Abstract
Background: Athanasios Koukopoulos proposed the primacy of mania hypothesis (PoM) in a 2006 book chapter and later, in two peer-reviewed papers with Nassir Ghaemi and other collaborators. This hypothesis supports that in bipolar disorder, mania leads to depression, while depression does not lead to mania.
Objective: To identify evidence in literature that supports or falsifies this hypothesis.
Method: We searched the medical literature (PubMed, Embase, PsycINFO, and the Cochrane Library) for peer-reviewed papers on the primacy of mania, the default mode function of the brain in normal people and in bipolar disorder patients, and on illusion superiority until 6 June, 2016. Papers resulting from searches were considered for appropriateness to our objective. We adopted the PRISMA method for our review. The search for consistency with PoM was filtered through the neurobiological results of superiority illusion studies.
Results: Out of a grand total of 139 records, 59 were included in our analysis. Of these, 36 were of uncertain value as to the primacy of mania hypothesis, 22 favoured it, and 1 was contrary, but the latter pooled patients in their manic and depressive phases, so to invalidate possible conclusions about its consistency with regard to PoM. All considered studies were not focused on PoM or superiority illusion, hence most of their results were, as expected, unrelated to the circuitry involved in superiority illusion. A considerable amount of evidence is consistent with the hypothesis, although indirectly so. Limitations. Only few studies compared manic with depressive phases, with the majority including patients in euthymia.
Conclusion: It is possible that humans have a natural tendency for elation/optimism and positive self-consideration, that are more akin to mania; the depressive state could be a consequence of frustrated or unsustainable mania. This would be consistent with PoM.
Keywords: Mania, Bipolar Disorder, Depression, Superiority Illusion, Primacy of Mania Hypothesis, functional Magnetic Resonance Imaging (fMRI)
INTRODUCTION
Athanasios Koukopoulos used to say “Mania is fire, depression its ashes” [1, 2]. He also reduced stigma in his patients by correctly informing them about their illness by stating “It’s not you, it’s life that is bipolar”. These two statements are the beginning and the conclusion of this paper. Put simply, ashes are produced by fire, but fire not always sparks from ashes, unless some other conditions are met. So if you quench fire, the amount of ash will be less, whereas if you are unable to set-off the fire, ash will ensue. We will use neurobiological evidence, mainly based on neuroimaging data, to support or to confute these points.
The starting lyrics of a 1969 popular song by the band “Blood, Sweat and Tears” were “What goes up must come down, Spinnin' wheel, got to go round”. This suits the issue of bipolar disorder (BD) better than the ca. XVIII Century idiom that had been attributed to Isaac Newton (1642-1727), that appeared also in Theodore Sedgwick's “Hints to my Countrymen” of 1826: «When one boy among a dozen throws a stone into the air, crying out, that “what goes up must come down,” it is very likely so to happen. » Presumably, this popular wisdom was used by Newton to support his gravitational theory, that later found experimental support, similarly to Einstein’s gravitational waves, the proof for which was only recently obtained. This story tells us that “On a Clear Day You Can See Forever”.1
Athanasios Koukopoulos was not a man of clairvoyance, but had things clear in his mind. He was likely to simplify problems, no matter how complex they were. This is how he came to theorise the primacy of mania (PoM). To reach his theorisation, he conjectured on writings of past masters and combined these with his own observations. During the late 18th Century, John Brown, a Scottish physician remarked «There is no life without excitement and no excitement without stimulus» [3]. Later, German psychiatrists [4,5] supported the notion that mania was the basic mental illness and that excitement was underpinned by neural tissue excitatory processes (astonishing intuition, given that Camillo Golgi’s neuronal doctrine at these times was still to come forth); this was reflected by Heinroth’s classification of melancholia saltans among the manias [4]. These views were increasingly strengthened in mid-20th Century literature [6]. Athanasios Koukopoulos with his life-long collaborator Daniela Reginaldi observed that when lithium reduced manic phenomena, it suppressed the expression of subsequent depressions. Conversely, when it was ineffective, depression regularly ensued after mania [7]. This led them to hypothesise that mania leads the dance and is the entity to suppress, so to reach remission. These are apparently naïve, simple observations, but simple as they may be, they were lacking in literature. Summarising, the PoM hypothesis holds that in the context of BD (the unhappy term that substituted manic-depressive illness or madness), manic excitement ensues in depression unless it is suppressed, while depression is not invariably followed by mania. This matches what is known about the neurobiology of excitement, that relies on neural excitatory activity; the end of a neuronal discharge is followed by a refractory period during which neurones are refractory to further excitatory stimulation, similarly to epileptic crises that are followed by a refractory period. This refractory period is an analogue to depression, that is characterised by inability to respond to stimuli. A paradigm of it may be represented by anhedonia, i.e., the inability to obtain pleasure from activities that in other times would have induced it. Thus, the patient is in a sort of refractory-to-pleasurable-stimulation period. When neurones are refractory to excitation, they will not respond to stimulation for a while; they should wait a millisecond to get back to the resting state, so to recover their excitability. Then, they will not immediately discharge, unless they receive a stimulus or until the conditions for a spontaneous excitation are fulfilled. This involves complex oscillatory phenomena which are intrinsic to individual neurones as well as emergent properties in groups of neurones, such as synchronisation. Matters become even more complicated when an individual is concerned in his/her totality. A person who is in a manic phase will go on to develop depression, whereas one in a depressive phase will not invariably jump to hypomania or mania, although in some cases, hitting the bottom may allow someone to rise above a standard mood measure. It depends on individual system properties, i.e., plasticity vs. elasticity, resilience, and the related ability to use stressful stimulation to grow through adversity.
Athanasios Koukopoulos and Nassir Ghaemi [2] offered their PoM model to testing, providing ways to prove it or falsify it. The PubMed search “primacy of mania” yielded 12 papers as of 6th June, 2016, but remarkably, no new record was added after December 2013. This speaks in favour of a general indifference for this concept, despite the possibility to test it easily. The same search identified 5 records on Embase as keyword, 6 on PsycINFO and 0 on the Cochrane Library. There were no additional records with respect to the PubMed search. The focused records of the search were two [2,8].
In March 2013, a group of Japanese investigators published a paper on “superiority illusion” that was supported by a reverberating circuit that is active during the resting state [9]. They used a combined functional magnetic resonance imaging (fMRI) and proton emission tomography (PET) technique and identified a reverberating circuit between the sensorimotor striatum and the dorsal anterior cingulate whose function is supported by dopamine. “Superiority illusion refers to the cognitive bias that most people rate themselves as above-average. The senior author believes this illusory self-evaluation to have been central in human evolution, as it helps people’s positive future outlook [10]; consequently, it can explain why individuals are attached to life, but also why some people may become megalomanic. In fact, people with mania have heightened dopaminergic transmission, and antidopaminergic drugs, such as the antipsychotics, are efficacious in reducing manic symptoms. Yamada et al. [9, 10] found that blocking the dopamine transporter, which enhanced the level of dopamine, increased the degree of the superiority illusion. The circuit is under the control of inhibitory cortical activity that is set-off by dopamine acting on striatal dopamine receptors, and this in turn leads to “disinhibited, heuristic, approaches to positive self-evaluation” [10], i.e., some degree of psychosis, if we define so a belief endorsed in the absence of adequate evidence. We then hypothesised that a tendency towards excitement is a natural human phenomenon and that depression may ensue as a result of reality testing, the more so when the positive self-consideration is far too excessive. So finding this reverberating circuitry hyperactive in the resting state in fMRI studies of individuals with bipolar disorder during their manic phase would support the plausibility of Koukopoulos’ PoM hypothesis.
The search “superiority illusion” OR “illusion of superiority” in PubMed yielded 23 records on 6th June 2016; of these 4 were relevant. The same search strategy in Embase produced 2 records, both relevant, 0 records in Cochrane Library, while in PsycINFO it yielded 15 records, 5 of which were relevant and overlapped with PubMed and Embase for only 1 record. Other 3 papers were retrieved serendipitously thanks to the Elsevier system’s and King’s College London procedures. The only record that had used neuroimaging to investigate superiority illusion was Yamada et al. [8]. Apart from the two papers from Yamada and from his group, the most meaningful study on this issue is Wolpe et al. [11]. This study found that people tend to judge their own performances better than performances of observed others; this is unaffected by learning and supports that the superiority illusion bias is based on optimistic traits and inbuilt “priors” that turn down objectivity when considering one’s own actions. The lack of objectivity for one’s own actions may be the basis of psychotic behaviour, that if it does not exceed too much objectivity, it may prove to be adaptive and appropriate in certain social conditions.
Our objective was to validate or disprove Athanasios Koukopoulos’ PoM hypothesis using literature findings of activation or deactivation maps or network connectivity strengths or FC in BD patients, and comparing them to the pattern of activity of the self-overvaluing network, as reported in Yamada et al. [9]. We guessed that if the results of brain activity studies in BD during mania matched those of the Yamada network and during depression were associated with reduced activity of the network, the PoM hypothesis could be valid and further testable, and if contrary evidence was obtained, the hypothesis would probably be not valid.
METHOD
The PubMed and Cochrane Library databases was searched for records on “resting state fMRI AND bipolar disorder”. The general, hyperinclusive nature of this search had the purpose to sample views of the subject that were as wide as possible and then to restrict to our focus. This method would ensure that no relevant papers were missed. Inclusion criteria were: original studies in peer-reviewed journals using fMRI during the resting state in patients with bipolar disorders and providing data of hyperactivations or hypoactivations and/or functional connectivity. Excluded were reviews and meta-analyses, opinion papers, editorials, and studies not providing data per group of subjects/patients, but only correlations of activations in groups considered according to indexes (for example, patients considered according to “bipolar index”, but not specifically belonging to a given diagnostic group). However, we used these papers’ reference lists for possible additional studies to include. We also excluded retracted papers and those papers by the same authors whose paper had been retracted. Studies that reported on the same groups of participants were considered as duplicates and excluded, unless different analyses were reported (for example analysing or not functional connectivity). Studies were also excluded when they investigated task-related activations or focused on only one region during the resting state. Congress abstracts were excluded, as they are not peer-reviewed. In performing this review, we used the Prisma method [12].
Apparently relevant records were all obtained and considered for appropriateness according to the above criteria using a Delphi method, i.e., all authors viewed obtained records and classified them according to how they provided the data, proposing inclusion or exclusion and the reasons of both. GDK, CR, GS and ADC gathered impressions and communicated approval or further questioning to all authors; when 100% agreement between authors was reached (this occurred at the end of the second round of consultations), all included papers were analysed and tabulated.
RESULTS
As of the 6th of June, 2016, 81 papers were found on PubMed on the resting state fMRI in bipolar disorder and 3 records on Cochrane Library, not overlapping with PubMed. All three Cochrane records were congress abstracts, with one duplicate of another, and were consequently excluded. Using the same key-words, Embase added 42 papers not overlapping with PubMed search, of which 40 were congress/conference abstracts (and 8 were duplicates) and 2 were included (however, 1 of these were contained in PubMed, but not identified by search strategy). PsycINFO added three more records, that were all excluded; in fact, one of them was a review, another was a comment to other papers, and the third used an attentional task, i.e., it was not resting-state. Hand searching of reference lists and Elsevier/King’s College additional proposals yielded 11 additional records, with the final output of studies to consider further reaching the amount of 93 records. Of these, 64 were relevant, i.e., they provided data that could refer to patients with a given phase of bipolar disorder. One study had been retracted, so we disregarded another paper by this group of authors. Three studies has been excluded because they reported the same data on the same patient and healthy control groups of other studies, two subsequent and one preceding, that were considered more complete. Hence, the final number of included studies was 60. Fig. (1) graphically depicts the results of our Prisma search. Included papers are listed in Table 1 that reports their relevant results, in particular those related to the primacy of mania hypothesis [13-72]. An overall judgement of each article’s concordance with the PoM hypothesis is provided at the fifth column of the Table (please, avoid conjectures). The evidence has been classified as favourable, contrary, or uncertain/unclear/not relevant, according to whether the network identified by Yamada et al. [9, 10] was found to be involved in the direction predicted by Yamada for superiority illusion; if the network strength or activation was found to be lower in mania than in depression, this was considered evidence against the hypothesis, and when reports focused or reported activations/deactivations or increased/decreased functional connectivity in circuits other than or unrelated to the one identified by Yamada et al., we considered the evidence neutral and uncertain as to the validity of the hypothesis. On these grounds, 23 studies were found to be consistent with the primacy of mania hypothesis, 1 was against, and 37 were uncertain/unclear/impossible to assess. The papers identified as possibly interesting for inclusion spanned from 2006 to 2016, but the first included was published in 2009 [13]. Reasons for exclusion are detailed in Fig. (1). A great majority of studies (79.66%) used functional connectivity (FC), and among those that did not, an equal number of studies used amplitude of low-frequency fluctuation (four) and regional homogeneity (four). The latter was also used in a study of FC as well.
Fig. (1).
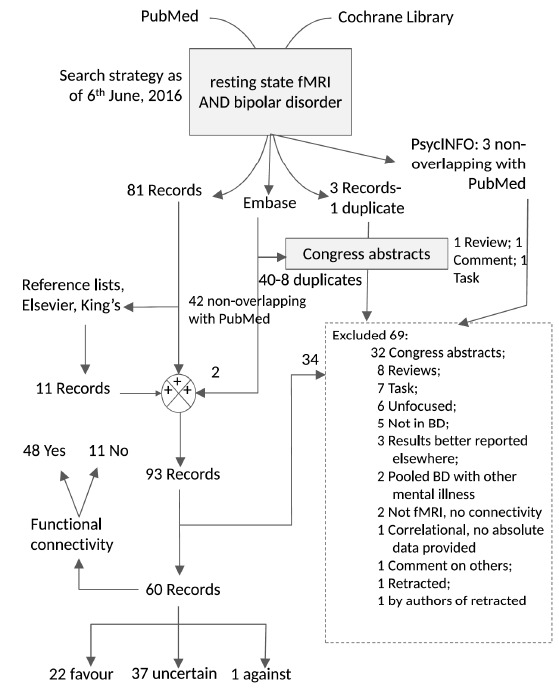
PRISMA search results with specific database contributions and reasons for exclusion. The cumulative figures for searches in the various databases were as follows: resting state fMRI AND bipolar disorder → PubMed 6.6.2016: 81 Records (1 retracted), Embase 1947-2016 Week 23-June 3: 64 Records (8 duplicates), 42 nonoverlapping with PubMed; 14 overlapping with PsycINFO; 3 overlapping with Cochrane; PsycINFO/PsycARTICLES 1806-June Week 1, 2016: 18 Records; 3 nonoverlapping with PubMed and Embase, 15 ovelapping with both PubMed and Embase; Cochrane Issue 5, May 2016: 3 overlapping with Embase, all congress abstracts, 1 duplicate.
Table 1. Included studies of fMRI in patients with bipolar disorder.
| Study | Sample | FC | Relevant Results | Relevance with PoM Hypothesis | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Anand et al. (2009) [13]* | 11 BD (6 manic and 5 depressive) vs. 15 MDD, all unmedicated, vs. 15 HC | Yes | ↓FC between striatum and subgenual anterior cingulate and between subgenual anterior cingulate and dorsomedial thalamus in both BD and MDD, compared to HC |
Against | |||||||||||||
| Chepenik et al. (2010) [14] | 15 BD (8 euthymic, 5 mixed/hypomanic/ manic, 2 depressive) vs. 10 HC |
Yes | left vPFC- left amygdala: greater neg. corr. in HC than in BD; left mvPFC- right hemisphere: greater pos. corr. in BD than in HC; left vPFC- dorsofrontal and parietal regions: greater neg. corr. in BD than in HC | Uncertain | |||||||||||||
| Dickstein et al. (2010) [15] | 15 pædiatric BD vs. 15 HC | Yes | left DLPFC-right STG: greater neg. corr. in BD than in HC; right STG-left MFG, right SFG, left thalamus/caudate: greater neg. corr. in BD than in HC; right STG-right parahippocampal gyrus: greater pos. corr in BD than in HC | Uncertain | |||||||||||||
| Chai et al. (2011) [16] | 14 BD with mania vs. 15 HC |
Yes | mPFC- bilateral dlPFC: neg. corr. in HC, NS corr. in BD; mPFC-right vlPFC: pos. corr. in BD, neg. corr. in HC; mPFC-left insula: pos. corr. in BD, neg. corr. in HC | Uncertain | |||||||||||||
| C.-H. Liu et al. (2012) [17] | 21 BD-I vs. 21 matched MDD | No | ↓ ALFF in left superior parietal lobule and left posterior insula; ↓ ALFF in right dorsal anterior insula in BD vs. MDD | Uncertain | |||||||||||||
| C.-H. Liu et al. (2012) [18] | 26 BD vs. 26 matched HC | Yes | ↑ ReHo in left medial frontal gyrus and left inferior parietal lobe in MDD vs. HC; no differences between BD and HC | Uncertain | |||||||||||||
| C.-H. Liu et al. (2012) [19] | 26 BD with depression vs. 26 matched HC | No | ↑ ALFF in left insula, right caudate, temporal gyrus, bilateral IFG, and posterior cerebellar lobe and ↓ ALFF in left postcentral gyrus, left parahippocampal gyrus, and cerebellum in BD with depression vs. HC; implies dysfunction in prefrontal-limbic networks and associated striatal systems | Favours | |||||||||||||
| C.-H. Liu et al. (2013) [20] | 21 BD with depression vs. 21 MDD vs. 26 matched HC | No | ↓ ReHo in right posterior cingulate cortex, right ventral anterior insular, and right parahippocampal gyrus, and ↑ ReHo in right MFG, right dorsal anterior insular, right posterior cerebellar gyrus, and left anterior cerebellar gyrus in BD with depression vs. MDD; ↓ ReHo in right parahippocampal gyrus in BD with depression vs. HC; ↓ ReHo in right MFG, right dorsal anterior insula, and right posterior cerebellar gyrus, and ↑ ReHo in right posterior cingulate in MDD vs. HC | Uncertain | |||||||||||||
| Liang et al. (2013) [21] | 17 BD vs. 16 MDD vs. 16 HC |
No | ↑ ReHo in right insular cortex, left middle frontal gyrus, left precuneus, left occipital lobe, left parietal, left superior frontal gyrus and left thalamus in BD; ↓ ReHo in right anterior cerebellar lobe, pons, right precentral gyrus, left postcentral gyrus, left IFG, and right cingulate. ↑ ReHo in left middle occipital lobe, right inferior parietal lobule, right precuneus and left convolution; ↓ ReHo in left parahippocampal gyrus, right precentral gyrus, left postcentral gyrus, left precentral gyrus, and left cingulate | Uncertain | |||||||||||||
| Xiao et al. (2013) [22] | 15 medicated pædiatric BD with mania vs. 15 matched HC |
No | ↑ ReHo in bilateral hippocampus, right anterior cingulate, right parahippocampal gyrus, and left caudate; ↓ ReHo in bilateral precuneus, bilateral precentral gyrus, bilateral SFG, bilateral superior parietal lobe, right OFC, and right STG | Favours | |||||||||||||
| Teng et al. (2013) [23] | 15 BD-I vs. 16 HC | Yes | ↑ FC from basal ganglia to left thalamus (visual network), to right parahippocampal gyrus (ventral default-mode network), to left parietal gyrus, precuneus, and angular gyrus (executive control network), and to the left anterior insula in BD vs. HC; ↓FC from basal ganglia to precuneus, to right retrosplenial cortex and posterior cingulate cortex (ventral default mode network), and to right caudate (right executive control) | Favours | |||||||||||||
| Reinke et al. (2013) [24] | 21 euthymic BD vs. 20 matched HC |
Yes | ↓FC between Heschl's gyrus/planum temporale and left STG/MTG in euthymic BD vs. HC; ↑ FC between frontal and temporal cortices and right IFG/precentral gyrus and insula in euthymic BD vs. HC | Uncertain | |||||||||||||
| Torrisi et al. (2013) [25] | 20 euthymic BD-I vs. 20 matched HC |
Yes | ↑ FC between right amygdala and right vlPFC in BD vs. HC; activity in BD partially mediated through the anterior cingulate | Favours | |||||||||||||
| Study | Sample | FC | Relevant Results | Relevance with PoM Hypothesis | |||||||||||||
| Mamah et al. (2013) [26] | 35 BD vs. 25 schizophrenia vs. 33 HC |
Yes | ↓FC in cingulo-opercular and cerebellar-salience networks in BD; ↓FC in schizophrenia more than in BD and in the salience and frontoparietal-cerebellar networks | Uncertain; more favours than not | |||||||||||||
| Meda et al. (2012) [27]# | 70 schizophrenia vs. 70 unaffected first-degree relatives vs. 64 BD with psychosis vs. 52 unaffected first-degree relatives vs. 118 matched HC | Yes | ↓FC in schizophrenia and BD with psychosis between fronto-occipital and default mode-prefrontal networks; ↓FC in schizophrenia between mesoparalimbic and sensorimotor networks; ↑ FC between frontotemporal-paralimbic and mesoparalimbic networks | Uncertain | |||||||||||||
| Meda et al. (2014) [28]# | 296 schizophrenia vs. 179 unaffected first-degree relatives vs. 300 BD with psychosis vs. 206 unaffected first-degree relatives vs. 324 matched HC |
Yes | ↓FC in schizophrenia and BD with psychosis within default mode networks with foci mPFC and anterior cingulate; abnormal connectivity between striatum and anterior cingulate in schizophrenia and BD with psychosis; abnormalities were similar between schizophrenia and their first-degree relatives, although less in the latter; no abnormalities in first-degree relatives of BD with psychosis |
Uncertain; slightly favours | |||||||||||||
| Anticevic et al. (2013) [29] | 68 euthymic BD with history of psychosis vs. 51 matched HC |
Yes | ↓intra-mPFC connectivity, ↑ connectivity between mPFC and amygdala, and ↓ connectivity between dlPFC and amygdala in BD, compatible with striatal suppression of cortical inhibition | Favours | |||||||||||||
| Anticevic et al. (2014) [30] | 47 euthymic BD (16.5% unmedicated) vs. matched HC | Yes | ↑ fronto-thalamic connectivity in BD | Uncertain | |||||||||||||
| Anticevic et al. (2014) [31] | 90 schizophrenia vs. 73 (33 with history of psychosis) euthymic BD vs. 146 HC | Yes | Mediodorsal thalamic-prefrontal and lateral geniculate-occipital dysconnectivity in schizophrenia, less consistently in BD, more severe in BD with history of psychosis; dysconnectivity with cerebellum present in schizophrenia and absent in BD | Uncertain | |||||||||||||
| Anticevic et al. (2015) [32] | 73 schizophrenia vs. 73 (33 with history of psychosis) euthymic BD vs. 56 HC |
Yes | ↓ FC in anterior cingulate-PFC in schizophrenia and BD with history of psychosis; ↓ FC in anterior cingulate-PFC and ↑ anterior cingulate-amygdalar FC in BD with or without history of psychosis | Favours | |||||||||||||
| Argyelan et al. (2014) [33] | 18 schizophrenia vs. 19 BD vs. 32 HC |
Yes | ↓ FC in paracingulate gyrus and right thalamus in schizophrenia and BD; schizophrenia showed additional ↓ FC in other areas | Uncertain | |||||||||||||
| Favre et al. (2014) [34] | 20 euthymic BD vs. 20 HC | Yes | mPFC-dlPFC: neg. corr. in HC, NS corr. in BD; mPFC-amygdala: pos. corr. in BD, NS corr. in HC | Uncertain | |||||||||||||
| Gao et al. (2014) [35] | 17 pædiatric BD with depression vs. 18 HC (age 10-18 years) |
No | ↓ ReHo in medial frontal gyrus, bilateral middle frontal gyrus, middle temporal gyrus, right putamen in BD vs. HC | Favours | |||||||||||||
| Rashid et al. (2014) [36] | 60 schizophrenia or schizoaffective disorder vs. 38 euthymic BD vs. 61 matched HC differing for gender composition |
Yes | Aberrant FC patterns in various default-mode components including dmPFC, bilateral angular gyrus, and bilateral precuneus in schizophrenia/schizoaffective disorder and BD | Uncertain | |||||||||||||
| Rashid et al. (2016) [37] | 60 schizophrenia or schizoaffective disorder vs. 38 euthymic BD vs. 61 matched HC differing for gender composition |
Yes | Functional network connectivity analysis of the previous results; the analysis allowed to classify subjects as BD, HC, and schizophrenia/schizoaffective disorder similarly to previous study |
Uncertain | |||||||||||||
| Singh et al. (2014) [38] | 24 healthy offspring of BD parents (8-17-year-old) vs. matched HC with no affected family members |
Yes | ↑ FC in vlPFC and other frontoparietal regions of the left executive control network in BD-risk vs. HC; ↓FC between left amygdala and pregenual cingulate, between subgenual cingulate and supplementary motor cortex, and between left vlPFC and left caudate |
Favours | |||||||||||||
| Study | Sample | FC | Relevant Results | Relevance with PoM Hypothesis | |||||||||||||
| H. Liu et al. (2014) [39] | 18 schizophrenia vs. 18 BD vs. 18 HC |
Yes | ↓ FC between laterobasal/centromedial amygdala and left dlPFC/left middle cingulate and ↑ FC between laterobasal amygdala and left rostral PFC in schizophrenia vs. HC; ↓ FC between laterobasal amygdala and right ventral and middle cingulate in BD vs. HC; ↓ FC between laterobasal/centromedial amygdala and right dlPFC in schizophrenia vs. BD; ↓ FC between laterobasal amygdala and pregenual anterior cingulate/rostral PFC in BD vs. schizophrenia; ↓ FC between centromedial amygdala and right ventral anterior cingulate/left pregenual cingulate/bilateral rostral PFC in BD vs. HC; ↓ FC between superficial amygdala and bilateral dlPFC/left middle cingulate and ↑ FC between superficial amygdala and left rostral/dlPFC in schizophrenia vs. HC; ↓ FC between superficial amygdala and left ventral anterior cingulate/right rostral PFC in BD vs. HC; ↓ FC between superficial amygdala and bilateral dlPFC in schizophrenia vs. BD; ↓ FC between superficial amygdala and pregenual/ventral cingulate/rostral PFC/bilateral OFC extending to the temporal pole in BD vs. schizophrenia | Uncertain | |||||||||||||
| Lu et al. (2014) [40] | 18 BD-I with mania vs. 18 matched HC |
No | ↑ ALFF in bilateral caudate and left pallidum, ↓ALFF in left precuneus, left superior parietal lobule, and bilateral inferior occipital gyrus in BD-I vs. HC | Favours | |||||||||||||
| Xu et al. (2014) [41] | 29 BD vs. 29 matched HC | No | ↑ ALFF in vm/vlPFC, dlPFC, frontal visual field, insula, and putamen extending to ventral striatum in BD vs. HC ↓ ALFF in lingual gyrus in BD vs. HC | Favours | |||||||||||||
| Yang et al. (2014) [42] | Independent samples with chronic schizophrenia (90 and 71 patients) vs. 73 BD vs. 220 HC |
Yes | Wide resting-state fluctuations in schizophrenia samples, but not in BD or HC (analysis of global brain signal) | Uncertain, not relevant | |||||||||||||
| Yip et al. (2014) [43] | 15 drug-naïve euthymic BD-II vs. 20 matched HC | Yes | ↑ FC in the temporo-insular resting-state network (↑ engagement across regions of the right caudate, left pre- and post-central gyri, left IFG, left supplementary motor area, bilateral putamen, and bilateral insula) in euthymic BD-II vs. HC | Uncertain | |||||||||||||
| Das et al. (2014) [44] | 16 BD vs. 14 with Borderline Personality Disorder vs. 13 HC | Yes | ↑ FC between the social salience network (dorsal anterior cingulate, OFC-insula, IFG, posterior superior temporal sulcus, inferior parietal lobule, temporoparietal junction) and each of the right frontoparietal network, the precuneus, and the vmPFC, and between the default-mode network and the precuneus in BD vs. borderline personality disorder; ↑ FC between default-mode and precuneus, and between social salience and vmPFC in BD vs. HC (differences did not survive multiple comparisons testing) | Favours | |||||||||||||
| Knöchel et al. (2014) [45] | 21 BD vs. 21 schizophrenia vs. 21 HC |
Yes | ↓ FC between hippocampus and left frontal lobe in BD vs. HC; ↓ FC between hippocampus and left frontal lobe/right lenticular nucleus/putamen, and left thalamus and ↑ FC in bilateral parahippocampal gyrus and bilateral cingulate in schizophrenia vs. HC | Uncertain | |||||||||||||
| Oertel-Knöchel et al. (2015) [46] | 21 euthymic BD vs. 21 matched HC |
Yes | ↓FC within frontal areas and between frontal and temporal/hippocampal/limbic regions in BD vs. HC; ↑ frontal-limbic FC in BD vs. HC | Not relevant; uncertain | |||||||||||||
| Lois et al. (2015) [47] | 30 euthymic BD-I vs. 35 matched HC |
Yes | ↑ FC between meso/paralimbic (amygdala, hippocampus, parahippocampal gyrus, and temporal lobes) and right frontoparietal networks in BD-I vs. HC | Uncertain | |||||||||||||
| Lui et al. (2015) [48] | 37 schizophrenia vs. 38 unaffected first-degree relatives vs. 57 psychotic BD vs. 28 unaffected first-degree relatives vs. 59 HC |
Yes | ↓ALFF in OFC and cingulate in schizophrenia and less in BD and their unaffected relatives; ↑ ALFF in striatal-thalamo-cortical networks in BD; ↑ FC between thalamus and bilateral insula in BD |
Favours | |||||||||||||
| Y. Liu et al. (2015) [49] | 17 BD vs. 17 MDD | Yes | ↑ connection strength between mPFC and posterior cingulate, and between right inferior parietal cortex and left hippocampus/right insula in BD vs. MDD; ↓/unapparent connection strength between mPFC/right insula and bilateral hippocampus in BD |
Uncertain | |||||||||||||
| Study | Sample | FC | Relevant Results | Relevance with PoM Hypothesis | |||||||||||||
| Du et al. (2015) [50] | 20 BD vs. 20 schizophrenia vs. 20 schizoaffective with mania vs. 13 schizoaffective with depression vs. 20 HC | No | ↑activity in right precuneus in BD correlating negatively with BPRS scores; activity in BD more similar to HC than other groups | Uncertain | |||||||||||||
| Jie et al. (2015) [51] | 21 BD vs. 25 MDD vs. 23 HC | No | ↑ activity in anterior cingulate in BD vs. MDD and vs. HC; differences were reported also in other structures | Favours | |||||||||||||
| Jie et al. (2015) [52] | 21 BD vs. 25 MDD vs. 23 HC | Yes | ↑ FC in hypothesis-related structures, like default-mode in BD vs. MDD; differences were reported also in the IFG, the dlPFC and the cerebellum | Slightly favours | |||||||||||||
| Li C.-T. et al. (2015) [53] | 20 euthymic BD vs. 20 unaffected siblings vs. 20 matched HC |
Yes | ↓ dlPFC-amygdalar FC in BD vs. siblings and in BD and siblings vs. HC; ↓ top down control from dlPFC to limbic structures | Favours | |||||||||||||
| Li M. et al. (2015) [54] | 18 BD with mania vs. 10 BD with depression vs. 28 HC | Yes | ↓ amygdala-IFG (orbital)/striatum/right lingual gyrus/posterior cerebellar lobe FC in BD vs. HC. ↑ amygdala-hippocampal FC in BD with mania vs. BD with depression | Favours | |||||||||||||
| Satterthwaite et al. (2015) [55] | 27 BD (21 BD-I) with depression vs. 25 MDD vs. 33 HC |
Yes | Nodal connectivity strength of left ventral striatum correlated inversely with depression severity (BDI scores) in both BD and MDD; diminished edge network connectivity with greater depression between ventral striatum and thalamus, and between ventral tegmental area and vmPFC | Favours | |||||||||||||
| Z. Wang et al. (2015) [56]# | 220 schizophrenia vs. 150 first-degree relatives vs. 147 with schizoaffective disorder vs. 126 first-degree relatives vs. 180 with psychotic BD vs. 134 first-degree relatives vs. 242 HC |
No | Structural MRI and resting-state fMRI scans were combined to provide “fused” data of combined structural/functional alterations. Combined structural abnormalities in default-mode network and functional prefrontal–striatal–thalamic–cerebellar network alterations in schizophrenia, schizoaffective disorder and BD vs. HC; fused alteration in temporal lobe in schizophrenia and schizoaffective disorder, but not in BD; no alterations detected in groups of relatives | Uncertain | |||||||||||||
| Stoddard et al. (2015) [57] | 14 BD vs. 19 with severe mood dysregulation vs. 20 HC in the 9–18.5 years age range | Yes | ↑ FC between left basolateral amygdala-medial left frontal pole and basolateral amygdala-posterior cingulate/precuneus in BD vs. mood dysregulation vs. HC (focused only connectivity concerning basolateral, centromedial, and superficial amygdala) | Unclear | |||||||||||||
| Stoddard et al. (2016) [58] | 39 BD (22 aged 10-22; 17 aged >22-50) (27 euthymic, 3 mixed/hypomanic, 7 depressed) vs. 78 HC (36 aged 10-22; 42 aged >22-50) |
Yes | ↑ FC in right inferior parietal lobule right posterior cingulate, left STG, and left precentral gyrus in BD vs. HC | Unclear | |||||||||||||
| Y. Wang et al. (2015) [59] | 26 unmedicated BD-II with depression vs. 40 HC | Yes | ↓FC in mPFC (core hub of default-mode network) and ITG (interhemispheric synchronisation) in BD-II with depression vs. HC | Uncertain | |||||||||||||
| Y. Wang et al. (2015) [60] | 36 BD-II with depression (24 unmedicated) vs. 32 MDD with depression (23 unmedicated) vs. 40 HC |
Yes | No differences in FC between BD-II with depression and MDD with depression; ↓FC in fusiform/lingual gyrus, and anterior and posterior cerebellar lobes in BD-II with depression vs. HC; ↓FC in posterior cingulate, fusiform/lingual gyrus, and posterior cerebellar lobe in MDD with depression vs. HC. Both BD-II and MDD show interhemispheric abnormalities |
Uncertain | |||||||||||||
| Y. Wang et al. (2016) [61] | 37 unmedicated BD-II with depression vs. 37 HC | Yes | ↓FC strength in bilateral mPFC, bilateral MTG, left precuneus, and right posterior cingulate (default-mode network), right supramarginal gyrus and angular gyrus, right SFG, and right superior parietal gyrus in BD-II with depression vs. HC; ↑ FC strength in bilateral temporal pole (including parahippocampal gyrus and amygdalæ), left anterior cingulate, left STG, right lingual gyrus, and left anterior cerebellar lobe in BD-II with depression vs. HC; results consistent with decreased default-mode and limbic function during depression in BD-II | Uncertain | |||||||||||||
| Study | Sample | FC | Relevant Results | Relevance with PoM Hypothesis | |||||||||||||
| Altinay et al. (2016) [62] | 30 BD with mania/hypomania vs. 30 BD with depression, all unmedicated, vs. 30 HC |
Yes | ↑ FC between left dorsal striatum and midbrain; ↑ FC between ventral striatum and thalamus in BD with mania/hypomania; ↑ FC between dorsal striatum and insula and temporal cortex in BD with depression; extensive abnormalities in BD connectivity between striatum on one hand, and cortex, midbrain, and limbic structures on the other | Favours | |||||||||||||
| Goya-Maldonado et al. (2016) [63] | 20 BD with depression vs. 20 MDD | Yes | ↑ FC in the frontoparietal network in BD; ↑ FC in default network in MDD; ↓ FC in anterior, pregenual and subgenual cingulate in MDD | Slightly favours | |||||||||||||
| He et al. 2016 [64] | 13 BD vs. 40 MDD vs. 33 HC, all unmedicated |
Yes | ↑ FC in dlPFC and vlPFC with anterior cingulate in BD compared to MDD | Favours | |||||||||||||
| Lv et al. (2016) [65] | 23 BD with depression, 19 euthymic BD (medicated with benzodiazepines) vs. 28 matched HC | Yes | ↓FC strength in language regions (left triangular part of IFG, left opercular part of IFG, left middle temporal gyrus, and left angular gyrus) in BD with depression vs. HC | Uncertain | |||||||||||||
| Magioncalda et al. (2016) [66] | 40 BD (11 in manic phase, 11 in depressive, 7 in mixed state, and 11 euthymic) vs. 40 matched HC |
Yes | ↓FC between perigenual anterior cingulate to posterior cingulate and ITG (default mode network) and in salience network (supragenual anterior cingulate and vlPFC) in BD vs. HC. Uncoupling between perigenual anterior cingulate FC and variability within target regions in BD; ↓ FC between perigenual anterior cingulate and supragenual cingulate in BD with depression and between perigenual anterior cingulate and posterior cingulate in BD with mania | Slightly favours | |||||||||||||
| Martino et al. (2016) [67] | 20 BD with depression vs. 20 BD with mania vs. 20 BD in euthymia vs. 40 HC | Yes | ↑ FC in default mode vs. sensorimotor networks in BD with depression; ↑ FC in sensorimotor vs. default mode networks in BD with mania; balanced activity in BD with euthymia; whereas the opposite topographical pattern was observed in mania | Uncertain | |||||||||||||
| Rey et al. (2016) [68]* | 27 BD (15 euthymic vs. 12 non-euthymic: 7 with depression, 3 with hypomania, and 2 with mixed state) vs. 27 matched HC | Yes | ↑ FC in left amygdala-left subgenual anterior cingulate and posterior cingulate in BD vs. HC; ↓FC between right amygdala and subgenual anterior cingulate in non-euthymic BD; ↓FC between posterior cingulate and subgenual anterior in euthymic BD; ↑ FC between subgenual anterior cingulate and right vlPFC in euthymic BD | Impossible to assess due to lumping depressive with hypomanic/mixed | |||||||||||||
| Skåtun et al. (2016) [69] | 71 schizophrenia vs. 43 BD vs. 196 HC |
Yes | ↓FC in high-centrality clusters, i.e., sensory regions in schizophrenia and subcortical regions in both schizophrenia and BD; ↑ FC in frontal and parietal clusters in schizophrenia, intermediate alterations in BD | Uncertain | |||||||||||||
| Solé-Padullés et al. (2016) [70] | 27 offspring of patients with schizophrenia vs. 39 offspring of patients with BD vs. 40 offspring of HC (all three samples in the 7-19 year-old range) |
Yes | No difference in FC between offspring of patients with BD and offspring of HC; ↓FC in offspring of patients with schizophrenia in left basal ganglia network vs. HC. FC correlated with left caudate grey matter volume (i.e., the greater the grey matter loss, the less the connectivity). The patient-related sample drove the results | Uncertain | |||||||||||||
| Souza-Queiroz et al. (2016) [71] | 32 BD (most were medicated) vs. 47 HC |
Yes | No difference in FC between vmPFC and amygdala or hippocampus and in general fractional anisotropy in the uncinate fasciculus in BD vs. HC | Unclear-uncertain | |||||||||||||
| Brady et al. (2016) [72] | 28 manic BD vs. 24 euthymic BD (all medicated) vs. 23 matched HC | Yes | ↓ FC between amygdala and anterior cingulate in BD with mania vs. eythymic BD; ↑ FC between amygdala and supplementary motor area in BD with mania vs. euthymic BD; ↑ FC between amygdala and left cerebellum in BD with mania vs. HC; ↑ FC between left vlPFC and anterior cingulate in euthymic BD vs. manic BD; ↑ FC between OFC and cerebellum in manic BD vs. euthymic BD and HC | Uncertain | |||||||||||||
*These studies did not distinguish between BD patients with opposite phases. Caution needed in interpreting their results. #This group found different results with the same methodology in these two studies. Two further studies by the same group reported on schizophrenia spectrum and involved large numbers of schizoaffective disorder patients. The diagnostic consistency of these studies, that reported on samples without defining whether the patients among across the three studies were different or partially overlapping, is doubtful.
Six of the 23 studies that showed consistency with PoM were not FC studies (26.09%), whereas only 5 of the 36 uncertain studies were not FC (13.89%). However, the difference was not statistically significant (χ2= 1.377; p=0.241; Fisher’s Exact Test=0.310; not significant).
DISCUSSION
Based on superiority illusion data, we plotted against them results regarding resting-state activation and connectivity patterns in BD during specific phases as related to PoM hypothesis. We found that this hypothesis is more likely than not, as the striate-anterior cingulate connection was often found to be strengthened in BD, especially during elevated mood phases, and the prefrontal control was found to be weakened, with almost opposite results during the depressive phase. The fact that we found only one contrary evidence that was marred by the indiscriminate, pooled processing of data of patients in their manic or depressive phase (in about equal numbers) [13] shifted this study among uncertain studies, as regards the PoM hypothesis.
The first author to mention the term “primacy of mania” was the American psychoanalyst Philip Weissman [73]. Weissman viewed the depressive phase as a failure of ego defences (denial, in particular) during the manic phase resulting from increased environmental stimulation or confrontation with reality; so he framed the wish to regain health as an unconscious wish to return to an idealised manic phase, thus assigning mania a driving role. However, Athanasios Koukopoulos’ view is psychodynamic theorisation-free and more clinically-based. If a system’s function moves away from its biologically set values, we observe a drive towards restoring of the previous condition, that Cannon called homœostasis [74] and needs no psychoanalytical speculations to be framed. If the resting-state activity is towards self-overvaluing, as predicted by the Yamada et al. model [9, 10], we may guess that the trend of brain activity
Abbreviations: ALFF, amplitude of low-frequency fluctuation; BD, patients with bipolar disorder; BD-I/II, bipolar disorder, type I or II; BDI, Beck Depression Inventory; BPRS, Brief Psychiatric Rating Scale; dmPFC, dorsomedial prefrontal cortex; dlPFC, dorsolateral prefrontal cortex; FC, Functional connectivity; HC, healthy controls; IFG, inferior frontal gyrus; ITG, inferior temporal gyrus; MDD, patients with major depressive disorder (unipolar depression); MFG, medial frontal gyrus; mPFC, medial prefrontal cortex; neg. corr., negative correlation; MTG, middle temporal gyrus; NS, not significant; pos. corr., positive correlation; OFC, orbitofrontal cortex; PoM, Primacy of Mania; ReHo, regional homogeneity; SFG, superior frontal gyrus; STG, superior temporal gyrus; vlPFC, ventrolateral prefrontal cortex; vmPFC, ventromedial prefrontal cortex.
transitions will be directed at this condition, hence implicitly recognising its intrinsic driving force. However, the depressive condition, no matter how strong or unconscious is the desire for getting back to elation, as Weissman supported, is not per se a spur pushing towards the desired direction, nobody may assure this. On the other hand, an increased dopaminergic activity with high striatal-anterior cingulate connectivity that sets-off or weakens executive cortical control results in an energetically highly expensive state, so it is plausible that its excessive activity cannot be maintained for a long time and if there is not much to counter the quenching of the fire, ashes will ensue. So we decided to consider strengthened connectivity or higher activity of the strio-thalamic-cingulate network activity as indicative of a mood more shifted toward the manic, while the opposite we considered as consistent with the existence of depressed mood; both these conditions are compatible with the PoM hypothesis.
To test adequately the PoM hypothesis, some conditions should be met that were not in this account. First, it should be shown that the Yamada et al. [9] circuit is overactive in mania and hypoactive in depression, and then, that prolonged hyperactivity in this circuit is invariably followed by hypoactivity, and also that hypoactivity is not invariably followed by hyperactivity. In this review of ours, we obtained partial evidence for the first condition. However, the conspicuous number of studies that were consistent with PoM, although not representing the great majority, prompts us to consider the evidence gathered so far as an encouraging starting point to proceed to further investigations.
The method employed to investigate brain network activity and brain area-related activations or deactivations did not influence the final judgement of consistency with PoM hypothesis. Generally, the studies that were not consistent with PoM focused on ROIs other than those involved in the network identified by Yamada et al. [9]. Most of these studies did not specifically report no involvement of the striatum, the anterior cingulate or the ventromedial prefrontal cortex; their results involved other structures. Studies compatible with the hypothesis generally investigated these areas and related networks, finding changes in BD patients that matched the prediction of the model, i.e., increased strio-anterior cingulate connectivity [24, 32, 48] or decreased cortical-striatal-control [23] in the manic BD phase and even in euthymia [25, 29, 53] and in unaffected first-degree relatives [38]. Opposite results were found in depressive phases, with decreased activity in the cingulate [63]. The Yamada model-network was more active in patients with BD compared to those with major depressive disorder [64]; although this is not a strong evidence in favour of the PoM hypothesis, it is indicative. The decreased intracingulate (anterior-posterior) FC found in Magioncalda et al. [64] may reflect sample heterogeneity, with equal numbers of patients in manic and depressive phases. Taken together, the data indicate that the PoM hypothesis may be valid.
Limitations
The data we considered here do not allow for definite conclusions. This is due to many factors, including the possible influence of drug treatment, that differed across studies or was not always addressed. Other factors may be the small sample sizes of most studies and the possible diagnostic inaccuracy of large studies. No study focused on the reverberating circuit identified by Yamada et al. [9], hence the evidence we detailed here is not direct. The focus on other areas also limited the possibility to obtain significant results for Yamada’s circuitry. Furthermore, several other circuits and areas emerged to differ between BD patients and controls, pointing to a greater complexity of the disorder. Moreover, a complex developing brain, with an inbuilt self-overvaluing, reverberating circuit, which is active normally at the resting state, may modulate the function of this circuit through interactions with other inbuilt systems, like the circuit underpinning the egalitarian drive [75], which originates mirror-neurone/representation-related phenomena like empathy and perspective taking, cooperativeness, social value orientation, altruism, compassion, and emotional contagion, that are subject to refinement through both evolution and personal development [76-81]. Interestingly, an empathy substrate is activity in insula and inferior frontal gyrus (IFG) [82, 83]; reduced IFG activity was found in patients with mania [54] and euthymic BD in an uncertain study as to the PoM hypothesis [65] and reduced insular activity in patients with mania [17], while insular activity and connectivity was found to be increased in patients with BD and depression [19, 62] and in euthymic BD [24]. It is possible that the empathy network negatively modulates the self-overvaluing circuit, so that some “uncertain” results we found relative to the PoM hypothesis could be due to interference carried by other networks. It is also worth considering that IFG, especially right, is a brain region involved in cognitive control and impulse control in general [84]. Therefore, its altered function in mania and/or euthymia may be consistent with this, possibly representing an impulse dyscontrol-related endophenotype, that was shown to be active during manic/mixed phases [85]. Another possible connection could be the social salience network, to which insular-IFG connectivity contributes [44]. However, social salience is little focused upon compared to generally-speaking salience, that does not share all social salience connectivities [86], and the few studies that found reduced salience network FC, did not distinguished between the various phases of BD, and were uncertain [26] or slightly favoured PoM [66]. Finally, a certain naiveté of our approach and our enthusiasm about the PoM hypothesis may have biased our judgements. However, the high number of people involved in this paper provided a buffer that held our speculations down to the ground, and the Delphi method we employed, the intense debate among group members controlled for the objectivity of our final decisions.
CONCLUSION
We obtained here preliminary evidence for the possible validity of the PoM hypothesis. Direct testing of the hypothesis that the Yamada circuitry is hyperactive during mania may be obtained by future, targeted studies. Observing the activity of this circuit during various phases of BD diachronically in the same patient will be another way to test the model. The final proof may be obtained by testing how lithium treatment affects this circuit’s function.
Summarising, it’s true that what goes up, must come down, but after touching bottom, one will rise is not always true, it may happen or not; it depends on how bouncing-back ability and energy one has. Hence, “it can’t go worse” is not followed suit by recovery, because “al peggio non c’è mai fine” (the worst never has an end). So it must be mania that drives depression, while the opposite is only seldom the case. The fact that a reverberating circuit is involved brings forth the idea of oscillation. Reverberating circuits are “a theory of periodic conduction through the cerebral cortex of trains of impulses traveling in circuits of neurons” [87]. Periodicity and oscillation are consequences of waveform activity, which is ubiquitous in the universe, and in life, which is part of it. So, as Athanasios Koukopoulos used to tell his patients, “it’s life that is bipolar”.
FUNDING
This study has been partially supported by an Educational Grant of the Ministry of Research of Italy (RBFR12LD0W_002).
ACKNOWLEDGEMENT
The authors wish to thank Ms Mimma Ariano, Ms Ales Casciaro, Ms Teresa Prioreschi, and Ms Susanna Rospo, Librarians of the Sant’Andrea Hospital, School of Medicine and Psychology, Sapienza University, Rome, for rendering precious bibliographical material accessible, as well as their Secretary Lucilla Martinelli for her assistance during the writing of the manuscript.
CONFLICT OF INTEREST
No author or any family member has financial relationships with any commercial organization that might appear to represent a conflict of interest with the material presented in this paper.
REFERENCES
- 1.Koukopoulos A. The primacy of mania. In: Akiskal H.S., Tohen M., editors. Bipolar Psycho- pharmacotherapy: Caring for the Patient. Hoboken, New Jersey: John Wiley and Sons; 2006. pp. 169–191. [http://dx.doi.org/10.1002/0470017953.ch10] [Google Scholar]
- 2.Koukopoulos A., Ghaemi S.N. The primacy of mania: a reconsideration of mood disorders. Eur. Psychiatry. 2009;24(2):125–134. doi: 10.1016/j.eurpsy.2008.07.006. [http://dx.doi.org/10.1016/j.eurpsy.2008.07.006]. [PMID: 18789854]. [DOI] [PubMed] [Google Scholar]
- 3.Brown J., Beddoes T., Blake W. The Elements of Medicine: Of John Brown, M. D. Translated From the Latin, With Comments and Illustrations By the Author. A new ed., rev. and corr.(Brown’s translation of Elementa Medicinæ Brunois, 1780); J. Johnson: London; 1795. [Google Scholar]
- 4.Heinroth J.C. Lehrbuch der Störungen des Seelenlebens oder der Seelenstörungen und ihrer Behandlung. Erster oder theoretischer Theil; Zweyter oder praktischer Theil. Leipzig: Fr. Chr. Wilh. Vogel; 1818. [Google Scholar]
- 5.Griesinger W. Pathologie und Therapie der psychischen Krankheiten für Ärzte und Studirenden, 1. Stuttgart: Verlag von Adolph Krabbe; 1845. [Google Scholar]
- 6.Dorcus R.M., Shaffer G.W. Textbook of Abnormal Psychology. 3rd ed. Baltimore: Williams & Wilkins; 1945. [Google Scholar]
- 7.Kukopulos A., Reginaldi D. Does lithium prevent depressions by suppressing manias? Int. Pharmacopsychiatry. 1973;8(3):152–158. doi: 10.1159/000467986. [PMID: 4803866]. [DOI] [PubMed] [Google Scholar]
- 8.Koukopoulos A., Sani G., Koukopoulos A.E., Albert M.J., Girardi P., Tatarelli R. Endogenous and exogenous cyclicity and temperament in bipolar disorder: review, new data and hypotheses. J. Affect. Disord. 2006;96(3):165–175. doi: 10.1016/j.jad.2006.08.031. [http://dx.doi.org/10.1016/ j.jad.2006.08.031]. [PMID: 16997381]. [DOI] [PubMed] [Google Scholar]
- 9.Yamada M., Uddin L.Q., Takahashi H., Kimura Y., Takahata K., Kousa R., Ikoma Y., Eguchi Y., Takano H., Ito H., Higuchi M., Suhara T. Superiority illusion arises from resting-state brain networks modulated by dopamine. Proc. Natl. Acad. Sci. USA. 2013;110(11):4363–4367. doi: 10.1073/pnas.1221681110. [http://dx.doi.org/10.1073/pnas. 1221681110]. [PMID: 23440209]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamada M. Brain Nerve. 2014;66(1):49–55. [Neuromolecular mechanism of the superiority illusion]. [PMID: 24371131]. [PubMed] [Google Scholar]
- 11.Wolpe N., Wolpert D.M., Rowe J.B. Seeing what you want to see: priors for ones own actions represent exaggerated expectations of success. Front. Behav. Neurosci. 2014;8:232. doi: 10.3389/fnbeh.2014.00232. [http://dx.doi. org/10.3389/fnbeh.2014.00232]. [PMID: 25018710]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J. Clin. Epidemiol. 2009;62(10):1006–1012. doi: 10.1016/j.jclinepi.2009.06.005. [http://dx.doi.org/10.1016/j.jclinepi.2009.06.005].[PMID: 19631508]. [DOI] [PubMed] [Google Scholar]
- 13.Anand A., Li Y., Wang Y., Lowe M.J., Dzemidzic M. Resting state corticolimbic connectivity abnormalities in unmedicated bipolar disorder and unipolar depression. Psychiatry Res. 2009;171(3):189–198. doi: 10.1016/j.pscychresns.2008.03.012. [http://dx.doi.org/10.1016/j.pscychresns.2008.03. 012]. [PMID: 19230623]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chepenik L.G., Raffo M., Hampson M., Lacadie C., Wang F., Jones M.M., Pittman B., Skudlarski P., Blumberg H.P. Functional connectivity between ventral prefrontal cortex and amygdala at low frequency in the resting state in bipolar disorder. Psychiatry Res. 2010;182(3):207–210. doi: 10.1016/j.pscychresns.2010.04.002. [http://dx.doi.org/10.1016/ j.pscychresns.2010.04.002]. [PMID: 20493671]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dickstein D.P., Gorrostieta C., Ombao H., Goldberg L.D., Brazel A.C., Gable C.J., Kelly C., Gee D.G., Zuo X.N., Castellanos F.X., Milham M.P. Fronto-temporal spontaneous resting state functional connectivity in pediatric bipolar disorder. Biol. Psychiatry. 2010;68(9):839–846. doi: 10.1016/j.biopsych.2010.06.029. [http://dx.doi.org/10.1016/ j.biopsych.2010.06.029]. [PMID: 20739018]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chai X.J., Whitfield-Gabrieli S., Shinn A.K., Gabrieli J.D., Nieto Castañón A., McCarthy J.M., Cohen B.M., Ongür D. Abnormal medial prefrontal cortex resting-state connectivity in bipolar disorder and schizophrenia. Neuropsychopharmacology. 2011;36(10):2009–2017. doi: 10.1038/npp.2011.88. [http://dx.doi.org/10.1038/npp.2011.88]. [PMID: 21654735]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu C.H., Ma X., Wu X., Li F., Zhang Y., Zhou F.C., Wang Y.J., Tie C.L., Zhou Z., Zhang D., Dong J., Yao L., Wang C.Y. Resting-state abnormal baseline brain activity in unipolar and bipolar depression. Neurosci. Lett. 2012;516(2):202–206. doi: 10.1016/j.neulet.2012.03.083. [http://dx.doi.org/10.1016/j.neulet.2012.03.083]. [PMID: 22503728]. [DOI] [PubMed] [Google Scholar]
- 18.Liu C.H., Ma X., Li F., Wang Y.J., Tie C.L., Li S.F., Chen T.L., Fan T.T., Zhang Y., Dong J., Yao L., Wu X., Wang C.Y. Regional homogeneity within the default mode network in bipolar depression: a resting-state functional magnetic resonance imaging study. PLoS One. 2012;7(11):e48181. doi: 10.1371/journal.pone.0048181. [http://dx.doi.org/10.1371/ journal.pone.0048181]. [PMID: 23133615]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu C.H., Li F., Li S.F., Wang Y.J., Tie C.L., Wu H.Y., Zhou Z., Zhang D., Dong J., Yang Z., Wang C.Y. Abnormal baseline brain activity in bipolar depression: a resting state functional magnetic resonance imaging study. Psychiatry Res. 2012;203(2-3):175–179. doi: 10.1016/j.pscychresns.2012.02.007. [http://dx.doi.org/10.1016/j.pscychresns.2012.02.007]. [PMID: 23017873]. [DOI] [PubMed] [Google Scholar]
- 20.Liu C.H., Ma X., Wu X., Zhang Y., Zhou F.C., Li F., Tie C.L., Dong J., Wang Y.J., Yang Z., Wang C.Y. Regional homogeneity of resting-state brain abnormalities in bipolar and unipolar depression. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2013;41:52–59. doi: 10.1016/j.pnpbp.2012.11.010. [http://dx.doi.org/10.1016/j.pnpbp.2012.11.010]. [PMID: 23200830]. [DOI] [PubMed] [Google Scholar]
- 21.Liang M.J., Zhou Q., Yang K.R., Yang X.L., Fang J., Chen W.L., Huang Z. Identify changes of brain regional homogeneity in bipolar disorder and unipolar depression using resting-state FMRI. PLoS One. 2013;8(12):e79999. doi: 10.1371/journal.pone.0079999. [http://dx.doi.org/10.1371/journal. pone.0079999]. [PMID: 24324588]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiao Q., Zhong Y., Lu D., Gao W., Jiao Q., Lu G., Su L. Altered regional homogeneity in pediatric bipolar disorder during manic state: a resting-state fMRI study. PLoS One. 2013;8(3):e57978. doi: 10.1371/journal.pone.0057978. [http://dx.doi.org/10.1371/journal.pone.0057978]. [PMID: 23526961]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Teng S., Lu C.F., Wang P.S., Hung C.I., Li C.T., Tu P.C., Su T.P., Wu Y.T. Classification of bipolar disorder using basal-ganglia-related functional connectivity in the resting state. 2013. [DOI] [PubMed]
- 24.Reinke B., Ven Vv., Matura S., Linden D.E., Oertel-Knöchel V. Altered intrinsic functional connectivity in language-related brain regions in association with verbal memory performance in euthymic bipolar patients. Brain Sci. 2013;3(3):1357–1373. doi: 10.3390/brainsci3031357. [http://dx.doi.org/10.3390/brainsci3031357]. [PMID: 24961532]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Torrisi S., Moody T.D., Vizueta N., Thomason M.E., Monti M.M., Townsend J.D., Bookheimer S.Y., Altshuler L.L. Differences in resting corticolimbic functional connectivity in bipolar I euthymia. Bipolar Disord. 2013;15(2):156–166. doi: 10.1111/bdi.12047. [http://dx.doi.org/10.1111/bdi.12047]. [PMID: 23347587]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mamah D., Barch D.M., Repovš G. Resting state functional connectivity of five neural networks in bipolar disorder and schizophrenia. J. Affect. Disord. 2013;150(2):601–609. doi: 10.1016/j.jad.2013.01.051. [http://dx. doi.org/10.1016/j.jad.2013.01.051]. [PMID: 23489402]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meda S.A., Gill A., Stevens M.C., Lorenzoni R.P., Glahn D.C., Calhoun V.D., Sweeney J.A., Tamminga C.A., Keshavan M.S., Thaker G., Pearlson G.D. Differences in resting-state functional magnetic resonance imaging functional network connectivity between schizophrenia and psychotic bipolar probands and their unaffected first-degree relatives. Biol. Psychiatry. 2012;71(10):881–889. doi: 10.1016/j.biopsych.2012.01.025. [http://dx.doi.org/10.1016/j.biopsych.2012.01.025]. [PMID: 22401986]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meda S.A., Ruaño G., Windemuth A. ONeil, K.; Berwise, C.; Dunn, S.M.; Boccaccio, L.E.; Narayanan, B.; Kocherla, M.; Sprooten, E.; Keshavan, M.S.; Tamminga, C.A.; Sweeney, J.A.; Clementz, B.A.; Calhoun, V.D.; Pearlson, G.D. Multivariate analysis reveals genetic associations of the resting default mode network in psychotic bipolar disorder and schizophrenia. Proc. Natl. Acad. Sci. USA. 2014;111(19):E2066–E2075. doi: 10.1073/pnas.1313093111. [http://dx. doi.org/10.1073/pnas.1313093111]. [PMID: 24778245]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anticevic A., Brumbaugh M.S., Winkler A.M., Lombardo L.E., Barrett J., Corlett P.R., Kober H., Gruber J., Repovs G., Cole M.W., Krystal J.H., Pearlson G.D., Glahn D.C. Global prefrontal and fronto-amygdala dysconnectivity in bipolar I disorder with psychosis history. Biol. Psychiatry. 2013;73(6):565–573. doi: 10.1016/j.biopsych.2012.07.031. [http:// dx.doi.org/10.1016/j.biopsych.2012.07.031]. [PMID: 22980587]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anticevic A., Cole M.W., Repovs G., Murray J.D., Brumbaugh M.S., Winkler A.M., Savic A., Krystal J.H., Pearlson G.D., Glahn D.C. Characterizing thalamo-cortical disturbances in schizophrenia and bipolar illness. Cereb. Cortex. 2014;24(12):3116–3130. doi: 10.1093/cercor/bht165. [http://dx.doi.org/10.1093/cercor/bht165]. [PMID: 23825317]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anticevic A., Yang G., Savic A., Murray J.D., Cole M.W., Repovs G., Pearlson G.D., Glahn D.C. Mediodorsal and visual thalamic connectivity differ in schizophrenia and bipolar disorder with and without psychosis history. Schizophr. Bull. 2014;40(6):1227–1243. doi: 10.1093/schbul/sbu100. [http://dx.doi.org/10.1093/schbul/sbu100]. [PMID: 25031221]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anticevic A., Savic A., Repovs G., Yang G., McKay D.R., Sprooten E., Knowles E.E., Krystal J.H., Pearlson G.D. [DOI] [PMC free article] [PubMed]; Glahn D.C. Ventral anterior cingulate connectivity distinguished nonpsychotic bipolar illness from psychotic bipolar disorder and schizophrenia. Schizophr. Bull. 2015;41(1):133–143. doi: 10.1093/schbul/sbu051. [http://dx. doi.org/10.1093/schbul/sbu051]. [PMID: 24782562]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Argyelan M., Ikuta T., DeRosse P., Braga R.J., Burdick K.E., John M., Kingsley P.B., Malhotra A.K., Szeszko P.R. Resting-state fMRI connectivity impairment in schizophrenia and bipolar disorder. Schizophr. Bull. 2014;40(1):100–110. doi: 10.1093/schbul/sbt092. [http://dx.doi.org/ 10.1093/schbul/sbt092]. [PMID: 23851068]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Favre P., Baciu M., Pichat C., Bougerol T., Polosan M. fMRI evidence for abnormal resting-state functional connectivity in euthymic bipolar patients. J. Affect. Disord. 2014;165:182–189. doi: 10.1016/j.jad.2014.04.054. [http://dx.doi.org/10.1016/j.jad.2014.04.054]. [PMID: 24882198]. [DOI] [PubMed] [Google Scholar]
- 35.Gao W., Jiao Q., Lu S., Zhong Y., Qi R., Lu D., Xiao Q., Yang F., Lu G., Su L. Alterations of regional homogeneity in pediatric bipolar depression: a resting-state fMRI study. BMC Psychiatry. 2014;14:222. doi: 10.1186/s12888-014-0222-y. [http://dx.doi.org/10.1186/s12888-014-0222-y]. [PMID: 25095790]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rashid B., Damaraju E., Pearlson G.D., Calhoun V.D. Dynamic connectivity states estimated from resting fMRI Identify differences among Schizophrenia, bipolar disorder, and healthy control subjects. Front. Hum. Neurosci. 2014;8:897. doi: 10.3389/fnhum.2014.00897. [http://dx.doi.org/10.3389/fnhum.2014.00897]. [PMID: 25426048]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rashid B., Arbabshirani M.R., Damaraju E., Cetin M.S., Miller R., Pearlson G.D., Calhoun V.D. Classification of schizophrenia and bipolar patients using static and dynamic resting-state fMRI brain connectivity. Neuroimage. 2016;134:645–657. doi: 10.1016/j.neuroimage.2016.04.051. [http://dx.doi. org/10.1016/j.neuroimage.2016.04.051]. [PMID: 27118088]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Singh M.K., Chang K.D., Kelley R.G., Saggar M., Reiss A.L., Gotlib I.H. Early signs of anomalous neural functional connectivity in healthy offspring of parents with bipolar disorder. Bipolar Disord. 2014;16(7):678–689. doi: 10.1111/bdi.12221. [http://dx.doi.org/10.1111/ bdi.12221]. [PMID: 24938878]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu H., Tang Y., Womer F., Fan G., Lu T., Driesen N., Ren L., Wang Y., He Y., Blumberg H.P., Xu K., Wang F. Differentiating patterns of amygdala-frontal functional connectivity in schizophrenia and bipolar disorder. Schizophr. Bull. 2014;40(2):469–477. doi: 10.1093/schbul/sbt044. [http://dx.doi.org/10.1093/schbul/sbt044]. [PMID: 23599250]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lu D., Jiao Q., Zhong Y., Gao W., Xiao Q., Liu X., Lin X., Cheng W., Luo L., Xu C., Lu G., Su L. Altered baseline brain activity in children with bipolar disorder during mania state: a resting-state study. Neuropsychiatr. Dis. Treat. 2014;10:317–323. doi: 10.2147/NDT.S54663. [PMID: 24570585]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu K., Liu H., Li H., Tang Y., Womer F., Jiang X., Chen K., Zhou Y., Jiang W., Luo X., Fan G., Wang F. Amplitude of low-frequency fluctuations in bipolar disorder: a resting state fMRI study. J. Affect. Disord. 2014;152-154:237–242. doi: 10.1016/j.jad.2013.09.017. [http://dx.doi. org/10.1016/j.jad.2013.09.017]. [PMID: 24120087]. [DOI] [PubMed] [Google Scholar]
- 42.Yang G.J., Murray J.D., Repovs G., Cole M.W., Savic A., Glasser M.F., Pittenger C., Krystal J.H., Wang X.J., Pearlson G.D., Glahn D.C., Anticevic A. Altered global brain signal in schizophrenia. Proc. Natl. Acad. Sci. USA. 2014;111(20):7438–7443. doi: 10.1073/pnas.1405289111. [http://dx.doi.org/10.1073/pnas.1405289111]. [PMID: 24799682]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yip S.W., Mackay C.E., Goodwin G.M. Increased temporo-insular engagement in unmedicated bipolar II disorder: an exploratory resting state study using independent component analysis. Bipolar Disord. 2014;16(7):748–755. doi: 10.1111/bdi.12206. [http://dx.doi.org/ 10.1111/bdi.12206]. [PMID: 24725219]. [DOI] [PubMed] [Google Scholar]
- 44.Das P., Calhoun V., Malhi G.S. Bipolar and borderline patients display differential patterns of functional connectivity among resting state networks. Neuroimage. 2014;98:73–81. doi: 10.1016/j.neuroimage.2014.04.062. [http://dx. doi.org/10.1016/j.neuroimage.2014.04.062]. [PMID: 24793833]. [DOI] [PubMed] [Google Scholar]
- 45.Knöchel C., Stäblein M., Storchak H., Reinke B., Jurcoane A., Prvulovic D., Linden D.E., van de Ven V., Ghinea D., Wenzler S., Alves G., Matura S., Kröger A., Oertel-Knöchel V. Multimodal assessments of the hippocampal formation in schizophrenia and bipolar disorder: evidences from neuro- behavioral measures and functional and structural MRI. Neuroimage Clin. 2014;6:134–144. doi: 10.1016/j.nicl.2014.08.015. [http://dx.doi.org/10.1016/j.nicl.2014.08. 015]. [PMID: 25379425]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Oertel-Knöchel V., Reinke B., Matura S., Prvulovic D., Linden D.E., van de Ven V. Functional connectivity pattern during rest within the episodic memory network in association with episodic memory performance in bipolar disorder. Psychiatry Res. 2015;231(2):141–150. doi: 10.1016/j.pscychresns.2014.11.014. [http://dx.doi.org/10.1016/j.pscychresns.2014.11. 014]. [PMID: 25575881]. [DOI] [PubMed] [Google Scholar]
- 47.Lois G., Linke J., Wessa M. Altered functional connectivity between emotional and cognitive resting state networks in euthymic bipolar I disorder patients. PLoS One. 2014;9(10):e107829. doi: 10.1371/journal.pone.0107829. [http://dx.doi.org/10.1371/journal.pone.0107829]. [PMID: 25343370]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lui S., Yao L., Xiao Y., Keedy S.K., Reilly J.L., Keefe R.S., Tamminga C.A., Keshavan M.S., Pearlson G.D., Gong Q., Sweeney J.A. Resting-state brain function in schizophrenia and psychotic bipolar probands and their first-degree relatives. Psychol. Med. 2015;45(1):97–108. doi: 10.1017/S003329171400110X. [http://dx.doi.org/10.1017/ S003329171400110X]. [PMID: 25066779]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu Y., Wu X., Zhang J., Guo X., Long Z., Yao L. Altered effective connectivity model in the default mode network between bipolar and unipolar depression based on resting-state fMRI. J. Affect. Disord. 2015;182:8–17. doi: 10.1016/j.jad.2015.04.009. [http://dx.doi.org/10.1016/j.jad. 2015.04.009]. [PMID: 25942576]. [DOI] [PubMed] [Google Scholar]
- 50.Du Y., Pearlson G.D., Liu J., Sui J., Yu Q., He H., Castro E., Calhoun V.D. A group ICA based framework for evaluating resting fMRI markers when disease categories are unclear: application to schizophrenia, bipolar, and schizoaffective disorders. Neuroimage. 2015;122:272–280. doi: 10.1016/j.neuroimage.2015.07.054. [http://dx.doi.org/10.1016/ j.neuroimage.2015.07.054]. [PMID: 26216278]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jie N.F., Zhu M.H., Ma X.Y., Osuch E.A., Wammes M., Théberge J., Li H.D., Zhang Y., Jiang T.Z., Sui J., Calhoun V.D. Discriminating bipolar disorder from major depression based on SVM-FoBa: efficient feature selection with multimodal brain imaging data. IEEE Trans. Auton. Ment. Dev. 2015;7(4):320–331. doi: 10.1109/TAMD.2015.2440298. [http://dx.doi.org/10.1109/TAMD.2015.2440298]. [PMID: 26858825]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jie N-F., Osuch E.A., Zhu M-H., Ma X-Y., Wammes M., Jiang T-Z., Sui J., Calhoun V.D. Discriminating bipolar disorder from major depression using whole-brain functional connectivity: A feature selection analysis with SVM-FoBA algorithm IEEE 25th International Workshop on Machine Learning for Signal Processing (MLSP)17-20 September, Boston,; 2015. pp. 1–6. [Google Scholar]
- 53.Li C.T., Tu P.C., Hsieh J.C., Lee H.C., Bai Y.M., Tsai C.F., Wang S.J., Hsu J.W., Huang K.L., Hong C.J., Su T.P. Functional dysconnection in the prefrontal-amygdala circuitry in unaffected siblings of patients with bipolar I disorder. Bipolar Disord. 2015;17(6):626–635. doi: 10.1111/bdi.12321. [http://dx.doi.org/10.1111/bdi.12321]. [PMID: 26291695]. [DOI] [PubMed] [Google Scholar]
- 54.Li M., Huang C., Deng W., Ma X., Han Y., Wang Q., Li Z., Guo W., Li Y., Jiang L., Lei W., Hu X., Gong Q., Merikangas K.R., Palaniyappan L., Li T. Contrasting and convergent patterns of amygdala connectivity in mania and depression: a resting-state study. J. Affect. Disord. 2015;173:53–58. doi: 10.1016/j.jad.2014.10.044. [http://dx.doi.org/ 10.1016/j.jad.2014.10.044]. [PMID: 25462396]. [DOI] [PubMed] [Google Scholar]
- 55.Satterthwaite T.D., Kable J.W., Vandekar L., Katchmar N., Bassett D.S., Baldassano C.F., Ruparel K., Elliott M.A., Sheline Y.I., Gur R.C., Gur R.E., Davatzikos C., Leibenluft E., Thase M.E., Wolf D.H. Common and dissociable dysfunction of the reward system in bipolar and unipolar depression. Neuropsychopharmacology. 2015;40(9):2258–2268. doi: 10.1038/npp.2015.75. [http://dx.doi.org/10. 1038/npp.2015.75]. [PMID: 25767910]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang Z., Meda S.A., Keshavan M.S., Tamminga C.A., Sweeney J.A., Clementz B.A., Schretlen D.J., Calhoun V.D., Lui S., Pearlson G.D. Large-scale fusion of gray matter and resting-state functional MRI reveals common and distinct biological markers across the psychosis spectrum in the B-SNIP Cohort. Front. Psychiatry. 2015;6:174. doi: 10.3389/fpsyt.2015.00174. [http://dx.doi.org/10. 3389/fpsyt.2015.00174]. [PMID: 26732139]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stoddard J., Hsu D., Reynolds R.C., Brotman M.A., Ernst M., Pine D.S., Leibenluft E., Dickstein D.P. Aberrant amygdala intrinsic functional connectivity distinguishes youths with bipolar disorder from those with severe mood dysregulation. Psychiatry Res. 2015;231(2):120–125. doi: 10.1016/j.pscychresns.2014.11.006. [http://dx.doi.org/10.1016/j.pscychresns. 2014.11.006]. [PMID: 25544024]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stoddard J., Gotts S.J., Brotman M.A., Lever S., Hsu D., Zarate C., Ernst M., Pine D.S., Leibenluft E. Aberrant intrinsic functional connectivity within and between corticostriatal and temporal-parietal networks in adults and youth with bipolar disorder. Psychol. Med. 2016;46(7):1509–1522. doi: 10.1017/S0033291716000143. Epub ahead of print [http:// dx.doi.org/10.1017/S0033291716000143]. [PMID: 26924633]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang Y., Zhong S., Jia Y., Zhou Z., Zhou Q., Huang L. Reduced interhemispheric resting-state functional connectivity in unmedicated bipolar II disorder. Acta Psychiatr. Scand. 2015;132(5):400–407. doi: 10.1111/acps.12429. [http://dx.doi.org/10.1111/acps.12429]. [PMID: 25929680]. [DOI] [PubMed] [Google Scholar]
- 60.Wang Y., Zhong S., Jia Y., Zhou Z., Wang B., Pan J., Huang L. Interhemispheric resting state functional connectivity abnormalities in unipolar depression and bipolar depression. Bipolar Disord. 2015;17(5):486–495. doi: 10.1111/bdi.12315. [http://dx.doi.org/10.1111/bdi.12315]. [PMID: 26241359]. [DOI] [PubMed] [Google Scholar]
- 61.Wang Y., Zhong S., Jia Y., Sun Y., Wang B., Liu T., Pan J., Huang L. Disrupted resting-state functional connectivity in nonmedicated bipolar disorder. Radiology. 2016;280(2):529–536. doi: 10.1148/radiol.2016151641. Epub ahead of print [http://dx.doi.org/10.1148/radiol.2016151641]. [PMID: 26909649]. [DOI] [PubMed] [Google Scholar]
- 62.Altinay M.I., Hulvershorn L.A., Karne H., Beall E.B., Anand A. Differential resting-state functional connectivity of striatal subregions in bipolar depression and hypomania. Brain Connect. 2016;6(3):255–265. doi: 10.1089/brain.2015.0396. Epub ahead of print [http://dx.doi.org/10. 1089/brain.2015.0396]. [PMID: 26824737]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Goya-Maldonado R., Brodmann K., Keil M., Trost S., Dechent P., Gruber O. Differentiating unipolar and bipolar depression by alterations in large-scale brain networks. Hum. Brain Mapp. 2016;37(2):808–818. doi: 10.1002/hbm.23070. [http://dx.doi.org/10.1002/hbm.23070]. [PMID: 26611711]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.He H., Yu Q., Du Y., Vergara V., Victor T.A., Drevets W.C., Savitz J.B., Jiang T., Sui J., Calhoun V.D. Resting-state functional network connectivity in prefrontal regions differs between unmedicated patients with bipolar and major depressive disorders. J. Affect. Disord. 2016;190:483–493. doi: 10.1016/j.jad.2015.10.042. [http://dx.doi.org/ 10.1016/j.jad.2015.10.042]. [PMID: 26551408]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lv D., Lin W., Xue Z., Pu W., Yang Q., Huang X., Zhou L., Yang L., Liu Z. Decreased functional connectivity in the language regions in bipolar patients during depressive episodes but not remission. J. Affect. Disord. 2016;197:116–124. doi: 10.1016/j.jad.2016.03.026. [http://dx.doi. org/10.1016/j.jad.2016.03.026]. [PMID: 26991366]. [DOI] [PubMed] [Google Scholar]
- 66.Magioncalda P., Martino M., Conio B., Escelsior A., Piaggio N., Presta A., Marozzi V., Rocchi G., Anastasio L., Vassallo L., Ferri F., Huang Z., Roccatagliata L., Pardini M., Northoff G., Amore M. Functional connectivity and neuronal variability of resting state activity in bipolar disorderreduction and decoupling in anterior cortical midline structures. Hum. Brain Mapp. 2015;36(2):666–682. doi: 10.1002/hbm.22655. [http://dx.doi.org/10.1002/hbm.22655]. [PMID: 25307723]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Martino M., Magioncalda P., Huang Z., Conio B., Piaggio N., Duncan N.W., Rocchi G., Escelsior A., Marozzi V., Wolff A., Inglese M., Amore M., Northoff G. Contrasting variability patterns in the default mode and sensorimotor networks balance in bipolar depression and mania. Proc. Natl. Acad. Sci. USA. 2016;•••:201517558. doi: 10.1073/pnas.1517558113. [Epub ahead of print]. [http://dx.doi.org/10.1073/ pnas.1517558113]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rey G., Piguet C., Benders A., Favre S., Eickhoff S.B., Aubry J.M., Vuilleumier P. Resting-state functional connectivity of emotion regulation networks in euthymic and non-euthymic bipolar disorder patients. Eur. Psychiatry. 2016;34:56–63. doi: 10.1016/j.eurpsy.2015.12.005. [http://dx. doi.org/10.1016/j.eurpsy.2015.12.005]. [PMID: 26945530]. [DOI] [PubMed] [Google Scholar]
- 69.Skåtun K.C., Kaufmann T., Tønnesen S., Biele G., Melle I., Agartz I., Alnæs D., Andreassen O.A., Westlye L.T. Global brain connectivity alterations in patients with schizophrenia and bipolar spectrum disorders. J. Psychiatry Neurosci. 2016;41(5):331–341. doi: 10.1503/jpn.150159. [http://dx.doi.org/10.1503/jpn.150159]. [PMID: 26854755]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Solé-Padullés C., Castro-Fornieles J., de la Serna E., Romero S., Calvo A., Sánchez-Gistau V., Padrós-Fornieles M., Baeza I., Bargalló N., Frangou S., Sugranyes G. Altered cortico-striatal connectivity in offspring of schizophrenia patients relative to offspring of bipolar patients and controls. PLoS One. 2016;11(2):e0148045. doi: 10.1371/journal.pone.0148045. [http://dx.doi.org/10.1371/journal.pone.0148045]. [PMID: 26885824]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Souza-Queiroz J., Boisgontier J., Etain B., Poupon C., Duclap D. dAlbis, M.A.; Daban, C.; Hamdani, N.; Le Corvoisier, P.; Delavest, M.; Bellivier, F.; Guevara, P.; Leboyer, M.; Henry, C.; Houenou, J. Childhood trauma and the limbic network: a multimodal MRI study in patients with bipolar disorder and controls. J. Affect. Disord. 2016;200:159–164. doi: 10.1016/j.jad.2016.04.038. [http://dx.doi.org/ 10.1016/j.jad.2016.04.038]. [PMID: 27136413]. [DOI] [PubMed] [Google Scholar]
- 72.Brady R.O., Jr, Masters G.A., Mathew I.T., Margolis A., Cohen B.M., Öngür D., Keshavan M. State dependent cortico-amygdala circuit dysfunction in bipolar disorder. J. Affect. Disord. 2016;201:79–87. doi: 10.1016/j.jad.2016.04.052. [http://dx.doi.org/10.1016/j.jad.2016.04.052]. [PMID: 27177299]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Weissman P. The primacy of mania in some forms of cyclothymic states. J. Hillside Hosp. 1961;10(1):34–52. [Google Scholar]
- 74.Cannon W.B. The Wisdom of the Body. New York: W.W. Norton; 1932. [Google Scholar]
- 75.Gavrilets S. On the evolutionary origins of the egalitarian syndrome. Proc. Natl. Acad. Sci. USA. 2012;109(35):14069–14074. doi: 10.1073/pnas.1201718109. [http://dx.doi.org/10.1073/pnas.1201718109]. [PMID: 22891349]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Greimel E., Schulte-Rüther M., Fink G.R., Piefke M., Herpertz-Dahlmann B., Konrad K. Development of neural correlates of empathy from childhood to early adulthood: an fMRI study in boys and adult men. J. Neural. Transm (Vienna) 2010;117(6):781–791. doi: 10.1007/s00702-010-0404-9. [http://dx.doi.org/10.1007/s00702-010-0404-9]. [PMID: 20411397]. [DOI] [PubMed] [Google Scholar]
- 77.Decety J., Norman G.J., Berntson G.G., Cacioppo J.T. A neurobehavioral evolutionary perspective on the mechanisms underlying empathy. Prog. Neurobiol. 2012;98(1):38–48. doi: 10.1016/j.pneurobio.2012.05.001. [http:// dx.doi.org/10.1016/j.pneurobio.2012.05.001]. [PMID: 22580447]. [DOI] [PubMed] [Google Scholar]
- 78.Decety J., Bartal I.B., Uzefovsky F., Knafo-Noam A. Empathy as a driver of prosocial behaviour: highly conserved neuro- behavioural mechanisms across species. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1686;2016(371):20150077. doi: 10.1098/rstb.2015.0077. [http://dx.doi.org/ 10.1098/rstb.2015.0077]. [PMID: 26644596]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Catmur C., Cross E.S., Over H. Understanding self and others: from origins to disorders. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1686;2016(371):20150066. doi: 10.1098/rstb.2015.0066. [http://dx.doi.org/10.1098/rstb. 2015.0066]. [PMID: 26644602]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mathur V.A., Cheon B.K., Harada T., Scimeca J.M., Chiao J.Y. Overlapping neural response to the pain or harm of people, animals, and nature. Neuropsychologia. 2016;81:265–273. doi: 10.1016/j.neuropsychologia.2015.12.025. [http://dx.doi.org/10.1016/j.neuropsychologia.2015.12.025]. [PMID: 26727304]. [DOI] [PubMed] [Google Scholar]
- 81.Silk J.B., House B.R. The evolution of altruistic social preferences in human groups. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1687;2016(371):20150097. doi: 10.1098/rstb.2015.0097. [http://dx.doi.org/10.1098/rstb. 2015.0097]. [PMID: 26729936]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Singer T. The neuronal basis and ontogeny of empathy and mind reading: review of literature and implications for future research. Neurosci. Biobehav. Rev. 2006;30(6):855–863. doi: 10.1016/j.neubiorev.2006.06.011. [http://dx. doi.org/10.1016/j.neubiorev.2006.06.011]. [PMID: 16904182]. [DOI] [PubMed] [Google Scholar]
- 83.Betti V., Aglioti S.M. Dynamic construction of the neural networks underpinning empathy for pain. Neurosci. Biobehav. Rev. 2016;63:191–206. doi: 10.1016/j.neubiorev.2016.02.009. [http://dx.doi.org/10.1016/j.neubiorev. 2016.02.009]. [PMID: 26877105]. [DOI] [PubMed] [Google Scholar]
- 84.Tops M., Boksem M.A. A potential role of the inferior frontal gyrus and anterior insula in cognitive control, brain rhythms, and event-related potentials. Front. Psychol. 2011;2:330. doi: 10.3389/fpsyg.2011.00330. [http://dx.doi.org/10.3389/fpsyg.2011.00330]. [PMID: 22084637]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Strakowski S.M., Fleck D.E., DelBello M.P., Adler C.M., Shear P.K., Kotwal R., Arndt S. Impulsivity across the course of bipolar disorder. Bipolar Disord. 2010;12(3):285–297. doi: 10.1111/j.1399-5618.2010.00806.x. [http://dx.doi. org/10.1111/j.1399-5618.2010.00806.x]. [PMID: 20565435]. [DOI] [PMC free article] [PubMed] [Google Scholar]


