ABSTRACT
Hepatitis E virus (HEV) causes substantial morbidity and mortality in developing countries and is considered an emerging foodborne pathogen in developed countries in which it was previously not endemic. To investigate genetic association between human HEV infection and HEV-contaminated high-risk food in Hong Kong, we compared local virus strains obtained from hepatitis E patient sera with those surveyed from high-risk food items during 2014 to 2016. Twenty-four cases of laboratory-confirmed human HEV infections were identified from January 2014 to March 2016 in our hospitals. Five types of food items at risk of HEV contamination were purchased on a biweekly basis from April 2014 to March 2016 in two local market settings: supermarkets (lamb, oyster, and pig liver) and wet markets (oyster, pig blood curd, pig large intestine, and pig liver). HEV RNA detection was performed by a real-time reverse transcription-PCR assay. HEV RNA was detected in pig liver, pig intestine, and oyster samples with prevalences of 1.5%, 0.4%, and 0.2%, respectively. Neighbor-joining phylogenetic inference showed that all human and swine HEV strains belonged to genotype 4. HEV subtype distributions in humans and swine were highly comparable: subtype 4b predominated, while subtype 4d was the minority. Local human and swine HEV genotype 4 strains shared over 95% nucleotide identity and were genetically very similar, implicating swine as an important foodborne source of autochthonous human HEV infections in Hong Kong. Action should be taken to raise the awareness among public and health care professionals of hepatitis E as an emerging foodborne disease.
KEYWORDS: foodborne infection, genotype distribution, hepatitis E virus, oyster, pig intestine, pig liver, swine
INTRODUCTION
Hepatitis E virus (HEV) is a nonenveloped, positive-sense, single-stranded RNA virus in the family Hepeviridae and genus Orthohepevirus. A global disease burden study by the World Health Organization estimated that HEV accounts for 20 million cases of infection and leads to 3.4 million symptomatic cases every year (1). HEV infections are usually asymptomatic, and symptoms of hepatitis are self-limiting and last for a few weeks in otherwise healthy individuals (2). However, HEV may cause fulminant hepatitis in those with other underlying liver diseases, while chronic hepatitis may develop in organ transplant recipients (3, 4). Pregnant females are at risk of fatal outcomes (5). It was estimated that HEV causes 70,000 deaths and 3,000 stillbirths globally per year (1).
HEV is transmitted primarily through the fecal-oral route. In recent years, hepatitis E has come to be considered an emerging disease due to increasing reports of non-travel-associated, locally acquired cases in developed countries (6, 7). Seven genotypes of HEV are proposed (HEV-1 to HEV-7) that are further subdivided into more than 40 subtypes (e.g., subtype 3a) (8, 9). HEV-3 and HEV-4 are responsible for most HEV infections in developed countries (10). Since HEV-3 and HEV-4 can be detected in both humans and pigs, the foodborne route of virus acquisition from consumption of high-risk food items such as contaminated pig liver sausage (11, 12) and zoonotic transmission via close contact with infected pigs (13) have been implicated. HEV is also present in common vehicles of enteric viruses, such as shellfish (14).
In Hong Kong, hepatitis E is a notifiable disease, and the number of cases has been on an upward trend since 2001 (15). An earlier study conducted by the Hong Kong Centre for Food Safety reported HEV in local and imported fresh liver of pigs aged <4 months collected in slaughterhouses (16). However, virus subtype data were not available, and genetic relatedness of HEV detected in human and food remains elusive. In this study, we compared HEV from local clinical cases and contaminated food products from 2014 to 2016. We obtained molecular evidence suggesting that contaminated pig livers were one of the sources of autochthonous human HEV infections in Hong Kong.
RESULTS
Demographic characteristics of hepatitis E patients.
There were 24 patients testing positive for HEV IgM from January 2014 to March 2016 in our hospitals. The median age was 57 years, and the male-to-female ratio was 5:1. A winter seasonality was observed in which 14 (58%) cases were admitted to hospitals and diagnosed with hepatitis E during winter months, i.e., January to March. In contrast, there was only one (4%) case during summer months, from July to September. For these 24 patients, 22 archived HEV IgM-positive sera had sufficient volume for further testing, and 18 (82%) of them tested HEV RNA positive. The median cycle threshold (CT) value was 30.6, with an interquartile range of 27.6 to 32.8.
HEV prevalence in food samples.
A total of 240 lamb, 479 oyster, 240 pig blood curd, 240 pig intestine, and 479 pig liver samples were collected from 1 April 2014 to 31 March 2016 and tested for HEV RNA by quantitative reverse transcription-PCR (RT-qPCR). To monitor for the RNA extraction efficiency and PCR inhibition, 382 food samples were randomly selected for spike RNA detection and 377 (98.7%) of them tested positive, indicating satisfactory recovery and PCR efficiency at a 96% confidence level. HEV RNA was detected in 7 pig liver, 1 pig intestine, and 1 oyster sample from 4 out of 5 districts (except for New Territories West) that we have tested (Table 1). The prevalences of HEV, in decreasing order, in pig liver, pig intestine, oyster, lamb, and pig blood curd samples were 1.5% (95% confidence interval [CI], 0.6% to 3.0%), 0.4% (0.0% to 2.3%), 0.2% (0.0% to 1.2%), 0% (0.0% to 1.5%), and 0% (0.0% to 1.5%), respectively (Table 1). HEV was detected in pig liver samples from both local supermarkets and wet markets, and there was no significant difference in detection rate between retail settings. Food samples testing HEV RNA positive were collected year-round, and no observable seasonality was noted (see Fig. S1 in the supplemental material). The median RT-qPCR CT value of positive samples was 37.7. The median CT value of pig liver samples tended to be lower (indicating higher viral load) than that of non-pig liver samples, although the difference was not statistically significant (Fig. S2).
TABLE 1.
Prevalence of HEV in food samples
| Sample type | No. of samples | No. of samples testing HEV positive | % of samples testing HEV positive (95% confidence interval) |
|---|---|---|---|
| Pig liver | 479 | 7 | 1.5 (0.6–3.0) |
| Pig intestine | 240 | 1 | 0.4 (0.0–2.3) |
| Oyster | 479 | 1 | 0.2 (0.0–1.2) |
| Lamb | 240 | 0 | 0.0 (0.0–1.5) |
| Pig blood | 240 | 0 | 0.0 (0.0–1.5) |
HEV genotype distribution in human sera and pig livers.
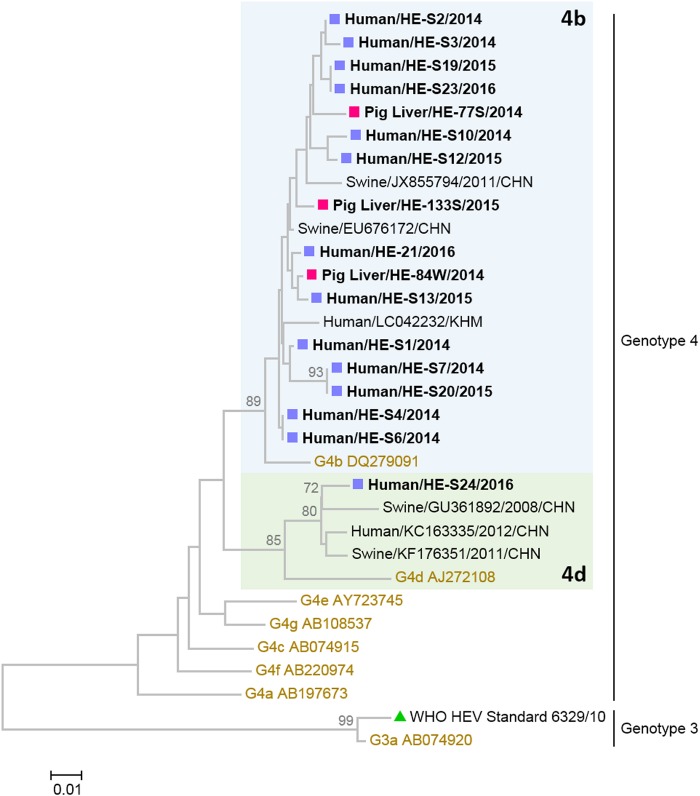
Among the 18 HEV RNA-positive human serum samples, 14 (78%) were successfully genotyped by separate nested PCRs targeting open reading frame 1 (ORF1; 133 nucleotides) and the ORF2/3 junction (97 nucleotides). The remaining 4 human serum samples with the lowest HEV viral load were genotyped by nested ORF2/3 PCR only. Genotyping failure was associated with lower HEV viral load (P < 0.05; Mann-Whitney U test). Among the 9 HEV-positive food samples, 3 samples with the highest viral load (lowest CT value), all collected from pig livers, were successfully genotyped by both nested ORF1 and ORF2/3 PCRs. In addition, a partial ORF1 sequence was determined from a pig liver sample that had the fourth highest viral load. Phylogenetic neighbor-joining trees were constructed using concatenated and individual partial ORF1 and ORF2/3 sequences (Fig. 1; see also Fig. S3). All HEV from human sera and pig liver samples clustered into genotype 4, with the best BLAST hits corresponding to human and swine HEV sequences from other parts of China and neighboring countries collected in earlier years (Fig. 1). For human sera, all but one HEV strain were assigned to subtype 4b and another strain was assigned to subtype 4d. Pairwise nucleotide identity in concatenated ORF1 and ORF2/3 of human HEV in subtype 4b ranged from 96.5% to 100.0%. Similarly, all but one swine HEV strain belonged to subtype 4b and the remaining strain grouped into subtype 4d based on partial ORF1 sequence. We did not detect any recombination between HEV ORF1 and ORF2/3 (Fig. S3). Pairwise nucleotide identity in concatenated ORF1 and ORF2/3 of swine HEV in subtype 4b ranged from 97.4% to 98.7%. The mean pairwise nucleotide identity between human and swine HEV strains was 97.8%. Both human and swine HEV strains were interspersed in the subtype 4b lineage, and there was no species-specific sublineage observed from the phylogenetic tree. Of note, human HEV strain HE-S13 (collected on 16 February 2015) clustered closest (99.6% nucleotide identity) to swine HEV strain HE-84W, which was collected nearly 3 months earlier, on 24 November 2014. Three pairs of identical human HEV strains were observed (HE-S4 and -S6, HE-S7 and -S20, and HE-S19 and -S23). Subsequent sequencing of longer amplicons (647 bp) showed heterogeneity, with 93.0% to 99.2% pairwise nucleotide identity, indicating the absence of laboratory contamination. Robustness of HEV genotyping using concatenated partial sequences was confirmed by high bootstrap values, 70% to 89%, and correct clustering of the WHO HEV RNA standard 6329/10 to the preassigned subtype, 3a, in the phylogenetic tree (Fig. 1; see also Fig. S3).
FIG 1.
Neighbor-joining phylogenetic tree of human and swine hepatitis E virus strains obtained in this study from 2014 to 2016 in Hong Kong. The tree was constructed using the Kimura 2-parameter distance method with 1,000 bootstrap replicates. Sequences used were concatenated partial open reading frame 1 (ORF1) and ORF2/3 junction sequences of 230 nucleotides in length. Strains for which sequences were obtained in this study are shown in bold. Magenta squares indicate pig liver samples, and those collected in supermarkets and wet markets are labeled with suffixes S and W, respectively. Purple squares indicate human serum samples. The best three hits in the BLAST search are included. The green triangle refers to the World Health Organization HEV RNA standard 6329/10 (subtype 3a). Proposed reference sequences of HEV subtypes are shown in brown and labeled in the following format: genotype and subtype, followed by GenBank accession number (9). Bootstrap values above a cutoff value of 70% are shown at nodes on the phylogenetic tree. The tree is midpoint rooted. The scale bar indicates the number of nucleotide substitutions per site.
DISCUSSION
In this study, we have provided molecular evidence supporting a close genetic relatedness, to the subtype level, between human and swine HEV genotype 4 strains detected during the same period in Hong Kong. Over the past decade, an increasing number of hepatitis E cases has been reported in developed countries in which hepatitis E is not endemic (17, 18). Most of these cases were locally acquired without a recent history of travel to an area where hepatitis E is endemic, suggesting a local source of infection and foodborne and zoonotic transmission from pigs (19, 20). In our study, the prevalence of HEV RNA in pig liver samples from markets was 1.5%, and there was no observable seasonality. The local HEV RNA prevalence in retail pig livers is comparable to those reported by others in Asia. In China, where HEV genotype 4 strains are predominant (21), HEV RNA prevalence in pig herds is around 5% (22, 23). In Japan, where HEV genotypes 3 and 4 cocirculate, HEV RNA prevalence in retail pig liver products ranges from 2% to 5% (24, 25). In Thailand, HEV RNA was reported in 0.28% of pig liver samples from markets (26). In sharp contrast, the rate of detection of HEV in pig livers outside Asia, where HEV genotype 3 predominates (27), is much higher. In North America, the prevalence of HEV contamination in retail pig livers typically ranges from 5% to 10% (28, 29). In Europe, the prevalence is even higher and can reach up to over 40% in very-high-risk pig liver-derived food products, such as figatellu (12, 30, 31). It is of interest to know whether HEV prevalence in pig livers is genotype dependent.
Phylogenetic analysis showed that all sequenced local human and swine HEV strains were genotype 4. Among both human and swine HEV strains, subtype 4b is predominant, while subtype 4d is the minority. This concordant HEV subtype distribution indicates possible direct association between human and swine HEV strains. Close genetic relatedness is further supported by molecular genetic evidence that the interspecies nucleotide percentage difference (<5%) between human and swine HEV strains is within that of intrahuman HEV strains among clinical cases. Human and swine HEV strains detected in Hong Kong were genetically very similar. In other words, it is impossible to tell whether one HEV strain is from swine or humans based solely on genetic sequence information in the absence of other linked demographic and epidemiological data. Similar observations were reported from other parts of China (32). Interestingly, HEV subtype distribution seems to vary in different cities in China (21). For instance, subtype 4b is found to be the predominant type in both humans and pigs in southern China as in our study. However, the predominant HEV subtypes are 4a and 4i in eastern China, while subtype 4g predominates in northeast part in both humans and pigs. Such a geography-specific, highly matched HEV genotype and subtype distribution between human and swine HEV strains strongly suggests that the pig is one main reservoir of human HEV infections.
HEV RNA was present in retailing pig intestine, which is a popular food in Chinese cuisine. It is not surprising to have HEV detected in pig intestine, as extrahepatic HEV dissemination is common in naturally infected pigs (33). Furthermore, a Japanese study reported that nearly 80% HEV patients had consumed undercooked pig liver or intestine during the incubation period (34). Indeed, limited reports showed that HEV contamination may not be uncommon in chitterlings in the United States (35). Of note, pig liver harbored a higher viral load than other food items, indicating a higher risk of transmission. We also detected HEV RNA in oysters but at a very low frequency, 0.2%. This is in good agreement with studies from most countries, such as Croatia, France, and Japan, that detected no HEV in oysters (36–38), possibly reflecting mild contamination of sewage from limited fecal shedding of HEV in clinical cases. In contrast, a higher HEV prevalence, 8.7%, in oysters was reported in the coastal region of South Korea (39). Considering that oysters are common vehicles of other foodborne viruses, such as norovirus and hepatitis A virus, and are consumed raw, the risk of foodborne HEV transmission from oysters and other shellfish should not be underestimated (40). Although seroprevalence studies indicated high anti-HEV positivity in ovine (10% to 35%) and porcine (nearly 100%) species in China (41, 42), we did not find any HEV RNA in lamb and pig blood curd samples. This suggests that extragastrointestinal spread of and viremia with HEV may be rare and that the risk of transmission from these food items could be considered minimal compared with that of pig liver products.
Our study has several strengths and limitations. Most studies with a similar design either compared new human HEV strains with previously reported swine HEV strains in GenBank or vice versa. In our studies, human sera and food samples were collected in the same period within one city. This allows us to better elucidate possible association between HEV infections and consumption of HEV-contaminated food items, because HEV genotype and subtype distribution may differ between cities and change over time (21). For example, although a direct link has yet to be established, we found that swine HEV strain HE-84W clustered closest with human HEV strain HE-S23. These strains were collected 2 to 3 months apart (the hepatitis E incubation period ranges from 2 to 10 weeks) and had almost identical sequences, providing strong molecular evidence of zoonotic transmission of HEV. Our study is limited by difficulties in genotyping HEV in food items, such as oyster and pig intestine samples, that had very low viral load. Genetic relatedness of human HEV with those from oyster and pig intestine remains elusive. Low viral load also precluded us from using longer regions to perform phylogenetic analysis. It should be noted that swine HEV spread from animals to humans may also act through the environment. Considering the relatively low detection rate of HEV RNA in different food items tested, the role of HEV spread via the food production network other than at the retail points (e.g., transport vehicles in slaughterhouse) should be evaluated in the future (43).
In summary, we found that human and swine HEV genotype 4 strains detected during the same period from local hospitalized hepatitis E cases and pig liver products at retail markets, respectively, are genetically very close to each other, suggesting that contaminated pigs are one of the sources of autochthonous human HEV infections in Hong Kong. The foodborne route plays an important role in HEV infections. Taking into account that HEV infections are increasingly recognized as more common than previously known in developed countries where HEV is not endemic, action should be taken to raise the awareness among public and health care professionals of hepatitis E as an emerging foodborne disease (19, 20).
MATERIALS AND METHODS
Clinical specimens from hepatitis E patients.
Archived human sera collected from hospitalized patients who tested positive for HEV IgM from 1 January 2014 to 31 March 2016 in our hospitals were retrieved and studied. Viral RNA was extracted and purified by a QIAamp viral RNA minikit according to the manufacturer's instructions. HEV RNA detection was performed as described below. Ethics approval for the use of archived human specimens was obtained from the Joint CUHK-NTEC Clinical Research Ethics Committee (reference number CRE-2016.167).
Food sampling strategy.
From 1 April 2014 to 31 March 2016, five types of food items at risk of HEV contamination were purchased in two local market settings: supermarkets (lamb [44], oysters [39], and pig liver [24]) and wet markets (oysters and pig blood curd [45], pig large intestine [35], and pig liver) at 5 widely spread districts (Hong Kong Island, Kowloon East, Kowloon West, New Territories East, and New Territories West) on a biweekly basis (Table S1). These items were selected based on earlier evidence of HEV contamination and the way they are consumed in Chinese cuisine. Oysters are usually consumed raw, whereas pig liver, pig blood curd, and pig large intestine are commonly served in congee and hot pot, with a higher risk of undercooking. Purchased food items were transported on ice packs to the laboratory within 2 to 4 h. For pig and lamb samples, 250 mg of tissue was excised by sterile forceps and disposable surgical blades. For oyster samples, 2 g of digestive tissue was excised. Dissected tissues were stored at −70°C until viral RNA extraction. All food items were processed on the day of purchase.
Tissue homogenization and viral RNA extraction and purification from food samples.
Food tissues were homogenized in 1 ml (except oyster tissues, for which 3 ml was used) of TRIzol (Life Technologies, USA) with 2.8-mm zirconium oxide beads using Precellys Minilys (Bertin Technologies, France). Homogenization was performed by two cycles of 5,000 rpm for 30 s, separated by a 30-s pause, at room temperature. To monitor for viral RNA extraction efficiency and carryover of PCR inhibitors, each food sample was spiked with 2 × 107 copies of TATAA Universal RNA Spike I (TATAA Biocenter, Sweden). After homogenization, samples were incubated for 5 min at room temperature, followed by chloroform extraction and centrifugation at 12,000 × g at 4°C for 15 min. Aqueous supernatant containing total RNA was aspirated and precipitated with 70% ethanol in a 1:1 (vol/vol) ratio. The RNA-ethanol mixture was loaded onto spin columns of a QIAamp viral RNA minikit (Qiagen, Germany) and purified according to the manufacturer's instructions. Purified RNA was stored at −70°C until further processing.
HEV RNA detection and quantification in human and food samples.
HEV RNA detection was performed by a broadly reactive one-step RT-qPCR assay as described by Jothikumar et al. (46). Each 20-μl reaction mixture contained 5 μl of TaqMan Fast Virus 1-Step master mix, 0.8 μl of 10 μM forward primer JVHEVF, 0.8 μl of 10 μM reverse primer JVHEVR, 0.2 μl of 10 μM TaqMan TAMRA probe JVHEVP (Table S2), and 0.4 μl of purified sample RNA (except for human sera and pig blood curd, for which 2 μl of purified sample RNA was used) and was topped up to 20 μl with UltraPure DNase/RNase-free distilled water. Reverse transcription was carried out at 50°C for 5 min, followed by denaturation at 95°C for 20 s. cDNA amplification was then carried out with 45 PCR cycles at 95°C for 3 s and 55°C for 30 s on a StepOne system (Life Technologies, USA). CT value was determined using a threshold line set at 0.01 in StepOne software v2.0 (Life Technologies).
HEV genotyping PCR and phylogenetic analysis.
HEV RNAs from human sera and food samples were reverse transcribed to cDNA using SuperScript III/IV Reverse Transcriptase (Invitrogen, USA) and random primer according to the manufacturer's instructions. Nested PCRs targeting HEV open reading frame 1 (ORF1; 133 nucleotides) and the ORF2/3 junction (97 nucleotides) were then performed separately using Phusion high-fidelity DNA polymerase (Thermo Fisher, USA)/Titanium Taq DNA polymerase (TaKaRa, Japan). Primers used are listed in Table S2 (47). Precautionary measures to minimize cross-contamination were implemented: (i) master mix preparation, DNA/RNA template addition, thermocycling, and post-PCR steps (e.g., gel electrophoresis) were performed in four separate rooms, and (ii) filtered pipette tips were used. Identical sequences were subjected to a longer PCR (647 bp) against ORF2 and sequencing to rule out contamination (48). WHO HEV RNA standard 6329/10 was used as a positive control in the genotyping assays. PCR products were Sanger sequenced bidirectionally using inner PCR primers. DNA chromatograms were inspected and assembled by ChromasPro v1.7.6 (Technelysium, Australia). Primer sequences were trimmed before phylogenetic analysis. Reference nucleotide sequences of HEV subtypes proposed by an international consortium were downloaded from GenBank (9). Nucleotide sequences were aligned using ClustalW, and phylogenetic inference was made using neighbor-joining clustering method with 1,000 bootstrap replications using MEGA v6.
Spike RNA detection and quantification in food samples.
Spike RNA detection and quantification were performed with one-step RT-qPCR on a subset of food samples selected randomly according to the ANSI/ASQ standard Z1.4-2003 for acceptance sampling. Each 20-μl reaction mixture contained the following: 5 μl of TaqMan Fast Virus 1-Step master mix (Life Technologies, USA), 0.8 μl of 10 μM spike primer mix (TATAA Biocenter, Sweden), 0.2 μl of 10 μM spike probe (TATAA Biocenter, Sweden), 13.6 μl of UltraPure DNase/RNase-free distilled water (Life Technologies, USA), and 0.4 μl of purified sample RNA. Reverse transcription and denaturation were carried as described above. cDNA was then amplified with 45 PCR cycles at 95°C for 3 s and 60°C for 30 s. Thermal cycling was carried out on StepOne System (Life Technologies). CT value was determined as for HEV RNA detection.
Statistical analysis.
The percentage of samples testing HEV positive was calculated for each food type, and the 95% confidence interval was determined by binomial exact test using Sampsize, available online at http://sampsize.sourceforge.net/iface/index.html. Statistical tests were performed using Prism 7 (GraphPad, USA). A two-tailed P value of <0.05 was considered statistically significant.
Accession number(s).
Sequences obtained in this study have been deposited in GenBank under accession numbers KX752737 to KX752775 and KY510921 to KY510926.
Supplementary Material
ACKNOWLEDGMENTS
We declare no conflict of interest.
We thank Angel Ng, Cathy Lam, and Maggie Tam for logistical support in food sampling, and we thank Angela Kwok, Tracy Chung, Serena Kwok, and Edith Tin for technical assistance in the initial phase of sample processing.
This study was jointly supported by the Health and Medical Research Fund of Food and Health Bureau of the HKSAR government (to M.C.W.C.; reference number 13120172) and departmental research fund.
The funder had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
M.C.W.C. and P.K.S.C. conceived and designed the study. M.C.W.C. supervised the study. K.K. and T.-N.H. performed experiments. M.C.W.C. and K.K. analyzed data and drafted the manuscript. All authors reviewed, commented on, and approved the submitted version of the manuscript. M.C.W.C. has access to all data and is responsible for the research integrity of this study.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/JCM.02020-16.
REFERENCES
- 1.Rein DB, Stevens GA, Theaker J, Wittenborn JS, Wiersma ST. 2012. The global burden of hepatitis E virus genotypes 1 and 2 in 2005. Hepatology 55:988–997. doi: 10.1002/hep.25505. [DOI] [PubMed] [Google Scholar]
- 2.Guillois Y, Abravanel F, Miura T, Pavio N, Vaillant V, Lhomme S, Le Guyader FS, Rose N, Le Saux JC, King LA, Izopet J, Couturier E. 2016. High proportion of asymptomatic infections in an outbreak of hepatitis E associated with a spit-roasted piglet, France, 2013. Clin Infect Dis 62:351–357. doi: 10.1093/cid/civ862. [DOI] [PubMed] [Google Scholar]
- 3.Dalton HR, Hazeldine S, Banks M, Ijaz S, Bendall R. 2007. Locally acquired hepatitis E in chronic liver disease. Lancet 369:1260. doi: 10.1016/S0140-6736(07)60595-9. [DOI] [PubMed] [Google Scholar]
- 4.Kamar N, Selves J, Mansuy JM, Ouezzani L, Peron JM, Guitard J, Cointault O, Esposito L, Abravanel F, Danjoux M, Durand D, Vinel JP, Izopet J, Rostaing L. 2008. Hepatitis E virus and chronic hepatitis in organ-transplant recipients. N Engl J Med 358:811–817. doi: 10.1056/NEJMoa0706992. [DOI] [PubMed] [Google Scholar]
- 5.Labrique AB, Sikder SS, Krain LJ, West KP Jr, Christian P, Rashid M, Nelson KE. 2012. Hepatitis E, a vaccine-preventable cause of maternal deaths. Emerg Infect Dis 18:1401–1404. doi: 10.3201/eid1809.120241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arends JE, Ghisetti V, Irving W, Dalton HR, Izopet J, Hoepelman AI, Salmon D. 2014. Hepatitis E: an emerging infection in high income countries. J Clin Virol 59:81–88. doi: 10.1016/j.jcv.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 7.Ijaz S, Arnold E, Banks M, Bendall RP, Cramp ME, Cunningham R, Dalton HR, Harrison TJ, Hill SF, Macfarlane L, Meigh RE, Shafi S, Sheppard MJ, Smithson J, Wilson MP, Teo CG. 2005. Non-travel-associated hepatitis E in England and Wales: demographic, clinical, and molecular epidemiological characteristics. J Infect Dis 192:1166–1172. doi: 10.1086/444396. [DOI] [PubMed] [Google Scholar]
- 8.Smith DB, Simmonds P, Izopet J, Oliveira-Filho EF, Ulrich RG, Johne R, Koenig M, Jameel S, Harrison TJ, Meng XJ, Okamoto H, Van der Poel WH, Purdy MA. 2016. Proposed reference sequences for hepatitis E virus subtypes. J Gen Virol 97:537–542. doi: 10.1099/jgv.0.000393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith DB, Simmonds P, Jameel S, Emerson SU, Harrison TJ, Meng XJ, Okamoto H, Van der Poel WH, Purdy MA. 2014. Consensus proposals for classification of the family Hepeviridae. J Gen Virol 95:2223–2232. doi: 10.1099/vir.0.068429-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kamar N, Bendall R, Legrand-Abravanel F, Xia NS, Ijaz S, Izopet J, Dalton HR. 2012. Hepatitis E. Lancet 379:2477–2488. doi: 10.1016/S0140-6736(11)61849-7. [DOI] [PubMed] [Google Scholar]
- 11.Colson P, Borentain P, Queyriaux B, Kaba M, Moal V, Gallian P, Heyries L, Raoult D, Gerolami R. 2010. Pig liver sausage as a source of hepatitis E virus transmission to humans. J Infect Dis 202:825–834. doi: 10.1086/655898. [DOI] [PubMed] [Google Scholar]
- 12.Di Bartolo I, Angeloni G, Ponterio E, Ostanello F, Ruggeri FM. 2015. Detection of hepatitis E virus in pork liver sausages. Int J Food Microbiol 193:29–33. doi: 10.1016/j.ijfoodmicro.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 13.Meng XJ, Wiseman B, Elvinger F, Guenette DK, Toth TE, Engle RE, Emerson SU, Purcell RH. 2002. Prevalence of antibodies to hepatitis E virus in veterinarians working with swine and in normal blood donors in the United States and other countries. J Clin Microbiol 40:117–122. doi: 10.1128/JCM.40.1.117-122.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crossan C, Baker PJ, Craft J, Takeuchi Y, Dalton HR, Scobie L. 2012. Hepatitis E virus genotype 3 in shellfish, United Kingdom. Emerg Infect Dis 18:2085–2087. doi: 10.3201/eid1812.120924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tai AL, Cheng PK, Ip SM, Wong RM, Lim WW. 2009. Molecular epidemiology of hepatitis E virus in Hong Kong. J Med Virol 81:1062–1068. doi: 10.1002/jmv.21497. [DOI] [PubMed] [Google Scholar]
- 16.Centre for Food Safety, Food and Environmental Hygiene Department, The Government of the Hong Kong Special Administrative Region. 2010. Hepatitis E virus in fresh pig livers. Centre for Food Safety, Food and Environmental Hygiene Department, The Government of the Hong Kong Special Administrative Region, Hong Kong: http://www.cfs.gov.hk/english/programme/programme_rafs/files/RA_44_HEV_pig_liver_e.pdf Accessed 13 August 2016. [Google Scholar]
- 17.Ijaz S, Said B, Boxall E, Smit E, Morgan D, Tedder RS. 2014. Indigenous hepatitis E in England and wales from 2003 to 2012: evidence of an emerging novel phylotype of viruses. J Infect Dis 209:1212–1218. doi: 10.1093/infdis/jit652. [DOI] [PubMed] [Google Scholar]
- 18.Sayed IM, Vercouter AS, Abdelwahab SF, Vercauteren K, Meuleman P. 2015. Is hepatitis E virus an emerging problem in industrialized countries? Hepatology 62:1883–1892. doi: 10.1002/hep.27990. [DOI] [PubMed] [Google Scholar]
- 19.Meng XJ. 2016. Expanding host range and cross-species infection of hepatitis E virus. PLoS Pathog 12:e1005695. doi: 10.1371/journal.ppat.1005695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dalton HR, Bendall R, Ijaz S, Banks M. 2008. Hepatitis E: an emerging infection in developed countries. Lancet Infect Dis 8:698–709. doi: 10.1016/S1473-3099(08)70255-X. [DOI] [PubMed] [Google Scholar]
- 21.Liu P, Li L, Wang L, Bu Q, Fu H, Han J, Zhu Y, Lu F, Zhuang H. 2012. Phylogenetic analysis of 626 hepatitis E virus (HEV) isolates from humans and animals in China (1986–2011) showing genotype diversity and zoonotic transmission. Infect Genet Evol 12:428–434. doi: 10.1016/j.meegid.2012.01.017. [DOI] [PubMed] [Google Scholar]
- 22.Li W, She R, Wei H, Zhao J, Wang Y, Sun Q, Zhang Y, Wang D, Li R. 2009. Prevalence of hepatitis E virus in swine under different breeding environment and abattoir in Beijing, China. Vet Microbiol 133:75–83. doi: 10.1016/j.vetmic.2008.06.026. [DOI] [PubMed] [Google Scholar]
- 23.Li W, Shu X, Pu Y, Bi J, Yang G, Yin G. 2011. Seroprevalence and molecular detection of hepatitis E virus in Yunnan Province, China. Arch Virol 156:1989–1995. doi: 10.1007/s00705-011-1089-6. [DOI] [PubMed] [Google Scholar]
- 24.Yazaki Y, Mizuo H, Takahashi M, Nishizawa T, Sasaki N, Gotanda Y, Okamoto H. 2003. Sporadic acute or fulminant hepatitis E in Hokkaido, Japan, may be food-borne, as suggested by the presence of hepatitis E virus in pig liver as food. J Gen Virol 84:2351–2357. doi: 10.1099/vir.0.19242-0. [DOI] [PubMed] [Google Scholar]
- 25.Okano H, Takahashi M, Isono Y, Tanaka H, Nakano T, Oya Y, Sugimoto K, Ito K, Ohmori S, Maegawa T, Kobayashi M, Nagashima S, Nishizawa T, Okamoto H. 2014. Characterization of sporadic acute hepatitis E and comparison of hepatitis E virus genomes in acute hepatitis patients and pig liver sold as food in Mie, Japan. Hepatol Res 44:E63–E76. doi: 10.1111/hepr.12216. [DOI] [PubMed] [Google Scholar]
- 26.Intharasongkroh D, Sa-Nguanmoo P, Tuanthap S, Thongmee T, Duang-In A, Klinfueng S, Chansaenroj J, Vongpunsawad S, Theamboonlers A, Payungporn S, Chirathaworn C, Poovorawan Y. 31 August 2016. Hepatitis E virus in pork and variety meats sold in fresh markets. Food Environ Virol doi: 10.1007/s12560-016-9258-0. [DOI] [PubMed] [Google Scholar]
- 27.Pérez-Gracia MT, Garcia M, Suay B, Mateos-Lindemann ML. 2015. Current knowledge on hepatitis E. J Clin Transl Hepatol 3:117–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feagins AR, Opriessnig T, Guenette DK, Halbur PG, Meng XJ. 2007. Detection and characterization of infectious hepatitis E virus from commercial pig livers sold in local grocery stores in the USA. J Gen Virol 88:912–917. doi: 10.1099/vir.0.82613-0. [DOI] [PubMed] [Google Scholar]
- 29.Wilhelm BJ, Leblanc D, Avery B, Pearl DL, Houde A, Rajic A, McEwen SA. 2016. Factors affecting detection of hepatitis E virus on Canadian retail pork chops and pork livers assayed using real-time RT-PCR. Zoonoses Public Health 63:152–159. doi: 10.1111/zph.12216. [DOI] [PubMed] [Google Scholar]
- 30.Pavio N, Merbah T, Thebault A. 2014. Frequent hepatitis E virus contamination in food containing raw pork liver, France. Emerg Infect Dis 20:1925–1927. doi: 10.3201/eid2011.140891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Szabo K, Trojnar E, Anheyer-Behmenburg H, Binder A, Schotte U, Ellerbroek L, Klein G, Johne R. 2015. Detection of hepatitis E virus RNA in raw sausages and liver sausages from retail in Germany using an optimized method. Int J Food Microbiol 215:149–156. doi: 10.1016/j.ijfoodmicro.2015.09.013. [DOI] [PubMed] [Google Scholar]
- 32.Zhang W, He Y, Wang H, Shen Q, Cui L, Wang X, Shao S, Hua X. 2010. Hepatitis E virus genotype diversity in eastern China. Emerg Infect Dis 16:1630–1632. doi: 10.3201/eid1610.100873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Choi C, Chae C. 2003. Localization of swine hepatitis E virus in liver and extrahepatic tissues from naturally infected pigs by in situ hybridization. J Hepatol 38:827–832. doi: 10.1016/S0168-8278(03)00115-6. [DOI] [PubMed] [Google Scholar]
- 34.Mizuo H, Yazaki Y, Sugawara K, Tsuda F, Takahashi M, Nishizawa T, Okamoto H. 2005. Possible risk factors for the transmission of hepatitis E virus and for the severe form of hepatitis E acquired locally in Hokkaido, Japan. J Med Virol 76:341–349. doi: 10.1002/jmv.20364. [DOI] [PubMed] [Google Scholar]
- 35.Cossaboom CM, Heffron CL, Cao D, Yugo DM, Houk-Miles AE, Lindsay DS, Zajac AM, Bertke AS, Elvinger F, Meng XJ. 2016. Risk factors and sources of foodborne hepatitis E virus infection in the United States. J Med Virol 88:1641–1645. doi: 10.1002/jmv.24497. [DOI] [PubMed] [Google Scholar]
- 36.Prpić J, Cerni S, Skoric D, Keros T, Brnic D, Cvetnic Z, Jemersic L. 2015. Distribution and molecular characterization of hepatitis E virus in domestic animals and wildlife in Croatia. Food Environ Virol 7:195–205. doi: 10.1007/s12560-015-9193-5. [DOI] [PubMed] [Google Scholar]
- 37.Grodzki M, Schaeffer J, Piquet JC, Le Saux JC, Cheve J, Ollivier J, Le Pendu J, Le Guyader FS. 2014. Bioaccumulation efficiency, tissue distribution, and environmental occurrence of hepatitis E virus in bivalve shellfish from France. Appl Environ Microbiol 80:4269–4276. doi: 10.1128/AEM.00978-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ishida S, Yoshizumi S, Ikeda T, Miyoshi M, Goto A, Matsubayashi K, Ikeda H. 2012. Detection and molecular characterization of hepatitis E virus in clinical, environmental and putative animal sources. Arch Virol 157:2363–2368. doi: 10.1007/s00705-012-1422-8. [DOI] [PubMed] [Google Scholar]
- 39.Song YJ, Jeong HJ, Kim YJ, Lee SW, Lee JB, Park SY, Song CS, Park HM, Choi IS. 2010. Analysis of complete genome sequences of swine hepatitis E virus and possible risk factors for transmission of HEV to humans in Korea. J Med Virol 82:583–591. doi: 10.1002/jmv.21730. [DOI] [PubMed] [Google Scholar]
- 40.Bellou M, Kokkinos P, Vantarakis A. 2013. Shellfish-borne viral outbreaks: a systematic review. Food Environ Virol 5:13–23. doi: 10.1007/s12560-012-9097-6. [DOI] [PubMed] [Google Scholar]
- 41.Geng J, Wang L, Wang X, Fu H, Bu Q, Liu P, Zhu Y, Wang M, Sui Y, Zhuang H. 2011. Potential risk of zoonotic transmission from young swine to human: seroepidemiological and genetic characterization of hepatitis E virus in human and various animals in Beijing, China. J Viral Hepat 18:e583–590. doi: 10.1111/j.1365-2893.2011.01472.x. [DOI] [PubMed] [Google Scholar]
- 42.Wu J, Si F, Jiang C, Li T, Jin M. 2015. Molecular detection of hepatitis E virus in sheep from southern Xinjiang, China. Virus Genes 50:410–417. doi: 10.1007/s11262-015-1194-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nantel-Fortier N, Letellier A, Lachapelle V, Fravalo P, L'Homme Y, Brassard J. 2016. Detection and phylogenetic analysis of the hepatitis E virus in a Canadian swine production network. Food Environ Virol 8:296–304. doi: 10.1007/s12560-016-9252-6. [DOI] [PubMed] [Google Scholar]
- 44.Wang Y, Ma X. 2010. Detection and sequences analysis of sheep hepatitis E virus RNA in Xinjiang autonomous region. Acta Microbiologica Sinica 50:937–941. (In Chinese.) [PubMed] [Google Scholar]
- 45.Crossan C, Grierson S, Thomson J, Ward A, Nunez-Garcia J, Banks M, Scobie L. 2015. Prevalence of hepatitis E virus in slaughter-age pigs in Scotland. Epidemiol Infect 143:2237–2240. doi: 10.1017/S0950268814003100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jothikumar N, Cromeans TL, Robertson BH, Meng XJ, Hill VR. 2006. A broadly reactive one-step real-time RT-PCR assay for rapid and sensitive detection of hepatitis E virus. J Virol Methods 131:65–71. doi: 10.1016/j.jviromet.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 47.La Rosa G, Fratini M, Muscillo M, Iaconelli M, Taffon S, Equestre M, Chionne P, Madonna E, Pisani G, Bruni R, Ciccaglione AR. 2014. Molecular characterisation of human hepatitis E virus from Italy: comparative analysis of five reverse transcription-PCR assays. Virol J 11:72. doi: 10.1186/1743-422X-11-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dai X, Dong C, Zhou Z, Liang J, Dong M, Yang Y, Fu J, Tian H, Wang S, Fan J, Meng J, Purdy MA. 2013. Hepatitis E virus genotype 4, Nanjing, China, 2001–2011. Emerg Infect Dis 19:1528–1530. doi: 10.3201/eid1909.130013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.