Abstract
Context:
Antiphospholipid antibodies (aPL) are related with a high risk of pregnancy morbidity (PM) and also of vascular thrombosis. On the basis of recent studies, we expect that in women with PM associated with antiphospholipid syndrome (APS), further factors may be deregulated and involved in pathophysiology of the disease. Such factors may have the potential to become novel biomarkers for APS and its stages.
Settings and Design:
Descriptive study from a recurrent pregnancy loss program.
Aims:
To study the protein expression in sera from women with PM with or without aPL.
Materials and Methods:
Protein profiles were determined by surface enhanced laser desorption and ionization − time of flight mass spectrometry (SELDI-TOF MS) in the serum samples from women with PM, 10 of them with aPL and 12 without aPL. On the basis of the mass-to-charge ratio (m/z) of the protein, signals differentially expressed between the two groups were compared with data banks to approach candidate proteins.
Statistical Analysis Used:
To determine the differential expression of each protein, a no paired t-test was performed using Ciphergen Express Client 3.1 software.
Results:
SELDI-TOF analysis makes it possible to discriminate between several proteins in women with PM with and without aPL, although it does not allow protein identification. Nine proteins were found in significantly higher levels in aPL-positive women.
Conclusion:
The results underline that further factors beyond autoantibodies are involved in PM associated with APS and might lead to the development of new biomarkers.
KEYWORDS: Antiphospholipid antibodies, pregnancy loss, proteomics, SELDI-TOF MS
INTRODUCTION
Antiphospholipid syndrome (APS) is characterized by clinical manifestations of thrombosis (arterial, venous, or small vessel) in different vascular territories, pregnancy morbidities [early or late gestational losses, intrauterine growth restriction, and preterm labor or preeclampsia (PE)], and the persistent presence of antiphospholipid antibodies (aPL).[1] aPL are a heterogeneous auto-antibody subset directed against negatively charged phospholipids, and they can also recognize phospholipid binding proteins such as annexin-V, proteins C and S, prothrombin, and β2 glycoprotein I (β2GPI).[2] Cell surfaces can potentially bind β2GPI from the bloodstream while negative phospholipids are exposed; however, trophoblasts can permanently expose these phospholipids, thus allowing β2GPI binding, and in addition, they can synthesize their own β2GPI and express it in their plasma membrane, turning placenta into a target of anti-β2GPI antibodies.[2,3,4]
aPL could induce its deleterious effects on the cells through antigen–antibody complex formation on cell surfaces. This complex induces activation of several signaling pathways, transcription processes, and expression of adhesion molecules and pro-coagulant proteins, leading to a thrombogenic and inflammatory state. These effects have been demonstrated using endothelial cells, monocytes, and platelets.[5] In the pregnancy context, current reports suggest that thrombotic mechanisms are not sufficient to explain the pregnancy losses associated with APS. Consequently, it is presently thought that pregnancy failure in women with obstetric APS involves alterations on normal trophoblast functions such as migration, invasion, differentiation, or hormone production that leads to an impaired placental development.[2,3,6,7,8]
Currently, the laboratory criteria involved in APS diagnosis are the positive test for anticardiolipin antibodies (aCL), anti-β2GPI antibodies, or lupus anticoagulant (LA).[1] However, another group of aPL, such as antiphosphatidic acid (aPA), antiphosphatidylinositol (aPI), and antiphosphatidylserine (aPS) antibodies, have been associated to APS and are known as non-criteria aPL.[9] The most extensively investigated aPL in thrombosis and pregnancy morbidity (PM)-related APS are aPS antibodies, and some investigators have suggested that testing for these non-criteria aPL may help identify women with recurrent pregnancy loss who could benefit of the treatment for PM associated to APS.[9]
The awareness for pregnancy losses associated with APS is rising because it has often remained undiagnosed and untreated with unfortunate consequences.[10] The search for biomarkers for the diagnosis of APS is increasing, while clinical applications of proteomics have been focused on cardiovascular risk and thrombosis.[11,12,13,14] Although there are very few qualified biomarkers for APS diagnosis that have already been identified through proteomics assessment,[13] Ripoll et al.[15] recently reported the upregulated expression of six proteins in human monocytes treated with polyclonal immunoglobulin G (IgG) from APS patients [both vascular thrombosis (VT) and PM groups]. In the human reproductive field, we recently used a surface enhanced laser desorption and ionization − time of flight (SELDI-TOF) approach to identify protein expression in seminal plasma from fertile and infertile men.[16] Since it is still expected that advances in proteomic technology could help identify potential biomarkers of the disease and lead to improvement of their management and prognosis, in this study, we investigated serum protein targets associated with obstetric APS using the proteomic mass spectrometry technology, SELDI-TOF.
MATERIALS AND METHODS
The serum samples were obtained from 22 women who attended a recurrent pregnancy loss program in our group. The Ethics Review Committee of our University approved the collection of their serum, and written consent was obtained. All of them fulfilled clinical criteria for PM. In all the samples, aCL were tested using an in-house Enzyme-linked immunosorbent assay (ELISA),[17,18] and were also tested using a commercial aCL ELISA kit (BioSystems, Barcelona, Spain). Anti-β2GPI antibodies were detected using the Quanta Lite ELISA kit (INOVA Diagnostics, San Diego, USA). LA was measured using diluted Russell’s Viper Venom Time test (Rochem Biocare, Bogotá, Colombia). All antibodies were tested twice, at least 12 weeks apart. In addition, other aPL were detected by in-house ELISA [aPA, aPI, aPS, and also anti-phosphatidylglycerol (aPG)]. Finally, 10 of them were positive to the presence of aPL (aPL-positive group), and 12 women presented PM in the absence of aPL (aPL negative group).
Stored serum samples were centrifuged at 14,000×g for 10 min at 4°C. The amount of total serum proteins was measured using a NanoDrop® spectrophotometer, and then the samples were diluted to 0.5 mg/ml in binding buffer (0.1 M Tris–HCl, 0.02% Tween 20%, pH 9.0). Anionic Q10 ProteinChip® (Ciphergen Biosystems, CA, USA) was used here. Protein chip analysis was performed according the manufacturer’s specifications. Briefly, the spots were equilibrated three times with 5 μl binding buffer for 5 min and then 5 μl of each sample was placed on each spot. After incubation for 90 min in a humid chamber, each spot was washed twice with 5 μl binding buffer and twice with 5 μl distilled water. After air-drying, the spots were coated with 0.5 μl of energy-absorbing matrix (EAM: 20 mg/ml sinapinic acid in 50% acetonitrile and 0.5% trifluoroacetic acid), to facilitate desorption and ionization of proteins. Chips were air-dried again during 5 min, and then another 0.5 μl of EAM was applied.
The protein chip arrays thus prepared were placed in a ProteinChip SELDI System Series 4000 (Ciphergen) for reading. Arrays were exposed to 2200 nJ laser energy to detect proteins of low molecular weights (2000–20,000 Da), as well as to 3500 nJ for proteins in the high molecular weight range (20,000–200,000 Da) at a pressure of <150 μPa, according a protocol established in Placentalabor − University Hospital, Jena-Germany. The sample reading was performed randomly to avoid bias handling of the samples or the protein chips. All these SELDI-TOF MS analyses were performed at the Institute for Human Genetics − University Hospital Jena.
The spectra were analyzed using the Ciphergen Express Client 3.1 software (Ciphergen) that generates a consistent peak for comparison between two groups, with automatic data analysis. Mean values were evaluated by the Student’s t-test. Receiver operating characteristic curve analysis was also performed to assess the predictive value of the peaks observed and the results are expressed as area under the curve, and a cutoff value was selected for optimal sensitivity and specificity.
RESULTS
Demographic characteristics of the women from both groups are described in Table 1. There were no significant differences in the age or number of pregnancy losses between the groups. Sera samples had an average protein concentration of 88.5 mg/ml. In aPL-positive group, two women presented VT in addition to their pregnancy losses and other women did not fit the international laboratory criteria of APS because they had other aPL such as aPA, aPI, aPG, and aPS antibodies. However, they were included because it is important to evaluate the relationship between the presence of other aPL and PM (i.e., non-criteria aPL).[9] In addition, we recently found that sera from these women induced decreased trophoblast migration and trophoblast–endothelial cell interaction.[19] Even though our research group is a reference center for women with recurrent pregnancy losses in Medellín, few women have been well characterized with international clinical and laboratory diagnostic criteria for APS; this is the reason for the low number of patients in the present study.
Table 1.
Summary of clinical and laboratory characteristics among the women included
A representative spectrum in the range of 12,500–15,000 Da of a serum sample from an aPL-positive woman exposed to 2200 nJ laser energy to detect proteins in the high molecular weight range is shown in Figure 1. Two peaks in the low molecular weight range corresponding to small proteins (2000–20,000 Da) and seven peaks in the high-molecular weight range corresponding to large proteins (20,000–200,000 Da) were consistently present and at increased levels in the aPL-positive group [Figure 2], and their respective mass/charge (m/z) value was obtained. Differential expression of all the proteins was analyzed using a heat map. Figure 3a and b shows a hierarchically organized dendrogram based on protein similarities (y-axis) in each serum sample.
Figure 1.
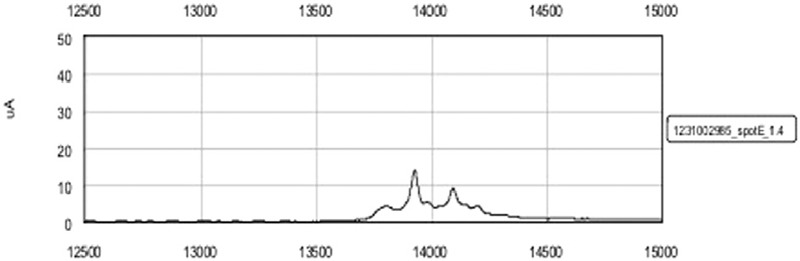
Representative spectrum with peaks in the range between 12,500 and 13,500 Da of one serum sample of an aPL-positive woman
Figure 2.
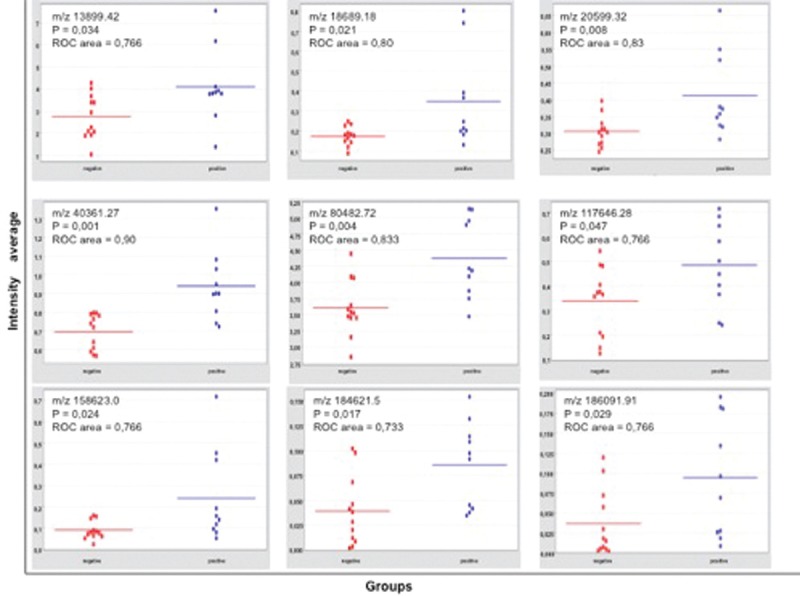
Dot plots of differentially expressed proteins in sera of both groups of women. All protein expression was increased in serum from the aPL-positive group
Figure 3.
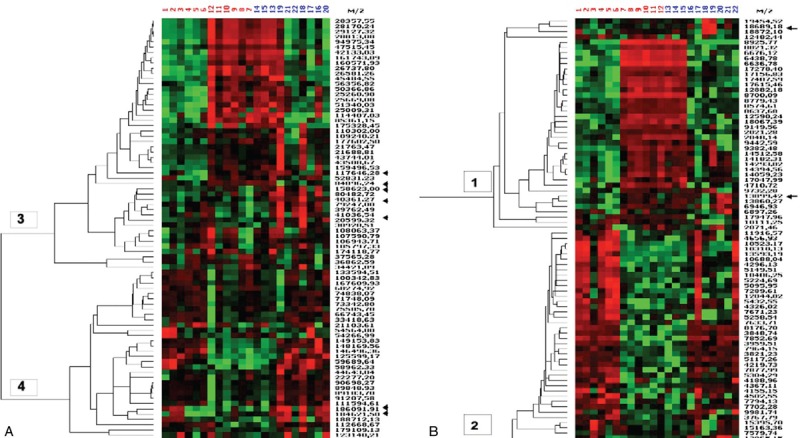
Heat map represents the relative expression of proteins in the serum samples of aPL-negative (numbers 1–12) and aPL-positive (numbers 13–22) women. Red represents overexpression, and green represents downregulated expression. Arrows identify the differentially expressed proteins. (a) Heat map for small proteins (2000–20,000 Da) and (b) heat map for large proteins (20,000–200,000 Da)
SELDI-TOF analysis provides a good discrimination of distinct proteins in a complex mixture such as human serum. Here, the protein profiles of women with or without aPL antibodies who suffered pregnancy losses were compared. The data reveal that several proteins are differentially expressed in these groups. These differences can be found only in distinct protein signals and not in the entire profile, as clearly demonstrated in the heat map. Moreover, SELDI-TOF analyses show that most of the m/z values of significantly different peaks are concentrated in clusters 1 and 3. This reflects the close relationship of these protein-based m/z signals and finally also of the proteins themselves.
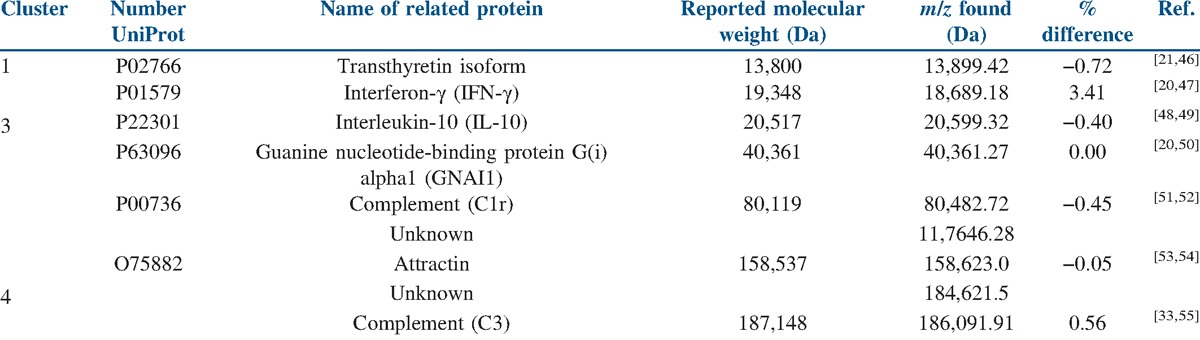
With the aim of identifying these proteins, a search in several databases (Plasma Proteome Database, neXtProt, Phosphosite, PRIDE, and UniprotKB) and in the scientific literature was performed using the m/z value for these differentially expressed peaks, and allowed us to propose some proteins as summarized in Table 2.
Table 2.
Approach to protein identification
DISCUSSION
Most of these proposed proteins have been described in a plasma proteome map generated by a multidimensional strong cation exchange − phase liquid chromatography − ion mobility spectrometry − mass spectrometry experiment combined with a database assignment approach.[20]
Transthyretin (TTR)
Thyroid hormone-binding protein is a small protein highly abundant in plasma (200–400 μg/ml) and the cerebrospinal fluid (CSF) (10–40 μg/ml). This 13.8 kDa protein is synthesized in the liver as well as in the choroid plexus, but it is also secreted by placental trophoblasts. It forms a homotetramer that transports thyroxine and retinol in both blood and CSF. TTR also plays an important role in the transport of thyroxine and retinol from maternal to fetal circulation that is critical for normal development of the human fetus. Moreover, about 100 point mutations in the TTR gene are known, and several of them are harmless but many play crucial roles in variants of hereditary TTR amyloidosis.[21] Defects in TTR are a cause of hyperthyroxinemia dystransthyretinemic euthyroidal. It is a condition characterized by the elevation of total and free thyroxine in healthy, euthyroid people without detectable binding protein abnormalities.[22] There are about nine TTR isoforms, due to numerous S-thiolation in a single cysteine residue at position ten.[21] Chen and Zhang[23] published a hypothesis concerning the relationship between PE and the deposit of TTR amyloid fibrils, and also Kalkunte et al.[24] recently reported its dysregulation in women with PE using SELDI-TOF and a mouse model in addition.
Interferon-gamma (IFN-γ) and interleukin-10 (IL-10)
IFN-γ is produced by lymphocytes activated by specific antigens or mitogens. In addition to having antiviral activity, IFN-γ has important immunoregulatory functions. It is a potent activator of macrophages, it has antiproliferative effects on transformed cells, and it can potentiate the antiviral and antitumor effects of type I interferons. It belongs to the type II (or gamma) interferon family.[22] IL-10 is a cytokine with pleiotropic effects in immunoregulation and inflammation. It downregulates the expression of cytokines from type I T helper cells Th1 cytokines, major histocompatibility complex (MHC) class II antigens, and co-stimulatory molecules on macrophages. It acts in concert with IL-4 to induce activated B lymphocytes to grow, switch isotype, and ultimately differentiate into antibody-producing plasma cells.[25]
Cytokines such as IFN-γ, IL-6, IL-17, and IL-21 are elevated in the plasma of mouse models of lupus, arthritis, and multiple sclerosis, and in subsets of patients with autoimmune diseases.[26] IFN-γ is intimately connected to autoimmunity and, specifically, has long been associated with lupus. Systemic autoimmunity owing to overactivity of follicular helper T cells (Tfh) and dysregulated germinal centers (GC) has been described in mice and humans. Although GC-driven autoimmunity probably results from the association of many dysregulated processes, cytokine imbalance plays a key role particularly centered around IFN-γ, IL-6, IL-17, and IL-21.[26]
On the other hand, it is currently widely accepted that phagocytosis of apoptotic cells by bacterial lipopolysaccharide LPS-activated macrophages induces secretion of IL-10 and decreases the secretion of other inflammatory cytokines such as TNF-α, IL-1β, and IL-12.[27] It has been reported that accumulation of dead cells containing nuclear autoantigens in sites of immune selection may provide survival signals for autoreactive B-cells, and that the production of antibodies against nuclear structures determines the initiation of chronic autoimmunity in systemic lupus erythematosus.[27] Moreover, high levels of IL-10 were found in cases of autoimmune lymphoproliferative syndrome (ALPS) and this could provide useful tools for ALPS diagnosis.[28,29] Furthermore, plasma levels of IFN-γ and IL-10 among other cytokines were recently measured in patients with colorectal adenomas, and it was found that IFN-γ could contribute to cancer development.[30]
Complement components
C1r is a serine protease that combines with C1q and C1s to form C1, the first component of the classical pathway of the complement system.[22] Congenital defects in C1 and C4 are strongly associated with lupus, and this pattern along with laboratory evidence suggests that the activity of this classical pathway is highly important in safely eliminating and preventing immune complexes diseases.[31] Similar immunological mechanisms might account for the vast majority of autoimmune diseases.
Likewise, C3 plays a central role in the activation of the complement system. Its processing by C3 convertase is the central reaction in both classical and alternative complement pathways. C3a anaphylatoxin derived from proteolytic degradation of C3 is a mediator of the local inflammatory process. It induces the contraction of smooth muscle, increases vascular permeability, and causes histamine release from mast cells and basophilic leukocytes.[22] Several studies have demonstrated that aPL can activate the complement system through the classical activation pathway, and also showed that C3 activation was required in aPL-induced fetal losses.[32] Moreover, altered C3 expression was found in plasma samples from patients with polycystic ovary syndrome compared with healthy controls, using a Matrix-assisted laser desorption/ionization-time of flight (MALDI-TOF/TOF) approach.[33]
Guanine nucleotide-binding protein G(i) alpha 1 (GNAI1)
Guanine nucleotide-binding proteins (G proteins) participate as modulators or transducers in various transmembrane signaling systems. GNAI1 is involved in biological processes as platelet activator, a response to peptide hormone stimulus, synaptic transmission, G protein coupled receptor protein signaling pathway, cell division, and blood coagulation.[22] Recently, it was demonstrated that GNAI1 inhibits the class III β-tubulin isotype (βIII-tubulin), a predictive biomarker in ovarian cancer and other solid tumor malignancies.[34]
Attractin
Attractin is a protein involved in initial immune cell clustering during inflammatory response, and it may regulate the chemotactic activity of chemokines. Attractin may play a role in melanocortin signaling pathways that regulate energy homeostasis and pigmentation, and it may also be important in normal myelination in the central nervous system.[35] It was first discovered as a circulating secreted molecule expressed by activated T lymphocytes; it was then examined as a potential marker of immune activity.[36] Mass spectrometry and liquid chromatography analysis of plasma samples from patients with S-adenosylhomocysteine hydrolase (AHCY) deficiency revealed attractin as a potential biomarker, useful for routine diagnosis and management of AHCY deficiency.[37] Mutations at the attractin locus were described as the cause of juvenile-onset neurodegeneration in animal mutants. The reassessment of earlier attractin mutants demonstrated that neurodegeneration, alterations in pigmentation regulation, and basal metabolic rates were common to all allelic variants.[36]
The proposed proteins have been identified as biomarkers for other disorders, and some of them are proteins associated with the inflammatory response, which is not surprising given the APS characteristics.[38,39] Even though the Giles group demonstrated changes in signaling pathways and proteins in human monocytes treated with IgG of different clinical types of APS patients,[15,40] to our knowledge, this is one of the first reports about the protein profile in sera of women with pregnancy losses associated with APS. It is also interesting that some of the proteins detected could be cytokines, given the elevated levels of inflammatory cytokines in autoimmune diseases. These cytokines play a role in promoting germinal centers that give rise to autoantibodies, so it is possible that monoclonal antibodies against these molecules will be effective in selected groups of patients, such as women with obstetric APS.[26] In addition, our finding of two target proteins related with the complement system is interesting, since it has been widely demonstrated that complement activity mediates the aPL-induced pregnancy loss in APS patients.[41,42,43,44,45]
Therefore, the best strategy to improve early diagnosis of obstetric APS could reside in the association between cytokine levels and the detection of other proteins. Maybe, the target candidates shown here raise the possibility of finding useful biomarkers in sera, since our findings highlight the need for additional investigation in this field. Novel research could be addressed to select some of these proteins and perform a complete identification of them by several proteomics methods, and then explore their expression in patients groups presented in this study. In addition, it will be interesting to explore protein post-translational modifications such enzymatic glycosylation. The search for glycan’s patterns of proteins involved in immune response could be a novel pathway to understand this disease and support its diagnosis.
Financial support and sponsorship
Administrative Department of Science, Technology and Innovation − Colciencias, Colombia (Grant #1115-493-26157). Universidad de Antioquia − UdeA. AMA is a fellow of Colciencias.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
The authors thank Prof. F.v. Eggeling for his advice with the SELDI-TOF technique and Anne-Lise Haenni for her critical review.
REFERENCES
- 1.Miyakis S, Lockshin MD, Atsumi T, Branch DW, Brey RL, Cervera R, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS) J Thromb Haemost. 2006;4:295–306. doi: 10.1111/j.1538-7836.2006.01753.x. [DOI] [PubMed] [Google Scholar]
- 2.Di Simone N, Luigi MP, Marco D, Fiorella DN, Silvia D, Clara DM, et al. Pregnancies complicated with antiphospholipid syndrome: The pathogenic mechanism of antiphospholipid antibodies: A review of the literature. Ann N Y Acad Sci. 2007;1108:505–14. doi: 10.1196/annals.1422.054. [DOI] [PubMed] [Google Scholar]
- 3.Abrahams VM. Mechanisms of antiphospholipid antibody-associated pregnancy complications. Thromb Res. 2009;124:521–5. doi: 10.1016/j.thromres.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 4.La Rosa L, Meroni PL, Tincani A, Balestrieri G, Faden D, Lojacono A, et al. Beta 2 glycoprotein I and placental anticoagulant protein I in placentae from patients with antiphospholipid syndrome. J Rheumatol. 1994;21:1684–93. [PubMed] [Google Scholar]
- 5.Poulton K, Rahman A, Giles I. Examining how antiphospholipid antibodies activate intracellular signaling pathways: A systematic review. Semin Arthritis Rheum. 2012;41:720–36. doi: 10.1016/j.semarthrit.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 6.Pierangeli SS, Chen PP, González EB. Antiphospholipid antibodies and the antiphospholipid syndrome: An update on treatment and pathogenic mechanisms. Curr Opin Hematol. 2006;13:366–75. doi: 10.1097/01.moh.0000239710.47921.d2. [DOI] [PubMed] [Google Scholar]
- 7.Meroni PL, Borghi MO, Raschi E, Tedesco F. Pathogenesis of antiphospholipid syndrome: Understanding the antibodies. Nat Rev Rheumatol. 2011;7:330–9. doi: 10.1038/nrrheum.2011.52. [DOI] [PubMed] [Google Scholar]
- 8.Blank M, Shoenfeld Y. Antiphospholipid antibody-mediated reproductive failure in antiphospholipid syndrome. Clin Rev Allergy Immunol. 2010;38:141–7. doi: 10.1007/s12016-009-8146-x. [DOI] [PubMed] [Google Scholar]
- 9.Bertolaccini ML, Amengual O, Atsumi T, Binder WL, de Laat B, Forastiero R, et al. ‘Non-criteria’ aPL tests: Report of a task force and preconference workshop at the 13th International Congress on Antiphospholipid Antibodies, Galveston, TX, USA, April 2010. Lupus. 2011;20:191–205. doi: 10.1177/0961203310397082. [DOI] [PubMed] [Google Scholar]
- 10.Raising awareness of antiphospholipid antibody syndrome. Lancet. 2010;375:778. doi: 10.1016/S0140-6736(10)60326-1. [DOI] [PubMed] [Google Scholar]
- 11.López-Pedrera C, Barbarroja N, Villalba JM. Novel biomarkers of atherosclerosis and cardiovascular risk in autoimmune diseases: Genomics and proteomics approaches. Proteomics Clin Appl. 2009;3:213–25. doi: 10.1002/prca.200800147. [DOI] [PubMed] [Google Scholar]
- 12.López-Pedrera C, Cuadrado MJ, Herandez V, Buendïa P, Aguirre MA, Barbarroja N, et al. Proteomic analysis in monocytes of antiphospholipid syndrome patients: Deregulation of proteins related to the development of thrombosis. Arthritis Rheum. 2008;58:2835–44. doi: 10.1002/art.23756. [DOI] [PubMed] [Google Scholar]
- 13.Cuadrado MJ, Aguirre MA, Barbarroja N, Khamashta MA, Lopez-Pedrera C. Proteomics in antiphospholipid syndrome: A review. Lupus. 2010;19:385–8. doi: 10.1177/0961203309360986. [DOI] [PubMed] [Google Scholar]
- 14.Teixeira PC, Ferber P, Vuilleumier N, Cutler P. Biomarkers for cardiovascular risk assessment in autoimmune diseases. Proteomics Clin Appl. 2015;9:48–57. doi: 10.1002/prca.201400125. [DOI] [PubMed] [Google Scholar]
- 15.Ripoll VM, Lambrianides A, Pierangeli SS, Poulton K, Ioannou Y, Heywood WE, et al. Changes in regulation of human monocyte proteins in response to IgG from patients with antiphospholipid syndrome. Blood. 2014;124:3808–16. doi: 10.1182/blood-2014-05-577569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cadavid JA, Alvarez A, Markert UR, Cardona Maya W. Differential protein expression in seminal plasma from fertile and infertile males. J Hum Reprod Sci. 2014;7:206–11. doi: 10.4103/0974-1208.142485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kwak JY, Gilman-Sachs A, Beaman KD, Beer AE. Autoantibodies in women with primary recurrent spontaneous abortion of unknown etiology. J Reprod Immunol. 1992;22:15–31. doi: 10.1016/0165-0378(92)90003-m. [DOI] [PubMed] [Google Scholar]
- 18.Peña RB, Cadavid AP, Botero JH, García GP, Gallego MI, Ossa JE. The production of MLR-blocking factors after lymphocyte immunotherapy for RSA does not predict the outcome of pregnancy. Am J Reprod Immunol. 1998;39:120–4. doi: 10.1111/j.1600-0897.1998.tb00343.x. [DOI] [PubMed] [Google Scholar]
- 19.Alvarez AM, Mulla MJ, Chamley LW, Cadavid AP, Abrahams VM. Aspirin-triggered lipoxin prevents antiphospholipid antibody effects on human trophoblast migration and endothelial cell interactions. Arthritis Rheumatol. 2015;67:488–97. doi: 10.1002/art.38934. [DOI] [PubMed] [Google Scholar]
- 20.Liu X, Valentine SJ, Plasencia MD, Trimpin S, Naylor S, Clemmer DE. Mapping the human plasma proteome by SCX-LC-IMS-MS. J Am Soc Mass Spectrom. 2007;18:1249–64. doi: 10.1016/j.jasms.2007.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Poulsen K, Bahl JM, Tanassi JT, Simonsen AH, Heegaard NH. Characterization and stability of transthyretin isoforms in cerebrospinal fluid examined by immunoprecipitation and high-resolution mass spectrometry of intact protein. Methods. 2012;56:284–92. doi: 10.1016/j.ymeth.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 22.Hornbeck PV, Kornhauser JM, Tkachev S, Zhang B, Skrzypek E, Murray B, et al. PhosphoSitePlus: A comprehensive resource for investigating the structure and function of experimentally determined post-translational modifications in man and mouse. Nucleic Acids Res. 2012;40:D261–70. doi: 10.1093/nar/gkr1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen Y, Zhang Z. Does transthyretin function as one of contributors for preeclampsia? Med Hypotheses. 2011;76:8–10. doi: 10.1016/j.mehy.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kalkunte SS, Neubeck S, Norris WE, Cheng SB, Kostadinov S, Vu Hoang D, et al. Transthyretin is dysregulated in preeclampsia, and its native form prevents the onset of disease in a preclinical mouse model. Am J Pathol. 2013;183:1425–36. doi: 10.1016/j.ajpath.2013.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Banchereau J. Converging and diverging properties of human interleukin-4 and interleukin-10. Behring Inst Mitt. 1995:58–77. [PubMed] [Google Scholar]
- 26.Sweet RA, Lee SK, Vinuesa CG. Developing connections amongst key cytokines and dysregulated germinal centers in autoimmunity. Curr Opin Immunol. 2012;24:658–64. doi: 10.1016/j.coi.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 27.Chaurio RA, Janko C, Muñoz LE, Frey B, Herrmann M, Gaipl US. Phospholipids: Key players in apoptosis and immune regulation. Molecules. 2009;14:4892–914. doi: 10.3390/molecules14124892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Magerus-Chatinet A, Stolzenberg MC, Loffredo MS, Neven B, Schaffner C, Ducrot N, et al. FAS-L, IL-10, and double-negative CD4− CD8− TCR alpha/beta+ T cells are reliable markers of autoimmune lymphoproliferative syndrome (ALPS) associated with FAS loss of function. Blood. 2009;113:3027–30. doi: 10.1182/blood-2008-09-179630. [DOI] [PubMed] [Google Scholar]
- 29.Rodrigues V, Conde M, Figueiredo A, Vasconcelos J, Dias A. Autoimmune lymphoproliferative syndrome. Acta Med Port. 2011;24:833–6. [PubMed] [Google Scholar]
- 30.Kang M, Edmundson P, Araujo-Perez F, McCoy AN, Galanko J, Keku TO. Association of plasma endotoxin, inflammatory cytokines and risk of colorectal adenomas. BMC Cancer. 2013;13:91. doi: 10.1186/1471-2407-13-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arason GJ, Jorgensen GH, Ludviksson BR. Primary immunodeficiency and autoimmunity: Lessons from human diseases. Scand J Immunol. 2010;71:317–28. doi: 10.1111/j.1365-3083.2010.02386.x. [DOI] [PubMed] [Google Scholar]
- 32.Holers VM, Girardi G, Mo L, Guthridge JM, Molina H, Pierangeli SS, et al. Complement C3 activation is required for antiphospholipid antibody-induced fetal loss. J Exp Med. 2002;195:211–20. doi: 10.1084/jem.200116116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Insenser M, Martinez-Garcia MA, Montes R, San-Millán JL, Escobar-Morreale HF. Proteomic analysis of plasma in the polycystic ovary syndrome identifies novel markers involved in iron metabolism, acute-phase response, and inflammation. J Clin Endocrinol Metab. 2010;95:3863–70. doi: 10.1210/jc.2010-0220. [DOI] [PubMed] [Google Scholar]
- 34.De Donato M, Mariani M, Petrella L, Martinelli E, Zannoni GF, Vellone V, et al. Class III β-tubulin and the cytoskeletal gateway for drug resistance in ovarian cancer. J Cell Physiol. 2012;227:1034–41. doi: 10.1002/jcp.22813. [DOI] [PubMed] [Google Scholar]
- 35.Malík R, Mares V, Kleibl Z, Pohlreich P, Vlasicová K, Sedo A. Expression of attractin and its differential enzyme activity in glioma cells. Biochem Biophys Res Commun. 2001;284:289–94. doi: 10.1006/bbrc.2001.4956. [DOI] [PubMed] [Google Scholar]
- 36.Duke-Cohan JS, Kim JH, Azouz A. Attractin: Cautionary tales for therapeutic intervention in molecules with pleiotropic functionality. J Environ Pathol Toxicol Oncol. 2004;23:1–11. doi: 10.1615/jenvpathtoxoncol.v23.i1.10. [DOI] [PubMed] [Google Scholar]
- 37.Sedic M, Kraljevic Pavelic S, Cindric M, Vissers JP, Peronja M, Josic D, et al. Plasma biomarker identification in S-adenosylhomocysteine hydrolase deficiency. Electrophoresis. 2011;32:1970–5. doi: 10.1002/elps.201000556. [DOI] [PubMed] [Google Scholar]
- 38.Salmon JE, Girardi G. Antiphospholipid antibodies and pregnancy loss: A disorder of inflammation. J Reprod Immunol. 2008;77:51–6. doi: 10.1016/j.jri.2007.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Salmon JE, Girardi G, Lockshin MD. The antiphospholipid syndrome as a disorder initiated by inflammation: Implications for the therapy of pregnant patients. Nat Clin Pract Rheumatol. 2007;3:140–7. doi: 10.1038/ncprheum0432. quiz 1 p following 87. [DOI] [PubMed] [Google Scholar]
- 40.Lambrianides A, Carroll CJ, Pierangeli SS, Pericleous C, Branch W, Rice J, et al. Effects of polyclonal IgG derived from patients with different clinical types of the antiphospholipid syndrome on monocyte signaling pathways. J Immunol. 2010;184:6622–8. doi: 10.4049/jimmunol.0902765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Salmon JE, Girardi G, Holers VM. Complement activation as a mediator of antiphospholipid antibody induced pregnancy loss and thrombosis. Ann Rheum Dis. 2002;61(Suppl 2):ii46–50. doi: 10.1136/ard.61.suppl_2.ii46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Salmon JE, Girardi G, Holers VM. Activation of complement mediates antiphospholipid antibody-induced pregnancy loss. Lupus. 2003;12:535–8. doi: 10.1191/0961203303lu397oa. [DOI] [PubMed] [Google Scholar]
- 43.Girardi G, Berman J, Redecha P, Spruce L, Thurman JM, Kraus D, et al. Complement C5a receptors and neutrophils mediate fetal injury in the antiphospholipid syndrome. J Clin Invest. 2003;112:1644–54. doi: 10.1172/JCI18817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Girardi G, Salmon JB. The role of complement in pregnancy and fetal loss. Autoimmunity. 2003;36:19–26. doi: 10.1080/0891693031000067322. [DOI] [PubMed] [Google Scholar]
- 45.Girardi G, Redecha P, Salmon JE. Heparin prevents antiphospholipid antibody-induced fetal loss by inhibiting complement activation. Nat Med. 2004;10:1222–6. doi: 10.1038/nm1121. [DOI] [PubMed] [Google Scholar]
- 46.Kolialexi A, Anagnostopoulos AK, Papantoniou N, Vougas K, Antsaklis A, Fountoulakis M, et al. Potential biomarkers for Turner in maternal plasma: Possibility for noninvasive prenatal diagnosis. J Proteome Res. 2010;9:5164–70. doi: 10.1021/pr100459q. [DOI] [PubMed] [Google Scholar]
- 47.Tu CJ, Dai J, Li SJ, Sheng QH, Deng WJ, Xia QC, et al. High-sensitivity analysis of human plasma proteome by immobilized isoelectric focusing fractionation coupled to mass spectrometry identification. J Proteome Res. 2005;4:1265–73. doi: 10.1021/pr0497529. [DOI] [PubMed] [Google Scholar]
- 48.Bouwman FG, de Roos B, Rubio-Aliaga I, Crosley LK, Duthie SJ, Mayer C, et al. 2D-electrophoresis and multiplex immunoassay proteomic analysis of different body fluids and cellular components reveal known and novel markers for extended fasting. BMC Med Genomics. 2011;4:24. doi: 10.1186/1755-8794-4-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Qian WJ, Jacobs JM, Camp DG, 2nd, Monroe ME, Moore RJ, Gritsenko MA, et al. Comparative proteome analyses of human plasma following in vivo lipopolysaccharide administration using multidimensional separations coupled with tandem mass spectrometry. Proteomics. 2005;5:572–84. doi: 10.1002/pmic.200400942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jin WH, Dai J, Li SJ, Xia QC, Zou HF, Zeng R. Human plasma proteome analysis by multidimensional chromatography prefractionation and linear ion trap mass spectrometry identification. J Proteome Res. 2005;4:613–9. doi: 10.1021/pr049761h. [DOI] [PubMed] [Google Scholar]
- 51.Jeong SK, Lee EY, Cho JY, Lee HJ, Jeong AS, Cho SY, et al. Data management and functional annotation of the Korean reference plasma proteome. Proteomics. 2010;10:1250–5. doi: 10.1002/pmic.200900371. [DOI] [PubMed] [Google Scholar]
- 52.Sinclair J, Timms JF. Quantitative profiling of serum samples using TMT protein labelling, fractionation and LC-MS/MS. Methods. 2011;54:361–9. doi: 10.1016/j.ymeth.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 53.Bortner JD, Jr, Richie JP, Jr, Das A, Liao J, Umstead TM, Stanley A, et al. Proteomic profiling of human plasma by iTRAQ reveals down-regulation of ITI-HC3 and VDBP by cigarette smoking. J Proteome Res. 2011;10:1151–9. doi: 10.1021/pr100925p. [DOI] [PubMed] [Google Scholar]
- 54.Misek DE, Kuick R, Wang H, Galchev V, Deng B, Zhao R, et al. A wide range of protein isoforms in serum and plasma uncovered by a quantitative intact protein analysis system. Proteomics. 2005;5:3343–52. doi: 10.1002/pmic.200500103. [DOI] [PubMed] [Google Scholar]
- 55.Ignjatovic V, Lai C, Summerhayes R, Mathesius U, Tawfilis S, Perugini MA, et al. Age-related differences in plasma proteins: How plasma proteins change from neonates to adults. PLoS One. 2011;6:e17213. doi: 10.1371/journal.pone.0017213. [DOI] [PMC free article] [PubMed] [Google Scholar]


