Abstract
Aim:
To evaluate the role of uterine natural killer (uNK) CD56dim and CD16+ cells in patients with refractory antiphospholipid, antibody-mediated, recurrent, pregnancy loss.
Settings and Design:
A case–control study was conducted between 2012 and 2015 at a university hospital.
Patients and Methods:
A group of 118 women with a history of antiphospholipid antibody syndrome experiencing fetal loss in spite of low dose aspirin (LDA) and low molecular weight heparin (LMWH) treatment in the current pregnancy were included in this study. A group of 32 patients undergoing an elective termination of viable pregnancies before 20 weeks were taken as controls. Suction evacuation was performed to collect abortus specimens, and uterine wall curettage was performed to collect decidua specimens, which were then stained using monoclonal antibodies specific to CD56 and CD16.
Statistics:
Statistical analyses were performed using the Statistical Package for the Social Sciences version 18 software. Chi-square and Fisher exact tests were used for making comparison between the groups.
Results:
Abnormal fetal karyotype was found in nine (9/97) cases of the study group, which means that abnormal karyotype accounts for only 9.3% of the causes of failure of treatment. Abnormal karyotype was found in four cases of the control group. Only cases with normal karyotyping were subjected to decidual uNK cells analysis. We found that CD56dim and CD16+ were found in the decidua of 79 cases (79/97), which means that aberrant natural killer cells expression might account for 81.4% of the cases of refractory antiphospholipid antibody (APA)-mediated recurrent pregnancy loss.
Conclusion:
CD56dim and CD16+ uNK cells might be correlated with refractory APA-mediated recurrent pregnancy loss.
KEYWORDS: Antiphospholipid antibodies, recurrent miscarriage, uNK, uterine natural killer cells
INTRODUCTION
Recurrent spontaneous abortion (RSA) refers to the loss of pregnancy for ≥2 times in patients before the 20 weeks of gestation or a fetal weight of <500 g.[1,2] Although it has been estimated to affect 5% of women in the reproductive age, it is believed that as per these statistics, the incidence of RSA is even higher because of the exclusion of subclinical and under diagnosed RSAs.[3]
The etiology of RSA includes chromosomal, anatomic, endocrine, and autoimmune abnormalities, as well as the infections of the reproductive tract. In at least 35–44% of the patients, no cause can be identified, and they are referred to as cases of an unexplained recurrent spontaneous abortion (URSA). However, it has been shown that URSA might be alloimmune in origin with failure of the fetal–maternal immunologic tolerance.[4,5,6]
Up to 15% of the patients with recurrent miscarriage have been found to be positive for antiphospholipid antibody syndrome (APS), and both low-dose aspirin and low molecular weight heparin have been recommended for the cases of obstetric APS.[7] Unfortunately, 30% of the cases continue to experience pregnancy loss in spite of treatment with no obvious cause and no effective treatment.[8]
Chromosomal aberrations in the embryo account only for 30% of the miscarriages in APS.[9,10] There is much evidence on the underlying inflammatory mechanisms, as the complement-mediated tissue injury and the infiltration of placental bed with high concentration of inflammatory cells suggest another mechanism for the pregnancy loss in pregnancies affected by APS.[11,12]
The pathophysiologic basis of obstetric APS is not fully understood. The pathological features of antiphospholipid antibodies (APAs) on the trophoblast include the following: decreased vasculosyncytial membranes, increased syncytial knots, fibrosis, and infarcts than in women without APS.[13] The changes in syncytial membranes may be secondary to thrombosis or secondary to placental damage by the antibodies themselves, which can inhibit placental human chorionic gonadotropin secretion,[14] cause complement activation,[15] and impair cytokine levels, but all of which may be responsible for fetal loss in obstetric APS. Cytokine imbalances are particularly relevant, as cytokines may affect the activation or inhibition of natural killer (NK) cells.[16]
The presence of APAs was shown to be associated with increased peripheral blood NK cells number, proportions, and cytotoxicity especially in a patient with RSA.[17]
APAs augment NK cell numbers and cytotoxicity, and result in an increased recruitment of decidual NK cells. Under these conditions, noncytotoxic decidual NK cells might change to cytotoxic CD56+/16+ NK cells, which in turn act via several mechanisms such as the mediation of trophoblastic tissue apoptosis and the secretion of various proinflammatory cytokines causing decidual microvessel thrombosis and fetal loss.[18]
This study aimed at evaluating the decidual NK cells in obstetric APS patients experiencing fetal loss in spite of low dose aspirin (LDA) and low molecular weight heparin (LMWH) treatment.
PATIENTS AND METHODS
Selection of participants
The study was designed over a 4-year period from January 2012 to December 2015. One hundred eighteen cases having a history of obstetric APS and experiencing fetal loss in their current pregnancy in spite of low-dose aspirin (81 mg/day) and low molecular weight heparin (enoxaparin 40 mg/day) treatment were included in this study. The study was conducted at the recurrent miscarriage clinic of a university hospital. Another 32 patients undergoing elective termination of viable pregnancy before 20 weeks because of heart disease New York heart assiciation (NYHA) III and IV stages and due to lethal congenital anomalies as bilateral polycystic kidneys, renal agenesis, and anencephaly were taken as controls.
In our study, unexplained recurrent miscarriage was defined as ≥2 confirmed successive spontaneous miscarriages (<20 weeks’ gestation). APS was diagnosed according to the Sydney criteria.[19] All the patients had their antibody status checked at the enrollment.
In our study, the women who proved to have septic miscarriage, documented with endocrinopathies (diabetes, thyroid disorders, and hyperprolactinemia), other autoimmune syndromes, mullerian anomalies, metabolic disorder, thrombophilia, abnormal karyotype in one or both parents, the history of hormonal contraception, and the history of intrauterine contraceptive device application within the last 3 months preceding current pregnancy were excluded from the study. The women more than 35 years old and those with body mass index (BMI) >30 were also excluded.
The women were included in the study after signing a written informed consent. The study was conducted in compliance with the declaration of Helsinki and approved by the hospital research ethical committee.
Technical information
For each patient, a thorough medical and obstetric history was taken, and physical examination was performed.
Evacuation and curettage were performed for all women included in this study; these two operations were performed under regional anesthesia, using suction evacuation to collect abortus specimens after cervical dilatation, followed by uterine wall curettage to collect decidua specimens. Abortus specimens were collected on a special medium for a long-term monolayer cell culture and subsequent chromosome analysis using a conventional Giemsa banding technique. Decidua specimens of cases with normal karyotyping of the abortus were subjected to an immunohistochemical staining using monoclonal antibodies specific to uterine natural killer (uNK) cells, namely CD56+ and CD16+.
Reagents and materials used included the following:
-
(1)
Primary antibodies: monoclonal mouse antibody (MoAb) against CD56+ and CD16+ expressed on NK cells.
-
(2)
Universal kits: immunodetection system from Biogenex Laboratories, Switzerland contained the following: (a) negative control antibody, (b) biotinylated antiimmunoglobulin for mouse antibody, (c) label: streptavidine peroxidase complex, (d) chromogen: 2,3-diaminobenzidine (DAB) chromogen solution, ready to use substrate buffer and H2O2 substrate for use with liquid DAB chromogen and substrate buffer, and (e) blocking reagent to block endogenous peroxidase activity.
-
(3)
Lyophilized pepsin powder, phosphate buffer saline, counter stain (Mayer’s hematoxylin), distilled water, and mounting media (Canada balsam).
-
(4)
Staining jars, microscopic positively charged slides, cover slips for slides, and immune stainer.
-
(5)
Light microscope: with 100× and 400× magnification.
Immunohistochemical procedure: Decidua specimens were fixed in 10% neutral buffered formalin for a period not more than 24 h, routinely processed and embedded in paraffin wax. Three-micrometer-thick sections were mounted onto 3-aminopropyltriethoxysilane (Sigma Chemical Co., Poole, UK) coated slides, and serial sections were stained for uNK cells (CD56+ and CD16+). All the primary antibodies were incubated for 60 min for CD56+ and for 120 min for CD16+ at room temperature, and the reaction was developed with 3,3-diaminobenzidine (2,3 diaminobenzidine (DAB); Sigma Chemical Co., Poole, UK) containing 0.01% H2O2 to give a brown reaction product; staining intensity was noted; sections were lightly counterstained with Mayer’s hematoxylin, dehydrated, cleared, and mounted with DPX synthetic resin (Raymond A, Lamb Ltd., London, UK), and were then examined by using an ordinary light microscopy. Appropriate positive controls (neuroblastoma for CD56+ and tonsils for CD16+) were performed in each staining run, and negative controls were performed for each sample by replacing the primary antibody with mouse immunoglobulin-G.
Ethics
The study was conducted in accordance with the ethical standards of Helsinki declaration 1973 (revised 2000) and was approved by the local ethical committee of Ain Shams University Maternity Hospital.
Statistical analysis
Statistical analyses were performed using the Statistical Package for the Social Sciences version 18 software (SPSS Inc., Chicago, IL, United States). Numerical variables were presented as mean and standard deviation (±SD), whereas categorical variables were presented as number (n) and percentage (%). Chi-square and Fisher exact tests were used for making comparison regarding the qualitative variables between the groups. A difference with a P value <0.05 was considered statistically significant.
Required sample size was calculated using G*Power software version 3.17 for the sample size calculation (Heinrich Heine Universität, Düsseldorf, Germany), setting α-error probability at 0.05, power (1-β error probability) at 0.95%, and effective sample size (w) at 0.3. The effective size (w) was calculated as follows: 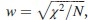 , where χ2 is the chi-square test, and N is the total sample size. The number of women participants needed to produce a statistically acceptable figure was 100.
, where χ2 is the chi-square test, and N is the total sample size. The number of women participants needed to produce a statistically acceptable figure was 100.
RESULTS
Among the one hundred eighteen women recruited for the study, 21 women were excluded. Among the excluded women, four women were excluded for having anatomical uterine defects, 10 women for having age >35 or BMI >30, four women for having history of chronic anovulation suggestive of polycystic ovarian disease, and three women with hyperprolactinemia and hypothyroidism. Abnormal fetal karyotype was found in nine (9/97) cases of the study group, which means that abnormal karyotype accounts for only 9.3% of the causes of failure of treatment. Abnormal karyotype was found in four cases of the control group. Only cases with the normal karyotype were subjected to the decidual uNK cells analysis. We found that CD56dim and CD16+ cells were found in the decidua of 79 cases with chromosomally intact abortuses (79/88), which means that aberrant NK cells expression might account for 81.4% of the cases of refractory APA-mediated pregnancy loss.
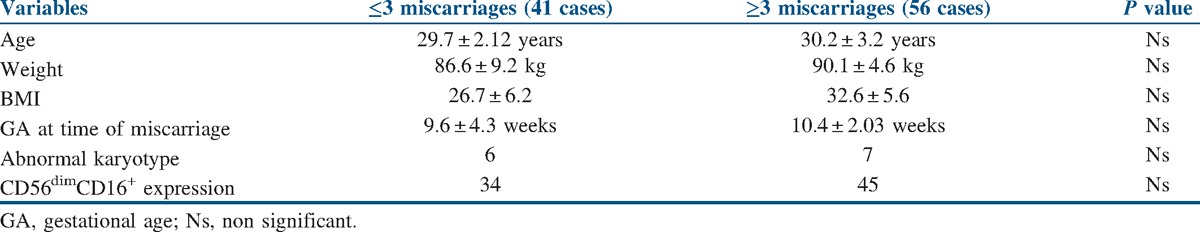
The mean age of the women included in the analysis was 31.25 ± 2.09 years, and mean BMI was 27.34 ± 3.41 kg/m2 [Table 1]. A karyotyping study of the abortus specimens showed normal karyotyping in 89.9% (116/129) of the studied specimens and abnormal karyotyping in 10.1% (13/129) of the studied specimens [Table 2]. From the 13 women with abnormal karyotype, nine were in the studied group (they are excluded) and four in the control group [Tables 3 and 4].
Table 1.
Descriptive characteristics of the study group

Table 2.
Karyotyping analysis of the studied specimens
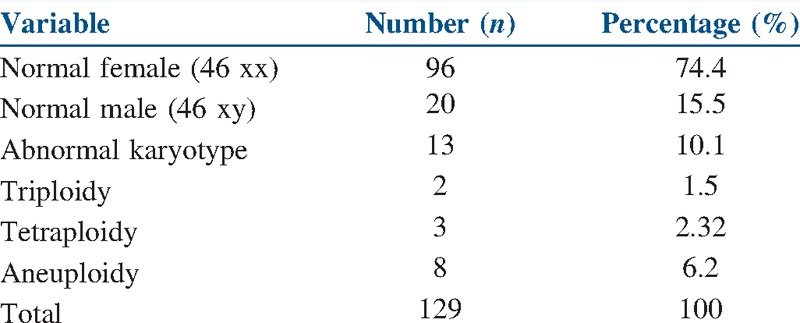
Table 3.
Descriptive characteristics of the study and control groups

Table 4.
Characteristics of the study group according to the number of previous miscarriages
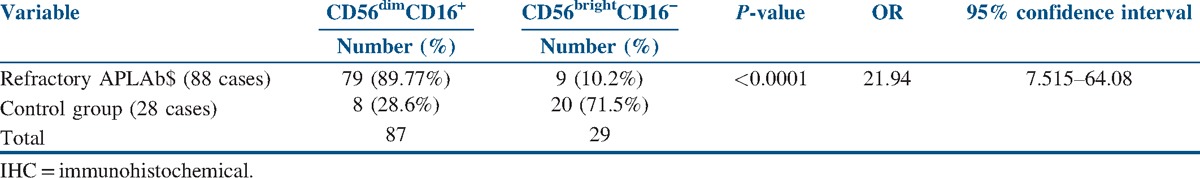
We found a significant difference between the expression of CD56dim and CD16+ in the decidua of cases and controls with an odds ratio (OR) of 21.94, as shown in Table 5.
Table 5.
IHC results of decidua specimens in cases with chromosomally intact abortuses and controls
DISCUSSION
With proper management, more than 70% of the patients with obstetric APS will have a live birth. The goals of treatment in obstetric APS are to improve maternal, fetal, and neonatal outcomes by decreasing the risks of the known complications of the disorder, including maternal thrombosis, fetal loss, preeclampsia, placental insufficiency, and fetal growth restriction.[20]
Earliest treatment for recurrent pregnancy loss associated with obstetric APS was a combination of high-dose prednisone and low-dose aspirin, with successful outcome in 75% of the cases but with high maternal and fetal morbidity due to gestational diabetes, hypertension, and premature rupture of membranes. The combination of prednisone and aspirin was compared with the combination of heparin, and both were found to be equally efficacious with less morbidity in the heparin group.[21] However, up to 30% of women with an obstetric APS experience RSA in spite of the use of LDA and LMWH in treatment, and this failure might be due to the underlying inflammatory mechanisms including complement-mediated tissue injury and the higher concentration of various inflammatory cells in the deciduas of those patients.[8]
The most abundant immune cells in the uterine decidua around the time of implantation and early placental development are the uNK cells. Altered numbers of uNK cells have been associated with several human reproductive disorders, including recurrent miscarriage and recurrent implantation failure.[22]
This study was designed to evaluate the role of uNK cells in patients with refractory APA-mediated RSA. In the study, karyotyping results showed normal karyotyping in 90.7% and abnormal karyotyping in 9.3% of the studied abortus specimens. A 29–57% rate of chromosomal abnormality was previously reported during analysis of miscarried tissue from the women suffering RSA,[9,23] and the higher percent of normal chromosomal results in miscarried tissue of women with RSA confirms that there may be other factors other than chromosomal abnormalities associated with RSA. However, the relatively lower percentage of chromosomal abnormalities in our study might be due to our inclusion criteria and the use of conventional G-banding technique in the analysis, which is less accurate than comparative genomic hybridization, and this might have caused some sort of bias in our analysis.
In our study, we found an overexpression of CD56dim and CD16+ uNK cells in the decidua specimens of women with refractory APA-mediated RSA. The findings of this study suggest that uNK cells might play an important role in the pathogenesis of refractory APA-mediated RSA. It is to be noted that Perricone et al., in 2007, found that the peripheral NK cells were elevated in the patients with RSA and APS, and these cells might be the source of uterine natural killer cells. In their study, they hypothesized that there is a subcategory of APS that is associated with elevated NK cells, and this group is the one suffering from pregnancy losses during the first 10 weeks of gestation.[24] Our results confirm this hypothesis, as it proves the elevated levels of uNK cells in resistant APS raising the hypothesis that NK cells augment the effect of APAs on trophoblastic tissue and also raises a question on the benefit of steroids in the treatment of such patients. Prednisolone has been suggested as a suitable treatment for RSA women with high uNK cells,[25] and it has been tried with encouraging results in the cases of resistant obstetric APS.[8] The beneficial effects of steroids might be due to its suppressive effect on uNK cells.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
The authors wish to thank all the women participated and included in this study, and the study was funded by the authors themselves.
REFERENCES
- 1.Mei S, Tan J, Chen H, Chen Y, Zhang J. Changes of CD4+CD25high regulatory T cells and FOXP3 expression in unexplained recurrent spontaneous abortion patients. Fertil Steril. 2010;94:2244–7. doi: 10.1016/j.fertnstert.2009.11.020. [DOI] [PubMed] [Google Scholar]
- 2.Beaman KD, Ntrivalas E, Mallers TM, Jaiswal MK, Kwak-Kim J, Gilman-Sachs A. Immune etiology of recurrent pregnancy loss and its diagnosis. Am J Reprod Immunol. 2012;67:319–25. doi: 10.1111/j.1600-0897.2012.01118.x. [DOI] [PubMed] [Google Scholar]
- 3.Stephenson M, Kutteh W. Evaluation and management of recurrent early pregnancy loss. Clin Obstet Gynecol. 2007;50:132–45. doi: 10.1097/GRF.0b013e31802f1c28. [DOI] [PubMed] [Google Scholar]
- 4.Christiansen OB, Nielsen HS, Pedersen B. Active or passive immunization in unexplained recurrent miscarriage. J Reprod Immunol. 2004;62:41–52. doi: 10.1016/j.jri.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 5.Kwak-Kim J, Park JC, Ahn HK, Kim JW, Gilman-Sachs A. Immunological modes of pregnancy loss. Am J Reprod Immunol. 2010;63:611–23. doi: 10.1111/j.1600-0897.2010.00847.x. [DOI] [PubMed] [Google Scholar]
- 6.Leber A, Zenclussen ML, Teles A, Brachwitz N, Casalis P, El-Mousleh T, et al. Pregnancy: Tolerance and suppression of immune responses. Methods Mol Biol. 2011;677:397–417. doi: 10.1007/978-1-60761-869-0_25. [DOI] [PubMed] [Google Scholar]
- 7.Bates SM, Greer IA, Pabinger I, Sofaer S, Hirsh J. Venous thromboembolism, thrombophilia, antithrombotic therapy, and pregnancy: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition) Chest. 2008;133:844–86S. doi: 10.1378/chest.08-0761. [DOI] [PubMed] [Google Scholar]
- 8.Bramaham K, Thomas M, Nelson-Piercy C, Khamashta M, Hunt BJ. First-trimester low-dose prednisolone in refractory antiphospholipid antibody-related pregnancy loss. Blood. 2011;117:6948–51. doi: 10.1182/blood-2011-02-339234. [DOI] [PubMed] [Google Scholar]
- 9.Ogasawara M, Aoki K, Okada S, Suzumori K. Embryonic karyotype of abortuses in relation to the number of previous miscarriages. Fertil Steril. 2000;73:300–4. doi: 10.1016/s0015-0282(99)00495-1. [DOI] [PubMed] [Google Scholar]
- 10.Takakuwa K, Asano K, Arakawa M, Yasuda M, Hasegawa I, Tanaka K. Chromosome analysis of aborted conceptuses of recurrent aborters positive for anticardiolipin antibody. Fertil Steril. 1997;68:54–8. doi: 10.1016/s0015-0282(97)81475-6. [DOI] [PubMed] [Google Scholar]
- 11.van Horn JT, Craven C, Ward K, Branch DW, Silver RM. Histologic features of placentas and abortion specimens from women with antiphospholipid and antiphospholipis-like syndromes. Placenta. 2004;25:642–8. doi: 10.1016/j.placenta.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 12.Miyakis S, Lockshin MD, Atsumi T, Branch DW, Brey RL, Cervera R, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS) J Thromb Haemost. 2006;4:295–306. doi: 10.1111/j.1538-7836.2006.01753.x. [DOI] [PubMed] [Google Scholar]
- 13.Out HJ, Kooijman CD, Bruinse HW, Derksen RH. Histo-pathological findings from patients with intrauterine fetal death and antiphospholipid antibodies. Eur J Obstet Gynecol. 1991;41:179–86. doi: 10.1016/0028-2243(91)90021-c. [DOI] [PubMed] [Google Scholar]
- 14.Shurtz-Swirsky R, Inbar O, Blank M, Cohen J, Bakimer R, Barnea ER, et al. In vitro effect of anticardiolipin autoantibodies upon total and pulsatile placental hCG secretion during early pregnancy. Am J Reprod Immunol. 1993;29:206–10. doi: 10.1111/j.1600-0897.1993.tb00588.x. [DOI] [PubMed] [Google Scholar]
- 15.Salmon JE, Girardi G. The role of complement in the antiphospholipid syndrome. Curr Dir Autoimmun. 2004;7:133–48. doi: 10.1159/000075690. [DOI] [PubMed] [Google Scholar]
- 16.Fishman P, Falach-Vaknine E, Zigelman R, Bakimer R, Sredni B, Djaldetti M, et al. Prevention of fetal loss in experimental antiphospholipid syndrome by in vivo administration of recombinant interleukin-3. J Clin Invest. 1993;91:1834–7. doi: 10.1172/JCI116396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lash GE, Bulmer JN. Do uterine natural killer (uNK) cells contribute to female reproductive disorders? J Reprod Immunol. 2011;88:156–64. doi: 10.1016/j.jri.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 18.Quenby S, Farquharson R. Uterine natural killer cells, implantation failure and recurrent miscarriage. Reprod Biomed Online. 2006;13:24–8. doi: 10.1016/s1472-6483(10)62012-3. [DOI] [PubMed] [Google Scholar]
- 19.The American College of Obstetricians and Gynecologists. Antiphospholipid syndrome. ACOG Pract Bull. 2011;118:1–8. [Google Scholar]
- 20.Branch DW, Khamashta MA. Antiphospholipid syndrome: Obstetric diagnosis, management, and controversies. Obstet Gynecol. 2003;101:1333–44. doi: 10.1016/s0029-7844(03)00363-6. [DOI] [PubMed] [Google Scholar]
- 21.Cowchock FS, Reece EA, Balaban D, Branch DW, Plouffe L. Repeated fetal losses associated with antiphospholipid antibodies: A collaborative randomized trial comparing prednisone with low dose heparin treatment. Am J Obstet Gynecol. 1992;166:1318–27. doi: 10.1016/0002-9378(92)91596-3. [DOI] [PubMed] [Google Scholar]
- 22.Seshadri S, Sunkara SK. Natural killer cells in female infertility and recurrent miscarriage: A systematic review and meta-analysis. Hum Reprod Update. 2014;20:429–38. doi: 10.1093/humupd/dmt056. [DOI] [PubMed] [Google Scholar]
- 23.Carp H, Toddler V, Aviram A, Daniely M, Mashiach S, Barkai G. Karyotype of the abortus in recurrent miscarriage. Fertil Steril. 2001;75:678–82. doi: 10.1016/s0015-0282(00)01801-x. [DOI] [PubMed] [Google Scholar]
- 24.Perricone C, De Carolis C, Giacomelli R, Zaccari G, Cipriani P, Bizzi E, et al. High levels of NK cells in the peripheral blood of patients affected with anti-phospholipid syndrome and recurrent spontaneous abortion: A potential new hypothesis. Rheumatology (Oxford) 2007;46:1574–8. doi: 10.1093/rheumatology/kem197. Epub 2007 Aug 17. [DOI] [PubMed] [Google Scholar]
- 25.Quenby S, Kalumbi C, Bates M, Farquharson R, Vince G. Prednisolone reduces preconceptual endometrial natural killer cells in women with recurrent miscarriage. Fertil Steril. 2005;84:980–4. doi: 10.1016/j.fertnstert.2005.05.012. [DOI] [PubMed] [Google Scholar]


