Abstract
Background:
Renal diseases are important causes of morbidity and mortality in children worldwide particularly in the resource-poor countries of sub-Saharan Africa. Adequate data on these diseases in children in our setting are limited as a result of late/nonpresentation.
Aim:
The aim of the study is to review the pattern and outcome of pediatric renal admissions at the Federal Teaching Hospital (FETH) Abakaliki over a 3-year period.
Subjects and Methods:
This was a retrospective observational review of all childhood renal admissions in FETH, Abakaliki, Ebonyi state between 2011 and 2013. Relevant clinical data extracted from the hospital records included patients' biodata, presenting symptom(s), examination finding (s), laboratory investigation results as well as treatment and outcome using a semi-structured questionnaire. Data collected were analyzed using SPSS software package version 16.0. The differences in proportions were tested for statistical significance using the Chi-square statistics. Statistical significance was based on P < 0.05.
Results:
In the period under review, 1780 children were admitted, of which 4.4% (79/1780) had renal disorders. The mean age of the children was 8.37 (5.1) years. Nephrotic syndrome 32.9% (26/79) was the most common while on individual basis, meatal stenosis, acute kidney injury and end-stage renal disease, respectively, 1.3% (1/79) were the least renal disorders in the study population. The association between treatment mode and outcome of the treatment was statistically significant (P = 0.03), whereas other variables, such as age (P = 0.42), sex (P = 0.28), socioeconomic status (P = 0.33), and type of renal disease (P = 1.00) were not statistically significant. The case fatality rate was 3.8% (3/79).
Conclusion:
The prevalence of individual renal cases in the current study appears to be high. Nephrotic syndrome was the most common with the majority having favorable outcome. There is need to encourage early presentation as the outcome of some of these renal diseases is encouraging, especially when diagnosis and effective management are possible.
Keywords: Children, Burden and Kidney disease
Introduction
Renal diseases are important causes of morbidity and mortality in children globally particularly in the sub-Saharan Africa where a dearth of workforce and institutional infrastructure as well as high level of poverty further compromise the clinical outcomes of such cases.
Early diagnosis and management are the cornerstone of renal disorders management to stall further progress to renal complications, such as chronic kidney disease (CKD) and end-stage renal failure (ESRF).[1,2,3,4] In the sub-Saharan Africa particularly in Nigeria, early diagnosis is a problem, especially with congenital renal disorders which have been attributed to various reasons. These reasons include absence (due to cost) of routine pre- and post-natal screening which ideally identifies these congenital renal tract abnormalities, poor spread of the pediatric nephrologists who are mostly found in tertiary hospitals and late presentation of cases to specialized centers. Most of these late presentations most often are primarily attended to by primary health workers who are not trained to manage such cases thereby resulting in misdiagnosis/poor reporting and often complications, including CKD and ESRF. Unfortunately, management of these complications demand the use of advanced technology, which is not available and when available is not affordable or easily accessible.[1,2,3,4] In addition, the cost of management is high and often incurred by the family (caregivers) who usually pay out of their pockets compared to some middle- and high-income economies with universal health insurance policy. Hence, it becomes an arduous task managing such pediatric renal cases given the cost of their treatment. This is further compounded by the poor infrastructure for managing such cases in our environment with regard to renal replacement therapy.
On the part of the attending pediatric nephrologist, with little or no standard means/tools to work with, management becomes quite frustrating.
It is worthwhile to note that a majority of renal disorders in children result from preventable causes such as urinary tract infection (UTI) with the good outcome when the patients present early before the onset of end-stage renal disease requiring permanent renal replacement therapy.[3,4,5]
Studies on individual childhood renal disorders, such as nephrotic syndrome, CKD, and acute renal failure (ARF) among others abound and have been reported in other centers in Nigeria.[1,2,3,4,5] However, the exact or overall burden and outcome of renal disorders among Nigerian Children, especially in Abakaliki, Ebonyi state has not been reported.
It is, therefore, imperative that the burden, pattern, and outcome of renal disorders be known in our environment so that planning can be instituted to prevent renal complications as well as offer appropriate and adequate care for our ailing populace.
This study, therefore, is aimed at reviewing the prevalence, pattern, and outcome of pediatric renal admissions at the Federal Teaching Hospital (FETH) Abakaliki over 3 years period.
Subjects and Methods
Study design
This retrospective observational study was carried out at the children's out-patient department and the general children's ward of FETH, Abakaliki, Ebonyi state between 2011 and 2013.
Study setting
The hospital which was first established in the 1940s as a general hospital and upgraded into the tertiary hospital in 2011 is situated within the Abakaliki metropolis. It is a major referral center for other health institutions, including three general hospitals, and two major missionary hospitals and a host of private hospitals all located within and around Abakaliki metropolis. It serves an estimated population of about two million people who are mainly farmers, civil servants, and small to medium-scale people in business.[6] The hospital attends to about 60,000 patients out of which children constitute about 10% on an out-patient basis annually.[6]
This retrospective observational study reviewed children with renal disorders who attended FETH Abakaliki and were admitted into the renal unit of the Department of Pediatrics.
The general pediatric ward is a seventy-bed facility which houses children from the various subspecialties of pediatrics as they receive in-patient care. The renal unit is one of the major units in the Department of Pediatrics of the hospital. It is manned by consultants, residents and nursing staff who are not yet formally trained in this specialty. The consultants on their own try to get a lot of exposure from frequent attendance at nephrology workshops and conferences. They also liaise with the formally trained paediatric nephrologists in a nearby tertiary hospital.
The children out-patient department is under the Paediatric Department run by the residents but supervised by the consultant in-charge. It is the first port of call for sick children brought in by their parents/caregivers on self-referrals, verbal referrals or by paper documented referrals from private hospitals and primary care health centers. Those who require admissions are taken into the children emergency room or pediatric ward depending on the severity of the problem where investigations and treatment are commenced. The less serious ones are treated and sent home from there.
For the purpose of this study, the Medical Records Department of the hospital was visited and a review of all pediatric admissions with renal disorders was carried out and the folders of all the patients who fit the inclusion criteria were retrieved. Data of the patients, including patients' biodata, presenting symptoms, examination findings, laboratory investigation results as well as treatment mode, were extracted from these hospital records.
Ethical approval
Approval to retrieve the folders of patients for this study was obtained from the Ethics and Research Committee of FETH Abakaliki.
Definition of terms
Renal disorders were defined as abnormalities in the clinical, urinary, serum biochemical, ultrasonic scan, or radiological findings in the renal tract of these study patients. They are defined as follows.
Hypertension
Blood pressure (BP) values are based on gender, height, and age of children, thus BP readings below the 90th percentile for age, gender, and height percentile are considered normal. Hypertension for the purpose of this study was taken as systolic or diastolic BP above the 90th percentile.[7]
Edema
The presence of body swelling due to excessive fluid accumulation in the interstitial spaces.[7]
Nitrituria
Positive nitrite test (presence of nitrite in urine) resulting from the conversion of urinary nitrate to nitrite by the bacterial enzyme nitrate reductase. Often positive on suspicion of UTI but usually not confirmatory.[8,9]
Proteinuria
Urinary protein excretion that is approximately more than 150 mg/dl (1+) depending on the age of the child.[10,11]
Hematuria
The presence of at least five red blood cells (RBCs) per microliter of urine.[11,12]
Urinary tract infection
An infection that affects any part of the urinary tract and can be confirmed with urinalysis which shows the presence of urinary nitrites, white blood cells (WBCs; leukocytes), or leukocyte esterase, and microscopy which shows the presence of a high number of WBCs, RBCs, and white cell casts.[8,9,12]
Nephrotic syndrome
Nephrotic syndrome a manifestation of the glomerular disease, is characterized by heavy proteinuria and the triad of clinical findings of hypoalbuminemia, edema, and hyperlipidemia. Nephrotic range proteinuria is defined as protein excretion of >40 mg/m 2/h or a first-morning protein: creatinine ratio of >2–3:1 mg:g or dipstick urine protein of ≥3+.[13]
Renal failure
Renal failure could be acute or chronic. ARF is a clinical syndrome in which a sudden deterioration in renal function results in the inability of the kidneys to maintain fluid and electrolyte homeostasis. CKD is defined as renal injury (proteinuria) and/or a glomerular filtration rate <60 mL/min/1.73 m 2 for >3 months.[14]
Inclusion criteria
All children below the age of 18 years
The presence of a renal disorder as defined above.
Exclusion criteria
Children whose parents or caregivers did not give consent
Children above the age 18 years.
Data analysis
The data collected were entered into the data editor of [SPSS software package version 16 (IBM Chicago, IL, USA)] The analysis was based on percentages, proportions, charts, and tables. The influence of sex, age, socioeconomic status (SES) of the parents, and disease type on the outcome of renal disease was assessed. Proportions were compared. The differences in proportions were tested for statistical significance using the Chi-square statistics. Statistical significance was based on P < 0.05.
Results
In the period under review, 1780 children were admitted, of which 79 had renal disorders, giving a prevalence rate of 4.4% (79/1780). The majority of the patients seen were males 62% (49/79) with a male:female ratio of 1.6:1.0.
Fever, dysuria, and generalized body swelling were the most common symptoms recorded accounting for 30.4% (24/79), 20.3% (16/79), and 12.7% (10/79), respectively, of the study population. Family history of hypertension or renal disease was obtained in 10.1% (8/79) and 6.3% (5/79) of the cases, respectively.
Majority 32.9% (26/79) of the patients were aged between 10 and 14 years, whereas 29.1% (23/79) were aged <5 years, 58.2% (46/79) belonged to the lower social class, and 72.2% (57/79) resided in the rural areas [Table 1].
Table 1.
Sociodemographic details of the study children

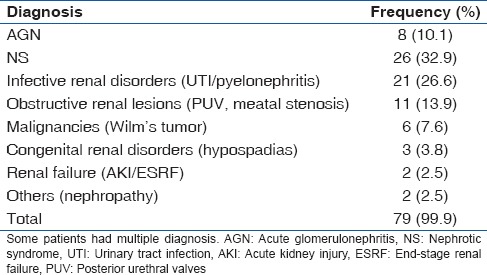
The major renal disorders seen were classified into various groups for ease of identification and management [Table 2]. They included nephrotic syndrome 32.9% (26/79), infective renal disorders (including UTIs/pyelonephritis) – 26.6% (21/79), acute glomerulonephritis (AGN) 10.1% (8/79), obstructive renal conditions (including posterior urethral valves [PUV], meatal stenosis) – 13.9% (11/79). Others include congenital causes (hypospadias) – 3.8% (3/79), malignancies (Wilms tumor) – 7.6% (6/79), renal failure (acute kidney injury [AKI]/ESRF) – 2.5% (2/79), and others (nephropathy) in 2.5% (2/79).
Table 2.
Renal diagnoses of study subjects
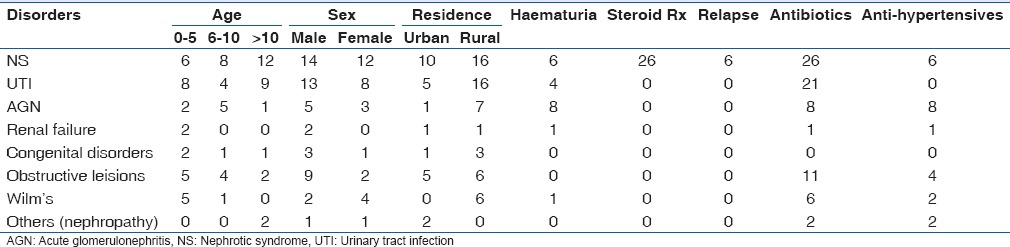
Approximately 33% (26/79) of the children had nephrotic syndrome, 53.9% (14/26) were males and the disorder occurred more in children below 10 years of age, especially among those aged 6–10 years (38.5%), whereas 23.1% (6/26) of the children also had hematuria and hypertension, respectively [Table 3].
Table 3.
Clinicodemographic features of the different renal disorders among the study patients
The serum creatinine level was normal (<100 mg/dl) in all the patients, whereas the serum albumin levels were low with a mean value of 20.2 (1.5) g/l. The dipstick test (the only method used) demonstrated urine protein of ≥3+ in all the patients.
On presentation, all the patients were admitted to the ward for stabilization before the commencement of steroid treatment. All 26 (100%) patients received corticosteroids and diuretics for 6 weeks at a daily dose of 2 mg/kg and tapered down afterward for another 6 weeks. The average response time to steroid treatment among the study population was 14 (1.7) days and the average length of stay in the hospital was 18 (2.3) days. Patients who were found to be clinically stable and responding positively to steroid therapy were discharged home and followed up on 2 weekly basis and subsequently 4 weekly basis until they achieved a complete remission or were recommended to undergo renal biopsy.
Remission was taken as being a proteinuric for 3 consecutive days or more while being on treatment. Twenty-four (92.3%) of the patients initially achieved remission and were discharged home after having been educated on how to do a daily urine dipstick test. Six (25%) of them relapsed while on treatment and were re-admitted. The underlying cause for the relapse was investigated and appropriately managed before re-commencing steroids at the dose of 2 mg/kg/day till remission was re-achieved.
Four (16.7%) responded with re-initiation of steroid treatment, whereas 2 (8.3%) did not and had to undergo renal biopsy. The biopsy results showed minimal change lesion in one and focal segmental glomerulosclerosis in the other.
The treatment outcome demonstrated that 84.6% (22/26) of the patients achieved remission, were discharged home and followed up regularly. One (3.9%) was referred out on parents' request, 7.7% (2/26) were discharged against medical advice (DAMA) and 3.9% (1/26) died from complications arising from congestive cardiac failure despite all decongestive treatment administered. The association between nephrotic syndrome and outcome of treatment was not statistically significant (P = 1.00)
AGN was diagnosed in 10.1% (8/79) of the study children. Of this number, 62.5% (5/8) were males with children aged 6–10 years (62.5%) most affected. All (100%) the children had hematuria and hypertension, respectively [Table 3].
The serum creatinine value was noted to be deranged in 4 (50%) of the patients, whereas the mean value of 89.4 (2.4) mg/dl was obtained. The serum albumin levels were normal in 3 (37.5%) and low in 5 (62.5%) with a mean value of 21.2 (1.3) g/l. The urine protein levels using the dipstick method ranged between 1+ and 2+.
On presentation, all the patients were admitted to the ward on account of hypertension and oliguria, and the average length of stay in the hospital was 18.0 (1.0) days.
All the patients 100% (8/8) with AGN received diuretics and antihypertensives, most often calcium channel blockers (amlodipine) in addition to antibiotics for those who had underlying bacterial infections. No patient underwent renal biopsy and as such no histological pattern was described. Seven (87.5%) improved and were discharged home for further monthly follow-up visits, whereas 1 (12.5%) was referred out on account of non-response to all instituted management protocols.
UTI another common renal disease among the study group accounted for 26.6% (21/79) of the cases. UTIs were more frequently seen in the males 66.7% (14/21) and appeared to be evenly spread among the age-groups but occurring more in 7 (35%) children <5 years of age. All had UTI either alone or as comorbidity with other renal disorders [Table 3].
The serum creatinine level was normal in 20 (95.2%), whereas 1 (4.8%) had increased values. The mean number of WBCs in the urine was 8.5 (1.2), whereas 6 (28.6%) had positive nitrite in their urine. The most common infecting organisms cultured were Klebsiella, Staphylococcus, Pseudomonas, and Escherichia coli while gentamicin, ceftriaxone, and ciprofloxacin were the antibiotics that were most sensitive and effective.
Fifteen (71.4%) of these patients with UTI were admitted with a mean hospitalization period of 7 (2.1) days. All received intravenous antibiotics for 5 days followed by oral antibiotics for the remaining 5–9 days depending on the isolated organism. Thirteen of the admitted patients (86.7%) were discharged home following recovery, while 2 (13.3%) were DAMA.
The remaining 6 (28.6%) patients were treated on an out-patients basis with oral antibiotics based on drug sensitivity report. Both groups were to be followed up on completion of their antibiotics. The association between the disease (UTI) and the outcome of treatment was not statistically significant (P = 0.56).
Two (2.5%) patients had renal failure existing as AKI (1.3%) and ESRF (1.3%), respectively. The AKI was secondary to hypovolemia which was treated with fluid replacement therapy and frusemide and was discharged home. The other patient with ESRF was however referred out [Table 3].
The 11 (13.9%) patients with obstructive lesions were seen and initially managed by the pediatric nephrologists with mostly antibiotics, anti-hypertensives (for the hypertensive ones) and placement of a urinary catheter for temporary relief of obstruction and monitoring of the urine output. Following stabilization, the patients were referred to the pediatric surgeons for surgical relief of the obstruction and were followed up postsurgically by the pediatric nephrologists. Three (27.3%) of these patients recovered and were discharged home, 1 (9.1%) died, whereas 6 (54.5%) were referred out and 1 (9.1%) was DAMA.
The association between the other renal disease variables (AGN [P = 1.00), Obstructive lesions [P = 0.17], congenital lesions [P = 1.00), renal failure [P = 1.00) and the outcome of treatment was not statistically significant.
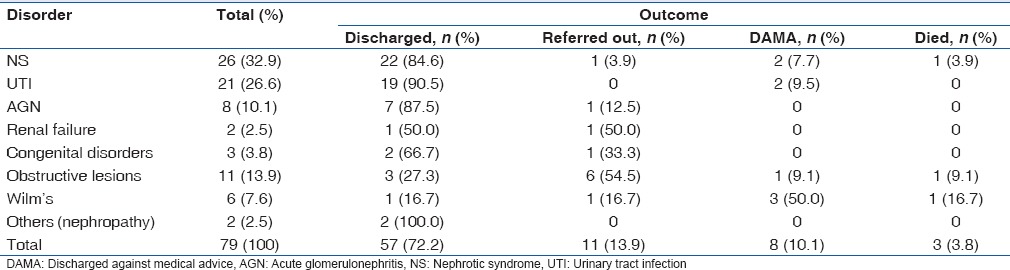
In general, 72.2% (57/79) of the study patients recovered and were discharged home to be followed up regularly, 13.9% (11/79) were referred out to other tertiary hospitals mostly due to the lack of proper equipment for treatment as well as on parental request.
Ten percent (8/79) signed against medical advice and left while 3/79 died giving a case fatality rate of 3.8%. The underlying causes of death were congestive cardiac failure secondary to nephrotic syndrome, recalcitrant electrolyte imbalance with sepsis secondary to PUV and malignant complications in the child with Wilm's tumor [Table 4].
Table 4.
Treatment outcome of the various renal disorders
The association between the outcome of treatment and some variables, such as the sociodemographic factors age, (P = 0.42), sex,(P = 0.28), SES (P = 0.33), place of residence, (P = 0.56), family history of renal disease (P = 0.18), and family history of hypertension,(P = 1.00) was not statistically significant.
The reverse is the case with the association between the outcome of treatment and the mode of treatment which was found to be statistically significant,(P = 0.03).
Discussion
This study has demonstrated the pattern and outcome of childhood renal diseases seen in FETH Abakaliki, Ebonyi state. A 4.4% prevalence of renal disorders was obtained in the study. This finding is comparable with some similar Nigerian studies conducted in Benin[1], Lagos[2] and Enugu[3] as well as in some African countries, including Libya.[15] However, our findings are at variance with that obtained from Niger Delta,[5] Port Harcourt,[16] and Sudan.[17] This variation in prevalence rates may be related to the socioeconomic, genetic and/or environmental factors which are said to play major roles in the etiology of renal diseases globally.[3] Some Nigerian studies have associated prevailing local conditions, such as malnutrition, poor environmental and personal hygiene and poverty with the development of renal diseases in children in Nigeria.[3] From this study, most of the patients with these renal diseases appear to reside more in the rural areas as well as coming from low socioeconomic background. Although, poverty and place of residence (probably related to lack of information and ignorance) were important factors that may have supported the development of renal disease among the patients, its association with the outcome of treatment was however not found to be statistically significant (χ2 = 1.29, P = 0.56).
Poor infrastructure and inequitable distribution of medical workforce to rural areas, patronage of other alternative health care particularly traditional healers and patent medicine dealers were other possible reasons for development of renal diseases among children, especially in sub-saharan Africa.
Renal diseases have been shown to cut across all age groups, however, an estimated 70% of cases of kidney diseases in childhood are congenital with a likely genetic basis.[18,19] This was also the finding in the present study as more children under the age of 10 were affected. This may be explained by the manner of presentation, especially among the under-fives as they often generally present with vague symptoms which may not resemble typical renal features. This in turn results in underdiagnosis and/or misdiagnosis which subsequently lead to late presentation.
In general, there was a demonstrable male predominance in most of the renal disorders which agree with the findings of other authors.[2,3,20] No plausible reason was offered for this finding; although, it is possible that cultural beliefs may have in a subtle way influenced it, as male children are valued more than their female counterparts and as such given more attention when sick in most African and Asian communities.
The demonstration of the pattern of renal disease in the present study showed that nephrotic syndrome (32.9%) was the most frequently presenting diagnosis. A similar pattern was observed in other Nigerian studies such as seen in Ibadan,[10] Lagos[2] Enugu,[3] Ilorin,[20] and Niger Delta (Oghara and Warri).[5] However, it came a close second after UTI in the Sudan and Libyan studies.[15,17] Proper pathophysiological classification of the disorder was, however, not carried out in all the patients with seeming steroid resistance on account of financial constraints. The results of the 2 patients who were able to undergo renal biopsy showed minimal change lesion in one and Focal segmental glomerulosclerosis in the other under light microscopy.
From the study, there was a noticeable high rate of remission (74.1%) among patients who received steroids either alone or in combination with other drugs. This appeared higher than that demonstrated by the Enugu study[3] (64%) but similar to the rates demonstrated by other studies.[5,21,22,23,24]
The lower remission rate in the other African studies was attributed to poor or no response to steroid therapy as again demonstrated by the 55% steroid resistance rate recorded by the Niger Delta study.[5] The association between the mode of treatment and outcome of treatment appeared to be statistically significant (χ2 = 10.67, P = 0.03) showing that most cases of the Nephrotic syndrome among the study children were quite responsive to the use of steroids alone or in combination during treatment.
UTI ranked second highest in this study (25.3%), whereas it was the most common cause of childhood renal diseases in Port Harcourt[16] (68.9%), Benin[1] (32.8%), and Sudan[17] (30%). The lower rate found in the study could be explained by the fact that most of the patients who presented with fever may not have been screened for UTI and so the diagnosis was missed out. On the other hand, false negative laboratory results may have been obtained despite the presence of positive clinical symptoms. A possible explanation for this could be that these patients may have been on antibiotic treatment before presentation - a common practice among these people.
AGN was the third most common renal disorder in the current study. However, it was the most common in studies from Zaria (39.1%) North West and Jos[25] (37.7%) North Central Nigeria, respectively and Libya (40.2%),[15] Northern Africa. Its low prevalence in the current study may have some environmental or genetic factors involvement. Uncontrolled use of antibiotics leading to a low streptococcal infection rate may be an additional factor in this study as most of the patients are often primarily managed at home or in patient medical clinics and are only referred to orthodox medical institutions when severe.
In this study, the prevalence of renal tract anomalies alone was relatively low, and comparable to a similar study observed by Barakat.[19] This seemingly low rate could be attributable to certain factors such as lack of advanced diagnostic facilities for early diagnosis, lack of screening programs for antenatal diagnosis and possibly poor index of suspicion among health care givers.
Renal tumors (nephroblastoma) accounted for 7.6% of the renal cases was remarkably higher than all other studies cited in the current study.[3,16,22] This higher prevalence of nephroblastoma documented may be attributable to different genetic predispositions as well other environmental factors in the different populations studied. Moreover, the current study was carried out in a center with a well-established oncology unit.
Renal failure presenting either as AKI or ESRF was seen in two children, respectively. It was quite surprising finding only one patient with AKI in the face of occasional outbreaks of AGN among babies in the study area. It is possible that the aggressive teaching on diarrhea management among antenatal patients may have improved the outlook of consequences of gastroenteritis among the study population. It is also possible that a percentage of these children may have died even before presenting at the hospital.
A remarkable number of renal cases DAMA was observed to be higher in the current study than that reported in Niger Delta.[5] DAMA is a recognized phenomenon in developing countries which is frequently attributable to financial constraints of patients.[5] In addition, many patients in resource-poor settings believe in supernatural causes to diseases, especially for such chronic diseases such as renal diseases and would only seek orthodox help when they are overtly ill. This could equally account for the comparably lower mortality rate observed in this study.
A good number (13.9%) of renal patients in this study were referred out to other centers. This is attributable to the limited resources available for the management of renal cases in this center. No patient demanded to be referred out to other hospitals.
This finding highlights prevailing circumstances which exist in resource-poor countries pertaining to patient management. Therefore, the need for increased provision of material as well as human resources in our locality to diagnose and manage renal diseases without a referral is desirable.
The case fatality rate of 3.8% recorded was noted to be much lower than that observed in the study from Niger Delta. Causes of mortality in this study include congestive cardiac failure complications in the nephrotic syndrome patient, complications arising from the advanced stage of Wilm's tumor and the severe electrolyte imbalance that followed surgical correction of the PUV in the deceased patients.
The majority of the patients recovered and were discharged home probably because most of them had the treatable disease as diagnosis. This shows that majority of renal cases are treatable especially when presentation and diagnosis are made early. Similar findings were made in Niger Delta area of Nigeria where a discharge rate of 80% was obtained.[5]
Conclusion
There is a high prevalence of renal disorders in our locality with a remarkable proportion having poor outcome probably due to late presentation. Based on this, it is pertinent that awareness campaigns be embarked on so that patients may seek orthodox help early and that resources be made readily available for diagnosis and treatment of renal diseases in our locale.
Limitations of the study
The retrospective nature of the current study limited access to some valuable patients' clinical data while some useful laboratory investigations were not carried out in some of the study patients.
Financial support and sponsorship
Nil.
Conflicts of interest
The concept of the study was conceived by the corresponding author, data obtained, analysed and finally reviewed by all the authors.
References
- 1.Michael IO, Gabriel OE. Pattern of renal diseases in children in Midwestern zone of Nigeria. Saudi J Kidney Dis Transpl. 2003;14:539–44. [PubMed] [Google Scholar]
- 2.Onifade EU. A ten-year review of childhood renal admissions into Lagos University Teaching Hospital Nigeria. Nig Q J Hosp Med. 2003;13:1–5. [Google Scholar]
- 3.Okoro BA, Okafor HU. Pattern of childhood renal disorders in Enugu. Niger J Paediatr. 1999;26:14–8. [Google Scholar]
- 4.Anochie I, Eke F. Chronic renal failure in children: A report from Port Harcourt, Nigeria (1985-2000) Pediatr Nephrol. 2003;18:692–5. doi: 10.1007/s00467-003-1150-0. [DOI] [PubMed] [Google Scholar]
- 5.Ugwu GI, Nwajei G, Chinemelu U. Pattern of renal diseases among children in the Niger delta region, Nigeria. Arab J Nephrol Transplant. 2014;7:49–50. [PubMed] [Google Scholar]
- 6.Muoneke UV. Severe Anaemia in Children Aged 6 Months to 5 Years Seen at Ebonyi State University Teaching Hospital (EBSUTH) Abakaliki Ebonyi State, Nigeria. A Dissertation Submitted to the National Postgraduate Medical College of Nigeria in Part Fulfilment of the Requirement for the Fellowship of the Faculty of Paediatrics. 2009 May [Google Scholar]
- 7.McNiece KL, Portman RJ. Hypertension: Epidermiology and evaluation. In: Kher K, Schaper WH, Makker SP, editors. Clinical Pediatric Nephrology. 2nd ed. Oxon: Informa Healthcare; 2006. pp. 459–66. [Google Scholar]
- 8.Gorelick MH, Shaw KN. Screening tests for urinary tract infection in children: A meta-analysis. Pediatrics. 1999;104:e54. doi: 10.1542/peds.104.5.e54. [DOI] [PubMed] [Google Scholar]
- 9.Scheer KA, Segert LA, Grammers GL. Urine leukocyte esterase and nitrite tests as an aid to predict urine culture results. Lab Med. 1984;15:186–7. [Google Scholar]
- 10.Anigilaje EA, Adedoyin OT. Correlation between dipstick urinalysis and urine sediment microscopy in detecting haematuria among children with sickle cell anaemia in steady state in Ilorin, Nigeria. Pan Afr Med J. 2013;15:135. doi: 10.11604/pamj.2013.15.135.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moxey-Mims M. Proteinuria and haematuria. In: Kher K, Schaper WH, Makker SP, editors. Clinical Pediatric Nephrology. 2nd ed. Oxon: Informa Healthcare; 2006. pp. 129–30. [Google Scholar]
- 12.Jantausch B, Kanwal K. Urinary tract infection. In: Kher K, Schaper WH, Makker SP, editors. Clinical Pediatric Nephrology. 2nd ed. Oxon: Informa Healthcare; 2006. pp. 553–74. [Google Scholar]
- 13.Pais P, Ellis A. Nephrotic syndrome. In: Kliegman RM, Stanton BF, St Geme JW, Schor NF, Behrman RE, editors. Nelson Textbook of Pediatrics. 19th ed. Philadelphia: Elsevier Saunders; 2011. pp. 521–3. [Google Scholar]
- 14.Craig W, Robert M. Chronic kidney disease. In: Kher K, Schaper WH, Makker SP, editors. Clinical Pediatric Nephrology. 2nd ed. Oxon: Informa Healthcare; 2006. pp. 337–41. [Google Scholar]
- 15.Elzouki AY, Amin F, Jaiswal OP. Prevalence and pattern of renal disease in Eastern Libya. Arch Dis Child. 1983;58:106–9. doi: 10.1136/adc.58.2.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eke FU, Eke NN. Renal disorders in children: A Nigerian study. Pediatr Nephrol. 1994;8:383–6. doi: 10.1007/BF00866371. [DOI] [PubMed] [Google Scholar]
- 17.Ali el TM, Rahman AH, Karrar ZA. Pattern and outcome of renal diseases in hospitalized children in Khartoum state, Sudan. Sudan J Paediatr. 2012;12:52–9. [PMC free article] [PubMed] [Google Scholar]
- 18.Bockenhauer D, Medlar AJ, Ashton E, Kleta R, Lench N. Genetic testing in renal disease. Pediatr Nephrol. 2012;27:873–83. doi: 10.1007/s00467-011-1865-2. [DOI] [PubMed] [Google Scholar]
- 19.Barakat AJ. Presentation of the child with renal disease and guidelines for referral to the pediatric nephrologist. Int J Pediatr. 2012;2012:978673. doi: 10.1155/2012/978673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adedoyin OT, Adesiyun OA, Mark F, Adeniyi A. Childhood renal disorders in Ilorin, North central Nigeria. Niger Postgrad Med J. 2012;19:88–91. [PubMed] [Google Scholar]
- 21.Hendrickse RG, Gilles HM. The nephrotic syndrome and other renal diseases in children in Western Nigeria. East Afr Med J. 1963;40:186–201. [PubMed] [Google Scholar]
- 22.Abdurrahman MB, Babaoye FA, Aikhionbare HA. Childhood renal disorders in Nigeria. Pediatr Nephrol. 1990;4:88–93. doi: 10.1007/BF00858449. [DOI] [PubMed] [Google Scholar]
- 23.Bhimma R, Coovadia HM, Adhikari M. Nephrotic syndrome in South African children: Changing perspectives over 20 years. Pediatr Nephrol. 1997;11:429–34. doi: 10.1007/s004670050310. [DOI] [PubMed] [Google Scholar]
- 24.Olowu WA, Adelusola AK, Adefehinti O. Childhood idiopathic steroid resistant nephrotic syndrome in South Western Nigeria. Saudi J Kidney Dis Transpl. 2010;21:979–90. [PubMed] [Google Scholar]
- 25.Ocheke IE, Okolo SN, Bode-Thomas F, Agaba EI. Pattern of childhood renal diseases in Jos, Nigeria: A preliminary report. J Med Trop. 2010;12:52–5. [Google Scholar]


