ABSTRACT
Matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) decreases the time to organism identification and improves clinical and financial outcomes. The purpose of this study was to evaluate the impact of MALDI-TOF MS alone versus MALDI-TOF MS combined with real-time, pharmacist-driven, antimicrobial stewardship (AMS) intervention on patient outcomes. This single-center, pre-post, quasiexperimental study evaluated hospitalized patients with positive blood cultures identified via MALDI-TOF MS combined with prospective AMS intervention compared to a control cohort with MALDI-TOF MS identification without AMS intervention. AMS intervention included: real-time MALDI-TOF MS pharmacist notification and prospective AMS provider feedback. The primary outcome was the time to optimal therapy (TTOT). A total of 252 blood cultures, 126 in each group, were included in the final analysis. MALDI-TOF MS plus AMS intervention significantly reduced the overall TTOT (75.17 versus 43.06 h; P < 0.001), the Gram-positive contaminant TTOT (48.21 versus 11.75 h; P < 0.001), the Gram-negative infection (GNI) TTOT (71.83 versus 35.98 h; P < 0.001), and the overall hospital length of stay (LOS; 15.03 versus 9.02 days; P = 0.021). The TTOT for Gram-positive infection (GPI) was improved (64.04 versus 41.61 h; P = 0.082). For GPI, the hospital LOS (14.64 versus 10.31 days; P = 0.002) and length of antimicrobial therapy 24.30 versus 18.97 days; P = 0.018) were reduced. For GNI, the time to microbiologic clearance (51.13 versus 34.51 h; P < 0.001), the hospital LOS (15.40 versus 7.90 days; P = 0.027), and the intensive care unit LOS (5.55 versus 1.19 days; P = 0.035) were reduced. To achieve optimal outcomes, rapid identification with MALDI-TOF MS combined with real-time AMS intervention is more impactful than MALDI-TOF MS alone.
KEYWORDS: MALDI-TOF MS, antimicrobial stewardship, rapid molecular diagnostics
INTRODUCTION
Despite advances in antimicrobial therapy, bloodstream infections (BSIs) remain a threat to hospitalized patients. A significant proportion of health care-associated infections result from multidrug-resistant organisms (MDROs). These infection rates continue to uptrend, posing a substantial public health risk by driving providers to utilize broad-spectrum antimicrobials and potentiating the cycle that creates MDROs (1–3). To minimize these threats, early administration of appropriate antimicrobial therapy is critical, as are antimicrobial streamlining to optimal therapy and discontinuation of inappropriate therapy, particularly in the setting of contaminated cultures (4–7). Utilizing time-consuming conventional methods of organism identification can delay the time to optimal therapy (TTOT) and increase patient exposure to unnecessary antimicrobials.
Matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) rapidly and accurately identifies isolated organisms to the genus and species levels. Compared to conventional methods, MALDI-TOF MS decreases the time to organism identification by approximately 1.2 to 1.5 days (8–10). Several studies have established that the combination of MALDI-TOF MS identification and real-time antimicrobial stewardship (AMS) intervention provided by AMS teams (ASTs) improves patient outcomes compared to traditional methods of organism identification (9–12). However, there are limited data evaluating AMS by comparing rapid organism identification via MALDI-TOF MS alone with MALDI-TOF MS combined with real-time AMS intervention. Preliminary data at the study location suggested that implementation of MALDI-TOF MS without stewardship intervention did not have an impact on the time to optimal antimicrobial therapy. Therefore, the purpose of this study was to evaluate the impact of rapid organism identification via MALDI-TOF MS alone versus MALDI-TOF MS combined with real-time AMS intervention on the time to optimal antimicrobial therapy for patients with positive blood cultures.
RESULTS
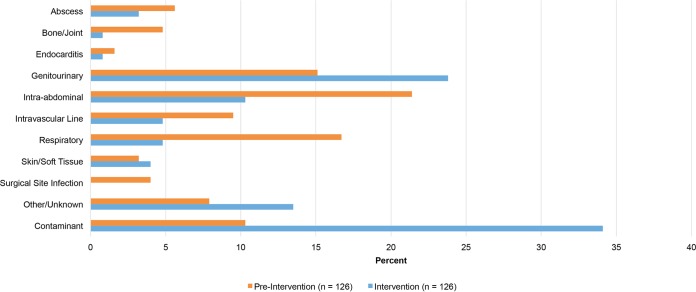
A total of 252 blood cultures from 239 patients were included in the final analysis: 126 from 116 patients in the preintervention group and 126 from 123 patients in the intervention group. Of the 126 positive blood cultures in each arm, 113 from 103 patients in the preintervention group and 83 from 80 patients in the intervention group had true (i.e., noncontaminant) bacteremia. One bacteremia was evaluated per patient. Polymicrobial BSIs were evaluated by organism, as the optimal therapy may vary. There were no statistically significant differences in baseline demographic data (Table 1) between the two groups. There were higher observed Pitt bacteremia scores and Charlson comorbidity indices in the intervention group, although the difference was not statistically significant. Hospice and palliative care consults were numerically higher in the intervention group, but interventions encouraging earlier consults were implemented between study periods. The most common sources of infection were genitourinary, intraabdominal, and respiratory (Fig. 1). There was no difference in infection-causing organisms between the groups; however, significantly more contaminants were noted in the intervention group (Table 1).
TABLE 1.
Patient baseline demographics
| Patient demographic | Preintervention (n = 116) | Intervention (n = 123) | P value |
|---|---|---|---|
| Mean age (yr) ± SD (n) | |||
| All | 58.33 ± 28.3 | 63.5 ± 23.4 | 0.219 |
| Adult | 68.43 ± 17.8 (97) | 68.59 ± 16.4 (113) | 0.946 |
| Pediatric | 6.78 ± 7.5 (19) | 6 ± 6.5 (10) | 0.783 |
| No. (%) of females | 63 (54.3) | 66 (53.7) | 0.920 |
| No. (%) with following clinical status: | |||
| General medicine | 77 (66.3) | 82 (66.7) | 0.962 |
| ICU | 39 (33.6) | 41 (33.3) | 0.962 |
| Hospice or palliative care consult | 12 (10.3) | 22 (17.9) | 0.095 |
| Mean Pitt bacteremia score ± SD | 1.91 ± 1.96 | 2.39 ± 2.44 | 0.096 |
| No. (%) with hemodynamic instability requiring vasopressors | 20 (17.2) | 19 (15.4) | 0.708 |
| Mean Charlson comorbidity index ± SD | 4.81 ±3.76 | 5.15± 3.47 | 0.468 |
| No. (%) with following no. of MDRO risk factorsa | |||
| 0 | 23 (19.8) | 27 (21.9) | 0.558 |
| 1 | 29 (25) | 39 (31.7) | |
| 2 | 29 (25) | 29 (23.6) | |
| 3 | 21 (18.1) | 20 (16.3) | |
| 4 | 10 (8.6) | 7 (5.7) | |
| 5 | 4 (3.4) | 1 (0.8) | |
| No./total (%) with MDRO infections | 27/113 (25.3) | 21/83 (23.9) | 0.996 |
| No. (%) of organisms | |||
| All Gram positive | 56 (44.4) | 78 (61.9) | 0.005 |
| Gram-positive infection | 43 (76.8) | 35 (44.9) | 0.340 |
| Gram-positive contaminant | 13 (23.2) | 43 (55.1) | <0.001 |
| Gram negative | 59 (46.8) | 46 (36.5) | 0.097 |
MDRO risk factors include health care exposure (recent hospitalization for >48 h or nursing home/skilled nursing facility residence), prior antimicrobial (especially broad spectrum) use within 90 days, recent history of MDRO, hospitalization for >5 days, mechanical ventilation for ≥5 days, immunosuppression, chronic dialysis within 30 days, a recent invasive procedure, home wound care, intravenous drug use, and structural lung disease.
FIG 1.
Sources of positive blood cultures.
MALDI-TOF MS plus AMS intervention, which had an 88% acceptance rate (Table 2), resulted in a significantly shorter TTOT than MALDI-TOF MS alone (75.17 versus 43.06 h; P < 0.001). Further evaluation of the results on the basis of organism type demonstrated that MALDI-TOF MS plus AMS intervention led to a shorter TTOT for patients with contaminated cultures (48.21 versus 11.75 h; P < 0.001) and Gram-negative infections (71.83 versus 35.98 h; P < 0.001). There was faster optimization for patients with Gram-positive infections, although the difference was not statistically significant (64.04 versus 41.61 h; P = 0.082) (Table 3).
TABLE 2.
AMS interventions
| Intervention type | No. (%) of interventionsa |
|---|---|
| Narrowed coverage | 25 (33.3) |
| Discontinued therapy | 24 (32) |
| Initiated/broadened coverage | 15 (20) |
| Other | 11 (14.7) |
| Total | 75 (100) |
Interventions were accepted 88% of the time (n = 66).
TABLE 3.
Primary endpoint outcomes
| Category | Mean TTOT (h) ± SD (n) |
P value | |
|---|---|---|---|
| Preintervention (n = 126) | Intervention (n = 126) | ||
| Overall | 75.17 ± 59.5 | 43.06 ± 35.3 | <0.001 |
| Gram-positive infection | 64.04 ± 63.3 (43) | 41.61 ± 44.9 (35) | 0.082 |
| Gram-positive contaminant | 48.21 ± 37.1 (13) | 11.75 ± 23.7 (43) | <0.001 |
| Gram-negative infection | 71.83 ± 61.5 (59) | 35.98 ± 30.9 (46) | <0.001 |
Ten patients in the preintervention group and eight in the intervention group were never on optimal therapy and were excluded from the final TTOT analysis. In the intervention group, the following reasons for the patients never having received optimal therapy were identified: four patients were severely immunocompromised, and providers were uncomfortable de-escalating therapy prior to confirmed susceptibility data; two patients had a multidrug-resistant Acinetobacter baumannii infection and did not receive one of the two AST-approved combination therapies; and two patients with penicillin allergies were initiated on aztreonam when cephalosporin therapy was more appropriate, in accordance with the institution's beta-lactam allergy guide. Due to the retrospective nature of evaluation of the preintervention group, it was difficult to evaluate the reasons why patients in this group were never on optimal therapy.
Optimization of MALDI-TOF MS through its pairing with prospective AMS intervention resulted in a significantly shorter hospital length of stay (LOS; 15.03 versus 9.02 days; P = 0.021), a significantly shorter ICU LOS for Gram-negative infections (5.55 versus 1.19 days; P = 0.035), a significantly shorter time to microbiologic clearance for Gram-negative infections (51.13 versus 34.51 h; P = 0.001), and a significantly shorter length of antimicrobial therapy for Gram-positive infections (24.30 versus 18.97; P = 0.018). Although the time to effective therapy (TTET; 16.8 versus 12.15 h; P = 0.082) and the overall ICU LOS (4.30 versus 1.22 days; P = 0.053) were not statistically significantly different, these outcomes are clinically significant in the setting of sepsis and health care cost reduction, respectively. Due to the significant reduction in the hospital LOS and ICU LOS, financial outcomes were assessed. Direct costs were reduced by half, and this resulted in an annual projected health care cost savings of approximately $6.3 million ($28,677 versus $15,784 per patient; P = 0.010) (Table 4).
TABLE 4.
Secondary endpoint outcomes
| Outcome | Preintervention | Intervention | P value |
|---|---|---|---|
| Mean TTET (h) ± SD (n) | 16.8 ± 19.59 (113) | 12.15 ± 17.2 (83) | 0.082 |
| No. of in-hospital deaths from any cause/total no. of patients (%) | |||
| Overall | 12/116 (10.3) | 15/123 (12.2) | 0.805 |
| Gram-positive infection | 3/116 (2.6) | 8/123 (6.5) | 0.256 |
| Gram-negative infection | 6/116 (5.2) | 7/123 (5.7) | 0.860 |
| Mean time (h) to microbiologic clearance ± SD | |||
| Overall | 55.07 ± 45.6 | 42.49 ± 46.2 | 0.059 |
| Gram-positive infection | 58.49 ± 56.1 | 53.94 ± 62.8 | 0.595 |
| Gram-negative infection | 51.13 ± 31.2 | 34.51 ± 26.5 | <0.001 |
| Mean hospital LOS (days) ± SD | |||
| Overall | 15.03 ± 22.7 | 9.02 ± 7.3 | 0.021 |
| Gram-positive infection | 14.64 ± 10.5 | 10.31 ± 7.89 | 0.002 |
| Gram-negative infection | 15.40 ± 30.1 | 7.90 ± 6.7 | 0.027 |
| Mean ICU LOS (days) ± SD | |||
| Overall | 4.30 ± 14.0 | 1.22 ± 3.8 | 0.053 |
| Gram-positive infection | 1.43 ± 4.2 | 1.32 ± 3.5 | 0.846 |
| Gram-negative infection | 5.55 ± 18.3 | 1.19 ± 4.13 | 0.035 |
| No. (%) of patients with recurrence of same bacteremia | |||
| Overall | 4 (3.5) | 1 (1.2) | 0.255 |
| Gram-positive infection | 0 | 0 | |
| Gram-negative infection | 4 (3.5) | 1 (1.2) | 0.255 |
| Mean length (days) of antimicrobial therapy ± SD | |||
| Overall | 18.57 ± 11.95 | 15.93 ± 11.11 | 0.117 |
| Gram-positive infection | 24.30 ± 16.0 | 18.97 ± 14.8 | 0.018 |
| Gram-negative infection | 14.25 ± 5.5 | 13.20 ± 4.5 | 0.156 |
| Avg direct costsa | $28,677 | $15,784 | 0.010 |
Difference between preintervention and intervention groups, $12,893. Projected annual cost savings, $6,291,784.
DISCUSSION
To our knowledge, this is the only study comparing the utility of MALDI-TOF MS alone with that of MALDI-TOF MS combined with prospective AMS intervention for adult and pediatric patients with positive blood cultures, highlighting the positive impact of incorporating pharmacist-driven AMS interventions to molecular rapid diagnostic testing (mRDT). Despite having the ability to obtain blood culture results up to 1.5 days faster than traditional identification methods, this study shows that without real-time AMS intervention, treatment optimization was significantly delayed. Several studies have established that the use of mRDT in settings with ASPs and microbiology result analysis improves the time to antimicrobial streamlining and various patient outcomes compared to traditional methods of organism identification (8–19).
Vlek and colleagues conducted a crossover study of 253 episodes (218 patients) of BSI that compared traditional identification methods to MALDI-TOF MS and found an 11.3% increase in the proportion of patients on appropriate antimicrobial therapy within 24 h (64% versus 75.3%; P = 0.001) (8). As the benefits of rapid antimicrobial administration became evident, studies were designed to evaluate patient outcomes. Perez and colleagues conducted a pre-post quasiexperimental study that evaluated 219 patients with Gram-negative BSIs and the impact of integrating rapid organism identification via MALDI TOF with AMS intervention. Similar to our data, their study demonstrated significant reductions in the length of hospitalization (9.3 versus 11.9 days; P = 0.01) and health care costs ($26,162 versus $45,709 per patient; P = 0.009) and nonsignificant reductions in the 30-day all-cause mortality rate (5.6% versus 10.7%; P = 0.19) and the ICU LOS (6.3 versus 7.3 days; P = 0.05) in the intervention group (9). In a subsequent study, Perez et al. evaluated the clinical outcomes for patients with antimicrobial-resistant Gram-negative BSIs and found significant improvements in the hospital LOS (15.3 versus 23.3 days; P = 0.0001) and the ICU LOS (10.7 versus 16 days; P = 0.008), as well as the 30-day all-cause mortality rate (8.9% versus 21%; P = 0.01), indicating that AMS intervention is critical when streamlining therapy for MDROs (16). Although it was not within the scope of the present study, several educational opportunities were discovered when optimizing treatment for patients infected with MDROs, particularly with regard to the treatment of beta-lactamase-producing organisms and susceptibility report interpretation.
While this study did not show a significant decrease in TTOT for patients with Gram-positive infections, we did note a trend toward faster optimization; of note, these patients were often empirically treated with vancomycin, which is the optimal therapy for many Gram-positive infections. Furthermore, despite a trend toward a shorter TTET, we did not see a significant reduction, which may be partly due to several interventions that ensure that patients receive rapid broad-spectrum therapy when presenting with sepsis and partly due to a small sample size. When evaluating a large sample of 501 patients, Huang and colleagues found that integration of real-time AMS intervention resulted in an improved TTET (20.4 versus 30.1 h; P = 0.021) and an improved TTOT (47.3 versus 90.3 h; P < 0.001). Furthermore, clinical outcomes demonstrated a significant reduction in the all-cause mortality rate (20.3% versus 12.7%; P = 0.021), the ICU LOS (8.3 versus 14.9 days; P = 0.014), and bacteremia recurrence (5.9% versus 2.0%; P = 0.038) and a nonsignificant reduction in the hospital LOS (11.4 versus 14.2; P = 0.066) (10). Despite not having a significant reduction in the TTET, we did note a significant reduction in the LOS in our study. A conceivable explanation for this may be that as a result of early pathogen notification and subsequent provider-pharmacist discussion of streamlining to optimal therapy, providers could confidently discharge patients earlier.
Interestingly, this study demonstrated a trend toward a reduction in the duration of antimicrobial therapy in the intervention group, with a significant reduction in the duration of antimicrobial therapy for patients with Gram-positive infections. Although the optimal length of therapy depends on the source of infection, a reduction in the duration of treatment may be the result of faster optimization. In addition to reducing the duration of therapy, our intervention significantly impacted early discontinuation of unnecessary antimicrobials, a critical intervention particularly as the rise in antimicrobial resistance threatens public health. Nagel and colleagues evaluated patients with coagulase-negative Staphylococcus-positive blood cultures. Consistent with our study, their findings showed a significant reduction in unnecessary antimicrobial use (3.0 versus 4.4 days; P = 0.015) (11).
Although the present study demonstrated faster antimicrobial optimization, a shorter LOS, and substantial cost savings, a significant difference between the mortality rates of the preintervention and intervention groups was not observed. Clinical studies evaluating larger sample sizes or narrowing their focus to MDROs did demonstrate significant reductions in the mortality rate (10, 16). Perhaps we did not observe this benefit because of our small sample size and broad inclusion criteria. Nonetheless, facilities with ASTs are more likely to observe mortality rate benefits. In a recent meta-analysis of 31 studies evaluating the effect of mRDT on clinical outcomes, Timbrook and colleagues found that the risk of death was lower when mRDT was done in facilities with ASTs providing intervention and not in facilities without ASTs, demonstrating an overall positive impact of ASTs on the mortality rate (15). However, literature evaluating the added benefit of AMS intervention alone is limited. The present study aimed to evaluate this addition of AMS intervention and resulted in an 88% intervention acceptance rate. The high success rate is likely attributable to the long-standing integration of clinical pharmacists within the institution. Specifically, our institution has had one full-time equivalent (FTE) infectious diseases clinical pharmacy specialist devoted to AMS-related activities for approximately 30 years. This pharmacist is responsible for chairing the AST, program outcomes, and providing daily prospective audit and feedback Monday through Friday. Despite the success, a challenge of this study included de-escalating empirical therapy prior to susceptibility results because of the perceived risk of MDROs regardless of antibiogram utilization.
Several limitations exist. This was a quasiexperimental study that utilized a convenience sample and therefore lacked randomization. Patients were not matched, and substantially more contaminants were present in the intervention group, although these were excluded from the analysis of secondary outcomes. This study included a small sample size, which may have contributed to a lack of difference in some of the secondary outcomes, including the mortality rate. Confounding factors, including the difference in the source of infection and other unaccounted for variables, cannot be ruled out and may have impacted the observed outcomes. Another major limitation of this study was the highly labor-intensive process, as this work was largely completed by one individual. Interventions were made on the basis of real-time MALDI-TOF MS results, and patients were evaluated via chart review or direct patient care before sensitivity data became available. All interventions were made by a pharmacy resident under the direct supervision of an infectious diseases clinical pharmacy specialist. For this intervention to be sustained, an additional FTE infectious disease clinical pharmacist would need to be supported.
At study completion, a new laboratory process was implemented in response to MALDI-TOF MS's inability to detect resistance markers. Although patients with a history of and risk factors for MDROs were carefully evaluated, absence of susceptibility data at the time of organism identification made streamlining of therapy particularly challenging. Walker et al. demonstrated that detection of resistance markers via Verigene BC-GN assay was valuable and optimized antimicrobial regimens faster, resulting in a significantly shorter ICU LOS (16.2 versus 12.0 days; P = 0.03) and a decreased 30-day mortality rate (19.2 versus 8.1%; P = 0.04), particularly for patients infected with extended-spectrum beta-lactamase-producing bacteria (20). The significant cost savings demonstrated in this study, as well as several others (8, 21, 22), may justify obtaining additional technology with the ability to detect resistance markers in the future. Meanwhile, susceptibility testing of bacterial dilutions made from 5-h cultures is performed via Vitek 2, reducing the time to susceptibility determination by at least 12 h. As a quality control measure, a purity plate from each sample that undergoes susceptibility testing is always set up since there is a possibility that the sample from the 5-h culture is contaminated with other bacteria. Susceptibility results are not released until purity plates are evaluated. Despite the aforementioned challenges, this 24-h microbiology laboratory initially purchased MALDI-TOF MS over other technologies, as it is more cost effective, fits well into the algorithms and workflows used for organism identification, and requires no additional equipment or training. Coupled with AMS intervention, MALDI-TOF MS further demonstrates a significant impact on patient care.
Conclusion.
This study demonstrated that combining rapid culture techniques and MALDI-TOF MS with real-time AMS intervention consistently provided more favorable outcomes than MALDI-TOF MS alone, highlighting the importance of real-time AMS. These data should be factored into budgetary considerations when preparing for the implementation of mRDT.
MATERIALS AND METHODS
Study design and patient population.
This was a single-center, pre-post, quasiexperimental study conducted in a 645-bed tertiary care teaching facility in a suburban setting. Adult and pediatric patients admitted to hospital inpatient services during a 3-month period (November 2015 to January 2016) with a positive blood culture identified via MALDI-TOF MS were included and compared to a control cohort during the same 3-month period of the previous year (November 2014 to January 2015). Preintervention patients were evaluated via retrospective chart review, while patients in the intervention group were prospectively reviewed as cultures became positive without blinding. All of the positive blood cultures of both groups during the study periods were included for review. Excluded were patients with active BSIs who were transferred from outside hospitals and patients who expired before a blood culture result was obtained.
Workflow procedures prior to intervention.
The workflow prior to intervention was implemented in 2012 when MALDI-TOF MS technology was purchased. Blood cultures were analyzed for the presence of microorganisms via the BacT/Alert Microbial detection system (bioMérieux, Durham, NC), which contains culture media with suitable nutritional and environmental conditions for the most common organisms found in blood. Inoculated bottles are placed into the instrument (BacT/Alert 3D), incubated, and continuously monitored for growth. Every 2 h, the BacT/Alert 3D is evaluated for culture positivity. Once an organism is flagged as positive, a Gram stain is performed and results are posted in the patient's electronic medical record (EMR) and promptly called to the charge nurse. Blood agar plates were preincubated in a 5% CO2 incubator at 35°C for 4 h prior to inoculation in the middle of the plate with 0.1-ml samples from positive blood culture bottles. Inoculated plates were incubated for 5 h in 5% CO2 at 35°C before bacterial growth on these plates was analyzed by MALDI-TOF MS (Vitek MS; bioMérieux). Once the MALDI-TOF MS identification data are available, results are posted in the EMR but they are not communicated directly to a health care provider.
New workflow and AMS interventions.
Prior to initiating the new workflow, the AST developed a comprehensive adult and pediatric blood culture and bacteremia guideline including indication for and timing of blood cultures, methods of obtaining blood cultures, duration of incubation and organism identification, interpretation of blood culture results, assessment of contamination versus true bacteremia, clinical pearls of BSI management, and organism-specific bacteremia treatment recommendations. The assessment of contamination versus true bacteremia and organism-specific bacteremia treatment recommendations can be found in Text S1 in the supplemental material. These guidelines were presented to and approved by the AST and the pharmacy and therapeutics committee.
Positive blood cultures were evaluated by using the same microbiologic procedures prior to this study. However, rather than passive verification of final culture results, a designated pharmacist was responsible for receiving real-time notification of all blood culture-positive MALDI-TOF MS results via pager 24 h a day, 7 days a week. Subsequently, this pharmacist promptly contacted the physician to provide recommendations based on the AST-approved, evidence-based protocol. Pages received between the hours of 10:00 pm and 6:00 am were evaluated and triaged by the designated pharmacist; however, with the exception of events requiring immediate attention, such as organism-antimicrobial mismatches, overnight pages were addressed immediately the following morning prior to antimicrobial administration.
Study endpoints.
The primary outcome was TTOT, which was determined by the AST on the basis of previously reported definitions (10). Optimal therapy was defined as the time from blood culture draw to the time of most appropriate antimicrobial therapy administration on the basis of the bacteremia guideline, patient-specific susceptibility, and source of infection. This included broadening coverage if necessary, de-escalating therapy to the narrowest-spectrum antimicrobial, or discontinuing inappropriate or duplicative antimicrobial therapy. Optimal therapy for contaminants included discontinuation of therapy, provided there was no other source of infection. Contaminants were adjudicated on the basis of several AST-approved factors (see Table S1 in the supplemental material). Patients who were never on optimal therapy were excluded from the TTOT analysis. Antimicrobial recommendations were based on MALDI-TOF MS results and local, institution-specific resistance patterns for adult and pediatric patients (see Tables S2 to S7). For true bacteremia only (i.e., contaminants excluded), the following secondary endpoints were evaluated: the TTET, defined as the time from blood culture draw to the time of first susceptible antimicrobial administration; the in-hospital all-cause mortality rate; the hospital LOS, the ICU LOS; the time to microbiologic clearance; the length of antimicrobial therapy (including inpatient and outpatient treatment durations); and the recurrence of bacteremia within 30 days of antimicrobial discontinuation. The time to microbiologic clearance was not analyzed for patients without surveillance cultures. Patients who expired were excluded from the analysis of LOS data. Financial data were evaluated by the finance department by evaluating direct cost savings based on the LOS for all patients.
Statistical analysis.
A sample size of 40 patients per group was needed to achieve 80% power at a 5% significance level with a true difference between the means of 1.50 and a standard deviation of 1.00, and the equivalence limits are −2.00 and 2.00. Descriptive statistics were performed for all continuous (mean ± standard deviation) and categorical (number [percent]) data. All normally distributed continuous variables were compared by using the Student t test, and all categorical variables were compared by χ2 analysis. Analyses were performed with SPSS for Windows, version 22.0 (SPSS Inc., Chicago, IL), and a two-tailed P value of 0.05 was considered statistically significant.
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge Ronda Oram, Pediatric Infectious Diseases, and Procopio LoDuca, Adult Infectious Diseases, for their insight and review of guidelines to facilitate this project and Ina Zamfirova and Sarah Kozmic, research coordinators, Patient Centered Outcomes Research at the Russell Institute for Research and Innovation, and the ACL Laboratories Microbiology staff for their assistance with this project.
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
We have no actual or potential conflicts of interest to disclose.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/JCM.02245-16.
REFERENCES
- 1.Hidron AI, Edwards JR, Patel J, Horan TC, Sievert DM, Pollock DA, Fridkin SK, National Healthcare Safety Network Team Participating National Healthcare Safety Network Facilities. 2008. NHSN annual update: antimicrobial resistant pathogens associated with healthcare-associated infections: annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006 2007. Infect Control Hosp Epidemiol 29:996–1011. doi: 10.1086/591861. [DOI] [PubMed] [Google Scholar]
- 2.Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. 2004. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis 39:309–317. doi: 10.1086/421946. [DOI] [PubMed] [Google Scholar]
- 3.Gaynes R. 1997. The impact of antimicrobial use on the emergence of antimicrobial-resistant bacteria in hospitals. Infect Dis Clin North Am 11:757–765. doi: 10.1016/S0891-5520(05)70388-3. [DOI] [PubMed] [Google Scholar]
- 4.Kumar A, Roberts D, Wood KE, Light B, Parrillo JE, Sharma S, Suppes R, Feinstein D, Zanotti S, Taiberg L, Gurka D, Kumar A, Cheang M. 2006. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med 34:1589–1596. doi: 10.1097/01.CCM.0000217961.75225.E9. [DOI] [PubMed] [Google Scholar]
- 5.Harbarth S, Garbino J, Pugin J, Romand JA, Lew D, Pittet D. 2003. Inappropriate initial antimicrobial therapy and its effect on survival in a clinical trial of immunomodulating therapy for severe sepsis. Am J Med 115:529–535. doi: 10.1016/j.amjmed.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 6.Ibrahim EH, Sherman G, Ward S, Fraser VJ, Kollef MH. 2000. The influence of inadequate antimicrobial treatment of bloodstream infections on patient outcomes in the ICU setting. Chest 118:146–155. doi: 10.1378/chest.118.1.146. [DOI] [PubMed] [Google Scholar]
- 7.Barenfanger J, Graham DR, Kolluri L, Sangwan G, Lawhorn J, Drake CA, Verhulst SJ, Peterson R, Ertmoed MM, Moja AB, Shevlin DW, Vautrain R, Callahan CD. 2008. Decreased mortality associated with prompt Gram staining of blood cultures. Am J Clin Pathol 130:870–876. doi: 10.1309/AJCPVMDQU2ZJDPBL. [DOI] [PubMed] [Google Scholar]
- 8.Vlek AL, Bonten MJ, Boel CH. 2012. Direct matrix-assisted laser desorption ionization time-of-flight mass spectrometry improves appropriateness of antibiotic treatment of bacteremia. PLoS One 7:e32589. doi: 10.1371/journal.pone.0032589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perez KK, Olsen RJ, Musick WL, Cernoch PL, Davis JR, Land GA, Peterson LE, Musser JM. 2013. Integrating rapid pathogen identification and antimicrobial stewardship significantly decreases hospital costs. Arch Pathol Lab Med 137:1247–1254. doi: 10.5858/arpa.2012-0651-OA. [DOI] [PubMed] [Google Scholar]
- 10.Huang AM, Newton D, Kunapuli A, Gandhi TN, Washer LL, Isip J, Collins CD, Nagel JL. 2013. Impact of rapid organism identification via matrix-assisted laser desorption/ionization time-of-flight combined with antimicrobial stewardship team intervention in adult patients with bacteremia and candidemia. Clin Infect Dis 57:1237–1245. doi: 10.1093/cid/cit498. [DOI] [PubMed] [Google Scholar]
- 11.Nagel JL, Huang AM, Kunapuli A, Gandhi TN, Washer LL, Lassiter J, Patel T, Newton DW. 2014. Impact of antimicrobial stewardship intervention on coagulase-negative Staphylococcus blood cultures in conjunction with rapid diagnostic testing. J Clin Microbiol 52:2849–2854. doi: 10.1128/JCM.00682-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clerc O, Prod'hom G, Vogne C, Bizzini A, Calandra T, Greub G. 2013. Impact of matrix-assisted laser desorption ionization time-of-flight mass spectrometry on the clinical management of patients with Gram-negative bacteremia: a prospective observational study. Clin Infect Dis 56:1101–1107. doi: 10.1093/cid/cis1204. [DOI] [PubMed] [Google Scholar]
- 13.Neuner EA, Pallotta AM, Lam SW, Stowe D, Gordon SM, Procop GW, Richter SS. 2016. Experience with rapid microarray-based diagnostic technology and antimicrobial stewardship for patients with Gram-positive bacteremia. Infect Control Hosp Epidemiol 37:1361–1366. doi: 10.1017/ice.2016.175. [DOI] [PubMed] [Google Scholar]
- 14.Doern CD. 2016. The confounding role of antimicrobial stewardship programs in understanding the impact of technology on patient care. J Clin Microbiol 54:2420–2423. doi: 10.1128/JCM.01484-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Timbrook TT, Morton JB, McConeghy KW, Caffrey AR, Mylonakis E, LaPlante KL. 2017. The effect of molecular rapid diagnostic testing on clinical outcomes in bloodstream infections: a systematic review and meta-analysis. Clin Infect Dis 64:15–23. doi: 10.1093/cid/ciw649. [DOI] [PubMed] [Google Scholar]
- 16.Perez KK, Olsen RJ, Musick WL, Cernoch PL, Davis JR, Peterson LE, Musser JM. 2014. Integrating rapid diagnostics and antimicrobial stewardship improves outcomes in patients with antibiotic-resistant Gram-negative bacteremia. J Infect 69:216–225. doi: 10.1016/j.jinf.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 17.Sango A, McCarter YS, Johnson D, Ferreira J, Guzman N, Jankowski CA. 2013. Stewardship approach for optimizing antimicrobial therapy through use of a rapid microarray assay on blood cultures positive for Enterococcus species. J Clin Microbiol 51:4008–4011. doi: 10.1128/JCM.01951-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sothoron C, Ferreira J, Guzman N, Aldridge P, McCarter YS, Jankowski CA. 2015. A stewardship approach to optimize antimicrobial therapy through use of a rapid microarray assay on blood cultures positive for Gram-negative bacteria. J Clin Microbiol 53:3627–3629. doi: 10.1128/JCM.02161-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.MacVane SH, Nolte FS. 2016. Benefits of adding a rapid PCR-based blood culture identification panel to an established antimicrobial stewardship program. J Clin Microbiol 54:2455–2463. doi: 10.1128/JCM.00996-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walker T, Dumadag S, Lee CJ, Lee SH, Bender JM, Cupo Abbott J, She RC. 2016. Clinical impact after laboratory implementation of the Verigene Gram-negative bacteria microarray for positive blood cultures. J Clin Microbiol 54:1789–1796. doi: 10.1128/JCM.00376-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaki R, Elligsen M, Walker S, Simor A, Palmay L, Daneman N. 2011. Impact of antimicrobial stewardship in critical care: a systematic review. J Antimicrob Chemother 66:1223–1230. doi: 10.1093/jac/dkr137. [DOI] [PubMed] [Google Scholar]
- 22.Nowak MA, Nelson RE, Breidenbach JL, Thompson PA, Carson PJ. 2012. Clinical and economic outcomes of a prospective antimicrobial stewardship program. Am J Health Syst Pharm 69:1500–1508. doi: 10.2146/ajhp110603. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.