ABSTRACT
Leprosy is an important cause of disability in the developing world. Early diagnosis is essential to allow for cure and to interrupt transmission of this infection. MicroRNAs (miRNAs) are important factors for host-pathogen interaction and they have been identified as biomarkers for various infectious diseases. The expression profile of 377 microRNAs were analyzed by TaqMan low-density array (TLDA) in skin lesions of tuberculoid and lepromatous leprosy patients as well as skin specimens from healthy controls. In a second step, 16 microRNAs were selected for validation experiments with reverse transcription-quantitative PCR (qRT-PCR) in skin samples from new individuals. Principal-component analysis followed by logistic regression model and receiver operating characteristic (ROC) curve analyses were performed to evaluate the diagnostic potential of selected miRNAs. Four patterns of differential expression were identified in the TLDA experiment, suggesting a diagnostic potential of miRNAs in leprosy. After validation experiments, a combination of four miRNAs (miR-101, miR-196b, miR-27b, and miR-29c) was revealed as able to discriminate between healthy control and leprosy patients with 80% sensitivity and 91% specificity. This set of miRNAs was also able to discriminate between lepromatous and tuberculoid patients with a sensitivity of 83% and 80% specificity. In this work, it was possible to identify a set of miRNAs with good diagnostic potential for leprosy.
KEYWORDS: biomarker, leprosy, miRNA
INTRODUCTION
Leprosy is a chronic infectious disease caused by the bacillus Mycobacterium leprae. The infection affects primarily the skin and can cause damage to peripheral nerves, mucosa, and other organs, including liver and eyes. Leprosy is classified as a neglected tropical disease and remains one of the main causes of disability in the world (1–3). According to a World Health Organization (WHO) report including data from 138 countries, 211,974 newly diagnosed patients were notified in the year 2015 and 96% of them were reported from 22 countries, including Brazil (4).
Leprosy presents a spectrum of clinical manifestations depending on the host immune response against M. leprae. It can be classified according to histopathological (Ridley-Jopling) criteria into different forms across two opposing poles: a so-called resistance pole responsible for a localized form of the disease (tuberculoid tuberculoid [TT]) and a susceptibility pole that is a disseminated form, which leads to the development of more severe clinical manifestations (lepromatous leprosy [LL]). There are also three intermediate and unstable forms: borderline tuberculoid (BT), borderline borderline (BB), and borderline lepromatous (BL) (6). The immunological profile in leprosy is also spectral. In the TT form, there is a predominant cell-mediated immunity characterized by greater production of Th1 cytokines (7–9). This form of leprosy is also characterized by the activation of a vitamin D-dependent antimicrobial pathway by macrophages (10). On the other hand, the LL form is characterized mainly by humoral immune responses and presence of Th2 cytokines (7–9). In this way, clinical manifestation of infection by M. leprae is defined by the balance of lymphocyte subsets, cytokines, chemokines, and their receptors in response to the microorganism.
Human beings are recognized as the main source of the infection, and transmission is thought to occur via contact of susceptible individuals with bacillary patients without treatment. After diagnosis, leprosy can be treated with multidrug therapy, which allows cure and prevents disabilities. Time and type of treatment depend on the form of the disease. Therefore, early and accurate diagnosis is very important for interruption of transmission and control of dissemination (5, 7, 11). Accurate diagnosis is particularly challenging in the case of patients with borderline forms of leprosy who have unstable features.
Leprosy diagnosis is based on the number and type of lesions, nerve involvement, bacterial index, and histopathological examination, which reflects the underlying immunological profile. This approach requires biopsy and bacilloscopy of skin samples obtained from an active lesion, which is the “gold standard” for leprosy diagnosis (11, 12). However, this is associated with delayed diagnoses and presents low sensitivity in mild forms of the disease in addition to requiring experienced technicians to perform histopathological and bacilloscopic evaluations (11, 13). Due to the impossibility of growing M. leprae in vitro, other alternatives have been evaluated to increase the reliability of leprosy diagnoses.
Molecular techniques using PCR technology and serological tests were developed; however, specificity and sensitivity were limited (11, 14–18, 21). In this context, the identification of biomarkers that allow early diagnosis of leprosy and differentiation between forms of the disease with an adequate sensitivity and specificity is still required.
MicroRNAs (miRNAs) are small (18 to 25 nucleotides [nt]), endogenous, stable, and highly conserved noncoding RNAs that act as post-transcriptional gene expression modulators even in physiological or pathological conditions and have been implicated in the regulation of innate and adaptive immune responses (22–30). Accordingly, miRNAs may be involved in the complex host immune response modulation in leprosy, influencing its outcome. miRNAs have also been identified as endogenous molecules with differential expression profiles under pathological conditions. Due to their characteristic and stability, they have been suggested as potential disease biomarkers (31, 32).
In our study aiming to identify possible miRNAs with a potential to be used in the diagnosis of leprosy, a wide analysis of miRNA expression in skin lesions of patients with polar forms of leprosy (TT and LL) and controls was carried out. Our results reveal a set of differentially expressed miRNAs as potential biomarkers of leprosy per se and of their clinical forms with high levels of sensitivity and specificity.
RESULTS
Leprosy patient characteristics.
Clinical characteristics of LL and TT patients are listed in Table 1. A total of 36 individuals were recruited for this study (12 LL patients, 12 TT patients, and 12 healthy controls). Leprosy patients were classified according to Ridley-Jopling criteria taking into consideration the histological pattern (6). There was no statistically significant difference in relation to gender and age of individuals between the LL and TT groups (P > 0.05, Fisher exact test). LL patients had positive bacilloscopy results, while the bacilloscopy results were negative in TT patients, which is in accordance with the expected characteristics of these leprosy subforms. The majority of LL patients exhibited more than six skin lesions, whereas the majority of patients with the TT subform exhibited less than six skin lesions (P < 0.05, Fisher exact test) (Table 1).
TABLE 1.
Clinical characteristics of lepromatous and tuberculoid leprosy patientsa
| Variable | No. of patients |
P valueb | |
|---|---|---|---|
| LL | TT | ||
| Sex | |||
| Male | 10 | 5 | 0.0894 |
| Female | 2 | 7 | |
| Bacilloscopy result | |||
| Positive | 12 | 0 | <0.0001 |
| Negative | 0 | 12 | |
| Number of lesions | |||
| ≤6 | 1 | 11 | 0.0001 |
| >6 | 11 | 1 | |
Ridley-Jopling classification. LL, lepromatous leprosy; TT, tuberculoid leprosy.
Fisher exact test.
Expression profiling of miRNAs in skin lesions of LL and TT leprosy patients by TLDA.
For this first step, 18 individuals were recruited (six LL patients, six TT patients, and six healthy controls). Large-scale miRNA screening was performed by using a TaqMan low-density array (TLDA) in order to identify miRNAs differentially expressed between skin lesions of leprosy patients and skin samples of healthy controls and/or skin lesions of LL and TT patients. After tissue sample RNA extraction, cDNA was synthesized and TLDA was carried out generating the miRNA expression profile of these samples.
A total of 311 from the 377 miRNAs included in the array were detected in lesions of LL patients, 275 in lesions of TT patients, and 294 in skin samples of healthy controls. To be included in further analyses, miRNA expression levels were required to have reliable detection with a cycle threshold (CT) of <35.
Among the detected genes, 188 were expressed differentially between the three groups (P < 0.05) and were included in the subsequent analysis. Hierarchical clustering analysis of miRNA expression data revealed a differential pattern of expression with four main clusters in which LL, TT, and healthy controls were segregated. The first cluster is constituted of 29 miRNA genes with an expression pattern of lower expression level in the TT group. In the second cluster, a differential expression pattern was observed with 43 positively regulated genes in leprosy patients. The third cluster presents three positively regulated genes in TT patients. Finally, the fourth cluster is constituted of 112 genes with an expression pattern of positive regulation in LL patients (Fig. 1).
FIG 1.
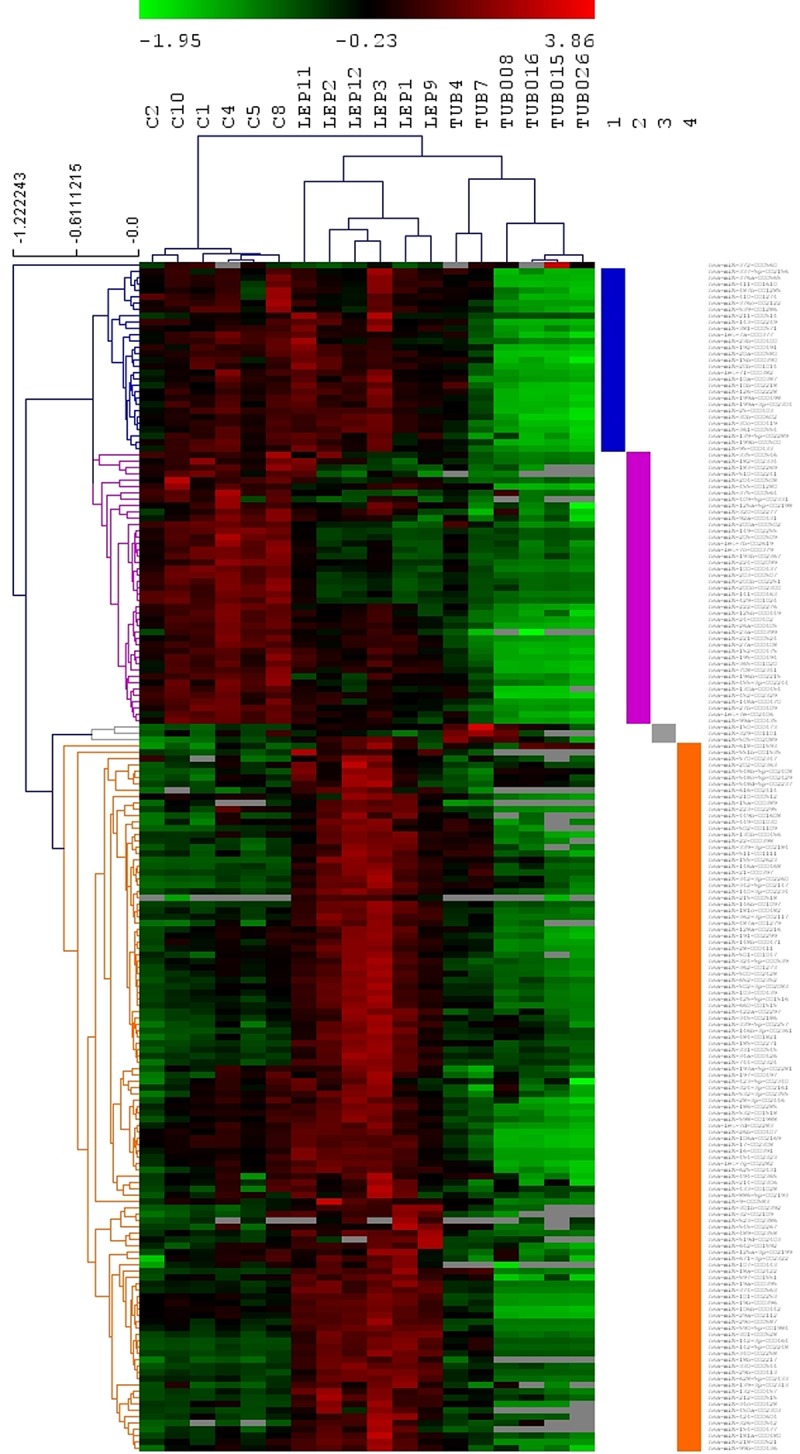
miRNA expression profiles in leprosy patients and healthy controls. Hierarchical clustering analysis of miRNA expression data. Sample labels are on the top. C, healthy control; LEP, lepromatous; TUB, tuberculoid. On the right side, each of the four clusters are highlighted.
Among the detected miRNAs, 16 miRNAs were randomly selected for further analysis. These miRNAs are miR-539 from cluster 1; miR-125b, miR-196b, miR-26a, miR-27a, miR-27b, miR-452, miR-455-3p, miR-92a, and miR-99a from cluster 2; and miR-101, miR-29c, miR-34c, miR-425-5p, miR-502-3p, and miR-660 from cluster 4. One of these miRNAs (miR-92a), which was not differentially expressed between the groups, was included in the validation step in order to confirm the results, acting as a negative internal control.
qRT-PCR analysis of differential miRNA expression in skin lesions of leprosy patients.
The set of 16 selected miRNAs was quantified by reverse transcription-quantitative PCR (qRT-PCR) using TaqMan miRNA assays in a validation step. For this purpose, a new group of 18 individuals was recruited (six LL patients, six TT patients, and six healthy controls), and RNA was extracted from the lesions of leprosy patients and skin samples from healthy control individuals as was done for the TLDA analysis. For expression level normalization, U6 snRNA was used as an endogenous control.
The validation results showed that both techniques are reproducible and comparable for seven of the analyzed miRNAs. The miRNAs miR-125b, miR-196b, miR-27b, miR-29c, miR-425-5p, and miR-502-3p were differentially expressed between the groups and miR-92a was not differentially expressed between the groups as was observed in the TLDA experiment (P < 0.05) (Fig. 2). The expression values (2−ΔCT) from the validation experiment are presented in Table S1 in the supplemental material.
FIG 2.
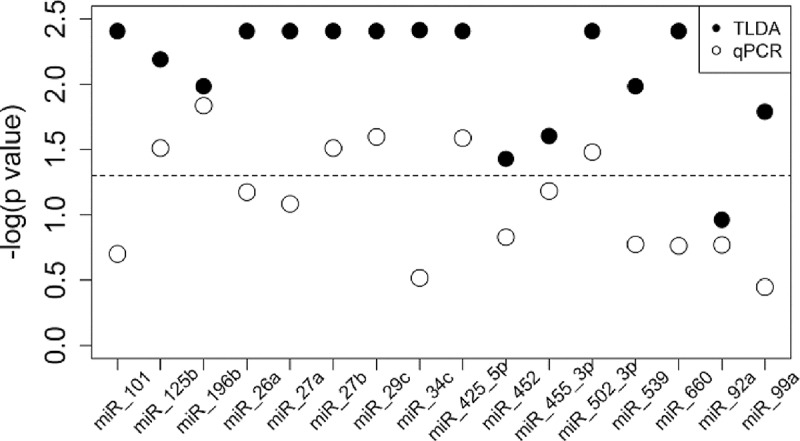
miRNA expression median differences in TLDA and qRT-PCR experiments. Distribution of −log(P value) (Kruskal-Wallis) of comparison between miRNA expression median differences in the three groups (LL and TT patients and healthy controls). Filled circles represent TLDA experiments and unfilled circles represent qRT-PCR experiments. The dashed line indicates threshold of statistical significance (P value of 0.05). LL, lepromatous leprosy; TT, tuberculoid leprosy; TLDA, TaqMan low-density array.
Diagnostic potential evaluation of miRNAs for leprosy and their polar subforms.
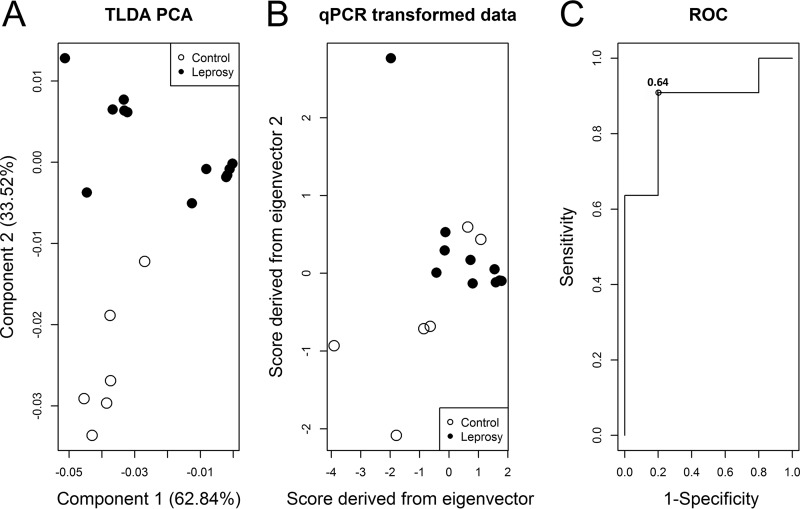
Initially, all 16 qPCR-evaluated miRNAs were included in the principal-component analysis (PCA) (see Fig. S1 and S2). However, results with greater sensitivity were found using a subset of four miRNAs (miR-101, miR-196b, miR-27b, and miR-29c).
In a first step, PCA was employed on miR-101, miR-196b, miR-27b, and miR-29c TLDA expression for discrimination between the leprosy group and healthy controls. The first two components explained 96.4% of the total variance. The normalized eigenvectors were −0.54, −0.48, −0.48, and −0.49 for the first component and 0.43, −0.52, −0.51, and 0.53 for the second component. Graphical representation of component scores indicated separation of each subject subgroup (Fig. 3A). qPCR-transformed data indicated that values on both axes were highly correlated (Fig. 3B), and only the first score was used as a covariate in the logistic regression. Receiver operating characteristic (ROC) analysis indicated that at a cutoff of 0.64, 80% sensitivity and 91% specificity (area under the curve [AUC], 87.3%; 95% confidence interval [CI], 68.6% to 100.0%) were achieved by the four selected miRNAs used in combination to discriminate between leprosy and healthy individuals (Fig. 3C). The cutoff should be compared to the value from the equation 2.79 + 2.88 × [−0.54(miR-101) − 0.48(miR-196b) − 0.48(miR-27b) − 0.49(miR-29c)].
FIG 3.
Diagnostic power evaluation of miRNAs for discrimination between leprosy patients and healthy controls using a subset of four miRNAs. (A) Principal component scores of TLDA expression data of miR-101, miR-196b, miR-27b, and miR-29c indicate separation between the leprosy and healthy control groups. (B) Normalized eigenvectors were used to transform qRT-PCR data revealing a separation between the groups and high correlation of the data. (C) The first qRT-PCR-transformed score was evaluated in a ROC analysis after logistic regression revealing 100% sensitivity and 80% specificity with the cutoff of 0.64 and an AUC of 87.3% (95% CI, 66.8% to 100.0%) for discrimination of leprosy patients and healthy controls. TLDA, TaqMan low-density array.
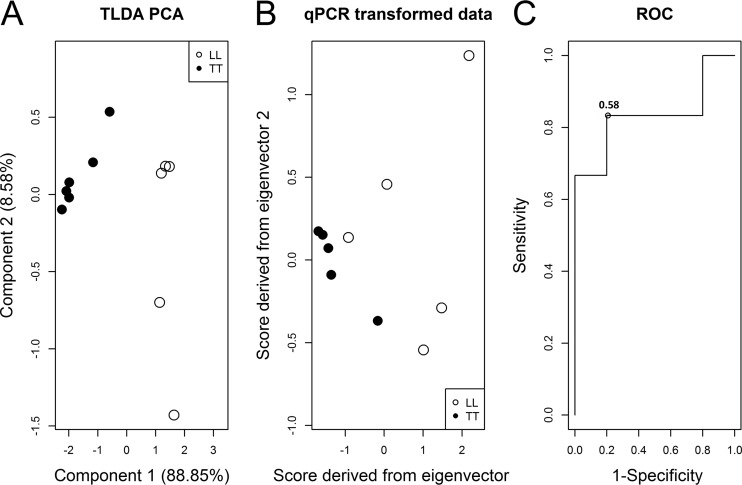
Aiming to discriminate between the polar forms of the disease, LL and TT, PCA was employed on miR-101, miR-196b, miR-27b, and miR-29c TLDA expression in a similar way. The first two components explained 97.4% of the total variance. The normalized eigenvectors were 0.52, 0.46, 0.49, and 0.52 for the first component and −0.12, 0.81, −0.57, and −0.06 for the second component. The graphical representation of component scores did not indicate a high separation of each subject subgroup (Fig. 4A). Due to high variance as explained by the first component, only the first qPCR-transformed score was used as the covariate in logistic regression (Fig. 4B). ROC analysis indicated that a cutoff of 0.58 reached 83% sensitivity and 80% specificity (AUC, 83.3%; 95% CI, 55.7% to 100.0%) (Fig. 4C) for discrimination between LL and TT patients using the four selected miRNAs. The cutoff should be compared to the value of the equation 1.21 + 0.42 × [0.52(miR-101) + 0.46(miR-196b) + 0.49(miR-27b) + 0.52(miR-29c)].
FIG 4.
Diagnostic power evaluation of miRNAs for discrimination between LL and TT patients using a subset of four miRNAs. (A) Principal component scores of TLDA expression data of miR-101, miR-196b, miR-27b, and miR-29c indicate separation between LL and TT groups. (B) Normalized eigenvectors were used to transform qRT-PCR data revealing a separation between the groups. (C) The first qRT-PCR-transformed score was evaluated in a ROC analysis after logistic regression revealing 83.3% sensitivity and 80% specificity with the cutoff of 0.58 and an AUC of 83.3% (95% CI, 55.7% to 100.0%) for discrimination of LL and TT patients. TLDA, TaqMan low-density array.
Together, these results indicate that miR-101, miR-196b, miR-27b, and miR-29c, when used in combination, are good biomarkers of leprosy with 80% sensitivity and 91% specificity and of leprosy polar subforms with 83% sensitivity and 80% specificity.
DISCUSSION
Early and accurate diagnosis of leprosy is very important to control the disease and to allow preventive measures to minimize the associated disability (7, 11). Currently, the gold standard for leprosy diagnosis is based on clinical examination and skin biopsy. Techniques based on PCR technology and serological analysis have been developed but were not able to diagnose leprosy with acceptable sensitivity and specificity taking into consideration the different clinical forms and/or bacterial burden (11, 14–16, 18–21). Accordingly, identification of biomarkers that allow the diagnosis of leprosy with a greater sensitivity and specificity is still needed.
miRNAs have been extensively investigated as biomarkers for a variety of diseases (31, 32). These molecules are responsible for the regulation of various physiological processes and alterations in their expression levels can reflect different pathological conditions. Moreover, miRNAs are stable, and they can be quantified by different molecular techniques (32, 33), such as qRT-PCR (33, 34).
Due to their diagnostic potential, miRNAs were evaluated as biomarkers in several pathological conditions like infectious diseases (38, 39). Using different detection and data analysis approaches, some authors were able to identify miRNAs with a diagnostic power ranging from 82% to 100% in tuberculosis diagnosis (40–43).
In leprosy, Liu et al. identified 13 differentially regulated miRNAs in skin lesions of LL patients compared with lesions of TT patients using microarray. These miRNAs were related to immune gene targets. Among them, miR-21 was demonstrated as upregulated in the LL form and targeting the vitamin D-dependent antimicrobial pathway (26), which is preferentially activated in TT leprosy (10). Curiously, Liu et al. found that differentiation between LL and TT patients was not possible by using miRNA expression data (26).
In our study, we were able to generate a discriminative set of miRNA expression data by investigating 377 miRNAs in skin lesions from LL and TT patients by using TLDA technology. The hierarchical clustering analysis revealed 188 miRNAs with a differential expression pattern between the groups segregated into four well-defined clusters. This highly interesting pattern suggests a possible role of miRNAs in leprosy susceptibility to be involved in differential regulation of host immune response with different outcomes. Moreover, these miRNAs identified in skin lesions may be originally expressed in different cell types, like epithelial cells, lymphocytes, macrophages, plasma cells, neutrophils, dendritic cells, and mast cells (35, 36), contributing to generation of a specific immune/inflammatory microenvironment in the context of M. leprae infection.
It is important to highlight that alterations of host miRNA expression levels have been recognized as associated with intracellular bacterial survival, including the genera Mycobacterium, Salmonella, Listeria, and Francisella (37). In leprosy, the role of miRNAs in bacterial survival and clearance still needs to be addressed.
After the large-scale screening of miRNAs in leprosy lesions with TLDA, a validation step was performed using qRT-PCR to verify the expression levels of 16 randomly selected miRNAs. This step confirmed the expression levels of seven miRNAs (miR-125b, miR-196b, miR-27b, miR-29c, miR-425-5p, miR-502-3p, and miR-92a).
The use of a combination of several miRNAs as biomarkers, instead of a unique miRNA, has been indicated as a more powerful option due to the possible overlap in miRNA targeting (31). In this way, we sought to use a combination of miRNAs with good diagnostic performance that was also able to discriminate between the two polar forms of leprosy. PCA was employed to reduce the data and, after a sensitivity and specificity evaluation for discriminating between the groups, four miRNAs (miR-101, miR-196b, miR-27b, and miR-29c) were chosen that allowed higher sensitivity than that achieved using higher numbers of miRNAs. This set of miRNAs was evaluated for their ability to distinguish between leprosy and healthy control groups in a first step and between LL and TT groups in a second step. For these purposes, the first two components were sufficient to explain most of the total variance. PCA revealed that even miR-101, which did not present a statistical difference between the groups in the validation step, contributed to discrimination between them when used in combination with the three other miRNAs (miR-196b, miR-27b, and miR-29c).
The combination of miR-101, miR-196b, miR-27b, and miR-29c achieved 80% sensitivity and 91% specificity in discriminating between leprosy and healthy individuals with an AUC of 87%, revealing a high diagnostic power of this set of miRNAs. These four miRNAs were also able to discriminate between the leprosy polar forms, LL and TT. A sensitivity of 83% and 80% specificity were reached, which gives an AUC of 83%, representing a high diagnostic power (Fig. 4).
Our study represents a preliminary investigation of host response modulation by miRNAs associated with leprosy. Borderline forms of leprosy were not included in the current study, because the high variability of clinical presentation of these intermediate forms would make it more difficult to identify particular subsets of miRNAs to be used in further studies. Such analysis of intermediate forms should be the object of another study validating the 4 miRNAs identified here. To our knowledge, this is the first study to successfully identify miRNAs with good potential in diagnosing leprosy and identifying the most severe forms of the disease.
MATERIALS AND METHODS
Ethics statement.
This study was approved by the human ethics committee (COEP, 147/08). All patients and controls signed a written informed consent form prior to their participation in this study.
Patients and healthy controls.
Skin biopsy specimens were obtained from active lesions of LL and TT patients at the time of diagnosis. Accordingly, all the samples were obtained from patients before treatment. The patients were classified according to Ridley-Jopling criteria (20). Twelve TT patients and 12 LL patients were recruited at the university hospitals from the Federal University of Minas Gerais (Brazil) and the Federal University of Sergipe (Brazil). Six individuals from each group were included in a first step of the study in which the expression profiles of miRNAs were investigated by using a large-scale approach, TaqMan low-density array (TLDA). The other six individuals from each group were further included in the validation step.
Patients under 18 or over 60 years old, HIV positive, with other mycobacterial infections, or using corticosteroids were excluded from the study. Skin specimens from 12 controls were obtained during plastic surgery. The specimens were maintained in RNAlater solution at −20°C until RNA extraction.
RNA extraction and miRNA expression profile by a TaqMan low-density array.
Total RNA was isolated from skin specimens using a Qiagen miRNeasy minikit (Qiagen, Germany) according to the manufacturer's instructions. Briefly, approximately 50 mg of tissue was added to 700 μl of QIAzol lysis reagent, disrupted, and homogenized. The extracted RNA was eluted with nuclease-free water to a final concentration of approximately 100 ng/μl. A NanoDrop spectrophotometer (ND-1000; Thermo Scientific, DE, USA) was used to measure the quantity and purity of RNA.
The extracted RNA was used to synthesize cDNA using a TaqMan microRNA reverse transcription kit (Applied Biosystems, USA). Briefly, 300 ng of RNA was added to a Megaplex RT primer solution (10× pool A primers v2.1, 0.2 μl deoxynucleoside triphosphate [dNTP; 100 mM], 0.9 μl MgCl2 [25 mM], 0.1 μl RNase inhibitor [20 U/μl], 1.5 μl MultiScribe reverse transcriptase [50 U/μl], 10× RT buffer, and nuclease-free water to a 4.5 μl final volume). RT reactions were carried out according to the manufacturer's instructions.
After RT, a preamplification step was performed to increase the sensitivity of the miRNA detection using TaqMan PreAmp master mix and Megaplex PreAmp primers, pool A v2.1 (Applied Biosystems, USA), and 2.5 μl of RT product according to the manufacturer's instructions. For TLDA experiments, the preamplification product was 4-fold diluted in nuclease-free water and 9 μl of the diluted product was combined with 450 μl 2× TaqMan universal PCR master mix (Applied Biosystems, USA) and 441 μl nuclease-free water. The mixtures for each sample (100 μl) were loaded into each port of the TLDA (Applied Biosystems, USA), and each card was centrifuged and sealed. The reactions were carried out in duplicates in a ViiA 7 PCR real-time system (Applied Biosystems, USA) according to the manufacturer's instructions. The real-time PCR data were analyzed using Expression Suite Software v1.0.3.
Validation step by qRT-PCR.
Validation was done by qRT-PCR. RNA extraction, cDNA synthesis, and preamplification were done as described above. The real-time PCR assays were performed in a final volume of 10 μl containing 1 μl of diluted cDNA (1:5), 5 μl 2× TaqMan universal PCR master mix, no AmpErase UNG (Applied Biosystems, USA), and 0.5 μl 20× TaqMan miRNA assay primers (Applied Biosystems, USA) using a 7500 Fast real-time PCR system according to the manufacturer's instructions. Each sample was run in duplicate and data were analyzed using 7500 Software v2.0.6 (Applied Biosystems, USA).
Data processing.
The expression data of miRNAs were normalized to U6 snRNA and CT values more than 35 were considered undetectable. The relative expression levels were defined using the 2−ΔΔCT method (44). The 2−ΔCT values (CT miRNA − CT U6 snRNA) were used to compare the expression levels between the three study groups (healthy control, LL, and TT).
Hierarchical clustering analysis comparing the study groups by means of miRNA expression level was performed using MeV software (J. Craig Venter Institute Microarray Software Suite 4) with data from the TLDAs. The miRNA expression differences were evaluated by Mann-Whitney U or Kruskal-Wallis tests with Tukey post hoc tests using R software (version 3.2.5).
Principal-component analysis (PCA) and logistic regression were carried out using R software (version 3.2.5). The first two normalized eigenvectors created a decomposing TLDA expression correlation matrix (PCA), which was used to transform qPCR expression results. qPCR-transformed values were used as covariates in two logistic regression models. The first model attempted to classify subjects as healthy controls or leprosy patients, while the second model aimed to distinguish between LL and TT patients. Receiver operating characteristic (ROC) curves were used to find cutoff values to maximize the sum of sensitivity and specificity for each model.
Supplementary Material
ACKNOWLEDGMENTS
We would like to thank Matthieu Pesant, Humanitas Clinical and Research Center, Italy, for assisting with the TLDA analysis.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/JCM.02408-16.
REFERENCES
- 1.Mathers CD, Ezzati M, Lopez AD. 2007. Measuring the burden of neglected tropical diseases: the global burden of disease framework. PLoS Negl Trop Dis 1:e114. doi: 10.1371/journal.pntd.0000114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lockwood DN, Suneetha S. 2005. Leprosy: too complex a disease for a simple elimination paradigm. Bull World Health Organ 83:230–235. doi: 10.1590/S0042-96862005000300018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hotez PJ, Bottazzi ME, Franco-Paredes C, Ault SK, Periago MR. 2008. The neglected tropical diseases of Latin America and the Caribbean: a review of disease burden and distribution and a roadmap for control and elimination. PLoS Negl Trop Dis 2:e300. doi: 10.1371/journal.pntd.0000300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization. 2016. Leprosy fact sheet (updated February 2017). World Health Organization, Geneva, Switzerland: http://www.who.int/mediacentre/factsheets/fs101/en/. [Google Scholar]
- 5.van Beers SM, Hatta M, Klatser PR. 1999. Patient contact is the major determinant in incident leprosy: implications for future control. Int J Lepr Other Mycobact Dis 67:119–128. [PubMed] [Google Scholar]
- 6.Ridley DS, Jopling WH. 1966. Classification of leprosy according to immunity. A five-group system. Int J Lepr Other Mycobact Dis 34:255–273. [PubMed] [Google Scholar]
- 7.Mazini PS, Alves HV, Reis PG, Lopes AP, Sell AM, Santos-Rosa M, Visentainer JE, Rodrigues-Santos P. 2016. Gene association with leprosy: a review of published data. Front Immunol 6:658. doi: 10.3389/fimmu.2015.00658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamamura M, Uyemura K, Deans RJ, Weinberg K, Rea TH, Bloom BR, Modlin RL. 1991. Defining protective responses to pathogens: cytokine profiles in leprosy lesions. Science 254:277–279. doi: 10.1126/science.1925582. [DOI] [PubMed] [Google Scholar]
- 9.Salgame P, Abrams JS, Clayberger C, Goldstein H, Convit J, Modlin RL, Bloom BR. 1991. Differing lymphokine profiles of functional subsets of human CD4 and CD8 T cell clones. Science 254:279–282. doi: 10.1126/science.1681588. [DOI] [PubMed] [Google Scholar]
- 10.Montoya D, Cruz D, Teles RMB, Lee DJ, Ochoa MT, Krutzik SR, Chun R, Schenk M, Zhang X, Ferguson BG, Burdick AE, Sarno EN, Rea TH, Haewison M, Adams JS, Cheng G, Modlin RL. 2009. Divergence of macrophage phagocytic and antimicrobial programs in leprosy. Cell Host Microbe 6:343–353. doi: 10.1016/j.chom.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reibel F, Cambau E, Aubry A. 2015. Update on the epidemiology, diagnosis, and treatment of leprosy. Med Mal Infect 45:383–393. doi: 10.1016/j.medmal.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 12.World Health Organization. 2012. WHO expert committee on leprosy. World Health Organ Tech Rep Ser 2012:1–61. [PubMed] [Google Scholar]
- 13.Scollard DM, Adams LB, Gillis TP, Krahenbuhl JL, Truman RW, Williams DL. 2006. The continuing challenges of leprosy. Clin Microbiol Rev 19:338–381. doi: 10.1128/CMR.19.2.338-381.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martinez AN, Talhari C, Moraes MO, Talhari S. 2014. PCR-based techniques for leprosy diagnosis: from the laboratory to the clinic. PLoS Negl Trop Dis 8:e2655. doi: 10.1371/journal.pntd.0002655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheah ESG, Malkin J, Free RC, Lee S-M, Perera N, Woltmann G, Patel H, Kimmitt PT, Smith RJ, Rajakumar K, Barer MR. 2010. A two-tube combined TaqMan/SYBR Green assay to identify mycobacteria and detect single global lineage-defining polymorphisms in Mycobacterium tuberculosis. J Mol Diagn 12:250–256. doi: 10.2353/jmoldx.2010.090030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Foongladda S, Pholwat S, Eampokalap B, Kiratisin P, Sutthent R. 2009. Multi-probe real-time PCR identification of common Mycobacterium species in blood culture broth. J Mol Diagn 11:42–48. doi: 10.2353/jmoldx.2009.080081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cambau E, Bonnafous P, Perani E, Sougakoff W, Ji B, Jarlier V. 2002. Molecular detection of rifampin and ofloxacin resistance for patients who experience relapse of multibacillary leprosy. Clin Infect Dis 34:39–45. doi: 10.1086/324623. [DOI] [PubMed] [Google Scholar]
- 18.Torres P, Camarena JJ, Gomez JR, Nogueira JM, Gimeno V, Navarro JC, Olmos A. 2003. Comparison of PCR mediated amplification of DNA and the classical methods for detection of Mycobacterium leprae in different types of clinical samples in leprosy patients and contacts. Lepr Rev 74:18–30. [PubMed] [Google Scholar]
- 19.Duthie MS, Raychaudhuri R, Tutterrow YL, Misquith A, Bowman J, Casey A, Balagon MF, Maghanoy A, Beltran-Alzate JC, Romero-Alzate M, Cardona-Castro N, Reed SG. 2014. A rapid ELISA for the diagnosis of MB leprosy based on complementary detection of antibodies against a novel protein-glycolipid conjugate. Diagn Microbiol Infect Dis 79:233–239. doi: 10.1016/j.diagmicrobio.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 20.Sinha S, Kannan S, Nagaraju B, Sengupta U, Gupte MD. 2004. Utility of serodiagnostic tests for leprosy: a study in an endemic population in South India. Lepr Rev 75:266–273. [PubMed] [Google Scholar]
- 21.Moura RS, Calado KL, Oliveira MLW, Bührer-Sékula S. 2008. Leprosy serology using PGL-I: a systematic review. Rev Soc Bras Med Trop 41(Suppl 2):11–18. doi: 10.1590/S0037-86822008000700004. [DOI] [PubMed] [Google Scholar]
- 22.Felekkis K, Touvana E, Stefanou C, Deltas C. 2010. microRNAs: a newly described class of encoded molecules that play a role in health and disease. Hippokratia 14:236–240. [PMC free article] [PubMed] [Google Scholar]
- 23.Dai R, Ahmed SA. 2011. microRNA, a new paradigm for understanding immunoregulation, inflammation, and autoimmune diseases. Transl Res 157:163–179. doi: 10.1016/j.trsl.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Winter J, Jung S, Keller S, Gregory RI, Diederichs S. 2009. Many roads to maturity: microRNA biogenesis pathways and their regulation. Nat Cell Biol 11:228–234. doi: 10.1038/ncb0309-228. [DOI] [PubMed] [Google Scholar]
- 25.Perry MM, Williams AE, Tsitsiou E, Larner-Svensson HM, Lindsay MA. 2009. Divergent intracellular pathways regulate interleukin-1beta-induced miR-146a and miR-146b expression and chemokine release in human alveolar epithelial cells. FEBS Lett 583:3349–3355. doi: 10.1016/j.febslet.2009.09.038. [DOI] [PubMed] [Google Scholar]
- 26.Liu PT, Wheelwright M, Teles R, Komisopoulou E, Edfeldt K, Ferguson B, Mehta MD, Vazirnia A, Rea TH, Sarno EN, Graeber TG, Modlin RL. 2012. MicroRNA-21 targets the vitamin D-dependent antimicrobial pathway in leprosy. Nat Med 18:267–273. doi: 10.1038/nm.2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park H, Huang X, Lu C, Cairo MS, Zhou X. 2015. MicroRNA-146a and microRNA-146b regulate human dendritic cell apoptosis and cytokine production by targeting TRAF6 and IRAK1 proteins. J Biol Chem 290:2831–2841. doi: 10.1074/jbc.M114.591420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang J, Wu M, Wen J, Yang K, Li M, Zhan X, Feng L, Li M, Huang X. 2014. MicroRNA-155 induction by Mycobacterium bovis BCG enhances ROS production through targeting SHIP1. Mol Immunol 62:29–36. doi: 10.1016/j.molimm.2014.05.012. [DOI] [PubMed] [Google Scholar]
- 29.Kumar M, Sahu SK, Kumar R, Subuddhi A, Maji RK, Jana K, Gupta P, Raffetseder J, Lerm M, Ghosh Z, van Loo G, Beyaert R, Gupta UD, Kundu M, Basu J. 2015. MicroRNA let-7 modulates the immune response to Mycobacterium tuberculosis infection via control of A20, an inhibitor of the NF-κB pathway. Cell Host Microbe 17:345–356. doi: 10.1016/j.chom.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 30.Lou Z, Casali P, Xu Z. 2015. Regulation of B cell differentiation by intracellular membrane-associated proteins and microRNAs: role in the antibody response. Front Immunol 6:537. doi: 10.3389/fimmu.2015.00537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ajit SK. 2012. Circulating microRNAs as biomarkers, therapeutic targets, and signaling molecules. Sensors (Basel) 12:3359–3369. doi: 10.3390/s120303359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Faruq O, Vecchione A. 2015. microRNA: diagnostic perspective. Front Med (Lausanne) 2:51. doi: 10.3389/fmed.2015.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Planell-Saguer M, Rodicio MC. 2013. Detection methods for microRNAs in clinic practice. Clin Biochem 46:869–878. doi: 10.1016/j.clinbiochem.2013.02.017. [DOI] [PubMed] [Google Scholar]
- 34.Schmittgen TD, Lee EJ, Jiang J, Sarkar A, Yang L, Elton TS, Chen C. 2008. Real-time PCR quantification of precursor and mature microRNA. Methods 44:31–38. doi: 10.1016/j.ymeth.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sieling PA, Modlin RL. 1992. T cell and cytokine patterns in leprosy skin lesions. Springer Semin Immunopathol 13:413–426. [DOI] [PubMed] [Google Scholar]
- 36.Fachin LRV, Soares CT, Belone AFF, Trombone APF, Rosa PS, Guidella CC, Franco MF. 2016. Immunohistochemical assessment of cell populations in leprosy-spectrum lesions and reactional forms. Histol Histopathol 32:385–396. doi: 10.14670/HH-11-804. [DOI] [PubMed] [Google Scholar]
- 37.Das K, Garnica O, Dhandayuthapani SPD. 2016. Modulation of host miRNAs by intracellular bacterial pathogens. Front Cell Infect Microbiol 6:79. doi: 10.3389/fcimb.2016.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leylabadlo HE, Kafil HS, Yousefi M, Aghazadeh M, Asgharzadeh M. 2016. Pulmonary tuberculosis diagnosis: where we are? Tuberc Respir Dis (Seoul) 79:134–142. doi: 10.4046/trd.2016.79.3.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goletti D, Petruccioli E, Joosten SA, Ottenhoff THM. 2016. Tuberculosis biomarkers: from diagnosis to protection. Infect Dis Rep 8:6568. doi: 10.4081/idr.2016.6568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fu Y, Yi Z, Wu X, Li J, Xu F. 2011. Circulating microRNAs in patients with active pulmonary tuberculosis. J Clin Microbiol 49:4246–4251. doi: 10.1128/JCM.05459-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu J, Lu C, Diao N, Zhang S, Wang S, Wang F, Gao Y, Chen J, Shao L, Lu J, Zhang X, Weng X, Wang H, Zhang W, Huang Y. 2012. Analysis of microRNA expression profiling identifies miR-155 and miR-155* as potential diagnostic markers for active tuberculosis: a preliminary study. Hum Immunol 73:31–37. doi: 10.1016/j.humimm.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 42.Qi Y, Cui L, Ge Y, Shi Z, Zhao K, Guo X, Yang D, Yu H, Cui L, Shan Y, Zhou M, Wang H, Lu Z. 2012. Altered serum microRNAs as biomarkers for the early diagnosis of pulmonary tuberculosis infection. BMC Infect Dis 12:384. doi: 10.1186/1471-2334-12-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miotto P, Mwangoka G, Valente IC, Norbis L, Sotgiu G, Bosu R, Ambrosi A, Codecasa LR, Goletti D, Matteelli A, Ntinqinya EN, Aloi F, Heinrich N, Reither K, Cirillo DM. 2013. miRNA signatures in sera of patients with active pulmonary tuberculosis. PLoS One 8:e80149. doi: 10.1371/journal.pone.0080149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) Method. Methods 25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.