Abstract
Aiming to combine the flexibility of Brucella lumazine synthase (BLS) to adapt different protein domains in a decameric structure and the capacity of BLS and flagellin to enhance the immunogenicity of peptides that are linked to their structure, we generated a chimeric protein (BLS‐FliC131) by fusing flagellin from Salmonella in the N‐termini of BLS. The obtained protein was recognized by anti‐flagellin and anti‐BLS antibodies, keeping the oligomerization capacity of BLS, without affecting the folding of the monomeric protein components determined by circular dichroism. Furthermore, the thermal stability of each fusion partner is conserved, indicating that the interactions that participate in its folding are not affected by the genetic fusion. Besides, either in vitro or in vivo using TLR5‐deficient animals we could determine that BLS‐FliC131 retains the capacity of triggering TLR5. The humoral response against BLS elicited by BLS‐FliC131 was stronger than the one elicited by equimolar amounts of BLS + FliC. Since BLS scaffold allows the generation of hetero‐decameric structures, we expect that flagellin oligomerization on this protein scaffold will generate a new vaccine platform with enhanced capacity to activate immune responses.
Keywords: BLS, flagellin, scaffold, TLR5
Abbreviations
- BAL
bronchoalveolar lavage
- BLS
Brucella lumazine synthase
- CD
circular dichroism
- DCs
dendritic cells
- DTT
dithiothreitol
- IPTG
isopropyl β‐D‐1‐thiogalactopyranoside
- LPS
lipopolysaccharides
- NLR
NOD‐like receptor
- NOD
nucleotide oligomerization domain
- PMSF
phenylmethanesulfonyl fluoride or phenylmethylsulfonyl fluoride
- SEC–SLS
static light scattering assay coupled to size‐exclusion chromatography
- Tm
thermal denaturation
- UV
ultraviolet
Introduction
The increasing understanding on microbial biology, pathogenesis, and host immunobiology in combination with development of powerful screening tools and predictive bioinformatics has shifted vaccine development from exclusively empirical approaches toward rational design.1, 2 Initial vaccines were mainly based on the use of attenuated or inactivated whole microorganisms. A new generation of vaccines based on recombinant components has followed, moving toward chemically defined vaccines. The ideal vaccine should keep the highest protective capacity eliciting as few adverse effects as possible. Chemically defined vaccines have the best safety profiles, but they have to face the challenge of eliciting protection using just one or a few components of a pathogen.3 Immunogenicity of single proteins or peptides is much lower than whole microorganisms. To override this disadvantage, the selection of the best immunogen(s) for each pathogen as well as combining it with appropriate adjuvants becomes crucial. The discovery of several families of innate response receptors has refreshed the adjuvant field, providing a rational background for the selection of compounds with adjuvant properties.4
Flagellin is the major structural protein of Gram‐negative bacteria, being also a ligand of TLR55 and of intracytosolic innate receptors of the nucleotide oligomerization domain (NOD)‐like receptor (NLR) family.6 It exhibits strong adjuvant properties when administered by different routes7 being much more efficient when is covalently linked to the immunogen.8 Consequently, flagellin has been proposed as a platform for vaccine development being successfully used in proof of concept studies using several model infections, including Yersinia pestis, Influenza, Clostridium difficile, and Plasmodium vivax among others.9, 10, 11, 12 Considerable experimental evidence supports the role of flagellin in the enhancement of immunogenicity. It has been hard to accurately define the structure of flagellin monomer, since they tend to polymerize in aqueous solution. This was overcome using a fragment of flagellin lacking the N and C termini (called F41 in Salmonella).13
The enzyme lumazine synthase from Brucella spp. (BLS) is a highly immunogenic protein14, 15, 16 and behaves as a potent oral or systemic immunogen when injected as a protein or as a DNA vaccine.17 BLS has been extensively used as a carrier for peptides and proteins being a proven successful platform for antigen presentation to the immune system for vaccine development.16, 17, 18, 19 Several BLS features make this molecule an attractive protein scaffold for multiple display. First, the oligomer is highly stable to chemical and thermal denaturation. Second, it can be reversibly unfolded and reassembled in different conditions. Third, its 10 N‐termini are excellent acceptors for foreign polypeptides given their exposed and flexible nature. Finally, recombinant BLS can be expressed at high levels in prokaryotic and eukaryotic cells and is highly immunogenic, an essential property for vaccine development. BLS folds as stable dimers of pentamers20 and has been shown to allow the insertion of foreign peptides or proteins at its N‐terminus without disrupting its general folding.18, 19, 21 The presentation of BLS to the immune system in a highly ordered three‐dimensional array enhances the immunogenicity of the heterologous peptides.18, 19, 22 BLS induces the functional and phenotypic maturation of dendritic cells (DCs) via TLR4, stimulating the secretion of proinflammatory cytokines and chemokines.23 BLS induces the cross presentation of covalently attached peptides and generates a strong and long‐lasting humoral immune response without adjuvants.22 BLS elicits an antitumor immune response via TLR4 and has a direct effect on tumor growth and mice survival dependent on TLR4 from B16 melanoma.24
With the aim of combining the flexibility of BLS to adapt different protein domains in a heterodecameric structure25 and the capacity of BLS and flagellin to enhance the immunogenicity of its attached peptides, we generated a chimeric protein (BLS‐FliC131) by fusing flagellin from Salmonella in the 10 N‐termini of BLS. Since the anti‐flagellin antibodies generated upon several administrations could hamper its adjuvanticity,26 we used a flagellin with a deletion in the D2‐D3 antigenic domains.27 Overall, this construct keeps the oligomerization capacity of BLS, without affecting the folding of the monomeric protein components. Furthermore, this fusion protein retains the capacity of triggering TLR5 enhancing the humoral response against BLS. We expect that flagellin oligomerization on this protein scaffold will generate a new vaccine platform with enhanced capacity to activate immune responses.
Results
BLS‐FliC131 chimeric protein keeps folding properties of FliC C131 and BLS and is decameric in solution
Using the same strategy described in Laplagne et al.18 for the construction of chimeras of BLS and small peptides, we replaced the coding sequence of the first eight residues of the N‐terminal end of BLS with the coding sequence of FliC C131. The production and purification of the chimera was carried out as described in the Methods section. SDS–PAGE analysis along expression and purification process of the chimeric protein BLS‐FliC131 indicated that the monomeric protein has the expected molecular weight of 52 kDa. The BLS‐FliC131 chimeric protein is recognized by previously described anti‐flagellin and anti‐BLS monoclonal antibodies,14, 28 as shown by Western blot (Fig. 1).
Figure 1.

BLS‐FliC131 chimeric protein conserves immunochemical reactivity. Western blot analysis using anti‐FliC and anti‐BLS monoclonal antibodies. Lane 1: BLS‐FliC131 chimera, Lane 2: FliC C131, Lane 3: BLS. MW: molecular weight marker.
Considering the biophysical properties of BLS, we expected the BLS‐FliC131 chimera to be decameric. Based on the structure of the F41 fragment of Salmonella enterica flagellin and of BLS, we constructed a schematic three‐dimensional model of the BLS‐FliC131 chimera (Fig. 2). In order to determine the oligomeric state of the BLS‐FliC131 chimera, we performed a static light scattering assay coupled to size‐exclusion chromatography (SEC–SLS) of BLS, FliC C131, and BLS‐FliC131 (Fig. 3). BLS eluted as two peaks. The major one corresponds to a molecular weight of 176 kDa, which is compatible with the expected value for the decamer, 174 kDa. The presence of the extra peak of higher molecular weight (not determined) indicates a tendency to form higher n‐oligomers in the conditions of the experiment, not reported previously. FliC C131 eluted as a single and symmetric peak with a molecular weight of 30 kDa. As the theoretical molecular weight is 35 kDa, the result indicates that FliC C131 is a monomer in solution. Taking into account that FliC C131 lacks the D3 domain that favors the protofilament formation, we expect FliC C131 to be a monomer or a low n‐oligomer. As for the BLS‐FliC131 chimera, there is a peak around 500 kDa compatible with the chimeric decamer, whereas most part of the sample was not resolved by the column, possibly corresponding to high molecular weight aggregates. For all in vivo experiments performed afterward, 500 kDa fraction was used.
Figure 2.
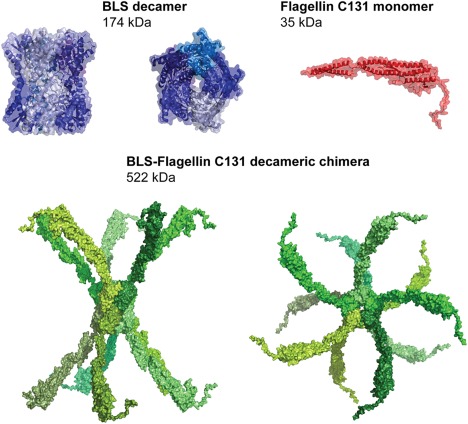
Three‐dimensional structure of the decameric BLS (PDB code: 1T13), the flagellin 131 (PDB code: 3A5X), and a model of BLS‐FliC131 chimera. The structures are represented as surface and for BLS and flagellin also as cartoon. The expected MW values are indicated.
Figure 3.
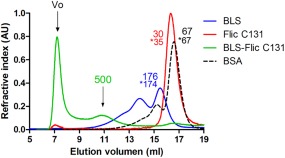
Static light scattering coupled to size exclusion chromatography. BLS (blue), FliC C131 (red), and BLS‐FliC131 (green) were subjected to SEC coupled to a light scattering instrument connected in tandem to a differential refractometer detector. The MW values estimated by the relation of scattering/RI signals are indicated above each curve. The values following after an asterisk correspond to the expected MW.
The secondary structure of BLS and FliC C131 are partially preserved in the BLS‐FliC131 chimera
We compared the far ultraviolet (UV)‐circular dichroism spectra of BLS, FliC, C131, and BLS‐FliC131 (Fig. 4). As already reported,20 BLS presents two minima, the main at 220 nm and the other at 211 nm. FliC C131 also presents two minima, being the main located at 208 nm and the other at 220 nm. The results show that the spectrum of BLS‐FliC131 chimera partially resembles the sum of spectra of the isolated BLS and FliC C131. The experimental and expected spectra are practically identical from 215 to 254 nm, while they differ in the range from 200 to 215 nm. Taking into account that most of the signal within this last range correspond to FliC C131, the results indicate that BLS structure seems to be preserved in the BLS‐FliC131 chimera while FliC C131 does not fully fold in the context of the chimera as the isolated FliC C131.
Figure 4.
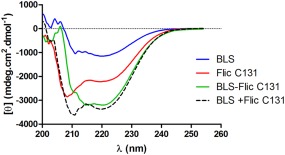
Far UV circular dichroism. Comparison of the far UV‐CD spectra of BLS (blue), FliC C131 (red), BLS‐FliC131 (green), and the sum of BLS and FliC C131 spectra (black dashed). Results are representative from three different experiments.
The BLS‐FliC131 chimera keeps the thermal denaturation and the reversibility of the thermal unfolding behaviors of the isolated BLS and FliC C131
The stability of the BLS‐FliC131 chimera to thermal denaturation was followed by measuring the molar ellipticity at 220 nm as a function of increasing temperature, compared to the isolated BLS and FliC C131 (Fig. 5). As stated before,20 BLS shows a sharp decrease of ellipticity between 85 and 95°C with an apparent midpoint of the thermal denaturation (Tm) of 88.6°C. The loss of secondary structure is not recovered after cooling of the samples back to 25°C (Fig. 6), indicating that an irreversible unfolding of BLS takes place under these conditions. On the other hand, FliC C131 presents an apparent Tm of 34.9°C, however preserves most of the secondary structure after denaturation. BLS‐FliC131 chimera presents two phases in the thermal denaturation, with apparent Tm similar to the ones of the isolated BLS and FliC C131, 87.4 and 35.7°C. Interestingly, the chimera presents a partial reversibility of the thermal unfolding. The results indicate that the BLS‐FliC131 chimera keeps the thermal denaturation and the reversibility of the thermal unfolding behaviors of the isolated BLS and FliC C131.
Figure 5.
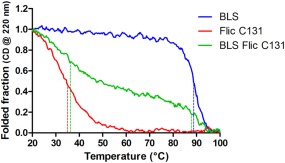
Thermal denaturation monitored by circular dichroism. The heat‐induced denaturation curve of BLS (blue), FliC C131 (red), BLS‐FliC131 (green). CD signal was measured at 220 nm. Dashed lines indicate the inflection points of each curve. Results are representative from two different experiments.
Figure 6.

Reversibility of the thermal unfolding monitored by circular dichroism. CD spectra of BLS (blue), FliC131 (red), BLS‐FliC131 (green) at 25°C before (continuous line) and after (dashed lines) the thermal denaturation experiment. Results are representative from two different experiments.
BLS‐FliC131 chimeric protein can activate innate immunity either in vitro or in vivo
In order to assess the biological properties of the generated chimeric protein, we first used a Caco2 luciferase reporter cell line generated in our laboratory.26 This system is highly sensitive to TLR5 activation, whereas TLR4 stimulation does not induce changes in this cell type.26, 29 It was observed that 5 µg/mL of BLS‐FliC131 chimera as well as the equimolar amount of FliC C131 (3.5 µg/mL) are strong agonists of this in vitro system [Fig. 7(A)], whereas treatment with BLS or lipopolysaccharides (LPS) did not induce changes to the basal activity.
Figure 7.
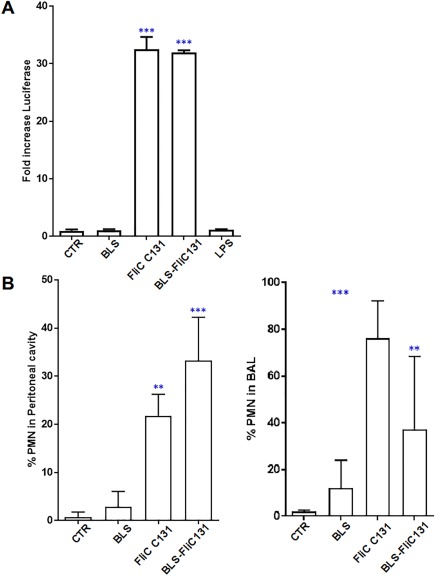
Fusion protein keeps the capacity to trigger innate response either in vitro or in vivo. (A) Activity on Caco2‐luciferase reporter cell line. Cells were treated with different stimuli and luciferase activity was measured on cell lysate 6 h post‐stimulation. Results are expressed as fold‐increase with respect to non‐treated control. Results are representative from three different experiments. (***P < 0.005 two way ANOVA followed by Tuckey test). (B) Recruitment of neutrophils when administered either i.p. or i.n. C57BL/6J mice were treated with different stimuli and cells recovered by broncheoalveolar or peritoneal wash and analyzed by flow cytometry upon immunostaining. Results are expressed as percentage of polymorphonuclear cells (CD11c‐ CD11b+Ly6G+ GR1+). Results are representative from three different experiments. (***P < 0.005 two way ANOVA followed by Tuckey test).
The capacity of BLS‐FliC131 fusion protein to trigger an innate response was also tested in vivo in a mouse model. BLS‐FliC131 chimeric protein induced a significant recruitment of neutrophils to the peritoneal cavity or the bronchoalveolar space 24 h post‐treatment [Fig. 7(B)]. Comparable results were obtained upon FliC C131 treatment whereas BLS treatment showed a modest, statistically not significant change. These results indicate that BLS‐FliC131 fusion protein retains the capacity to trigger TLR activation comparable to their individual components.
BLS‐FliC131 capacity to induce innate response markers in vivo is highly dependent on TLR5
In order to determine the capacity of BLS‐FliC131 fusion protein to activate the innate response in vivo, we treated mice i.v. and measured levels of selected innate response‐associated cytokines in plasma 2 h after stimulation.
IL6 levels were increased in wt animals treated with LPS, FliC C131, or BLS‐FliC131 chimera compared with control group [Fig. 8(A)]. Interestingly, in TLR4‐deficient mice, FliC C131 and BLS‐FliC131 treatments, but not LPS, enhanced serum IL6. As expected, in TLR5‐deficient mice treated with FliC C131 there was not change in IL6 levels whereas opposite results were obtained in those animals treated with LPS and BLS‐FliC131.
Figure 8.
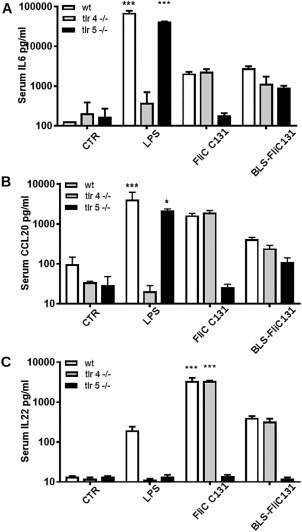
BLS‐FliC131 has the capacity to trigger innate response activation in vivo in TLR4 and TLR5‐deficient mice. Animals were stimulated i.v. with different treatments. Cytokines were determined in serum samples 2 h upon treatment by ELISA. Results are representative from two different experiments. (***P < 0.005 two way ANOVA followed by Tuckey test).
Similar results were obtained when detecting CCL20 serum levels, which are mainly contributed by liver stimulation.30 BLS‐FliC131 treatment induced a rise in CCL20 serum levels in wt and TLR4‐deficient mice, with only slight changes in TLR5‐deficient mice. As expected, no changes in CCL20 levels were observed by treatment with LPS and FliC C131 in TLR4 and TLR5‐deficient mice, respectively [Fig. 8(B)].
In the case of IL22, it was reported that flagellin treatment generates a peak in IL22 serum levels in a TLR5‐dependent fashion, this being dependent of mucosal and splenic CD11c+ DCs and innate lymphoid cells. On the other hand, LPS administration generates less important changes in IL22 serum levels.31 In agreement with this report, no changes were observed by any treatment in TLR5‐deficient mice [Fig. 8(C)], whereas FliC C131 and BLS‐FliC131 induced a rise in this cytokine in wt as well as in TLR4‐deficient mice. In the case of LPS, a rise in IL22 was observed only for wt mice, whereas no response was measurable in TLR4‐deficient animals [Fig. 8(C)]. Altogether, these results indicate that in vivo BLS‐FliC131 retains its capacity to signal through TLR5 since their activating capacities were conserved in TLR4 or wt mice, while being mainly affected in TLR5‐deficient mice.
Immunogenicity of BLS is enhanced in the BLS‐FliC131 fusion protein
Anti‐BLS antibody titers were determined in mice immunized twice in different conditions, using an equimolar low amount of antigen in each treatment. Notably, animals immunized with BLS‐FliC131 fusion protein elicited higher anti‐BLS IgG than the other groups (P < 0.05; Fig. 9), showing that physical attachment of BLS to flagellin enhances the capacity of BLS to generate antibody response.
Figure 9.
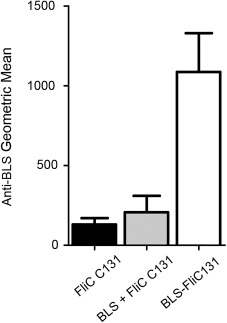
Immunogenicity of BLS is enhanced in the BLS‐FliC131 fusion protein. Anti‐BLS antibodies were determined by indirect ELISA in animals immunized twice with the different antigenic mixtures. Results are expressed as geometric mean of each group (n = 5). Results are representative from two different experiments.
Discussion
We report the generation of a fusion protein between FliC from Salmonella and BLS from Brucella that has conserved the main features of each of its constitutive polypeptides, basically the capacity to generate a decameric structure and to activate innate response. We selected a flagellin variant devoid of exposed D2 and D3 domains (FliC C131) which are highly antigenic regions.27 The trigger of anti‐flagellin antibodies may constitute a problem for repeated in vivo treatment, since it has been shown that this response may impair TLR5 activation.26 BLS has the capacity to act as a vaccine carrier, having a compact decameric quaternary structure and allowing the addition of antigenic peptide/proteins in its N‐terminus.18, 19, 21 We have shown here that the BLS‐FliC131 chimera (a) can adopt decameric structure; (b) fully keeps the secondary structure of BLS and partially the one of FliC C131; (c) has a thermal denaturation behavior that keeps the features of both isolated BLS and FliC C131; (d) presents a partial reversibility of the thermal unfolding, behaving as an intermediate case between the isolate BLS and FliC C131, (e) keeps the capacity to trigger TLR5‐activation, and is highly immunogenic.
It has been shown that a stronger response is obtained if the antigen is attached to BLS,17 and this have been the basis for the development of several applications using BLS as vaccine carrier.17, 19, 22, 24, 32, 33, 34, 35 Furthermore, it has been shown that through changes in urea concentration, reassociation between different BLS‐peptide fusion proteins, a heterodecameric structure can be obtained.25 This may be an interesting application for the fusion protein described here, allowing to produce mixed BLS chimeras containing BLS‐FliC and BLS‐antigen of interest fusion proteins.
Beyond the structural analysis indicating that FliC C131 domain in the fusion protein has partially retained the folding of native FliC C131 (Fig. 4), we could demonstrate that it has kept the capacity to trigger innate immunity due to its interaction with TLR5 (Fig. 7). It has been recently shown that TLR5 signaling is triggered by a 2:2 interaction with two different flagellin subunits that are not physically linked.36, 37 We are currently performing experiments to determine the stoichiometry of the interaction established between the BLS‐FliC131 fusion protein and TLR5. Different in vivo results indicate that this capacity to trigger TLR5 signaling is also functional in this situation (Fig. 8), specially the capacity to trigger IL22 rise in serum, which has been associated to TLR5‐dependent dendritic cell activation in vivo.31
There is extensive evidence showing that covalent attachment of antigens to flagellin enhances the antibody and cellular response against the antigen.8, 38, 39, 40 This is also the case of the construction reported herein, since enhanced anti‐BLS IgG response was observed in animals immunized with BLS‐FliC131 chimera using protein amounts that have shown weak antibody response against BLS.16 Notably, when BLS was not attached to flagellin (BLS + FliC C131 condition), anti‐BLS antibody response was lower than in the case of BLS‐FliC131 immunized animals (Fig. 9). It has been shown that uptake and processing is enhanced when the antigen is attached to a TLR ligand,41 although the cellular mechanisms responsible for this activity has not been fully elucidated. BLS‐FliC131 fusion protein is also an interesting tool to study the contribution of TLRs to these processes, which is subject of current investigations.
In conclusion, we have generated a chimeric BLS‐FliC131 protein that keeps structural and biological features and that could be exploited as vaccine carrier/adjuvant combining the characteristics of two promising candidate molecules.
Materials and Methods
Gene cloning and protein expression and purification
Flagellin C131 variant from Salmonella typhimurium
The flagellin C131 from Salmonella typhimurium (strain LT2/SGSC1412/ATCC 700720) corresponds to the deletion version FliCΔ202‐369 of flagellin (Uniprot code: P06179). The construct was cloned in pET11a vector (Novagen). Escherichia coli BL21(DE3) competent cells (Stratagene, La Jolla, CA) were transformed with the expression plasmid mentioned above. A preculture was grown overnight in LB medium with 100 µg/mL ampicillin at 37°C with agitation (200 rev/min) and then diluted to 1:100 and grown to an absorbance (at 600 nm) of 0.6. At this point, isopropyl β‐D‐1‐thiogalactopyranoside (IPTG) was added to a final concentration of 1 mM and the cultures were further incubated overnight at 20°C with agitation (200 rev/min). The flagellin C131 variant was purified as previously described.1 Briefly, the bacteria were centrifuged at 5000g for 10 min at 4°C. Pellets were resuspended and sonicated. The cytosolic fraction was filtered and saturated with 60% ammonium sulfate (Sigma–Aldrich). The precipitated materials were recovered by centrifugation, solubilized in 20 mM Tris–HCl pH 7.5 and then dialyzed. The protein was further purified by anion exchange chromatography (Bio‐Rad).
BLS (Brucella lumazine synthase)
The cloning, recombinant expression, and purification of BLS were described previously.18, 42 Briefly, the BLS gene cloned in pET11a vector (Novagen) was transformed and expressed as inclusion bodies on E. coli BL21 (DE3) strain. The inclusion bodies were solubilized by overnight agitation in 50 mM Tris–HCl, 5 mM EDTA, 8M urea, pH 8.0 buffer at room temperature. The solubilized material was refolded by dialysis against PBS buffer (20 mM phosphate pH 7.4, 150 mM sodium chloride) containing 1 mM dithiothreitol (DTT). This preparation was purified in a Mono‐Q column in a fast performance liquid chromatography system (Amersham Bioscience, Uppsala, Sweden) using a linear gradient of sodium chloride between 0 and 1M in 50 mM Tris–HCl pH 8.5 buffer. The BLS‐enriched peak was further purified on a Superdex‐200 column by elution with PBS containing 1 mM phenylmethanesulfonyl fluoride or phenylmethylsulfonyl fluoride (PMSF) and 1 mM DTT. The purity of the BLS preparation was determined by SDS–PAGE 15% (w/v).
BLS‐FliC131 fusion protein
To produce the plasmid pFliC‐BLS the FliCΔ202‐369 coding sequence was cloned upstream to the BLS gene in a previously generated pET11a vector containing the BLS sequence (Gen‐Script Corporation). FliC C131 and BLS sequences were linked through a pentapeptide linker of sequence glycine–serine–glycine–serine–glycine–serine. E. coli BL21(DE3) competent cells (Stratagene, La Jolla, CA) were transformed with the expression plasmid mentioned above. A preculture was grown overnight in LB medium with 100 µg/mL ampicillin at 37°C with agitation (200 rev/min) and then diluted to 1:100 and grown to an absorbance (at 600 nm) of 0.6. At this point, IPTG was added to a final concentration of 1 mM and the cultures were further incubated overnight at 20°C with agitation (200 rev/min). The recombinant chimera was purified from inclusion bodies. The inclusion bodies were solubilized by overnight agitation in 50 mM Tris–HCl, 5 mM EDTA, 8M urea, pH 8.0 buffer at room temperature. Afterward, urea was gently removed by stepwise dialysis against Tris–HCl–EDTA buffer containing 4M urea and then against PBS buffer. The solubilized protein was purified by affinity chromatography using monoclonal anti‐flagellin antibodies.28 Briefly, the 2B3C5 antibody was conjugated to an activated resin (NHS‐Activated Sepharose 4 Fast Flow G & E) according to the manufacturer's instructions. The protein was incubated within a TBS buffer (50 mM Tris–HCl pH 8.4, 150 mM sodium chloride) with the resin at 4°C, centrifuged at 2500 rpm for a minute, washed with TBS, eluted with 100 mM glycine pH 3 and neutralized with 1M Tris pH 8.
Lastly, the three proteins were depleted of LPS using a polymyxin B column (Pierce) and dialyzed in PBS buffer and 1 mM PMSF. The quality of the final preparations was checked by SDS–PAGE and UV spectrophotometry. The concentration of the protein was estimated using the absorbance at 280 nm and the molar extinction coefficient calculated from the sequence using the ProtParam tool from the Expasy server (http://web.expasy.org/protparam/).
Measurement of endotoxin activity
The Limulus amebocyte lysate test was performed using the Gel‐clot method for the detection and quantification of Gram‐negative bacterial endotoxins (LPS) (Pyrotell), following the manufacturer's instructions. The detection limit of the assay was 0.03 EU/mL (0.003 ng/mL).
Western blot analysis
Ten microgram of total proteins were separated by 12% SDS–PAGE and transferred to nitrocellulose membrane (Amersham; GE Healthcare). Mouse monoclonal antibody to flagellin (2B3C5) or to BLS were used as the primary antibody;14, 28 and appropriate HRP‐conjugated anti‐mouse (Bio‐Rad) was used as the secondary antibody.
Caco2‐CCL20‐luc reporter cell assays
Caco‐2 cells stably transfected with a luciferase reporter construction under the control of CCL20 promoter (Caco‐2 ccl20:luc) were previously described.26 Confluent Caco‐2 ccl20:luc cells growth on 48‐well plates were treated with equimolar concentrations of FliC C131 (0.7 µg/mL), BLS (0.3 µg/mL), or BLS‐FliC131 (1 µg/mL). After 6 h cells were collected and luciferase activity was evaluated using the Luciferase Assay Kit (Promega, USA) following manufacturer's instructions using a Luminoskan TL Plus luminometer.
Size exclusion chromatography–static light scattering measurements
The average molecular weight (MW) of BLS, FliC C131, and BLS‐BLS‐FliC131 were determined on a Precision Detectors PD2010 90° light scattering instrument tandemly connected to a high‐performance liquid chromatography and a LKB 2142 differential refractometer. The column used was Superose 6 GL 10/300 (GE Healthcare). Five‐hundred microliter of each purified protein were injected into the column, and the chromatographic runs were performed with PBS buffer (20 mM phosphate pH 7.4, 300 mM sodium chloride) under isocratic conditions at a flow rate of 0.4 mL/min at 25°C. The concentration of the injected samples was ∼1 mg/mL. The MW of each sample was calculated relating its 90° and RI signals, using the software Discovery32. BSA (MW: 66.5 kDa) was used as a standard.
Circular dichroism
The circular dichroism (CD) spectra of FliC C131, BLS, and BLS‐FliC131 were recorded in the far UV (200–254 nm) using a Jasco J‐815 spectropolarimeter equipped with a Peltier temperature control system, using a 2 mm path length quartz cell. A scanning speed of 100 nm/min, a response time of 2 s, a data pitch of 0.2 nm, and a band width of 2 nm were set. For each sample, three spectra were recorded and the result presented as the average spectrum to reduce background noise. Data were collected at 25°C in PBS buffer containing 1 mM PMSF. Protein concentration was 15 µM. The samples were incubated for 15 min at 25°C before taking CD measurements. Raw data were converted to molar ellipticity using the following relationship:
where θ is the raw signal in millidegrees, L is the path length in cm, and [M] is the protein concentration in molar units.
Thermal denaturation monitored by CD
The heat‐induced denaturation of FliC C131, BLS, and BLS‐FliC131 in PBS buffer containing 1 mM PMSF was followed by measuring the CD signal at 220 nm on the spectropolarimeter mentioned above at different temperatures. The samples were slowly heated by increasing the temperature with the Peltier system. The range of temperature scanning was 20–100°C at a speed of 4°C/min. The molar ellipticity at 220 nm was measured every 0.1°C. The data was fitted to sigmoidal functions and inflection points were calculated using GraphPad Prism software.
Model of the BLS‐FliC131 structure
A schematic model of the BLS‐FliC131 chimera was performed based on the crystallographic coordinates of BLS, PDB code: 1T13 and the electron microscopy structure of the Salmonella enterica flagellin, PDB code: 3A5X. The model was made by simply putting in close proximity the C‐terminus of the portion of the 3A5X coordinates that correspond to the FliC C131 to the N‐terminal end of the 1T13 coordinates, using the program PyMOL (http://www.pymol.org).
Cell recruitment assays
Bronchoalveolar lavage (BAL) was performed as described.43 Briefly, C57BL/6 mice were stimulated with equimolar amounts of FliC C131 (0.7 µg), BLS (0.3 µg), or BLS‐FliC131 (1 µg) diluted in PBS given by intranasal route. After 6 h post‐treatment, animals were euthanized by intraperitoneal pentobarbital injection and the thoracic cavity was dissected, the blood in the lungs was cleared by perfusing PBS at room temperature through the heart's right lobe until the lungs became pale. To perform the BAL, the trachea was partially cut and 1 mL of sterile PBS containing 0.1% BSA was flushed into the lungs and then withdrawn. This procedure was repeated 3 times.
For analysis of cell recruitment to the abdominal cavity, animals were treated i.p. with equimolar amounts of FliC C131 (0.7 µg), BLS (0.3 µg), or BLS‐FliC131 (1 µg) diluted in PBS. After 6 h, peritoneal fluid was obtained from mice by peritoneal wash and centrifuged at 450g for 10 min. The supernatant fluid was discarded and pelleted cells were analyzed by flow cytometry.
Cells from the BAL or peritoneal wash were stained with fluorescent antibodies for 1 h at 4°C, followed by flow cytometry analysis using a FACSCalibur from Becton Dickinson. The FITC‐, PE‐, APC‐, and PerCP‐conjugated monoclonal‐specific antibodies for CD11c (clone N418, hamster IgG: eBioscience, San Diego, CA), Gr1 (clone RB6‐8C5, Rat IgG2b: eBioscience), CD11b (clone M1/70.15, Rat IgG2b: Caltag Laboratories, CA), Ly‐6G (clone 1A8, Rat IgG2a: BD Pharmingen) and Ly6C (AL‐21, Rat IgM: BD Pharmingen) were used to label the cells. Polymorphonuclear cells were identified as CD11c‐CD11b + Ly6G+ GR1+ cells.
Serum cytokine analysis
Female C57BL/6J (6–8 weeks old) mice were obtained from Janvier laboratories (St. Berthevin, France). TLR424 and TLR544 knockout mice were maintained in a specific pathogen‐free facility (Leloir Institute and Institut Pasteur de Lille, respectively). Equimolar amounts of FliC C131 (0.7 µg), BLS (0.3 µg), or BLS‐FliC131 (1 µg) diluted in PBS were given by the intravenous route. LPS (1 µg) from E. coli (serotype 0111:B4, Invivogen) or PBS were used as control. Blood samples were collected 2 h after treatment and clotted at room temperature, with the serum then being separated by centrifugation. All samples were stored at −80°C prior to analysis. IL‐6, IL‐22, and CCL20 concentration in serum were measured using ELISA kits (eBioscience) following manufacturer's instructions. All experiments complied with current national and institutional regulations and ethical guidelines (B59‐350009, Institut Pasteur de Lille).
Immunization and anti‐BLS antibody determination
C57BL/6J mice were vaccinated i.p. on Days 1 and 21 with LPS‐depleted BLS (0.3 µg) ± LPS‐depleted FliC C131 (0.7 µg) or with LPS‐depleted BLS‐FliC131 fusion protein (1 µg). At Day 30, blood was extracted from the submandibular sinus and anti‐BLS antibodies were determined by indirect ELISA in serum as previously described.16
Statistical analysis
Results were expressed as the mean + SD. Levels of significance were determined using two‐tailed Student's t test, and a confidence level of greater than 95% (P < 0.05) was used to establish statistical significance.
Acknowledgments
M.E.B., Y.I., and A.H.R. had fellowships from Argentinian National Research Council (CONICET), P.B., G.M., F.G., M.R., and A.J.E. are members of CONICET. A.E. received a Bernardo Houssay fellowship from CONICET. The work was supported by grants from Agencia Nacional de Promoción Científica y Tecnológica (ANPCYT), INSERM, CNRS, Institut Pasteur de Lille, Université de Lille, the Argentinian Ministry of Science, Technology and Innovation, and the French Ministry for Research and Higher Education (grant: ECOS A12B03).
P. Berguer and M. Rumbo contributed equally to this work.
Y. Hiriart, A.H. Rossi and M.E. Biedma are co‐first authors.
References
- 1. Berzofsky JA, Ahlers JD, Belyakov IM (2001) Strategies for designing and optimizing new generation vaccines. Nat Rev Immunol 1:209–219. [DOI] [PubMed] [Google Scholar]
- 2. Plotkin SA (2009) Vaccines: the fourth century. Clin Vaccine Immunol 16:1709–1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rothbard JB (1992) Synthetic peptides as vaccines. Biotechnology 20:451–465. [DOI] [PubMed] [Google Scholar]
- 4. Monie TP, Bryant CE, Gay NJ (2009) Activating immunity: lessons from the TLRs and NLRs. Trends Biochem Sci 34:553–561. [DOI] [PubMed] [Google Scholar]
- 5. Rumbo M, Nempont C, Kraehenbuhl JP, Sirard JC (2006) Mucosal interplay among commensal and pathogenic bacteria: lessons from flagellin and Toll‐like receptor 5. FEBS Lett 580:2976–2984. [DOI] [PubMed] [Google Scholar]
- 6. Miao EA, Andersen‐Nissen E, Warren SE, Aderem A (2007) TLR5 and Ipaf: Dual sensors of bacterial flagellin in the innate immune system. Semin Immunopathol 29:275–288. [DOI] [PubMed] [Google Scholar]
- 7. Mizel SB, Bates JT (2010) Flagellin as an adjuvant: cellular mechanisms and potential. J Immunol 185:5677–5682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Huleatt JW, Jacobs AR, Tang J, Desai P, Kopp EB, Huang Y, Song L, Nakaar V, Powell TJ (2007) Vaccination with recombinant fusion proteins incorporating Toll‐like receptor ligands induces rapid cellular and humoral immunity. Vaccine 25:763–775. [DOI] [PubMed] [Google Scholar]
- 9. Mizel SB, Graff AH, Sriranganathan N, Ervin S, Lees CJ, Lively MO, Hantgan RR, Thomas MJ, Wood J, Bell B (2009) Flagellin‐F1‐V fusion protein is an effective plague vaccine in mice and two species of nonhuman primates. Clin Vaccine Immunol 16:21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ghose C, Verhagen JM, Chen X, Yu J, Huang Y, Chenesseau O, Kelly CP, Ho DD (2013) Toll‐like receptor 5‐dependent immunogenicity and protective efficacy of a recombinant fusion protein vaccine containing the nontoxic domains of Clostridium difficile toxins A and B and Salmonella enterica serovar typhimurium flagellin in a mouse model of Clostridium difficile disease. Infect Immun 81:2190–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Leal MT, Camacho AG, Teixeira LH, Bargieri DY, Soares IS, Tararam CA, Rodrigues MM (2013) Immunogenicity of recombinant proteins consisting of Plasmodium vivax circumsporozoite protein allelic variant‐derived epitopes fused with Salmonella enterica Serovar Typhimurium flagellin. Clin Vaccine Immunol 20:1418–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Stepanova LA, Kotlyarov RY, Kovaleva AA, Potapchuk MV, Korotkov AV, Sergeeva MV, Kasianenko MA, Kuprianov VV, Ravin NV, Tsybalova LM, Skryabin KG, Kiselev, OI (2015) Protection against multiple influenza A virus strains induced by candidate recombinant vaccine based on heterologous M2e peptides linked to flagellin. PLoS One 10:e0119520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Samatey FA, Imada K, Nagashima S, Vonderviszt F, Kumasaka T, Yamamoto M, Namba K (2001) Structure of the bacterial flagellar protofilament and implications for a switch for supercoiling. Nature 410:331–337. [DOI] [PubMed] [Google Scholar]
- 14. Goldbaum FA, Leoni J, Wallach JC, Fossati CA (1993) Characterization of an 18‐kilodalton Brucella cytoplasmic protein which appears to be a serological marker of active infection of both human and bovine brucellosis. J Clin Microbiol 31:2141–2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Baldi PC, Wanke MM, Loza ME, Monachesi N, Fossati CA (1997) Diagnosis of canine brucellosis by detection of serum antibodies against an 18 kDa cytoplasmic protein of Brucella spp. Vet Microbiol 57:273–281. [DOI] [PubMed] [Google Scholar]
- 16. Velikovsky CA, Goldbaum FA, Cassataro J, Estein S, Bowden RA, Bruno L, Fossati CA, Giambartolomei GH (2003) Brucella lumazine synthase elicits a mixed Th1‐Th2 immune response and reduces infection in mice challenged with Brucella abortus 544 independently of the adjuvant formulation used. Infect Immun 71:5750–5755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rosas G, Fragoso G, Ainciart N, Esquivel‐Guadarrama F, Santana A, Bobes RJ, Ramirez‐Pliego O, Toledo A, Cruz‐Revilla C, Meneses G, Berguer P, Goldbaum FA, Sciutto E (2006) Brucella spp. lumazine synthase: a novel adjuvant and antigen delivery system to effectively induce oral immunity. Microbes Infect 8:1277–1286. [DOI] [PubMed] [Google Scholar]
- 18. Laplagne DA, Zylberman V, Ainciart N, Steward MW, Sciutto E, Fossati CA, Goldbaum FA (2004) Engineering of a polymeric bacterial protein as a scaffold for the multiple display of peptides. Proteins 57:820–828. [DOI] [PubMed] [Google Scholar]
- 19. Craig PO, Berguer PM, Ainciart N, Zylberman V, Thomas MG, Martinez Tosar LJ, Bulloj A, Boccaccio GL, Goldbaum FA (2005) Multiple display of a protein domain on a bacterial polymeric scaffold. Proteins 61:1089–1100. [DOI] [PubMed] [Google Scholar]
- 20. Zylberman V, Craig PO, Klinke S, Braden BC, Cauerhff A, Goldbaum FA (2004) High order quaternary arrangement confers increased structural stability to Brucella sp. lumazine synthase. J Biol Chem 279:8093–8101. [DOI] [PubMed] [Google Scholar]
- 21. Alvarez P, Zylberman V, Ghersi G, Boado L, Palacios C, Goldbaum F, Mattion N (2013) Tandem repeats of the extracellular domain of Matrix 2 influenza protein exposed in Brucella lumazine synthase decameric carrier molecule induce protection in mice. Vaccine 31:806–812. [DOI] [PubMed] [Google Scholar]
- 22. Berguer PM, Alzogaray VA, Rossi AH, Mundinano J, Piazzon I, Goldbaum FA (2012) A polymeric protein induces specific cytotoxicity in a TLR4 dependent manner in the absence of adjuvants. PLoS One 7:e45705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Berguer PM, Mundinano J, Piazzon I, Goldbaum FA (2006) A polymeric bacterial protein activates dendritic cells via TLR4. J Immunol 176:2366–2372. [DOI] [PubMed] [Google Scholar]
- 24. Rossi AH, Farias A, Fernandez JE, Bonomi HR, Goldbaum FA, Berguer PM (2015) Brucella spp. lumazine synthase induces a TLR4‐mediated protective response against B16 melanoma in mice. PLoS One 10:e0126827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Craig PO, Alzogaray V, Goldbaum FA (2012) Polymeric display of proteins through high affinity leucine zipper peptide adaptors. Biomacromolecules 13:1112–1121. [DOI] [PubMed] [Google Scholar]
- 26. Nempont C, Cayet D, Rumbo M, Bompard C, Villeret V, Sirard JC (2008) Deletion of flagellin's hypervariable region abrogates antibody‐mediated neutralization and systemic activation of TLR5‐dependent immunity. J Immunol 181:2036–2043. [DOI] [PubMed] [Google Scholar]
- 27. Malapaka RR, Adebayo LO, Tripp BC (2007) A deletion variant study of the functional role of the Salmonella flagellin hypervariable domain region in motility. J Mol Biol 365:1102–1116. [DOI] [PubMed] [Google Scholar]
- 28. Hiriart Y, Serradell M, Martinez A, Sampaolesi S, Maciel DG, Chabalgoity JA, Yim L, Algorta G, Rumbo M (2013) Generation and selection of anti‐flagellin monoclonal antibodies useful for serotyping Salmonella enterica . Springerplus 2:640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Romanin D, Serradell M, Gonzalez Maciel D, Lausada N, Garrote GL, Rumbo M (2010) Down‐regulation of intestinal epithelial innate response by probiotic yeasts isolated from kefir. Int J Food Microbiol 140:102–108. [DOI] [PubMed] [Google Scholar]
- 30. Affo S, Morales‐Ibanez O, Rodrigo‐Torres D, Altamirano J, Blaya D, Dapito DH, Millan C, Coll M, Caviglia JM, Arroyo V, Caballería J, Schwabe RF, Ginès P, Bataller R, Sancho‐Bru P (2014) CCL20 mediates lipopolysaccharide induced liver injury and is a potential driver of inflammation and fibrosis in alcoholic hepatitis. Gut 63:1782–1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Van Maele L, Carnoy C, Cayet D, Songhet P, Dumoutier L, Ferrero I, Janot L, Erard F, Bertout J, Leger H, Sebbane F, Benecke A, Renauld JC, Hardt WD, Ryffel B, Sirard JC (2010) TLR5 signaling stimulates the innate production of IL‐17 and IL‐22 by CD3(neg)CD127+ immune cells in spleen and mucosa. J Immunol 185:1177–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sciutto E, Toledo A, Cruz C, Rosas G, Meneses G, Laplagne D, Ainciart N, Cervantes J, Fragoso G, Goldbaum FA (2005) Brucella spp. lumazine synthase: a novel antigen delivery system. Vaccine 23:2784–2790. [DOI] [PubMed] [Google Scholar]
- 33. Bellido D, Craig PO, Mozgovoj MV, Gonzalez DD, Wigdorovitz A, Goldbaum FA, Dus Santos MJ (2009) Brucella spp. lumazine synthase as a bovine rotavirus antigen delivery system. Vaccine 27:136–145. [DOI] [PubMed] [Google Scholar]
- 34. Mejias MP, Cabrera G, Fernandez‐Brando RJ, Baschkier A, Ghersi G, Abrey‐Recalde MJ, Miliwebsky E, Meiss R, Goldbaum F, Zylberman V, Rivas M, Palermo MS (2014) Protection of mice against Shiga toxin 2 (Stx2)‐associated damage by maternal immunization with a Brucella lumazine synthase‐Stx2 B subunit chimera. Infect Immun 82:1491–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mejias MP, Ghersi G, Craig PO, Panek CA, Bentancor LV, Baschkier A, Goldbaum FA, Zylberman V, Palermo MS (2013) Immunization with a chimera consisting of the B subunit of Shiga toxin type 2 and Brucella lumazine synthase confers total protection against Shiga toxins in mice. J Immunol 191:2403–2411. [DOI] [PubMed] [Google Scholar]
- 36. Ivicak‐Kocjan K, Panter G, Bencina M, Jerala R (2013) Determination of the physiological 2:2 TLR5:flagellin activation stoichiometry revealed by the activity of a fusion receptor. Biochem Biophys Res Commun 435:40–45. [DOI] [PubMed] [Google Scholar]
- 37. Yoon SI, Kurnasov O, Natarajan V, Hong M, Gudkov AV, Osterman AL, Wilson IA (2012) Structural basis of TLR5‐flagellin recognition and signaling. Science 335:859–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Schulke S, Burggraf M, Waibler Z, Wangorsch A, Wolfheimer S, Kalinke U, Vieths S, Toda M, Scheurer S (2011) A fusion protein of flagellin and ovalbumin suppresses the TH2 response and prevents murine intestinal allergy. J Allergy Clin Immunol 128:1340–1348. [DOI] [PubMed] [Google Scholar]
- 39. Karam MR, Oloomi M, Mahdavi M, Habibi M, Bouzari S (2013) Assessment of immune responses of the flagellin (FliC) fused to FimH adhesin of Uropathogenic Escherichia coli . Mol Immunol 54:32–39. [DOI] [PubMed] [Google Scholar]
- 40. Asadi Karam MR, Oloomi M, Mahdavi M, Habibi M, Bouzari S (2013) Vaccination with recombinant FimH fused with flagellin enhances cellular and humoral immunity against urinary tract infection in mice. Vaccine 31:1210–1216. [DOI] [PubMed] [Google Scholar]
- 41. Khan S, Bijker MS, Weterings JJ, Tanke HJ, Adema GJ, van Hall T, Drijfhout JW, Melief CJ, Overkleeft HS, van der Marel GA, Filippov DV, van der Burg SH, Ossendorp F (2007) Distinct uptake mechanisms but similar intracellular processing of two different toll‐like receptor ligand‐peptide conjugates in dendritic cells. J Biol Chem 282:21145–21159. [DOI] [PubMed] [Google Scholar]
- 42. Goldbaum FA, Velikovsky CA, Baldi PC, Mortl S, Bacher A, Fossati CA (1999) The 18‐kDa cytoplasmic protein of Brucella species—an antigen useful for diagnosis—is a lumazine synthase. J Med Microbiol 48:833–839. [DOI] [PubMed] [Google Scholar]
- 43. Moreno G, Errea A, Van Maele L, Roberts R, Leger H, Sirard JC, Benecke A, Rumbo M, Hozbor D (2013) Toll‐like receptor 4 orchestrates neutrophil recruitment into airways during the first hours of Bordetella pertussis infection. Microbes Infect 15:708–718. [DOI] [PubMed] [Google Scholar]
- 44. Uematsu S, Jang MH, Chevrier N, Guo Z, Kumagai Y, Yamamoto M, Kato H, Sougawa N, Matsui H, Kuwata H, Hemmi H, Coban C, Kawai T, Ishii KJ, Takeuchi O, Miyasaka M, Takeda K, Akira S (2006) Detection of pathogenic intestinal bacteria by Toll‐like receptor 5 on intestinal CD11c+ lamina propria cells. Nat Immunol 7:868–874. [DOI] [PubMed] [Google Scholar]


