on C. difficile test utilization, incidence of HO-CDI, and oral vancomycin utilization. (A to C) C. difficile test utilization rate in hospitalized patients (A), HO-CDI rate (B), and oral vancomycin days of therapy (vanco DOT) (C) in hospitalized patients. Dotted lines show average rates during the preintervention (red) and postintervention (green) periods. Pt, patient; Jan, January; Mar, March; Jul, July; Sep, September.
Clinical outcomes.
In 329 patients with 375 episodes of canceled orders (40 patients had 2 or more orders) due to not meeting the intervention testing criteria, rates for white blood count (WBC) increase to ≥15,000 cells/ml within 7 days, admission to an intensive care unit (ICU) within 7 days, and 30-day all-cause mortality were not significantly different compared with the rates seen with 678 patients with 869 episodes of accepted orders (130 patients had 2 or more orders) that met the intervention testing criteria but tested C. difficile-negative (Table 2). The levels of overlap in 30-day follow-up in duplicate patients were 1.3% and 9.7% of rejected and accepted orders, respectively. The rate of occurrence of another episode of diarrhea within 7 days was higher in patients with accepted orders. Similar results were obtained after excluding patients empirically treated with oral vancomycin and metronidazole and those who qualified for testing within 24 h of cancellation (see Table S2 in the supplemental material). In a subanalysis of 356 patients with canceled orders and stool samples available for C. difficile testing, there were no significant differences in complications between PCR-positive (n = 53) and PCR-negative (n = 303) patients for diarrhea within 7 days (62.3% [33/53] [CI, 49.3% to 75.4%] versus 59.4% [180/303] [CI, 53.9% to 64.9%]; P = 0.76), WBC rise above 15,000 cells/ml within 7 days (10.7% [3/28] [CI, 0% to 22.2%] versus 13.2% [23/174] [CI, 8.2% to 18.2%]; P = 1.00), ICU admission within 7 days (11.3% [6/53] [CI, 2.8% to 19.8%] versus 13.5% [41/303] [CI, 9.7% to 17.4%]; P = 0.83), and 30-day all-cause mortality (11.8% [6/51] [CI, 3.0% to 20.7%] versus 9.8% [28/285] [CI, 6.4% to 13.3%]; P = 0.61).
TABLE 2.
Clinical outcomes in patients with canceled C. difficile orders
| Clinical outcome | % of patients with indicated outcome (no. of patients with indicated outcome/total no. of patients), 95% CIa |
P value | |
|---|---|---|---|
| Canceled orders (n = 375) | Accepted orders, C. difficile negative (n = 869) | ||
| Diarrhea in 7 days | 63.2 (237/375), 58.3–68.1 | 73.7 (640/869), 70.7–76.6 | <0.001 |
| WBC rise to >15,000 cells/ml in 7 days | 12.5 (27/216), 8.1–16.9 | 13.1 (73/557), 10.3–15.9 | 0.91 |
| ICU admission in 7 days | 13.1 (49/375), 9.7–16.5 | 10.5 (91/869), 8.4–12.5 | 0.20 |
| 30-day all-cause mortality | 10.3 (34/329), 7.0–13.6 | 8.3 (65/783), 6.4–10.2 | 0.30 |
Canceled-order data represent canceled orders for patients without diarrhea or with laxative intake. Accepted-order data represent accepted orders for patients with diarrhea and no laxative intake.
Empirical CDI therapy.
Empirical oral vancomycin or metronidazole therapy rates within 24 h of a canceled order in 274 patients whose orders were canceled as a consequence of not meeting the intervention testing criteria were significantly different from those seen with 790 patients who met testing criteria but tested C. difficile negative (1.8% [5/274] [CI, 0.2% to 3.4%] versus 0.3% [3/1,079] [CI, −0.1% to 0.6%); P = 0.014) (Table 3).
TABLE 3.
Analysis of empirical anti-C. difficile therapy given to patients within 24 h of order cancellation or test result
| Therapy | % (no.) of patients with indicated outcome, 95% CI |
||||
|---|---|---|---|---|---|
| Canceled orders (n = 274) | Accepted orders |
||||
| C. difficile negative (n = 790) | P value | C. difficile positive (n = 103) | P value | ||
| Vancomycin | 1.8 (5), 0.2–3.4 | 0.1 (1), −0.1–0.4 | 0.005 | 41.7 (43), 32.2–51.2 | <0.001 |
| Metronidazole | 0.7 (2), −0.3–1.7 | 0.1 (1), −0.1–0.4 | 0.17 | 68.9 (71), 60.0–77.8 | <0.001 |
| Vancomycin or metronidazole | 1.8 (5), 0.2–3.4 | 0.3 (2), −0.1–0.6 | 0.014 | 96.1 (99), 92.5–99.9 | <0.001 |
a Canceled-order data represent canceled orders for patients without diarrhea or with laxative intake after excluding patients who qualified for testing within 24 h of cancellation and patients who were on any empirical therapy within 24 h before cancellation. Accepted-order data represent accepted orders for patients with diarrhea and no laxative intake after excluding C. difficile-negative and C. difficile-positive patients who were on any empirical therapy within 24 h before resulting. P values are for comparisons to canceled orders.
DISCUSSION
We describe implementation of real-time electronic patient data tracking to verify the presence of clinical diagnostic criteria in patients with C. difficile test orders. Our institution was able to enforce the recommended case definition for CDI (11) by limiting C. difficile testing to patients with evidence of diarrhea (i.e., ≥3 unformed stools in 24 h) and, additionally, in patients with diarrhea, excluding those with evidence of laxative intake. Using real-time electronic clinical data tracking, the laboratory reached >90% compliance with the intervention testing criteria. Given the high frequency with which patients without diarrhea have been reported to undergo C. difficile testing (13, 14, 16, 17), this achievement has significant implications with regard to the elimination of unnecessary and misleading testing. The overall effect of our intervention was a significant decrease in health care-onset health care facility-associated CDI (HO-CDI) rates and vancomycin utilization without a significant increase in CDI-related complications in patients with tests canceled for not meeting the intervention testing criteria. Studies indicate that 4.4% to 21% of hospitalized patients are asymptomatically colonized with toxigenic C. difficile (3–10, 30). Given the inability of C. difficile diagnostics to distinguish between asymptomatic carriage and disease state, our intervention represents an important step toward reducing CDI overdiagnosis and lowering the inflated HO-CDI rates.
The intervention described in this study is complementary to prior interventions shown to reduce HO-CDI rates (20, 21) and is independent of the diagnostic method used for testing. It is also an alternative approach to the multistep testing algorithms recommended by the European guidelines, which do not require three unformed stools but instead base treatment on detection of C. difficile toxins in stool of symptomatic patients (12). Although we educated providers regarding the pending intervention, a fraction of the members of the population of physicians and nursing staff were noncompliant with the intervention testing criteria and the provider compliance rate did not improve over the course of study. This is consistent with a prior study that showed that nurse and physician education alone is insufficient for reducing inappropriate C. difficile testing and treatment in patients without diarrhea (16). This finding underscores the importance of enforcement of the criteria via electronic data tracking. Although our intervention was effective, it did require manual interpretation of the electronic data tracking report, which took approximately 90 s per order, and manual enforcement of testing criteria. Manual handling is prone to human error and subject to noncompliance as is evident by the spike in the laboratory noncompliance rate during the Christmas holiday, when the laboratory is typically short staffed. Planned immediate improvements to our intervention include automation of enforcement of the criteria during electronic order entry. Electronic alerts have previously been shown to be effective for reducing unnecessary C. difficile repeat testing (22, 23).
The reduction in HO-CDI incidence is likely to have significant antibiotic stewardship and health economic benefits, although the comprehensive measurement of these effects was beyond the scope of this study. A significant decrease in vancomycin utilization was observed during the postintervention period despite the finding of higher rates of empirical therapy in patients with canceled orders than in those with negative results, which reflects reduced testing of patients not meeting the intervention testing criteria. The decrease in vancomycin usage has implications for the spread of antibiotic-resistant organisms such as vancomycin-resistant enterococcus, which has been shown to be associated with high vancomycin usage in health care settings (24, 25). Reducing vancomycin usage may avert selection and spread of antibiotic-resistant organisms. Reducing unnecessary antibiotic therapy should also prevent disruption of gut microbiota (26), which is important for gut homeostasis and health (27). The decrease in HO-CDI rates also has health economic benefits. Over the past decade, many North American hospitals, including ours, have switched from C. difficile toxin-based immunoassays to highly sensitive stand-alone nucleic acid amplification tests (NAAT) for their ability to confidently rule out CDI (28, 29). However, NAAT testing has resulted in higher CDI diagnosis rates (28), now threatening to trigger penalties in the framework of value-based Medicare reimbursement. Many hospitals are currently searching for strategies to reduce recorded HO-CDI rates in order to avoid penalties and a bad reputation associated with public reporting. The reduction in HO-CDI incidence achieved at our hospital by avoiding detection of colonized patients may be sufficient to avoid the Medicare reimbursement penalty, and adoption of our intervention may assist other institutions seeking to reduce their HO-CDI rates.
The findings of this study are promising, but we acknowledge several limitations. First, the preintervention/postintervention design could have influenced the outcomes attributed to the intervention. However, no other interventions, hospital directives, diagnostic modifications, or documented changes in hand hygiene compliance, environmental cleaning service compliance, or isolation compliance that could explain the study findings was introduced over the study period. There was also no change in non-CDI antibiotic use. Second, the intervention was evaluated at a single institution. Validation of the intervention at multiple centers is needed to confirm our findings. Third, it was assumed that nurses accurately reported bowel movement consistency. The fact that 5.6% of stool samples sent to the laboratory were formed suggests that the documentation of unformed stool in electronic health records (EHR) might have been inflated, leading to overqualification of patients for testing. Further efforts to improve accurate documentation by nursing staff members may result in a further reduction of unwarranted testing. Fourth, the subanalysis of C. difficile-related complications in patients with canceled orders was underpowered. Longer follow-up is needed to confirm the initial findings. Fifth, other approaches not requiring real-time data tracking were not assessed in this study. For example, although the procedure would be laborious, antibiotic stewardship staff members might be able to assess patients with C. difficile test orders and cancel inappropriate tests while educating nursing staff members. Last, the impact of our intervention on compliance with the testing criteria could not be directly compared to the results from the preintervention period as electronic tracking of bowel movement data was not previously available. However, using manual chart review, we have shown that 66.6% of patients with positive C. difficile results at our institution did not have diarrhea and that half had received laxatives (14). Therefore, the compliance achieved with the intervention testing criteria is highly significant in benchmarking against our historical data and was likely the result of reduced provider C. difficile ordering in nondiarrhea patients and laboratory cancellation of orders in patients that did not meet testing criteria.
In summary, real-time electronic patient data tracking is an effective tool for verification of C. difficile clinical testing criteria, resulting in reductions in inflated HO-CDI rates and vancomycin utilization. Real-time electronic data tracking may have a broader application for appropriate utilization of diagnostics and therapeutics.
MATERIALS AND METHODS
Study design.
We conducted a quasiexperimental study to evaluate the efficacy of electronic patient data tracking to enforce the intervention testing criteria and to evaluate the impact on C. difficile test utilization, CDI rates, antibiotic utilization, and clinical outcomes. Clinical outcomes were evaluated during the first 48 weeks of postintervention (12 4-week periods), while the other outcomes were compared between preintervention (14 3-month periods) and postintervention (4 3-month periods). The study population included consecutive adult patients hospitalized at Stanford Hospital.
Ethics.
This study was approved by the Stanford University Internal Review Board. A waiver of the informed consent requirement was obtained for the use of discarded stool samples.
Definitions.
A CDI case was considered a health care-onset health care facility-associated (HO-CDI) case per the National Healthcare Safety Network (NHSN) definition if unformed stool first tested positive for toxigenic C. difficile on or after the fourth day after admission. Intervention criteria for permitting C. difficile testing included the presence of diarrhea (i.e., 3 or more unformed stools in 24 h) and the absence of laxative intake during the 48 h prior to testing.
Intervention. (i) Real-time data tracking.
Stanford Health Care uses Epic electronic health record (EHR) software. A dropdown menu was built in Epic for non-free text documentation of stool occurrence and consistency (e.g., formed/solid, unformed/liquid, and mucus) in a formatted field. Between May 2015 and June 2015, nursing staff members were educated on using the new dropdown menu to document bowel movements at least once per shift in hospitalized patients and their compliance was documented and monitored (data not shown). Next, a real-time data tracking report, here referred to as the C. difficile Testing Criteria Report, was developed in Epic to show the date and time of bowel movement occurrences, stool consistency, and laxative administration, as well as the presence of a rectal tube where appropriate (see Fig. S1 in the supplemental material). The report documents the exact times that bowel movements were documented; however, a nurse may choose to report a sum number. Laxatives were defined as those classified under the “laxatives” and “laxatives and cathartics” drug classes in Epic (Table S1). The stool softener docusate was not considered a laxative. Between May 2015 and June 2015, laboratory staff members were trained with respect to viewing and interpreting the C. difficile Testing Criteria Report, which is generated automatically when the patient's chart is opened. The report can be viewed per 24 h, per 8 h, or per entry.
(ii) C. difficile testing criteria.
The Stanford Health Care clinical laboratory rejects formed stool samples, with the exception of those from patients with ileus and megacolon, data concerning which have to be communicated to our laboratory by their provider. With the assistance of an existing electronic alert (22), the laboratory also rejects repeat C. difficile orders within a 7-day interval. Starting on 22 June 2015, laboratory technologists used the C. difficile Testing Criteria Report to reject C. difficile orders from hospitalized patients with <3 unformed stools (excreted mucous was considered to represent unformed stool) in 24 h and in patients that received a laxative in the 48 h prior to stool collection. This step took approximately 90 s per order. When criteria for testing were not met, the order was canceled and the provider was notified. The intervention criteria were not applied to patients hospitalized less than 24 h prior to stool collection or to patients with a rectal tube and ostomy. Physicians could verbally request overriding of the criteria in patients with diarrhea and laxative intake and patients with ileus and megacolon. Rollout of the intervention was communicated to physicians and nurses between May 2015 and June 2015.
Laboratory testing. (i) C. difficile assay.
Stool samples were tested for toxigenic C. difficile using the GeneXpert C.diff tcdB PCR assay (Cepheid, Sunnyvale, CA). Testing was performed according to package insert instructions. Stool samples not meeting the intervention testing criteria were tested using this assay, but results were not reported in the EHR or to the ordering physician.
(ii) Data analysis.
A daily EHR report and manual chart review were used to evaluate the compliance of laboratory staff with the intervention testing criteria. Between 22 June 2015 and May 22, 2016 (the first 48 weeks of postintervention), laboratory and provider noncompliance with the testing criteria was analyzed per 4-week period for a total of 12 4-week periods. During the same postintervention period, the clinical outcomes of patients with canceled orders due to their not meeting the intervention testing criteria were compared with those of patients who met the criteria but tested C. difficile negative. Within the former group, outcomes were also compared between C. difficile-positive and C. difficile-negative patients. The following outcomes were measured using an EHR report: development of diarrhea documented by nursing per intervention criteria within 7 days of cancellation or testing; WBC rise to ≥15,000 cells/ml within 7 days of cancellation or testing; admission to ICU within 7 days of cancellation or testing; and 30-day all-cause mortality. We also analyzed empirical therapy with oral vancomycin and oral or intravenous metronidazole directed at C. difficile (via chart review for metronidazole) up to 24 h after test cancellation compared with completed orders. Patients who qualified for testing within 24 h of cancellation and patients receiving any empirical therapy within 24 h prior to cancellation or test results were excluded from analysis. Between 1 January 2012 (14 preintervention 3-month periods) and 30 June 2016 (4 postintervention 3-month periods), the C. difficile test utilization rate in hospitalized patients, HO-CDI incidence, and oral vancomycin days on therapy in hospitalized patients were analyzed per 3-month period. Postintervention rates (4 periods) were compared to preintervention rates (14 periods).
(iii) Statistical analysis.
The Mann-Whitney one-sided test was used to analyze preintervention/postintervention HO-CDI and utilization rates. The Fisher's exact test was used to analyze differences between proportions. This study was powered at 80% to detect a difference of at least 2.3% for each individual outcome between canceled orders and accepted C. difficile-negative orders based on intervention testing criteria at an expected 13.1% or higher complication rate and on baseline data from our institution, assuming 2,000 C. difficile orders with 70% meeting the intervention criteria for cancellation or acceptance and 30% automatically accepted (patients hospitalized for less than 24 h and patients with a rectal tube and ostomy) or rejected (formed stool and repeat test within a 7-day period) for other reasons, a negativity rate of 85%, and a 40% cancellation rate (13, 16, 17). The statistical software packages GraphPad Prism 5.0 and R 3.2.3 were used for all analyses. A type 1 error rate of 5% was used in all statistical tests.
Supplementary Material
ACKNOWLEDGMENTS
We thank John Tamaresis for assistance with statistical analysis.
This study was partly sponsored by Stanford Health Care and Stanford Department of Pathology.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/JCM.02319-16.
For a commentary on this article, see https://doi.org/10.1128/JCM.00147-17.
REFERENCES
- 1.Lessa FC, Mu Y, Bamberg WM, Beldavs ZG, Dumyati GK, Dunn JR, Farley MM, Holzbauer SM, Meek JI, Phipps EC, Wilson LE, Winston LG, Cohen JA, Limbago BM, Fridkin SK, Gerding DN, McDonald LC. 2015. Burden of Clostridium difficile infection in the United States. N Engl J Med 372:825–834. doi: 10.1056/NEJMoa1408913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Desai K, Gupta SB, Dubberke ER, Prabhu VS, Browne C, Mast TC. 2016. Epidemiological and economic burden of Clostridium difficile in the United States: estimates from a modeling approach. BMC Infect Dis 16:303. doi: 10.1186/s12879-016-1610-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alasmari F, Seiler SM, Hink T, Burnham CA, Dubberke ER. 2014. Prevalence and risk factors for asymptomatic Clostridium difficile carriage. Clin Infect Dis 59:216–222. doi: 10.1093/cid/ciu258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnson S, Clabots CR, Linn FV, Olson MM, Peterson LR, Gerding DN. 1990. Nosocomial Clostridium difficile colonisation and disease. Lancet 336:97–100. doi: 10.1016/0140-6736(90)91605-A. [DOI] [PubMed] [Google Scholar]
- 5.Leekha S, Aronhalt KC, Sloan LM, Patel R, Orenstein R. 2013. Asymptomatic Clostridium difficile colonization in a tertiary care hospital: admission prevalence and risk factors. Am J Infect Control 41:390–393. doi: 10.1016/j.ajic.2012.09.023. [DOI] [PubMed] [Google Scholar]
- 6.Samore MH, DeGirolami PC, Tlucko A, Lichtenberg DA, Melvin ZA, Karchmer AW. 1994. Clostridium difficile colonization and diarrhea at a tertiary care hospital. Clin Infect Dis 18:181–187. doi: 10.1093/clinids/18.2.181. [DOI] [PubMed] [Google Scholar]
- 7.Brazier JS, Fitzgerald TC, Hosein I, Cefai C, Looker N, Walker M, Bushell AC, Rooney P. 1999. Screening for carriage and nosocomial acquisition of Clostridium difficile by culture: a study of 284 admissions of elderly patients to six general hospitals in Wales. J Hosp Infect 43:317–319. doi: 10.1016/S0195-6701(99)90431-0. [DOI] [PubMed] [Google Scholar]
- 8.Hutin Y, Casin I, Lesprit P, Welker Y, Decazes JM, Lagrange P, Modai J, Molina JM. 1997. Prevalence of and risk factors for Clostridium difficile colonization at admission to an infectious diseases ward. Clin Infect Dis 24:920–924. doi: 10.1093/clinids/24.5.920. [DOI] [PubMed] [Google Scholar]
- 9.Kyne L, Warny M, Qamar A, Kelly CP. 2000. Asymptomatic carriage of Clostridium difficile and serum levels of IgG antibody against toxin A. N Engl J Med 342:390–397. doi: 10.1056/NEJM200002103420604. [DOI] [PubMed] [Google Scholar]
- 10.Loo VG, Bourgault AM, Poirier L, Lamothe F, Michaud S, Turgeon N, Toye B, Beaudoin A, Frost EH, Gilca R, Brassard P, Dendukuri N, Beliveau C, Oughton M, Brukner I, Dascal A. 2011. Host and pathogen factors for Clostridium difficile infection and colonization. N Engl J Med 365:1693–1703. doi: 10.1056/NEJMoa1012413. [DOI] [PubMed] [Google Scholar]
- 11.Cohen SH, Gerding DN, Johnson S, Kelly CP, Loo VG, McDonald LC, Pepin J, Wilcox MH; Society for Healthcare Epidemiology of America; Infectious Diseases Society of America. 2010. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the infectious diseases society of America (IDSA). Infect Control Hosp Epidemiol 31:431–455. doi: 10.1086/651706. [DOI] [PubMed] [Google Scholar]
- 12.Crobach MJ, Planche T, Eckert C, Barbut F, Terveer EM, Dekkers OM, Wilcox MH, Kuijper EJ. 2016. European Society of Clinical Microbiology and Infectious Diseases: update of the diagnostic guidance document for Clostridium difficile infection. Clin Microbiol Infect 22(Suppl 4):S63–S81. doi: 10.1016/j.cmi.2016.03.010. [DOI] [PubMed] [Google Scholar]
- 13.Dubberke ER, Han Z, Bobo L, Hink T, Lawrence B, Copper S, Hoppe-Bauer J, Burnham CA, Dunne WM Jr. 2011. Impact of clinical symptoms on interpretation of diagnostic assays for Clostridium difficile infections. J Clin Microbiol 49:2887–2893. doi: 10.1128/JCM.00891-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Banaei N, Anikst V, Schroeder LF. 2015. Burden of Clostridium difficile infection in the United States. N Engl J Med 372:2368–2369. doi: 10.1056/NEJMc1505190#SA2. [DOI] [PubMed] [Google Scholar]
- 15.Guerrero DM, Chou C, Jury LA, Nerandzic MM, Cadnum JC, Donskey CJ. 2011. Clinical and infection control implications of Clostridium difficile infection with negative enzyme immunoassay for toxin. Clin Infect Dis 53:287–290. doi: 10.1093/cid/cir361. [DOI] [PubMed] [Google Scholar]
- 16.Buckel WR, Avdic E, Carroll KC, Gunaseelan V, Hadhazy E, Cosgrove SE. 2015. Gut check: Clostridium difficile testing and treatment in the molecular testing era. Infect Control Hosp Epidemiol 36:217–221. doi: 10.1017/ice.2014.19. [DOI] [PubMed] [Google Scholar]
- 17.Peterson LR, Manson RU, Paule SM, Hacek DM, Robicsek A, Thomson RB Jr, Kaul KL. 2007. Detection of toxigenic Clostridium difficile in stool samples by real-time polymerase chain reaction for the diagnosis of C. difficile-associated diarrhea. Clin Infect Dis 45:1152–1160. doi: 10.1086/522185. [DOI] [PubMed] [Google Scholar]
- 18.Anikst VE, Gaur RL, Schroeder LF, Banaei N. 2016. Organism burden, toxin concentration, and lactoferrin concentration do not distinguish between clinically significant and nonsignificant diarrhea in patients with Clostridium difficile. Diagn Microbiol Infect Dis 84:343–346. doi: 10.1016/j.diagmicrobio.2015.11.022. [DOI] [PubMed] [Google Scholar]
- 19.Polage CR, Solnick JV, Cohen SH. 2012. Nosocomial diarrhea: evaluation and treatment of causes other than Clostridium difficile. Clin Infect Dis 55:982–989. doi: 10.1093/cid/cis551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sickbert-Bennett EE, DiBiase LM, Willis TM, Wolak ES, Weber DJ, Rutala WA. 2016. Reduction of healthcare-associated infections by exceeding high compliance with hand hygiene practices. Emerg Infect Dis 22:1628–1630. doi: 10.3201/eid2209.151440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Longtin Y, Paquet-Bolduc B, Gilca R, Garenc C, Fortin E, Longtin J, Trottier S, Gervais P, Roussy JF, Levesque S, Ben-David D, Cloutier I, Loo VG. 2016. Effect of detecting and isolating Clostridium difficile carriers at hospital admission on the incidence of C difficile infections: a quasi-experimental controlled study. JAMA Intern Med 176:796–804. doi: 10.1001/jamainternmed.2016.0177. [DOI] [PubMed] [Google Scholar]
- 22.Luo RF, Spradley S, Banaei N. 2013. Alerting physicians during electronic order entry effectively reduces unnecessary repeat PCR testing for Clostridium difficile. J Clin Microbiol 51:3872–3874. doi: 10.1128/JCM.01724-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Otto CC, Shuptar SL, Milord P, Essick CJ, Nevrekar R, Granovsky SL, Seo SK, Babady NE, Martin SC, Tang YW, Pessin MS. 2015. Reducing unnecessary and duplicate ordering for ovum and parasite examinations and Clostridium difficile PCR in immunocompromised patients by using an alert at the time of request in the order management system. J Clin Microbiol 53:2745–2748. doi: 10.1128/JCM.00968-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tacconelli E. 2009. Antimicrobial use: risk driver of multidrug resistant microorganisms in healthcare settings. Curr Opin Infect Dis 22:352–358. doi: 10.1097/QCO.0b013e32832d52e0. [DOI] [PubMed] [Google Scholar]
- 25.Fridkin SK, Edwards JR, Courval JM, Hill H, Tenover FC, Lawton R, Gaynes RP, McGowan JE Jr; Intensive Care Antimicrobial Resistance Epidemiology (ICARE) Project and the National Nosocomial Infections Surveillance (NNIS) System Hospitals. 2001. The effect of vancomycin and third-generation cephalosporins on prevalence of vancomycin-resistant enterococci in 126 U.S. adult intensive care units. Ann Intern Med 135:175–183. doi: 10.7326/0003-4819-135-3-200108070-00009. [DOI] [PubMed] [Google Scholar]
- 26.Isaac S, Scher JU, Djukovic A, Jiménez N, Littman DR, Abramson SB, Pamer EG, Ubeda C. 5 October 2016. Short- and long-term effects of oral vancomycin on the human intestinal microbiota. J Antimicrob Chemother doi: 10.1093/jac/dkw383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clemente JC, Ursell LK, Parfrey LW, Knight R. 2012. The impact of the gut microbiota on human health: an integrative view. Cell 148:1258–1270. doi: 10.1016/j.cell.2012.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cohen J, Limbago B, Dumyati G, Holzbauer S, Johnston H, Perlmutter R, Dunn J, Nadle J, Lyons C, Phipps E, Beldavs Z, Clark LA, Lessa FC, CDC's Clostridium difficile Infection Surveillance Investigators. 2014. Impact of changes in Clostridium difficile testing practices on stool rejection policies and C. difficile positivity rates across multiple laboratories in the United States. J Clin Microbiol 52:632–634. doi: 10.1128/JCM.02177-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deshpande A, Pasupuleti V, Rolston DD, Jain A, Deshpande N, Pant C, Hernandez AV. 2011. Diagnostic accuracy of real-time polymerase chain reaction in detection of Clostridium difficile in the stool samples of patients with suspected Clostridium difficile infection: a meta-analysis. Clin Infect Dis 53:e81–90. doi: 10.1093/cid/cir505. [DOI] [PubMed] [Google Scholar]
- 30.Truong C, Schroeder LF, Gaur R, Anikst VE, Komo I, Watters C, McCalley E, Kulik C, Pickham D, Lee NJ, Banaei N. 2017. Clostridium difficile rates in asymptomatic and symptomatic hospitalized patients using nucleic acid testing. Diagn Microbiol Infect Dis 87:365–370. doi: 10.1016/j.diagmicrobio.2016.12.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


