Abstract
Necrotizing fasciitis was recognized centuries ago by physicians. It is a rapidly progressive and potentially fatal soft-tissue infection that is typified by soft-tissue necrosis, especially affecting the subcutaneous tissues and fascia. Cervico-facial necrotizing fasciitis is said to be uncommon, but when it occurs, it is often of odontogenic origin and has severe consequences if not promptly treated. Possible underlying systemic diseases and the source of infection should be addressed and treated appropriately. We present two cases of extensive cervicofacial necrotizing fasciitis, one of which was idiopathic in origin and the other with gross involvement of the chest and abdominal walls. Both were treated successfully. Immediate resuscitation of the patients, administration of empirical antibiotics, treatment of underlying systemic conditions and early, aggressive and serial debridement were the bedrock of management in these cases.
Keywords: Idiopathic, Necrotizing fasciitis, Odontogenic
Introduction
Necrotizing fasciitis was recognized centuries ago by physicians who gave it different names, including “malignant ulcer,” “gangrenous ulcer,” “flesh eating bacteria,” and “Fournier gangrene.”[1] However, the term “Necrotizing fasciitis” was first used by Wilson in 1952.[2,3]
It is a rapidly progressive and potentially fatal soft-tissue infection that is typified by soft-tissue necrosis, especially affecting the subcutaneous tissues and fascia.[4,5,6] It is said to involve the fascia primarily and other subcutaneous tissues and skin secondarily.[1] Relative sparing of the muscle and bone is often observed though reports of bone involvement exist. It also has a potential for producing severe disfigurement.[7] It is said to be uncommon in the head and neck region, perhaps because of its relatively high vascularity.[2,5] Nevertheless, when cervico-facial necrotizing fasciitis occurs, it is most common cause are dental infections.[2,8] Cervico-facial necrotizing fasciitis is often polymicrobial in nature, with streptococci predominance.[9] Underlying systemic factors such as uncontrolled diabetes mellitus and nutritional deficiencies have been implicated as predisposing factors.[5,6]
Patients often require serial surgical debridement and aggressive medical therapy to have a good chance of surviving.[4,5] Adjunctive hyperbaric oxygen therapy has also been successfully used by some investigators.[10] Rehabilitation of the patients is also of immense importance in the overall management of this disease.[11] We report two cases of extensive cervico-facial necrotizing fasciitis.
Case Reports
Case report 1
A 25-year-old female presented at our clinic with foul smelling, pus discharging anterolateral cervical ulceration of 2 weeks duration. She was referred from a primary health-care facility. Dental and medical history revealed no relevant information. The patient's vital signs are as follows: blood pressure (BP) - 110/70 mmHg, pulse rate (PR) - 80 bpm, respiratory rate (RR) - 20 cpm, and temperature (T) - 38.1°C. On general examination, the patient was clinically pale, febrile, and dehydrated. There were two ulcerations in the cervical region separated by a tract of skin [Figure 1]. The superior ulcer was oval in shape, measuring about 5.3 by 2.8 cm while the second larger ulceration measured about 12.8 by 21 cm. The edges were undermined, with exposed friable fascia and a putrid odor emanated from it.
Figure 1.
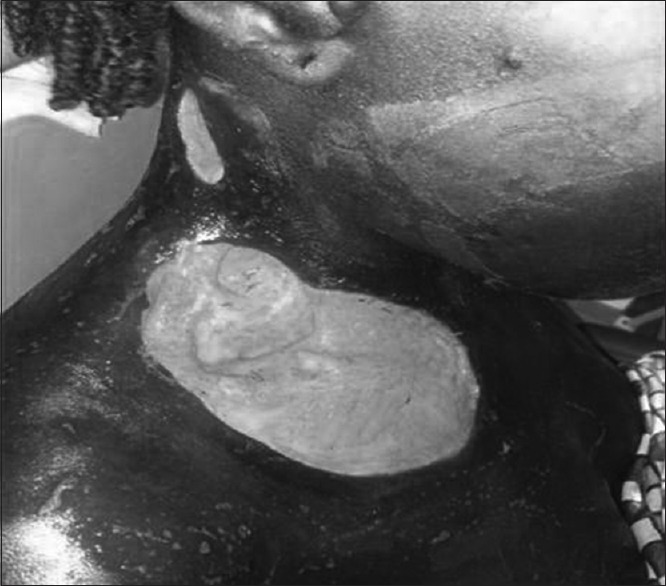
Cervical ulceration with exposure of underlying muscles
The surrounding skin was darkened and sloughed off with minimal effort to reveal the underlying necrotic fascia and relatively healthy looking muscles. Crepitus was elicited on palpation, and cutaneous pinprick test revealed anesthesia of the immediate surrounding skin up to about 1 cm from the margins of the ulceration. Mouth opening was about 2.5 cm, oral hygiene was fair with no obvious sign of ongoing infective process, or carious tooth was identified. All the teeth present were neither mobile nor tender, indicating a nonodontogenic origin [Figures 2 and 3]. In addition, she had pitting pedal edema which extended superiorly to about 3 cm above the malleoli.
Figure 2.
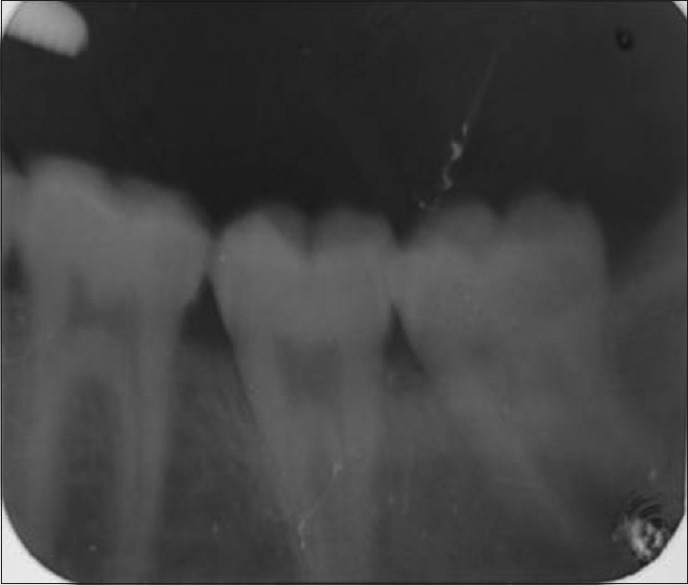
Periapical radiograph of the right mandibular molars
Figure 3.
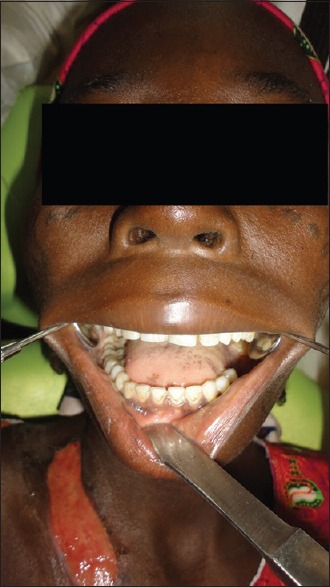
Photograph showing complete set of teeth in the right mandibular quadrant and a healing ulcer
The patient was admitted immediately and preliminary investigations, including electrolyte and urea, random plasma glucose, retroviral screening, serum albumin, full blood count, and microscopy culture and sensitivity (MCS) were requested. The results for MCS yielded growth of normal flora. The hematocrit, mean corpuscular hemoglobin, and erythrocyte sedimentation rate were 10%, 24.6 pg and 13 mm/h, respectively. Reports of requested investigations revealed that the random plasma glucose was within normal range and retroviral screening was negative. Nevertheless, the patient was also found to have low serum albumin at 2.4 g/dl. The total white cell count was 3.9 × 103/ᶷL with granulocytes, monocytes, and lymphocytes making up 66.9%, 7.7%, and 25.4%, respectively.
The patient was started on initial aggressive fluid resuscitation with concurrent serial surgical debridement and empirical antibiotic administration (intravenous ceftriaxone 2 g stat; then 1 g daily, metronidazole infusion 500 mg 8 hourly, intramuscular diclofenac sodium 75 mg 12 hourly; 300 mg of regular release tablets ferrous sulfate 8 hourly; Vitamin C tablets 500 mg 12 hourly). She was also transfused with three pints of blood and placed on nutritional supplements. Serial debridement was initiated, which was done under local anesthesia and ketamine sedation (0.5 mg/kg). This was done daily until all visually identified necrotic tissue was removed. Copious amounts of dark colored; foul-smelling fluid was drained from the site. Necrotic cutaneous and subcutaneous tissues were removed, sometimes with the aid of a scalpel under profuse irrigation with normal saline until fresh bleeding tissues were encountered.
Initial wound dressings were done with absorbent sandwich of gauze and cotton wool, which was later changed to iodine-gauze dressings as the fluid exudation reduced in volume. The wound dressings (povidone iodine-soaked gauze dressings) were changed daily initially and then on alternate days as wound healing progressed. The drug regimen remained unchanged throughout the course of treatment because of the vague MCS result and the fact that infection started abating with the empirical antibiotic regimen.
On the 7th day post admission, the patient complained of abdominal ache for which the general surgeons were invited. She had abdomino-pelvic ultrasound, chest X-ray, upper gastrointestinal tract endoscopy, and barium meal done. A diagnosis of gastric outlet obstruction secondary to gastric peptic ulcer disease was made, and she was treated appropriately. The patient was discharged after 6 weeks of admission. She declined having the healing ulcers grafted due to financial reasons. Thus, healing was by secondary intention. Subsequent reviews were satisfactory.
Case report 2
A 35-year-old male farmer presented at our clinic with a 6-month history of recurrent toothache and a 1 week history of anterolateral neck swelling which produced a purulent discharge. He had applied local concoctions intraorally which did not relieve him of his symptoms. Medical history did not reveal any contributory information. His vital signs at presentation were as follows: BP - 120/80 mmHg, PR - 104 bpm, RR - 22 cpm, and T - 38.0°C. On general examination, he was febrile, and the submandibular lymph nodes were palpable bilaterally and tender.
The neck swelling was diffuse, fluctuant, tender on deep palpation, and it discharged a foul smelling grayish/black fluid spontaneously. The surrounding skin appeared dull and darkened in coloration. Crepitations were felt on palpation and needle prick test was negative in the immediate surrounding skin up to 1.5 cm away from the neck swelling. However, tenderness was elicited on palpation of the skin at an average of about 4.5 cm from the neck mass [Figure 4]. Mouth opening was about 2.3 cm. The #36, #37, and #38 teeth were carious, tender, and mobile.
Figure 4.
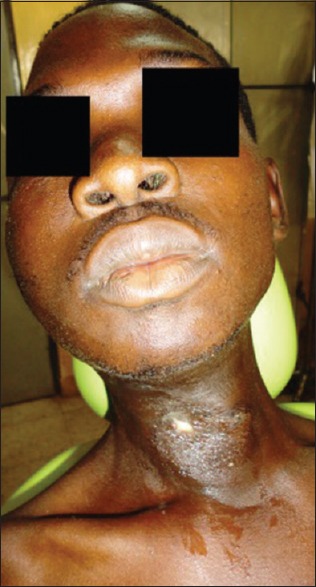
Anterior neck swelling with skin discoloration
A clinical diagnosis of necrotizing fasciitis was made and immediate aggressive fluid resuscitation and debridement instituted. Preliminary investigations including electrolyte and urea, random plasma glucose, retroviral screening, serum albumin, full blood count, and MCS were done. The hematocrit and total protein were 20% and 5.5 g/dL, respectively; all other investigation results were satisfactory. We commenced incision, dependent drainage, and aggressive serial debridement under copious irrigation with normal saline while awaiting results of the requested investigations.
The patient was placed on empirical antibiotics and analgesics (intravenous ceftriaxone 1 g daily; intravenous metronidazole 500 mg 8 hourly; intramuscular paracetamol 600 mg; intramuscular diclofenac 75 mg; 300 mg tablets of regular release ferrous sulfate 8 hourly; Vitamin C tablets 500 mg 12 hourly). The implicated teeth were extracted immediately. Notably, 12 hourly debridement was performed until active infection was controlled. MCS isolated Escherichia coli and Staphylococcus aureus after incubation, which was sensitive to ciprofloxacin, imipenem, and ofloxacin. Therefore, ciprofloxacin was added to the patient's drug regimen. Initial dressings were done with alginate, which was later replaced with the use of gauze/iodine dressing. In addition, the patient was transfused with 1 pint of whole blood.
On the 4th day of admission, examination revealed that pus had tracked subcutaneously through the left pectoral region to collect in the left hypochondriac region. Following this discovery, incision and drainage with the placement of a dependent drain were done. By the 6th day of admission, the skin over the anterior chest wall and the left hypochondriac region began to slough off leaving a bridge of intact skin between the second emerging ulcer and the first [Figure 5]. Extensive debridement was done and affected necrotic skin excised until healthy bleeding tissues were encountered.
Figure 5.
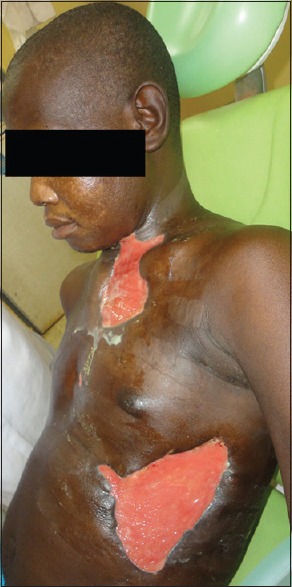
Healing ulcers with healthy granulation tissue awaiting skin grafting
Consequently, there were two large ulcerations; one of which was located on the left anterolateral aspect of the neck, extending inferiorly to the upper anterior chest wall, while the other was located on the left inferolateral aspect of the chest wall. They measured an average of 6.5 by 13 cm and 8.5 by 16 cm, respectively. They had extensively undermined edges and necrotic floors lined with grayish, delicate tissue residue. Both ulcers discharged copious amounts of foul-smelling dark colored fluid akin to the typical dish water discharge. Thereafter, he had chest X-ray done which revealed no mediastinal involvement.
Serial debridement, antibiotic administration, and fluid resuscitation continued during his stay at our hospital until all signs of active infection subsided. Daily alginate dressing was done for the 1st week of hospital admission, followed by daily gamgee dressing for the next 10 days. Povidone iodine-soaked gauze dressing was used subsequently. By the 25th day of his admission in the hospital, healthy granulation tissue had formed significantly. However, the patient declined skin grafting despite due counseling by the maxillofacial and plastic surgery teams. Wound healing was by secondary intention with consequent scar formation. Follow-up was satisfactory.
Discussion
Necrotizing fasciitis often displays a fulminant course.[5,9] Two classical types of necrotizing fasciitis have been described; Type 1 is a polymicrobial infection involving mixed aerobes and anaerobes, while Type 2 is a monomicrobial infection.[12] Group A Streptococcus are the typical pathogens.[2,4] However, this trend is changing as other organisms are increasingly being identified.[12,13,14] This may be due to abuse of antibiotics, which may have led to the emergence of virulent strains of microorganisms and the increasing prevalence of immunocompromising conditions. Recently, reports of necrotizing fasciitis caused by methicillin-resistant S. aureus have been made.[14] Other commonly implicated organisms include E. coli, Pseudomonas spp., Bacteroides spp., and Klebsiella pneumonia among others.[2,14,15]
The bacterial invasion of soft tissues culminates in vascular thrombosis and subsequent necrosis of the fat, fascia, and overlying skin.[16] Oftentimes, significant underlying systemic or local conditions such as diabetes mellitus, chronic alcoholism, and prolonged cannulation may exist which predispose the patient to the development of this disease.[5,6] The majority of cervico-facial necrotizing fasciitis are of odontogenic origin.[6] However, hematogenous spread of infection, though not common, has also been suggested.[17] The first case presented had an obscure origin as there was no obvious odontogenic or nonodontogenic source; hence, it was labeled as being of idiopathic origin. Notably, the patient was anemic and also malnourished as evidenced by the hematocrit and serum albumin level of 10% and 2.4 g/dL, respectively.
These are systemic conditions that may predispose to development of this disease condition, from seemingly innocuous lesions or injuries such as folliculitis, furuncles, carbuncles, needle prick injuries, skin abrasions, and insect bite. These may serve as initiating factors especially in the presence of underlying systemic conditions. Moreover, gastric outlet obstruction secondary to peptic ulcer disease as diagnosed in this patient may have predisposed the patient to malnutrition or worsened an existing state of malnutrition. Malnutrition is a known predisposing factor to the development of necrotizing fasciitis, thus improvement of the patient's nutritional status was of immense importance. The hematogenous route of infection is improbable in this case, as no distant focus of infection was identified.
The patient should also be assessed for predisposing systemic conditions such as diabetes mellitus and malnutrition. Treatment must be instituted immediately with immediate hydration of the patient and commencement of empirical antibiotics. Following this, aggressive serial debridement should be embarked upon.
Most odontogenic infections are nonspecific in nature, with affected patients often presenting with symptoms of pain and swelling.[5] They are often mild in nature and respond well to treatment. However, complications are observed in up to 12.5% of them.[5] In patients where these complications occur, underlying predisposing factors are often present although reports of them occurring in nonimmunocompromised patients exist.[18] Poor glycemic control is the most common predisposing systemic factor having been implicated in 20%–78% of cases reported.[8] Other commonly implicated systemic conditions are chronic alcoholism, drug abuse, and malnutrition. These may exist alone or in combinations.[4,5,6]
Patients often present with a history of recurrent pain of odontogenic origin, commonly involving the molars.[2] Systemic signs and symptoms should not be overlooked; patients may become septic as the disease progresses. They frequently present with hyperesthesia, especially in the initial course of the disease; areas of pale anesthetized skin surrounded by an area of concentric erythema may be seen in the latter stages of the disease.[2,4,5] In addition, subcutaneous crepitation may be felt.[6] Dark, foul smelling, necrotic fascia which does not bleed on incising it may be seen in advanced stages. Simple abscesses have a central necrotic area, in contrast to necrotizing fasciitis which has a broad front and undermines the overlying skin, separating it from the underlying muscles.[6,8] It initiates vascular thrombosis in the subcutaneous vessels with resultant cutaneous ischemia and subsequent necrosis.[9,16] The infection classically spreads along the subcutaneous fascial layer because of its relatively poor blood supply. Remarkably, the observed cutaneous changes often belie the extent of the underlying infection.
Although the diagnosis can be made clinically, several adjuncts in its diagnosis and management have been described.[19] These include the use of imaging techniques in the management of necrotizing fasciitis which is controversial. Magnetic resonance imaging and computed tomography scan have some importance in the management of this disease; however, cost and availability limit use.[6] Others include the use of transcutaneous tissue oxygen saturation monitoring, rapid streptococcal diagnostic kits, polymerase chain reactions, and ultrasound.[4,6,19] Debridement was done under local anesthesia and sedation in both cases. This was because of the limited resources available in our environment and the inability of the patients to afford treatment under general anesthesia.
Although both patients survived, necrotizing fasciitis is known to have high mortality rates (21.9%–30%). Malnutrition is a known predisposing factor to the development of necrotizing fasciitis, and it may also worsen treatment outcome. Therefore, immediate institution of empirical antibiotic therapy, early rehydration, and nutritional rehabilitation done in these cases may have improved their chances of survival. Broad spectrum antibiotics should be administered empirically because of the frequent polymicrobial nature of the disease. A combination of a penicillin and metronidazole has proven value in the patient management.[2,20,21] In addition, blood transfusion and serial debridement were done. This may have improved circulatory efficiency and tissue oxygenation, thus enhancing ability to combat infection; serial debridement ensures repeated the removal of necrotic tissue which otherwise would worsen outcome.
Management should be directed at immediate resuscitation of patients, removal of the source of infection, correction of underlying systemic anomalies, commencement of empirical antibiotics directed at the commonly implicated organisms, and subsequent rehabilitation of patients.[2,5] Evaluation of the nutritional and immune status of patients should be done routinely. Aggressive serial debridement and early institution of empirical antibiotics regimen are of utmost importance.[5,6] Alginate dressing, which is available, absorbent, and comparatively affordable is a viable option for dressing the ulcers, especially in the initial stages of the disease. Skin grafts may be placed after infection has subsided, and adequate healthy granulation tissue has formed.[4] Furthermore, physiotherapy has been advocated for patients with necrotizing fasciitis, especially those who would not benefit from skin grafts to reduce the effects of contracture formation.[22]
Conclusion
Necrotizing fasciitis is a rapidly progressing disease with the potential to create severe morbidities or even death. Early diagnosis and aggressive medical and surgical intervention are of utmost importance. In addition, there is a need to remove the source of infection when there is any, as well as to screen for underlying predisposing systemic conditions.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Hakkarainen TW, Kopari NM, Pham TN, Evans HL. Necrotizing soft tissue infections: Review and current concepts in treatment, systems of care, and outcomes. Curr Probl Surg. 2014;51:344–62. doi: 10.1067/j.cpsurg.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maria A, Rajnikanth K. Cervical necrotizing fasciitis caused by dental infection: A review and case report. Natl J Maxillofac Surg. 2010;1:135–8. doi: 10.4103/0975-5950.79215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rapoport Y, Himelfarb MZ, Zikk D, Bloom J. Cervical necrotizing fasciitis of odontogenic origin. Oral Surg Oral Med Oral Pathol. 1991;72:15–8. doi: 10.1016/0030-4220(91)90181-b. [DOI] [PubMed] [Google Scholar]
- 4.Adigun IA, Abdulrahaman LO. Necrotizing fasciitis in a plastic surgery unit: A report of ten patients from Ilorin. Niger J Surg Res. 2004;6:1–2. [Google Scholar]
- 5.Camino Junior R, Naclerio-Homem MG, Cabral LM, Luz JG. Cervical necrotizing fasciitis of odontogenic origin in a diabetic patient complicated by substance abuse. Braz Dent J. 2014;25:69–72. doi: 10.1590/0103-6440201302336. [DOI] [PubMed] [Google Scholar]
- 6.Lambade PN, Dolas RS, Virani N, Lambade DP. Cervicofacial necrotising fasciitis of odontogenic origin: A Review. Sci Rep. 2012;1:414. [Google Scholar]
- 7.Salins PC, Saxena S, John JK. Reconstruction of mandible and surrounding soft tissues in patient with necrotizing fasciitis. Int J Oral Maxillofac Surg. 1996;25:98–100. doi: 10.1016/s0901-5027(96)80049-x. [DOI] [PubMed] [Google Scholar]
- 8.McMahon J, Lowe T, Koppel DA. Necrotizing soft tissue infections of the head and neck: Case reports and literature review. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;95:30–7. doi: 10.1067/moe.2003.15. [DOI] [PubMed] [Google Scholar]
- 9.Vaid N, Kothadiya A, Patki S, Kanhere H. Necrotising fasciitis of the neck. Indian J Otolaryngol Head Neck Surg. 2002;54:143–5. doi: 10.1007/BF02968735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Langford FP, Moon RE, Stolp BW, Scher RL. Treatment of cervical necrotizing fasciitis with hyperbaric oxygen therapy. Otolaryngol Head Neck Surg. 1995;112:274–8. doi: 10.1016/S0194-59989570250-4. [DOI] [PubMed] [Google Scholar]
- 11.Balbierz JM, Ellis K. Streptococcal infection and necrotizing fasciitis – Implications for rehabilitation: A report of 5 cases and review of the literature. Arch Phys Med Rehabil. 2004;85:1205–9. doi: 10.1016/j.apmr.2003.12.024. [DOI] [PubMed] [Google Scholar]
- 12.Cheng NC, Yu YC, Tai HC, Hsueh PR, Chang SC, Lai SY, et al. Recent trend of necrotizing fasciitis in Taiwan: Focus on monomicrobial Klebsiella pneumoniae necrotizing fasciitis. Clin Infect Dis. 2012;55:930–9. doi: 10.1093/cid/cis565. [DOI] [PubMed] [Google Scholar]
- 13.Wong CH, Chang HC, Pasupathy S, Khin LW, Tan JL, Low CO. Necrotizing fasciitis: Clinical presentation, microbiology, and determinants of mortality. J Bone Joint Surg Am. 2003;85-A:1454–60. [PubMed] [Google Scholar]
- 14.Miller LG, Perdreau-Remington F, Rieg G, Mehdi S, Perlroth J, Bayer AS, et al. Necrotizing fasciitis caused by community-associated methicillin-resistant Staphylococcus aureus in Los Angeles. N Engl J Med. 2005;352:1445–53. doi: 10.1056/NEJMoa042683. [DOI] [PubMed] [Google Scholar]
- 15.Ndukwe KC, Fatusi OA, Ugboko VI. Craniocervical necrotizing fasciitis in Ile-Ife, Nigeria. Br J Oral Maxillofac Surg. 2002;40:64–7. doi: 10.1054/bjom.2001.0715. [DOI] [PubMed] [Google Scholar]
- 16.Abramo A, Ferri E, Spinato G, Tirelli G. Necrotizing fasciitis of the posterior cervical compartment: An atypical case due to Streptococcus agalactiae. Otolaryngol Online J. 2012;2:4. [Google Scholar]
- 17.Green RJ, Dafoe DC, Raffin TA. Necrotizing fasciitis. Chest. 1996;110:219–29. doi: 10.1378/chest.110.1.219. [DOI] [PubMed] [Google Scholar]
- 18.Sangamesh NC, Vidya KC, Roopa GS, Sakri SB. Necrotizing fasciitis of odontogenic origin in a nonimmunocompromised patient: A rare case report. J Sci Soc. 2014;41:179. [Google Scholar]
- 19.Jason W, David J. Diagnosis and management of necrotizing fasciitis. Orthopedics. 2011;34:196–202. doi: 10.3928/01477447-20110124-20. [DOI] [PubMed] [Google Scholar]
- 20.Lille ST, Sato TT, Engrav LH, Foy H, Jurkovich GJ. Necrotizing soft tissue infections: Obstacles in diagnosis. J Am Coll Surg. 1996;182:7–11. [PubMed] [Google Scholar]
- 21.Legbo JN, Shehu BB. Necrotizing fasciitis: A comparative analysis of 56 cases. J Natl Med Assoc. 2005;97:1692–7. [PMC free article] [PubMed] [Google Scholar]
- 22.Kovacs LH, Kloeppel M, Papadopulos NA, Reeker W, Biemer E. Necrotizing fasciitis. Ann Plast Surg. 2001;47:680–2. doi: 10.1097/00000637-200112000-00022. [DOI] [PubMed] [Google Scholar]


