Abstract
Single domain antibodies (sdAbs) from camels or sharks comprise only the variable heavy chain domain. Human sdAbs comprise the variable domain of the heavy chain (VH) or light chain (VL) and can be selected from human antibodies. SdAbs are stable, nonaggregating molecules in vitro and in vivo compared to complete antibodies and scFv fragments. They are excellent novel inhibitors of cytosolic/nuclear proteins because they are correctly folded inside the cytosol in contrast to scFv fragments. SdAbs are unique because of their excellent specificity and possibility to target posttranslational modifications such as phosphorylation sites, conformers or interaction regions of proteins that cannot be targeted with genetic knockout techniques and are impossible to knockdown with RNAi. The number of inhibiting cytosolic/nuclear sdAbs is increasing and usage of synthetic single pot single domain antibody libraries will boost the generation of these fascinating molecules without the need of immunization. The most frequently selected antigenic epitopes belong to viral and oncogenic proteins, followed by toxins, proteins of the nervous system as well as plant‐ and drosophila proteins. It is now possible to select functional sdAbs against virtually every cytosolic/nuclear protein and desired epitope. The development of new endosomal escape protein domains and cell‐penetrating peptides for efficient transfection broaden the application of inhibiting sdAbs. Last but not least, the generation of relatively new cell‐specific nanoparticles such as polymersomes and polyplexes carrying cytosolic/nuclear sdAb‐DNA or –protein will pave the way to apply cytosolic/nuclear sdAbs for inhibition of viral infection and cancer in the clinic.
Keywords: intrabodies, single domain antibodies, scFv fragment, cytosolic/nuclear intrabodies, camelid VHHs, shark vNARs, human VH, human VL
Abbreviations
- Nb
nanobody
- NLS
nucleus leader sequence
- sdAb
single domain antibody
- vNAR
variable domain of the new antigen antigen receptor (IgNAR) from sharks.
Introduction
New insights into the function of proteins can be obtained with nucleotide‐based knockout and knockdown methods at the DNA or mRNA level respectively. Knockdown and knockout methods are essential to analyze the function of proteins.
Knockdown of proteins complement these methods and is essential if post‐translational modifications, conformations and interaction regions of proteins have to be studied. Regarding knockdown at the mRNA level this cannot be achieved with siRNA technology. Today, protein inhibitors are dominant negative mutants, small molecule inhibitors including peptides, allosteric modulators, short single‐stranded DNA or RNA oligonucleotides (aptamers, intramers), neutralizing antibodies and intrabodies and nonimmunoglobulin binder scaffolds such as cysteine‐knot proteins, adnectins, anticalins, affibodies, and DARPins.1, 2
Another class of promising knockdown molecules recently gaining importance are intracellular antibodies (intrabodies), i.e. antibodies that are expressed within the target cell that contains the corresponding antigen. Intrabodies are recombinant antibody fragments and the main advantages are their high specificity such that post‐translational modifications, interaction regions, conformers, splice variants and isoforms can be targeted without off target effects. Long lasting functional inhibition of intracellular proteins can be achieved, which is not possible with classical neutralizing antibodies. Intrabodies can be specifically transported to the cytosol, nucleus or ER to bind their antigens at specific loci only (Fig. 1).
Figure 1.
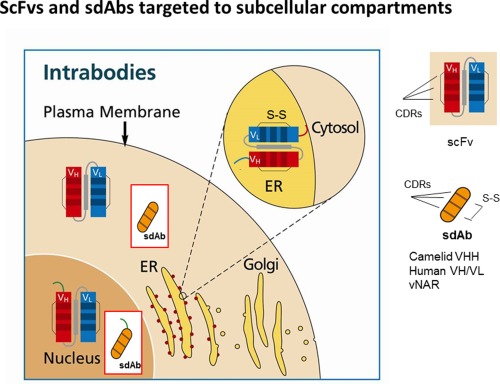
ScFvs and sdAbs targeted to subcellular compartments. Intrabodies can be targeted to the ER, mainly as scFv fragment, where they are retained by the KDEL sequence (red line). Binding of the specific antigen will lead to retention of the antigen‐ER‐intrabody complex inside the ER (not shown). Alternatively, scFvs or sdAbs can be targeted to the nucleus or cytosol via a nucleus leader peptide (green line) or without leader sequence, respectively. Complementarity‐determing regions are shown by lines. sdAbs from shark have only CDR1 and CDR3.
The development of cytosolic/nuclear intrabodies was hampered by the fact that intrabodies in the scFv and Fab format cannot form disulfide bridges within the reducing environment of the cytosol and are therefore unstable.3, 4
Specific strategies were developed such as the yeast two‐hybrid system5 to express functional cytosolic/nuclear scFv intrabodies and usage of these methods led to some remarkable functional cytosolic/nuclear scFv intrabodies.6 A breakthrough in antibody engineering was the discovery of stable expressed cytosolic/nuclear sdAbs derived from camel or shark antibodies comprising only the variable domain of the heavy chain. The variable domain of camelid heavy chain antibodies is called VHH, the variable domain of the new antigen receptor (IgNAR) from sharks vNAR. Camelid VHHs recognizing their antigens are called nanobodies.
Both single domain antibodies derived from camels or sharks constitute intact functional antigen‐binding sites, the single domain VHH domain with 3 complementarity determining CDR regions7 and the vNAR domain with CDR1, CDR3 and HV2 and HV4.8, 9, 10 Camelid and shark single domain antibodies possess good solubility, high thermal stability and refolding capability.7, 10, 11 In addition, stable human VH and VL domains can be constructed from human antibodies after framework mutations and randomization of CDRs.12, 13, 14, 15, 16, 17, 18 Camelid nanobodies can be stably expressed inside the cytoplasm even if the disulfide bridge is not formed.2, 6 Folded nanobodies can find their antigen and are then stabilized by binding to the antigen.
As an analytical tool camelid antibodies have been used to illuminate in vivo trafficking of target molecules, to detect conformational states and post‐translational modification of proteins. In addition, they have been employed as chaperons for crystallization in cases where unstable proteins are sensitive to degradation, unfolding or aggregation.19, 20, 21, 22, 23
In general nanobodies are selected from naïve, immune, synthetic camelid VHH single domain antibody repertoires24, 25, 26, 27 whereas shark sdAbs and human VH and VL sdAbs are mainly selected from synthetic antibody libraries.12, 13, 14, 15, 16, 17, 18, 28, 29, 30, 31, 32, 33, 34
Antibody fragments are displayed on filamentous phages or yeast. Selected sdAbs can then be converted to cytosolic/nuclear intrabodies with a single cloning step (Figs. 2 and 3). In particular, universal synthetic nanobody libraries are very valuable because no immunization with the target antigen is needed. Low affinity binders can be affinity‐maturated by introducing CDR mutations, randomization of mutational hotspots or by error prone PCR.35, 36, 37
Figure 2.
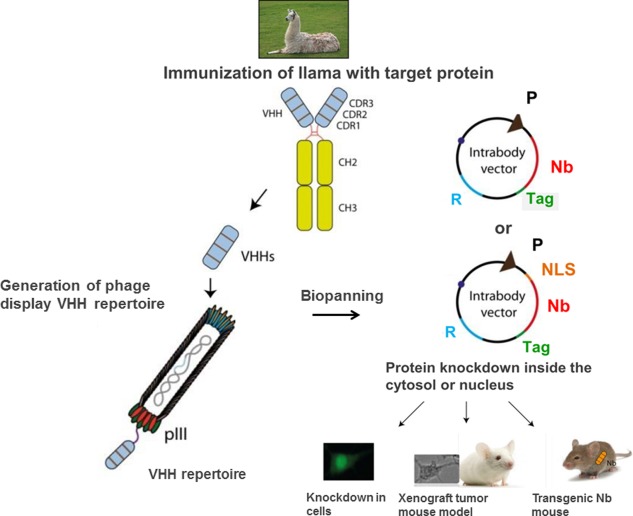
Construction and application of cytosolic/nuclear camelid nanobodies. Until now, most cytosolic camelid nanobodies have been constructed after immunization of camels. The VHH domains will be amplified from the cDNA isolated from the peripheral blood lymphocytes of immunized camels. Then a phage display repertoire is generated to display the VHH repertoire on the surface. Biopanning with the antigen will deliver specific recombinant nanobody phages from which the genes can be cloned into an appropriate targeting vector for expression in the cytosol or nucleus. The function of the intrabody can be tested in cell culture or applied in a xenograft tumor mouse model. Alternatively, a transgenic mouse expressing the nanobody can be generated. (P, promoter; R, resistant gene; Nb, nanobody; NLS, nucleus leader sequence).
Figure 3.
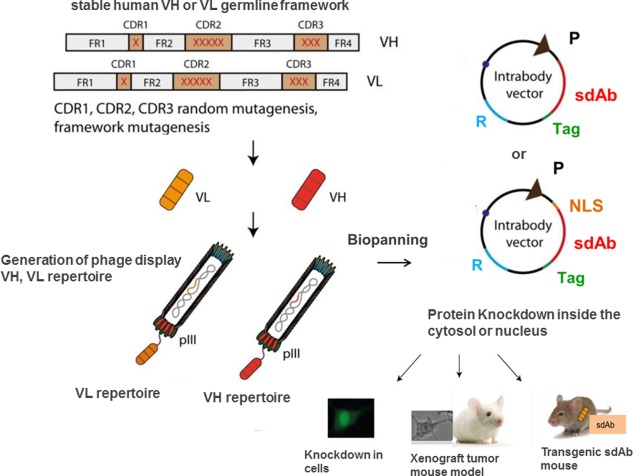
Construction and application of cytosolic/nuclear human VH and VL domains. Synthetic human VH/VL libraries are constructed from a stable human VH or VL germline framework or from a VH/VL scaffold of an existing antibody. After randomization of the CDRs (mainly CDR3) and possible introduction of framework mutations, human VH and VL are displayed on phages. Specific sdAbs are selected by biopanning and the sdAb‐coding region is subsequently cloned into a targeting vector for expression inside the cytosol or nucleus. In vivo application follows. (P, promoter; R, resistant gene; NLS, nucleus leader sequence; sdAb, single domain antibody).
The number of available inhibiting nanobodies against different cytosolic/nuclear proteins is increasing. So far most cytosolic/nuclear single domain intrabodies have been derived from camels, followed by some single domains VH and VL of human origin (Table 1).
Table 1.
Reports of Cytosolic/nuclear sdAb Mediated Knockdown
| Target | Source | Characterization, cells and mice | Physiological knockdown readout | Ref. |
|---|---|---|---|---|
| Viral Proteins | ||||
| HIV Rev | Llama immunized with recombinant HIV Rev. |
HeLa cells and human embryonal kidney 293T cells were cotransfected with Rev‐GFP and intrabody‐expression plasmid. Localization of REV‐GFP and intrabody was determined by immunofluorescence. Cells stable expressing intrabody. |
Intrabody interferes with Rev multimerization and inhibits HIV‐1 production inside cells. Suppression of the replication of different HIV subtypes. |
Vercruysse et al.38
Boons et al.39 |
| HIV Vpr | Llama immunized with synthetic Vpr peptide, selection of nanobodies by cytoplasmic yeast two‐hybrid system. | Yeast cells co‐transfected with intrabody and HA‐Vpr‐ expression plasmids. Analyzation of the localization of Vpr by immunofluorescence. | Delocalization of Vpr from the nucleus to the cytoplasm. | Matz et al.40 |
| HIV Vif |
Rabbit immunized with HIV‐1 Vif protein. Construction of camelized rabbit‐derived VH single domain from anti‐HIV‐1 scFv. |
Permissive Jurkat and non‐permissive H9 cells transfected with recombinant HIV‐1 virus encoding single domain intrabody. HIV‐1 GAG p24 levels were determined by ELISA. | Inhibition of HIV‐1 replication in non‐permissive H9 cells and reduction of viral infectivity. | Aires da Silva et al.41 |
| HIV‐1 Nef | Llama immunized with purified recombinant Nef protein. |
HPB‐ALL CD4+ T cells cotransfected with Nef‐GFP and intrabody ‐expression plasmid. Cell surface expression of CD4 was determined by flow cytometry. In vivo: fetal liver cells from CD4+/HIVNEF transgenic mice retroviral infected with nanobody were injected into lethally irradiated host animals. Analysis of CD4+ T‐cell function. |
Intrabody inhibits Nef‐induced down‐regulation and endocytosis of CD4. Intrabody was able to rescue Nef‐mediated thymic CD4+ T‐cell maturation defect and peripheral CD4+ T‐cell activation phenotypes of the transgenic mouse model. |
Bouchet et al.42 |
| HIV‐1 Nef | Nanobody against HIV‐1 Nef was modified by fusion of a Nef SH3 binding peptide. | HPB‐ALL T cells and RAW264.7 Macrophages transiently transfected with Nef‐GFP and modified intrabodies (Neffins). | Neffins inhibit Nef‐induced MHC‐I and CD4 downregulation in T cells and restores efficient phagocytosis. Down regulation of MHC I were more efficiently inhibited by Neffins compared to the parental intrabodies. | Bouchet et al.43 |
| Influenza virus Nucleoprotein (NP) | Llama immunized with alcohol‐fixed influenza virus PR8. | MDCK cells stable transfected with intrabody expression plasmid were infected with influence virus and nuclear localization of viral ribonucleoprotein complex analyzed. | Inhibition of nuclear import of incoming viral ribonucleoprotein complex. | Ashour et al.44 |
|
HCV NSB4 |
Usage of a naïve camelid VH/VHH phage display antibody repertoire. Biopanning was performed with recombinant NSB4. | Analysis of intrabody mediated inhibition of HCV replication using Huh7 cells electroporated with HCV RNA and transfected with VH/VHH fused with penetratin. | 4 clones inhibited HCV replication. | Glab‐Ampai et al.45 |
|
HCV NS3 helicase |
Usage of a naïve camelid VH/VHH phage display antibody repertoire. Biopanning was performed with C‐terminal NS3 helicase. VH/VHH gene sequences were fused to cell penetrating peptide penetratin. | Analysis of intrabody mediated inhibition of HCV replication performed as by Glab‐Ampai et al.45 | VH/VHH clones inhibited HCV replication by interfering with the virus helicase activity. | Phalaphol et al.46 |
| HCV RNA dependent RNA polymerase NS5B | Usage of a naïve camelid VH/VHH phage display antibody repertoire. Biopanning was performed with recombinant NS5B. VH/VHH gene sequences were fused to cell penetrating peptide penetratin. | Analysis of intrabody mediated inhibition of HCV replication performed as by K. Glab‐ampai. | 4 clones inhibited HCV replication. | Thueng‐in et al.47 |
|
HCV Core antigen |
Nanobodies were constructed from a mouse mAb. | Analysis of the HCV titer in HCV and recombinant intrabody adenovirus transfected CD81‐defective Huh7‐25 cells. | Intrabody expression reduced intracellular and extracellular infectious titres. Furthermore intrabody expression suppressed HCV‐core induced NFκB promoter activity. | Suzuki et al.48 |
|
Hepatitis B Core antigen (HBcAg) |
Llama immunized with recombinant HBcAg. | Hepatocyte cell line HepG2 transfected with intrabody and HBcAg expression plasmid. Localization of HBcAg was determined by immunofluorescence. | Cotransfection with the intrabody directed to the nucleus showed a speckled HBcAg pattern demonstrating that nuclear nanobodies interferes with HBcAg trafficking. | Serruys et al.49 |
|
Porcine reproductive and respiratory syndrome virus (PRRSV) Nonstructural protein 9 (Nsp9) |
Llama immunized with Nsp9. | Monkey kidney MARC‐145 cells stable expressing nanobody were infected with PRRSV. | Nanobody protected cells from virus‐induced cytopathic effect and fully block PRRSV replication. | Liu et al.50 |
|
Porcine reproductive and respiratory syndrome virus Nsp4 |
Llama immunized with Nsp4. | MARC‐145 cells stable expressing nanobody‐EGFP fusionprotein infected with PRRSV. | Nanobodies inhibit PRRSV replication. | Liu et al.51 |
|
Porcine endogenous retrovirus p15 Matrix Protein |
Llama immunized with recombinant p15 Matrix protein. | PK 15 cells for production of porcine endogenous retrovirus PERV‐A/B transfected with nanobody. | PERV‐A/B Virus production is blocked upon expression of intrabody. | Dekker et al.52 |
| Toxins | ||||
| SpvB toxin of Salmonella typhimurium | Llama immunized with SpvB Toxin of Salmonella typhimurium. | Raw 264.7 macrophages transiently transfected with intrabody were infected with Salmonella typhimurium. Vero cells transiently transfected with nanobody were infected with binary chimeric C2IN‐C/SpvB fusionprotein. Cytopathic effects were analyzed by immunofluorescence. | Single domain intrabody protect cells from cytopathic effects of Salmonella infection. | Alzogaray et al.53 |
| Clostridium botulinum neurotoxin protease | Llama immunized with recombinant Clostridium botulinum neurotoxin A and B proteases. | Neuronal cells, transfected with nanobody recognizing neurotoxin A protease was infected with botulinum neurotoxin A. Cleavage of SNAP25 was analyzed by Western Blot. | Cleavage of endogenous synaptic protein SNAP25 was inhibited. | Tremblay et al.54 |
| Mycotoxin 15‐Acetyldeoxynivalenol (15‐AcDON) | Llama immunized with 15‐AcDON. | P. pastoris transformed with nanobody and cultured with Mycotoxin 15‐Acetyldeoxynivalenol. Analysis of the influence of Nb on cytotoxicity. | Intracellular expression of mycotoxin‐specific VHH in P. pastoris causes significant resistance to 15‐AcDON. | Doyle et al.55 |
| Oncogenic Proteins | ||||
| C‐MYC | Human VH libraries with randomized CDR3. Human VHs were selected using the yeast and mammalian two hybrid system. | Affinity maturation was achieved by additional stepwise randomization of CDR2 and CDR1. | Isolation of human single domain intrabodies recognizing the basic‐helix‐loop‐helix and leucine zipper region of CMYC. | Zeng et al.56 |
| LIM domain only 2 (LMO2) | Human single domain VH and VL library with randomized CDR3 regions generated from two human scFv genes recognizing the RAS oncogenic protein. Selection of appropriate single domain antibody using yeast two hybrid system. | In vivo: Neoplastic T cells from a LcK‐Lmo2 transgenic mouse thymoma were transfected with retrovirus‐VH and transplanted into nude mice. The amount of retrovirus comprising reporter GFP transfected T‐cells was compared with retrovirus comprising reporter GFP and single domain intrabody transfected cells. | Anti‐LMO2 VH inhibits T‐cell neoplasia in a nude mouse. LMO2 is necessary for T‐cell tumorigenesis. | Tanaka et al.57 |
| Endothelial and epithelial kinase (Etk) | Human single domain VL library (Domantis) derived from a single human framework of a ĸ light chain variable region. Complete randomization at 13 residues of the CDR1 ‐CDR3 was performed. | NSR cells (mouse NIH3T3 cells overexpressing v‐SRC) were transfected with single domain intrabodies and inhibition of the function of endogenous Etk assayed with [ϒ‐33P] ATP. Analyzing inhibition of cellular transformation by estimating colony formation of intrabody transfected NSR cells. | Significant inhibition of Etk function and cellular transformation by single domain intrabodies. | Paz et al.58 |
| Protein kinase Cɛ | Camels were immunized with the kinase Cɛ. | One inhibitory VHH domain with a mCherry tag was cotransfected with PKCɛ‐EGFP in HeLa cells and the translocation of the kinase to the cell membrane tested. | The inhibitory VHH inhibits PKCɛ translocation from the cytosol to the cell membrane. | Summanen et al.59 |
| F‐actin capping protein CapG | Llama immunized with human recombinant CapG. |
MDA‐MB‐231 breast tumor cells transfected with intrabodies. Analysis of the inhibition of migration, matrigel invasion, multinucleation. In vivo: Breast tumor metastasis mouse model with immunodeficient NOD‐SCID mice and MDA‐MB‐231 cells stable expressing single domain antibody GFP fusionprotein and luciferase reporter. |
Significant inhibition of migration, matrigel invasion, multinucleation. Significant inhibition of lungmetastasis in immunodeficient NOD‐SCID mice. |
Van Impe et al.60 |
| RAS | Human single domain VH library was constructed from a consensus framework sequence of an anti‐RAS scFv fragment. After randomization of the CDRs specific single domain VHs were selected using the yeast two hybrid system with mutated oncogenic RAS as antigen. |
RAS transformed mouse NIH3T3 cells, RAS transformed human fibrosarcoma and RAS transformed human colorectal adenocarcinoma cells. Cells were lentiviral transfected with a selected nanobody. In vivo: Lung metastasis tumor mouse model. RAS transformed cells retroviral stable transfected with selected nanobody were injected subcutaneously into nude mice and tumor size was analyzed. |
Nanobody reverts the RAS transformed phenotype of the cells. Furthermore it was shown that the nanobody prevents effector molecules binding to RAS. The variable domain binds to the regions where the effector ligands bind, demonstrated with the crystal structure of antibody‐RAS. Intrabody inhibits tumorigenesis and metastasis of RAS transformed cells in mice. |
Tanaka et al.61 |
| Caspase‐3 | VHH library derived from non‐immunized llamas. Selection of nanobodies against recombinant purified Caspase‐3. |
SHSY‐5Y cells transiently transfected with VHH antibodies. After induction of oxidative stress with H2O2 the influence of the VHHs on apoptosis was analyzed microscopically. Activity assays with purified caspase‐3 in the presence of purified VHHs. |
Two VHHs were selected. One VHH inhibit apoptosis and activity of caspase‐3, the other clone was an antagonist of apoptosis. | McGonical et al.62 |
| Heterogenous nuclear ribonucleoprotein K (hnRNP‐K) | Construction of a VHH library with randomized CDR 3 starting from a VHH recognizing hen egg white lysozyme. The aim was to use the library to identify targets involved in metastasis. | Establishment of a loss of cell migration screening procedure: (1) transfection of VHH library into metastatic human fibrosarcoma HT1080 cells, (2) separation of non‐migrating cells from migrating cells, (3) isolation of antibody plasmids from non‐migrating cells and transformation in E.coli. |
After repeating the screening procedure three times one clone was selected that inhibits cell migration of HT 1080 cells. Further it was shown that the targeted protein is hnRNP‐K. Nanobody lead to cytoplasmic accumulation of the protein. |
Inoue et al.63 |
| Bax | VHH library derived from non‐immunized llamas. Panning was performed with recombinant Bax. |
Isolation of mitochondria from SHSY‐5Y cells and analyzing reactive oxygen species (ROS) and cytochrome c release in the presence of Bax and nanobodies. SHSY‐5Y cells stable transfected with VHH‐GFP fusionproteins. Induction of oxidative stress with H2O2 and measurement of Caspase 3/7. |
Bax activity was inhibited in isolated mitochondria. Caspase 3/7 activation was prevented by anti‐Bax intrabodies. Anti‐Bax VHHs can be applied for studying the role of Bax in oxidative‐stress‐induced apoptosis. |
Gueorguieva et al.64 |
| L‐plastin | Llama immunized with recombinant L‐plastin |
Usage of human prostate cancer cell line PC‐3 cells. Studying the effect of L‐plastin on Actin binding and Actin bundling in the presence of VHHs. Analysis of cell migration and matrigel invasion of PC‐3 cells transiently transfected with nanobodies. THP‐1 monocytic leukemia cells lentiviral stable transfected with anti‐L‐plastin nanobodies. Analysis of actin bundling and stability of the podosome. Jurcat T cells transfected with nanobodies and activated with anti‐CD3/CD28 antibodies. Incubation of Jurcat T cells transfected with nanobody with Staphylococcus enterotoxin stimulated antigen presenting Raji cells. |
VHHs inhibit binding of L‐plastin to actin and actin bundling. Cell migration and matrigel invasion was inhibited by anti‐L‐plastin nanobodies. Nanobodies inhibit actin bundling leading to podosome instability. Nanobodies reduce proliferation and IL‐2 secretion after primary T cell activation. Anti‐L‐plastin antibodies decrease T cell‐Raji cell contact area. One nanobody inhibits phosphorylation of L‐plastin and LFA‐1. |
Delanote et al.65
De Clercq et al.66 De Clercq et al.67 |
| Fascin and cortactin | Llama immunized with fascin or cortactin. | MDA‐MB‐231 breast cancer cells and PC‐3 prostate cancer cells transient or retroviral transfected with nanobodies. Analysis of the influence of the anti‐fascin nanobodies on actin‐bundling and anti‐cortractin nanobodies on WASP‐interacting protein (WIP) recruitment toward the plasma membran. Analysis of the filopodium formation. | Fascin‐actin bundling is affected by anti‐fascin nanobodies and they have an effect on invadopodium array organization. WIP recruitment is inhibited by anti‐cortactin antibodies and they inhibit invadopodium array organization as well as formation of invadopodium. The nanobodies prevent matrix degradation. | Van Audenhove et al.68 |
| Gelsolin | Llamas immunized with human recombinant gelsolin. |
MDA‐MB‐231 breast cancer cells stable lentiviral transfected with VHHs. Binding assays to analyze if VHHs inhibits binding of gelsolin to actin. Migration of PC‐3 cells transfected with VHH was analyzed. |
One nanobody inhibits interaction of gelsolin with actin and prevents migration of PC‐3 cells. | Van den Abbeele et al.69 |
| Proteins of the nervous system | ||||
| Hungtington |
ScFv fragments against exon 1 of huntingtin were isolated from a yeast‐displayed non‐immune human scFv library. It was demonstrated that the paratope was only defined by VL. The function of affinity maturated light chain was analyzed. The cysteine residues of yeast‐displayed VL (see D W Colby, P Garg above) were changed to valine and alanine and the variant affinity maturated by Error Prone PCR. |
Analyzing huntingtin aggregation in a cell‐free in vitro assay in the presence of affinity maturated VL or in HEK293 cells transiently transfected with affinity maturated VL and hungtingtin exon 1 (httex1) fragment with pathological‐length of 67 poly‐glutamine residues‐fused to GFP (httex1‐Q67 ‐ GFP). Human embryonic ST14A cells and human neuroblastoma SH‐SY5Y cells co‐transfected with disulfide bond‐free VL and httex1Q97‐GFP were analyzed for hungtingtin aggregation. Cytotoxicity assay with sdAb transfected ST14A and yeast cells. |
Single domain VL inhibits huntingtin aggregation in vitro and in mammalian cells. Disulfide bond‐free mutant of VL, affinity maturated by Error Prone PCR, blocked hungtingtin aggregation and inhibited toxicity. |
Colby et al.70
Colby et al.71 |
| α‐Synuclein |
Selection of anti‐ α Synuclein scFv fragments and VH sdAbs from a yeast displayed non‐immune human scFv library. VH single domain antibody derived from a yeast‐displayed non‐immune human scFv library was not soluble. The PEST sequence was fused to the sdAb to favour solubility and intracellular degradation of the formed antigen‐sdAb complex. |
Transfection of a truncated version of WTsyn‐EGFP into stable sdAb expressing ST14A cells. Inhibition of aggregation of WTsyn‐EGFP was tested. (WTsyn=wildtype Synuclein). In human embryonic ST14A cells co‐transfected with sdAb‐Pest and α‐synuclein‐GFP, degradation of α‐Synuclein‐GFP was analyzed. |
Aggregation of α‐Synuclein was inhibited. Fusion of the PEST sequence to the nanobody significantly increased steady‐state soluble intrabody levels and α‐synuclein‐GFP is significantly degraded in presence of nanobody‐PEST. |
Lynch et al.72
Joshi et al.73 |
| Other Targets | ||||
| β‐catenin | Llama immunized with recombinant β‐catenin. | Wnt induced luciferase bioassay was performed with HEK293 cell transfected with anti‐β‐catenin VHHs. | 4 nanobodies inhibited β‐catenin activity. | Newnham et al.74 |
| Nuclear poly(A)‐binding protein 1 (PABPN1) | A non‐immune llama nanobody repertoire was screened with recombinant PABPN1. | HeLa cells were co‐transfected with nuclear targeted VHH‐NLS and aggregation prone mutant PABPN1 comprising 17 alanines. Aggregation of mutant PABPN1 in the presence of nanobody was analyzed by immunofluorescence. | Nanobody inhibits aggregation of mutant PABPN1 and cleared existing aggregates. | Verheesen et al.75 |
|
2,5 dihydroxy pyridine dioxygenase (NicX) |
Llamas immunized with NicX. | Bacterial strain P.putida transformed with VHH fused to a thioredoxin domain, which function as chaperon. Bacterial growth and NicX activity was measured. | Nanobodies inhibited bacterial growth and enzyme activity of NicX. | Jimenez et al.76 |
| ß2 – adrenergic Receptor | Llamas immunized with a truncated form of ß2 – adrenergic receptor. | HEK293 cells transiently transfected with nanobodies were tested for G protein activation and β‐arrestin recruitment. | Approximately half of the intrabodies showed inhibitory effects on G protein activation and β‐arrestin recruitment. | Staus et al.21 |
| Plants | ||||
| Potato starch branching enzyme A (SBE A) | A naive single‐domain llama antibody library with randomized CDRs was screened with recombinant SBE A. | VHHs were cloned into plant transformation vectors, introduced into Agrobacterium tumefaciens and transgenic potato lines generated. SBE A activity was measured. | VHHs inhibited significantly enzyme activity. | Jobling et al.77 |
| Nla protease of potato virus Y | Llama immunized with Nla protease. | The expression plasmid was transferred into potato plants via Agrobacterium tumefaciens mediated transformation. Transgenic plants were inoculated with potato virus Y. Effect of virusinfection in the presence of nanobody was tested. | Expression of nanobody leads to partial protection against the virus. | Bouaziz et al.78 |
| Drosophila | ||||
| Myosin regulatory light chain (Sqh)‐GFP and transcriptionfactor apterous (ap)‐GFP from Drosophila | Llama immunized with GFP. To selected nanobody was fused the F‐box domain contained in the N‐terminal part of Slmb (F Box Protein) from Drosophila. Fusion protein NSlmb‐VHHGFP leads to recruitment of E2 which fuse Ubiquitinin to the nanobody target. The target is degradated inside the proteasome. | Transgenic Drosophila knock in lines carrying the NSlmb‐VHHGFP gene and the Sqh‐GFP‐gene or ap‐GFP‐gene were generated and the phenotype analyzed. |
Degradation of Sqh and apterous protein fused to GFP mediated through ubiquitination led to significant phenotyp of transgenic intrabody flies. This method is universal and the GFP tag can be used to monitor the protein knockout in living cells. |
Caussinus et al.7 9 |
| Drosophila Decapentaplegic‐(Dpp) GFP | Into the framework of an anti‐ß‐lactamase VHH were transplanted the CDRs of an anti‐GFP VHH |
An anti‐eGFP VHH ‐ CD8 transmembrane domain ‐ cytoplasmic mCherry fluorescent tag‐fusion protein (morphotrap) was expressed in a transgenic mouse expressing DPP‐GFP to trap GFP‐Dpp on the cell surface. The wing disc development was analyzed. |
When Dpp is immobilized on the cell surface, wing disc patterning is lost and lateral cells still devide at normal rates. | Harmansa et al.80 |
The targets comprise
intracellular enzymes including kinases:53, 54, 58, 59, 62, 76, 77, 78, 81
toxins53 −55
virus proteins38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52
Most cytosolic/nuclear sdAbs have been evaluated in living cells but in vivo application of single domain intrabodies in mice is well documented, too.57, 60, 81 In the future, generation of transgenic mice expressing cytosolic/nuclear sdAb is possible and very useful if a corresponding genetic knockout is lethal.82
ER intrabodies versus cytosolic/nuclear intrabodies
ER‐intrabody knockdown function is based on retention of molecules passing the secretory pathway via the KDEL sequence fused to the C‐terminal end of the intrabody.6, 83, 84, 85 In the past ER‐intrabodies have been the most promising molecules because they are correctly folded in the ER environment.86 Contrary to ER intrabodies which have only to bind to the antigen inside the ER, cytosolic/nuclear intrabodies have to inactivate their targets by binding to the antigen and inducing a conformational change or interfering with the binding of the target protein to its specific binding partner. Intrabodies targeted to the ER (ER‐intrabodies) are mostly expressed as scFv fragment, cytosolic/nuclear intrabodies as scFv or recently as much more stable single domain antibody.6, 87, 88 Due to the high stability of sdAbs in the cytosol/nucleus compared to scFv fragments and efficient reliable selection techniques based on phage and yeast display, the knockdown of cytosolic/nuclear proteins by sdAbs is very promising.
Cytosolic/nuclear intrabodies
To select stable correctly folded scFv fragments, specific in vivo selection in eukaryotes such as yeast or in prokaryotes such as E.coli has to be performed.6 Possible options are also expression of scFv‐protein fusions and CDR grafting or introduction of synthetic CDRs in stable frameworks.6 The most frequently used method to select functional scFv's is the Antibody Capture Technology (IACT) based on the yeast 2‐hybrid system.5 The next paragraph summarizes the targets against which scFv fragments have been selected by IACT and describes some new scFv's inhibiting the degradation of p53 and restoring the activity of mutated p53, respectively.
ScFv format
Many different scFv's have been isolated by IACT, for example against Tau, amyloid‐ß peptide, a‐synuclein, proNGF, gephyrin, Syk, EGFR, BCR‐ABL, caspase 3, aggrecanase‐2 and NSP5 from rotavirus.6
Recently, a scFv fragment against the E6 oncoprotein of HPV selected by IACT was targeted to the nucleus where it inhibits the ability of HPV16 E6 (16E6) to mediate p53 degradation and causes cell death of HPV16‐positive cells.89 Additionally, the nucleus‐targeted intrabody inhibited tumor activity of HPV16‐positive tumor cells in two pre‐clinical mouse models. Interestingly, two bispecific scFv fragments have been constructed that (1) comprises a scFv that targets the nucleus and a scFv fragment that restored the function of mutated p53 and (2) comprises the intracellular nuclear transport scFv fragment and a scFv fragment that binds Mdm2 and prevents destruction of p53 by Mdm2.90 All three scFv fragments were constructed from corresponding hybridoma clones.
Single domain format
In contrast to the time‐consuming strategies for selection of functional cytosolic/nuclear scFv's, nanobodies can be directly selected from immune, naïve or synthetic libraries displayed on phage or yeast which significantly shortens the isolation of binders. Single domain antibodies are composed of only one V region (variable domain of the heavy or light chain) and in numerous examples it was shown that sdAbs can be stably expressed inside the cytoplasm or nucleus (Table 1). In the next paragraph, current methods for construction and selection of camelid, shark and human sdAbs including new developments will be summarized.
Generation of cytosolic/nuclear single domain intrabodies
Single domains antibodies can be generated from camelid heavy‐chain IgGs (VHHs), from cartilaginous fish IgNARs ( ) or from conventional human IgGs (VH and VL). The antibody repertoires can be derived after immunization of camels or sharks, build up from naïve natural antibody sequences or synthetically constructed based on stable scaffolds.10, 24, 56, 57, 58, 61, 63, 81 However, the process of immunization of sharks is demanding in comparison to immunization of mammals. Therefore, shark nanobodies have mostly been selected from synthetic repertoires. Human single domain VH and VL domains were selected from synthetic human VH or VL antibody repertoires.
Strategies to Construct Camelid VHH Repertoires
Construction of immune camelid VHH libraries
Camelid VHHs repertoires for the selection of single domain antibodies are mostly generated after immunization of camels. PCR amplification of VHH can be performed with primers binding to the N‐terminus and CH2 region of camel heavy chain IgG cDNA, followed by nested PCR amplifying the VHH coding region. The product is cloned into a phagemid vector and a phage antibody repertoire established91 (Fig. 2).
Examples of cytosolic/nuclear nanobodies from immune camelid VHH libraries
So far, most nanobodies and cytosolic/nuclear nanobodies have been selected after immunization of llamas with the target protein. Nanobodies inhibited the function of HIV Rev,38, 39 HIV Vpr,40 HIV‐1 Nef,42, 43 influenza virus nucleoprotein,44 hepatitis B Core antigen (HBcAg),49 nonstructural proteins 9 and 4 of porcine reproductive and respiratory syndrome virus (PRRSV),50, 51 p15 matrix protein of porcine endogenous retrovirus,52 SpvB toxin of Salmonella typhimurium,53 neurotoxine protease of Clostridium botulinum,54 mycotoxin 15‐Acetyldeoxynivalenol (15‐AcDON),55 protein kinase Cɛ,59 F‐actin capping protein CapG,60 L‐plastin,65 fascin and cortactin,68 gelsolin,69 β‐catenin,74 2.5 dihydroxy pyridine dioxygenase (NicX),76 β2 –adrenergic receptor,21 Nla protease of potato virus Y78 and GFP for depletion of target GFP‐fusions in eukaryotics.79
Naïve and synthetic single domains libraries have the advantage that no immunization of animals is needed. A disadvantage is that the selected sdAbs are not affinity maturated. Nevertheless, if these libraries contain a high number of clones with high diversity, sdAbs against all possible targets and epitops should be identifyable. Selected sdAbs can then easily be converted into cytosolic/nuclear antibody fragments by one cloning step (Fig. 3). In the next paragraphs, the principles of the generation of naïve and synthetic libraries together with some new developments are shown.
Construction of naïve camelid VHH libraries
Naïve llama VHH libraries are single pot or universal libraries generated from blood samples from several animals for the selection of a large number of different specificities.92 Naïve libraries with different sizes have been constructed ranging from 107 to 5 ×109 clones.25, 93 Recently, ribosome display with nanobodies has been performed with an expression cassette that can easily be used for in vitro synthesis of ribosome‐VHH complexes in different expression systems.94
Examples of cytosolic/nuclear naive VHHs
Some interesting naïve cytosolic/nuclear nanobodies have been generated particularly against non‐structural membrane protein NSB4 of HCV,45 Bax,64 NS3 helicase of HCV,46 RNA dependent RNA polymerase NS5B,47 nuclear poly(A)‐binding protein 1 (PABPN1)75 and Caspase‐3.62
Construction of synthetic VHH libraries
Synthetic camelid VHH libraries have been constructed based on natural VHH frameworks. Antigen binding diversity has been introduced by randomization of the CDRs, mainly CDR3.95 Interestingly, a semi‐synthetic library has been constructed from 3 unimmunized llamas. In this case, the CDRs and frameworks were diversified by error‐prone PCR and then these sequences were spliced together through the common framework overlaps by PCR.96, 97
Examples of synthetic cytosolic/nuclear nanobodies
A specific nanobody that leads to cytoplasmic accumulation of heterogenous nuclear ribonucleoprotein K (hnRPN‐K) has been selected.63 Furthermore, a nanobody that significantly inhibits the enzyme activity of potato starch branching enzyme has been generated.77 Recently, a very stable variant of an anti‐GFP nanobody was constructed by transplantation of the CDRs from an anti‐GFP VHH to a stable framework of an anti‐ß lactamase VHH.98 This antibody was part of the morphotrap that was used to analyze the function of Drosophila Decapentaplegic (Dpp) on wing disc development.80
Non camelid sdAb libraries
Construction of synthetic human VH/VL libraries
Synthetic human VH/VL libraries are constructed from a stable human VH/VL germline framework or from a VH/VL scaffold of a human monoclonal antibody. Randomization of the CDRs, especially of CDR3, leads to synthetic human VH libraries with high diversity.6, 12 −16, 99 VL single domain antibody libraries have been constructed similarly16 −18 (Fig. 3). Recently a synthetic library was constructed comprising the Ig1 domain of human neural cell adhesion molecule 1 (NCAM) in which randomized CDR1 and CDR3 from a shark clone were transplanted.100 Chimeric antibodies were selected that block the signaling activity of chemokine receptor CXCR4.
Examples of synthetic human VH/VL cytosolic/nuclear sdAbs
Anti‐myc human VH single domain antibodies have been selected from a synthetic human VH library based on a VH consensus framework of an anti‐RAS scFv. Stepwise randomization of CDR3, CDR2 and CDR1 followed by yeast two hybrid system selection led to specific anti‐cmyc clones.56, 61 Similarly, an anti‐RAS human VH and anti‐LIM domain only 2 (LMO2) VH domain was selected.57, 81 The anti‐RAS VH inhibited RAS‐dependent tumorigenesis in a mouse tumor model and the anti‐LM02 antibody fragment inhibits T‐cell neoplasia in a nude mouse. An anti‐epithelial kinase (Etk) human VL was selected from a synthetic library based on a human ĸ domain framework with randomized CDR3s.58
Furthermore, camelization of human VHs can be performed by introduction of point mutations in the VH framework of an human antibody, mainly framework 2 to render the overall structure more hydrophilic.88 Such camelized human VH frameworks have been used to generate CDR3 randomized single domain antibody repertoires displayed on phages for selection of new binders with improved solubility and refolding efficiency.101, 102 Interestingly a rabbit‐derived anti‐HIV Vif VH single‐domain intrabody was active in its camelized format.41
Construction of synthetic IgNAR variable domain (vNAR) libraries
In general, vNAR libraries have been selected from semi‐synthetic libraries based on naturally occurring vNAR sequences and randomized CDR 3 loops.28 −32 A shark anti‐hen egg‐white lysozyme IgNAR VH clone was used as the scaffold for the generation of a semi‐synthetic library with randomized CDR 3 loops.33 Interestingly, a neutralizing VNAR single‐domain antibody against BAFF that blocked the binding of BAFF to all three of its receptors was selected recently. In this case, the shark antibody repertoire was based on Type 2 nurse shark VNAR frameworks with a number of framework mutations and partial randomization of CDR3.34 At present, no shark cytosolic/nuclear sdAb have been reported in the literature.
Selection of cytosolic/nuclear sdAbs
For selection of antigen‐specific sdAbs, the sdAb repertoires are displayed on phages or on the cell surface of yeast cells. Then, by incubation of the recombinant phage antibody repertoire with immobilized antigen (biopanning), specific binders will be selected and cloned into a cytosolic or nuclear targeting vector (Figs. 2 and 3). The cytosolic or nuclear intrabodies will be evaluated in target cells, in xenograft tumor mouse models or transgenic sdAb mice.
Recently, a promising screening method to identify functional sdAbs has been developed.103 Alpacas were immunized with a mixture of inactivated influenza A virus (IAV) and vesicular stomatitis virus (VSV). Generated VHH cDNAs were subsequently directly cloned into a retroviral vector to produce lentivirus particles. Then, A549 cells were infected with recombinant viruses and after 48 h treated with a lethal dosis of IAV and VSV. Surviving colonies were further characterized and the sequences of the VHHs were determined. After this screen, 19 VHHs that specifically block infection of cells with IAV or VSV were isolated. This screening approach should be applicable to identify inhibitors of any pathogen or biological pathway.
Most sdAbs have been selected by phage display without additional usage of the bacterial‐, yeast‐ or mammalian‐two hybrid system. This approach is usually reliable and can be used for selection of cytosolic/nuclear scFv fragments as well as sdAbs because cytosolic/nuclear selection will favor well‐folded scFv fragments or sdAbs rather than the high affinity ones. However, it is still unclear if the affinity of antibodies is the only prerequisite for efficient intrabody engineering. Usage of bacterial‐, yeast‐ or mammalian‐two hybrid systems enables direct in vivo selection of functional cytosolic/nuclear intrabodies without the need of purified antigen.104 – 106 Another two‐hybrid assay that allows studying the interaction of two proteins in living cells directly has been developed.107 Here, a fluorescently labeled prey protein and the corresponding bait protein fused to fluorescently labeled lacI are coexpressed in mammalian cells that contain chromosomally integrated lac operator. Interaction of both molecules results in co‐localization of two different fluorescent signals at the lac operator, which can be observed as a spot within the nucleus. The next paragraph provides a list of the selection and description of all known cytosolic/nuclear sdAbs derived from camels and human antibodies. Cytosolic sdAbs are descripted which have been successfully applied in xenograft tumor models and in addition a nanobody‐GFP fusion protein is highlighted which is universally applicable for monitoring protein knockout in living cells.
Examples of cytosolic/nuclear single domain antibodies
Cytosolic/nuclear nanobodies that interfere with the function of approximately 40 cytosolic/nuclear targets have been generated successfully (Table 1: reports of cytosolic/nuclear sdAb mediated knockdown). Compared to this number of cytosolic/nuclear targets ER‐intrabodies against approximately 35 transitory proteins have been reported. Most sdAb‐targets belong to viral or oncogenic proteins, followed by toxins and proteins of the nervous system.
Cytosolic intrabodies have also successfully been expressed in plants and in Drosophila. Single domain intrabodies partially protected potato plants against potato virus Y.78 A universal method has been developed to monitor protein knockout in living cells.79 To achieve this anti‐GFP VHHs were fused to an F‐box domain of Drosophila Slmb for degradation of targets fused to GFP via the ubiquitin depletion pathway. The technique was applied for the degradation of a transcription factor and a protein involved in morphogenesis of transgenic Drosophila monogaster flies expressing GFP‐target protein and anti‐GFP VHH. In addition an anti‐eGFP – CD8 transmembrane domain – cytoplasmic mCherry‐fusion protein (morphotrap) was developed to study wing disc development in transgenic flies expressing eGFP‐morphogenetic protein Decapentaplegic (Dpp) and the morphotrap. The morphotrap immobilized eGFP‐Dpp on the cell surface, leading to inhibition of wing disc patterning.80 Even gene silencing using sequence‐selective and nucleic‐acid hydrolyzing nanobodies is now possible.108
Most sdAbs have been tested in cell culture and only few have been investigated in mice as an animal model. SdAbs against LIM domain only 2 (LMO2), F‐Actin capping protein CapG and RAS were tested in appropriate xenograft tumor mouse models by transplanting tumor cells expressing the corresponding intrabody in nude mice or NOD‐SCID mice.57, 60, 81 In addition an anti‐HIV‐1 Nef intrabody was able to rescue Nef‐mediated thymic CD4+ T‐cell maturation defect and peripheral CD4+ T‐cell activation phenotypes in CD4+/HIVNEF transgenic mice.42 Interestingly, fusion of a loop of a SH3 domain to one selected nanobody improved some inhibiting HIV‐1 Nef functions.43 Furthermore a cysteine‐free human VL has been selected71 with better activity as the original antibody fragment. Interestingly changing the cysteine residues to valine and alanine led to lower affinity as the original antibody domain with the cysteins. An improved cysteine‐free variant could be selected after random mutagenesis using error‐prone PCR.
Several nanobodies against viral proteins, toxins, oncogenic proteins and proteins of the nervous system have therapeutic potential (table: reports of cytosolic/nuclear sdAb mediated knockdown). In the next paragraph new developments in protein delivery of scFv fragments/sdAbs and model proteins into the cytosol/nucleus of target cells will be shown.
Protein transfection with cytosolic/nuclear sdAbs
Alternatively to viral transfection or plasmid transfection, intrabodies can be delivered as proteins into the cytosol (profection). Delivery of intrabodies in the scFv or nanobody format into the cytosol of a target cell has the advantage that the intrabodies retain their disulfide bridges for correct folding. Further, this method might be a safe alternative to viral gene transfer for clinical approaches.
The delivery of antibodies to the cytosol using different profection reagents has been studied in detail and was compared to electroporation, which was found to be the most effective reliable technique.109
Alternatively, fusion of cell penetrating peptides or targeting proteins (ligands or antibodies) together with translocation domains or usage of cell penetrating peptides fused to endosome escape domains has been investigated.109, 110 Fusion of peptides and proteins to reach the cytoplasm is often not very efficient because of inefficient endosomal escape.110 However, a cell penetrating anti‐DNA antibody localizes to the nucleus through a nucleoside salvage pathway and is used as a delivery vehicle and combined with specific targeting scFvs.90
Some highlights referring to cell‐penetrating peptides should be mentioned. Highly efficient delivery was achieved using a cell‐penetrating peptide‐adaptor system built of TAT‐calmodulin fusion and a fusion consisting of calmodulin binding site and cargo.111 TAT‐calmodulin non‐covalently binds, delivers and releases cargo into the cytoplasm. Another system uses cell‐penetrating peptides binding to heparan sulfate glycosaminoglycan fused to a protein transduction domain. Cell types that are generally difficult to transduce, such as mouse embryonic stem cells (mESCs), human ESCs and human induced pluripotent stem cells (hiPSCs), have been efficiently transfected.112 Furthermore, camelid nanobodies against HCV proteins have been fused to cell penetrating penetrin and were successfully applied in HVC infected cells.45, 46, 47, 113
It was shown that the osteoblast transcription factor Runx2 can be targeted to the nucleus of mesenchymal cells by the peptide dentin phosphophoryn, bypassing the lysosomal degradation pathway.114 Recently, an efficient serum‐stable and low‐toxic molecular transporter comprising one rigid planar core and four flexible arms with one guanidinium group on each arm was constructed to efficiently deliver small cargoes and large active proteins into cytosol.115 A very interesting approach combined the TAT cell penetrating peptide to an endosomal escape sequence and GFPß11 peptide. Endosomal escape can be measured by binding of GFPß11 to non‐fluorescent GFP ß1‐10, inducing chemical formation of the GFP fluorescent chromophore. The assay enables screening of the next generation of endosomal escape domains.116
In addition, highly efficient cyclic cell‐penetrating peptides have been designed. These cyclic peptides are characterized by efficient release from the endosome.117 Last but not least, the solvent exposed residues of the framework region of nanobodies have been mutated to positively charged arginines or lysines (cationic resurfacing), leading to cell penetration.118 This approach has the potential to build a general applicable scaffold to transport nanobodies into the cell.
A very promising additional approach is the usage of the type VI secretion pathway of pathogenic bacteria to transport sdAbs into human cells.119 The injection of the sdAbs does not require bacterial invasion or the transfer of genetic material.
The approach to deliver drugs or toxins specifically to tumors mediated by nanoparticles decorated with nanobodies has been demonstrated in many in vitro examples and is now beginning to be tested in vivo.88 Polymersomes and polyplexes are relatively new artificial vesicles that can be modified simply by modifying the polymer‐building blocks.120, 121 It might be very attractive to encapsule recombinant cytosolic sdAb or sdAb expression plasmids into nanoparticles decorated with an specific anti‐receptor nanobody (for example EGFR122 to deliver cytosolic anti‐cancer single domain antibodies to tumor cells. Furthermore, nanoparticles/nanotubes coated with transferrin or Angiopep‐2, a ligand for the low‐density lipoprotein receptor‐related protein‐1 (LRP1), demonstrated translocation across the blood‐brain barrier.123, 124 The delivery of cytosolic/nuclear single domain intrabodies into the brain (for example recognizing hungtington protein or α‐Synuclein)71, 72 might be possible in the future.
Conclusion
Single domain antibodies from camels, shark and humans are very useful to efficiently and specifically knock down cytosolic and nuclear proteins in vivo.
The option to specifically target interaction regions, conformers, splice variants, isoforms and even posttranslational modifications such as phosphorylation sites21, 22, 34, 58, 125 – 127 makes intrabodies very unique and valuable. RNAi, on the other hand, has the disadvantage of demonstrating off‐target effects128, 129 even if allele specific inhibition of Hungtington protein and inactivation of oncogenic mutations of p53 by RNAi have been reported.130, 131
The availability of synthetic universal, single‐pot camelid, shark and human single domain antibody libraries displayed on phages or yeast cells enables selection of a huge number of different single domain intrabodies theoretically directed against any antigen and desired epitope.12 −18, 28 −31, 33, 34, 95, 96, 132 By using antibody affinity maturation strategies, binders with high affinity can be isolated from these nonaffinity‐maturated universal libraries by introducing CDR mutations, randomization of mutational hotspots or error prone PCR.35 −37 Usage of a recently published method based on selection of specific inhibitory dAbs by recombinant lentiviruses to omit phage display selection will be attractive in the future.103
Strategies to apply cytosolic/nuclear single domain intrabodies in the clinic may become possible with cell penetrating peptides,45, 46, 47, 113 new optimized endosomal escape domains,116 sdAb expression plasmids or recombinant sdAbs embedded into nanoparticles such as polymersomes and polyplexes.88 For cell‐ and tissue‐specific transductional targeting, nanoparticles can be decorated with anti‐receptor sdAbs. Application of mRNA coding for cytosolic/nuclear single domain intrabodies might also be possible in the future.133, 134
In summary, cytosolic/nuclear sdAbs of camelid, shark and human origin can be applied to clarify the function of uncharacterized proteins such as virus proteins and host cell factors, oncogenic proteins and cofactors, proteins of the nervous system, intracellular enzymes involved in signaling, transcription factors and proteins involved in differentiation and development.
Acknowledgments
Figures 1 and 2 were prepared by Oliver Backhaus. I thank Peter P Müller for reading the manuscript. Special thanks to S. Muyldermans and Wulf Blankenfeldt for critical reading the manuscript and giving very helpful comments.
References
- 1. Böldicke T (2015) Protein targeting compounds. Prediction, Selection and Activity of Specific Inhibitors. Springer Cham Heidelberg, New York. [PubMed] [Google Scholar]
- 2. Helma J, Cardoso MC, Muyldermans S, Leonhardt H (2015) Nanobodies and recombinant binders in cell biology. J Cell Biol 209:633–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Biocca S, Ruberti F, Tafani M, Pierandrei‐Amaldi P, Cattaneo A (1995) Redox state of single chain Fv fragments targeted to the endoplasmic reticulum, cytosol and mitochondria. Biotechnology 13:1110–1115. [DOI] [PubMed] [Google Scholar]
- 4. Wörn A, Plückthun A (2001) Stability engineering of antibody single‐chain Fv fragments. J Mol Biol 305:989–1010. [DOI] [PubMed] [Google Scholar]
- 5. Visintin M, Settanni G, Maritan A, Graziosi S, Marks JD, Cattaneo A (2002) The intracellular antibody capture technology (IACT): towards a consensus sequence for intracellular antibodies. J Mol Biol 317:73–83. [DOI] [PubMed] [Google Scholar]
- 6. Marschall AL, Dübel S, Böldicke T (2015) Specific in vivo knockdown of protein function by intrabodies. mAbs 7:1010–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Muyldermans S (2013) Nanobodies: natural single‐domain antibodies. Annu Rev Biochem 82:775–797. [DOI] [PubMed] [Google Scholar]
- 8. Greenberg AS, Avila D, Hughes M, Hughes A, McKinney EC, Flajnik MF (1995) A new antigen receptor gene family that undergoes rearrangement and extensive somatic diversification in sharks. Nature 374:168–173. [DOI] [PubMed] [Google Scholar]
- 9. Flajnik MF, Deschacht N, Muyldermans S (2011) A case of convergence: why did a simple alternative to canonical antibodies arise in sharks and camels?. PLoS Biol 9:e1001120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zielonka S, Empting M, Grzeschik J, Konning D, Barelle CJ, Kolmar H (2015) Structural insights and biomedical potential of IgNAR scaffolds from sharks. MAbs 7:15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liu JL, Zabetakis D, Brown JC, Anderson GP, Goldman ER (2014) Thermal stability and refolding capability of shark derived single domain antibodies. Mol Immunol 59:194–199. [DOI] [PubMed] [Google Scholar]
- 12. Hairul Bahara NH, Chin ST, Choong YS, Lim TS (2016) Construction of a semisynthetic human VH single‐domain antibody library and selection of domain antibodies against alpha‐crystalline of Mycobacterium tuberculosis. J Biomol Screen 21:35–43. [DOI] [PubMed] [Google Scholar]
- 13. Moutel S, Bery N, Bernard V, Keller L, Lemesre E, de Marco A, Ligat L, Rain JC, Favre G, Olichon A, Perez F (2016) NaLi‐H1: A universal synthetic library of humanized nanobodies providing highly functional antibodies and intrabodies. Elife 5 pii: e16228. doi: 10.7554/eLife.16228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Christ D, Famm K, Winter G (2007) Repertoires of aggregation‐resistant human antibody domains. Protein Eng Des Sel 20:413–416. [DOI] [PubMed] [Google Scholar]
- 15. Mandrup OA, Friis NA, Lykkemark S, Just J, Kristensen P (2013) A novel heavy domain antibody library with functionally optimized complementarity determining regions. PLoS One 8:e76834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ignatovisch O, Jespers, L. , Tomlinson, I.M. , de Wildt, R.M.T. , (2012) Creation of the Large and Highly Functional Synthetic Repertoire of Human VH and Vĸ Domain Antibodies, in Saerens D., Muyldermans (eds.) Single Domain Antibodies: Methods and Protocols, Springer, Methods in Molecular Biology, vol. 911, pp.39−63. [DOI] [PubMed] [Google Scholar]
- 17. Hussack G, Keklikian A, Alsughayyir J, Hanifi‐Moghaddam P, Arbabi‐Ghahroudi M, van Faassen H, Hou ST, Sad S, MacKenzie R, Tanha J (2012) A V(L) single‐domain antibody library shows a high‐propensity to yield non‐aggregating binders. Protein Eng Des Sel 25:313–318. [DOI] [PubMed] [Google Scholar]
- 18. Soderlind E, Vergeles M, Borrebaeck CA (1995) Domain libraries: synthetic diversity for de novo design of antibody V‐regions. Gene 160:269–272. [DOI] [PubMed] [Google Scholar]
- 19. Traenkle B, Emele F, Anton R, Poetz O, Haeussler RS, Maier J, Kaiser PD, Scholz AM, Nueske S, Buchfellner A, Romer T, Rothbauer U (2015) Monitoring interactions and dynamics of endogenous beta‐catenin with intracellular nanobodies in living cells. Mol Cell Proteomics 14:707–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Panza P, Maier J, Schmees C, Rothbauer U, Sollner C (2015) Live imaging of endogenous protein dynamics in zebrafish using chromobodies. Development 142:1879–1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Staus DP, Wingler LM, Strachan RT, Rasmussen SG, Pardon E, Ahn S, Steyaert J, Kobilka BK, Lefkowitz RJ (2014) Regulation of beta2‐adrenergic receptor function by conformationally selective single‐domain intrabodies. Mol Pharmacol 85:472–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rajan M, Mortusewicz O, Rothbauer U, Hastert FD, Schmidthals K, Rapp A, Leonhardt H, Cardoso MC (2015) Generation of an alpaca‐derived nanobody recognizing gamma‐H2AX. FEBS Open Bio 5:779–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Desmyter A, Spinelli S, Roussel A, Cambillau C (2015) Camelid nanobodies: killing two birds with one stone. Curr Opin Struct Biol 32:1–8. [DOI] [PubMed] [Google Scholar]
- 24. Revets H, De Baetselier P, Muyldermans S (2005) Nanobodies as novel agents for cancer therapy. Expert Opin Biol Ther 5:111–124. [DOI] [PubMed] [Google Scholar]
- 25. Monegal A, Ami D, Martinelli C, Huang H, Aliprandi M, Capasso P, Francavilla C, Ossolengo G, de Marco A (2009) Immunological applications of single‐domain llama recombinant antibodies isolated from a naive library. Protein Eng Des Sel 22:273–280. [DOI] [PubMed] [Google Scholar]
- 26. Yan J, Wang P, Zhu M, Li G, Romao E, Xiong S, Wan Y (2015) Characterization and applications of Nanobodies against human procalcitonin selected from a novel naive Nanobody phage display library. J Nanobiotechnology 13:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yau KY, Groves MA, Li S, Sheedy C, Lee H, Tanha J, MacKenzie CR, Jermutus L, Hall JC (2003) Selection of hapten‐specific single‐domain antibodies from a non‐immunized llama ribosome display library. J Immunol Methods 281:161–175. [DOI] [PubMed] [Google Scholar]
- 28. Konning D, Zielonka S, Sellmann C, Schroter C, Grzeschik J, Becker S, Kolmar H (2016) Isolation of a pH‐Sensitive IgNAR Variable Domain from a Yeast‐Displayed, Histidine‐Doped Master Library. Mar Biotechnol 18:161–167. [DOI] [PubMed] [Google Scholar]
- 29. Liu JL, Anderson GP, Goldman ER (2007) Isolation of anti‐toxin single domain antibodies from a semi‐synthetic spiny dogfish shark display library. BMC Biotechnol 7:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nuttall SD, Krishnan UV, Doughty L, Pearson K, Ryan MT, Hoogenraad NJ, Hattarki M, Carmichael JA, Irving RA, Hudson PJ (2003) Isolation and characterization of an IgNAR variable domain specific for the human mitochondrial translocase receptor Tom70. Eur J Biochem 270:3543–3554. [DOI] [PubMed] [Google Scholar]
- 31. Nuttall SD, Krishnan UV, Hattarki M, De Gori R, Irving RA, Hudson PJ (2001) Isolation of the new antigen receptor from wobbegong sharks, and use as a scaffold for the display of protein loop libraries. Mol Immunol 38:313–326. [DOI] [PubMed] [Google Scholar]
- 32. Ohtani M, Hikima J, Jung TS, Kondo H, Hirono I, Aoki T (2013) Construction of an artificially randomized IgNAR phage display library: screening of variable regions that bind to hen egg white lysozyme. Mar Biotechnol 15:56–62. [DOI] [PubMed] [Google Scholar]
- 33. Shao CY, Secombes CJ, Porter AJ (2007) Rapid isolation of IgNAR variable single‐domain antibody fragments from a shark synthetic library. Mol Immunol 44:656–665. [DOI] [PubMed] [Google Scholar]
- 34. Hasler J, Flajnik MF, Williams G, Walsh FS, Rutkowski JL (2016) VNAR single‐domain antibodies specific for BAFF inhibit B cell development by molecular mimicry. Mol Immunol 75:28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Van Roy M, Ververken C, Beirnaert E, Hoefman S, Kolkman J, Vierboom M, Breedveld E, t Hart B, Poelmans S, Bontinck L, Hemeryck A, Jacobs S, Baumeister J, Ulrichts H (2015) The preclinical pharmacology of the high affinity anti‐IL‐6R Nanobody(R) ALX‐0061 supports its clinical development in rheumatoid arthritis. Arthritis Res Ther 17:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yau KY, Dubuc G, Li S, Hirama T, Mackenzie CR, Jermutus L, Hall JC, Tanha J (2005) Affinity maturation of a V(H)H by mutational hotspot randomization. J Immunol Methods 297:213–224. [DOI] [PubMed] [Google Scholar]
- 37. Shahi B, Mousavi Gargari SL, Rasooli I, Rajabi Bazl M, Hoseinpoor R (2014) Random mutagenesis of BoNT/E Hc nanobody to construct a secondary phage‐display library. J Appl Microbiol 117:528–536. [DOI] [PubMed] [Google Scholar]
- 38. Vercruysse T, Pardon E, Vanstreels E, Steyaert J, Daelemans D (2010) An intrabody based on a llama single‐domain antibody targeting the N‐terminal alpha‐helical multimerization domain of HIV‐1 rev prevents viral production. J Biol Chem 285:21768–21780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Boons E, Li G, Vanstreels E, Vercruysse T, Pannecouque C, Vandamme AM, Daelemans D (2014) A stably expressed llama single‐domain intrabody targeting Rev displays broad‐spectrum anti‐HIV activity. Antiviral Res 112:91–102. [DOI] [PubMed] [Google Scholar]
- 40. Matz J, Herate C, Bouchet J, Dusetti N, Gayet O, Baty D, Benichou S, Chames P (2014) Selection of intracellular single‐domain antibodies targeting the HIV‐1 Vpr protein by cytoplasmic yeast two‐hybrid system. PLoS One 9:e113729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Aires da Silva F, Santa‐Marta M, Freitas‐Vieira A, Mascarenhas P, Barahona I, Moniz‐Pereira J, Gabuzda D, Goncalves J (2004) Camelized rabbit‐derived VH single‐domain intrabodies against Vif strongly neutralize HIV‐1 infectivity. J Mol Biol 340:525–542. [DOI] [PubMed] [Google Scholar]
- 42. Bouchet J, Basmaciogullari SE, Chrobak P, Stolp B, Bouchard N, Fackler OT, Chames P, Jolicoeur P, Benichou S, Baty D (2011) Inhibition of the Nef regulatory protein of HIV‐1 by a single‐domain antibody. Blood 117:3559–3568. [DOI] [PubMed] [Google Scholar]
- 43. Bouchet J, Herate C, Guenzel CA, Verollet C, Jarviluoma A, Mazzolini J, Rafie S, Chames P, Baty D, Saksela K, Niedergang F, Maridonneau‐Parini I, Benichou S (2012) Single‐domain antibody‐SH3 fusions for efficient neutralization of HIV‐1 Nef functions. J Virol 86:4856–4867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ashour J, Schmidt FI, Hanke L, Cragnolini J, Cavallari M, Altenburg A, Brewer R, Ingram J, Shoemaker C, Ploegh HL (2015) Intracellular expression of camelid single‐domain antibodies specific for influenza virus nucleoprotein uncovers distinct features of its nuclear localization. J Virol 89:2792–2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Glab‐Ampai K, Malik AA, Chulanetra M, Thanongsaksrikul J, Thueng‐In K, Srimanote P, Tongtawe P, Chaicumpa W (2016) Inhibition of HCV replication by humanized‐single domain transbodies to NS4B. Biochem Biophys Res Commun 476:654–664. [DOI] [PubMed] [Google Scholar]
- 46. Phalaphol A, Thueng‐In K, Thanongsaksrikul J, Poungpair O, Bangphoomi K, Sookrung N, Srimanote P, Chaicumpa W (2013) Humanized‐VH/VHH that inhibit HCV replication by interfering with the virus helicase activity. J Virol Methods 194:289–299. [DOI] [PubMed] [Google Scholar]
- 47. Thueng‐in K, Thanongsaksrikul J, Srimanote P, Bangphoomi K, Poungpair O, Maneewatch S, Choowongkomon K, Chaicumpa W (2012) Cell penetrable humanized‐VH/V(H)H that inhibit RNA dependent RNA polymerase (NS5B) of HCV. PLoS One 7:e49254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Suzuki R, Saito K, Matsuda M, Sato M, Kanegae Y, Shi G, Watashi K, Aizaki H, Chiba J, Saito I, Wakita T, Suzuki T (2016) Single‐domain intrabodies against hepatitis C virus core inhibit viral propagation and core‐induced NFkappaB activation. J Gen Virol 97:887–892. [DOI] [PubMed] [Google Scholar]
- 49. Serruys B, Van Houtte F, Farhoudi‐Moghadam A, Leroux‐Roels G, Vanlandschoot P (2010) Production, characterization and in vitro testing of HBcAg‐specific VHH intrabodies. J Gen Virol 91:643–652. [DOI] [PubMed] [Google Scholar]
- 50. Liu H, Wang Y, Duan H, Zhang A, Liang C, Gao J, Zhang C, Huang B, Li Q, Li N, Xiao S, Zhou EM (2015) An intracellularly expressed Nsp9‐specific nanobody in MARC‐145 cells inhibits porcine reproductive and respiratory syndrome virus replication. Vet Microbiol 181:252–260. [DOI] [PubMed] [Google Scholar]
- 51. Liu H, Liang C, Duan H, Zhang X, Wang X, Xiao S, Zhou EM (2016) Intracellularly expressed nanobodies against non‐structural protein 4 of porcine reproductive and respiratory syndrome virus inhibit virus replication. Biotechnol Lett 38:1081–1088. [DOI] [PubMed] [Google Scholar]
- 52. Dekker S, Toussaint W, Panayotou G, de Wit T, Visser P, Grosveld F, Drabek D (2003) Intracellularly expressed single‐domain antibody against p15 matrix protein prevents the production of porcine retroviruses. J Virol 77:12132–12139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Alzogaray V, Danquah W, Aguirre A, Urrutia M, Berguer P, Garcia Vescovi E, Haag F, Koch‐Nolte F, Goldbaum FA (2011) Single‐domain llama antibodies as specific intracellular inhibitors of SpvB, the actin ADP‐ribosylating toxin of Salmonella typhimurium. FASEB J 25:526–534. [DOI] [PubMed] [Google Scholar]
- 54. Tremblay JM, Kuo CL, Abeijon C, Sepulveda J, Oyler G, Hu X, Jin MM, Shoemaker CB (2010) Camelid single domain antibodies (VHHs) as neuronal cell intrabody binding agents and inhibitors of Clostridium botulinum neurotoxin (BoNT) proteases. Toxicon 56:990–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Doyle PJ, Saeed H, Hermans A, Gleddie SC, Hussack G, Arbabi‐Ghahroudi M, Seguin C, Savard ME, Mackenzie CR, Hall JC (2009) Intracellular expression of a single domain antibody reduces cytotoxicity of 15‐acetyldeoxynivalenol in yeast. J Biol Chem 284:35029–35039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zeng J, Li HC, Tanaka T, Rabbitts TH (2015) Selection of human single domain antibodies recognizing the CMYC protein using enhanced intracellular antibody capture. J Immunol Methods 426:140–143. [DOI] [PubMed] [Google Scholar]
- 57. Tanaka T, Sewell H, Waters S, Phillips SE, Rabbitts TH (2011) Single domain intracellular antibodies from diverse libraries: emphasizing dual functions of LMO2 protein interactions using a single VH domain. J Biol Chem 286:3707–3716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Paz K, Brennan LA, Iacolina M, Doody J, Hadari YR, Zhu Z (2005) Human single‐domain neutralizing intrabodies directed against Etk kinase: a novel approach to impair cellular transformation. Mol Cancer Ther 4:1801–1809. [DOI] [PubMed] [Google Scholar]
- 59. Summanen M, Granqvist N, Tuominen RK, Yliperttula M, Verrips CT, Boonstra J, Blanchetot C, Ekokoski E (2012) Kinetics of PKCepsilon activating and inhibiting llama single chain antibodies and their effect on PKCepsilon translocation in HeLa cells. PLoS One 7:e35630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Van Impe K, Bethuyne J, Cool S, Impens F, Ruano‐Gallego D, De Wever O, Vanloo B, Van Troys M, Lambein K, Boucherie C, Martens E, Zwaenepoel O, Hassanzadeh‐Ghassabeh G, Vandekerckhove J, Gevaert K, Fernandez LA, Sanders NN, Gettemans J (2013) A nanobody targeting the F‐actin capping protein CapG restrains breast cancer metastasis. Breast Cancer Res 15:R116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Tanaka T, Rabbitts TH (2003) Intrabodies based on intracellular capture frameworks that bind the RAS protein with high affinity and impair oncogenic transformation. embo J 22:1025–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. McGonigal K, Tanha J, Palazov E, Li S, Gueorguieva‐Owens D, Pandey S (2009) Isolation and functional characterization of single domain antibody modulators of Caspase‐3 and apoptosis. Appl Biochem Biotechnol 157:226–236. [DOI] [PubMed] [Google Scholar]
- 63. Inoue A, Sawata SY, Taira K, Wadhwa R (2007) Loss‐of‐function screening by randomized intracellular antibodies: identification of hnRNP‐K as a potential target for metastasis. Proc Natl Acad Sci USA 104:8983–8988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Gueorguieva D, Li S, Walsh N, Mukerji A, Tanha J, Pandey S (2006) Identification of single‐domain, Bax‐specific intrabodies that confer resistance to mammalian cells against oxidative‐stress‐induced apoptosis. FASEB J 20:2636–2638. [DOI] [PubMed] [Google Scholar]
- 65. Delanote V, Vanloo B, Catillon M, Friederich E, Vandekerckhove J, Gettemans J (2010) An alpaca single‐domain antibody blocks filopodia formation by obstructing L‐plastin‐mediated F‐actin bundling. FASEB J 24:105–118. [DOI] [PubMed] [Google Scholar]
- 66. De Clercq S, Boucherie C, Vandekerckhove J, Gettemans J, Guillabert A (2013) L‐plastin nanobodies perturb matrix degradation, podosome formation, stability and lifetime in THP‐1 macrophages. PloS one 8:e78108. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 67. De Clercq S, Zwaenepoel O, Martens E, Vandekerckhove J, Guillabert A, Gettemans J (2013) Nanobody‐induced perturbation of LFA‐1/L‐plastin phosphorylation impairs MTOC docking, immune synapse formation and T cell activation. Cell Mol Life Sci 70:909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Van Audenhove I, Boucherie C, Pieters L, Zwaenepoel O, Vanloo B, Martens E, Verbrugge C, Hassanzadeh‐Ghassabeh G, Vandekerckhove J, Cornelissen M, De Ganck A, Gettemans J (2014) Stratifying fascin and cortactin function in invadopodium formation using inhibitory nanobodies and targeted subcellular delocalization. FASEB J 28:1805–1818. [DOI] [PubMed] [Google Scholar]
- 69. Van den Abbeele A, De Clercq S, De Ganck A, De Corte V, Van Loo B, Soror SH, Srinivasan V, Steyaert J, Vandekerckhove J, Gettemans J (2010) A llama‐derived gelsolin single‐domain antibody blocks gelsolin‐G‐actin interaction. Cell Mol Life Sci 67:1519–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Colby DW, Garg P, Holden T, Chao G, Webster JM, Messer A, Ingram VM, Wittrup KD (2004) Development of a human light chain variable domain (V(L)) intracellular antibody specific for the amino terminus of huntingtin via yeast surface display. J Mol Biol 342:901–912. [DOI] [PubMed] [Google Scholar]
- 71. Colby DW, Chu Y, Cassady JP, Duennwald M, Zazulak H, Webster JM, Messer A, Lindquist S, Ingram VM, Wittrup KD (2004) Potent inhibition of huntingtin aggregation and cytotoxicity by a disulfide bond‐free single‐domain intracellular antibody. Proc Natl Acad Sci USA 101:17616–17621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Lynch SM, Zhou C, Messer A (2008) An scFv intrabody against the nonamyloid component of alpha‐synuclein reduces intracellular aggregation and toxicity. J Mol Biol 377:136–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Joshi SN, Butler DC, Messer A (2012) Fusion to a highly charged proteasomal retargeting sequence increases soluble cytoplasmic expression and efficacy of diverse anti‐synuclein intrabodies. MAbs 4:686–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Newnham LE, Wright MJ, Holdsworth G, Kostarelos K, Robinson MK, Rabbitts TH, Lawson AD (2015) Functional inhibition of beta‐catenin‐mediatedWnt signaling by intracellular VHHantibodies. MAbs 7:180–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Verheesen P, de Kluijver A, van Koningsbruggen S, de Brij M, de Haard HJ, van Ommen GJ, van der Maarel SM, Verrips CT (2006) Prevention of oculopharyngeal muscular dystrophy‐associated aggregation of nuclear polyA‐binding protein with a single‐domain intracellular antibody. Hum Mol Genet 15:105–111. [DOI] [PubMed] [Google Scholar]
- 76. Jimenez JI, Fraile S, Zafra O, de Lorenzo V (2015) Phenotypic knockouts of selected metabolic pathways by targeting enzymes with camel‐derived nanobodies (V(HH)s). Metab Eng 30:40–48. [DOI] [PubMed] [Google Scholar]
- 77. Jobling SA, Jarman C, Teh MM, Holmberg N, Blake C, Verhoeyen ME (2003) Immunomodulation of enzyme function in plants by single‐domain antibody fragments. Nat Biotechnol 21:77–80. [DOI] [PubMed] [Google Scholar]
- 78. Bouaziz D, Ayadi M, Bidani A, Rouis S, Nouri‐Ellouz O, Jellouli R, Drira N, Gargouri‐Bouzid R (2009) A stable cytosolic expression of VH antibody fragment directed against PVY NIa protein in transgenic potato plant confers partial protection against the virus. Plant Sci 176:489–496. [DOI] [PubMed] [Google Scholar]
- 79. Caussinus E, Kanca O, Affolter M (2012) Fluorescent fusion protein knockout mediated by anti‐GFP nanobody. Nat Struct Mol Biol 19:117–121. [DOI] [PubMed] [Google Scholar]
- 80. Harmansa S, Hamaratoglu F, Affolter M, Caussinus E (2015) Dpp spreading is required for medial but not for lateral wing disc growth. Nature 527:317–322. [DOI] [PubMed] [Google Scholar]
- 81. Tanaka T, Williams RL, Rabbitts TH (2007) Tumour prevention by a single antibody domain targeting the interaction of signal transduction proteins with RAS. EMBO J 26:3250–3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Marschall AL, Single FN, Schlarmann K, Bosio A, Strebe N, van den Heuvel J, Frenzel A, Dübel S (2014) Functional knock down of VCAM1 in mice mediated by endoplasmatic reticulum retained intrabodies. MAbs 6:[PAGE #]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Marschall AL, Dubel S, Boldicke T (2016) Recent advances with ER targeted intrabodies. Adv Exp Med Biol 917:77–93. [DOI] [PubMed] [Google Scholar]
- 84. Böldicke T (2007) Blocking translocation of cell surface molecules from the ER to the cell surface by intracellular antibodies targeted to the ER. J Cell Mol Med 11:54–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Böldicke T, Somplatzki S, Sergeev G, Mueller PP (2012) Functional inhibition of transitory proteins by intrabody‐mediated retention in the endoplasmatic reticulum. Methods 56:338–350. [DOI] [PubMed] [Google Scholar]
- 86. van Anken E, Braakman I (2005) Versatility of the endoplasmic reticulum protein folding factory. Crit Rev Biochem Mol Biol 40:191–228. [DOI] [PubMed] [Google Scholar]
- 87. De Meyer T, Muyldermans S, Depicker A (2014) Nanobody‐based products as research and diagnostic tools. Trends Biotechnol 32:263–270. [DOI] [PubMed] [Google Scholar]
- 88. Van Audenhove I, Gettemans J (2016) Nanobodies as versatile tools to understand, diagnose, visualize and treat cancer. EBioMedicine 8:40–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Amici C, Visintin M, Verachi F, Paolini F, Percario Z, Di Bonito P, Mandarino A, Affabris E, Venuti A, Accardi L (2016) A novel intracellular antibody against the E6 oncoprotein impairs growth of human papillomavirus 16‐positive tumor cells in mouse models. Oncotarget 7:15539–15553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Chan G, Jordaan G, Nishimura RN, Weisbart RH (2016) Combining intracellular antibodies to restore function of mutated p53 in cancer. Int J Cancer 138:182–186. [DOI] [PubMed] [Google Scholar]
- 91. Arbabi Ghahroudi M, Desmyter A, Wyns L, Hamers R, Muyldermans S (1997) Selection and identification of single domain antibody fragments from camel heavy‐chain antibodies. FEBS Lett 414:521–526. [DOI] [PubMed] [Google Scholar]
- 92. Verheesen P, Roussis A, de Haard HJ, Groot AJ, Stam JC, den Dunnen JT, Frants RR, Verkleij AJ, Theo Verrips C, van der Maarel SM (2006) Reliable and controllable antibody fragment selections from Camelid non‐immune libraries for target validation. Biochim Biophys Acta 1764:1307–1319. [DOI] [PubMed] [Google Scholar]
- 93. Sabir JS, Atef A, El‐Domyati FM, Edris S, Hajrah N, Alzohairy AM, Bahieldin A (2014) Construction of naive camelids VHH repertoire in phage display‐based library. C R Biol 337:244–249. [DOI] [PubMed] [Google Scholar]
- 94. Bencurova E, Pulzova L, Flachbartova Z, Bhide M (2015) A rapid and simple pipeline for synthesis of mRNA‐ribosome‐V(H)H complexes used in single‐domain antibody ribosome display. Mol Biosyst 11:1515–1524. [DOI] [PubMed] [Google Scholar]
- 95. Yan J, Li G, Hu Y, Ou W, Wan Y (2014) Construction of a synthetic phage‐displayed Nanobody library with CDR3 regions randomized by trinucleotide cassettes for diagnostic applications. J Transl Med 12:343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Goldman ER, Anderson GP, Liu JL, Delehanty JB, Sherwood LJ, Osborn LE, Cummins LB, Hayhurst A (2006) Facile generation of heat‐stable antiviral and antitoxin single domain antibodies from a semisynthetic llama library. Anal Chem 78:8245–8255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Sherwood LJ, Hayhurst A (2013) Ebolavirus nucleoprotein C‐termini potently attract single domain antibodies enabling monoclonal affinity reagent sandwich assay (MARSA) formulation. PLoS One 8:e61232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Saerens D, Pellis M, Loris R, Pardon E, Dumoulin M, Matagne A, Wyns L, Muyldermans S, Conrath K (2005) Identification of a universal VHH framework to graft non‐canonical antigen‐binding loops of camel single‐domain antibodies. J Mol Biol 352:597–607. [DOI] [PubMed] [Google Scholar]
- 99. Reiter Y, Schuck P, Boyd LF, Plaksin D (1999) An antibody single‐domain phage display library of a native heavy chain variable region: isolation of functional single‐domain VH molecules with a unique interface. J Mol Biol 290:685–698. [DOI] [PubMed] [Google Scholar]
- 100. Griffiths K, Dolezal O, Cao B, Nilsson SK, See HB, Pfleger KD, Roche M, Gorry PR, Pow A, Viduka K, Lim K, Lu BG, Chang DH, Murray‐Rust T, Kvansakul M, Perugini MA, Dogovski C, Doerflinger M, Zhang Y, Parisi K, Casey JL, Nuttall SD, Foley M (2016) i‐bodies, human single domain antibodies that antagonize chemokine receptor CXCR4. J Biol Chem 291:12641–12657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Tanha J, Nguyen TD, Ng A, Ryan S, Ni F, Mackenzie R (2006) Improving solubility and refolding efficiency of human V(H)s by a novel mutational approach. Protein Eng Des Sel 19:503–509. [DOI] [PubMed] [Google Scholar]
- 102. Tanha J, Xu P, Chen Z, Ni F, Kaplan H, Narang SA, MacKenzie CR (2001) Optimal design features of camelized human single‐domain antibody libraries. J Biol Chem 276:24774–24780. [DOI] [PubMed] [Google Scholar]
- 103. Schmidt FI, Hanke L, Morin B, Brewer R, Brusic V, Whelan SP, Ploegh HL (2016) Phenotypic lentivirus screens to identify functional single domain antibodies. Nat Microbiol 1:16080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Tanaka T, Rabbitts TH (2012) Intracellular antibody capture (IAC) methods for single domain antibodies. Methods Mol Biol 911:151–173. [DOI] [PubMed] [Google Scholar]
- 105. Tanaka T, Rabbitts TH (2010) Protocol for the selection of single‐domain antibody fragments by third generation intracellular antibody capture. Nat Protoc 5:67–92. [DOI] [PubMed] [Google Scholar]
- 106. Pellis M, Pardon E, Zolghadr K, Rothbauer U, Vincke C, Kinne J, Dierynck I, Hertogs K, Leonhardt H, Messens J, Muyldermans S, Conrath K (2012) A bacterial‐two‐hybrid selection system for one‐step isolation of intracellularly functional Nanobodies. Arch Biochem Biophys 526:114–123. [DOI] [PubMed] [Google Scholar]
- 107. Zolghadr K, Mortusewicz O, Rothbauer U, Kleinhans R, Goehler H, Wanker EE, Cardoso MC, Leonhardt H (2008) A fluorescent two‐hybrid assay for direct visualization of protein interactions in living cells. Mol Cell Proteomics 7:2279–2287. [DOI] [PubMed] [Google Scholar]
- 108. Lee WR, Jang JY, Kim JS, Kwon MH, Kim YS (2010) Gene silencing by cell‐penetrating, sequence‐selective and nucleic‐acid hydrolyzing antibodies. Nucleic Acids Res 38:1596–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Marschall AL, Zhang C, Frenzel A, Schirrmann T, Hust M, Perez F, Dübel S (2014) Delivery of antibodies to the cytosol: debunking the myths. MAbs 6:943–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Marschall AL, Frenzel A, Schirrmann T, Schüngel M, Dübel S (2011) Targeting antibodies to the cytoplasm. MAbs 3:3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Salerno JC, Ngwa VM, Nowak SJ, Chrestensen CA, Healey AN, McMurry JL (2016) Novel cell‐penetrating peptide‐adaptors effect intracellular delivery and endosomal escape of protein cargos. J Cell Sci 129:893–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Dixon JE, Osman G, Morris GE, Markides H, Rotherham M, Bayoussef Z, El Haj AJ, Denning C, Shakesheff KM (2016) Highly efficient delivery of functional cargoes by the synergistic effect of GAG binding motifs and cell‐penetrating peptides. Proc Natl Acad Sci USA 113:E291–E299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Jittavisutthikul S, Thanongsaksrikul J, Thueng‐In K, Chulanetra M, Srimanote P, Seesuay W, Malik AA, Chaicumpa W (2015) Humanized‐VHH transbodies that inhibit HCV protease and replication. Viruses 7:2030–2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Ravindran S, Snee PT, Ramachandran A, George A (2013) Acidic domain in dentin phosphophoryn facilitates cellular uptake: implications in targeted protein delivery. J Biol Chem 288:16098–16109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Zhang N, Yan Z, Zhao X, Chen Q, Ma M (2016) An efficient mini‐transporter for cytosolic protein delivery. ACS Appl Mater Interfaces [VOL:PAGE #S]. [DOI] [PubMed] [Google Scholar]
- 116. Lonn P, Kacsinta AD, Cui XS, Hamil AS, Kaulich M, Gogoi K, Dowdy SF (2016) Enhancing endosomal escape for intracellular delivery of macromolecular biologic therapeutics. Sci Rep 6:32301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Qian Z, Martyna A, Hard RL, Wang J, Appiah‐Kubi G, Coss C, Phelps MA, Rossman JS, Pei D (2016) Discovery and mechanism of highly efficient cyclic cell‐penetrating peptides. Biochemistry 55:2601–2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Bruce VJ, Lopez‐Islas M, McNaughton BR (2016) Resurfaced cell‐penetrating nanobodies: A potentially general scaffold for intracellularly targeted protein discovery. Protein Sci 25:1129–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Blanco‐Toribio A, Muyldermans S, Frankel G, Fernandez LA (2010) Direct injection of functional single‐domain antibodies from E. coli into human cells. PLoS One 5:e15227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Zou T, Dembele F, Beugnet A, Sengmanivong L, Trepout S, Marco S, de Marco A, Li MH (2015) Nanobody‐functionalized PEG‐b‐PCL polymersomes and their targeting study. J Biotechnol 214:147–155. [DOI] [PubMed] [Google Scholar]
- 121. Sadeqzadeh E, Rahbarizadeh F, Ahmadvand D, Rasaee MJ, Parhamifar L, Moghimi SM (2011) Combined MUC1‐specific nanobody‐tagged PEG‐polyethylenimine polyplex targeting and transcriptional targeting of tBid transgene for directed killing of MUC1 over‐expressing tumour cells. J Control Release 156:85–91. [DOI] [PubMed] [Google Scholar]
- 122. Bian X, Wu P, Sha H, Qian H, Wang Q, Cheng L, Yang Y, Yang M, Liu B (2016) Anti‐EGFR‐iRGD recombinant protein conjugated silk fibroin nanoparticles for enhanced tumor targeting and antitumor efficiency. Onco Targets Ther 9:3153–3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Kafa H, Wang JT, Rubio N, Klippstein R, Costa PM, Hassan HA, Sosabowski JK, Bansal SS, Preston JE, Abbott NJ, Al‐Jamal KT (2016) Translocation of LRP1 targeted carbon nanotubes of different diameters across the blood‐brain barrier in vitro and in vivo. J Control Release 225:217–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Yan F, Wang Y, He S, Ku S, Gu W, Ye L (2013) Transferrin‐conjugated, fluorescein‐loaded magnetic nanoparticles for targeted delivery across the blood‐brain barrier. J Mater Sci Mater Med 24:2371–2379. [DOI] [PubMed] [Google Scholar]
- 125. Oku T, Ando Y, Ogura M, Tsuji T (2016) Development of splice variant‐specific monoclonal antibodies against human alpha3 integrin. Monoclon Antib Immunodiagn Immunother 35:12–17. [DOI] [PubMed] [Google Scholar]
- 126. D'Amico AG, Maugeri G, Reitano R, Cavallaro S, D'Agata V (2016) Proteomic analysis of parkin isoforms expression in different rat brain areas. Protein J [VOL:PAGE #S]. [DOI] [PubMed] [Google Scholar]
- 127. Koo MY, Park J, Lim JM, Joo SY, Shin SP, Shim HB, Chung J, Kang D, Woo HA, Rhee SG (2014) Selective inhibition of the function of tyrosine‐phosphorylated STAT3 with a phosphorylation site‐specific intrabody. Proc Natl Acad Sci USA 111:6269–6274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Dorsett Y, Tuschl T (2004) siRNAs: applications in functional genomics and potential as therapeutics. Nat Rev Drug Discov 3:318–329. [DOI] [PubMed] [Google Scholar]
- 129. Behlke MA (2008) Chemical modification of siRNAs for in vivo use. Oligonucleotides 18:305–319. [DOI] [PubMed] [Google Scholar]
- 130. Hu J, Liu J, Corey DR (2010) Allele‐selective inhibition of huntingtin expression by switching to an miRNA‐like RNAi mechanism. Chem Biol 17:1183–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Martinez LA, Naguibneva I, Lehrmann H, Vervisch A, Tchenio T, Lozano G, Harel‐Bellan A (2002) Synthetic small inhibiting RNAs: efficient tools to inactivate oncogenic mutations and restore p53 pathways. Proc Natl Acad Sci USA 99:14849–14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Wei G, Meng W, Guo H, Pan W, Liu J, Peng T, Chen L, Chen CY (2011) Potent neutralization of influenza A virus by a single‐domain antibody blocking M2 ion channel protein. PLoS One 6:e28309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Kallen KJ, Thess A (2014) A development that may evolve into a revolution in medicine: mRNA as the basis for novel, nucleotide‐based vaccines and drugs. Ther Adv Vaccines 2:10–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Dolgin E (2015) Business: The billion‐dollar biotech. Nature 522:26–28. [DOI] [PubMed] [Google Scholar]


