ABSTRACT
The immigration of Latin American women of childbearing age has spread the congenital transmission of Chagas disease to areas of nonendemicity, and the disease is now a worldwide problem. Some European health authorities have implemented screening programs to prevent vertical transmission, but the lack of a uniform protocol calls for the urgent establishment of a new strategy common to all laboratories. Our aims were to (i) analyze the trend of passive IgG antibodies in the newborn by means of five serological tests for the diagnosis and follow-up of congenital Trypanosoma cruzi infection, (ii) assess the utility of these techniques for diagnosing a congenital transmission, and (iii) propose a strategy for a prompt, efficient, and cost-effective diagnosis of T. cruzi infection. In noninfected newborns, a continuous decreasing trend of passive IgG antibodies was observed, but none of the serological assays seroreverted in any the infants before 12 months. From 12 months onwards, serological tests achieved negative results in all the samples analyzed, with the exception of the highly sensitive chemiluminescent microparticle immunoassay (CMIA). In contrast, in congenitally infected infants, the antibody decline was detected only after treatment initiation. In order to improve the diagnosis of congenital T. cruzi infection, we propose a new strategy involving fewer tests that allows significant cost savings. The protocol could start 1 month after birth with a parasitological test and/or a PCR. If negative, a serological test would be carried out at 9 months, which if positive, would be followed by another at around 12 months for confirmation.
KEYWORDS: congenital Chagas disease, serology, flowchart, countries of endemicity, immigrant population, Spain, Europe
INTRODUCTION
Chagas disease, or American trypanosomiasis, is a zoonotic and parasitic infection caused by Trypanosoma cruzi, affecting around six million people in Latin America (1), where it is estimated that approximately two million women of childbearing age are infected (2, 3). In areas of endemicity, the infection is mainly due to vectorial transmission, while in areas where the vector is absent, T. cruzi can be transmitted by alternative routes, such as blood transfusion, organ transplant, and vertical transmission (4). In areas of nonendemicity, strict protocols exist in blood banks and transplants so mother-to-child transmission is the main route for T. cruzi transmission, and its control represents an important challenge (5–7).
The immigration of Latin American women of childbearing age has spread the congenital transmission of Chagas disease to areas of nonendemicity (2), especially Europe and the United States (8–10), and the disease has become a worldwide health problem. A total of approximately 1.7 million people from countries endemic for Chagas disease live in Spain, and around 50% of them are women of reproductive age (11).
In areas where T. cruzi is endemic, its prevalence in pregnant women is highly varied (from 5 to 40%), depending on their geographical origin, and the congenital transmission rate from mother to child is about 1 to 12% (12–16). Cases of congenital T. cruzi infection have also been described in Spain (17–22). The rates of seroprevalence and transmission in pregnant Latin American women living in Spain reported by Muñoz et al. (21) are 3.4% and 7.3%, respectively. Nevertheless, a point to consider is the high index of underdiagnosis (23).
Congenital infection with T. cruzi is mostly asymptomatic and may progress to chronic Chagas disease, with cardiac and/or gastrointestinal disorders occurring years later (24–26). In cases of symptomatic Chagas disease, there is a spectrum of clinical manifestations, commonly nonspecific, and they can appear at birth or days later (24, 27). Severe cases have a mortality rate of approximately 5% and are associated with myocarditis and meningoencephalitis (7, 24).
Benznidazole and nifurtimox are the only approved trypanocidal drugs with proven efficacy for the treatment of Chagas disease (28). Both compounds are better tolerated in infancy and more effective during the acute phase of the infection (28–30). Treatment within the first year of life is close to 100% effective and without the adverse reactions seen in adults (29, 31, 32). Treating infected women of childbearing age could be a useful strategy to prevent the congenital transmission of T. cruzi (16, 33). In Spain, a study also reported that the screening of pregnant Latin American women and their infants to detect and treat congenital cases at an early stage is cost-effective (11).
During the first weeks of life, diagnosis relies on the microscopic observation of bloodstream parasites, usually with concentration techniques, like the microhematocrit method or micromethod (14). The micromethod has been used for many years in areas of endemicity for the early detection of parasites in newborn blood, although its lack of sensitivity can miss up to 50% of infected infants (34). The sensitivity of the technique varies according to the parasitemia (35). Parasite detection in blood by molecular techniques, such as PCR, may improve early diagnosis (27, 31, 36, 37). The parasite burden is at its maximum 1 month after birth (25, 38, 39), so it would be preferable to perform the PCR at this time and also to avoid false-positive diagnosis in case of parasite DNA transmission from mother to fetus (25). Amniotic fluid is not useful for the diagnosis of congenital T. cruzi infection (40), and there is disagreement about the utility of umbilical cord blood (41).
Serological tests are useful for chronic diagnosis and for the follow-up of newborns if the direct diagnosis has not been conclusive (31). However, to date, no single serological test is considered the reference standard for the diagnosis of Chagas disease (42). Consequently, confirmation of the infection requires coincident positive results of two tests in infants age >8 months in order to avoid the detection of anti-T. cruzi IgG antibodies of maternal origin (31, 42). A third technique should be performed when the results are conflicting (42). The detection of anti-T. cruzi IgM antibodies in the newborn is controversial (36, 41). Rodríguez et al. (43) reported that IgM antibodies were not useful for diagnosis because they could appear in response to the excretion/secretion of T. cruzi antigens, which cross the placenta.
In order to prevent vertical transmission, some European health authorities have implemented screening programs for pregnant Latin American women and their babies (44–51). Early diagnosis and treatment of the newborns have high priority in control programs (14). However, the existence of procedural differences between protocols urgently calls for the establishment of a new and more efficient strategy common to all laboratories.
The aims of this study were to (i) analyze the trend of passive IgG antibodies in the newborn by means of five serological tests for the diagnosis and follow-up of congenital T. cruzi infection, (ii) assess the utility of these techniques in the diagnosis of a congenital transmission, and (iii) propose a suitable strategy for a prompt, efficient, and cost-effective diagnosis of T. cruzi infection.
RESULTS
From the 81 newborns included in the study, it was possible to obtain a sample at birth to 1 month in 78 cases, and the percentage of seropositivity in this first sample was 97.4%. Fourteen out of 81 newborns were excluded from the follow-up study, as they were unavailable for further sampling, resulting in a final panel of 67 infants.
Four out of the 67 infants were diagnosed as congenitally infected by T. cruzi based on positive real-time PCR (RT-PCR) results at different times during the follow-up and a constant level of IgG antibodies during the first year of life (Table 1). The remaining 63 infants were considered not infected due to negative RT-PCR results and a drop in IgG antibodies.
TABLE 1.
Test results with congenitally infected infants (n = 4)a
| Case | Tested groupb | PCR resultc | IFAT (dilution) | ELISAc (U) | ELISAr (Abs/CO) | CMIA (S/CO) | WB (kDa bands) |
|---|---|---|---|---|---|---|---|
| 1 | Mother | ND | ≥1:5,120 | 169 | 8.39 | 12.31 | 6/6 |
| Birth–1 mo | ND | 1:2,560 | ND | 8.52 | ND | ND | |
| >1–6 mo | ND | 1:640 | 76 | 6.82 | 7.97 | 5/6 (28/32/38/39/40) | |
| >6–9 mo | ND | 1:640 | ND | 7.47 | ND | ND | |
| >9–12 mo | Pos | 1:640 | ND | 6.67 | ND | ND | |
| 1 mo Pt. | ND | 1:640 | ND | 8.70 | ND | ND | |
| 12 mo Pt. | ND | 1:80 | 42 | 1.63 | 3.57 | 0/6 | |
| 2 | Mother | Pos | 1:1,280 | 200 | 7.60 | 11.07 | 5/6 (28/32/38/39/40) |
| >12 mo | Pos | 1:1,280 | 162 | 5.99 | 12.04 | 5/6 (28/32/38/39/40) | |
| During Tr. | Neg | ND | 164 | 5.96 | 11.78 | 3/6 (28/32/38) | |
| 5 mo Pt. | Neg | ND | 116 | 5.11 | 10.29 | 3/6 (28/32/38) | |
| 16 mo Pt. | Neg | 1:160 | 89 | 3.87 | 8.25 | 1/6 (32) | |
| 3 yr Pt. | Neg | ND | 66 | 2.07 | 8.07 | 1/6 (32) | |
| 4 yr Pt. | Neg | 1:80 | 54 | 1.35 | 7.66 | 0/6 | |
| 3 | Mother | Pos | 1:2,560 | 178 | 5.78 | 10.9 | 5/6 (28/32/38/39/40) |
| Birth–1 mo | Pos | 1:1,280 | 279 | 5.56 | 11.22 | 5/6 (28/32/38/39/40) | |
| 1 day Pt. | ND | 1:320 | 95 | 3.52 | 5.02 | 3/6 (32/38/40) | |
| 2 yr Pt. | Neg | ND | 8 | 0.2 | ND | ND | |
| 4 | Mother | Pos | 1:1,280 | 159 | 2.65 | 12.81 | 5/6 (28/32/38/39/40) |
| Birth–1 mo | Neg | 1:1,280 | 141 | 3.1 | ND | ND | |
| >12 mo | Pos | 1:1,280 | 143 | 6.41 | 11.64 | 5/6 (28/32/38/39/40) | |
| 7 mo Pt. | Neg | 1:320 | 105 | 5.77 | 7.21 | 5/6 (28/32/38/39/40) |
ND, not done.
Pt., posttreatment; Tr., treatment.
Pos, positive; Neg, negative.
In the four congenitally infected newborns (see Table 1), a trend toward a reduction in antibodies was not perceived until treatment initiation, after which antibodies began to decline, even disappearing in one infant (case 3); additionally, RT-PCR turned from positive to negative in all cases in which the assay was done. A negative RT-PCR result was observed at birth in one of the infected infants (case 4), although the infection was confirmed at 12 months with both a positive RT-PCR result and the maintenance of IgG antibody titers.
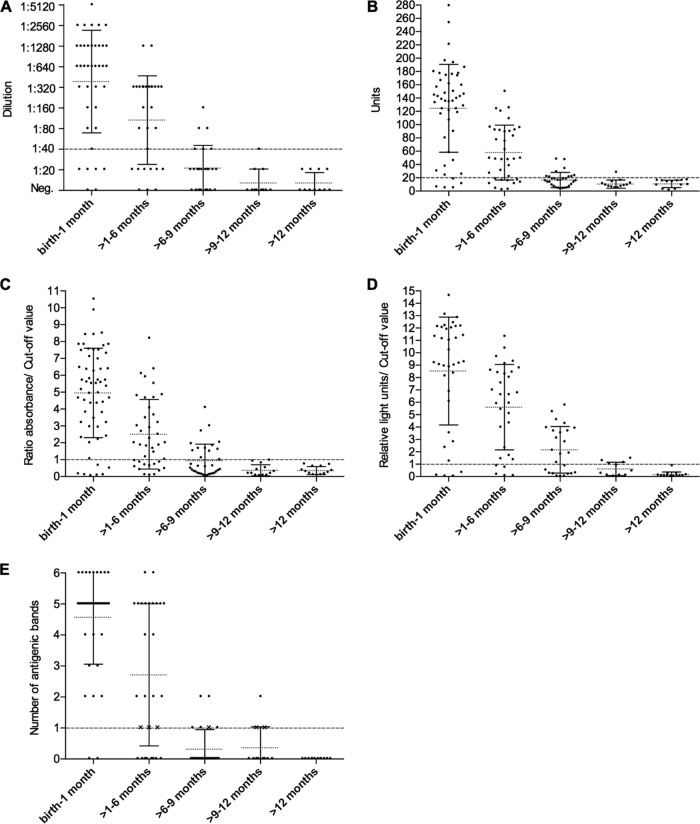
For all newborns, samples were grouped according to the age of the newborn when the sample was collected. The percentages of sera with positive and gray-zone results obtained by the five serological assays during the five follow-up periods of the noninfected newborns are shown in Fig. 1. The details of the results obtained by each serological assay during the follow-up of the noninfected newborns are shown in Fig. 2.
FIG 1.

Percentages of sera with positive and gray-zone results obtained by the five serological assays during the follow-up of the noninfected newborns. n, number of sera analyzed.
FIG 2.
Results obtained by each serological assay during the five follow-up periods of the noncongenital newborns: IFAT (A), ELISAc (B), ELISAr (C), CMIA (D), and WB (E). Dashed lines represent the cutoff values established for each test: 1:40 dilution for IFAT (A), 20 units for ELISAc (B), 1 absorbance/cutoff value for ELISAr (C), 1 relative light unit/cutoff value for CMIA (D), and a single band of the pattern (28, 32, 38, 39, 40, or 48 kDa) when it is as intense as the same band in the positive control for WB (E). Dotted lines indicate the mean of the results obtained in each period of the follow-up, and solid lines represent the standard deviation (SD). Crosses (X) in panel E indicate samples considered negative because they showed a band of the pattern but not as strong as in the positive control. Neg., negative.
Although a continuous decreasing trend of the passive IgG antibodies in noninfected newborns was observed over time, all serological assays still obtained at least one gray-zone (BioELISA [ELISAr] and chemiluminescent microparticle immunoassay [CMIA]) and/or positive result (indirect fluorescent antibody test [IFAT], in-house enzyme-linked immunosorbent assay [ELISA] [ELISAc], CMIA, and Western blotting [WB]) at >9 to 12 months of age. Maternal antibodies disappeared completely at >12 months of age, except for one remaining gray-zone sample detected by the CMIA.
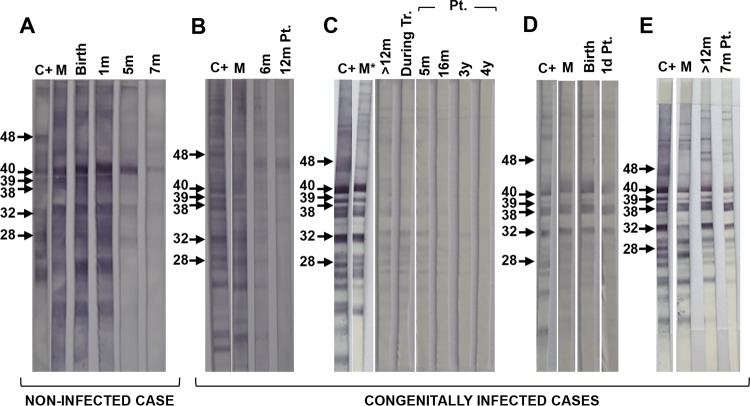
The WB pattern observed in mothers and noninfected and infected children was very homogeneous (Fig. 3). In noninfected infants tested at birth, the serum pattern did not show any differences with the mother (Fig. 3A), and the number of bands and their intensity decreased during the follow-up, with 80% testing negative at >6 to 9 months and all of them negative at 12 months. In the congenital cases, no differences were observed in the patterns between mother and newborn at birth, while some variation was apparent after a few months. In case 1, the bands showed a heterogeneous intensity 6 months after birth (Fig. 3B), and in case 4, these differences were observed at 15 months (Fig. 3E).
FIG 3.
Examples of the WB patterns of bands recognized in the newborns studied and their mothers. Noninfected (A) and congenitally infected children: case 1 (B), case 2 (C), case 3 (D), and case 4 (E). C+, positive control; M, mother; m, month; Pt., posttreatment; y, years; d, days. Molecular weights in kilodaltons are shown on the left in each panel. *, samples of the mother and newborn could not be analyzed in parallel in the same nitrocellulose membrane due to insufficient volume.
A proposed new strategy for the diagnosis of congenital Chagas disease is presented as a flowchart in Fig. 4.
FIG 4.
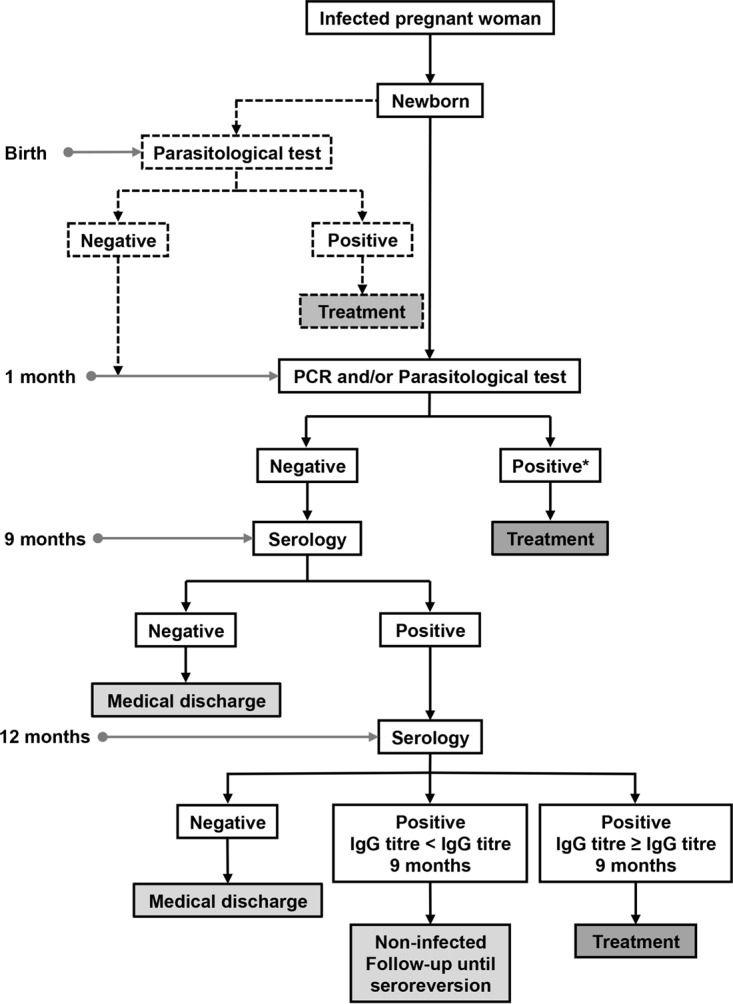
Proposal of a new strategy for the diagnosis of congenital Chagas disease. *, Infection is considered congenital with a positive result in any of the tests (PCR and/or parasitological test). Dashed lines indicate that a test is included as optional in the flowchart.
DISCUSSION
Screening programs for Chagas disease in pregnant Latin American women and their children are still uncommon in areas of nonendemicity (7, 52). Several European regions have implemented official prevention strategies to avoid the transmission of congenital Chagas disease (44): Tuscany in Italy (45) and three autonomous communities of Spain (Catalonia, Galicia, and Valencia) (46, 48, 49). The official screening programs implemented by European health authorities are summarized in Table 2. Other regions, namely, Andalusia and the Basque Country in Spain, recommend the screening of all pregnant women susceptible to the disease (50, 51). The diversity of procedures used in the control programs calls for the establishment of a uniform strategy suitable for all laboratories and that would allow a prompt, efficient, and cost-effective diagnosis of congenital T. cruzi infection.
TABLE 2.
Prevention strategies against the transmission of congenital Chagas disease implemented by European regionsa
| Protocol by region | Parasitological test |
PCR |
Serology test |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Birth | 1 mo | Birth | 1 mo | 7 mo | 9 mo | Birth | 1 mo | 7 mo | 9 mo | 12 mo | |
| Spain | |||||||||||
| Catalonia, 2010 (46) | ✓ M | X | X | X | X | X | X | X | X | ✓ | X |
| Valencia, 2009 (49) | ✓ M,S | ○ | ✓ | ○ | ○ | ? | X | ✓ | ✓ | ||
| Galicia, 2014 (48) | ✓ | ✓ | ✓ | ✓ | X | ✓ | X | X | X | ✓ | ✓ |
| Italy | |||||||||||
| Tuscany, 2012 (45) | ✓ T | ✓ | ✓ | ✓ | X | X | ✓ | X | X | ✓ | ✓ |
| Proposal | ○ | ✓ | X | ✓ | X | X | X | X | X | ✓ | ✓* |
Check marks (✓) indicate the tests and periods included in the protocols. Crosses (X) indicate the tests and periods not included in the protocols. Open circles (○) indicate the tests and periods included as optional in the protocols. M, micromethod; S, Strout method; T, thin or thick blood smear. *, only in case of a positive serology at 9 months of age.
Since most of the samples included in the present study were obtained retrospectively, not all patients could be followed during the first year of life, and not all the samples were analyzed by the five serological tests due to absent or insufficient volume. As expected, the proportion of positive sera in the first sample (birth to 1 month) was very high, in accordance with the findings of other authors (21), due to the detection of IgG antibodies of maternal origin. On the contrary, 2.6% (2/78) of the samples were negative, and all belonged to uninfected children. Of all the samples analyzed, congenital Chagas disease was confirmed in only 6%. This rate of congenital transmission is similar to others reported in different regions of Spain: Barcelona (7.3%), Madrid (2.6%), Valencia (3.7%), and Biscay (5.8%) (21, 53–55). These results do not significantly differ from those in areas of endemicity (12, 13, 16), although Howard et al. (15) consider that countries endemic for the disease have higher rates of congenital transmission.
All the official protocols published and implemented in Europe agree on the inclusion of a parasitological test at birth and, with the exception of Catalonia, if the result is negative, a new sample is analyzed 1 month later. In the Valencian protocol, the retest at 1 month is optional. The disadvantages of microscopic examination are low sensitivity and high subjectivity, since it depends on the ability and training of the observer to detect the parasite in the blood sample (39, 56). Both factors could lead to parasites being missed in congenitally infected newborns (57). Another important point is that the sample quality and storage play key roles in the results (35). In areas of nonendemicity, immediate observation of the sample is rare, and the low level of parasites and their lack of mobility, directly related to the storage time, might contribute to false-negative results. The highly varied parasitic burden in neonates, the number of replicates, and the time of observation are other important factors for consideration (38, 39). It should be also considered that congenital infection at any stage of pregnancy, as well as the perinatal transmission of T. cruzi, may affect parasitemia (57, 58). Thus, more sensitive methods are needed. These inconveniences for parasitological analysis have also been described in primary health care facilities in rural areas of endemicity (31).
The capacity of molecular techniques, such as PCR, to detect a small amount of parasites in blood can provide an early diagnosis (2, 59, 60). All the current screening programs include a PCR at birth, except in Catalonia, and the test is repeated 1 month later if the result is negative, except in Valencia, where it is optional. Additionally, in the case of negative results, the protocols of Valencia and Galicia recommend another PCR test between 7 and 9 months and at 9 months of age, respectively. At present, the screening program of Catalonia is being updated, and a PCR during the first month of life has been included in the new revised protocol. The maximum parasitic load is found 1 month after birth, when T. cruzi DNA from maternal transmission has disappeared (25, 38, 39). Therefore, we propose a single PCR to be performed only at this age.
False-negative results by PCR at birth cannot be disregarded: in our study, one of the four infants with congenital T. cruzi infection tested negative with RT-PCR at birth but was serologically positive. In this particular case, the serology test remained positive at 12 months, and for this reason, an additional RT-PCR was performed, which obtained a positive result. Hence, a positive RT-PCR result from 1 month onwards confirms T. cruzi infection, while a negative result at birth does not rule it out. This observation is in accordance with the findings of Fumadó et al. (61), who describe a confirmed case of congenital Chagas disease in a newborn with a negative RT-PCR.
Accordingly, the diagnostic algorithm could start directly at 1 month after birth with a parasitological test or a PCR or both, depending on the laboratory facilities. This would facilitate parasite detection and also avoid false positives resulting from the presence of parasite DNA of maternal origin (7, 24, 62) (see Table 2 and Fig. 4). However, the loss of contact with the mother and newborn after the postdelivery discharge represents a major problem in areas without well-organized health care and also when circuits between the different levels of health care (primary and hospital) are not properly established (7). Consequently, our proposed algorithm includes the optional performance of a parasitological test at birth.
In reference to the serology testing, only the protocol of Tuscany takes into account a sample at birth. In Valencia, the protocol does not clearly stipulate if this is required. Alternatively, the protocols of Catalonia and Galicia choose to include the first serum sample from the newborn at 9 months, unless the direct diagnosis was conclusive at birth or 1 month. If positive, all protocols, except that of Catalonia, include a follow-up at 12 months. Available data indicate that maternal antibodies have disappeared from the serum of the infant from 9 months after birth (31, 42, 61). A negative serology result at birth rules out infection (63), but as mentioned previously (57), the probability of this result is low (2.6% in the present study). In the only two cases of newborns with a negative serology result at birth reported here, their mothers yielded a low level of antibody titers. On the other hand, a positive result at birth, detected in most cases (97.4% in this study), does not confirm the congenital infection (31, 64) and requires a second serological test at 9 months. Since the inclusion of a basal serology test for all newborns from Chagas-infected mothers would lead to a fairly high cost-effective ratio, we propose performing the first serology test at 9 months, an age when a negative result rules out Chagas disease. On the contrary, the finding of antibody titers could mean congenital infection, which should be confirmed at around 12 months. Protocols that include a sample only at 9 months cannot give conclusive results in case of seropositivity. The adoption of this protocol would result in important savings due to the low number of infants requiring second sample testing. In this study, positive values in nonchagasic infants remained in at least 20% of the samples analyzed by all the serological assays at >6 to 9 months, except for CMIA, which gave 60% positive samples. In the following period, >9 to 12 months, positive serology results declined considerably. It should be noted that, despite a declining trend in passive antibodies, none of the serological assay results seroreverted in all of the nonchagasic infants. Finally, at >12 months of age, CMIA was the only test to give a sample in the gray zone.
In a previous study (65), our group reported that the CMIA Architect Chagas is a highly effective assay for the diagnosis of chronic Chagas disease, with 100% sensitivity, allowing most samples to be correctly diagnosed when applied as a single technique. Other authors have reported similar sensitivity after evaluating the assay (66–68). Nevertheless, precisely because of its high sensitivity, CMIA is not the most suitable technique for the diagnosis of congenital Chagas disease. According to our results, in comparison with the other tests, CMIA can detect more maternal IgG antibodies in the serology of the newborn and for a longer term (see Fig. 1 and 2), thus delaying the negativization of passive antibodies and impeding an early diagnosis or discarding of the disease. Therefore, the age at which maternal antibodies disappear from the serology results of the newborn varies according to the test: the greater its sensitivity, the longer passive antibodies are detectable (14).
In the present study, none of the serological methods were able to differentiate between infected and noninfected newborns with the analysis of a single serum sample. In the case of CMIA, however, it should be emphasized that in noninfected children at >6 to 9 months of age, at which point we propose the first serological analysis, all samples yielded results below a sample RLU/cutoff value (S/CO) of 6. This value was the cutoff that we established for chronic Chagas disease when all results with an S/CO of >6 are true positives, whereas only gray zone and positive sera with a result of S/CO ≤6 need to be confirmed by another serological assay (65). In our study, CMIA results for the four congenitally infected newborns at >6 to 9 months were not available, but in two of the cases at >12 months, they were far higher than an S/CO of 6. Further studies in this field involving a higher number of congenitally infected newborns are needed.
The application of WB analysis did not provide any characteristic band to distinguish between newborns with or without congenital T. cruzi infection, as in the study by Riera et al. (17). In the current study, a clear differentiation between patterns was lacking, probably due to the low number of samples of congenitally infected newborns analyzed in parallel with their mothers. The antibodies transferred from mother to child may also have hidden the antibody production in the newborn (69) (Fig. 3D). Nevertheless, differences in the intensity of bands and/or in the general pattern between mother and newborn were evident after 6 months, when passive antibodies are already scarce (Fig. 3B, C, and E). Hence, WB analysis could be a useful tool to diagnose congenital disease some months after birth in those laboratories where it is available.
In conclusion, in noninfected newborns, a continuous decreasing trend of passive IgG antibodies was observed, but none of the serological assays seroreverted in all of the infants before 12 months. From 12 months onwards, all serological tests achieved negative results in the totality of the samples analyzed, with the exception of the highly sensitive CMIA. On the contrary, in infants with congenital T. cruzi infection, the antibody decline was detected only after the initiation of the treatment.
In order to improve the diagnosis of congenital T. cruzi infection, we propose a new more cost-effective strategy with a reduced number of tests. The protocol could start at 1 month of age with a parasitological test and/or a PCR. If negative, a serology test would be carried out at 9 months, followed by a confirmatory serological testing at around 12 months in case of positive results.
MATERIALS AND METHODS
Ethics approval.
This study was approved by the Clinical Research Ethics Committee (CEIC) of the Hospital de la Santa Creu i Sant Pau in Barcelona (project code IIBSP-CHA-2013-33; CEIC no. 53/2013). All samples were anonymized before being evaluated and included in the study.
Study population and samples.
Peripheral blood samples and sera from 81 newborns of Latin American Chagas-infected mothers admitted to three hospitals in Barcelona (Spain) were analyzed. Samples were collected during the period from April 2003 to December 2015 and stored at −40°C. The mothers of the newborns were identified by two serological assays, according to the World Health Organization (WHO) recommendations (42).
DNA extraction and real-time PCR.
DNA was extracted from 200 μl of EDTA-blood with the High Pure PCR template preparation kit (Roche, Mannheim, Germany) and eluted in 200 μl of elution buffer (EB), according to the manufacturer's instructions. Five microliters of the extracted DNA was amplified in triplicate by a real-time PCR (RT-PCR) targeted at the T. cruzi satellite DNA (SatDNA), as described by Pirón et al. (70). The amplification was carried out in an ABI7900 device (Applied Biosystems), and the RNase P human gene (Life Technologies, Austin, TX) was included as an internal control of the RT-PCR amplification. A sample was considered valid when the RNase P human gene was efficiently amplified and was considered positive when the cycle threshold (CT) was ≤40 in at least one of the three replicates.
Serological assays.
Sera were tested by five serological assays: an indirect fluorescent antibody test (IFAT), two enzyme-linked immunosorbent assays (ELISAs), a chemiluminescent microparticle immunoassay (CMIA), and Western blotting (WB).
IFAT.
The IFAT (Trypanosomiasis IFA test system; Trinity Biotech, Bray Country, Wicklow, Ireland) was performed according to the manufacturer's instructions, with some modifications. Epimastigotes of T. cruzi (Corpus Christi strain) were used as the antigen. Double dilutions of sera from 1:20 to 1:5,120 were used, and a dilution of ≥1:40 was established as the cutoff.
ELISAc.
ELISAc was performed as previously described by Riera et al. (71). Sonicated epimastigotes of T. cruzi (Maracay strain) were used as the antigen. Sera were diluted 1:200 in phosphate-buffered saline (PBS)–Tween 20 (0.05%)–skimmed milk (1%). The reaction was quantified as units (U) that relate the optical density at 492 nm (OD492) obtained from the problem sera with that of the mean of three replicates of a calibrator serum arbitrarily set at 100 U. The cutoff was established at 20 U.
ELISAr.
ELISAr was performed according to the manufacturer's instructions (BioELISA Chagas; Biokit, Lliçà d'Amunt, Spain). This test contains the recombinant antigen TcF, which is a T. cruzi fusion protein. The TcF antigen consists of a linear assembly of four serologically active peptides, PEP-II, TcD, TcE, and TcLo1.2. Sera were diluted 1:20 in the sample diluent supplied with the commercial kit. Results with a sample ratio absorbance/cutoff value (Abs/CO) of <0.9 were considered negative, with an Abs/CO of ≥1 considered positive and the gray zone with an Abs/CO of ≥0.9 to <1.
CMIA.
The CMIA was performed according to the manufacturer's instructions (Architect Chagas; Abbott Laboratories, Wiesbaden, Germany). This fully automated assay is based on four hybrid recombinant proteins, FP3, FP6, FP10, and TcF, which in aggregate represent 14 distinct antigenic regions (72, 73). Sera were not diluted. The chemiluminescent reaction is measured in relative light units (RLU). Results with a sample RLU/cutoff value (S/CO) of <0.8 were considered negative, with S/CO of ≥1 considered positive and the gray zone with an S/CO of ≥0.8 to <1.
WB.
The test was performed as described elsewhere (74). A total extract of T. cruzi (Maracay strain) lysate epimastigotes was used as the antigen. Sera were diluted 1:50 in TS (20 mM Tris-0.13 mM NaCl, pH 7.6) with 1% skimmed milk and 0.2% Tween 20. The antigenic bands of the T. cruzi profile are 28, 32, 38, 39, 40, and 48 kDa. A serum was considered positive when at least two bands of the pattern were recognized and also when a single band appeared if it was as intense as the same band in the positive control, as used in assays for other infectious diseases, such as hepatitis C (INNO-LIA HCV score; Ghent, Belgium).
Criteria for the interpretation of results.
An infant was considered infected with T. cruzi when a positive RT-PCR was obtained and/or the level of IgG antibodies was maintained during the first year of life, as determined by at least two serological tests.
ACKNOWLEDGMENTS
We thank Pere Coll for his support and helpful scientific discussions.
This study was partially supported by Departament d'Universitats, Recerca i Societat de la Informació de la Generalitat de Catalunya (grant 2014SGR026), Institut d'Investigació Biomèdica Sant Pau (IIB Sant Pau) and by Plan Nacional de I+D+i and Instituto de Salud Carlos III, Subdirección General de Redes y Centros de Investigación Cooperativa, Ministerio de Economía y Competitividad, Spanish Network for Research in Infectious Diseases (grant REIPI RD12/0015); cofinanced by European Development Regional Fund (ERDF) “A way to achieve Europe.” A.A., S.T., C.B., M.-J.P., E.P., J.G., and M.G. belong to RICET, a Tropical Disease Cooperative Research Network in Spain (grant RD12/0018/0010).
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sector.
We declare no conflicts of interest.
REFERENCES
- 1.World Health Organization. 2015. Chagas disease in Latin America: an epidemiological update based on 2010 estimates. Wkly Epidemiol Rec 90:33–44. [PubMed] [Google Scholar]
- 2.Carlier Y, Truyens C, Deloron P, Peyron F. 2012. Congenital parasitic infections: a review. Acta Trop 121:55–70. doi: 10.1016/j.actatropica.2011.10.018. [DOI] [PubMed] [Google Scholar]
- 3.Organización Panamericana de la Salud. 2006. Estimación cuantitativa de la enfermedad de Chagas en las Américas. OPS/HDM/CD/425-06. Organización Panamericana de la Salud, Washington, DC. [Google Scholar]
- 4.Prata A. 2001. Clinical and epidemiological aspects of Chagas disease. Lancet Infect Dis 1:92–100. doi: 10.1016/S1473-3099(01)00065-2. [DOI] [PubMed] [Google Scholar]
- 5.Gascón J, Pinazo MJ. 2008. Control de la transmisión vertical de Trypanosoma cruzi en España: principal reto de la patología importada. Enferm Infecc Microbiol Clin 26:607–608. doi: 10.1016/S0213-005X(08)75275-3. [DOI] [PubMed] [Google Scholar]
- 6.Buekens P, Almendares O, Carlier Y, Dumonteil E, Eberhard M, Gamboa-Leon R, James M, Padilla N, Wesson D, Xiong X. 2008. Mother-to-child transmission of Chagas disease in North America: why don't we do more? Matern Child Health J 12:283–286. doi: 10.1007/s10995-007-0246-8. [DOI] [PubMed] [Google Scholar]
- 7.Oliveira I, Torrico F, Muñoz J, Gascón J. 2010. Congenital transmission of Chagas disease: a clinical approach. Expert Rev Anti Infect Ther 8:945–956. doi: 10.1586/eri.10.74. [DOI] [PubMed] [Google Scholar]
- 8.Schmunis GA. 2007. Epidemiology of Chagas disease in non-endemic countries: the role of international migration. Mem Inst Oswaldo Cruz 102(Suppl 1):75. [DOI] [PubMed] [Google Scholar]
- 9.Gascón J, Bern C, Pinazo MJ. 2010. Chagas disease in Spain, the United States and other non-endemic countries. Acta Trop 115:22–27. doi: 10.1016/j.actatropica.2009.07.019. [DOI] [PubMed] [Google Scholar]
- 10.Pinazo MJ, Gascón J. 2015. The importance of the multidisciplinary approach to deal with the new epidemiological scenario of Chagas disease (global health). Acta Trop 151:16–20. doi: 10.1016/j.actatropica.2015.06.013. [DOI] [PubMed] [Google Scholar]
- 11.Sicuri E, Muñoz J, Pinazo MJ, Posada E, Sánchez J, Alonso PL, Gascón J. 2011. Economic evaluation of Chagas disease screening of pregnant Latin American women and of their infants in a non endemic area. Acta Trop 118:110–117. doi: 10.1016/j.actatropica.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 12.Carlier Y, Torrico F. 2003. Congenital infection with Trypanosoma cruzi: from mechanisms of transmission to strategies for diagnosis and control. Rev Soc Bras Med Trop 36:767–771. doi: 10.1590/S0037-86822003000600024. [DOI] [PubMed] [Google Scholar]
- 13.Torrico F, Alonso-Vega C, Suárez E, Rodríguez P, Torrico MC, Dramaix M, Truyens C, Carlier Y. 2004. Maternal Trypanosoma cruzi infection, pregnancy outcome, morbidity, and mortality of congenitally infected and non-infected newborns in Bolivia. Am J Trop Med Hyg 70:201–209. [PubMed] [Google Scholar]
- 14.Bern C, Martin DL, Gilman RH. 2011. Acute and congenital Chagas disease. Adv Parasitol 75:19–47. doi: 10.1016/B978-0-12-385863-4.00002-2. [DOI] [PubMed] [Google Scholar]
- 15.Howard EJ, Xiong X, Carlier Y, Sosa-Estani S, Buekens P. 2014. Frequency of the congenital transmission of Trypanosoma cruzi: a systematic review and meta-analysis. BJOG 121:22–33. doi: 10.1111/1471-0528.12396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moscatelli G, Moroni S, García-Bournissen F, Ballering G, Bisio M, Freilij H, Altcheh J. 2015. Prevention of congenital Chagas through treatment of girls and women of childbearing age. Mem Inst Oswaldo Cruz 110:507–509. doi: 10.1590/0074-02760140347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Riera C, Guarro A, El Kassab H, Jorba JM, Castro M, Angrill R, Gállego M, Fisa R, Martín C, Lobato A, Portús M. 2006. Congenital transmission of Trypanosoma cruzi in Europe (Spain): a case report. Am J Trop Med Hyg 75:1078–1081. [PubMed] [Google Scholar]
- 18.Guarro A, El-Kassab H, Jorba JM, Lobato A, Martín C, Castro M, Angrill R, Corcoy F, Riera C. 2007. Un caso de transmisión congénita de la enfermedad de Chagas en Cataluña. Enferm Emerg 9(Suppl 1):S28–S30. [Google Scholar]
- 19.Muñoz J, Portús M, Corachan M, Fumadó V, Gascón J. 2007. Congenital Trypanosoma cruzi infection in a non-endemic area. Trans R Soc Trop Med Hyg 101:1161–1162. doi: 10.1016/j.trstmh.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 20.Flores-Chávez M, Faez Y, Olalla JM, Cruz I, Gárate T, Rodríguez M, Blanc P, Cañavate C. 2008. Fatal congenital Chagas' disease in a non-endemic area: a case report. Case J 1:302. doi: 10.1186/1757-1626-1-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muñoz J, Coll O, Juncosa T, Vergés M, del Pino M, Fumadó V, Bosch J, Posada EJ, Hernández S, Fisa R, Boguña JM, Gállego M, Sanz S, Portús M, Gascón J. 2009. Prevalence and vertical transmission of Trypanosoma cruzi infection among pregnant Latin American women attending 2 maternity clinics in Barcelona, Spain. Clin Infect Dis 48:1736–1740. doi: 10.1086/599223. [DOI] [PubMed] [Google Scholar]
- 22.Carrilero B, Quesada JJ, Alfayete S, Segovia M. 2009. Enfermedad de Chagas congénita en recién nacido de madre de origen boliviano. Enferm Infecc Microbiol Clin 27:486–487. doi: 10.1016/j.eimc.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 23.Basile L, Jansà JM, Carlier Y, Salamanca DD, Angheben A, Bartoloni A, Seixas J, Van Gool T, Cañavate C, Flores-Chávez M, Jackson Y, Chiodini PL, Albajar-Viñas P, Working Group on Chagas disease. 2011. Chagas disease in European countries: the challenge of a surveillance system. Euro Surveill: 16:pii=19968 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19968. [PubMed] [Google Scholar]
- 24.Cevallos AM, Hernández R. 2014. Chagas' disease: pregnancy and congenital transmission. Biomed Res Int 2014:401864. doi: 10.1155/2014/401864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carlier Y, Sosa-Estani S, Luquetti AO, Buekens P. 2015. Congenital Chagas disease: an update. Mem Inst Oswaldo Cruz 110:363–368. doi: 10.1590/0074-02760140405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grinnage-Pulley T, Scott B, Petersen CA. 2016. A mother's gift: congenital transmission of Trypanosoma and Leishmania species. PLoS Pathog 12:e1005302. doi: 10.1371/journal.ppat.1005302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bern C, Verastegui M, Gilman RH, LaFuente C, Galdos-Cárdenas G, Calderón M, Pacori J, Abastoflor MdC, Aparicio H, Brady MF, Ferrufino L, Angulo N, Marcus S, Sterling C, Maguire JH. 2009. Congenital Trypanosoma cruzi transmission in Santa Cruz, Bolivia. Clin Infect Dis 49:1667–1674. doi: 10.1086/648070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bern C, Montgomery SP, Herwaldt BL, Rassi A Jr, Marín-Neto JA, Dantas RO, Maguire JH, Acquatella H, Morillo C, Kirchhoff LV, Gilman RH, Reyes PA, Salvatella R, Moore AC. 2007. Evaluation and treatment of Chagas disease in the United States: a systematic review. JAMA 298:2171–2181. doi: 10.1001/jama.298.18.2171. [DOI] [PubMed] [Google Scholar]
- 29.Altcheh J, Moscatelli G, Moroni S, García-Bournissen F, Freilij H. 2011. Adverse events after the use of benznidazole in infants and children with Chagas disease. Pediatrics 127:e212–218. doi: 10.1542/peds.2010-1172. [DOI] [PubMed] [Google Scholar]
- 30.Bermudez J, Davies C, Simonazzi A, Real JP, Palma S. 2016. Current drug therapy and pharmaceutical challenges for Chagas disease. Acta Trop 156:1–16. doi: 10.1016/j.actatropica.2015.12.017. [DOI] [PubMed] [Google Scholar]
- 31.Carlier Y, Torrico F, Sosa-Estani S, Russomando G, Luquetti A, Freilij H, Albajar-Viñas P. 2011. Congenital Chagas disease: recommendations for diagnosis, treatment and control of newborns, siblings and pregnant women. PLoS Negl Trop Dis 5:e1250. doi: 10.1371/journal.pntd.0001250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.World Health Organization. 2012. Research priorities for Chagas disease, human African trypanosomiasis and leishmaniasis. World Health Organ Tech Rep Ser 975:1–100. [PubMed] [Google Scholar]
- 33.Fabbro DL, Danesi E, Olivera V, Codebó MO, Denner S, Heredia C, Streiger M, Sosa-Estani S. 2014. Trypanocide treatment of women infected with Trypanosoma cruzi and its effect on preventing congenital Chagas. PLoS Negl Trop Dis 8:e3312. doi: 10.1371/journal.pntd.0003312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Montes-Rincón LM, Galaviz-Silva L, González-Bravo FE, Molina-Garza ZJ. 2016. Trypanosoma cruzi seroprevalence in pregnant women and screening by PCR and microhaematocrit in newborns from Guanajuato, Mexico. Acta Trop 164:100–106. doi: 10.1016/j.actatropica.2016.08.029. [DOI] [PubMed] [Google Scholar]
- 35.Torrico MC, Solano MA, Córdova M, Suarez E, Torrico F. 2011. Diagnóstico parasitológico de la enfermedad de Chagas: de la teoría a la práctica. Enf Emerg 13(Suppl 1):S33–S38. [Google Scholar]
- 36.Schijman AG, Altcheh J, Burgos JM, Biancardi M, Bisio M, Levin MJ, Freilij H. 2003. Aetiological treatment of congenital Chagas' disease diagnosed and monitored by the polymerase chain reaction. J Antimicrob Chemother 52:441–449. doi: 10.1093/jac/dkg338. [DOI] [PubMed] [Google Scholar]
- 37.Burgos JM, Altcheh J, Petrucelli N, Bisio M, Levin MJ, Freilij H, Schijman AG. 2009. Molecular diagnosis and treatment monitoring of congenital transmission of Trypanosoma cruzi to twins of a triplet delivery. Diagn Microbiol Infect Dis 65:58–61. doi: 10.1016/j.diagmicrobio.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 38.Bua J, Volta BJ, Velázquez EB, Ruiz AM, De Rissio AM, Cardoni RL. 2012. Vertical transmission of Trypanosoma cruzi infection: quantification of parasite burden in mothers and their children by parasite DNA amplification. Trans R Soc Trop Med Hyg 106:623–628. doi: 10.1016/j.trstmh.2012.03.015. [DOI] [PubMed] [Google Scholar]
- 39.Bua J, Volta BJ, Perrone AE, Scollo K, Velázquez EB, Ruiz AM, De Rissio AM, Cardoni RL. 2013. How to improve the early diagnosis of Trypanosoma cruzi infection: relationship between validated conventional diagnosis and quantitative DNA amplification in congenitally infected children. PLoS Negl Trop Dis 10:e2476. doi: 10.1371/journal.pntd.0002476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Virreira M, Martínez S, Alonso-Vega C, Torrico F, Solano M, Torrico MC, Parrado R, Truyens C, Carlier Y, Svoboda M. 2006. Short report: amniotic fluid is not useful for diagnosis of congenital Trypanosoma cruzi infection. Am J Trop Med Hyg 75:1082–1084. [PubMed] [Google Scholar]
- 41.Flores-Chávez M, de Fuentes I, Gárate T, Cañavate C. 2007. Diagnóstico de laboratorio de la enfermedad de Chagas importada. Enferm Infecc Microbiol Clin 25(Suppl 3):S29–S37. [DOI] [PubMed] [Google Scholar]
- 42.WHO Expert Committee. 2002. Control of Chagas disease. World Health Organ Tech Rep Ser 905:1–109. [PubMed] [Google Scholar]
- 43.Rodríguez P, Truyens C, Alonso-Vega C, Flores A, Córdova M, Suárez E, Torrico F, Carlier Y. 2005. Serum levels for IgM and IgA antibodies to anti-Trypanosoma cruzi in samples of blood from newborns from mother with positive serology for Chagas disease. Rev Soc Bras Med Trop 38(Suppl 2):S62–S64. (In Spanish.) [PubMed] [Google Scholar]
- 44.Requena-Méndez A, Albajar-Viñas P, Angheben A, Chiodini P, Gascón J, Muñoz J, Chagas Disease COHEMI Working Group . 2014. Health policies to control Chagas disease transmission in European countries. PLoS Negl Trop Dis 8:e3245. doi: 10.1371/journal.pntd.0003245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Consiglio Regionale Regione Toscana. 2012. Programma regionale per la prevenzione e il controllo della malattia di Chagas congenita: indicazioni per l'assistenza in gravidanza. Servizio Sanitario della Toscana, Regione Toscana, Florence, Italy: http://servizi.salute.toscana.it/csr/img/getfile_img1.php?id=24147. [Google Scholar]
- 46.Generalitat de Catalunya. 2010. Protocolo de cribado y diagnóstico de la enfermedad de Chagas en mujeres embarazadas latinoamericanas y sus bebés. Departament de Salud, Generalitat de Catalunya, Barcelona, Spain: http://canalsalut.gencat.cat/web/.content/home_canal_salut/professionals/temes_de_salut/chagas/documents/arxius/chagas_espanyol.pdf. [Google Scholar]
- 47.Basile L, Oliveira I, Ciruela P, Plasencia A, Working Group For Developing the Catalonian Screening Programme for Congenital Transmission of Chagas Disease. 2011. The current screening programme for congenital transmission of Chagas disease in Catalonia, Spain. Euro Surveill 16:pii=19972 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19972. [DOI] [PubMed] [Google Scholar]
- 48.Xunta de Galicia. 2014. Protocolo de cribado da enfermidade de Chagas en mulleres embarazadas: Revisión ano 2014. Servizo Galego de Saúde, Consellería de Sanidade, Xunta de Galicia, Santiago de Compostela, Spain: www.sergas.es/Asistencia-sanitaria/Protocolo-de-cribado-da-enfermidade-de-chagas-en-mulleres-embarazadas-Actualizacion-2014. [Google Scholar]
- 49.Generalitat Valenciana. 2009. Enfermedad de Chagas importada. Protocolo de actuación en la Comunitat Valenciana. Conselleria de Sanitat, Generalitat Valenciana, Valencia, Spain: http://publicaciones.san.gva.es/publicaciones/documentos/V-5243-2008.pdf. [Google Scholar]
- 50.Junta de Andalucía. 2014. Embarazo, parto y puerperio: proceso asistencial integrado. Consejería de Igualdad, Salud y Políticas Sociales, Junta de Andalucía, Seville, Spain: http://www.juntadeandalucia.es/salud/export/sites/csalud/galerias/documentos/p_3_p_3_procesos_asistenciales_integrados/embarazo_parto_puerperio_nuevo/embarazo_parto_puerperio_septiembre_2014.pdf. [Google Scholar]
- 51.Gobierno Vasco. 2008. Recomendaciones para la asistencia médica del adulto inmigrante. Departamento de Sanidad, Gobierno Vasco, Vitoria, Spain: http://www.osakidetza.euskadi.eus/contenidos/informacion/osk_publicaciones/es_publi/adjuntos/primaria/asistenciaInmigrante.pdf. [Google Scholar]
- 52.Soriano-Arandes A, Angheben A, Serre-Delcor N, Treviño-Maruri B, Gómez i Prat J, Jackson Y. 2016. Control and management of congenital Chagas disease in Europe and other non-endemic countries: current policies and practices. Trop Med Int Health 21:590–596. doi: 10.1111/tmi.12687. [DOI] [PubMed] [Google Scholar]
- 53.Flores-Chávez MD, Merino FJ, García-Bujalance S, Martin-Rabadán P, Merino P, García-Bermejo I, Delgado A, Cuadros J, Working Group on Chagas Disease of Autonomous Community of Madrid. 2011. Surveillance of Chagas disease in pregnant women in Madrid, Spain, from 2008 to 2010. Euro Surveill 16:pii=19974 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19974. [DOI] [PubMed] [Google Scholar]
- 54.Barona-Vilar C, Giménez-Martí MJ, Fraile T, González-Steinbauer C, Parada C, Gil-Brusola A, Bravo D, Gómez MD, Navarro D, Pérez-Tamarit A, Fernández-Silveira L, Fullana-Montoro A, Borrás R. 2012. Prevalence of Trypanosoma cruzi infection in pregnant Latin American women and congenital transmission rate in a non-endemic area: the experience of the Valencian Health Programme (Spain). Epidemiol Infect 140:1896–1903. doi: 10.1017/S0950268811002482. [DOI] [PubMed] [Google Scholar]
- 55.Ávila Arzanegui O, Liendo Arenaza P, Martínez Indart L, Martínez Astorkiza T, Pocheville Guruceta MI, Egurbide Arberas MV. 2013. Prevalencia de la infección por Trypanosoma cruzi y transmisión vertical en mujeres gestantes latinoamericanas en un área de salud de Vizcaya. Enferm Infecc Microbiol Clin 31:210–216. doi: 10.1016/j.eimc.2012.01.029. [DOI] [PubMed] [Google Scholar]
- 56.Gebrekristos HT, Buekens P. 2014. Mother-to-child transmission of Trypanosoma cruzi. J Pediatric Infect Dis Soc 3(Suppl 1):S36–S40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zulantay I, Corral G, Guzmán MC, Aldunate F, Guerra W, Cruz I, Araya A, Tapia V, Márquez F, Muñoz C, Apt W. 2011. The investigation of congenital infection by Trypanosoma cruzi in an endemic area of Chile: three protocols explored in a pilot project. Ann Trop Med Parasitol 105:123–128. doi: 10.1179/136485911X12899838413583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Carlier Y, Truyens C. 2015. Congenital Chagas disease as an ecological model of interactions between Trypanosoma cruzi parasites, pregnant women, placenta and fetuses. Acta Trop 151:103–115. doi: 10.1016/j.actatropica.2015.07.016. [DOI] [PubMed] [Google Scholar]
- 59.Díez CN, Manattini S, Zanuttini JC, Bottasso O, Marcipar I. 2008. The value of molecular studies for the diagnosis of congenital Chagas disease in northeastern Argentina. Am J Trop Med Hyg 78:624–627. [PubMed] [Google Scholar]
- 60.Velázquez EB, Rivero R, De Rissio AM, Malagrino N, Esteva MI, Riarte AR, Ruiz AM. 2014. Predictive role of polymerase chain reaction in the early diagnosis of congenital Trypanosoma cruzi infection. Acta Trop 137:195–200. doi: 10.1016/j.actatropica.2014.05.016. [DOI] [PubMed] [Google Scholar]
- 61.Fumadó V, Juncosa T, Posada E, Fisa R, Gállego M, Gascón J. 2014. Chagas pediátrico en zona no endémica. Enferm Infecc Microbiol Clin 32:293–296. doi: 10.1016/j.eimc.2013.04.024. [DOI] [PubMed] [Google Scholar]
- 62.Norman FF, López-Vélez R. 2014. Mother-to-child transmission of Trypanosoma cruzi infection (Chagas disease): a neglected problem. Trans R Soc Trop Med Hyg 108:388–390. doi: 10.1093/trstmh/tru062. [DOI] [PubMed] [Google Scholar]
- 63.Luquetti AO, do Nascimiento Tavares SB, da Rocha Siriano L, de Oliveira RA, Campos DE, Alves de Morais C, Chaves de Oliveira E. 2015. Congenital transmission of Trypanosoma cruzi in central Brazil. A study of 1,211 individuals born to infected mothers. Mem Inst Oswaldo Cruz 110:369–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chippaux JP, Salas Clavijo AN, Santalla JA, Postigo JR, Schneider D, Brutus L. 2010. Antibody drop in newborns congenitally infected by Trypanosoma cruzi treated with benznidazole. Trop Med Int Health 15:87–93. [DOI] [PubMed] [Google Scholar]
- 65.Abras A, Gállego M, Llovet T, Tebar S, Herrero M, Berenguer P, Ballart C, Martí C, Muñoz C. 2016. Serological diagnosis of chronical Chagas disease: is it time for a change? J Clin Microbiol 54:1566–1572. doi: 10.1128/JCM.00142-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Praast G, Herzogenrath J, Bernhardt S, Christ H, Sickinger E. 2011. Evaluation of the Abbott ARCHITECT Chagas prototype assay. Diagn Microbiol Infect Dis 69:74–81. doi: 10.1016/j.diagmicrobio.2010.08.019. [DOI] [PubMed] [Google Scholar]
- 67.Iborra-Bendicho MA, Albert-Hernández M, Márquez-Contreras C, Segovia-Hernández M. 2012. ARCHITECT Chagas: a new diagnostic tool in Chagas disease. Enferm Infecc Microbiol Clin 30:463–465. (In Spanish.) doi: 10.1016/j.eimc.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 68.Holguín A, Norman F, Martín L, Mateos ML, Chacón J, López-Vélez R, Pérez-Molina JA. 2013. Dried blood as an alternative to plasma or serum for Trypanosoma cruzi IgG detection in screening programs. Clin Vaccine Immunol 20:1197–1202. doi: 10.1128/CVI.00221-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.López-Chejade P, Fisa R, Gállego M, Iniesta L, Riera C, Portús M, RIVEMTI. 2005. Diagnóstico de la enfermedad de Chagas en la población procedente de zona endémica en Barcelona. Valoración de las pruebas de diagnóstico utilizadas. Enferm Emerg 8(Suppl 1):S32–S34. [Google Scholar]
- 70.Pirón M, Fisa R, Casamitjana N, López-Chejade P, Puig L, Vergés M, Gascón J, Gómez i Prat J, Portús M, Sauleda S. 2007. Development of a real-time PCR assay for Trypanosoma cruzi detection in blood samples. Acta Trop 103:195–200. doi: 10.1016/j.actatropica.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 71.Riera C, Vergés M, López-Chejade P, Pirón M, Gascón J, Gállego M, Portús M. 2009. Desarrollo y evaluación de una técnica ELISA con antígeno crudo de Trypanosoma cruzi para el diagnóstico de la enfermedad de Chagas. Enferm Emerg 11:22–29. [Google Scholar]
- 72.Chang CD, Cheng KY, Jiang LX, Salbilla VA, Haller AS, Yem AW, Bryant JD, Kirchhoff LV, Leiby DA, Schochetman G, Shah DO. 2006. Evaluation of a prototype Trypanosoma cruzi antibody assay with recombinant antigens on a fully automated chemiluminescence analyzer for blood donor screening. Transfusion 46:1737–1744. doi: 10.1111/j.1537-2995.2006.00965.x. [DOI] [PubMed] [Google Scholar]
- 73.Cheng KY, Chang CD, Salbilla VA, Kirchhoff LV, Leiby DA, Schochetman G, Shah DO. 2007. Immunoblot assay using recombinant antigens as a supplemental test to confirm the presence of antibodies to Trypanosoma cruzi. Clin Vaccine Immunol 14:355–361. doi: 10.1128/CVI.00401-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Riera C, Vergés M, Iniesta L, Fisa R, Gállego M, Tebar S, Portús M. 2012. Short report: identification of a western blot pattern for the specific diagnosis of Trypanosoma cruzi infection in human sera. Am J Trop Med Hyg 86:412–416. doi: 10.4269/ajtmh.2012.11-0111. [DOI] [PMC free article] [PubMed] [Google Scholar]