ABSTRACT
Dichelobacter nodosus is a fastidious, strictly anaerobic bacterium, an obligate parasite of the ruminant hoof, and the essential causative agent of virulent ovine footrot. The clinical disease results from a complex interplay between the pathogen, the environment, and the host. Sheep flocks diagnosed with virulent but not benign footrot in Australia may be quarantined and required to undergo a compulsory eradication program, with costs met by the farmer. Virulence of D. nodosus at least partially depends on the elaboration of a protease encoded by aprV2 and manifests as elastase activity. Laboratory virulence tests are used to assist diagnosis because clinical differentiation of virulent and benign footrot can be challenging during the early stages of disease or when the disease is not fully expressed due to unfavorable pasture conditions. Using samples collected from foot lesions from 960 sheep from 40 flocks in four different geographic regions, we evaluated the analytical characteristics of qPCR tests for the protease gene alleles aprV2 and aprB2, and compared these with results from phenotypic protease (elastase and gelatin gel) tests. There was a low level of agreement between clinical diagnosis and quantitative PCR (qPCR) test outcomes at both the flock and sample levels and poor agreement between qPCR test outcomes and the results of phenotypic virulence tests. The diagnostic specificity of the qPCR test was low at both the flock and individual swab levels (31.3% and 18.8%, respectively). By contrast, agreement between the elastase test and clinical diagnosis was high at both the flock level (diagnostic sensitivity [DSe], 100%; diagnostic specificity [DSp], 78.6%) and the isolate level (DSe, 69.5%; DSp, 80.5%).
KEYWORDS: AprV2, Australia, diagnosis, elastase, footrot, protease, sheep, qPCR
INTRODUCTION
Dichelobacter nodosus (formerly Fusiformis nodosus, Bacteroides nodosus) (1, 2), is a fastidious, strictly anaerobic bacterium and an obligate parasite of the ruminant hoof (1). It is the essential causative agent of virulent ovine footrot. This is a major economic and animal welfare burden for sheep farmers in Australia (3) and many other sheep-producing countries, including the United Kingdom and the United States (4, 5). Clinical footrot is the result of a complex interplay between the pathogen, the environment, and the host. Depending on the inherent susceptibility of the host, an interaction between strains of D. nodosus and other microbial flora on the skin may induce dermatitis and degradation of the hoof, following environmental predisposition. Footrot initially presents as a mild dermatitis of the interdigital skin and can progress in susceptible individuals to separation of the sole and horn of the hoof (“underrunning”) encouraged by warm (average daily temperature ≥10°C [50°F]) moist environmental conditions (6, 7). For descriptive and classification purposes, two clinical forms of footrot are recognized by Australian regulatory authorities, namely, virulent and benign (8). The clinical severity of an outbreak of footrot is partially determined by the virulence of the infecting D. nodosus strain(s), which are also classified as virulent or benign according to their in vitro phenotypic characteristics (8), but in reality there is a continuum, which includes intermediate forms (7). Virulence of D. nodosus at least partially depends on the elaboration of a protease encoded by aprV2 that manifests as elastase activity (9).
Footrot lesions are graded using a simple scoring system ranging from 0 (clinically healthy) to 4 (severe underrunning of the hard horn of the hoof) (10). Score 3 and score 4 lesions are regarded severe and cause lameness; however, differentiation of virulent and benign outbreaks of footrot has been based on the prevalence of score 4 lesions (8, 11). Clinically virulent footrot is characterized by a high prevalence of sheep with score 4 lesions (≥10%), while score 4 lesions are rare (<1%) in outbreaks of clinically benign footrot (8, 11). Importantly, this classification scheme acknowledges that virulent and benign strains of D. nodosus are both capable of inducing severe lesions in a proportion of susceptible sheep under favorable environmental conditions.
Clinical differentiation of virulent and benign footrot is relatively straightforward when the disease is fully expressed; however, it can be difficult during the initial stages of infection or where environmental conditions do not enable the full expression of the disease (8, 11). In such circumstances, laboratory identification and virulence testing of the infecting D. nodosus strain(s) are used to assist diagnosis. Currently, virulent and benign strains of D. nodosus are differentiated using one or more phenotypic tests for proteolytic enzyme activity (12, 13). The elastase test, which measures temporal and quantitative variations in activity of the extracellular proteases expressed by virulent and benign D. nodosus strains (13), has been shown to correlate well with clinical diagnoses (14, 15). The gelatin gel test, which measures the differences in the thermostability of extracellular proteases between virulent and benign D. nodosus strains (12), is also used by Australian regulatory authorities but can be unreliable (14–16).
Phenotypic tests require microbiological culture of D. nodosus, a process that is expensive and laborious, requiring specialized equipment and training (8). Furthermore, there is evidence of discrepancies between the phenotypic virulence tests and clinical observations, with phenotypically virulent D. nodosus strains isolated from clinically benign outbreaks of footrot (13, 15, 16). These discrepancies may reflect the lack of reproducibility of the tests themselves, which are sensitive to culture conditions (12, 13), or the influences of other host, pathogen, or environmental factors on the expression of the disease. There is also evidence of discrepancies between the different phenotypic virulence tests and between these and a genotypic marker for virulence known as intA (15).
Virulent strains of D. nodosus secrete three subtilisin-like extracellular proteases, namely, acidic protease isoenzyme 2 (AprV2), acidic protease isoenzyme 5 (AprV5), and a basic protease (BprV), encoded by the genes aprV2, aprV5, and bprV, respectively (17, 18). Benign strains secrete the analogous proteases AprB2, AprB5, and BprB, encoded by the genes aprB2, aprB5, and bprB, respectively (17–19). Kennan et al. (9) reported that AprV2 is essential for virulence in vivo through the construction of an aprV2 gene mutant of virulent D. nodosus strain VSC1703A and established that the elastase activity of AprV2 forms the basis of the elastase test. Sequence analysis of aprV2 and its benign ortholog aprB2 has shown that the two alleles differ by a two base-pair substitution (TA/CG) (9, 20). Recently, two quantitative real-time PCR (qPCR) tests targeting this substitution were developed in Europe (21, 22). Both tests were reported to identify D. nodosus and differentiate virulent and benign strains. This has been the subject of rural media interest following press releases from the Departments of Primary Industries (23, 24).
In Australia, the means by which outbreaks of footrot are classified by regulatory authorities as virulent or benign differs between states. In NSW, for example, the diagnosis of virulent footrot is primarily based on clinical examination; laboratory tests may be used to assist with a diagnosis, but cannot be the sole basis of a diagnosis (8, 25). However, in Western Australia, the diagnosis of virulent footrot is based entirely on the results of laboratory virulence testing, irrespective of the clinical severity of an outbreak (25). Furthermore, although virulent footrot is a notifiable disease in some states, legislative approaches and the means by which footrot is controlled vary. In NSW, for example, flocks with clinically virulent footrot are quarantined and must undergo a compulsory eradication program, with costs met by the farmer. Allworth (26) estimated that the cost of eradicating virulent footrot from a flock can exceed $10 per head per annum. Benign footrot is not considered amendable to control (see Discussion).
To declare the protease gene-based qPCR tests as being suitable for use in Australian diagnostic laboratories, they must be evaluated using reference samples collected from representative Australian sheep flocks, using the appropriate case definitions (11). The aim of this study was to subject these tests to the important initial steps in the validation pathway outlined in chapter 1.1.6 of the Manual of Diagnostic Tests and Vaccines for Terrestrial Animals (27), which defines agreed international standards, including comparisons with clinical diagnosis and the currently used phenotypic protease virulence tests.
RESULTS
Flock selection and clinical diagnosis.
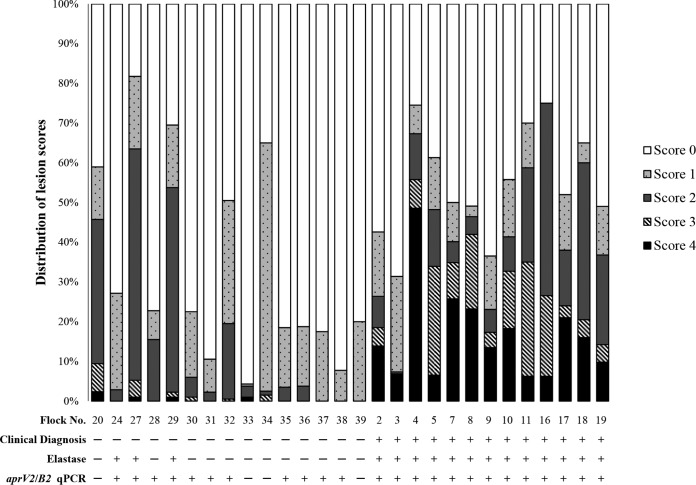
Forty Australian sheep flocks were selected for this study, including 24 flocks with clinically virulent footrot and 16 flocks with clinically benign footrot (Table 1). The flocks were selected from target populations in southeastern and southwestern Australia (Fig. 1). Three approaches to clinical diagnosis were used (Table 1). A summary of lesion scores was available for 28 flocks; the distribution of lesion scores observed in each of these flocks is illustrated in Fig. 2. Lesion swabs were collected for direct testing from 40 flocks, but lesion swabs for microbiological culture were collected from only 38 flocks.
TABLE 1.
Details of the Merino flocks sampled during this study
| Farm | State | Operator | Diagnostic approach | Season at time of inspection | No. of mobs inspected | No. of sheep inspected | No. or percentage of sheep with score 4 lesions | Clinical diagnosis | No. of sheep with lesions sampled | No. of swabs tested directly (n = 758) | No. of isolates collected (n = 469) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | SA | AHOa | 2 | Winter | 1 | NAb | ≥10% | Virulent | 11 | 12 | 12 |
| 2 | NSW | Authors | 3 | Winter | NA | 54 | 23 | Virulent | 14 | 4 | 12 |
| 3 | TAS | Authors | 3 | Winter | NA | 51 | 11 | Virulent | 24 | 11 | 11 |
| 4 | TAS | Authors | 3 | Winter | NA | 52 | 40 | Virulent | 50 | 10 | 14 |
| 5 | TAS | AHO | 3 | Winter | NA | 42 | 10 | Virulent | 20 | 23 | 23 |
| 6 | TAS | AHO | 2 | Winter | 1 | NA | ≥10% | Virulent | 20 | 23 | 23 |
| 7 | TAS | AHO | 3 | Winter | NA | 33 | 26 | Virulent | 20 | 20 | 20 |
| 8 | TAS | AHO | 3 | Winter | NA | 28 | 18 | Virulent | 20 | 19 | 19 |
| 9 | TAS | AHO | 3 | Winter | NA | 13 | 7 | Virulent | 13 | 13 | 13 |
| 10 | TAS | AHO | 3 | Spring | NA | 26 | 11 | Virulent | 20 | 15 | 15 |
| 11 | TAS | AHO | 3 | Spring | NA | 20 | 5 | Virulent | 20 | 8 | 8 |
| 12 | SA | AHO | 2 | Spring | 1 | NA | ≥10% | Virulent | 10 | 10 | 10 |
| 13 | SA | AHO | 2 | Spring | 1 | NA | ≥10% | Virulent | 6 | 9 | 9 |
| 14 | SA | AHO | 2 | Spring | 1 | NA | ≥10% | Virulent | 7 | 8 | 7 |
| 15 | SA | AHO | 2 | Spring | 1 | NA | ≥10% | Virulent | 10 | 13 | 13 |
| 16 | SA | AHO | 3 | Spring | NA | 16 | 3 | Virulent | 16 | 16 | 16 |
| 17 | TAS | Authors | 3 | Spring | NA | 25 | 14 | Virulent | 25 | 15 | 15 |
| 18 | TAS | Authors | 3 | Spring | NA | 50 | 19 | Virulent | 50 | 29 | 29 |
| 19 | NSW | Authors | 3 | Summer | NA | 51 | 15 | Virulent | 50 | 15 | 15 |
| 20 | NSW | AHO | 3 | Summer | NA | 50 | 4 | Benign | 14 | 14 | 14 |
| 21 | TAS | AHO | 2 | Summer | 1 | NA | ≥10% | Virulent | 11 | 11 | 11 |
| 22 | TAS | AHO | 2 | Summer | 1 | NA | ≥10% | Virulent | 17 | 17 | 17 |
| 23 | SA | AHO | 2 | Summer | 1 | NA | ≥10% | Virulent | 4 | 4 | 4 |
| 24 | NSW | Authors | 3 | Autumn | NA | 20 | 0 | Benign | 20 | 20 | 21 |
| 25 | NSW | Authors | 3 | Winter | NA | NA | ≥10% | Virulent | 10 | 14 | 14 |
| 26 | TAS | AHO | 2 | Winter | 1 | NA | ≥10% | Virulent | 12 | 5 | 5 |
| 27 | SA | Authors | 1 | Spring | 1 | 100 | 4 | Benign | 40 | 40 | 20 |
| 28 | SA | Authors | 1 | Spring | 1 | 100 | 0 | Benign | 40 | 40 | 11 |
| 29 | SA | Authors | 1 | Spring | 1 | 100 | 2 | Benign | 40 | 40 | 12 |
| 30 | SA | Authors | 1 | Spring | 1 | 100 | 0 | Benign | 40 | 40 | 6 |
| 31 | SA | Authors | 1 | Spring | 1 | 170 | 0 | Benign | 40 | 40 | 3 |
| 32 | SA | Authors | 1 | Spring | 1 | 100 | 0 | Benign | 40 | 40 | 7 |
| 33 | SA | AHO | 2 | Spring | 1 | 1716 | 42 | Benign | 50 | 0 | 15 |
| 34 | TAS | Authors | 1 | Winter | 2 | 100 | 0 | Benign | 30 | 30 | 6 |
| 35 | NSW | Authors | 1 | Winter | 2 | 120 | 0 | Benign | 21 | 21 | 10 |
| 36 | NSW | Authors | 1 | Winter | 1 | 100 | 0 | Benign | 28 | 20 | 6 |
| 37 | NSW | Authors | 1 | Winter | 1 | 100 | 0 | Benign | 22 | 20 | 1 |
| 38 | NSW | Authors | 1 | Winter | 1 | 100 | 0 | Benign | 20 | 20 | 2 |
| 39 | NSW | Authors | 1 | Winter | 1 | 100 | 0 | Benign | 25 | 23 | 0 |
| 40 | WA | AHO | 2 | Winter | 1 | NA | 0 | Benign | 30 | 27 | 0 |
AHO, animal health officer.
NA, not available.
FIG 1.
Distribution of Australian sheep flocks sampled in this present study. WA, Western Australia; NT, Northern Territory; SA, South Australia; QLD, Queensland; NSW, New South Wales; ACT, Australian Capital Territory; VIC, Victoria; TAS, Tasmania. Source: http://www.d-maps.com/carte.php?num_car=3293&lang=en.
FIG 2.
Summary of the proportions of feet with each lesion score for 28 of the flocks included in this study. Flocks were grouped according to clinical diagnosis. Lesion score summaries were not provided for 13 flocks inspected by animal health officers using method 3 (see Table 1). The number of sheep inspected in each flock is indicated in Table 1. Lesion scores were ordinal and based on Egerton and Roberts (10): clinically healthy feet were given a score of 0; mild lesions restricted to the interdigital skin were given a score of 1; if severe, a score of 2 was given; where underrunning of the posterior sole and soft horn of the heel were observed, a score of 3 was given; if the underrunning extended to the abaxial wall, a score of 4 was given. Flock-level clinical, elastase, and aprV2/B2 qPCR diagnoses of virulent (+) and benign (—) footrot are also provided.
Analytical characteristics of the qPCR tests.
Amplification efficiencies for aprV2 and aprB2 were 90.14 and 88.4, respectively, using the assay developed by Frosth et al. (21). Amplification efficiencies for aprV2 and aprB2 were 87.8 and 91.4, respectively, using the assay developed by Stäuble et al. (22). The limits of detection (LOD) of both qPCR tests for aprV2 and aprB2 were 0.005 and 0.05 pg, respectively.
Both assays were specific for the aprV2 and aprB2 alleles, and no amplification occurred for the 15 other bacterial species tested (Table 2).
TABLE 2.
Analytical specificities of the aprV2/V2 qPCR testsa
| Species or strain | Location | Host | ID no.b | qPCR test developed by: |
|||
|---|---|---|---|---|---|---|---|
| Stäuble et al. (22) |
Frosth et al. (21) |
||||||
| aprV2 | aprB2 | aprV2 | aprB2 | ||||
| Cardiobacterium hominis | NSW, Australia | Human | FD-3235 | — | — | — | — |
| Corynebacterium ovis | NSW, Australia | Ovine | FD-2798 | — | — | — | — |
| Dermatophilus congolensis | NSW, Australia | Ovine | FD-2839 | — | — | — | — |
| Enterococcus uberis | NSW, Australia | Bovine | NA | — | — | — | — |
| Erysipelothrix rhusiopathiae | QLD, Australia | Ovine | FD-2825 | — | — | — | — |
| Escherichia coli | NSW, Australia | Ovine | FD-2669 | — | — | — | — |
| Fusobacterium necrophorum | NSW, Australia | Ovine | FD-2842 | — | — | — | — |
| Klebsiella spp. | NSW, Australia | Bovine | NA | — | — | — | — |
| Moraxella bovis | NSW, Australia | Bovine | FD-2574 | — | — | — | — |
| Nocardia spp. | NSW, Australia | Bovine | 15-166 | — | — | — | — |
| Pseudomonas aeruginosa | NSW, Australia | Ovine | FD-2696 | — | — | — | — |
| Salmonella enterica serovar Typhimurium | VIC, Australia | Porcine | NA | — | — | — | — |
| Staphylococcus aureus | NSW, Australia | Bovine | 2793 | — | — | — | — |
| Streptococcus B | NSW, Australia | Ovine | 2438 | — | — | — | — |
| Suttonella indologenes | NSW, Australia | Human | FD-3234 | — | — | — | — |
| Dichelobacter nodosus A1001 | NSW, Australia | Ovine | A1001 | + | — | + | — |
| Dichelobacter nodosus JIR3528 | NSW, Australia | Ovine | JIR3528 | — | + | — | + |
Tests were evaluated using genomic DNA extracted from 15 bacterial species. Virulent (aprV2 positive) and benign (aprB2 positive) D. nodosus control strains were also included.
ID, identification.
The repeatability of the qPCR test developed by Frosth et al. (21) was evaluated for the aprV2 and aprB2 alleles with three concentrations of genomic DNA per reaction. The coefficients of variation (CVs) were similar (<5%) for each of the three DNA concentrations for both the aprV2 and aprB2 alleles.
The two qPCR tests were compared using 430 lesion swabs collected from 18 flocks (flocks 5 to 11, 17 to 18, and 24 to 32, as described in Table 1). The qPCR test developed by Frosth et al. (21) detected the aprV2 allele in 48 lesion swabs and the aprB2 allele in 26 lesion swabs that the test developed by Stäuble et al. (22) did not. Consequently, a decision was made to proceed with the assay developed by Frosth et al. (21) for a larger test evaluation.
Comparison of clinical diagnosis and virulence tests at the flock level.
aprV2/B2 qPCR test.
The qPCR test was evaluated using 758 lesion swabs collected from 40 Australian sheep flocks. An outbreak was more likely to be classified as virulent with the qPCR test than as classified clinically (P < 0.0009) (Table 3). The level of agreement between field diagnosis and the qPCR test was fair (kappa statistic, 0.353). At the flock level, the diagnostic sensitivity (DSe) and diagnostic specificity (DSp) of the qPCR test were 100% and 31.3%, respectively. The level of agreement was considerably lower at the foot swab level (kappa statistic, 0.096) (Table 4), where DSe and DSp were 98.1% and 18.8%, respectively.
TABLE 3.
Flock-level comparison of clinical diagnosis and aprV2/B2 qPCR using 758 lesion swabs collected from 40 Australian sheep flocksa
| Clinical diagnosisb | No. of flocks with a laboratory diagnosis (aprV2/B2 qPCR) of:c |
||
|---|---|---|---|
| Benign | Virulent | Total | |
| Benign | 5 | 11 | 16 |
| Virulent | 0 | 24 | 24 |
| Total | 5 | 35 | 40 |
McNemar's χ2 = 11.0, P = 0.0009; kappa statistic = 0.353 (95% CI, 0.105–0.601), DSe = 100% (95% CI, 87.5–100%), DSp = 31.3% (95% CI, 11.0–58.6%).
Clinical diagnoses are given in Table 1.
qPCR according to reference 21. Benign, swabs tested negative for the aprV2 allele; virulent, ≥1 swab tested positive for the aprV2 allele.
TABLE 4.
Sample-level comparison of clinical diagnosis and the aprV2/B2 qPCR using genomic DNA extracted directly from 758 foot swabs collected from 40 Australian sheep flocksa
| Clinical diagnosisb | No. of foot swabs with a laboratory diagnosis (aprV2/B2 qPCR) of:c |
||
|---|---|---|---|
| Benign | Virulent | Total | |
| Benign | 84 | 363 | 447 |
| Virulent | 6 | 305 | 311 |
| Total | 90 | 668 | 758 |
McNemar's χ2 = 345.39, P < 0.0001, kappa statistic = 0.096 (95% CI, 0.064–0.128), DSe = 98.1% (95% CI, 95.9–99.3%), DSp = 18.8% (95% CI, 15.3–22.7%).
Clinical diagnosis was at the flock level (Table 1).
qPCR according to reference 21. Benign, swabs that tested negative for the aprV2 allele; virulent, swabs that tested positive for the aprV2 allele.
Elastase test.
The elastase test was used to evaluate 469 D. nodosus isolates collected from 38 Australian sheep flocks (Table 5). There was no significant difference (P = 0.0833) between the proportion of outbreaks classified as virulent by clinical diagnosis or by the elastase test. The level of agreement between clinical diagnosis and the elastase test was almost perfect (kappa statistic, 0.822). At the flock level, the DSe and DSp of the elastase test were 100% and 78.6%, respectively.
TABLE 5.
Flock-level comparison of clinical diagnosis and the elastase test using 469 D. nodosus isolates collected from 38 Australian sheep flocksa
| Clinical diagnosis | No. of flocks with a laboratory diagnosis (elastase test) of:b |
||
|---|---|---|---|
| Benign | Virulent | Total | |
| Benign | 11 | 3 | 14 |
| Virulent | 0 | 24 | 24 |
| Total | 11 | 27 | 38 |
Lesion swabs were not collected for microbiological culture from flocks 39 and 40. McNemar's χ2 = 3.0, P = 0.0833; kappa statistic = 0.822 (95% CI, 0.633–1.000), DSe = 100% (95% CI, 85.8–100%), DSp = 78.6% (95% CI, 49.2–95.3%).
Benign, no D. nodosus isolates were elastase positive at ≤12 days; virulent, ≥1 D. nodosus isolate was elastase positive at ≤12 days.
Clinical diagnosis and the elastase test were also compared at the isolate level (Table 6). There was a significant difference (P < 0.0001) between the proportion of isolates from clinically virulent outbreaks that were elastase negative and those from clinically benign outbreaks that were elastase positive. The level of agreement was moderate (kappa statistic, 0.431). At the isolate level, DSe and DSp of the elastase test were 69.9% and 80.5%, respectively.
TABLE 6.
Isolate-level comparison of clinical diagnosis and the elastase test using 469 D. nodosus isolates collected from 38 Australian sheep flocksa
| Clinical diagnosis | No. of isolates with a laboratory diagnosis (elastase test) of:b |
||
|---|---|---|---|
| Benign | Virulent | Total | |
| Benign | 107 | 26 | 133 |
| Virulent | 101 | 235 | 336 |
| Total | 208 | 261 | 469 |
Lesion swabs were not collected for microbiological culture from flocks 39 and 40. McNemar's χ2 = 44.29, P < 0.0001, kappa statistic = 0.431 (95% CI, 0.352–0.51), DSe = 69.9% (95% CI, 64.7–74.8%), DSp = 80.5% (95% CI, 72.7–86.8%).
Benign, isolates that were elastase negative at ≤12 days; virulent, isolates that were elastase positive at ≤12 days.
Gelatin gel test.
The gelatin gel test was used to evaluate samples collected from six clinically benign outbreaks (flocks 27, 28, 29, 30, 31, and 32). Overall, 49.1% (28/57) of the D. nodosus isolates obtained from these flocks were heat stable (virulent).
Comparison of virulence tests at the isolate level.
aprV2/B2 qPCR test and elastase test.
There was a significant difference (P < 0.0001) between the results from the elastase test and those from the qPCR test. Only 52.7% (213/404) of D. nodosus isolates were classified as virulent by both tests (Table 7). There was considerable discrepancy between the elastase test and the qPCR test for isolates classified as benign by the elastase test, as 73.2% (139/190) of these isolates were classified as virulent by the qPCR test. These isolates were classified as benign as they were elastase negative at the cutoff point of 12 days; however, elastase activity was observed for 80.6% (112/139) of these isolates after 16 to 28 days of incubation. The level of agreement between the two tests was only fair (kappa statistic, 0.275).
TABLE 7.
Comparison of the elastase test and the aprV2/B2 qPCR test using 404 D. nodosus isolates obtained from 38 Australian sheep flocksa
| Laboratory diagnosis (elastase test) | No. of isolates with a laboratory diagnosis (aprV2/B2 qPCR) of:b |
||
|---|---|---|---|
| Benign | Virulent | Total | |
| Benign | 51 | 139 | 190 |
| Virulent | 1 | 213 | 214 |
| Total | 52 | 352 | 404 |
Lesion swabs were not collected for microbiological culture from flocks 39 and 40. McNemar's χ2 = 137.00, P < 0.0001, kappa statistic = 0.275 (95% CI, 0.207–0.343).
qPCR according to reference 21. Benign, isolates that were negative for the aprV2 allele; virulent, isolates that were positive for the aprV2 allele.
aprV2/B2 qPCR test and the gelatin gel test.
There was a significant difference (P < 0.0001) between the results from the gelatin gel test and those from the qPCR test, with 86.2% (25/29) of isolates classified as benign (unstable) by the gelatin gel test classified as virulent by the qPCR test (Table 8). The level of agreement between the two tests was poor (kappa statistic, 0.101, 95% confidence interval [CI] −0.024 to 0.244).
TABLE 8.
Comparison of the aprV2/B2 qPCR test and the gelatin gel test using 57 D. nodosus isolates obtained from flocks 27, 28, 29, 30, 31, and 32a
| Laboratory diagnosis (gelatin gel test) | No. of isolates with a laboratory diagnosis (aprV2/B2 qPCR) of:b |
||
|---|---|---|---|
| Benign | Virulent | Total | |
| Benign | 4 | 25 | 29 |
| Virulent | 1 | 27 | 28 |
| Total | 5 | 52 | 57 |
All of these flocks presented with clinically benign footrot (see Table 1). McNemar's χ2 = 23.04, P < 0.0001, kappa statistic = 0.101 (95% CI, −0.024 to 0.244).
qPCR according to reference 21. Benign, isolates that were negative for the aprV2 allele; virulent, isolates that were positive for the aprV2 allele.
Elastase test and the gelatin gel test.
Paired elastase and gelatin gel test results were available for 56 D. nodosus isolates (Table 9). There was a significant difference between the results from the two tests (P < 0.0001), with 42.9% (21/49) of isolates classified as benign by the elastase test classified as virulent by the gelatin gel test. The level of agreement between the tests was slight (kappa statistic, 0.193).
TABLE 9.
Comparison of the elastase test and the gelatin gel test using 56 D. nodosus isolates obtained from flocks 27, 28, 29, 30, 31, and 32a
| Laboratory diagnosis | No. of isolates with a laboratory diagnosis (elastase test) of:b |
||
|---|---|---|---|
| Benign | Virulent | Total | |
| Benign | 28 | 1 | 29 |
| Virulent | 21 | 6 | 27 |
| Total | 49 | 7 | 56 |
All of these flocks presented with clinically benign footrot (see Table 1). McNemar's χ2 = 18.18, P < 0.0001, kappa statistic = 0.193 (95% CI, 0.015–0.370).
Benign, isolates that were elastase negative at ≤12 days; virulent, isolates that were elastase positive at ≤12 days.
DISCUSSION
In this study, we undertook an evaluation of clinical diagnoses and microbial virulence tests with an emphasis on the qPCR tests developed by Stäuble et al. (22) and Frosth et al. (21), and we subjected the test developed by Frosth et al. (21) to a larger evaluation, in accordance with chapter 1.1.6 of the Manual of Diagnostic Tests and Vaccines for Terrestrial Animals (27), which states that a diagnostic test must be evaluated using appropriate reference samples from a defined target population to be declared fit for its intended purpose.
The analytical performance of the two qPCR tests was evaluated using a set of 430 samples collected from 18 Australian flocks. The test developed by Frosth et al. (21) detected the aprV2 allele in some samples and the aprB2 allele in other samples that the test developed by Stäuble et al. (22) did not. This discrepancy may have resulted from PCR inhibitors present in the DNA extract; Frosth et al. (21) include bovine serum albumin (BSA) in their reaction mixture, which has been shown to alleviate PCR inhibition (28). The lower sensitivity of the test developed by Stäuble et al. (22) may also be a consequence of variable primer binding, as there is known to be a single-nucleotide polymorphism (SNP) in the region of their forward primer in some D. nodosus strains (29). Consequently, we proceeded with a larger evaluation using the test developed by Frosth et al. (21).
Comparing the results from this qPCR test with clinical diagnoses, there was moderate to poor agreement at the flock and individual swab sample levels, and the diagnostic specificity of the qPCR was low overall (up to ∼30%).By contrast, there was much better agreement between clinical diagnoses and the results from the elastase test at the flock and individual sample levels with diagnostic specificity ∼80%. Isolate-level comparison of the qPCR test and elastase test revealed that 73.2% (139/190) of isolates that were deemed benign by the elastase test were deemed virulent by the qPCR test (aprV2 positive). The elastase test depends on visual assessment of the digestion of elastin particles in an agar matrix, and a cut point based on incubation time is applied, generally 10 to 12 days beyond which an isolate is deemed benign (8, 12). Thus, the amount of elastase activity and the rate at which it is elaborated influence the outcome of the test. Elastase activity was observed for 80.6% of the benign isolates after further incubation, confirming temporal and quantitative variations in the expression of the AprV2 enzyme between strains. Regardless, the results indicate that some strains that possess aprV2 did not express detectable elastase activity and may not be capable of inducing severe disease. Consequently, identification of the aprV2 allele may not be a reliable indicator of virulence.
Clinical diagnoses in this study were made using objective criteria that have been applied successfully in a state-wide eradication program in NSW (30). However, these criteria ignore the true spectrum of severity that is possible in ovine footrot, which ranges from inapparent through mild to severe (7). Forcing a dichotomous clinical classification was pragmatic from the perspective of disease control and, in the present study, enabled the comparison with dichotomous laboratory test outcomes. The authors acknowledge that this may lead to some inaccurate classifications of both clinical and laboratory results given that the latter could also be continuous variables. Nevertheless, the trends are very obvious, and the discrepancies between clinical and laboratory diagnoses are substantial. In Fig. 2, the frequency of sheep with foot lesions in each score category are shown for 28 flocks. It is clear that laboratory diagnoses of virulent footrot do not match the clinical patterns in flocks in which there were no sheep with severe lesions and where there was sufficient history to be very confident that virulent footrot was not present. Test outcomes like this undermine confidence and may lead to farmers disengaging from programs to control the disease.
These findings elaborate those of Stäuble et al. (22) and Frosth et al. (21) who reported a high level of agreement between the qPCR test and clinical diagnosis. The case definitions used by these authors, which differ from those used in Australia, may partially explain this discrepancy. Stäuble et al. (22) did not classify outbreaks as clinically virulent or benign; rather, flocks were classified as “nonaffected” (all feet assigned a score of 0) or “affected” (one or more feet assigned a score ≥1). The authors report that all lesion swabs from “affected” flocks were positive for the aprV2 allele and negative for the aprB2 allele and that >80% of samples from the “nonaffected” flocks were positive for the aprB2 allele. Therefore, the authors concluded that there was a high level of agreement between clinical diagnosis and the qPCR test. However, if the flocks are reclassified using the case definitions applied in Australia, at least two of the “affected” flocks would be regarded as having clinically benign footrot, as no score 4 lesions were observed in these flocks.
Similar discrepancies are apparent in the data provided by Frosth et al. (21). Each flock was assigned to one of four categories: (i) predominantly score 0 with some score 1 lesions, (ii) many score 1 lesions but no scores >1, (iii) at least one animal with a score 2 lesion, and (iv) at least one animal with a score 3 lesion. It is evident that under the Australian classification system, categories one and two could describe a flock with ovine interdigital dermatitis (OID), benign footrot, or the early stages of virulent footrot. Similarly, categories three and four could describe an outbreak of benign footrot or the early stages of an outbreak of virulent footrot. No category describes virulent footrot exclusively.
The clinical observations reported in this study support the use of a classification system that is based on the prevalence of score 4 lesions rather than the presence or absence of foot lesions of any grade. We observed that D. nodosus strains classified as benign by the qPCR test (aprB2 positive) are capable of inducing severe, underrun lesions in a small proportion of susceptible sheep (Table 1), a finding in keeping with prior knowledge of phenotypic protease tests (13). However, at the flock level, the impact of foot disease in these flocks was minor. In flock 33, for example, 42/1,716 sheep presented with score 4 lesions, but the overall prevalence of sheep with foot lesions of any grade in this flock was low (Fig. 2), despite all sheep being exposed to the same strain under the same environmental conditions. Thus, it would be inappropriate to subject these flocks to the same regulatory activity (quarantine and compulsory disease control) as those deemed to have virulent footrot, as the D. nodosus strains present in these outbreaks would most likely be incapable of inducing severe disease in a large proportion of sheep. In general, benign footrot is not considered amenable to control, and attempts to do so would expose farmers to ongoing costly measures (26, 31). Culling susceptible sheep that develop severe lesions when infected with benign strains is the most practical course of action (32). The experience in Australia using conventional phenotypic virulence tests is that after a control program directed at virulent footrot, benign strains of D. nodosus persist in flocks (33–35).
The identification of aprV2-positive strains of D. nodosus in clinically healthy flocks provides further evidence that the aprV2 may be an unreliable virulence marker. Stäuble et al. (22) reported that seven samples collected from “nonaffected” (clinically healthy) flocks were positive for the aprV2 allele, alone or in combination with the aprB2 allele. In Switzerland, Locher et al. (36) evaluated the qPCR test developed by Stäuble et al. (22) as a potential screening tool for identifying virulent D. nodosus isolates in clinically healthy flocks and reported that aprV2-positive isolates were identified in four flocks on one or more occasions, despite the flocks remaining clinically healthy for the duration of the study. This finding may also reflect differences in breed susceptibility, as the European breeds are inherently more resistant to footrot than the Merino breed (37).
In conclusion, strains of D. nodosus that may vary in virulence interact with other microbial flora on the skin of the foot after environmental predisposition and, depending on the inherent susceptibility of the host, may then induce dermatitis and degradation of the hoof. This complex interplay between the pathogen, the environment, and the host creates a difficult set of circumstances for diagnosticians. Nevertheless, virulence of D. nodosus at least partially depends on the elaboration of a protease coded by aprV2 and manifests as elastase activity. In this study, we demonstrated that aprV2-positive D. nodosus isolates are frequently isolated from outbreaks of clinically benign footrot and that phenotypic evidence of elastase activity was more closely related to clinical diagnosis than was the mere presence of the gene. As benign footrot is not associated with significant animal welfare concerns, and as attempts to control benign footrot are both expensive and ineffective, we conclude that the qPCR test is not fit for its intended purpose. There is a considerable risk that producers would be subject to unnecessary and costly regulatory activity if the aprV2/B2 qPCR was used as the sole basis for diagnosis. In this study, we demonstrated that there is a diversity of phenotypes among D. nodosus isolates that possess the aprV2 allele, though the basis of this diversity is currently unknown. We recommend further investigation of the molecular basis of virulence.
MATERIALS AND METHODS
Flock selection.
Forty Australian sheep flocks were included in this study from target populations in southern Australia, including 24 flocks with clinically virulent footrot and 16 flocks with clinically benign footrot (Table 1). Lesion swabs were collected from flocks 1 to 32 and 34 to 40 between June 2014 and August 2016 for diagnostic purposes. Lesion swabs were collected from flock 33 in 2006 during the course of a previous study (38).
Clinical examination and diagnosis.
Three methods of clinical diagnosis were used during this study as shown in Table 1. The method depended on whether the sheep were examined by the authors or an animal health officer (AHO), on the number of sheep or mobs examined, and on prior diagnostic investigations. On each farm, sheep were placed in dorsocaudal recumbency, and each foot was carefully examined, as described by Stewart and Claxton (8). A score was assigned to each foot according to the scoring system devised by Egerton and Roberts (10). This is a standardized systematic approach to grading footrot lesions, with a high level of repeatability (39). All observers were trained and highly experienced with foot scoring. For methods 1 and 2, the diagnosis of clinically virulent and benign footrot was based on the prevalence of score 4 lesions observed in ≥100 sheep selected from the flock by systematic random sampling, as described by Egerton (11), or after the producer had drafted off a proportion of the flock or mob as a convenience sample. A mob is a subset of a flock run separately for management purposes. To align with the dichotomous classification system used by regulatory authorities, outbreaks were classified as clinically benign when score 4 lesions were observed in <10% of the flock or mob or clinically virulent when score 4 lesions were observed in ≥10% of the flock or mob. This dichotomous system is pragmatic and was used during the NSW Footrot Strategic Plan to identify farms for the application of compulsory control and eradication measures, resulting in a reduction in the prevalence of farms affected with virulent footrot from 15% to <1% (30).
Method 1.
At least 100 sheep were examined by the authors or an AHO. The producer presented either (i) one mob for examination because it was the only one with clinical footrot on the farm or it was the mob with the most severe clinical signs of lameness or (ii) two mobs for examination (50 inspected per mob) because clinical signs of lameness were previously observed in both mobs or foot lesions were previously observed in both mobs during routine husbandry procedures. A flock history was provided by the producer at the time of inspection. Lesion swabs were collected at the time of examination by the authors.
In all flocks that appeared to have clinically benign footrot, additional criteria were used to support the diagnosis. (i) The flock/mob must have been examined previously on two or more occasions by the authors or an experienced AHO, according to the system described by Stewart and Claxton (1993). The disease must have been classified as clinically benign on each occasion, according to the system of Egerton (11). The retrospective foot score data were inspected by the authors. (ii) Environmental conditions must have been favorable for the transmission and expression of the disease in the 2 weeks prior to each of the examinations (average daily air temperature ≥10°C [50°F], consistent rainfall) (6). Climatic data were obtained from the nearest Bureau of Meteorology (BOM) weather station. (iii) The flock history was obtained from the producer and did not suggest clinically virulent footrot. There was no clinical evidence of virulent footrot having been present in the flock previously, i.e., old lesions (such as damage to the abaxial hoof wall indicative of underrun lesions having been present) were not observed. (iv) Topical treatments that may suppress or mask the severity of disease, such as antiseptic foot bathing, had not been used in the 4 weeks preceding each examination. (v) The sheep were all Merino, which are naturally susceptible to footrot (37).
Method 2.
A small number of animals were examined by an experienced AHO for the purpose of collecting lesion swabs. The sheep were sampled when convenient after the producer had drafted-off a proportion of the flock. The flock had been examined by the same AHO on two or more previous occasions and a clinical diagnosis made using method 1. As such, there was an interval between the time at which the clinical diagnoses were made and the time at which the lesion swabs were collected. The AHO informed the authors of his or her clinical diagnoses but did not provide the retrospective foot score data.
Method 3.
Sheep in a “hospital mob” were examined by the authors or an experienced AHO. Between 10 and 60 sheep from each hospital mob were examined on each farm, as indicated in Table 1. The sheep were sampled when convenient after the producer had drafted-off a proportion of the flock or the entire mob was sampled. The sheep were separated from the parent flock(s) by the producer because they had the most severe clinical signs of lameness or because they were the only sheep in the parent flock(s) with foot lesions. The sheep had not been examined previously by the authors or an experienced AHO, and retrospective foot scores were not available. However, a flock history was obtained from the producer describing the progression of the disease since it was first introduced to the flock. Clinical diagnosis was based on the severity of clinical disease observed in the hospital mob, on the number of sheep with score 4 lesions separated from the parent flocks(s), on the size of the parent flock (and therefore a rough estimate of apparent prevalence of sheep with severe lesions was possible), and on the flock history. Lesion swabs were collected at the time of examination by the authors or an AHO.
Collection of lesion swabs.
The interdigital skin or the active margin of an underrun lesion was swabbed with a sterile, cotton-tipped swab (CLASSIQSwabs, Copan Italia, Italy). In most cases, two swabs were collected from each lesion: the first was placed into a 5-ml serum vial containing modified Stuart's transport medium (mSTM) for microbiological culture, and the second was placed into a 1.5-ml microcentrifuge tube containing 500 μl of a lysis solution (buffer RLT [Qiagen] or nuclei lysis solution [Promega]) for DNA extraction and direct (culture-independent) testing. All swabs were transported on ice. Swabs collected for microbiological culture were processed immediately upon receipt at the laboratory. Swabs collected for direct testing were stored at 4°C prior to DNA extraction, which was undertaken 24 to 48 h after receipt.
Microbiological culture of D. nodosus.
Upon receipt at the laboratory, each lesion swab was removed from the mSTM and cultured anaerobically, as described previously (15).
Archival samples.
Lesion swabs were collected from flock 33 in 2006. The entire flock had been inspected on several occasions over a period of 3 years as part of a previous study (38), and the disease was classified as clinically benign on each occasion based on method 1. Individual D. nodosus isolates obtained in 2006 had been freeze-dried and stored at 4°C. For the present study, 15 randomly selected freeze-dried isolates were reconstituted in 100 μl phosphate-buffered saline (PBS) and spread-plated onto 4% hoof agar (HA) for microbiological culture, as described previously (15).
Control strains.
Virulent D. nodosus type strain A1001 and benign D. nodosus field strain JIR3528 were used as virulent (aprV2 positive) and benign (aprB2 positive) control strains, respectively.
DNA extraction.
Each pure culture of D. nodosus was harvested with a cotton-tipped swab, and DNA was extracted using the Wizard genomic DNA purification kit (Promega, WI, USA) in accordance with the protocol for Gram-negative bacteria. Extraction of DNA from lesion swabs was undertaken using a magnetic bead DNA purification kit (BioSprint 96 one-for-all vet kit, Qiagen) according to the BS96 Vet 100 protocol.
PCR identification of D. nodosus.
D. nodosus was identified via conventional PCR or real-time PCR amplification of a variable region of the D. nodosus 16S rRNA gene (40, 41). PCR products were visualized on 2% agarose gels stained with RedSafe (iNtRON Biotechnology, South Korea) and viewed under UV light, as described previously (15).
Phenotypic virulence testing.
Gelatin gel test.
Each pure culture of D. nodosus was evaluated using the gelatin gel test, as described previously (12). Known stable (A1001) and unstable (JIR3528) D. nodosus strains were included as controls.
Elastase test.
Each pure culture of D. nodosus was evaluated for elastase activity, as described previously (13). An isolate with known elastase activity (virulent D. nodosus type strain A1001, elastase positive at 4 to 8 days postinoculation) was included as a virulent control for each test.
aprV2/B2 qPCR test.
Primers, probes, and master mixes reported by Stäuble et al. (22) and Frosth et al. (21) were ordered from Thermo Fisher Scientific, Inc. Amplification was performed in a Stratagene Mx3000P thermocycler (Agilent Technologies, Santa Clara, CA). Reaction mixtures and cycling conditions were as described by Stäuble et al. (22). Reaction mixtures as described by Frosth et al. (21) were used; however, as the thermocycler used in this study was unable to accommodate the fast-cycling conditions described by the authors, the cycling conditions recommended in the TaqMan gene expression master mix protocol were used, consisting of a UNG activation step of 2 min at 50°C, an initial denaturation step of 10 min at 95°C, followed by 40 cycles of denaturation at 95°C for 15 s, and annealing/extension at 60°C for 60 s.
Virulent (aprV2 positive) and benign (aprB2 positive) controls were included in each run. A valid qPCR run was one in which: (i) there was amplification of both replicates of the aprV2 and aprB2 positive controls, with threshold cycle (CT) values falling within the range of the standard curve (0.005 pg to 5,000 pg); (ii) there was no amplification of the aprV2 and aprB2 negative controls; and (iii) given that the qPCR test was only used to determine the presence or absence of the two alleles during this study, amplification efficiencies of 85 to 110% were accepted. The fluorescence threshold was initially set automatically for each run by the MxPro software (Agilent Technologies, Santa Clara, CA). However, to ensure that comparable CT values were calculated for each run, the average fluorescence threshold was calculated for each target using the fluorescence threshold values set for all 20 runs, and applied retrospectively to each run.
Analytical performance of the qPCR tests.
Analytical sensitivity.
Amplification of the aprV2 and aprB2 alleles was analyzed separately using serial dilutions of genomic DNA prepared from pure cultures of virulent D. nodosus type strain A1001 and benign D. nodosus field strain JIR3528, respectively. The limits of detection (LOD) and amplification efficiencies were calculated for the aprV2 and aprB2 alleles for both qPCR tests. Data were collected from 20 individual experiments, with each reaction performed in duplicate (n = 40 data points per concentration). DNA template concentrations ranged from 0.0005 pg to 5,000 pg of D. nodosus genomic DNA per reaction. The LOD was defined as the lowest concentration of genomic DNA at which amplification occurred for 50% of the replicates (27).
Analytical specificity.
The analytical specificity of each qPCR test was evaluated using DNA extracted from 15 bacterial species along with the virulent and benign D. nodosus type strains (Table 2).
Repeatability.
The between-run repeatability of the qPCR test was determined for the aprV2 and aprB2 alleles using three different concentrations of D. nodosus genomic DNA: 5,000 pg, 50 pg, and 0.5 pg per reaction. The coefficient of variation (CV) was calculated for each concentration using CT values collected across 20 qPCR runs, with each reaction performed in duplicate (n = 40 data points per concentration). The coefficient of variation (CV) was calculated for each concentration of genomic DNA as CV = standard deviation of replications/mean of replicates × 100.
Diagnostic performance of each virulence test.
The levels of agreement between clinical diagnoses and laboratory diagnoses of virulent and benign footrot using each laboratory virulence test were compared. An outbreak of footrot was classified as virulent if one or more isolates were classified as virulent by a given laboratory virulence test.
Diagnostic sensitivity and specificity.
(i) qPCR test.
At the flock level, diagnostic sensitivity (DSe) was defined as the percentage of clinically virulent flocks in which one or more swabs tested positive for the aprV2 allele, while diagnostic specificity (DSp) was defined as the percentage of clinically benign flocks in which none of the swabs tested positive for the aprV2 allele. At the foot swab level, DSe was defined as the percentage of foot swabs collected from sheep in clinically virulent flocks that tested positive for the aprV2 allele, while DSp was defined as the percentage of foot swabs collected from clinically benign flocks that tested negative for the aprV2 allele.
(ii) Elastase test.
At the flock level, DSe was defined as the percentage of clinically virulent flocks from which one or more elastase-positive D. nodosus isolates were obtained, while DSp was defined as the percentage of clinically benign flocks from which no elastase-positive isolates were obtained. At the isolate level, DSe was defined as the percentage of isolates obtained from clinically virulent flocks that were elastase positive, while DSp was defined as the percentage of isolates obtained from clinically benign flocks that were elastase negative.
(iii) Gelatin gel test.
DSp was evaluated at both the flock and isolate levels. At the flock level, DSp was defined as the percentage of clinically benign flocks from which only heat-labile D. nodosus isolates were obtained. At the isolate level, DSp was defined as the percentage of isolates obtained from clinically benign flocks that were heat labile.
Statistical analysis.
The levels of agreement between clinical and laboratory diagnoses were evaluated using Cohen's kappa statistic (42). Kappa statistics were interpreted using the standards for strength of agreement proposed by Landis and Koch (43): ≤0, poor agreement; 0.01 to 0.20, slight agreement; 0.21 to 0.40, fair agreement; 0.41 to 0.60, moderate agreement; 0.61 to 0.80, substantial agreement; and 0.81 to 1.00, almost perfect agreement. McNemar's chi-square test for paired observations (44) was performed to establish if there were statistically significant differences between the proportions of outbreaks classified as virulent by clinical or laboratory diagnosis using each of the three virulence tests. The results of each individual laboratory virulence test were also compared using this approach. All statistical analyses were conducted in Microsoft Excel 2010. Exact 95% binomial confidence intervals were calculated for diagnostic sensitivities and specificities in GenStat 16th Edition (VSN International, UK).
ACKNOWLEDGMENTS
Funding for this study was provided by Australian sheep meat producers and the Commonwealth Government through Meat and Livestock Australia (MLA) and by the Ian (Peter) Wrigley Fund.
The authors wish to acknowledge Bruce Jackson, Andrew Ewers, Deb Lehmann, Scott Ison, and Neale Whitsed for identifying suitable flocks for inclusion in the present study and for their assistance with clinical examinations and collecting lesion swabs. The authors also wish to acknowledge Natalie Schiller, Nobel Toribio, Gina Attard, Karren Plain, and Paul Hick for their technical assistance.
The authors declare no conflict of interest.
REFERENCES
- 1.Beveridge WIB. 1941. Foot-rot in sheep: a transmissible disease due to infection with Fusiformis nodosus (n. sp.). Studies on its cause, epidemiology, and control. J Counc Sci Ind Res 140:1–56. [Google Scholar]
- 2.Dewhirst FE, Paster BJ, La Fontaine S, Rood JI. 1990. Transfer of Kingella indologenes (Snell and Lapage 1976) to the genus Suttonella gen. nov. as Suttonella indologenes comb. nov.; transfer of Bacteroides nodosus (Beveridge 1941) to the genus Dichelobacter gen. nov. as Dichelobacter nodosus comb. nov.; and assignment of the genera Cardiobacterium, Dichelobacter, and Suttonella to Cardiobacteriaceae fam. nov in the gamma division of Proteobacteria on the basis of 16S rRNA sequence comparisons. Int J Syst Bacteriol 40:426–433. [DOI] [PubMed] [Google Scholar]
- 3.Lane J, Jubb T, Shephard R, Webb-Ware J, Fordyce G. 2015. Priority list of endemic diseases for the red meat industries. GHD Pty Ltd., Meat and Livestock Australia, North Sydney, Australia [Google Scholar]
- 4.Gradin JL, Sonn AE, Petrovska L. 1993. Serogrouping of Bacteroides nodosus isolates from 62 sources in the United States. Am J Vet Res 54:1069–1073. [PubMed] [Google Scholar]
- 5.Nieuwhof GJ, Bishop SC. 2005. Costs of the major endemic diseases of sheep in Great Britain and the potential benefits of reduction in disease impact. Anim Sci 81:23–29. doi: 10.1079/ASC41010023. [DOI] [Google Scholar]
- 6.Graham NPH, Egerton JR. 1968. Pathogenesis of ovine foot-rot: the role of some environmental factors. Aust J Sci 44:235–240. [DOI] [PubMed] [Google Scholar]
- 7.Stewart DJ, Peterson JE, Vaughan JA, Clark BL, Emery DL, Caldwell JB, Kortt AA. 1986. The pathogenicity and cultural characteristics of virulent, intermediate and benign strains of Bacteroides nodosus causing ovine foot-rot. Aust Vet J 63:317–326. doi: 10.1111/j.1751-0813.1986.tb02875.x. [DOI] [PubMed] [Google Scholar]
- 8.Stewart DJ, Claxton PD. 1993. Ovine footrot: clinical diagnosis and bacteriology. In Corner LA, Bagust TJ (ed), Australian standard diagnostic tests for animal diseases. CSIRO, East Melbourne, Victoria, Australia. [Google Scholar]
- 9.Kennan RM, Wong W, Dhungyel OP, Han X, Wong D, Parker D, Rosado CJ, Law RHP, McGowan S, Reeve SB, Levina V, Powers GA, Pike RN, Bottomley SP, Smith AI, Marsh I, Whittington RJ, Whisstock JC, Porter CJ, Rood JI. 2010. The subtilisin-like protease AprV2 is required for virulence and uses a novel disulphide-tethered exosite to bind substrates. PLoS Pathog 6:e1001210. doi: 10.1371/journal.ppat.1001210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Egerton JR, Roberts DS. 1971. Vaccination against ovine foot-rot. J Comp Pathol 81:179–185. doi: 10.1016/0021-9975(71)90091-0. [DOI] [PubMed] [Google Scholar]
- 11.Egerton JR. 1989. Control and eradication of footrot at the farm level - the role of veterinarians, abstr proceedings 19th Annual Seminar, Society of Sheep & Beef Cattle Veterinarians, New Zealand Veterinary Association, Wellington, New Zealand. [Google Scholar]
- 12.Palmer MA. 1993. A gelatin test to detect activity and stability of proteases produced by Dichelobacter (Bacteroides) nodosus. Vet Microbiol 36:113–122. doi: 10.1016/0378-1135(93)90133-R. [DOI] [PubMed] [Google Scholar]
- 13.Stewart DJ. 1979. The role of elastase in the differentiation of Bacteroides nodosus infections in sheep and cattle. Res Vet Sci 27:99–105. [PubMed] [Google Scholar]
- 14.Links IJ, Morris S. 1996. Assessment of gelatin gel and elastase tests for detection of protease activity of Dichelobacter nodosus isolates from ovine footrot. Vet Microbiol 51:305–318. doi: 10.1016/0378-1135(96)00011-9. [DOI] [PubMed] [Google Scholar]
- 15.Dhungyel OP, Hill AE, Dhand NK, Whittington RJ. 2013. Comparative study of the commonly used virulence tests for laboratory diagnosis of ovine footrot caused by Dichelobacter nodosus in Australia. Vet Microbiol 162:756–760. doi: 10.1016/j.vetmic.2012.09.028. [DOI] [PubMed] [Google Scholar]
- 16.Cheetham BF, Tanjung LR, Sutherland M, Druitt J, Green G, McFarlane J, Bailey GD, Seaman JT, Katz ME. 2006. Improved diagnosis of virulent ovine footrot using the intA gene. Vet Microbiol 116:166–174. doi: 10.1016/j.vetmic.2006.04.018. [DOI] [PubMed] [Google Scholar]
- 17.Billington SJ, Johnston JL, Rood JI. 1996. Virulence regions and virulence factors of the ovine footrot pathogen, Dichelobacter nodosus. FEMS Microbiol Lett 145:147–156. doi: 10.1111/j.1574-6968.1996.tb08570.x. [DOI] [PubMed] [Google Scholar]
- 18.Lilley GG, Stewart DJ, Kortt AA. 1992. Amino acid and DNA sequences of an extracellular basic protease of Dichelobacter nodosus show that it is a member of the subtilisin family of proteases. Eur J Biochem 210:13–21. doi: 10.1111/j.1432-1033.1992.tb17385.x. [DOI] [PubMed] [Google Scholar]
- 19.Kortt AA, Stewart DJ. 1994. Properties of the extracellular acidic proteases of Dichelobacter nodosus. Stability and specificity of peptide bond cleavage. Biochem Mol Biol Int 34:1167–1176. [PubMed] [Google Scholar]
- 20.Riffkin MC, Wang L-F, Kortt AA, Stewart DJ. 1995. A single amino-acid change between the antigenically different extracellular serine proteases V2 and B2 from Dichelobacter nodosus. Gene 167:279–283. doi: 10.1016/0378-1119(95)00664-8. [DOI] [PubMed] [Google Scholar]
- 21.Frosth S, Koenig U, Nyman A-K, Pringle M, Aspan A. 2015. Characterisation of Dichelobacter nodosus and detection of Fusobacterium necrophorum and Treponema spp. in sheep with different clinical manifestations of footrot. Vet Microbiol 179:82–90. doi: 10.1016/j.vetmic.2015.02.034. [DOI] [PubMed] [Google Scholar]
- 22.Stäuble A, Steiner A, Frey J, Kuhnert P. 2014. Simultaneous detection and discrimination of virulent and benign Dichelobacter nodosus in sheep of flocks affected by foot rot and in clinically healthy flocks by competitive real-time PCR. J Clin Microbiol 52:1228–1231. doi: 10.1128/JCM.03485-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Department of Agriculture and Food, Western Australia. 2015. New test for virulent footrot looks promising. Department of Agriculture and Food, Western Australia, South Perth, WA. https://www.agric.wa.gov.au/news/media-releases/new-test-virulent-footrot-looks-promising. Accessed 28 August 2016.
- 24.Rawlin G. 2016. A new approach to virulent footrot diagnosis. Agriculture Victoria, Victoria, Australia. http://agriculture.vic.gov.au/agriculture/livestock/sheep/sheep-notes-newsletters/sheep-notes-spring-2016/a-new-approach-to-virulent-footrot-diagnosis Accessed August 28.
- 25.Buller N, Eamens G. 2014. Ovine Footrot. Australian and New Zealand standard diagnostic procedure. http://www.agriculture.gov.au/SiteCollectionDocuments/animal/ahl/ANZSDP-Ovine-footrot.pdf.
- 26.Allworth MB. 1990. Footrot. Control and eradication. Sheep Medicine Postgraduate Committee in Veterinary Science 141:443–454. [Google Scholar]
- 27.World Organization for Animal Health. 2016. Manual of diagnostic tests and vaccines for terrestrial animals, p 1–16. World Organization for Animal Health, Paris, France. [Google Scholar]
- 28.Kreader CA. 1996. Relief of amplification inhibition in PCR with bovine serum albumin or T4 gene 32 protein. Appl Environ Microbiol 62:1102–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Myers GSA, Parker D, Al-Hasani K, Kennan RM, Seemann T, Ren QH, Badger JH, Selengut JD, DeBoy RT, Tettelin H, Boyce JD, McCarl VP, Han XY, Nelson WC, Madupu R, Mohamoud Y, Holley T, Fedorova N, Khouri H, Bottomley SP, Whittington RJ, Adler B, Songer JG, Rood JI, Paulsen IT. 2007. Genome sequence and identification of candidate vaccine antigens from the animal pathogen Dichelobacter nodosus. Nat Biotechnol 25:569–575. doi: 10.1038/nbt1302. [DOI] [PubMed] [Google Scholar]
- 30.Scott-Orr H, Seaman JT. 2006. The New South Wales footrot strategic plan–an example of a long-term successful disease control program, p 196. Proceedings of the 11th International Symposium on Veterinary Epidemiology and Economics, Cairns, Australia. [Google Scholar]
- 31.Allworth MB. 2014. Challenges in ovine footrot control. Small Rumin Res 118:110–113. doi: 10.1016/j.smallrumres.2013.12.007. [DOI] [Google Scholar]
- 32.Raadsma HW, Egerton JR. 2013. A review of footrot in sheep: aetiology, risk factors and control methods. Livest Sci 156:106–114. doi: 10.1016/j.livsci.2013.06.009. [DOI] [Google Scholar]
- 33.Egerton JR, Parsonson IM. 1969. Benign foot-rot—a specific interdigital dermatitis of sheep associated with infection by less proteolytic strains of Fusiformis nodosus. Aust Vet J 45:345–349. doi: 10.1111/j.1751-0813.1969.tb06606.x. [DOI] [PubMed] [Google Scholar]
- 34.Egerton JR, Ghimire SC, Dhungyel OP, Shrestha HK, Joshi HD, Joshi BR, Abbott KA, Kristo C. 2002. Eradication of virulent footrot from sheep and goats in an endemic area of Nepal and an evaluation of specific vaccination. Vet Rec 151:290–295. doi: 10.1136/vr.151.10.290. [DOI] [PubMed] [Google Scholar]
- 35.Egerton JR, Raadsma HW. 1993. Unresolved questions about footrot eradication. Wool Technol Sheep Breed 41:99–107. [Google Scholar]
- 36.Locher I, Greber D, Holdener K, Luchinger R, Haerdi-Landerer C, Schupbach-Regula G, Frey J, Steiner A. 2015. Longitudinal Dichelobacter nodosus status in 9 sheep flocks free from clinical footrot. Small Rumin Res 132:128–132. doi: 10.1016/j.smallrumres.2015.10.021. [DOI] [Google Scholar]
- 37.Emery DL, Stewart DJ, Clark BL. 1984. The comparative susceptibility of five breeds of sheep to foot-rot. Aust Vet J 61:85–88. doi: 10.1111/j.1751-0813.1984.tb15524.x. [DOI] [PubMed] [Google Scholar]
- 38.Dhungyel OP, Lehmann DR, Whittington RJ. 2008. Pilot trials in Australia on eradication of footrot by flock specific vaccination. Vet Microbiol 132:364–371. doi: 10.1016/j.vetmic.2008.05.027. [DOI] [PubMed] [Google Scholar]
- 39.Conington J, Hosie B, Nieuwhof GJ, Bishop SC, Bunger L. 2008. Breeding for resistance to footrot–the use of hoof lesion scoring to quantify footrot in sheep. Vet Res Commun 32:583–589. doi: 10.1007/s11259-008-9062-x. [DOI] [PubMed] [Google Scholar]
- 40.Frosth S, Slettemeas JS, Jorgensen HJ, Angen O, Aspan A. 2012. Development and comparison of a real-time PCR assay for detection of Dichelobacter nodosus with culturing and conventional PCR: harmonisation between three laboratories. Acta Vet Scand 54:6. doi: 10.1186/1751-0147-54-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.La Fontaine S, Egerton JR, Rood JI. 1993. Detection of Dichelobacter nodosus using species-specific oligonucleotides as PCR primers. Vet Microbiol 35:101–117. doi: 10.1016/0378-1135(93)90119-R. [DOI] [PubMed] [Google Scholar]
- 42.Cohen J. 1960. A coefficient of agreement for nominal scales. Educ Psychol Meas 20:37–46. doi: 10.1177/001316446002000104. [DOI] [Google Scholar]
- 43.Landis JR, Koch GG. 1977. The measurement of observer agreement for categorical data. Biometrics 33:159–174. doi: 10.2307/2529310. [DOI] [PubMed] [Google Scholar]
- 44.McNemar Q. 1947. Note on the sampling error of the difference between correlated proportions or percentages. Psychometrika 12:153–157. doi: 10.1007/BF02295996. [DOI] [PubMed] [Google Scholar]