Abstract
Abstract: Background: Melatonin synchronizes central but also peripheral oscillators (fetal adrenal gland, pancreas, liver, kidney, heart, lung, fat, gut, etc.), allowing temporal organization of biological functions through circadian rhythms (24-hour cycles) in relation to periodic environmental changes and therefore adaptation of the individual to his/her internal and external environment. Measures of melatonin are considered the best peripheral indices of human circadian timing based on an internal 24-hour clock.
Methods: First, the pharmacology of melatonin (biosynthesis and circadian rhythms, pharmacokinetics and mechanisms of action) is described, allowing a better understanding of the short and long term effects of melatonin following its immediate or prolonged release. Then, research related to the physiological effects of melatonin is reviewed.
Results: The physiological effects of melatonin are various and include detoxification of free radicals and antioxidant actions, bone formation and protection, reproduction, and cardiovascular, immune or body mass regulation. Also, protective and therapeutic effects of melatonin are reported, especially with regard to brain or gastrointestinal protection, psychiatric disorders, cardiovascular diseases and oncostatic effects.
Conclusion: This review highlights the high number and diversity of major melatonin effects and opens important perspectives for measuring melatonin as a biomarker (biomarker of early identification of certain disorders and also biomarker of their follow-up) and using melatonin with clinical preventive and therapeutic applications in newborns, children and adults based on its physiological regulatory effects.
Keywords: Adaptation to external and internal environment, biological clocks, biomarker, clinical application, central and peripheral oscillators, melatonin, prevention and treatment, sleep-wake rhythm, synchronizer
1. INTRODUCTION
Melatonin or 5 methoxy-N-acetyltryptamine (Fig. 1) was discovered and isolated from bovine pineal in 1958 by Aaron Lerner [1]. Melatonin is the main hormone secreted by the pineal gland. Extrapineal sources of melatonin were reported in the retina, bone marrow cells, platelets, skin, lymphocytes, Harderian gland, cerebellum, and especially in the gastrointestinal tract of vertebrate species [2-9]. Indeed, melatonin is present but can also be synthesized in the enterochromaffin cells; the release of gastrointestinal melatonin into the circulation seems to follow the periodicity of food intake, particularly tryptophan intake [2, 10]. It is noteworthy that the concentration of melatonin in the gastrointestinal tract surpasses blood levels by 10-100 times and there is at least 400 times more melatonin in the gastrointestinal tract than in the pineal gland [2]. Melatonin in the gastrointestinal tract of newborn and infant mammals is of maternal origin given that melatonin penetrates easily the placenta and is after secreted into the mother’s milk [11-13]. It has even been suggested that melatonin is involved in the production of mekonium [2]. Melatonin in human breast milk follows a circadian rhythm in both preterm and term milk, with high levels during the night and undetectable levels during the day [14, 15]. No correlation was found between gestational age and concentration of melatonin. It is noteworthy that bottle milk composition does not contain melatonin in powder formula. Also, human colostrum, during the first 4 or 5 days after birth, contains immune – competent cells (colostral mononuclear cells) which are able to synthesize melatonin in an autocrine manner [16].
Melatonin is mainly synthesized by the pinealocytes from the amino acid tryptophan, which is hydroxylated (by the tryptophan-5-hydroxylase) in 5-hydroxytryptophan, then decarboxylated (by the 5-hydroxytryptophan decarboxylase) in serotonin. Two enzymes, found mainly in the pineal gland, transform serotonin to melatonin [17, 18]: serotonin is first acetylated to form N-acetylserotonin by arylalkylamine-N-acetyltransferase (AA-NAT, also called “Timezyme”, is the rate-limiting enzyme for melatonin synthesis), and then N-acetylserotonin is methylated by acetylserotonin-O-methyltransferase (ASMT, also called hydroxyindole-O- methyltransferase or HIOMT) to form melatonin (Fig. 2). Both AA-NAT and ASMT activities are controlled by noradrenergic and neuropeptidergic projections to the pineal gland [19]. Norepinephrine, also called noradrenaline, activates adenylate cyclase which in turn promotes the melatonin biosynthesis enzymes, especially AA-NAT [20]. Once synthesized, melatonin is quickly released into the systemic circulation to reach central and peripheral target tissues.
Melatonin synthesis and secretion is enhanced by darkness and inhibited by light (Fig. 3) [21]. Luminous information is transmitted from the retina to pineal gland through the suprachiasmatic nucleus (SCN) of the hypothalamus. In humans, its secretion starts soon after sundown, reaches a peak in the middle of the night (between 2 and 4 in the morning) and decreases gradually during the second half of the night [22]. Nearly 80% of the melatonin is synthesized at night, with serum concentrations varying between 80 and 120 pg/ml. During daylight hours, serum concentrations are low (10-20 pg/ml) [23].
Serum concentrations of melatonin vary considerably with age, and infants secrete very low levels of melatonin before 3 months of age. Melatonin secretion increases and becomes circadian along with child development: Sadeh [24] reported an association between melatonin secretion and organization of sleep-wake rhythm from 6 months of age. However, more recent studies suggest that melatonin rhythm is set around 3 months of age in typical development, at the same time that infants begin to have more regular sleep–wake cycles associated with nighttime sleep lasting 6-8 h [25]. In 3-years-old children, a stabilization of the sleep-wake rhythm is observed, which corresponds to a regular melatonin secretion rhythm [26]. Nocturnal concentration peaks are the highest between the 4th and 7th years of age [23], and then decline progressively [27].
2. PHARMACOKINETICS
After intravenous administration, melatonin is rapidly distributed (distribution half-life of 0.5 to 5.6 minutes) and eliminated [28]. After oral administration, plasma concentration peak arises within 60 minutes [29]. Plasma concentrations diminution is biphasic, with a half-life of respectively 2 and 20 minutes [30]. Intake of an usual dose (i.e., 1 to 5 mg), allows within the hour after ingestion, melatonin concentrations 10 to 100 times higher than the physiological nocturnal peak to be obtained, with a return to basal concentrations in 4 to 8 hours.
A bioavailability study in four male healthy volunteers [31] showed a plasma melatonin peak varying between 2 and 395 nmol/L and an elimination half-life of 47± 3 min (mean ± SD) after oral administration of a 500 µg dose. Bioavailability varied from 10 to 56% (mean 33%).
After intravenous or oral administration, melatonin is quickly metabolized, mainly in the liver and secondarily in the kidney. However, after intravenous administration, the hepatic bio-degradation is less important due to the absence of hepatic first pass. It undergoes hydroxylation to 6-hydroxymelatonin by the action of the cytochrome P450 enzyme CYP1A2, followed by conjugation with sulfuric acid (90%) or glucuronic acid (10%) and is excreted in the urine. About 5% of serum melatonin is excreted unmetabolized also in urine. The principal metabolite, the 6-sulfatoxy-melatonin (6-SM), is inactive, and its urinary excretion reflects melatonin plasma concentrations [32]. Plasma levels can be also measured directly or indirectly assessed through salivary measures. A reverse relation between bioavailability of melatonin and the 6-SM concentrations area under the curve has been shown, the low bioavailability being explained by an important hepatic first pass [31].
3. MECHANISMS OF ACTION
Melatonin acts through different molecular pathways. The best characterized pathway is the activation of two types of membrane specific receptors: high affinity ML1 sites and low affinity ML2 sites [33, 34]. The activation of ML1 receptors, which are G protein-coupled receptors [35], leads to an inhibition of the adenylate cyclase in target cells. The activation of ML2 receptors, currently called MT3, leads to phospho-inositides hydrolysis. MT3 is expressed in various brain areas and has been shown to be the enzyme quinone reductase 2 [36]. Two sub-types of the ML1 receptor have been described [37] Mel1a and Mel1b. Mel1a (or MT1) is encoded in human chromosome #4 (4q35.1) and consists of 351 amino acids. Mel1a is widely distributed in the pars tuberalis of the anterior pituitary and the SCN of the hypothalamus (which is the anatomic site of the circadian clock), and also in the cortex, thalamus, substantia nigra, nucleus accumbens, amygdala, hippocampus, cerebellum, cornea and retina [38]. Mel1b (or MT2) is encoded in human chromosome #11 (11q21-q22) and consists of 363 amino acids. Mel1b is distributed mainly in the retina and secondarily in the hippocampus, cortex, paraventricular nucleus, and cerebellum [39]. Melatonin has also an intracellular action by binding, on the one hand, to cytosolic calmodulin [40], and on the other hand, to two receptors of the Z retinoid nuclear receptors family [41]. Melatonin receptors have been found in several central but also in peripheral tissues, including heart and arteries, adrenal gland, kidney, lung, liver, gallbladder, small intestine, adipocytes, ovaries, uterus, breast, prostate, and skin [42]. Furthermore, they have also been detected in T and B lymphocytes. Evidence shows that there is a considerable variation in the density and location of the expression of melatonin receptors between species [43]. In a recent review, Liu et al. suggest, on the basis of affinity studies showing species-dependent fluctuations in vivo ligand selectivity, that there are considerable species differences in melatonin receptor pharmacology [44]. Models of mice with targeted disruption of either Mel1a or Mel1b receptor subtypes, or double mutants have been used, allowing the cellular mechanisms through which melatonin modulates circadian and photoperiodic rhythmicity to be understood [45, 46].
The effects of melatonin depend on the localization and types of melatonin receptors; those effects are described in the next section.
4. EFFECTS OF MELATONIN
4.1. Physiological Effects
Melatonin regulates circadian rhythms such as the sleep-wake rhythm, neuroendocrine rhythms or body temperature cycles through its action on MT1 and MT2 receptors [23, 42-49]. Ingestion of melatonin induces fatigue, sleepiness and a diminution of sleep latency [26]. Disturbed circadian rhythms are associated with sleep disorders and impaired health [50]. For example, children with multiple developmental, neuro- psychiatric and health difficulties often show melatonin deficiency [51]. When circadian rhythms are restored, behavior, mood, development, intellectual function, health, and even seizure control may improve [50, 52]. It should be noted that according to several studies, circadian rhythms are important for typical (normal) neurodevelopment and their absence suppresses neurogenesis in animal models [53-56].
Finally, melatonin may as well be involved in early fetal development, with direct effects on placenta, glial and neuronal development, and could play an ontogenic role in the establishment of diurnal rhythms and synchronization of the fetal biological clock [57-59]. Iwasaki et al. [57] investigated the expression of the two enzymes involved in the conversion of serotonin to melatonin (AA-NAT and ASMT) and found that transcripts of these enzymes were present in the first-trimester human placenta. Moreover, they found also that melatonin significantly potentiated hcg secretion at optimal concentrations on cultured human trophoblast cells. These results suggest that melatonin regulates in a paracrine/autocrine way human placental function with a potential role in human reproduction. In vitro studies have shown that neural stem/progenitor cells express MT1 receptors, and melatonin induces glial cell-line derived neurotrophic factor (GDNF) expression in neural stem cells, suggesting an early role for melatonin in central nervous system development. Indeed, as indicated previously, melatonin of maternal origin crosses the placenta and can therefore influence fetal development. Studies in humans have repeatedly confirmed that the cycle of melatonin in maternal blood occurs also in fetal circulation [60, 61]. The maturation and synchronization of the fetal circadian system have not been thoroughly studied. However, studies in nonhuman primate fetus have shown that maternal melatonin stimulates growth of the primate fetal adrenal gland and entrains fetal circadian rhythms, including SCN rhythms [62, 63]. Furthermore, in mice, the suppression of melatonin rhythm by maternal exposure to constant light changes the rhythmic expression in fetal clock genes; these changes are reversed when melatonin is injected daily to the mother [64]. These results document that the fetal clock is imprinted by melatonin, which under normal circumstances is of maternal origin. In addition, some studies in humans and nonhuman primates show 24h rhythms in fetal heart rate and respiratory movements during the latter half of pregnancy. Whether the circadian system of the human fetus, particularly in late pregnancy, is under the influence of maternal SCN remains to be better ascertained [65].
Besides the well-known effects of melatonin on the regulation of sleep-wake rhythms, melatonin is considered as an endogenous synchronizer and a chronobiotic molecule, i.e. a substance that reinforces oscillations or adjusts the timing of the central biological clock located in the suprachiasmatic nuclei of the hypothalamus to stabilize bodily rhythms [66]. Furthermore, Pevet and Challet [67] view melatonin as both the master clock output and internal time-giver in the complex circadian clocks network: as a major hormonal output, melatonin distributes, through its daily rhythm of secretion, temporal cues to the numerous tissue targets with melatonin receptors, driving circadian rhythms in some tissue structures such as the adenohypophysis or synchronizing peripheral oscillators such as the fetal adrenal gland but also many other peripheral tissues (pancreas, liver, kidney, heart, lung, fat, gut, etc.). Circadian rhythms, and more precisely the circadian clocks network, allow temporal organization of biological functions in relation to periodic environmental changes and therefore reflect adaptation to the environment. Thus, the sleep–wake rhythm associated with biological circadian rhythms can be seen as an adaptation to the day–night cycle. Moreover, the synchronization by melatonin of peripheral oscillators reflects adaptation of the individual to his/her internal and external environment (for example, the synchronized effects of melatonin on cortisol and insulin secretion allow the individual to be fully awake at 8am and able to start the day by eating and getting some energy from food intake). Given the major synchronizing effects of melatonin on central and peripheral oscillators, measures of melatonin are considered the best peripheral indices of human circadian timing [68].
Futhermore, melatonin is involved in blood pressure and autonomic cardiovascular regulation, immune system regulation but also various physiological functions such as retinal functions, detoxification of free radicals and antioxidant actions through its action on MT3 receptors protecting the brain from oxidative stress [12, 38, 68-74]. A through its action on MT3 receptors specific section is developed below on melatonin and brain protection. The antioxidant actions of melatonin protect also the gastrointestinal tract from ulcerations by reducing secretion of hydrochloric acid and the oxidative effects of bile acids on the intestinal epithelium, and by increasing duodenal mucosal secretion of bicarbonate through its action on MT2 receptors (this alkaline secretion is an important mechanism for duodenal protection against gastric acid); melatonin prevents also ulcerations of gastrointestinal tract by increasing microcirculation and fostering epithelial regeneration [2, 66]. Concerning the role of melatonin in immune regulation, melatonin has direct immuno-enhancement effects in animals and humans [75, 76]. Indeed, melatonin stimulates the production of cytokines and more specifically interleukins (IL-2, IL-6, IL-12) [77]. In addition, melatonin enhances T helper immune responses [76, 78]. Furthermore, the melatonin antioxidant actions contribute to its immuno-enhancing effects [77] and have also an indirect effect by reducing nitric oxide formation which facilitates the decrease of the inflammatory response [79]. As suggested by Esquifino et al. [80], melatonin might provide a time-related signal to the immune network.
In addition, effects of melatonin on body mass and bone mass regulation have been reported. Melatonin is known for its role in energy expenditure and body mass regulation in mammals by preventing the increase in body fat with age [81-83]. These effects are mediated by MT2 receptors in adipose tissue [84]. Moreover, melatonin increases bone mass by promoting osteoblast cell differentiation and bone formation [85, 86]. In humans, melatonin stimulates bone cell proliferation and Type I collagen synthesis in these cells, and inhibits bone resorption through down-regulation of the RANKL-mediated osteoclast formation and activation [87, 88]. Also, a deficit of melatonin has been found to be associated with animal scoliosis following pinealectomy and human idiopathic scoliosis [89, 90].
Finally, melatonin has physiological effects on reproduction and sexual maturation in mammals through down-regulation of gonadotropin-releasing hormone (GnRH) gene expression in a cyclical pattern over a 24-hour period [91-93]. The rhythmic release of GnRH controls luteinizing hormone (LH) and follicule-stimulating hormone (FSH) secretion. The daily profile of melatonin secretion conveys internal information used for both circadian and seasonal temporal organization [67]. The melatonin rhythmic pattern entrains the reproductive rhythm via the influence of photoperiod on LH pulsatile secretion and therefore mediates the seasonal fluctuations of reproduction clearly observed in animals (seasonal breading as species-specific seasons for reproduction) and moderately observed in humans [94-96].
4.2. Melatonin in Disorders and Therapeutic Effects
Therapeutic effects of melatonin have been reported in several disorders such as certain tumors, cardiovascular diseases or psychiatric disorders. The part concerning melatonin and psychiatric disorders is in particular developed given our past and current work on this topic.
Indeed, oncostatic effects of melatonin have been reported in several tumors (breast cancer, ovarian and endometrial carcinoma, human uveal melanoma, prostate cancer, hepatomas, and intestinal tumors) [97-106]. These oncostatic effects have been attributed to the anti-oxidative role of melatonin given that oxidative stress is involved in the initiation, promotion and progression of carcinogenesis [107, 108]. Also, decreased melatonin levels (measures of blood melatonin or urinary excretion of 6-SM) were reported in patients with cardiovascular diseases [109-112]. Inversely, melatonin treatment reduces blood pressure in patients with hypertension [69, 74, 113].
Concerning psychiatric disorders, secretion disturbances of the pineal gland have been described in child and adult psychiatry, with notably in most studies a decreased nocturnal melatonin secretion observed in major depressive disorder, bipolar disorder, schizophrenia or autism spectrum disorder [114-117].
Also, a phase-shift of melatonin has been reported in major depressive disorder and bipolar disorder, including in particular a delayed melatonin peak secretion [114, 118, 119]. It is noteworthy that increased melatonin levels (measures of blood melatonin and urinary excretion of 6-SM) were found when clinical therapeutic benefits were observed following the use of antidepressants [120-122]. Furthermore, significant improvement of major depressive disorder and anxiety was described following administration of 25mg per day of agomelatine, a MT1/MT2 melatonin agonist and selective antagonist of 5-HT2C receptors [123-125].
Concerning autism spectrum disorder (ASD), abnormalities in the serotoninergic system and sleep- wake rhythm disturbances observed in children with ASD suggest altered melatonin secretion in autism [126-128]. Sleep disorders (mostly increased sleep latency, reduced total sleep and nocturnal awakenings with insomnia) are observed in 50-80% of individuals with autism [129]. It is noteworthy that sleep problems are not specific of autism and are also observed in children with intellectual disability associated or not with autism [128]. However, melatonin measures in children with intellectual disability not associated with autism, such as some children with Down syndrome and Fragile X syndrome, showed respectively normal melatonin production despite delayed nocturnal melatonin peak secretion and increased levels of melatonin [130, 131], whereas decreased nocturnal melatonin secretion was mostly observed in children with autism [128]. We reported in two different large samples of children with autism significant relationships between decreased nocturnal urinary excretion and severity of autistic impairments in social communication [127, 132]. These results suggest that abnormalities in melatonin physiology might contribute not only to sleep problems in autism, but also to biological and psychopathological mechanisms involved in the development of ASD (for example, certain immunological abnormalities found in autism, such as a decrease number of T lympho- cytes, might be explained by the hypo-functioning of the melatonin system).
Concerning schizophrenia, as suggested by Morera-Fumero and Abreu-Gonzalez [116], a possible explanation for the “low melatonin syndrome” present in some individuals with schizophrenia may stem from the study of the melatonin-synthesizing enzymes, the AA-NAT and ASMT. Furthermore, according to some authors, MT3 might be involved in the melatonin disturbances observed in schiozophrenia [38]. Finally, melatonin secretion was also studied in obsessive compulsive disorder but no abnormalities in melatonin levels were reported.
4.3. Melatonin and Brain Protection
Neurological and neuropsychological disabilities caused by brain injuries are a major public health concern. Thus, reducing deficits after a stroke is a major issue. In this line, a number of recent studies have reported the important role of melatonin in neuroprotection in animal models of stroke [133]. Indeed, melatonin administration after an experimental stroke in animals reduces infarction volume [134, 135]. Such a protective effect can be seen in both gray and white matter [136] and melatonin reduces also inflammatory response [137], cerebral oedema formation [138], and blood-brain barrier permeability [139]. In addition, Kilic et al. [140] investigated how sub-acute delivery of melatonin, starting at 24 hours after stroke onset, and continuing for 29 days can influences neuronal survival, endogenous neurogenesis, motor recovery and locomotor activity in mice submitted to an occlusion of the middle cerebral artery during 30 minutes. Furthermore, melatonin improved neuronal survival and enhanced neurogenesis, even when applied one day after stroke. In addition, the authors showed both motor as well as behavioral improvements after melatonin administration. Indeed, the results indicate that cell survival was associated with a long-lasting improvement of motor and coordination deficits as well as with attenuation of hyperactivity and anxiety of the animals as revealed in open field tests. Its neuroprotective activity in animal models of ischemic stroke, as well as its lack of serious toxicity suggests that melatonin could be used for human stroke treatment in the future.
In addition to its protective effect after stroke, experimental data obtained in various independent animal models of brain lesions in neonates support the notion of a neuroprotective effect of melatonin in preterm neonates (see for a review, Biran et al., [141]). In infants, a major source of brain injury is preterm birth, often associated to long-term neurological, cognitive, educational, and social problems. Neurodevelopmental disorders are not only seen in extremely preterm birth [142] but also in late prematurity [143]. A large number of infants who survive very preterm birth develop cerebral palsy [144] with a high occurrence of associated motor, perceptual and cognitive deficiencies in childhood [145, 146]. Nowadays, the most common brain damage observed in preterm children is diffuse white matter damage as well as reduced neural connectivity [147, 148] in the context of infection, inflammation, and hypoxia-ischemia [149]. Although a number of treatments have been tested in preclinical animal models of perinatal brain injury, none of them had been proved to be efficient as a neuroprotector nor translated in clinical practice. Among the molecules proposed, melatonin is a very good candidate, given its effect on brain development, neuroprotection as well as regarding its absence of adverse effects [150]. As discussed by Biran et al., [141], in addition to its good safety profile, melatonin easily crosses the placenta as the blood-brain barriers and blocks oxidative, excitotoxic and inflammatory pathways, all involved in the pathogenesis of perinatal brain damage caused by preterm birth. However, only a few studies have looked at the synthesis of melatonin in preterm and term neonates. These studies point to a reduced urinary concentration of melatonin during the first 3 months after birth in preterm infants (see Biran et al. [141] for review and discussion). As these authors discussed, compared with term neonates, preterm neonates show a delayed secretion of melatonin which persists after correction for gestational age up to 8 to 9 months of age. In the absence of maternal melatonin, the appearance of circadian rhythms depends principally on neurological maturation, and very little on the environment [151].
Since, as above-mentioned, melatonin easily crosses the blood–brain and placental barriers, it can be administered antenatally in order to reduce or prevent the impact of brain lesions in preterm neonates. Currently, two therapeutic trials testing the neuroprotective properties of melatonin administration in the immediate prepartum period in very preterm infants are under way in France and in the United Kingdom (see Biran et al., [141] for description). The French trial aims to determine the dose of melatonin to be administered in prepartum by parenteral route to mothers at risk of preterm delivery, to decrease the extent of white matter damage detected by diffusion tensor imaging in infants born preterm. The objective of the English trial is to prove that melatonin is capable of reducing brain injury and white matter disease as defined by magnetic resonance imaging at term. These trials will probably lead to a clinical use of melatonin before preterm birth (in case of at risk mother) of just after birth in preterm neonates in order to prevent neurodevelopmental deficits in these children.
Interestingly, from a functional point of view, abnormalities in melatonin physiology associated with sleep disorders, and in particular sleep deprivation, are seen to endanger cerebral and more specifically hippocampal integrity, leading to cognitive dysfunction and contributing to the development of mood disorders [152]. The involvement of melatonin in the development of mood disorders was discussed in the previous section.
Finally, based on the brain protective role of melatonin against oxidative stress previously described in this article, there is also increased experimental evidence showing the therapeutic potential of melatonin in neurodegenerative conditions such as Alzheimer disease, Parkinson disease, Huntington's disease and amyotrophic lateral sclerosis (see Polimeni et al. [153] for a review). Additional studies and clinical trials are now required both in preterm neonates and aging adults to test the clinical efficacy of melatonin supplementation in such disorders, and to identify the specific therapeutic concentrations needed regarding the subject’s age, disease and brain lesion as well as the short and long-term effects of melatonin both on physiological, functional and cognitive outcomes.
5. CONCLUSION AND FUTURE DIRECTIONS
Regular physiological rhythms are important to ensure a stable internal environment by creating continuity from repetition of identical discontinuities. Several studies, based on animal models and human perinatal development, suggest that stable patterns of repeated stimuli in the form of maternal physiological rhythms, involving cross-modal perception such as regular cardiac rhythm, which provides the fetus with auditory and vibratory stimuli, allow the fetus to integrate sensory information facilitating prenatal perceptual learning and to develop a coherent representation of his or her internal and external environment [154-156]. Fluctuations in physiological rhythms (variants), such as variations in the maternal cardiac rhythm and also variations in hormone levels involved in the circadian rhythms that are already present during the fetus life (the fetus’ circadian rhythms are the mother’s ones), occurring in a background of regular repetition of identical sequences (invariants), may help the fetus to develop the ability to adapt to change in an environment characterized by high regularity. As previously emphasized by Tordjman [156], very early mother–infant relations provide a secure environment based on the repetition of invariants, while at the same time promoting adaptation to change through the presence of variants. It is through the regular repetition of identical sequences of discontinuity, such as circadian rhythms, that a continuum is constructed associated with the development of adaptation to changes. Melatonin plays a major role in the regularity and synchronization of central and peripheral oscillators allowing the development of harmonious internal functioning and adaptation of the internal environment to external environment. As highlighted by Arendt [50], melatonin might be the best peripheral biomarker of human circadian timing. This review shows the high number and diversity of melatonin effects opening important perspectives for measuring melatonin as a biomarker (biomarker of early identification of certain disorders and also biomarker of their follow-up) and using melatonin with therapeutic applications. Given the very large potential clinical applications of melatonin and its apparent absence of major side effects, it is amazing to see that the role of melatonin in human clinical trials has been mainly confined to sleep problems and focused on just a few disorders [157]. Future studies are required, such as studies of melatonin dose-response relationships, to extend the use of melatonin in clinical practice, prevention and treatment.
Fig. (1).
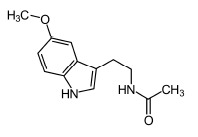
Melatonin chemical structure (N-acetyl-5-methoxytryptamine).
Fig. (2).
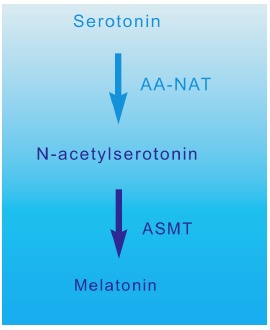
Melatonin synthesis (AA-NAT: arylalkylamine-N-acetyl- transferase; ASMT: acetylserotonin-O-methyltransferase).
Fig. (3).
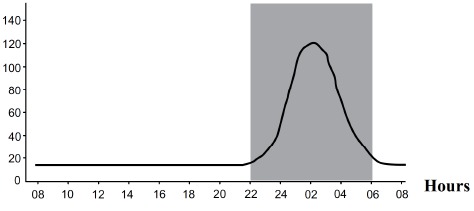
Circadian profile of melatonin plasma concentrations (in grey is represented the period of darkness).
ACKNOWLEDGEMENTS
ST and CF wrote the first draft of the manuscript; SC wrote the section on melatonin and brain protection; EB, NJ and RD revised the manuscript.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- 1.Lerner A.B., Case J.D., Takahashi Y., Lee T.H., Mori W. Isolation of melatonin, the pineal gland factor that lightens melanocytes. 1958.
- 2.Bubenik G.A. Gastrointestinal melatonin: localization, function, and clinical relevance. Dig. Dis. Sci. 2002;47:2336–2348. doi: 10.1023/a:1020107915919. [DOI] [PubMed] [Google Scholar]
- 3.Carrillo-Vico A., Calvo J.R., Abreu P., Lardone P.J., Garcia-Maurino S., Reiter R.J., Guerrero J.M. Evidence of melatonin synthesis by human lymphocytes and its physiological significance: possible role as intracrine, autocrine, and/or paracrine substance. FASEB J. 2004;18:537–539. doi: 10.1096/fj.03-0694fje. [DOI] [PubMed] [Google Scholar]
- 4.Champier J., Claustrat B., Besancon R., Eymin C., Killer C., Jouvet A., Chamba G., Fevre-Montange M. Evidence for tryptophan hydroxylase and hydroxyindol-O-methyl-transferase mRNAs in human blood platelets. Life Sci. 1997;60:2191–2197. doi: 10.1016/s0024-3205(97)00234-8. [DOI] [PubMed] [Google Scholar]
- 5.Conti A., Conconi S., Hertens E., Skwarlo-Sonta K., Markowska M., Maestroni J.M. Evidence for melatonin synthesis in mouse and human bone marrow cells. J. Pineal Res. 2000;28:193–202. doi: 10.1034/j.1600-079x.2000.280401.x. [DOI] [PubMed] [Google Scholar]
- 6.Martin M.T., Azpiroz F., Malagelada J.R. Melatonin and the gastrointestinal system. Therapy. 1998;53:453–458. [PubMed] [Google Scholar]
- 7.Reiter R.J., Richardson B.A., Hurlbut E.C. Pineal, retinal and Harderian gland melatonin in a diurnal species, the Richrdson’s ground squirrel (Spermophilus richardsonii). Neurosci. Lett. 1981;22:285–288. [Google Scholar]
- 8.Slominski A., Fischer T.W., Zmijewski M.A., Wortsman J., Semak I., Zbytek B., Slominski R.M., Tobin D.J. On the role of melatonin in skin physiology and pathology. Endocrine. 2005;27:137–148. doi: 10.1385/ENDO:27:2:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Slominski A., Wortsman J., Tobin D.J. the cutaneous serotoninergic/melatoninergic system securing a place under the sun. FASEB J. 2005;19:176–194. doi: 10.1096/fj.04-2079rev. [DOI] [PubMed] [Google Scholar]
- 10.Raikhlin N.T., Kvetnoy I.M. 1976.
- 11.Illerova H. Melatonin rhythm in the human milk. J. Clin. Endocrinol. Metab. 1993;77:838–841. doi: 10.1210/jcem.77.3.8370707. [DOI] [PubMed] [Google Scholar]
- 12.Reiter R.J., Tan D.X., Maldonado M.D. Melatonin as an antioxidant: physiology versus pharmacology. J. Pineal Res. 2005;39:215–216. doi: 10.1111/j.1600-079X.2005.00261.x. [DOI] [PubMed] [Google Scholar]
- 13.Reppert S.M., Klein D.C. Transport of maternal melatonin to suckling rats and the fate of melatonin in the neonatal rats. Endocrinology. 1978;102:582–588. doi: 10.1210/endo-102-2-582. [DOI] [PubMed] [Google Scholar]
- 14.Illnerova H., Buresova M., Presl J. Melatonin rhythm in human milk. J. Clin. Endocrinol. Metab. 1993;77(3):838–841. doi: 10.1210/jcem.77.3.8370707. [DOI] [PubMed] [Google Scholar]
- 15.Katzer D., Pauli L., Mueller A., Reutter H., Reinsberg J., Fimmers R., Bartmann P., Bagci S. Melatonin concentrations and antioxidative capacity of human breast milk according to gestational age and the time of the day. 2016. [DOI] [PubMed]
- 16.Pires-Lapa M.A., Tamura E.K., Salustiano E.M., Markus R.P. Melatonin synthesis in human colostrum mononuclear cells enhances dectin-1-mediated phagocytosis by mononuclear cells. 2013. [DOI] [PubMed]
- 17.Axelrod J., Weissbach H. Enzymatic O-methylation of N-acetylserotonin to melatonin. Science. 1960;131:1312. doi: 10.1126/science.131.3409.1312. [DOI] [PubMed] [Google Scholar]
- 18.Coon S.L., Roseboom P.H., Baler R., Weller J.L., Namboodiri M.A., Koonin E.V., Klein D.C. Pineal serotonin N-acetyltrans- ferase: expression cloning and molecular analysis. Science. 1995;270(5242):1681–1683. doi: 10.1126/science.270.5242.1681. [DOI] [PubMed] [Google Scholar]
- 19.Simonneaux V., Ribelayga C. Generation of the melatonin endocrine message in mammals: A review of the complex regulation of melatonin synthesis by norepinephrine, peptides, and other pineal transmitters. Pharmacol. Rev. 2003;55:325–395. doi: 10.1124/pr.55.2.2. [DOI] [PubMed] [Google Scholar]
- 20.Klein D.C. 2004.
- 21.Touitou Y. La mélatonine: hormone et médicament. C. R. Soc. Biol. 1998;192:643–657. [PubMed] [Google Scholar]
- 22.Brzezinski A. Melatonin in humans. N. Engl. J. Med. 1997;336(3):186–195. doi: 10.1056/NEJM199701163360306. [DOI] [PubMed] [Google Scholar]
- 23.Karasek K., Winczyk K. Melatonin in humans. J. Physiol. Pharmacol. 2006;57(5):19–39. [PubMed] [Google Scholar]
- 24.Sadeh A. Sleep and melatonin in infants: a preliminary study. Sleep. 1997;20(3):185–191. [PubMed] [Google Scholar]
- 25.Joseph D., Chong N.W., Shanks M.E., Rosato E., Taub N.A., Petersen S.A. Get- ting rhythm: how do babies do it? Arch. Dis. Child. Fetal Neonatal Ed. 2014;100(1):F50–F54. doi: 10.1136/archdischild-204-306104. [DOI] [PubMed] [Google Scholar]
- 26.Zhdanova I., Lynch H., Wurtman R. Melatonin: a sleep- promoting hormone. Sleep. 1997;20:899–907. [PubMed] [Google Scholar]
- 27.Waldhauser F., Weiszenbacher G., Frisch H., Zeitlhuber U., Waldhauser M., Wurtman R.J. Fall in nocturnal serum melatonin during prepuberty and pubescence. Lancet. 1984;1(8373):362–365. doi: 10.1016/s0140-6736(84)90412-4. [DOI] [PubMed] [Google Scholar]
- 28.Iguchi H., Kato K.I., Ibayashi H. Melatonin serum levels and metabolic clearance rate in patients with liver cirrhosis. 1982. [DOI] [PubMed]
- 29.Waldhauser F., Saletu B., Trinchard-Lugan I. Sleep laboratory investigations on hypnotic properties of melatonin. 1990. [DOI] [PubMed]
- 30.Claustrat B., Brun J., Chazot G. The basic physiology and pathophysiology of melatonin. Sleep Med. Rev. 2005;9(1):11–24. doi: 10.1016/j.smrv.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 31.Di W.L., Kadva A., Johnston A., Silman R. Variable bioavailability of oral melatonin. N. Engl. J. Med. 1997;336(14):1028–1029. doi: 10.1056/NEJM199704033361418. [DOI] [PubMed] [Google Scholar]
- 32.Lynch H.J., Wurtman R.J., Moskowitz M.A., Archer M.C., Ho M.H. Daily rhythm in human urinary melatonin. Science. 1975;187(4172):169–171. doi: 10.1126/science.1167425. [DOI] [PubMed] [Google Scholar]
- 33.Dubocovich M.L. Melatonin receptors: are there multiple subtypes? Trends Pharmacol. Sci. 1995;16(2):50–56. doi: 10.1016/s0165-6147(00)88978-6. [DOI] [PubMed] [Google Scholar]
- 34.Morgan P.J., Barrett P., Howell H.E., Helliwell R. Melatonin receptors: localization, molecular pharmacology and physiological significance. Neurochem. Int. 1994;24(2):101–146. doi: 10.1016/0197-0186(94)90100-7. [DOI] [PubMed] [Google Scholar]
- 35.Ebisawa T., Karne S., Lerner M.R., Reppert S.M. Expression cloning of a high-affinity melatonin receptor from Xenopus dermal melanophores. Proc. Natl. Acad. Sci. USA. 1994;91(13):6133–6137. doi: 10.1073/pnas.91.13.6133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cardinali D.P., Golombek D.A., Rosenstein R.E., Cutrera R.A., Esquifino A.I. 1997.
- 37.Li D.Y., Smith D.G., Hardeland R., Yang M.Y. XU, H.L.; Zhang, L.; Yin, H.D.; Zhu, Q. Melatonin receptor genes in vertebrates. Int. J. Mol. Sci. 2013;14(6):11208–11223. doi: 10.3390/ijms140611208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jockers R., Maurice P., Boutin J.A., Delagrange P. Melatonin receptors, heterodimerization, signal transduction and binding sites: what's new? Br. J. Pharmacol. 2008;154(6):1182–1195. doi: 10.1038/bjp.2008.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zawilska J.B., Skene D.J., Arendt J. Physiology and pharmacology of melatonin in relation to biological rhythms. 2009. [DOI] [PubMed]
- 40.Benitez-King G., Anton-Tay F. Calmodulin mediates melatonin cytoskeletal effects. Experientia. 1993;49(8):635–641. doi: 10.1007/BF01923944. [DOI] [PubMed] [Google Scholar]
- 41.Becker-Andre M., Wiesenberg I., Schaeren-Wiemers N., Andre E., Missbach M., Saurat J.H., Carlberg C. Pineal gland hormone melatonin binds and activates an orphan of the nuclear receptor superfamily. J. Biol. Chem. 1994;269(46):28531–28534. [PubMed] [Google Scholar]
- 42.Ekmekcioglu C. Melatonin receptors in humans: biological role and clinical relevance. Biomed. Pharmacother. 2006;60:97–108. doi: 10.1016/j.biopha.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 43.Morgan P., Barrett P., Howell H., Helliwell R. Melatonin receptors: Localization, molecular pharmacology and physiological significance. Neurochem. Int. 1994;24:101–146. doi: 10.1016/0197-0186(94)90100-7. [DOI] [PubMed] [Google Scholar]
- 44.Liu J., Clough S.J., Hutchinson A.J., Adamah-Biassi E.B., Popovska-Gorevski M., Dubocovich M.L. MT1 and MT2 melatonin receptors: a therapeutic perspective. Annu. Rev. Pharmacol. Toxicol. 2016;56:361–383. doi: 10.1146/annurev-pharmtox-010814-124742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jin X., Von Gall C., Pieschl R.L., Gribkoff V.K., Stehle J.H., Reppert S.M., Weaver D.R. Targeted Disruption of the Mouse Mel1b Melatonin Receptor. Mol. Cell. Biol. 2003;23(3):1054–1060. doi: 10.1128/MCB.23.3.1054-1060.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Von Gall C., Stehle J.H., Weaver D.R. Mammalian melatonin receptors: molecular biology and signal transduction. Cell Tissue Res. 2002;309:151–162. doi: 10.1007/s00441-002-0581-4. [DOI] [PubMed] [Google Scholar]
- 47.Axelrod J. The pineal gland: a neurochemical transducer. Science. 1974;184:1341–1348. doi: 10.1126/science.184.4144.1341. [DOI] [PubMed] [Google Scholar]
- 48.Dahlitz M., Alavarez B., Vignau J., English J., Arendt J., Parkes J.D. Delayed sleep phase syndrome. Lancet. 1991;337:1121–1124. doi: 10.1016/0140-6736(91)92787-3. [DOI] [PubMed] [Google Scholar]
- 49.Zisapel N. Circadian rhythm sleep disorders: pathophysiology and potential approaches to management. CNS Drugs. 2001;15:311–328. doi: 10.2165/00023210-200115040-00005. [DOI] [PubMed] [Google Scholar]
- 50.Arendt J. Melatonin: characteristics, concerns, and prospects. 2005. [DOI] [PubMed]
- 51.Jan J.E., Bax M.C., Owens J.A., Ipsiroglu O.S., Wasdell M.B. Neurophysiology of circadian rhythm sleep disorders of children with neurodevelopmental disabilities. Eur. J. Paediatr. Neurol. 2012;16(5):403–412. doi: 10.1016/j.ejpn.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 52.Mareš J., Stopka P., Nohejlová K., Rokyta R. Oxidative stress induced by epileptic seizure and its attenuation by melatonin. Physiol. Res. 2013;62(Suppl. 1):S67–S74. doi: 10.33549/physiolres.932576. [DOI] [PubMed] [Google Scholar]
- 53.Guzman-Marin R., Suntsova N., Methippara M., Greiffenstein R., Szymusiak R., McGinty D. Sleep deprivation suppresses neurogenesis in the adult hippocampus of rats. 2005. [DOI] [PubMed]
- 54.Kong X., Li X., Cai Z., Yang N., Liu Y., Shu J., Pan L., Zuo P. Melatonin regulates the viability and differentiation of rat midbrain neural stem cells. Cell. Mol. Neurobiol. 2008;28(4):569–579. doi: 10.1007/s10571-007-9212-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moriya T., Horie N., Mitome M., Shinohara K. Melatonin influences the proliferative and differentiative activity of neural stem cells. 2007. [DOI] [PubMed]
- 56.Reiter R.J. Neuroendocrine effects of light. Int. J. Biometeorol. 1991;35(3):169–175. doi: 10.1007/BF01049063. [DOI] [PubMed] [Google Scholar]
- 57.Iwasaki S., Nakazawa K., Sakai J., Kometani K., Iwashita M., Yoshimura Y., Maruyama T. Melatonin as a local regulator of human placental function. J. Pineal Res. 2005;39:261–265. doi: 10.1111/j.1600-079X.2005.00244.x. [DOI] [PubMed] [Google Scholar]
- 58.Kennaway D.J. 2000.
- 59.Niles L.P., Armstrong K.J., Rincón Castro L.M., Dao C.V., Sharma R., McMillan C.R., Doering L.C., Kirkham D.L. Neural stem cells express melatonin receptors and neurotrophic factors: colocalization of the MT1 receptor with neuronal and glial markers. BMC Neurosci. 2004;5:41. doi: 10.1186/1471-2202-5-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kennaway D.J., Goble F.C., Stamp G.E. Factors influencing the development of melatonin rhythmicity in humans. J. Clin. Endocrinol. Metab. 1996;81(4):1525–1532. doi: 10.1210/jcem.81.4.8636362. [DOI] [PubMed] [Google Scholar]
- 61.Okatani Y., Morioka N., Hayashi K. Changes in nocturnal pineal melatonin synthesis during the perimenopausal period: relation to estrogen levels in female rats. J. Pineal Res. 1999;27(2):65–72. doi: 10.1111/j.1600-079x.1999.tb00598.x. [DOI] [PubMed] [Google Scholar]
- 62.Torres-Farfan C., Valenzuela F.J., Germain A.M., Viale M.L., Campino C., Torrealba F., Valenzuela G.J., Richter H.G., Serón-Ferré M. Maternal melatonin stimulates growth and prevents maturation of the capuchin monkey fetal adrenal gland. 2006. [DOI] [PubMed]
- 63.Torres-Farfan C., Rocco V., Monsó C., Valenzuela F.J., Campino C., Germain A., Torrealba F., Valenzuela G.J., Seron-Ferre M. Maternal melatonin effects on clock gene expression in a nonhuman primate fetus. Endocrinology. 2006;147(10):4618–4626. doi: 10.1210/en.2006-0628. [DOI] [PubMed] [Google Scholar]
- 64.Torres-Farfan C., Serón-Ferré M., Dinet V., Korf H.W. Immunocytochemical demonstration of day/night changes of clock gene protein levels in the murine adrenal gland: differences between melatonin-proficient (C3H) and melatonin-deficient (C57BL) mice. J. Pineal Res. 2006;40(1):64–70. doi: 10.1111/j.1600-079X.2005.00279.x. [DOI] [PubMed] [Google Scholar]
- 65.Reiter R.J., Tan D.X., Korkmaz A., Rosales-Corral S.A. Melatonin and stable circadian rhythms optimize maternal, placental and fetal physiology. Hum. Reprod. Update. 2014;20(2):293–307. doi: 10.1093/humupd/dmt054. [DOI] [PubMed] [Google Scholar]
- 66.Pandi-Perumal S.R., Srinivasan V., Maestroni G.J., Cardinali D.P., Poeggeler B., Hardeland R. Melatonin: Nature’s most versatile biological signal? FEBS J. 2006;273:2813–2838. doi: 10.1111/j.1742-4658.2006.05322.x. [DOI] [PubMed] [Google Scholar]
- 67.Pevet P., Challet E. 2011.
- 68.Arendt J. Melatonin and human rhythms. Chronobiol. Int. 2006;29:21–37. doi: 10.1080/07420520500464361. [DOI] [PubMed] [Google Scholar]
- 69.Arangino S., Cagnacci A., Angiolucci M., Vacca A.M., Longu G., Volpe A., Melis G.B. Effects of melatonin an vascular reactivity, catecholamine levels, and blood pressure in healthy men. Am. J. Cardiol. 1999;83:1417. doi: 10.1016/s0002-9149(99)00112-5. [DOI] [PubMed] [Google Scholar]
- 70.Cagnacci A., Arangino S., Angiolucci M., Maschio E., Melis G.B. Influences of melatonin adminstration on the circulation of women. Am. J. Physiol. 1998;274:R335–R338. doi: 10.1152/ajpregu.1998.274.2.R335. [DOI] [PubMed] [Google Scholar]
- 71.Doolen S., Krause D.N., Dubocovich M.L., Duckles S.P. Melatonin mediates two distinct responses in vascular smooth muscle. 1998. [DOI] [PubMed]
- 72.Iuvone P.M., Tosini G., Pozdeyev N., Haque R., Klein D.C., Chaurasia S.S. Circadian clocks, clock networks, arylalkylamine N-acetyltransferase, and melatonin in the retina. 2005. [DOI] [PubMed]
- 73.Nishiyama K., Yasue H., Moriyama Y., Tsunoda R., Ogawa H., Yoshimura M., Kugiyama K. Acute effects of melatonin administration on cardiovascular autonomic regulation in healthy men. 2001. [DOI] [PubMed]
- 74.Scheer F.A., Van Montfrans G.A., Van Someren E.J., Mairuhu G., Buijs R.M. Daily night-time melatonin reduces blood pressure in male patients with essential hypertension. Hypertension. 2004;43:192–197. doi: 10.1161/01.HYP.0000113293.15186.3b. [DOI] [PubMed] [Google Scholar]
- 75.Pandi-Perumal S.R., Esquifino A.I., Cardinali D.P. The role of melatonin in immunoenhancement: Potential application in cancer. Int. J. Exp. Pathol. 2006;87:81–87. doi: 10.1111/j.0959-9673.2006.00474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Carrillo-Vico A., Guerrero J.M., Lardone P.J., Reiter R.J. A review of the multiple actions of melatonin on the immune system. 2005. [DOI] [PubMed]
- 77.Srinivasan V., Pandi-Perumal S.R., Maestroni G.J., Esquifino A.I., Hardeland R., Cardinali D.P. Role of melatonin in neurodegenerative diseases. Neurotox. Res. 2005;7:293–318. doi: 10.1007/BF03033887. [DOI] [PubMed] [Google Scholar]
- 78.Regodon S., Martin-Palomino P., Fernadez-Montesinos R., Herrera J.L., Carrascosa-Salmoral M.P., Piriz S., Vadillo S., Guerrero J.M., Pozo D. The use of melatonin as a vaccine agent. 2005. [DOI] [PubMed]
- 79.Hardeland R., Pandi-Perumal S.R. Melatonin, a potent agent in antioxidative defense: Actions as a natural food constituent, gastrointestinal factor, drug and prodrug. Nutr. Metab. 2005;2:22. doi: 10.1186/1743-7075-2-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Esquifino A.I., Pandi-Perumal S.R., Cardinali D.P. Circadian organization of the immune response: a role for melatonin. 2004.
- 81.Bartness T.J., Demas G.E., Song C.K. Seasonal changes in adiposity: the roles of the photoperiod, melatonin and other hormones, and sympathetic nervous system. Exp. Biol. Med. (Maywood) 2002;227:363–376. doi: 10.1177/153537020222700601. [DOI] [PubMed] [Google Scholar]
- 82.Ladizeski M.G., Boggio V., Albornoz L.E., Castrillon P.O., Mautalen C., Cardinali D.P. Melatonin increases oestradiol-induced bone formation in ovariectomized rats. J. Pineal Res. 2003;34:143–151. doi: 10.1034/j.1600-079x.2003.00021.x. [DOI] [PubMed] [Google Scholar]
- 83.Rasmussen D.D., Boldt B.M., Wilkinson C.W., Yellon S.M., Matsumoto A.M. Daily melatonin administration at middle age suppresses male rat visceral fat, plasma leptin, and plasma insulin to youthful levels. Endocrinology. 1999;140:1009–1012. doi: 10.1210/endo.140.2.6674. [DOI] [PubMed] [Google Scholar]
- 84.Brydon L., Petit L., Delagrange P., Strosberg A.D., Jockers R. Functional expression of mt2 (mel1b) melatonin receptors in human paz6 adipocytes. Endocrinology. 2001;142:4264–4271. doi: 10.1210/endo.142.10.8423. [DOI] [PubMed] [Google Scholar]
- 85.Ladizesky M.G., Cutrera R.A., Boggio V., Somoza J., Centrella J.M., Mautalen C., Cardinali D.P. Effect of melatonin on bone metabolism in ovariectomized rats. Life Sci. 2001;70:557–565. doi: 10.1016/s0024-3205(01)01431-x. [DOI] [PubMed] [Google Scholar]
- 86.Roth J.A., Kim B.G., Lin W.L., Cho M.I. Melatonin promotes osteoblast differentiation and bone formation. J. Biol. Chem. 1999;274:22041–22047. doi: 10.1074/jbc.274.31.22041. [DOI] [PubMed] [Google Scholar]
- 87.Koyama H., Nakade O., Takada Y., Kaku T., Lau K.H. Melatonin at pharmacologic doses increases bone mass by suppressing resorption throught downregulation of the RANKL-mediated osteoclast formation and activation. J. Bone Miner. Res. 2002;17:1219–1229. doi: 10.1359/jbmr.2002.17.7.1219. [DOI] [PubMed] [Google Scholar]
- 88.Nakade O., Koyama H., Ariji H., Yajima A., Kaku T. Melatonin stimulates proliferation and type I collagen synthesis in human bone cells in vitro. J. Pineal Res. 1999;27:106–110. doi: 10.1111/j.1600-079x.1999.tb00603.x. [DOI] [PubMed] [Google Scholar]
- 89.Fjelldal P.G., Grotmol S., Kryvi H., Gjerdet N.R., Taranger G.L., Hansen T., Porter M.J., Totland G.K. Pinealectomy induces malformation of the spine and reduces the mechanical strength of the vertebrae in Atlantic salmon, Salmo salar. J. Pineal Res. 2004;36:132–139. doi: 10.1046/j.1600-079x.2003.00109.x. [DOI] [PubMed] [Google Scholar]
- 90.Sadat-Ali M., Habdan I., Othman A. Adolescent idiopathic scoliosis. Is low melatonin a cause? Joint Bone Spine. 2000;67:62–64. [PubMed] [Google Scholar]
- 91.Balik A., Kretschmannova K., Mazna P., Svobodova I., Zemkova H. Melatonin action in neonatal gonadotrophs. Physiol. Res. 2004;53(1):S153–S166. [PubMed] [Google Scholar]
- 92.Glass J.D., Knotts L.K. 1987.
- 93.Roy D., Belsham D.D. Melatonin receptor activation regulates GnRH gene expression and secretion in GT1-7 GnRH neurons: Signal transduction mechanisms. J. Biol. Chem. 2001;277:251–258. doi: 10.1074/jbc.M108890200. [DOI] [PubMed] [Google Scholar]
- 94.Aleandri V., Spina V., Morini A. The pineal gland and reproduction. Hum. Reprod. Update. 1996;2:225–235. doi: 10.1093/humupd/2.3.225. [DOI] [PubMed] [Google Scholar]
- 95.Barrell G.K., Thrun L.A., Brown M.E., Viguie C., Karsch F.J. Importance of photoperiodic signal quality to entrainment of the circannual reproductive rhythm of the Ewe. Biol. Reprod. 2000;63:769–774. doi: 10.1095/biolreprod63.3.769. [DOI] [PubMed] [Google Scholar]
- 96.Kauppila A., Kivela A., Pakarinien A., Vakkuri O. Inverse seasonal relationship between melatonin and ovarian activity in humans in a region with a strong seasonal contrast in luminosity. 1987. [DOI] [PubMed]
- 97.Anisimov V.N., Popovich I.G., Shtylik A.V., Zabezhinski M.A., Ben Huh H., Gurevich P., Berman V., Tendler Y., Zusman I. Melatonin and colon carcinogenesis III. 2000. [DOI] [PubMed]
- 98.Anisimov V.N., Popovich I.G., Zabezhinski M.A. 1997.
- 99.Blask D.E., Dauchy R.T., Sauer L.A. Putting cancer to sleep at night: the neuroendocrine/circadian melatonin signal. Endocrine. 2005;27:179–188. doi: 10.1385/ENDO:27:2:179. [DOI] [PubMed] [Google Scholar]
- 100.Gilad E., Laufer M., Matzkin H., Zisapel N. Melatonin receptors in PC3 human prostate tumor cells. J. Pineal Res. 1999;26:211–220. doi: 10.1111/j.1600-079x.1999.tb00586.x. [DOI] [PubMed] [Google Scholar]
- 101.Hill S.M., Teplitzky S., Ram P.T., Kiefer T., Blask D.E., Spriggs L.L., Eck D.M. Melatonin synergizes with retinoic acid in the prevention and regression of breast cancer. Adv. Exp. Med. Biol. 1999;460:345–362. doi: 10.1007/0-306-46814-x_39. [DOI] [PubMed] [Google Scholar]
- 102.Hu D.N., McCormick S.A., Roberts J.E. Effects of melatonin, its precursors and derivates on the growth of cultured human uveal melanoma cells. Melanoma Res. 1998;8:205–210. doi: 10.1097/00008390-199806000-00002. [DOI] [PubMed] [Google Scholar]
- 103.Hu D.N., Roberts J.E. Melatonin inhibits growth of cultured human uveal melanoma cells. Melanoma Res. 1997;7:27–31. doi: 10.1097/00008390-199702000-00005. [DOI] [PubMed] [Google Scholar]
- 104.Kanashi Y., Kobayashi Y., Noda S., Ishizuka B., Saito K. Differential growth inhibitory effect of melatonin on two endometrial cancer cell lines. J. Pineal Res. 2000;28:227–233. doi: 10.1034/j.1600-079x.2000.280405.x. [DOI] [PubMed] [Google Scholar]
- 105.Kiefer T., Ram P.T., Yuan L., Hill S.M. Melatonin inhibits estrogen receptor transactivation and cAMP levels in breast cancer cells. Breast Cancer Res. Treat. 2002;71:37–45. doi: 10.1023/a:1013301408464. [DOI] [PubMed] [Google Scholar]
- 106.Petranka J., Baldwin W., Biermann J., Jayadev S., Barrett J.C., Murphy E. The oncostatic action of melatonin in an ovarian carcinoma cell line. J. Pineal Res. 1999;26:129–136. doi: 10.1111/j.1600-079x.1999.tb00574.x. [DOI] [PubMed] [Google Scholar]
- 107.Karbownik M., Lewinski A., Reiter R.J. Anticarcinogenic actions of melatonin which involve antioxidative processes: comparison with other antioxidants. Int. J. Biochem. Cell Biol. 2001;33:735–753. doi: 10.1016/s1357-2725(01)00059-0. [DOI] [PubMed] [Google Scholar]
- 108.Klaunig J.E., Xu Y., Isenberg J.S., Bachowski S., Kolaja K.L., Jiang J., Stevenson D.E., Walborg E.F., Jr The role of oxidative stress in chemical carcinogenesis. Environ. Health Perspect. 1998;106(1):289–295. doi: 10.1289/ehp.98106s1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Brugger P., Marktl W., Herold M. Impaired nocturnal secretion of melatonin in coronary heart disease. Lancet. 1995;345:1408. doi: 10.1016/s0140-6736(95)92600-3. [DOI] [PubMed] [Google Scholar]
- 110.Girotti L., Lago M., Ianovsky O., Carbajales J., Elizari M.V., Brusco L.I., Cardinali D.P. Low urinary 6-sulphatoxymelatonin levels in patients with coronary artery disease. J. Pineal Res. 2000;29:138–142. doi: 10.1034/j.1600-079x.2000.290302.x. [DOI] [PubMed] [Google Scholar]
- 111.Girotti L., Lago M., Ianovsky O., Elizari M.V., Dini A. ; Lloret S.P., Albornoz L.E., Cardinali D.P. 2003.
- 112.Yaprak M., Altun A., Vardar A., Aktoz M., Ciftci S., Ozbay G. Decreased nocturnal synthesis of melatonin in patients with coronary artery disease. Int. J. Cardiol. 2003;89:103–107. doi: 10.1016/s0167-5273(02)00461-8. [DOI] [PubMed] [Google Scholar]
- 113.Scheer F.A. Potential use of melatonin as adjunct antihypertensive therapy. 2005. [DOI] [PubMed]
- 114.Beck-Friis J., Kjellman B.F., Aperia B., Unden F., von Rosen D., Ljunggren J.G., Wetterberg L. Serum melatonin in relation to clinical variables in patients with major depressive disorder and a hypothesis of a low melatonin syndrome. 1985. [DOI] [PubMed]
- 115.Mayeda A., Mannon S., Hofstetter J., Adkins M., Baker R., Hu K., Nurnberger J.J. Effects of indirect light and propranolol on melatonin levels in normal human subjects. Psychiatry Res. 1998;81:9–17. doi: 10.1016/s0165-1781(98)00069-9. [DOI] [PubMed] [Google Scholar]
- 116.Morera-Fumero A.L., Abreu-Gonzalez P. Role of melatonin in schizophrenia. Int. J. Mol. Sci. 2013;14:9037–9050. doi: 10.3390/ijms14059037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Souetre E., Salvati E., Belugou J.L., Pringuey D., Candito M., Krebs B., Ardisson J.L., Darcourt G. Circadian rhythms in depression and recovery: evidence for blunted amplitude as the main chronobiological abnormality. Psychiatry Res. 1989;28:263–278. doi: 10.1016/0165-1781(89)90207-2. [DOI] [PubMed] [Google Scholar]
- 118.Milhiet V., Etain B., Boudebesse C., Bellivier F. Circadian biomakers, circadian genes and bipolar disorders. J. Physiol. 2011;105:183–189. doi: 10.1016/j.jphysparis.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 119.Nurnberger J.I., Jr, Adkins S., Lahiri D.K., Mayeda A., Hu K., Lewy A., Miller A., Bowman E.S., Miller M.J., Rau L., Smiley C., Davis-Singh D. Melatonin suppression by light in euthymic bipolar and unipolar patients. Arch. Gen. Psychiatry. 2000;57(6):572–579. doi: 10.1001/archpsyc.57.6.572. [DOI] [PubMed] [Google Scholar]
- 120.Golden R.N., Markey S.P., Risby E.D., Rudorfer M.V., Cowdry R.W., Potter W.Z. 1988. [DOI] [PubMed]
- 121.Thompson C., Mezey G., Corn T., Franey C., English J., Arendt J., Checkley S.A. The effect of desipramine upon melatonin and cortisol secretion in depressed and normal subjects. 1985. [DOI] [PubMed]
- 122.Venkoba rao, A.; Parvathi Devi, S.; Srinivasan, V. Urinary melatonin in depression. Indian J. Psychiatry. 1983;25:167–172. [PMC free article] [PubMed] [Google Scholar]
- 123.Den Boer J.A., Bosker F.J., Meesters Y. Clinical efficacy of agomelatine in depression: the evidence. 2006. [DOI] [PubMed]
- 124.Loo H., Dalery J., Macher J.P., Payen A. Pilot study comparing in blind the therapeutic effect of two doses of agomelatine, melatoninergic agonist and selective 5HT2C receptors antagonist, in the treatment of major depressive disorders. Encephale. 2002;28:356–362. [PubMed] [Google Scholar]
- 125.Olie P., Emsley R. Confirmed clinical efficacy of agomelatine (25-50 mg) in major depression; two randomized, double-blind controlled studies. Eur. Neuropsychopharmacol. 2005;15(3):S416. [Google Scholar]
- 126.Kotagal S., Broomall E. Sleep in children with autisms pectrum disorder. J. Pediatr. Neurol. 2012;47(4):242–251. doi: 10.1016/j.pediatrneurol.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 127.Tordjman S., Anderson G.M., Bellissant E., Botbol M., Charbuy H., Camus F., Graignic R., Kermarrec S., Fougerou C., Cohen D., Touitou Y. Day and nighttime excretion of 6-sulphatoxymelatonin in adolescents and young adults with autistic disorder. Psychoneuroendocrinology. 2012;37:1996–1997. doi: 10.1016/j.psyneuen.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 128.Tordjman S., Najjar I., Bellissant E., Anderson G.M., Barburoth M., Cohen D., Nemat Jaafari N., Schischmanoff O., Fagard R., Lagdas E., Kermarrec S., Ribardiere R., Botbol M., Fougerou C., Bronsard G., Vernay-Leconte J. Advances in the research of melatonin in autism spectrum disorders: literature review and new perspectives. Int. J. Mol. Sci. 2013;14(10):20508–20542. doi: 10.3390/ijms141020508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lai M.C., Lombardo M.V., Baron-Cohen S. Autism. Lancet. 2014;383:896–910. doi: 10.1016/S0140-6736(13)61539-1. [DOI] [PubMed] [Google Scholar]
- 130.Gould E.L., Loesch D.Z., Martin M.J., Hagerman R.J., Amstrong S.M., Huggins R.M. Melatonin profiles and sleep characteristics in boys with fragile X syndrome: a preliminary study. 2000. [PubMed]
- 131.Reiter R.J., Barlow-Walden L., Poeggeler B., Heiden S.M., Clayton R.J. 1996. [DOI] [PubMed]
- 132.Tordjman S., Anderson G.M., Pichard N., Charbuy H., Touitou Y. Nocturnal excretion of 6-sulphatoxymelatonin in children and adolescents with autistic disorder. Biol. Psychiatry. 2005;57:134–138. doi: 10.1016/j.biopsych.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 133.Kilic E., Ozdemir Y.G., Bolay H., Kelestimur H., Dalkara T. Pinealectomy aggravates and melatonin administration attenuates brain damage in focal ischemia. J. Cereb. Blood Flow Metab. 1999;19:511–516. doi: 10.1097/00004647-199905000-00005. [DOI] [PubMed] [Google Scholar]
- 134.Pei Z., Pang S.F., Cheung R.T. Administration of melatonin after onset of ischemia reduces the volume of cerebral infarction in a rat middle cerebral artery occlusion stroke model. Stroke. 2003;34:770–775. doi: 10.1161/01.STR.0000057460.14810.3E. [DOI] [PubMed] [Google Scholar]
- 135.Sinha K., Degaonkar M.N., Jagannathan N.R., Gupta Y.K. Effect of melatonin on ischemia reperfusion injury induced by middle cerebral artery occlusion in rats. Eur. J. Pharmacol. 2001;428:185–192. doi: 10.1016/s0014-2999(01)01253-5. [DOI] [PubMed] [Google Scholar]
- 136.Lee E.J., Lee M.Y., Chen H.Y., Hsu Y.S., Wu T.S., Chen S.T., Chang G.L. Melatonin attenuates gray and white matter damage in a mouse model of transient focal cerebral ischemia. J. Pineal Res. 2005;38:42–52. doi: 10.1111/j.1600-079X.2004.00173.x. [DOI] [PubMed] [Google Scholar]
- 137.Lee M.Y., Kuan Y.H., Chen H.Y., Chen T.Y., Chen S.T., Huang C.C., Yang I.P., Hsu Y.S., Wu T.S., Lee E.J. Intravenous administration of melatonin reduces the intracerebral cellular inflammatory response following transient focal cerebral ischemia in rats. 2007. [DOI] [PubMed]
- 138.Kondoh T., Uneyama H., Nishino H., Torii K. Melatonin reduces cerebral edema formation caused by transient forebrain ischemia in rats. Life Sci. 2002;72:583–590. doi: 10.1016/s0024-3205(02)02256-7. [DOI] [PubMed] [Google Scholar]
- 139.Chen T.Y., Lee M.Y., Chen H.Y., Kuo Y.L., Lin S.C., Wu T.S., Lee E.J. Melatonin attenuates the postischemic increase in blood-brain barrier permeability and decreases hemorrhagic transformation of tissue-plasminogen activator therapy following ischemic stroke in mice. J. Pineal Res. 2006;40:242–250. doi: 10.1111/j.1600-079X.2005.00307.x. [DOI] [PubMed] [Google Scholar]
- 140.Kilic E., Kilic U., Bacigaluppi M., Guo Z., Ben Abdallah N., Wolfer D.P., Reiter R.J., Hermann D.M., Bassetti C.L. Delayed melatonin administration promotes neuronal survival, neurogenesis and motor recovery, and attenuates hyperactivity and anxiety after mild focal cerebral ischemia in mice. J. Pineal Res. 2008;45:142–148. doi: 10.1111/j.1600-079X.2008.00568.x. [DOI] [PubMed] [Google Scholar]
- 141.Biran V., Phan Duy A., Decobert F., Bednarek N., Alberti C., Baud O. Is melatonin ready to be used in preterm infants as a neuroprotectant? Dev. Med. Child Neurol. 2014;56(8):717–723. doi: 10.1111/dmcn.12415. [DOI] [PubMed] [Google Scholar]
- 142.Arpino C., Compagnone E., Montanaro M.L., Cacciatore D., De Luca A., Cerulli A., Di Girolamo S., Curatolo P. 2010. [DOI] [PubMed]
- 143.Machado J. L.C.; Passini, Júnior, R.; Rodrigues, M. R. I. Late prematurity: a systematic review. J. Pediatr. (Rio J.) 2014;90(3):221–231. doi: 10.1016/j.jped.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 144.Allen M.C. Neurodevelopmental outcomes of preterm infants. 2008. [DOI] [PubMed]
- 145.Vincer M.J., Allen A.C., Allen V.M., Baskett T.F., O'Connell C.M. Trends in the prevalence of cerebral palsy among very preterm infants. Paediatr. Child Health. 2014;19(4):185–189. doi: 10.1093/pch/19.4.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kelly C.E., Cheong J.L., Molloy C., Anderson P.J., Lee K.J., Burnett A.C., Connelly A., Doyle L.W., Thompson D.K., Victorian Infant Collaborative Study Group 2014.
- 147.Dean J.M., Bennet L., Back S.A., McClendon E., Riddle A. What brakes the preterm brain? An arresting story. Pediatr. Res. 2014;75(1-2):227–233. doi: 10.1038/pr.2013.189. [DOI] [PubMed] [Google Scholar]
- 148.Pineda R.G., Neil J., Dierker D., Smyser C.D., Wallendorf M., Kidokoro H., Reynolds L.C., Walker S., Rogers C., Mathur A.M., Van Essen D.C., Inder T. Alterations in brain structure and neurodevelopmental outcome in preterm infants hospitalized in different neonatal intensive care unit environments. J. Pediatr. 2014;164(1):52–60.e2. doi: 10.1016/j.jpeds.2013.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Benders M.J., Kersbergen K.J., de Vries L.S. Neuroimaging of white matter injury, intraventricular and cerebellar hemorrhage. 2014. [DOI] [PubMed]
- 150.Reiter R.J., Tan D.X., Galano A. Melatonin: exceeding expectations. Physiology (Bethesda) 2014;29(5):325–333. doi: 10.1152/physiol.00011.2014. [DOI] [PubMed] [Google Scholar]
- 151.Gertner S., Greenbaum C.W., Sadeh A., Dolfin Z., Sirota L., Ben-Nun Y. Sleep-wake patterns in preterm infants and 6 month's home environment: implications for early cognitive development. 2002. [DOI] [PubMed]
- 152.Meerlo P., Mistlberger R.E., Jacobs B.L., Heller H.C., McGinty D. New neurons in the adult brain: the role of sleep and consequences of sleep loss. Sleep Med. Rev. 2009;13(3):187–194. doi: 10.1016/j.smrv.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Polimeni G., Esposito E., Bevelacqua V., Guarneri C., Cuzzocrea S. Role of melatonin supplementation in neurodegenerative disorders. Front. Biosci. (Landmark Ed.) 2014;19:429–446. doi: 10.2741/4217. [DOI] [PubMed] [Google Scholar]
- 154.Guedeney A., Guedeney N., Tereno S., Dugravier R., Greacen T., Welniarz B. Infant rhythms versus parental time: promoting parent-infant synchrony. J. Physiol. Paris. 2011;105(4-6):195–200. doi: 10.1016/j.jphysparis.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 155.Lickliter R., Bahrick L.E., Honeycutt H. 2002. [DOI] [PubMed]
- 156.Tordjman S. At the crossroads between psychoanalysis and neuroscience: the importance of subjectivity. J. Physiol. Paris. 2010;104(5):232–242. doi: 10.1016/j.jphysparis.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 157.Sanchez-Barcelo E.J., Mediavilla M.D., Tan D.X., Reiter R.J. 2010.


