ABSTRACT
The utility of a line probe assay (Genotype MTBDRplus) performed directly on 2-month sputa to monitor tuberculosis treatment response is unknown. We assessed if direct testing of 2-month sputa with MTBDRplus can predict 2-month culture conversion and long-term treatment outcome. Xpert MTB/RIF-confirmed rifampin-susceptible tuberculosis cases were recruited at tuberculosis diagnosis and followed up at 2 and 5 to 6 months. MTBDRplus was performed directly on 2-month sputa and on all positive cultured isolates at 2 and 5 to 6 months. We also investigated the association of a positive direct MTBDRplus at 2 months with subsequent unsuccessful tuberculosis treatment outcome (failure/death during treatment or subsequent disease recurrence). A total of 279 patients (62% of whom were HIV-1 coinfected) were recruited. Direct MTBDRplus at 2 months had a sensitivity of 78% (95% confidence interval [CI], 65 to 87) and specificity of 80% (95% CI, 74 to 84) to predict culture positivity at 2 months with a high negative predictive value of 93% (95% CI, 89 to 96). Inconclusive genotypic susceptibility results for both rifampin and isoniazid were seen in 26% of MTBDRplus tests performed directly on sputum. Compared to a reference of MTBDRplus performed on positive cultures, the false-positive resistance rate for direct testing of MTBDRplus on sputa was 4% for rifampin and 2% for isoniazid. While a positive 2-month smear was not significantly associated with an unsuccessful treatment outcome (adjusted odds ratio [aOR], 2.69; 95% CI, 0.88 to 8.21), a positive direct MTBDRplus at 2 months was associated with an unsuccessful outcome (aOR 2.87; 95% CI, 1.11 to 7.42). There is moderate utility of direct 2-month MTBDRplus to predict culture conversion at 2 months and also to predict an unfavorable outcome.
KEYWORDS: line probe assay, treatment outcome, drug resistance, bacterial biomarker, tuberculosis
INTRODUCTION
As part of the global strategy to diagnose, treat, and reduce the transmission of drug-sensitive (DS) and drug-resistant (DR) tuberculosis (TB), molecular diagnostic tests have reduced time to treatment (1). Interim outcome measures such as 2-month culture conversion have merit as a surrogate of long-term treatment outcomes and hence may expedite progression to testing of novel regimens in clinical trial settings (2). The good negative predictive value of a negative culture at 2 months for the long-term treatment outcomes of failure/relapse can be used by clinicians as a marker of treatment response, with a subsequent switch from a quadruple-drug regimen to dual therapy (3). Drug pressure may select for mutations conferring resistance or amplify resistance during treatment. This process can occur at different time points during treatment, ranging from within the first 2 months, when the bacterial load is highest, to later in treatment, and this can be screened for by using molecular or culture-based drug susceptibility tests on sputum obtained during treatment. Such screening is typically conducted at 2 months and at month 5 or 6 of treatment (at which point the patient is considered to have failed treatment if still culture positive) (4). Acquired drug resistance (ADR) is influenced by baseline drug susceptibility patterns, HIV-1 coinfection, baseline extent of disease, and treatment adherence (5).
The GenoType MTBDRplus line probe assay (LPA), version 2.0 (Hain Lifescience, Nehren, Germany), is a molecular diagnostic assay that has been widely used to identify resistance to first-line drugs rifampin and isoniazid among TB cases. It uses PCR to amplify regions specific to Mycobacterium tuberculosis complex, areas of the rpoB gene associated with rifampin resistance, and katG and inhA genes associated isoniazid resistance from smear-positive or smear-negative clinical samples (6, 7) or cultured isolates. Hybridization of amplified sequences to a membrane strip (with immobilized probes for both wild-type and mutant sequences) identifies specific mutant bands or the absence of wild-type bands. It appears to be more sensitive than Xpert MTB/RIF to detect heteroresistance, defined as the simultaneous presence of DS and DR populations in the same patient, thought likely to be an early step in the pathway of resistance amplification (8). ADR can be reliably detected only when drug-resistant mutants are present at greater than 65% and 5% of the overall bacterial population for GeneXpert MTB/RIF (9) and MTBDRplus, respectively (8, 10). Limitations of both tests in treatment monitoring include amplification of DNA from nonviable bacteria.
We prospectively determined the utility of MTBDRplus to predict culture conversion when carried out directly on a 2-month sputum specimen. We also assessed the potential of a direct MTBDRplus to detect early ADR/heteroresistance on a 2-month sputum specimen, compared with a reference of MTBDRplus on positive 2-month cultured isolates. We also determined the association between a positive 2-month MTBDRplus result and the composite long-term unsuccessful outcome of failure/death during treatment or TB recurrence.
RESULTS
Outcomes.
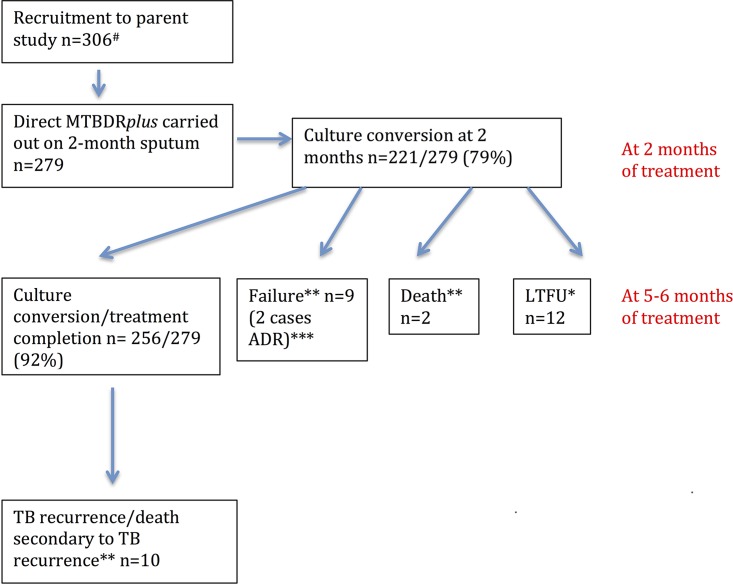
From the larger study of 306 participants, overall 279 participants (62% HIV coinfected, 51% with smear grade 1+ to 3+ at baseline, and 32% of retreatment status), who had a direct MTBDRplus test carried out on 2-month sputum, were included in the 2-month culture conversion analyses. Patients in the cohort had clinical follow-up for the duration of TB treatment and a follow-up at a median of 22 months (interquartile ratio [IQR], 18 to 28) to ascertain disease recurrence via electronic database searches. The smear and culture conversion rates at 2 months were 88% and 79%, respectively. There were no culture-confirmed cases of ADR at 2 months. At 5 to 6 months, the rate of culture conversion (the denominator including only those who produced sputum at 5 to 6 months) was 223/233 (95%). The culture conversion/treatment completion rate was 256/279 (92%). There were 267/279 participants who had both a 2-month direct MTBDRplus result and a known long-term treatment outcome. There were 8 individuals with a negative 2-month MTBDRplus result who defaulted on treatment and 3 who were lost to follow-up. There was 1 individual with a positive 2-month direct MTBDRplus result who had an unknown long-term treatment outcome. Two cases of ADR (1 with acquired isoniazid resistance and 1 with acquired rifampin resistance) were identified on MTBDRplus and phenotypic drug susceptibility testing (DST) of positive 5- to 6-month cultures. Overall, there were 21/267 (8%) individuals with unsuccessful outcomes (11 failures/deaths during treatment and 10 TB recurrences (see Fig. 2; see also Table S1 in the supplemental material).
FIG 2.
Study outcomes. #, 17 patients did not produce sputa at 2 months, 2 died before follow-up, and 8 produced only 1 sputum, which was erroneously used only for culture; *, 1 LTFU (lost to follow-up) participant had a positive MTBDRplus result, and 11 LTFU participants had a negative MTBDRplus at 2 months; **, unsuccessful outcomes (n = 21); ***, 1 case acquired isoniazid resistance, and 1 case acquired rifampin resistance.
Direct MTBDRplus for assessment for culture conversion at 2 months.
A positive direct MTBDRplus on a 2-month sputum sample (presence of M. tuberculosis complex band [TUB band], amplification and locus control) had a sensitivity of 78% (95% CI, 65 to 87) and specificity of 80% (95% CI, 74 to 85) to predict positivity at 2 months in liquid (MGIT) cultures. The negative predictive value of direct MTBDRplus on 2-month sputa to predict culture conversion was high, 93% (95% CI, 89 to 96) (Table 1). The sensitivity and specificity of a positive smear test carried out on the 2-month sample were 41% (95% CI, 29 to 55) and 96% (95% CI, 92 to 98), with a negative predictive value of 86% (95% CI, 81 to 90). The proportion of positive direct MTBDRplus results at 2 months was significantly higher in HIV-1-uninfected patients than in HIV-1-infected patients (P = 0.04), in patients with baseline smear grades 1+ to 3+, in smear-scanty/negative (P < 0.01) and retreatment patients, and in new patients (P = 0.04) (Table 2). However, when stratified by HIV-1 serostatus, there were no significant differences in sensitivity, specificity, or positive or negative predictive value of the direct MTBDRplus to predict 2-month culture conversion (Table 1). There were no associated clinical characteristics noted in the 13 individuals who were direct MTBDRplus negative and culture positive for whom a negative result did not correctly predict culture conversion at 2 months.
TABLE 1.
Utility of MTBDRplus directly on 2-month sputa to detect 2-month culture positivitya
| Assay characteristic | No. for MTBDRplus directly on 2-month sputa/no. for reference method (detection of culturable M. tuberculosis in liquid culture in MGIT) (%, 95% CI) |
||
|---|---|---|---|
| All (n = 279) | HIV infected (n = 172) | HIV uninfected (n = 107) | |
| Sensitivity | 45/58 (78, 65–87) | 23/30 (77, 58–90) | 22/28 (79, 59–92) |
| Specificity | 177/221 (80, 74–85) | 118/142 (83, 76–89) | 59/79 (75, 64–84) |
| Positive predictive value | 45/89 (51, 40–61) | 23/47 (49, 34–64) | 22/42 (52, 36–58) |
| Negative predictive value | 177/190 (93, 89–96) | 118/125 (94, 89–98) | 59/65 (90, 81–97) |
Comparison of characteristics of direct MTBDRplus assay using 2-month sputa (for predicting 2-month culture conversion) and of reference method (liquid culture in MGIT), with stratification of the cohort by HIV-1 serostatus to assess the effect of HIV-1 serostatus on the utility of the test.
TABLE 2.
Direct MTBDRplus results at 2 months stratified by HIV-1 serostatus, baseline smear grade, and retreatment status
| Direct MTBDRplus-positive results for M. tuberculosis complex (n = 89) | ||||||||
|---|---|---|---|---|---|---|---|---|
| No. (%) of patients: |
P value | No. (%) of patients with baseline smear grade: |
P value | No. (%) of patients who were: |
P value | |||
| Positive for HIV-1 infection (total n = 172) | Negative for HIV-1 infection (total n = 107) | Negative/scanty (total n = 136) | 1+ to 3+ (total n = 43) | New (total n = 189) | In retreatment (total n = 90) | |||
| 47 (27) | 42 (39) | 0.04 | 27 (20) | 62 (43) | <0.01 | 53 (28) | 36 (40) | 0.04 |
Direct MTBDRplus for assessment of long-term treatment outcomes.
The 2-month direct MTBDRplus test was more likely to be positive in those with unsuccessful outcomes, 12/21 (57%), than in those with successful outcomes, 76/246 (31%) (P = 0.01). The 2-month smear positivity rates were also significantly increased in those with unsuccessful outcomes, 6/21 (29%) compared with those with successful outcomes, 27/246 (11%) (P = 0.02). There was no significant difference in the proportion of unsuccessful treatment outcomes for new versus retreatment patients (see Table S2 in the supplemental material).
When adjusted for baseline extensive radiological disease and HIV-1 serostatus, a positive direct MTBDRplus at 2 months (adjusted odds ratio [aOR], 2.88; 95% CI, 1.11 to 7.42). A positive 2-month smear was not significantly associated with an unsuccessful treatment outcome (aOR, 2.69; 95% CI, 0.88 to 8.21).
Direct MTBDRplus for assessment of ADR.
The use of MTBDRplus directly on 2-month sputa did not identify any culture-proven cases of ADR. There were no cases in which both wild-type and mutant bands were simultaneously represented on the MTBDRplus strip, demonstrating heteroresistance. There were inconclusive rifampin and isoniazid genotypic susceptibility results in 26% of positive direct MTBDRplus tests. When this analysis was restricted to the 2-month samples that subsequently had paired positive cultures at 2 months, the proportions of inconclusive rifampin and isoniazid genotypic susceptibility results were reduced to 16% and 7%, respectively (Table 3). There were false rifampin resistance calls in 4% of cases and false isoniazid resistance calls in 2% of cases (Table 4). None of these cases showed heteroresistance on review of the MTBDRplus membrane strip. On repeat direct MTBDRplus tests performed on archived sputa, the result was either negative for MTB complex or rifampin/isoniazid susceptible. Only 3 sputa (of 11 with false-positive rifampin with or without isoniazid resistance) were subsequently culture positive, and DST on the culture showed rifampin/isoniazid susceptibility. All patients with false-positive resistance results completed standard anti-TB treatment and had successful treatment outcomes.
TABLE 3.
Utility of MTBDRplus directly on 2-month sputum to detect acquired drug resistance: inconclusive results
| MTBDRplus resulta direct on 2-mo sputa | No. (%) of results deemed positive (of a total of 89 samples) | No. (%) restricted to confirmatory positive cultureb (of a total of 58 samples) |
|---|---|---|
| Inconclusive INH genotypic susceptibility result | 23 (26) | 4 (7) |
| Inconclusive RIF genotypic susceptibility result | 23 (26) | 9 (16) |
INH, isoniazid; RIF, rifampin.
All positive cultures had confirmed rifampin and isoniazid susceptibilities.
TABLE 4.
Utility of MTBDRplus directly on 2-month sputum to detect acquired drug resistance: false-positive results
| MTBDRplus resulta | No. (%) of samples (total n = 279) | Repeat direct MTBDRplus test resultsb |
|---|---|---|
| False-positive isoniazid resistance | 6 (2) | Result was negative for MTB complex in 4/6 cases and isoniazid susceptible in 2/6 cases |
| False-positive rifampin resistance | 11 (4) | Result was negative for MTB complex in 7/11 cases and rifampin susceptible in 4/11 cases |
Compared with MTBDRplus and phenotypic drug susceptibility testing performed on culture if flagged positive. Only 3 patients (of 11 with false-positive rifampin with or without isoniazid resistance) were subsequently culture positive.
On archived sputa in cases of suspected false-positive resistance.
DISCUSSION
This is the first study to report the utility of MTBDRplus directly on sputum to predict 2-month positivity in liquid culture. The sensitivity was superior to that of a 2-month smear (78% versus 41%), and the specificity was inferior (80% versus 96%). The suboptimal sensitivity and positive predictive value of MDTBRplus directly on sputum do not support substitution of this assay for the conventional culture method to monitor interim treatment response.
Friedrich et al. assessed the utility of Xpert/MTB RIF (also carried out directly on 2-month sputum samples) to monitor treatment response against conventional smear and culture positivity in 177 HIV-1-uninfected patients. They reported a sensitivity and specificity of 95% (95% CI, 87 to 98) and 23% (95% CI, 15 to 32), respectively, for predicting 2-month culture positivity with a positive predictive value of 47% (95% CI, 39 to 56) and a negative predictive value of 85% (95% CI, 66 to 96) (11). Hence, the specificity of the direct MTBDRplus assay (80%) was much better than that of the results reported for the qualitative Xpert MTB/RIF test. This was not explained by potential differences in sputum bacillary load in HIV-1-infected and -uninfected individuals (12) (Friedrich et al. excluded HIV-coinfected individuals, whereas 62% of individuals in this study were HIV-1 coinfected). A negative MTBDRplus test had a high negative predictive value for 2-month culture conversion in both HIV-1-infected and HIV-1-uninfected individuals. There was a significantly lower sensitivity for culture positivity of direct MTBDRplus than for Xpert MTB/RIF at 2 months (78% versus 95%). Potentially, the former is less likely to be falsely positive (secondary to DNA from nonviable bacilli) several months following successful treatment of TB than has been reported with Xpert MTB/RIF (13). However, this hypothesis should be confirmed in a study directly comparing Xpert MTB/RIF and MTBDRplus for the same samples.
A study by Shenai et al. showed that by using Xpert MTB/RIF cycle threshold (CT) parameters to estimate bacterial load, a CT of 28.6 at month 2 of chemotherapy had a sensitivity of 88.6% (95% CI, 77 to 95) and a specificity of 76.3% (95% CI, 60 to 89%) for prediction of culture positivity. These results are close to our results obtained with MTBDRplus directly on sputum (14).
Preemptive detection of heteroresistance, which may give rise to ADR, can be challenging. While phenotypic or genotypic DST on a positive culture is currently the reference standard, it can take several weeks to culture M. tuberculosis from expectorated sputum, delaying the commencement of individualized treatment regimens in cases of ADR. In this study, neither of the cases of ADR detected by MTBDRplus and phenotypic DST at 5 to 6 months was detected as heteroresistance on direct MTBDRplus at 2 months. The majority of sputa at 2 months are smear negative, and this study demonstrated unacceptably high rates of inconclusive and false-positive resistance results using MTBDRplus directly on sputum compared to culture as the gold standard. The inconclusive results may be secondary to insufficient DNA in the context of increased amplification cycles included in the direct MTBDRplus test protocol. This interpretation is supported by the lower proportion of inconclusive direct MTBDRplus genotypic susceptibility results from samples that were culture positive versus culture negative. The false-positive resistance results may be secondary to handling PCR amplicons in open molecular diagnostic laboratories dealing with high volumes of routine clinical samples. Repeat direct MTBDRplus results for 2-month archived sputa from patients who had false-positive resistance results either were negative or showed rifampin/isoniazid susceptibility. Only 3/11 patients who had false-positive resistance results on direct MTBDRplus at 2 months were subsequently culture positive, and DST on these cultures also showed RIF/INH susceptibility.
Although this finding was not the primary aim of the study, in this cohort, a positive MTBDRplus at 2 months was associated with an approximately 3-fold-increased odds of the composite unsuccessful outcome of death/failure/relapse, having adjusted for baseline extensive disease on chest radiograph and HIV-1 status. MTBDRplus was better than 2-month smear at predicting unsuccessful outcomes.
A strength of this study was that the same 2-month sputum specimen was utilized for carrying out both direct MTBDRplus testing and liquid culture. This reduced the variability associated with cough efforts and M. tuberculosis inoculum size. A limitation of this study is that direct MTBDRplus testing was carried out at only a single time point during treatment, thus limiting its assessment as a biomarker of treatment response earlier or later in treatment. Archived sputa, used to repeat direct MTBDRplus tests for patients with false-positive resistance results, were frozen, and a smaller volume than the original sample was tested. Hence, this may not have been an ideal comparison. There was no clear explanation for the 13 individuals who were direct MTBDRplus negative and culture positive at 2 months. It is possible that the bacillary load was simply too low. In this cohort, there were only 2 culture-confirmed cases of ADR, neither of which were detected at 2 months by direct MTBDRplus. Hence, we were limited in our capacity to assess the ability of direct MTBDRplus as a screening tool for ADR. However, the inconclusive and false-positive genotypic resistance results are clearly of concern.
Conclusion.
In conclusion, the direct MTBDRplus may have moderate value in predicting 2-month culture conversion, its major value being that if negative, there is a 93% probability that the 2-month culture will be negative. Its potential as a biomarker of long-term treatment outcomes warrants further research. Use of direct MTBDRplus for routine treatment monitoring is not recommended. Its use in patient management should be carried out with caution, and the diagnostic utility and limitations should be interpreted in the appropriate clinical context.
MATERIALS AND METHODS
Setting.
This study was carried out as part of a larger study assessing the frequency and determinants of ADR in patients with baseline rifampin-susceptible pulmonary TB. Participant recruitment was at Site B Ubuntu Clinic, an integrated HIV-1/TB primary care clinic in Khayelitsha Western Cape, South Africa, from March 2013 to July 2014. All laboratory work was carried out at a routine diagnostic laboratory, National Health Laboratory Service (Groote Schuur Hospital), which annually receives approximately 9,000 respiratory samples for TB culture (13% positive). On average, 100 to 130 MTBDRplus LPAs are carried out per month.
Recruitment and procedures.
Participants with Xpert MTB/RIF-confirmed rifampin-sensitive pulmonary TB were recruited at the start of TB therapy. They were excluded if they were less than 18 years of age, refused HIV testing, had received more than 3 doses of treatment, or had received TB treatment in the previous 6 months. Treatment followed local TB program guidelines with 2 months of therapy with rifampin, isoniazid, pyrazinamide, ethambutol (7 days/week) followed by 4 months of rifampin and isoniazid (7 days/week). All participants had HIV-1 serology testing and chest radiography at baseline. Extensive disease on chest X-ray was noted as either involvement of both lungs or involvement of ≥1 of 3 (upper, middle, or lower) zones per lung. The study was approved by the University of Cape Town Human Research Ethics Committee (HREC Ref 568/2012).
At baseline (start of TB treatment) and at 2 and 5 to 6 months, 3 sputa were collected using sputum induction with 3% saline. The largest-volume sputum was sent for culture, and the other 2 samples were frozen and subsequently used for culture in the case of culture contamination. Standard NaOH-N-acetyl-l-cysteine decontamination (final NaOH concentration, 1.5%) and concentration were performed. One or 2 drops obtained directly from the pellet was used for microscopy, and then the pellet was resuspended in 0.5 ml of phosphate-buffered saline (PBS) prior to inoculation in mycobacterial growth indicator tube (MGIT) cultures as per the manufacturer's instruction. MTBDRplus LPA, version 2.0, was carried out on all baseline-positive MGIT cultures as per the manufacturer's instructions.
At 2 months, the largest-volume sputum was used for direct microscopy, the direct MTBDRplus test, and MGIT culture. The pellet was resuspended in 1 ml PBS, with a 0.5-ml aliquot used for direct MTBDRplus and a 0.5-ml aliquot inoculated into MGIT for culture. The manufacturer-recommended MTBDRplus protocol included an additional 20 amplification cycles when performed directly on clinical samples, compared with cultured samples. In cases of new isoniazid/rifampin resistance detected by direct MTBDRplus at 2 months, another direct MTBDRplus test was performed on an archived 2-month sputum from the same patient. All suspected cases of ADR were confirmed via both MTBDRplus and phenotypic drug susceptibility testing (DST) carried out on positive culture isolates of the paired 2-month sputum. Phenotypic DST was via the Bactec MGIT 960 system as previously described (15). When the result of MTBDRplus performed directly on sputum was internally valid (in terms of all control bands) but did not match the culture DST result, this was considered to be a false-positive result. MTBDRplus was also performed on positive cultures at 5 to 6 months of follow-up, with confirmation of cases of ADR with phenotypic DST.
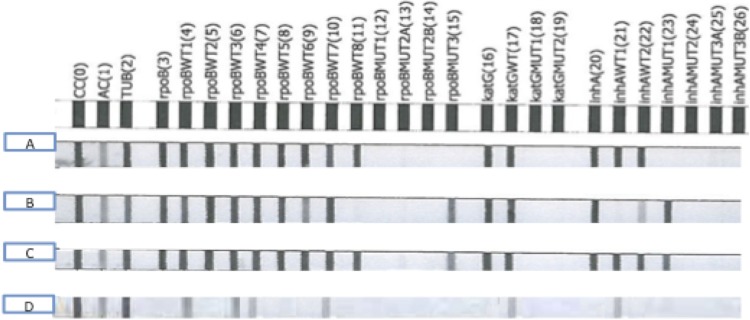
Figure 1 illustrates the interpretation of the MTBDRplus line probe assay. Study outcomes are illustrated in Fig. 2.
FIG 1.
Interpretation of MTBDRplus results. (A) Positive for M. tuberculosis complex (presence of TUB band while in the presence of both a conjugate control [CC] and an amplification control [AC]), RIF/INH susceptible (presence of all wild-type bands, absence of all mutation bands). (B) Positive for M. tuberculosis complex (presence of TUB band while in the presence of both a CC and an AC), RIF/INH resistant (absence of wild-type band rpoBWT8, presence of mutation band rpoBMUT3; absence of wild-type band inhAWT1 and presence of mutation band inhAMUT1). (C) Positive for M. tuberculosis complex (presence of TUB band while in the presence of both a CC and an AC), RIF heteroresistant, INH heteroresistant (presence of all wild-type bands; presence of mutation band rpoBMUT3; presence of mutation band inhAMUT1). (D) Positive for M. tuberculosis complex (presence of TUB band while in the presence of both a CC and an AC), inconclusive RIF and INH susceptibility (inconclusive because missing rpoB, inhA, and katG locus control regions [compared with amplification control] and numerous faint/missing wild-type bands).
Definition of treatment outcomes.
The 2-month culture conversion was defined by a negative 2-month MGIT culture. At 5 to 6 months of treatment, those who were culture negative were defined as cured. Those who were culture positive at 5 to 6 months were defined as treatment failures. Those who completed treatment and were symptom free but without microbiological ascertainment of cure were classified as treatment completers. Electronic database searches of (i) Western Cape Department of Health Data Repository and (ii) National Health Laboratory Service database were conducted to ascertain reported deaths and TB recurrences following completion of study clinical follow-up until the censor date (1 November 2015). “True recurrence” was defined as culture positivity and/or smear 2+/3+ positivity. “Possible recurrence” was defined as positive Xpert MTB/RIF result of a differing resistance profile to baseline and/or a smear grading of scanty/1+ positivity in the absence of culture confirmation.
All treatment failures, deaths during TB treatment, and true/possible TB recurrences were classified as “unsuccessful outcomes.” Deaths subsequent to treatment completion/cure that were not attributable to definite/possible TB recurrence were not defined as unsuccessful outcome. Those who had treatment cure/completion with no microbiologically confirmed TB recurrence (by censor date) were classified as “successful outcome.”
Statistical analyses.
The two-tailed chi-square test and multivariate logistic regression analysis were carried out in Stata/SE (13.1). In determining the association between a positive 2-month MTBDRplus or smear and the composite long-term unsuccessful outcome of failure/death during treatment/TB recurrence, it was elected a priori to adjust for the potential confounders HIV-1 serostatus and extensive radiological disease at baseline. Individuals who defaulted on treatment or were lost to follow-up or transferred care with unknown long-term treatment outcome were excluded from long-term treatment outcome analyses.
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge the clinical and administrative staff of the Western Cape Department of Health.
Robert J. Wilkinson is supported by the Wellcome Trust (WT104803; WT084323), Francis Crick Institute (10218), and the European Union (EU FP7 HEALTH-F3-2012-305578) and the National Research Foundation (NRF) of South Africa (96841). Graeme Meintjes was supported by the Wellcome Trust (WT098316), the South African Research Chairs Initiative of the Department of Science and Technology and National Research Foundation (NRF) of South Africa (64787), NRF incentive funding (UID: 85858), and the South African Medical Research Council through its TB and HIV Collaborating Centres Programme with funds received from the National Department of Health (RFA number SAMRC-RFA-CC: TB/HIV/AIDS-01-2014).
N.R., M.P.N., G.M., and R.J.W. conceived and designed the experiments; R.J.W. contributed materials and reagents; N.R. recruited, sampled, and collected data from patients; Y.G. did the MTBDRplus assays; N.R. analyzed the data; N.R., J.W., G.M., M.P.N., and R.J.W. contributed intellectual input; N.R., M.P.N., G.M., J.W., and R.J.W. drafted the manuscript and figures; all authors approved the final version of the manuscript.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/JCM.00025-17.
REFERENCES
- 1.Theron G, Zijenah L, Chanda D, Clowes P, Rachow A, Lesosky M, Bara W, Mungofa S, Pai M, Hoelscher M, Dowdy D, Pym A, Mwaba P, Mason P, Peter J, Dheda K, TB-NEAT team. 2014. Feasibility, accuracy, and clinical effect of point-of-care Xpert MTB/RIF testing for tuberculosis in primary-care settings in Africa: a multicentre, randomised, controlled trial. Lancet 383:424–435. doi: 10.1016/S0140-6736(13)62073-5. [DOI] [PubMed] [Google Scholar]
- 2.Wallis RS, Wang C, Meyer D, Thomas N. 2013. Month 2 culture status and treatment duration as predictors of tuberculosis relapse risk in a meta-regression model. PLoS One 8:e71116. doi: 10.1371/journal.pone.0071116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Horne DJ, Royce SE, Gooze L, Narita M, Hopewell PC, Nahid P, Steingart KR. 2010. Sputum monitoring during tuberculosis treatment for predicting outcome: systematic review and meta-analysis. Lancet Infect Dis 10:387–394. doi: 10.1016/S1473-3099(10)70071-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization. 2014. Definitions and reporting framework for tuberculosis–2013 revision. World Health Organization, Geneva, Switzerland: http://apps.who.int/iris/bitstream/10665/79199/1/9789241505345_eng.pdf Accessed 16 September 2015. [Google Scholar]
- 5.Rockwood N, Abdullahi LH, Wilkinson RJ, Meintjes G. 2015. Risk factors for acquired rifamycin and isoniazid resistance: a systematic review and meta-analysis. PLoS One 10:e0139017. doi: 10.1371/journal.pone.0139017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crudu V, Stratan E, Romancenco E, Allerheiligen V, Hillemann A, Moraru N. 2012. First evaluation of an improved assay for molecular genetic detection of tuberculosis as well as rifampin and isoniazid resistances. J Clin Microbiol 50:1264–1269. doi: 10.1128/JCM.05903-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barnard M, Gey van Pittius NC, van Helden PD, Bosman M, Coetzee G, Warren RM. 2012. The diagnostic performance of the GenoType MTBDRplus version 2 line probe assay is equivalent to that of the Xpert MTB/RIF assay. J Clin Microbiol 50:3712–3716. doi: 10.1128/JCM.01958-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Folkvardsen DB, Thomsen VO, Rigouts L, Rasmussen EM, Bang D, Bernaerts G, Werngren J, Toro JC, Hoffner S, Hillemann D, Svensson E. 2013. Rifampin heteroresistance in Mycobacterium tuberculosis cultures as detected by phenotypic and genotypic drug susceptibility test methods. J Clin Microbiol 51:4220–4222. doi: 10.1128/JCM.01602-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blakemore R, Story E, Helb D, Kop J, Banada P, Owens MR, Chakravorty S, Jones M, Alland D. 2010. Evaluation of the analytical performance of the Xpert MTB/RIF assay. J Clin Microbiol 48:2495–2501. doi: 10.1128/JCM.00128-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Folkvardsen DB, Svensson E, Thomsen VO, Rasmussen EM, Bang D, Werngren J, Hoffner S, Hillemann D, Rigouts L. 2013. Can molecular methods detect 1% isoniazid resistance in Mycobacterium tuberculosis? J Clin Microbiol 51:1596–1599. doi: 10.1128/JCM.00472-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Friedrich SO, Rachow A, Saathoff E, Singh K, Mangu CD, Dawson R, Phillips PPJ, Venter A, Bateson A, Boehme CC, Heinrich N, Hunt RD, Boeree MJ, Zumla A, McHugh TD, Gillespie SH, Diacon AH, Hoelscher M, Pan African Consortium for the Evaluation of Anti-tuberculosis Antibiotics (PanACEA). 2013. Assessment of the sensitivity and specificity of Xpert MTB/RIF assay as an early sputum biomarker of response to tuberculosis treatment. Lancet Respir Med 1:462–470. doi: 10.1016/S2213-2600(13)70119-X. [DOI] [PubMed] [Google Scholar]
- 12.Hanrahan CF, Theron G, Bassett J, Dheda K, Scott L, Stevens W, Sanne I, Van Rie A. 2014. Xpert MTB/RIF as a measure of sputum bacillary burden. Variation by HIV status and immunosuppression. Am J Respir Crit Care Med 189:1426–1434. doi: 10.1164/rccm.201312-2140OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boyles TH, Hughes J, Cox V, Burton R, Meintjes G, Mendelson M. 2014. False-positive Xpert(R) MTB/RIF assays in previously treated patients: need for caution in interpreting results. Int J Tuberc Lung Dis 18:876–878. doi: 10.5588/ijtld.13.0853. [DOI] [PubMed] [Google Scholar]
- 14.Shenai S, Ronacher K, Malherbe S, Stanley K, Kriel M, Winter J, Peppard T, Barry CE, Wang J, Dodd LE, Via LE, Barry CE III, Walzl G, Alland D. 2016. Bacterial loads measured by the Xpert MTB/RIF assay as markers of culture conversion and bacteriological cure in pulmonary TB. PLoS One 11:e0160062. doi: 10.1371/journal.pone.0160062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Springer B, Lucke K, Calligaris-Maibach R, Ritter C, Bottger EC. 2009. Quantitative drug susceptibility testing of Mycobacterium tuberculosis by use of MGIT 960 and EpiCenter instrumentation. J Clin Microbiol 47:1773–1780. doi: 10.1128/JCM.02501-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.