ABSTRACT
The absence of markers of inflammation in the cerebrospinal fluid (CSF) commonly predicts the absence of herpes simplex virus (HSV) central nervous system (CNS) infection. Consequently, multiple authors have proposed and validated criteria for deferring HSV PCR testing of CSF in immunocompetent hosts with normal CSF white blood cell and protein levels (≤5 cells/mm3 and ≤50 mg/dl, respectively). Hosts are considered immunocompetent if they are ≥2 years old and have not had HIV or an organ transplant. Adoption of the criteria may erroneously exclude HSV-infected persons from a necessary diagnostic test or, alternatively, reduce the costs associated with HSV tests with minimal to no effect on patient care. Little is known about the cost-effectiveness of this approach. A decision analysis model was developed to evaluate the adoption of criteria for screening HSV tests of CSF. Estimates of input parameter values combined available literature with a multiyear multisite review at two of the largest health care systems in the United States. Adoption of criteria to screen for HSV test need proved cost-effective when less than 1 in 200 patients deferred from testing truly had an HSV CNS infection. Similar to prior studies, none of the deferred cases had HSV encephalitis (n = 3120). Adoption of these criteria in the United States would save an estimated $127 million ($95 million to $158 million [±25%]) annually. The model calculations remained robust to variation in test cost, prevalence of HSV infection, and random variation to study assumptions. The adoption of criteria to screen HSV PCR tests in CSF represents a cost-effective approach.
KEYWORDS: cost-effectiveness, herpes simplex virus, polymerase chain reaction
INTRODUCTION
Multiple studies have identified herpes simplex virus (HSV) as the most common cause of encephalitis (1–3). Unlike treatment of other causes of viral encephalitis, treatment of herpes simplex virus with acyclovir (ACV) has the highest recommendation for treatment from the Infectious Diseases Society of America (4). The combination of relatively benign treatment and a poor prognosis without treatment justifies a laboratory workup for many more patients than have the disease. The gold standard test for herpes simplex has become nucleic acid amplification, such as HSV PCR. An alternative approach to PCR proposes to test only select patients who meet specific clinical and laboratory criteria.
Over the past 15 years, evidence for excluding herpes simplex virus without HSV PCR has accumulated (5–10). The authors of these studies cited five primary factors as their motivation. First, the spectrum of disease detected by PCR has become less severe and less treatable (e.g., Mollaret's meningitis, HSV-2 meningitis) (11). Second, PCR for HSV has a very low positive rate, between 1% and 3%. Most of the positive results are not encephalitis (12). Third, PCR has a high cost. HSV PCR ranks in the 90th percentile of costs in the Medicare-Medicaid laboratory fee schedule (13). Fourth, the exclusion of HSV with clinical features and routine lab tests has minimal to no monetary cost. Fifth, the criteria can limit unnecessary treatment, because clinical features and routine lab tests can be obtained prior to HSV PCR results.
Although the exclusion of HSV infection without PCR has advantages, it has a principal disadvantage as well. This approach could incorrectly exclude HSV, thereby delaying ACV treatment in a patient with PCR-positive HSV encephalitis (14). This worst-case scenario did not occur in the four largest studies. A review of the criteria proposed by Hanson et al. (Reller's criteria), the best-studied criteria, found only one anecdotal case report of a particularly unusual presentation of HSV encephalitis that would have been excluded from PCR testing. Other exclusion situations due to miscommunication about clinical criteria (8) and borderline elevations in CSF parameters also exist (6).
Given the advantages and disadvantages of PCR, we sought to evaluate the decision to selectively exclude HSV PCR with Reller's criteria compared to the decision to use PCR for all samples. We paid particular attention to the sensitivity of the model's conclusions to various estimates for the incorrect exclusion of HSV encephalitis from PCR testing. We also projected the amount of cost-savings in the United States with the adoption of Reller's criteria in clinical practice.
RESULTS
Baseline analysis.
The addition of criteria for screening HSV PCR outperformed the alternative strategy in our model using the baseline parameters. Compared to no criteria for screening for HSV PCR test need, the addition of criteria produced an identical quality-adjusted life-year (QALY) gain and saved $228 per person referred for HSV PCR. The baseline analysis assumed that a patient with no HSV CNS infection had normal CSF white blood cell (WBC) and protein levels, which reflects case series in the published literature and our institutional experience at Yale and the Veterans Health Administration (VHA). We estimate that using the criteria to screen for HSV PCR test need would save the United States $127,000,000 (range, $95 million to $158 million [2011 dollars]) per year.
Sensitivity analyses.
We performed multiple one-way sensitivity analyses to test the impact of parameter uncertainty. The probability of an HSV CNS infection in a person with normal CSF WBC and protein levels, and therefore not tested by the criteria to screen for HSV PCR test need, represents the most influential factor on the incremental cost-effectiveness ratio (ICER). The ICER represents the incremental cost (in US dollars) to incremental benefit (QALY gained) ratio of money spent on a strategy to perform HSV PCR in all patients compared to a strategy to screen for HSV PCR need. The indifference point between the two strategies with an ICER of $100,000/QALY, a commonly used threshold in cost-effectiveness studies, occurred at a probability of HSV infection with normal CSF WBC and CSF protein levels of 0.005 (Fig. 1). Therefore, the adoption of the criteria to screen for HSV PCR need represents a cost-effective alternative to the routine performance of HSV PCR if fewer than 1 in 200 eligible patients with normal CSF WBC and protein levels have HSV infection.
FIG 1.

One-way sensitivity analysis. As the probability of HSV infection in a patient with normal CSF WBC and protein levels increases, the criteria for screening for HSV PCR become less reliable. A less reliable screen for HSV PCR decreases the incremental cost-effectiveness ratio (ICER). The ICER declines because fewer dollars are required by the intervention (i.e., HSV PCR of all patients) to gain one quality-adjusted life year (QALY) relative to the strategy to screen for HSV PCR.
In two-way sensitivity analyses, we varied the probability of HSV CNS infection with normal CSF WBC and protein levels along with a second parameter. Variation in HSV PCR test cost from $220 to $450 had a modest effect on the indifference point between the two strategies (Fig. 2A). Likewise, variation in the probability of encephalitis in patients with a positive HSV PCR from 0.12 to 0.3 demonstrated model stability around the baseline estimates (Fig. 2B). Model stability was assessed by the observation that the proportions in the Fig. 2 graphs dedicated to either strategy remained relatively stable. For these analyses, we used a willingness-to-pay threshold of $100,000/QALY.
FIG 2.
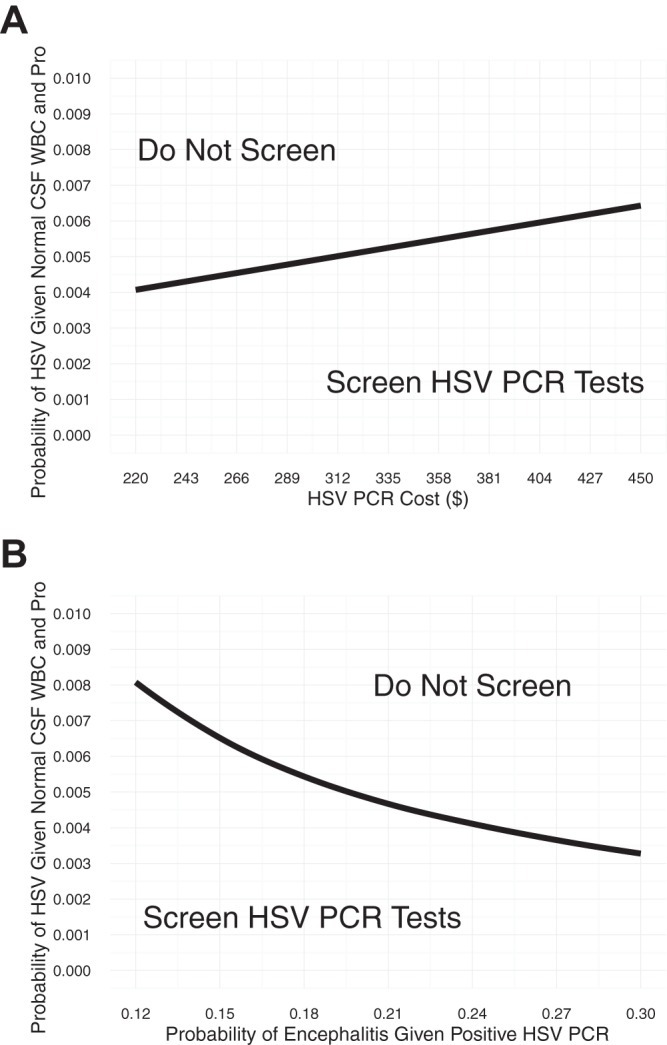
Two-way sensitivity analysis. (A and B) x- and y-axis parameters were varied over a range. For a given (x, y) point on the graph, the values of the two parameters listed on the two axes were used as inputs to compare using a strategy for screening for HSV PCR and not using a screening strategy. The shading indicates the most cost-effective strategy at a given (x, y) point. A standard value for the willingness to pay, $100,000/QALY, was used.
Tests of the model robustness to random variables showed that criteria for screening CSF for HSV PCR test need represented a cost-effective strategy in 98.7% of the Monte Carlo simulations. This estimate employs a $100,000/QALY threshold. Variations from $50,000 to $100,000 per QALY did not substantially change the estimate (Fig. 3).
FIG 3.

Probabilistic sensitivity analysis. At a given willingness to pay, the parameters listed in Table 1 were iteratively drawn from random distributions (i.e., beta distributions for probability parameters and utility estimates). For each iteration, the two strategies were compared to determine the most cost-effective strategy given the randomly drawn parameter values. For each strategy, the proportion of each iteration representing the most cost-effective strategy at a given willingness to pay is graphed.
DISCUSSION
Several studies have proposed laboratory screening criteria for CSF to determine the need for HSV PCR, but no analyses have compared the costs and benefits of adopting this approach. Compared to an approach testing all patients with suspicion of HSV in CSF, an approach selectively testing eligible patients with either abnormal CSF WBC or protein levels is cost-effective in our model if the probability of deferring a case of HSV encephalitis from empirical treatment and testing is lower than 1 in 200. Consistent with prior literature, patients eligible for the selective approach were defined as older than 2 years and without either HIV (any CD4 count) or solid organ transplant. These conclusions were generally unchanged by variations in the test cost, prevalence of HSV infection, and/or other input parameters in our model. Our results are particularly important given the current emphasis on value-based health care, because they suggest that the adoption of a screening protocol for HSV PCR CSF tests will have a minimal impact on patient care and will substantially decrease costs.
An estimation of the probability of HSV encephalitis in an eligible patient with normal CSF WBC and CSF protein levels can be inferred by interpreting our result in the context of the literature. The first paper to propose screening criteria found no exceptions to the screening criteria in 716 specimens, and a follow-up study found a similar result in 2,606 specimens (5, 6). Two other studies found no exceptions in 1,518 and 268 completed tests (7, 8). These papers did not specify the proportion of all HSV tests ordered for eligible patients, which totaled 88% at Yale. After the adjustment and combined with our results, 10,790 patients have undergone testing with the screening criteria. At a cost-efficacy cutoff of 1 in 200, more than 50 (10,790/200) missed cases of HSV infection would need to have occurred to make the rule equivalent, but the authors reported no cases of HSV infection excluded from testing by the rule. This suggests that adoption of HSV PCR test criteria represents a cost-effective approach.
The VHA and Yale have quite different patient populations, which we observed on chart review and reflect on in the study analysis. Compared to Yale, the VHA has a predominately male population with a higher incidence of HIV. HIV is an automatic exemption from test screening, which lowers the comparative benefit of screening over not screening. This is seen in the exclusion rate at the VHA compared to that at Yale (18% versus 12%, respectively). Females have a higher incidence of meningitis infection. In our review of the positive tests, we noticed a higher proportion of patients with encephalitis (compared to those with meningitis) in the male-predominant population at the VHA than at Yale (21% versus 12%, respectively) (see Fig. S3 in the supplemental material). An older average age of a patient with encephalitis would favor the strategy to screen for HSV PCR test need, since the model estimates disability costs from missed cases of encephalitis by age, although this factor appears relatively stable between our data and those from historical studies. Consequently, some patient populations (i.e., low incidence of HIV, female, older average age of encephalitis patients) may benefit slightly more than others by adoption of the screening criteria.
Cost-effectiveness represents one of many factors to consider when deciding to implement laboratory screening criteria for HSV testing of CSF. Institutional priorities, available diagnostic technology, and a relationship between ordering clinician and the performing laboratory also influence the criteria adoption decision. Clinicians may feel more comfortable endorsing an HSV CSF screening policy if they retain the right to opt out of the algorithm, particularly for severe cases near the eligibility cutoff criteria. Such cases may involve patients near 2 years of age or with alternative reasons for immunosuppression, such as bone marrow transplant, autoimmune disease, or undiagnosed HIV infection. Although undiagnosed HIV infection represents a concern, one large study found no cases of newly diagnosed HIV in patients eligible for the criteria (10). Test manufacturers and laboratories that perform the HSV test often receive direct or indirect compensation on a per-test basis, which may introduce a conflict of interest when deciding to implement the criteria, because the criteria will likely reduce test volume.
Adopters of the criteria will likely prefer an implementation approach that maximizes the role of available technology and minimizes time-consuming human review. We recommend the creation of a new order set for a CSF profile (i.e., obtain protein level and WBC count) with reflex to HSV PCR. (This order set would complement the existing order for HSV PCR without the reflex criteria.) As a comment in the new order set, clearly explain the indications for the order: (1) age of ≥2 years, (2) no HIV infection, and (3) no transplant-related immunosuppression. The comment should include relevant references (5–10). The clinical laboratory can build this new order as a “reflex test,” a test selectively performed based on the result of other tests. Most clinical laboratories likely already perform reflex tests, such as a manual differential, in response to an abnormality in an automated differential. In this way, an abnormality in the CSF WBC (≤5 cells/mm3) or protein (≤50 mg/dl) levels would trigger the performance of the HSV PCR test. Clinicians would remain responsible for the discontinuation of acyclovir treatment. Overall, the approach allows clinicians to opt out under special circumstances, allows administration to track adoption by order frequency, and allows the laboratory to avoid chart review.
As with all modeling studies, our model has limitations, because it attempts to overcome incomplete information by relying on the assumptions of the authors and their interpretation of the available literature. For example, we did not consider ACV treatment to modify the outcomes of HSV meningitis (11, 15). Information about the false-positive and false-negative results of the HSV PCR test was insufficient to incorporate into the model and we had to estimate long-term costs by synthesizing multiple sources of information. A randomized controlled trial would provide a more definitive answer, but HSV CNS infections are rare and sporadic, making the cost of such a trial prohibitive. The estimates derived from Yale and the VHA series may not apply in other health care settings. Nevertheless, our results were quite robust in sensitivity analyses, indicating that implementing these screening criteria would lead to substantial cost savings.
In conclusion, we believe that criteria for screening CSF for HSV PCR test need represents a cost-effective and safe intervention based on the experiences of the institutions reviewed and framed in the context of the present literature. Future research on the implementation of screening criteria for this test would further our ability to cost-effectively work up CNS infections.
MATERIALS AND METHODS
To assess the adoption of criteria for screening HSV PCR, we built a decision analysis model and evaluated the model's conclusions under multiple scenarios. First, we compared the presence and the absence of criteria to screen for HSV PCR test need using the best available estimates for the model parameters. Second, we varied an important parameter, the probability of HSV infection in a patient with normal CSF WBC and protein levels (≤5 cells/mm3 and ≤50 mg/dl, respectively) (8) and reassessed the model's conclusions. Third, we performed two-way sensitivity analyses. These analyses also varied the probability of HSV infection in a patient with normal CSF WBC and protein levels. In addition, one of the two-way analyses varied the cost of the HSV PCR test, while another varied the proportion of HSV PCR-positive patients with encephalitis. Fourth, we performed a probabilistic sensitivity analysis to evaluate the robustness of the model's conclusion to variation in many model parameters.
Decision analysis model.
We performed a cost-effectiveness analysis from the societal perspective over a lifetime horizon according to the Panel on Cost-Effectiveness in Health and Medicine recommendations (16). We developed a decision analysis model (Fig. 4) to compare the addition of HSV screening criteria with the current standard of routine HSV PCR. In the base-case scenario, the hypothetical patients had a clinical suspicion for HSV CNS infection evidenced by an order for HSV PCR from CSF. Eligible patients had an age of 50 years and neither HIV infection nor a solid organ transplant (i.e., heart, liver, or kidney) (17). The patients also received empirical ACV treatment and tests for CSF WBC and protein levels, in accordance with standard of care.
FIG 4.
Decision tree for evaluating the cost-effectiveness of herpes simplex virus (HSV) PCR testing strategies. Patients entering the decision tree have an age greater than 2 years and neither HIV nor solid organ transplant (i.e., heart, liver, or kidney). Components 1, 2, and 3 flow together in sequential order. Square nodes represent decisions, while circular nodes represent probabilistic events.
We considered two strategies for HSV PCR testing, depending on the levels of CSF WBC and protein. In the first strategy, all patients received HSV PCR testing. In the second strategy, patients who had normal tests for CSF WBC and protein levels did not undergo HSV PCR testing and stopped ACV treatment immediately when these test results become available. Only patients who had abnormal CSF tests would receive HSV PCR testing. The HSV PCR result determined ACV therapy. A positive HSV PCR led to continuation of ACV therapy, and a negative HSV PCR led to discontinuation of ACV.
These two strategies used a similar decision analysis model after the application of the criteria to screen for HSV PCR need. Patients with a positive HSV PCR had either meningitis or encephalitis. HSV meningitis patients were given a similar treatment and prognosis, whether with or without diagnostic confirmation by HSV PCR (11, 15). Treatment for HSV encephalitis consisted of 21 days of intravenous ACV.
Probabilities.
The probability estimates in the model were derived from published literature and institutional experience (Table 1). Parameter estimates originated from 1,227 HSV PCR CSF tests performed at Yale-New Haven Health System (Yale) and 6,357 HSV PCR CSF tests performed by the Veterans Health Administration (VHA) (10). This cohort represents the largest case series on criteria for screening HSV PCR in the present literature. The tests from Yale spanned from February 2010 to July 2012, and the tests from the VHA spanned from January 2000 to June 2013. The parameter estimates come from only those eligible for the study (age ≥ 2 years, no history of HIV or heart, lung, or liver transplant), which we determined through chart review of all HSV PCR-positive cases. To determine eligibility among HSV PCR-negative cases, we randomly reviewed 300 patients at Yale and 400 patients at the VHA who were tested by HSV PCR. From this sample, we estimated that 88% (1,080/1,227) of patients from Yale and 82% (5,213/6,357) of patients from the VHA were eligible. The Yale University and West Haven Veterans Affairs Medical Center institutional review boards approved this study.
TABLE 1.
Probabilities, costs, and ranges for cost-effectiveness analysis
| Variable | Baseline (range) | Reference or source |
|---|---|---|
| Probability estimates | ||
| Positive HSV PCR | 0.020 (0.014–0.027) | Fig. S1 |
| Normal CSF WBC and protein levels | 0.448 (0.324–0.578) | Fig. S2 |
| Positive HSV PCR, given normal CSF WBC and protein levels | 0 (0–0.01)a | 5–10 |
| Encephalitis, given positive HSV PCR | 0.189 (0.115–0.295) | Fig. S3 |
| Outcome probabilities of patients | 18 | |
| HSV encephalitis with acyclovir | ||
| Normal | 0.14 (0.06–0.25) | |
| Mild | 0.23 (0.15–0.32) | |
| Moderate | 0.28 (0.22–0.34) | |
| Severe | 0.2 (0.16–0.23) | |
| Death | 0.15 | |
| HSV encephalitis without acyclovir | 19 | |
| Normal | 0.057 (0.048–0.067) | |
| Mild | 0.093 (0.084–0.102) | |
| Moderate | 0.05 (0.042–0.059) | |
| Severe | 0.100 (0.037–0.190) | |
| Death | 0.7 | |
| HSV meningitis, with or without acyclovir | 20 | |
| Normal | 0.8 (0.69–0.89) | |
| Mild | 0.2 | |
| Moderate | 0 | |
| Severe | 0 | |
| Death | 0 | |
| No HSV infection | 21 | |
| Normal | 0.155 (0.072–0.264) | |
| Mild | 0.255 (0.176–0.340) | |
| Moderate | 0.19 (0.135–0.250) | |
| Severe | 0.21 (0.171–0.249) | |
| Death | 0.19 | |
| Life expectancy with severe sequelae (yr) | 3.8 | 18 |
| Life expectancy otherwise (yr) | 31 | 16 |
| Utility estimates | ||
| Utility preference by age | 22 | |
| 50 yr | 0.854 | |
| 60 yr | 0.829 | |
| 70 yr | 0.811 | |
| 80 yr | 0.755 | |
| Utility preference by neurological status | 25 | |
| Normal | 1 | |
| Mild | 0.82 (0.60–0.97) | |
| Moderate | 0.52 (0.33–0.71) | |
| Severe | 0.16 (0.02–0.39) | |
| Death | 0 | |
| Cost (2011 dollars) | ||
| Test, CSF HSV PCR | 330 (220–450) | |
| Acyclovir therapy, 30 mg/kg/day intravenously | 260 (150–400) | 27, 28 |
| Hospitalization, viral meningitis | 8,170 (5,200–11,000) | 29 |
| Hospitalization, viral encephalitis | 54,210 | 1, 2 |
| Normal | 33,920 (24,750–44,420) | 30 |
| Mild | 33,920 (24,750–44,420) | |
| Moderate | 43,630 (34,360–51,200) | |
| Severe | 100,420 (90,900–110,430) | |
| Death | 100,420 (90,900–110,430) | |
| Cost to care for disability, annual | 30 | |
| Normal | 2,790 (2,070–3,630) | |
| Mild | 2,790 (2,070–3,630) | |
| Moderate | 5,650 (4,530–6,870) | |
| Severe | 14,330 (7,740–23,220) | |
| Cost for end-of-life care | 39,640 (30,500–49,950) | 31 |
| Discount rate, utility and cost | 0.03 (0, 0.07)b | |
This value was 0.0013 (0.0001 to 0.0039) in probability sensitivity analysis.
One-way sensitivity only.
Consistent with case series in the published literature, our case series found no patients with HSV CNS infection and a normal CSF test (10). We observed an HSV PCR-positive rate of 2.0% in eligible patients (see Fig. S1 in the supplemental material). The criteria for screening for HSV PCR need deferred 44.8% of cases due to normal CSF WBC and protein levels (see Fig. S2 in the supplemental material). Patients with a positive HSV PCR had encephalitis listed as their discharge diagnosis in 18.9% of cases (see Fig. S3 in the supplemental material). Otherwise, we assumed that patients with a positive HSV PCR had meningitis. The presence of abnormal CSF WBC or protein levels did not change the probability of encephalitis in patients with a positive HSV PCR (10). We excluded estimates from literature sources when they included ineligible patients, such as infants or organ transplant recipients.
For the probabilistic sensitivity analysis, we altered the probability (given normal CSF tests) of HSV CNS infection because we could not model with a value of zero for the beta distribution. Instead, we chose 0.0013 (7/5,213) based on cases from the VHA of positive HSV PCR with normal CSF tests. These seven patients received neither a clinical diagnosis of HSV nor treatment with ACV.
We interpreted the available literature to determine the probability of various outcomes of HSV infection. Neurological outcomes of HSV infection included normal, mild, moderate, severe, and death. Raschilas et al. reported outcomes for patients with HSV encephalitis who received ACV (18). The Collaborative Antiviral Study Group reported outcomes for patients with HSV encephalitis who did not receive ACV (19). Omland et al. reported that 80% of patients with HSV meningitis returned to a normal state, and 20% had minor sequelae. This led us to assume that ACV did not alter the outcomes of HSV meningitis (20). Tebas et al. reported the outcomes for patients with a negative HSV PCR (21).
Quality-adjusted life expectancy.
We calculated quality-adjusted life expectancies based on estimates of mean life expectancy, declines in quality-of-life weights with age, and preference weights dependent on neurologic outcome (22). Our hypothetical patient had an age of 50 years, based on published literature (17) and institutional experience, with a mean life expectancy of 31.0 additional years (23). We estimated differences in quality-adjusted life expectancies between the two strategies as quality-adjusted life-years (QALYs) gained. Quality-adjusted life weights were derived from prior literature (24, 25). We assumed that patients with severe neurologic outcomes would die prematurely, with a 5-year survival rate of 31.2% (26).
Costs.
We incorporated the costs of HSV-related tests, treatment, and outcomes (Table 1). Costs were adjusted in 2011 U.S. dollars. Using a cost-to-charge ratio of 0.75, the HSV PCR test cost $330. ACV costs $260 per day for a body weight of 80 kg and 3 doses per day at 10 mg/kg of body weight per dose (27, 28). We assumed that clinicians had the HSV PCR result within 1 day; therefore, ACV was given for 1 day if HSV PCR was negative. Hospitalization for viral meningitis cost $8,170 (29). Hospitalization for encephalitis averaged $54,210 and varied based on the neurologic outcome (1, 2). We derived the cost estimates for HSV encephalitis disability from La Crosse virus encephalitis disability costs (30). End-of-life care costs $39,640 (31). Costs and outcomes of HSV infection had a 3% discount rate (range, 0% to 7%), applied following the recommendations of the Panel on Cost-Effectiveness in Health and Medicine (16).
Analyses.
We evaluated our models and performed sensitivity analyses with a commercial software package (TreeAge Pro, Williamstown, MA). Unless explicitly set as part of the sensitivity analysis, we used the range around point estimates presented in Table 1. The two-way sensitivity analyses used a willingness to pay of $100,000 per QALY (32). Willingness to pay represents the maximum amount of money to be paid per QALY, which facilities a comparison of the costs and benefits of either strategy by the model. The choice of distributions for the probabilistic sensitivity analyses followed the statistical framework suggested by previous literature using beta distributions for probability parameters and utility estimates, log-normal distribution for relative risk, and gamma distributions for the cost parameters (33).
We sought to extrapolate the difference in costs between the two strategies to the population of the United States. A 9-year review of encephalitis-associated hospitalizations listed HSV as one of the most common known pathogens, which accounted for 2,100 annual hospitalizations (2). We estimate that 18.9% of positive HSV PCR tests occur in encephalitis patients, 2% of HSV PCR tests have a positive result, and 85% of tested patients meet the eligibility requirements of the criteria for screening for HSV PCR test need. Thus, an estimated 555,000 tests occur in the United States each year (±25%; 417,000 to 694,000). To estimate the total cost savings, we multiplied the dollars saved per person referred for HSV PCR by the number of tests performed annually.
Supplementary Material
ACKNOWLEDGMENTS
We thank Douglas B. Quine, PhD (main laboratory, Bridgeport Hospital, Bridgeport, CT), for editorial assistance in the preparation of the manuscript.
The authors have no potential conflicts of interest.
Financial support for this study was provided entirely by the universities employing each author. The funding agreement ensured the authors' independence in designing the study, interpreting the data, and writing and publishing the report.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/JCM.00119-17.
REFERENCES
- 1.Vora NM, Holman RC, Mehal JM, Steiner CA, Blanton J, Sejvar J. 2014. Burden of encephalitis-associated hospitalizations in the United States, 1998-2010. Neurology 82:443–451. doi: 10.1212/WNL.0000000000000086. [DOI] [PubMed] [Google Scholar]
- 2.Khetsuriani N, Holman RC, Anderson LJ. 2002. Burden of encephalitis-associated hospitalizations in the United States, 1988-1997. Clin Infect Dis 35:175–182. doi: 10.1086/341301. [DOI] [PubMed] [Google Scholar]
- 3.Fodor PA, Levin MJ, Weinberg A, Sandberg E, Sylman J, Tyler KL. 1998. Atypical herpes simplex virus encephalitis diagnosed by PCR amplification of viral DNA from CSF. Neurology 51:554–559. doi: 10.1212/WNL.51.2.554. [DOI] [PubMed] [Google Scholar]
- 4.Tunkel AR, Glaser CA, Bloch KC, Sejvar JJ, Marra CM, Roos KL, Hartman BJ, Kaplan SL, Scheld WM, Whitley RJ. 2008. The management of encephalitis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 47:303–327. doi: 10.1086/589747. [DOI] [PubMed] [Google Scholar]
- 5.Tang YW, Hibbs JR, Tau KR, Qian Q, Skarhus HA, Smith TF, Persing DH. 1999. Effective use of polymerase chain reaction for diagnosis of central nervous system infections. Clin Infect Dis 29:803–806. doi: 10.1086/520439. [DOI] [PubMed] [Google Scholar]
- 6.Simko JP, Caliendo AM, Hogle K, Versalovic J. 2002. Differences in laboratory findings for cerebrospinal fluid specimens obtained from patients with meningitis or encephalitis due to herpes simplex virus (HSV) documented by detection of HSV DNA. Clin Infect Dis 35:414–419. doi: 10.1086/341979. [DOI] [PubMed] [Google Scholar]
- 7.Lopez Roa P, Alonso R, de Egea V, Usubillaga R, Munoz P, Bouza E. 2013. PCR for detection of herpes simplex virus in cerebrospinal fluid: alternative acceptance criteria for diagnostic workup. J Clin Microbiol 51:2880–2883. doi: 10.1128/JCM.00950-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hanson KE, Alexander BD, Woods C, Petti C, Reller LB. 2007. Validation of laboratory screening criteria for herpes simplex virus testing of cerebrospinal fluid. J Clin Microbiol 45:721–724. doi: 10.1128/JCM.01950-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilen CB, Monaco CL, Hoppe-Bauer J, Jackups R Jr., Bucelli RC, Burnham CA. 2015. Criteria for reducing unnecessary testing for herpes simplex virus, varicella-zoster virus, cytomegalovirus, and enterovirus in cerebrospinal fluid samples from adults. J Clin Microbiol 53:887–895. doi: 10.1128/JCM.03161-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hauser RG, Brandt C, Martinello R. 2017. Criteria to screen molecular tests for the diagnosis of herpes simplex virus in the central nervous system has no propensity to harm. J Pathol Inform, 8:4. doi: 10.4103/2153-3539.201113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Landry ML, Greenwold J, Vikram HR. 2009. Herpes simplex type-2 meningitis: presentation and lack of standardized therapy. Am J Med 122:688–691. doi: 10.1016/j.amjmed.2009.02.017. [DOI] [PubMed] [Google Scholar]
- 12.Afonso N, Gunasena S, Galla K, Podzorski R, Chandrasekar P, Alangaden G. 2007. Appropriate use of polymerase chain reaction for detection of herpes simplex virus 2 in cerebrospinal fluid of patients at an inner-city hospital. Diagn Microbiol Infect Dis 57:309–313. doi: 10.1016/j.diagmicrobio.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 13.Centers for Medicare and Medicaid Services. 2014. Clinical laboratory fee schedule. https://www.cms.gov/medicare/medicare-fee-for-service-payment/clinicallabfeesched/ Accessed 2 November 2014.
- 14.Boyapati R, Papadopoulos G, Olver J, Geluk M, Johnson PD. 2012. An unusual presentation of herpes simplex virus encephalitis. Case Rep Med 2012:241710. doi: 10.1155/2012/241710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aurelius E, Franzen-Rohl E, Glimaker M, Akre O, Grillner L, Jorup-Ronstrom C, Studahl M. 2012. Long-term valacyclovir suppressive treatment after herpes simplex virus type 2 meningitis: a double-blind, randomized controlled trial. Clin Infect Dis 54:1304–1313. doi: 10.1093/cid/cis031. [DOI] [PubMed] [Google Scholar]
- 16.Gold MR, Siegel JE, Russell LB, Weinstein MC (ed). 1996. Cost-effectiveness in health and medicine. Oxford University Press, New York, NY. [Google Scholar]
- 17.Whitley RJ, Alford CA, Hirsch MS, Schooley RT, Luby JP, Aoki FY, Hanley D, Nahmias AJ, Soong SJ. 1986. Vidarabine versus acyclovir therapy in herpes simplex encephalitis. N Engl J Med 314:144–149. doi: 10.1056/NEJM198601163140303. [DOI] [PubMed] [Google Scholar]
- 18.Raschilas F, Wolff M, Delatour F, Chaffaut C, De Broucker T, Chevret S, Lebon P, Canton P, Rozenberg F. 2002. Outcome of and prognostic factors for herpes simplex encephalitis in adult patients: results of a multicenter study. Clin Infect Dis 35:254–260. doi: 10.1086/341405. [DOI] [PubMed] [Google Scholar]
- 19.Soong SJ, Watson NE, Caddell GR, Alford CA Jr, Whitley RJ. 1991. Use of brain biopsy for diagnostic evaluation of patients with suspected herpes simplex encephalitis: a statistical model and its clinical implications. NIAID Collaborative Antiviral Study Group. J Infect Dis 163:17–22. [DOI] [PubMed] [Google Scholar]
- 20.Omland LH, Vestergaard BF, Wandall JH. 2008. Herpes simplex virus type 2 infections of the central nervous system: a retrospective study of 49 patients. Scand J Infect Dis 40:59–62. doi: 10.1080/00365540701509881. [DOI] [PubMed] [Google Scholar]
- 21.Tebas P, Nease RF, Storch GA. 1998. Use of the polymerase chain reaction in the diagnosis of herpes simplex encephalitis: a decision analysis model. Am J Med 105:287–295. doi: 10.1016/S0002-9343(98)00259-9. [DOI] [PubMed] [Google Scholar]
- 22.Nyman JA, Barleen NA, Dowd BE, Russell DW, Coons SJ, Sullivan PW. 2007. Quality-of-life weights for the US population: self-reported health status and priority health conditions, by demographic characteristics. Med Care 45:618–628. doi: 10.1097/MLR.0b013e31803dce05. [DOI] [PubMed] [Google Scholar]
- 23.US Census Bureau. 2014. Births, deaths, marriages, & divorces: life expectancy. http://www2.census.gov/library/publications/2011/compendia/statab/131ed/tables/vitstat.pdf Accessed July 22 2014.
- 24.Caviness AC, Demmler GJ, Swint JM, Cantor SB. 2008. Cost-effectiveness analysis of herpes simplex virus testing and treatment strategies in febrile neonates. Arch Pediatr Adolesc Med 162:665–674. doi: 10.1001/archpedi.162.7.665. [DOI] [PubMed] [Google Scholar]
- 25.Mennemeyer ST, Cyr LP, Whitley RJ. 1997. Antiviral therapy for neonatal herpes simplex virus: a cost-effectiveness analysis. Am J Manag Care 3:1551–1558. [PubMed] [Google Scholar]
- 26.Sacco RL, Shi T, Zamanillo MC, Kargman DE. 1994. Predictors of mortality and recurrence after hospitalized cerebral infarction in an urban community: the Northern Manhattan Stroke Study. Neurology 44:626–634. doi: 10.1212/WNL.44.4.626. [DOI] [PubMed] [Google Scholar]
- 27.Centers for Disease Control and Prevention. 2014. Body measurements. http://www.cdc.gov/nchs/fastats/body-measurements.htm Accessed 14 October 2014.
- 28.Pickering LK, Kimberlin DW, Long SS. 2012. Red book: 2012 report of the Committee on Infectious Diseases, 29th ed American Academy of Pediatrics, Elk Grove Village, IL: https://redbook.solutions.aap.org/DocumentLibrary/RB12_interior.pdf. [Google Scholar]
- 29.Holmquist L, Russo CA, Elixhauser A. 2008. Meningitis-related hospitalizations in the United States, 2006. Healthcare Cost and Utilization Project (HCUP) statistical brief no. 57. Agency for Healthcare Research and Quality, Rockville, MD: https://www.ncbi.nlm.nih.gov/books/NBK56046/. [PubMed] [Google Scholar]
- 30.Utz JT, Apperson CS, MacCormack JN, Salyers M, Dietz EJ, McPherson JT. 2003. Economic and social impacts of La Crosse encephalitis in western North Carolina. Am J Trop Med Hyg 69:509–518. [PubMed] [Google Scholar]
- 31.Riley GF, Lubitz JD. 2010. Long-term trends in Medicare payments in the last year of life. Health Serv Res 45:565–576. doi: 10.1111/j.1475-6773.2010.01082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gafni A, Birch S. 2006. Incremental cost-effectiveness ratios (ICERs): the silence of the lambda. Soc Sci Med 62:2091–2100. doi: 10.1016/j.socscimed.2005.10.023. [DOI] [PubMed] [Google Scholar]
- 33.Briggs AH, Goeree R, Blackhouse G, O'Brien BJ. 2002. Probabilistic analysis of cost-effectiveness models: choosing between treatment strategies for gastroesophageal reflux disease. Med Decis Making 22:290–308. doi: 10.1177/027298902400448867. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



