Abstract
Background
Modifiable cardiovascular risk factors elevate risk of subsequent depression in older adults, but the effect of their onset before or after age 65 on incident depression is unclear.
Methods
Participants were 1,190 male medical students without a diagnosis of depression, who matriculated in 1948–1964 and followed through 2011. Cox Proportional Hazards models were used to assess associations of vascular risk-factor burden, diabetes, hypertension, hyperlipidemia, smoking status, and overweight/obese status with onset of incident depression. Adjustment covariates were race, enrollment wave, baseline age, physical activity, and heavy alcohol use.
Results
The analysis included 44,175 person-years of follow-up. Among participants depression-free until age 65, vascular risk-factor burden after age 65 (Hazard Ratio, [HR]: 2.13, 95% Confidence Interval, [Cl]: 1.17, 3.90) was associated with incident depression risk after age 65. The magnitude of vascular risk-factor burden after age 65 on depression risk after age 65 is comparable to the effect of 8.2 additional years of age. Diabetes (HR: 2.79, 95% CI: 1.25, 6.26), hypertension (HR: 2.72, 95% CI: 1.52, 4.88), and hyperlipidemia (HR: 1.88, 95% CI: 1.05, 3.35) before age 65 were associated with incident depression risk after age 65. Men diagnosed with diabetes after age 65 had 2.87 times the risk of incident depression after age 65 (95% CI: 1.24, 6.62).
Limitations
Our findings are restricted to male former medical students, which may affect study generalizability.
Conclusions
Results support the vascular depression hypothesis. Depression screening in older adults with vascular risk-factor burden may provide an avenue for prevention of late-onset depression.
Keywords: vascular depression, depression, cardiovascular risk factors, hypertension, diabetes, hyperlipidemia
1. Introduction
Cardiovascular disease (CVD) is a common, growing public health challenge given increases in the older segments of the US population (Go et al., 2013). In 2013, 83.6 million Americans had one or more CVD, of whom 42.2 million were 60 years of age or older (Go et al., 2013). Prior research suggests CVD and depression have a reciprocal relationship: each increases the risk of developing the other (Alexopoulos, 2010; Alexopoulous et al., 1997; Barnes et al., 2012; Newberg et al., 2006). Despite being a top contributor to disability (García-Peña et al., 2013) and cognitive impairment (Diniz et al., 2013), depression in older adults is often undertreated (Licht-Strunk et al., 2009).
The vascular depression hypothesis has been used to explain how CVD promulgates late-onset depression (Alexopoulous et al., 1997). Cardinal features of late-onset depression, here taken to indicate depression with incidence after age 65, are diagnosis of incident depression at age 65 and older and presence of vascular disease or cardiovascular risk factors (Alexopoulous et al., 1997). Secondary features include greater psychomotor disturbance, apathy, executive dysfunction, lesions in basal ganglia, and white matter hyperintensities (Thomas et al., 2004). Modifiable cardiovascular risk factors include diabetes mellitus, hypertension, hyperlipidemia, obesity, and smoking (Barnes and Yaffe, 2011). The influence of cardiovascular risk factors on incident depression is likely more pronounced in older adults than in middleaged or younger adults, due to underlying physiological mechanisms (Taylor et al., 2013).
Previous studies of associations of midlife cardiovascular risk factors and late-onset depression have used cohorts with short durations of follow-up or follow-up without repeated measurement of midlife cardiovascular risk factors (Barnes et al., 2012; Sheline et al., 2006). The present investigation addresses these limitations by leveraging high-quality prospectively collected data on cardiovascular risk factors and depression from a long-standing cohort with extensive follow-up. The study is well-suited to examine cardiovascular risk factors occurring before vs. after age 65 and diagnosis of incident depression.
We evaluated the timing during life of the occurrence of modifiable cardiovascular risk factors and determined the extent to which they are associated with subsequent development of incident depression. Pursuant to the vascular depression hypothesis, we hypothesized vascular risk factor burden and cardiovascular risk factors occurring before age 65 are associated with increased risk of developing incident depression after age 65, not before age 65. We examined this hypothesis using data up to age 65 to examine the association of cardiovascular risk factors with incident depression before age 65. Among participants who survived depression-free up to age 65, we determined whether the presence of cardiovascular risk factors occurring before vs. after age 65 was associated with onset of incident depression after age 65 (Figure 1).
Figure 1.
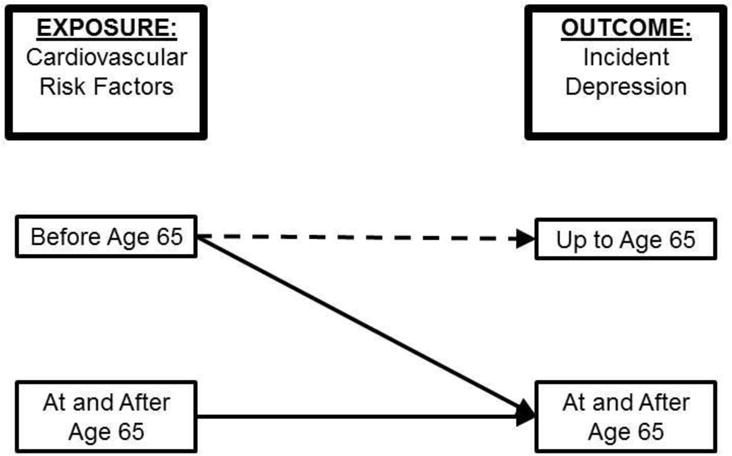
Conceptual framework for the analysis.
The dotted line illustrates the association of cardiovascular risk factors occurring before age 65 and the onset of incident depression up to age 65, using the full cohort. The solid lines show the association between cardiovascular risk factors occurring before age 65 or at and after age 65 and the onset of incident depression at and after age 65, using a subset of the cohort who survived up to age 65. Cardiovascular risk factors occurring after the onset of incident depression were excluded from the analysis. We used the cutoff of age 65 since onset after age 65 years was one of the cardinal features of vascular depression.
2. Methods
2.1 The Johns Hopkins Precursors Study
The Johns Hopkins Precursors Study, initiated in 1946, enrolled 1,337 medical school students in matriculating classes from 1948–1964 of The Johns Hopkins School of Medicine. Participants are followed approximately annually through mailed questionnaires to update morbidity and exposure information (Figure 2). The average 5-year period response rate is 90% (range 87% to 94%). Vital status is known for over 99% of the cohort. Self-reports of body mass index (BMI) and systolic blood pressure (SBP) have been validated externally (Klag et al., 1993). Study procedures are reviewed regularly and approved by the Johns Hopkins University School of Medicine Institutional Review Board.
Figure 2.
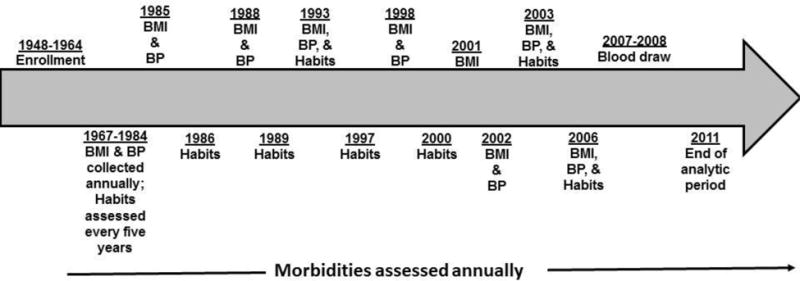
Brief timeline of data collection in the Johns Hopkins Precursors Study
Diagnoses for morbidities, cardiovascular disease, hypertension, and depression, were assessed approximately annually after graduation from medical school. Data about health behaviors (body mass index (BMI), blood pressure (BP) measurements) and habits (smoking status and frequency of alcohol consumption) were assessed at enrollment into the study around medical school graduation. These characteristics were assessed every five years from 1966–1984. After 1984, BMI and BP measurements were assessed in 1985, 1988, 1993, 1998, 2001 (BMI only), 2002, 2003, and 2006. Hypertension was defined as self-reported blood pressure ≥160/95 mm Hg on one annual questionnaire, ≥140/90 mm Hg on ≥2 annual questionnaires, or as hypertension requiring drug therapy.(Klag et al., 2002; Wang et al., 2008) Participants self-reported multiple blood pressure readings varying from one to seven per questionnaire on approximately annual questionnaires, so an average of the SBPs was taken for each age of reported blood pressures. After 1984, habits were assessed in 1986, 1989, 1993, 1997, 2000, 2003, and 2006. Blood draw measures consisted of a complete blood panel with measures on total and HDL cholesterol. Total cholesterol was collected during medical school and in 2007–2008. HDL cholesterol was only collected in 2007–2008.
We aimed to examine incident depression, hence excluded participants diagnosed with clinical depression before graduating from medical school (N=16), and those with no follow-up (N=9). The final sample size for this study was N=1, 190 participants.
2.2 Incident Depression
The primary outcome was first diagnosis of depression. Participants or family members submitted approximately annually mailed questionnaires inquiring about medical and psychiatric conditions in a checklist (Chang et al., 1997; Ford et al., 1998). Also, they answered questions about use of antidepressant medication multiple times and lifetime history of receiving care from a mental health specialist for an emotional problem in 1988 (Chang et al., 1997; Ford et al., 1998). Depression before medical school graduation were ascertained by history and physical examination, review of student health records for information about treatment and hospitalization for depression, and exit interviews with specific questions about depressive symptoms. Those who committed suicide were included in the case definition of depression.
Additionally, a committee of physician reviewers, unaware of the study’s hypothesis, adjudicated diagnoses alongside age of onset after reviewing participant self-reports (Chang et al., 1997; Ford et al., 1998). Strict adherence to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition was not possible, so the term, major depression, is not used. Questions about depression treatment assessed validity of the diagnosis of clinical depression (Chang et al., 1997; Ford et al., 1998).
2.3 Vascular risk factor burden
Vascular risk factor burden was defined by an adapted version of the Framingham CVD Risk Score (FCRS). FCRS is validated in participants aged 30–74 years without CVD, so we calculated scores within that age range (Cook et al., 2012; D’Agostino et al., 2008). The adapted version of the FCRS incorporates current smoking status, BMI, age, diabetes, and treated or untreated SBP (Cook et al., 2012). We used this version, since data for total and HDL cholesterol were unavailable for most visits. We assumed participants diagnosed as hypertensive were treated for hypertension because diagnostic criteria for hypertension in the study included drug therapy (Wang et al., 2008).
2.4 Cardiovascular risk factors
Cardiovascular risk factors were time-varying diabetes, hypertension, hyperlipidemia, overweight/obese status, and ever smoking status. Figure 2 provides the data collection schedule of these risk factors. Participants reported age and year of onset of diabetes, hypertension, and hyperlipidemia, which were confirmed by an adjudication committee. Overweight/obesity status was defined at each study interval by a cut-off of BMI>25 kg/m2 (Barone et al., 2006). For ever-smoking status, participants were classified as current/former smokers or never smokers in each year of follow-up (Figure 2).
2.5 Adjustment Covariates
All models were adjusted by white race, baseline age, enrollment cohort (1957–1964 vs. 1948–1956), heavy alcohol use (daily/almost daily vs. less than daily) and physical activity (none vs. some) at baseline and age 65.
2.6 Analytic strategy
We characterized the sample using means and percentages at baseline and age 65. Using Kaplan-Meier analysis, we compared the incidence of clinical depression developing at age 65 and older for time-invariant versions of each cardiovascular risk factor prior to age 65 (Kaplan and Meier, 1958). The difference in depression incidence between ever having and never having each cardiovascular risk factor before age 65 was tested using log-rank tests (Peto and Peto, 1972). This approach enabled us to assess whether the presence of cardiovascular risk factors before age 65 contributes to risk for incident depression after age 65.
We compared the incidence of depression before and after age 65 using discrete-time Cox proportional hazards survival models (Cox, 1972; Prentice and Gloeckler, 1978). We first modeled associations of time-varying indicators for vascular risk factor burden and cardiovascular risk factors occurring before age 65 with incident depression before age 65 (dotted line in Figure 1). We did not anticipate strong associations, since the vascular depression hypothesis posits association of cardiovascular risk factors with depression onset in late life. We tested the proportional hazards assumption through log-log plots (Cox, 1972).
Next, we modeled associations of time-varying indicators of vascular risk factor burden and cardiovascular risk factors occurring before and after age 65 with incident depression at age 65 or older using the subset that survived depression-free until age 65 (solid lines in Figure 1). In other models to tease apart the timing of the onset of vascular risk factor burden and each cardiovascular risk factor on risk for incident depression, we used a three-level time-varying indicator for each cardiovascular risk factor: (1) newly occurred at age 65 or older, (2) newly occurred before age 65, and (3) never occurred as the reference group.
Hazard ratios and 95% confidence intervals for associations were estimated using discrete-time Cox proportional hazards models (Cox, 1972; Prentice and Gloeckler, 1978). Participants contributed time from age at graduation from medical school until age of incident depression, censoring due to loss to follow-up or death, or age of administrative censoring in 2011. To maintain temporality, we excluded people with incident depression before each cardiovascular risk factor.
FCRS was standardized to two standard deviations of the baseline distribution to place effect estimates on approximately the same scale as those for the binary CVD indicators (Gelman, 2008). To assist clinical interpretation, we divided the hazard for vascular risk factor burden by the coefficient for baseline age to estimate how much older a person would have to be at baseline to have the same magnitude of risk for incident depression. Statistical significance was defined by α<0.05. Analyses were conducted using STATA 13.1 (StataCorp, 2013).
2.7 Sensitivity analysis
To examine potential reverse causation, we calculated percentages of those who switched cardiovascular risk factor status from absence to presence and vice versa prior to one-year of incident depression. We excluded these participants with potential co-occurring events to see if inferences changed. To evaluate the robustness of our findings for the selected age cutoff, we repeated the analysis using ages 70 and 75 instead of age 65. To evaluate the potential for death being responsible for associations with cardiovascular risk factors instead of depression, we used competing-risks regression based on proportional sub-hazards model to examine the association of cardiovascular risk factors with onset of death before incident depression (Fine and Gray, 1999). Despite small sample size, we repeated statistical analyses in women to evaluate robustness of associations by sex.
3. Results
3.1 Sample characteristics
Table 1 shows sample characteristics of 1,171 white and 19 non-white male participants at baseline (medical school graduation) and at age 65. Mean age at graduation from medical school was 26.3 years (standard deviation, [SD]=2.3 years). Fifty-two percent of the sample enrolled between 1948 and 1956 (Table 1). Mean SBP rose from 116.0 mmHg (SD=9.3 mmHg) at baseline to 127.6 mmHg (SD=13.7 mmHg) at age 65. The number of cases of diabetes, hypertension, and hyperlipidemia increased between baseline and age 65 (Table 1). Mean BMI was 23.1 kg/m2 (SD=2.6 kg/m2) at baseline and 25.0 kg/m2 (SD=3.4 kg/m2) at age 65. There were 556 (50.9%) current smokers at baseline and 60 (9.9%) at age 65 (Table 1).
Table 1.
Comparison of male participants at baseline and age 65 from the Johns Hopkins Precursors Study (1947–2011).
| Characteristics | Baseline n=1,190 |
Age 65 n=821 |
|---|---|---|
| Age at Graduation, mean (SD) | 26.3 (2.3) | — |
| Enrollment Wave | ||
| 1948–1956 | 623 (52.4) | — |
| 1957–1964 | 567 (47.7) | — |
| Total Cholesterol, mean (SD), mg/dL | 192.4 (29.2) | — |
| Systolic Blood Pressure, mean (SD), mm Hg | 116.0 (9.3) | 127.6 (13.7) |
| Diabetes, n (%) | 6 (0.6) | 50 (8.2) |
| Hypertension, n (%) | 28 (2.6) | 253 (41.2) |
| Hyperlipidemia, n (%) | 94 (8.7) | 251 (40.6) |
| BMI, mean (SD), kg/m2 | 23.1 (2.6) | 25.0 (3.4) |
| Current Smoker, n (%) | 556 (50.9) | 60 (9.9) |
| Lack of Physical Activity, n (%) | 501 (50.5) | 258 (32.7) |
| Heavy Alcohol Use, n (%) | 87 (8.3) | 161 (19.2) |
| Caucasian, n (%) | 1,171 (98.4) | — |
P-values based on chi-squared tests and t-tests. Tabulated values are means with standard deviations in parentheses, except where noted. mg/dL=milligrams per deciliter; mm Hg=millimeters of mercury; kg/m2=kilograms per meters-squared
There were 265 cases of incident depression (mean age of diagnosis = 57.1 years [SD=14.7 years]). The incidence of depression was 22.3% in the overall sample, 14.7% (n=175) before age 65 (N=1,190), and 11.0% (n=90) at age 65 and older (N=821). Among participants who died before age 65 (n=134, 11.3%), 28 (20.9%) had incident depression. Among participants who died after age 65 (n=321, 27.0%), 88 (27.4%) had incident depression.
3.2 Cardiovascular risk factors and depression up to age 65
Using data up to age 65, participants contributed 38,825 person-years between baseline and onset of incident depression or age 65 (median 56 years). Table 2 shows unadjusted and adjusted hazard ratios for associations of vascular risk factor burden and cardiovascular risk factors with onset of incident depression up to age 65. After adjustment, vascular risk factor burden before age 65 was not associated with onset of incident depression up to age 65 (Hazard Ratio [HR]:0.84, 95% Confidence Interval [CI]: 0.59, 1.21) (Table 2, top row). Overweight/obese men had 0.62 times the risk of depression before age 65 than men of normal BMI (95% CI: 0.42, 0.93). No other associations between cardiovascular risk factors and onset of incident depression up to age 65 were appreciably greater than null.
Table 2.
Associations of vascular risk factor burden and cardiovascular risk factors with incident clinical depression before age 65 (N=1,190). Data from the Johns Hopkins Precursors Study (1947–2011).
| Cardiovascular Risk Factors | Unadjusted
|
Adjusted+
|
||
|---|---|---|---|---|
| Hazard Ratio | 95% Confidence Interval | Hazard Ratio | 95% Confidence Interval | |
| Vascular risk factor burden (FCRS) | 0.83 | (0.59, 1.15) | 0.84 | (0.59, 1.21) |
|
| ||||
| Diabetes | 0.93 | (0.34, 2.51) | 1.00 | (0.37, 2.73) |
| Hypertension | 1.06 | (0.70, 1.61) | 1.01 | (0.64, 1.60) |
| Hyperlipidemia | 1.02 | (0.68, 1.54) | 0.80 | (0.50, 1.27) |
| Overweight or Obese Status** | 0.78 | (0.55, 1.11) | 0.62* | (0.42, 0.92) |
| Ever Smoking Status | 0.89 | (0.65, 1.21) | 0.87 | (0.61, 1.23) |
Overweight or obese defined as 25kg/m2 or greater.
Adjusted by race, baseline age, enrollment wave, baseline lack of physical activity, and baseline heavy alcohol use.
FCRS = Framingham Cardiovascular Disease Risk Score
This analysis refers to the dotted line in Figure 1. The hazard ratio associated with the FCRS represents a 2 standard deviation difference in score. The reference for individual conditions is never having the cardiovascular risk factor.
3.3 Cardiovascular risk factors and depression among those depression-free to age 65
Figure 3 shows Kaplan-Meier survival curves comparing risk of incident depression diagnosed after age 65 between the group with a cardiovascular risk factor before age 65 vs. those who did not. Participants who survived depression-free up to age 65 contributed 44,175 person-years between baseline and onset of incident depression, death, or end of follow-up in 2011.
Figure 3.
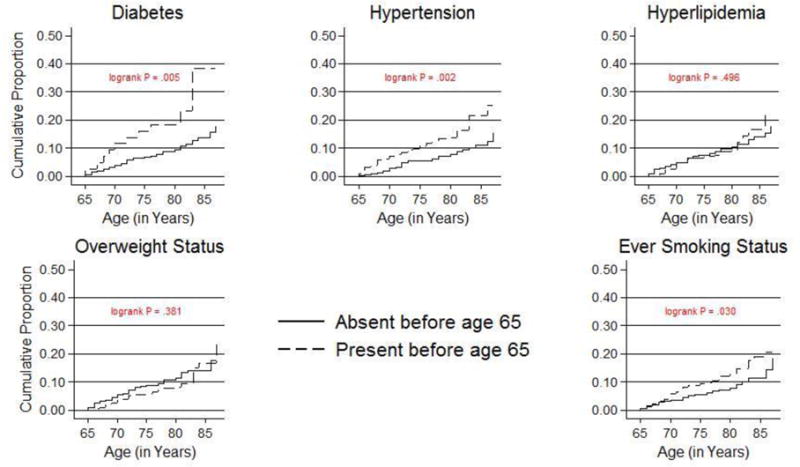
Kaplan-Meier plots of risk of incident clinical depression after age 65 by the presence of cardiovascular risk factors before age 65 (N=821).
The x-axis is age in years, and y-axis is the depression-free survival probability. These plots use data from participants who were depression-free before age 65.
Table 3 shows adjusted hazard ratios for the associations of vascular risk factor burden and cardiovascular risk factors present before vs. after age 65 with onset of incident depression among those who never developed depression prior to age 65. Analyses were adjusted for baseline age, enrollment wave, physical activity at age 65 and alcohol use at age 65. Vascular risk factor burden level after age 65 (HR: 2.13, 95% CI: 1.17, 3.90) were associated with onset of incident depression after age 65 (Table 3, top row). To provide context for this finding, we used the coefficient for baseline age in the hazards model to estimate how much older, on average, a person would be to have the same magnitude of elevated risk of incident depression for vascular risk factor burden. Among persons aged 65 and older, the elevated hazard of incident depression associated with a two SD higher vascular risk factor burden after age 65 is equivalent to being 8.2 years older.
Table 3.
Associations of vascular risk factor burden and cardiovascular risk factors with incident clinical depression after age 65 (N=821). Data from the Johns Hopkins Precursors Study (1947–2011).
| Cardiovascular Risk Factors | Occurrence of cardiovascular risk factors before age 65 Vs. Never
|
Occurrence of cardiovascular risk factors at age 65 and older Vs. Never
|
||
|---|---|---|---|---|
| Hazard Ratio | 95% Confidence Interval | Hazard Ratio | 95% Confidence Interval | |
| Vascular risk factor Burden (FCRS) | 1.78 | (0.22, 14.44) | 2.13* | (1.17, 3.90) |
|
| ||||
| Diabetes | 2.79* | (1.25, 6.26) | 2.87* | (1.24, 6.62) |
| Hypertension | 2.72* | (1.52, 4.88) | 2.01 | (0.87, 4.60) |
| Hyperlipidemia | 1.88* | (1.05, 3.35) | 1.48 | (0.69, 3.19) |
| Overweight or Obese Status** | 1.33 | (0.76, 2.33) | 1.46 | (0.42, 5.09) |
| Ever Smoking Status | 1.02 | (0.26, 3.96) | 1.35 | (0.77, 2.38) |
Statistically significant at P≤ 0.05.
Overweight or obese defined as 25kg/m2 or greater. All analyses were adjusted by race, baseline age, enrollment wave, lack of physical activity at age 65, and heavy alcohol use at age 65.
FCRS = Framingham Cardiovascular Disease Risk Score
This analysis refers to the two solid lines in Figure 1. The hazard ratio associated with the FCRS represents a 2 standard deviation difference in score. The reference for individual conditions is never having the cardiovascular risk factor.
Men with diabetes diagnosed before age 65 had 2.79 times the risk of developing incident depression after age 65 than those without diabetes (95% CI: 1.25, 6.26) after adjustment (Table 3). The same was true of hypertension (HR: 2.72, 95% CI: 1.52, 4.88) and hyperlipidemia (HR: 1.88, 95% CI: 1.05, 3.35). Men diagnosed with diabetes after age 65 had 2.87 times the risk of developing incident depression after age 65 than men without diabetes (95% CI: 1.24, 6.62) after adjustment (Table 3). No other cardiovascular risk factor after age 65 was associated with onset of incident depression after age 65 after covariate adjustment (Table 3).
3.4 Sensitivity analysis
Within one year of onset of incident depression (N=264, 22.5% of the sample), 3 (1.1%) started or stopped smoking, 22 (8.3%) developed diabetes, 6 (2.3%) developed hyperlipidemia, 0 (0.0%) experienced an increase in BMI to 25 kg/m2 or greater, and 20 (7.6%) developed hypertension. Inferences did not change after excluding these participants.
The adjusted sub-hazard ratios of associations of cardiovascular risk factors with onset of death before incident depression in both subsets are shown in Tables A1 and A2 in Appendix A. Among the overall cohort, cardiovascular risk factors were not associated with death more than depression up to age 65 (p >0.05) (Table A1 in Appendix A). Among the subset, diabetes before age 65 (Subhazard Ratio [SHR]=2.14, 95% CI: 1.17, 3.91) and ever smoking status after age 65 (SHR=2.23, 95% CI: 1.26, 3.91) were associated more with death than depression after age 65 (Table A2 in Appendix A).
Associations for women were in similar directions as those for men, although associations among women did not reach statistical significance (Tables B1 & B2 in Appendix B). Results were similar when using ages 70 and 75 as cutoffs (Tables C1 & C2 in Appendix C).
4. Discussion
In this longitudinal study of adults followed for up to 63 years after medical school, vascular risk factor burden and specifically, diabetes, hypertension, and hyperlipidemia were associated with increased risk of onset of incident depression among men who survived depression-free to age 65. Vascular risk factor burden accelerates onset of incident depression, since the magnitude of vascular risk factor burden after age 65 is comparable to the effect of 8.2 additional years of age. Vascular risk factor burden and cardiovascular risk factors, except for overweight/obese status, were not associated with onset of incident depression developing before age 65. These findings are consistent with the premise of the vascular depression hypothesis that cardiovascular risk factors may underlie depression in adults over age 65 (Alexopoulous et al., 1997).
Our study is broadly concurrent with past work distinguishing early-onset depression and late-onset depression pursuant to the vascular depression hypothesis (Bukh et al., 2011). There is substantial research suggesting greater roles medical and cardiovascular comorbidity in late-onset depression characterized as an initial depressive episode occurring later in life. Our study moves this research area by distinguishing between midlife and later-life cardiovascular risk factors.
The vascular depression hypothesis implies accumulation of small-vessel disease could compromise the integrity of subcortical regions of the brain involved in mood regulation (Taylor et al., 2013). Modifiable cardiovascular risk factors are associated with small-vessel brain disease (Taylor et al., 2013). As vascular risk factor burden increases with increasing age, small-vessel brain disease could accumulate, thus elevating the risk of the onset of clinical depression in older adults. Taylor et al. (2013) proposed that pro-inflammatory processes contribute to acceleration of vascular damage and deficits in perfusion, resulting in white matter lesions that disrupt structural and functional connectivity. Pro-inflammatory states arise in aging and chronic disease via cardiovascular risk factors.
Previous studies with shorter follow-up of one to two years have provided evidence in support of the vascular depression hypothesis, but none have examined these relationships throughout the life course as our study did (Lyness et al., 2000; Mast et al., 2004). Unlike previous studies of one cardiovascular risk factor (e.g., diabetes (Mezuk et al., 2008), hypertension (Meyer et al., 2004), hyperlipidemia (Chuang et al., 2014), and smoking status (Pasco et al., 2008)), the present study examined vascular risk factor burden and cardiovascular risk factors. Epidemiologic studies with incident cases of depression are rare, and the long-term follow-up of a single cohort confers a considerable advantage by sharpening the focus on the temporal relationship between the cardiovascular risk factors and lifetime depression onset.
We found an inverse association between overweight status and depression before age 65 among men. Evidence of an association between overweight status and depression has been mixed in other longitudinal studies (Palinkas et al., 1996; Roberts et al., 2000; Shelton and Miller, 2010). One potential biological mechanism is that carbohydrate cravings may temporarily relieve depressive symptoms via increased serotonergic activity (Lieberman et al., 1986). Further, we found a positive association between hyperlipidemia and depression onset after age 65, consistent with previous research (Chuang et al., 2014).
Our study has considerable strengths. The Johns Hopkins Precursors Study is a prospective cohort study with lengthy follow-up, high response rates, and repeated measurement of cardiovascular risk factors (Chang et al., 1997; Ford et al., 1998; Wang et al., 2008). One strength is the sample consists of former medical students who became physicians, likely increasing the accuracy of self-reported conditions, and the same participants are followed prospectively (Klag et al., 1993). Although the case definition for depression may have been more reliable if standard criteria were applied retrospectively, it would do so at the expense of validity if the presentation of depression changes with age. While new cases of depression identified in older age might be the most severe cases, if the participants reporting depression in later life were the most severe cases, then one would expect these participants would have reported depression earlier in life, not only in old age. Another advantage is the validity of self-reported cardiovascular risk factors compared to in-person measures, thereby ensuring minimal bias (Klag et al., 1993).
Several caveats of the study should be mentioned. Incidences of depression in our study are not comparable to nationally representative estimates because we excluded cases occurring before graduation, leading to lower incidence before age 65 than national estimates (Kessler and Wang, 2008). Additionally, physicians have higher rates of depression than the general population, thus the overall incidence rate in our sample of 22.3% is slightly higher than national estimates of 16.6% (Kessler and Wang, 2008).
Another caveat is other variables not incorporated into the analyses, i.e., genes and anxiety, could bias associations. We did not adjust models for mental status because medical school graduates are likely to perform at ceiling on screening tools developed to detect dementias later in the life course. Selection factors could also affect our study’s generalizability; particularly, high socioeconomic status could have a protective effect against depression, thus leading to a potentially conservative estimate of risk related to depression (Ford et al., 1998). This limitation is also an advantage because the sample’s homogeneity and selectivity ensures fewer unknown confounders. A third limitation is the small sample size and limited number of cases especially in the under-65 analysis, resulting in wide confidence intervals. Fourth, some participants (11%) died before age 65, thus death is a potentially competing outcome with depression after age 65 although sensitivity analyses showed no cardiovascular risk factors are associated with death prior to depression before age 65. A final limitation is that the sample and thus inferences are restricted to men. In a sensitivity analysis we repeated analyses in women; although associations were similar in magnitude, we were unable to conclude comparable relationships in women, due to limited sample size.
Depression is under-detected by primary care physicians (Licht-Strunk et al., 2009), yet related to earlier risk of disability and cognitive impairment (Diniz et al., 2013; García-Peña et al., 2013). The predictive associations of modifiable cardiovascular risk factors and vascular risk factor burden with onset of incident depression are consistent with the vascular depression hypothesis, and underscore the need to screen older adults with multiple cardiovascular risk factors for depressive symptoms. Depressive care management can reduce depressive symptoms, increase quality of life, increase antidepressant treatment adherence, decrease hospitalizations, and extend life (Gallo et al., 2013; Unutzer et al., 2002).
Supplementary Material
Highlights.
The vascular burden — depression association is comparable to 8.2 years of aging.
Results are consistent with the vascular depression hypothesis.
Diabetes, hypertension, and hyperlipidemia <age 65 increase depression risk ≥age 65.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexopoulos GS. The vascular depression hypothesis: 10 years later. Biological Psychiatry. 2010;60:1304–1305. doi: 10.1016/j.biopsych.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Alexopoulous GS, Meyers BS, Young RC, Campbell S, Silbersweig D, Charlson M. ’Vascular Depression’ Hypothesis. Archives of General Psychiatry. 1997;54:915–922. doi: 10.1001/archpsyc.1997.01830220033006. [DOI] [PubMed] [Google Scholar]
- Barnes DE, Yaffe K. The projected effect of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurology. 2011;10:819–828. doi: 10.1016/S1474-4422(11)70072-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes DE, Yaffe K, Byers AL, McCormick M, Schaefer C, Whitmer RA. Midlife vs. late-life depressive symptoms and the risk of dementia: differential effects for Alzheimer disease and vascular dementia. Archives of General Psychiatry. 2012;69:493–498. doi: 10.1001/archgenpsychiatry.2011.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barone BB, Clark JM, Wang NY, Meoni LA, Klag MJ, Brancati FL. Lifetime weight patterns in male physicians: the effects of cohort and selective survival. Obesity. 2006;14:902–908. doi: 10.1038/oby.2006.104. [DOI] [PubMed] [Google Scholar]
- Bukh JD, Bock C, Vinberg M, Gether U, Kessing LV. Differences Between Early and Late Onset Adult Depression. Clinical Practice and Epidemiology in Mental Health : CP & EMH. 2011;7:140–147. doi: 10.2174/1745017901107010140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang PP, Ford DE, Mead LA, Cooper-Patrick L, Klag MJ. Insomnia in young men and subsequent depression: The Johns Hopkins Precursors Study. American Journal of Epidemiology. 1997;146:105–114. doi: 10.1093/oxfordjournals.aje.a009241. [DOI] [PubMed] [Google Scholar]
- Chuang CS, Yang TY, Muo CH, Su HL, Sung FC, Kao CH. Hyperlipidemia, statin use and the risk of developing depression: a nationwide retrospective cohort study. General Hospital Psychiatry. 2014;36:497–501. doi: 10.1016/j.genhosppsych.2014.05.008. [DOI] [PubMed] [Google Scholar]
- Cook NR, Paynter NP, Eaton CB, Manson JE, Martin LW, Robinson JG, Rossouw JE, Wassertheil-Smoller S, Ridker PM. Comparison of the Framingham and Reynolds risk scores for global cardiovascular risk prediction in the Multiethnic Women’s Health Initiative. Circulation. 2012;125:1748–1756. doi: 10.1161/CIRCULATIONAHA.111.075929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox DR. Regression models and life tables (with discussion) Journal of the Royal Statistical Society, Series B. 1972;34:187–220. [Google Scholar]
- D’Agostino R, Vasan R, Pencina M, Wolf P, Cobain M, Massaro J, Kannel W. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117:743–753. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- Diniz BS, Butters MA, Albert SM, Dew MA, Reynolds CF. Late-life depression and risk of vascular dementia and Alzheimer’s disease: Systematic review and meta-analysis of community-based cohort studies. The British Journal of Psychiatry. 2013;202:329–335. doi: 10.1192/bjp.bp.112.118307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine J, Gray R. A proportional hazards model for the subdistribution of a competing risk. Journal of American Statistical Association. 1999;94:496–509. [Google Scholar]
- Ford D, Mead L, Chang P, Cooper-Patrick L, Wang N, Klag M. Depression is a risk factor for coronary artery disease in men: The Precursors Study. Archives of Internal Medicine. 1998;158:1422–1426. doi: 10.1001/archinte.158.13.1422. [DOI] [PubMed] [Google Scholar]
- Gallo J, Morales K, Bogner H, Raue P, Zee J, Bruce L, Reynolds C. Long term effect of depression care management on mortality in older adults: follow-up of cluster randomized clinical trial in primary care. BMJ. 2013;346:f2570. doi: 10.1136/bmj.f2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Peña C, Wagner FA, Sanchez-García S, Espinel-Bermudez C, Juarez-Cedillo T, Perez-Zepeda M, Arango-Lopera V, Franco-Marina F, Ramirez-Aldana R, Gallo JJ. Late-life depressive symptoms: Prediction models of change. Journal of Affective Disorders. 2013;150:886–894. doi: 10.1016/j.jad.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelman A. Scaling regression inputs by dividing by two standard deviations. Statistics in Medicine. 2008;27:2865–2873. doi: 10.1002/sim.3107. [DOI] [PubMed] [Google Scholar]
- Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Magid D, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, Moy CS, Mussolino ME, Nichol G, Paynter NP, Schreiner PJ, Sorlie PD, Stein J, Turan TN, Virani SS, Wong ND, Woo D, Turner MB, Subcommittee A.H.A.S.C.a.S.S Heart disease and stroke statistics-2013 update: A report from the American Heart Association. Circulation. 2013;127:e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan E, Meier P. Non-parametric estimation from incomplete observations. Journal of American Statistical Association. 1958;53:457–481. [Google Scholar]
- Kessler R, Wang P. The descriptive epidemiology of commonly occurring mental disorders in the United States. Annual Review of Public Health. 2008;29:115–129. doi: 10.1146/annurev.publhealth.29.020907.090847. [DOI] [PubMed] [Google Scholar]
- Klag MJ, He J, Mead LA, Ford DE, Pearson TA, Levine DM. Validity of physicians’ self-reports of cardiovascular disease risk factors. Annals of Epidemiology. 1993;3:442–447. doi: 10.1016/1047-2797(93)90074-e. [DOI] [PubMed] [Google Scholar]
- Klag MJ, Wang NY, Meoni LA, Brancati FL, Cooper LA, Liang KY, Young JH, Ford DE. Coffee intake and risk of hypertension: The Johns Hopkins Precursors Study. Archives of Internal Medicine. 2002;162:657–662. doi: 10.1001/archinte.162.6.657. [DOI] [PubMed] [Google Scholar]
- Licht-Strunk E, Beekman ATF, de Haan M, van Marwijk HW. The prognosis of undetected depression in older general practice patients: a one year follow-up study. Journal of Affective Disorders. 2009;114:310–315. doi: 10.1016/j.jad.2008.06.006. [DOI] [PubMed] [Google Scholar]
- Lieberman H, Wurtman J, Chew B. Changes in mood after carbohydrate consumption among obese individuals. The American Journal of Clinical Nutrition. 1986;44:772–778. doi: 10.1093/ajcn/44.6.772. [DOI] [PubMed] [Google Scholar]
- Lyness JM, King DA, Conwell Y, Cox C, Caine ED. Cerebrovascular risk factors and 1-year depression outcome in older primary care patients. American Journal of Psychiatry. 2000;157:1499–1501. doi: 10.1176/appi.ajp.157.9.1499. [DOI] [PubMed] [Google Scholar]
- Mast BT, Neufeld S, MacNeill SE, Lichtenberg PA. Longitudinal support for the relationship between vascular risk factors and late-life depressive symptoms. American Journal of Geriatric Psychiatry. 2004;12:93–101. [PubMed] [Google Scholar]
- Meyer C, Armenian H, Eaton W, Ford D. Incident hypertension associated with depression in the Baltimore Epidemiologic Catchment area follow-up study. Journal of Affective Disorders. 2004;83 doi: 10.1016/j.jad.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Mezuk B, Eaton WW, Albrecht S, Golden SH. Depression and type 2 diabetes over the lifespan: a meta-analysis. Diabetes Care. 2008;31:2383–2390. doi: 10.2337/dc08-0985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newberg A, Davydow D, Lee H. Cerebrovascular disease basis of depression: post-stroke depression and vascular depression. International Review of Psychiatry. 2006;18:433–441. doi: 10.1080/09540260600935447. [DOI] [PubMed] [Google Scholar]
- Palinkas LA, Wingard DL, Barrett-Connor E. Depressive symptoms in overweight and obese older adults: a test of the “jolly fat” hypothesis. Journal of Psychosomatic Research. 1996;40:59–66. doi: 10.1016/0022-3999(95)00542-0. [DOI] [PubMed] [Google Scholar]
- Pasco JA, Williams LJ, Jacka FN, Ng F, Henry MJ, Nicholson GC, Kotowicz MA, Berk M. Tobacco smoking as a risk factor for major depressive disorder: population-based study. The British Journal of Psychiatry. 2008;193:322–326. doi: 10.1192/bjp.bp.107.046706. [DOI] [PubMed] [Google Scholar]
- Peto R, Peto J. Asymptotically efficient rank invariant test procedures. (Series B).Journal of the Royal Statistical Society. 1972:135. [Google Scholar]
- Prentice R, Gloeckler L. Regression analysis of grouped survival data with application to breast cancer data. Biometrics. 1978;34:57–67. [PubMed] [Google Scholar]
- Roberts RE, Kaplan GA, Shema SJ, Strawbridge WJ. Are the obese at greater risk for depression? American Journal of Epidemiology. 2000;152:163–170. doi: 10.1093/aje/152.2.163. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Barch DM, Garcia K, Gersing K, Pieper C, Welsh-Bohmer K, Steffens DC, Doraiswamy PM. Cognitive function in late life depression: Relationships to depression severity, cerebrovascular risk factors and processing speed. Biological Psychiatry. 2006;60:58–65. doi: 10.1016/j.biopsych.2005.09.019. [DOI] [PubMed] [Google Scholar]
- Shelton RC, Miller AH. Eating ourselves to death (and despair): the contribution of adiposity and inflammation to depression. Progress in Neurobiology. 2010;91:275–299. doi: 10.1016/j.pneurobio.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- StataCorp. Stata Statistical Software: Release 13. StataCorp LP; College Station, TX: 2013. [Google Scholar]
- Taylor W, Aizenstein H, Alexopoulos GS. The vascular depression hypothesis: mechanisms linking vascular disease with depression. Molecular Psychiatry. 2013;18:963–974. doi: 10.1038/mp.2013.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas AJ, Kalaria RN, O’Brien JT. Depression and vascular disease: What is the relationship? Journal of Affective Disorders. 2004;79:81–95. doi: 10.1016/S0165-0327(02)00349-X. [DOI] [PubMed] [Google Scholar]
- Unutzer J, Katon W, Callahan C JW, Jr, Hunkeler E, Harpole L, Hoffing M, Penna RD, Noel P, Lin E, Arean P, Hegel M, Tang L, Belin T, Oishi S, Langston C, IMPACT Investigators. Improving Mood-Promoting Access to Collaborative Treatment Collaborative care management of late-life depression in the primary care setting: a randomized controlled trial. JAMA. 2002;288:2836–2845. doi: 10.1001/jama.288.22.2836. [DOI] [PubMed] [Google Scholar]
- Wang NY, Young JH, Meoni LA, Ford DE, Erlinger TP, Klag MJ. Blood pressure change and risk of hypertension associated with parental hypertension. Archives of Internal Medicine. 2008;168:643–648. doi: 10.1001/archinte.168.6.643. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


