Abstract
Background:
There is significant evidence among the general population regarding the impact of physical activity in improving the quality of life; however, evidence regarding the effect of physical activity in improving fertility and the quality of life in infertile women is inadequate. Existing medical literature shows that moderately regular physical activity positively influences ovarian reserve and assisted reproductive technology (ART) outcomes in overweight and obese women. It is not known whether moderate physical activity influences ovarian reserve in normal weight reproductive age women, and whether age has any influence on the physical activity related changes in ovarian reserve.
Objectives:
The objectives of the study were (1) to study the impact of moderate physical activity on ovarian reserve markers in normal weight reproductive age women and (2) to understand whether age influences the effect of physical activity on ovarian reserve markers in reproductive age women.
Methods:
This observational, cross-sectional study included 162 married women in the age group of 19–42 years, who were evaluated for ovarian reserve markers antimullerian hormone (AMH), follicular stimulating hormone (FSH), and antral follicle count (AFC) on the days 3–6 of the menstrual cycle. The study participants were divided into two age groups (above and below 30 years) and physically active and inactive groups. Ovarian reserve markers were compared among both the age groups and the physically active and inactive participants by analysis of variance.
Results:
When the study participants in both the age groups were compared for the effect of moderate exercise on ovarian reserve profile, better ovarian reserve profile was observed in the physically active participants in both the age groups. Significant differences were not seen with respect to FSH level (P = 0.371) and AFC (P = 0.483) in both the age groups, but significant difference was observed with respect to AMH level in the below 30 years age group compared to the above 30 years age group (P ≤ 0.001).
Conclusion:
This study demonstrated that moderate physical activity is associated with improved age-specific levels of ovarian reserve markers.
KEYWORDS: AFC, AMH, FSH, ovarian reserve, physical activity
INTRODUCTION
The importance of physical activity in the prevention of noncommunicable diseases cannot be overemphasized in the present era of obesogenic environment. It is estimated that globally physical inactivity resulted in more than 5 million deaths in the year 2012, and the global physical inactivity related healthcare costs exceeded 53 billion dollars in the year 2013.[1,2] There is significant evidence among the general population regarding the impact of physical activity in improving the quality of life; however, evidence regarding the effect of physical activity in improving fertility and the quality of life in infertile women is inadequate.[3,4,5] Various studies revealed that there is a finely tuned interrelationship between energy metabolism and reproduction, thereby affecting female fertility. Recent studies revealed that the link between energy homeostasis and fertility is mediated through the sex steroids, particularly estrogens and their cognate receptors. In the hypothalamus, which is the central regulator of energy homeostasis, estrogens through their action on estrogen receptors regulate the expression of orexigenic neuropeptides, such as neuropeptide Y and agouti-related protein, and the activity of anorexigenic neurons, such as proopiomelanocortin and cocaine- and amphetamine-regulated transcript. In addition, estrogens acting on the hypothalamus and the brain stem also attenuate the orexigenic signals coming from the peripheral peptides such as cholecystokinin, leptin, and ghrelin.[6] Recent research suggested that the adipokine leptin, a protein product of the obesity gene, might be an independent regulator of the metabolic rate and a mediator of the reproductive function. Leptin receptors were found on the hypothalamic neurons involved in the control of the GnRH pulse generator, and thus, leptin may be a critical factor involved in signaling low-energy availability to the reproductive axis. In some studies, strong correlations were seen between leptin pulsatility and leutinising hormone (LH) and estrogen levels in women with normal menstrual cycles, and a cross-talk between leptin and estrogen signaling pathways in the hypothalamus was postulated.[7] In fact, leptin and its receptors were implicated in maintaining normal female reproductive functions, including menstrual cycle regulation, folliculogenesis, ovarian steroid genesis, the development of dominant follicles and oocyte maturation, endometrial development, and endometrial receptivity. It is hypothesized that the beneficial effects of physical activity on energy metabolism and reproduction are mediated through the regulation of the hypothalamic pituitary gonadal axis and adipokine (leptin) function and the reduction of oxidative stress.[8,9,10]
Fertility rates started declining in India in the last few decades, and one in every sixth couple is seeking treatment for infertility.[11] This can be attributed in part to the demographic transition, as well as the increasing female literacy and employment with consequent delay in child bearing. It is a well-established fact that ovarian reserve decreases with increasing age, and decreased fertility rates can be due to declining ovarian reserve with increasing age. Adequate follicular development of the ovaries in response to gonadotrophin has been referred to as ovarian reserve. Follicular stimulating hormone (FSH), antimullerian hormone (AMH), and antral follicle count (AFC) are the commonly used ovarian reserve markers for assessing ovarian reserve in the women of reproductive age.[12,13,14] Existing medical literature shows that moderately regular physical activity positively influences ovarian reserve and ART outcomes in overweight and obese women.[15,16] It is not known whether moderate physical activity influences ovarian reserve in normal weight reproductive age women, and whether age has any influence on the physical activity related changes in ovarian reserve. This study was undertaken to examine the effect of moderate physical activity on ovarian reserve markers in the two age groups of normal weight reproductive age women. The objectives of the study were (1) to study the impact of moderate physical activity on ovarian reserve markers in normal weight reproductive age women and (2) to understand whether age influences the effect of physical activity on ovarian reserve markers in this study group.
MATERIALS AND METHODS
This observational, cross-sectional study included 162 married women in the age group of 19–42 years, who attended the gynecology department of a tertiary care teaching hospital from January 2014 to December 2015 and who satisfied the inclusion criteria.
Inclusion criteria
Healthy women with normal menstrual cycles (25–35 days).
Exclusion criteria
The participants using hormonal contraceptives, smokers, pregnant and lactating mothers, those who underwent hysterectomy, oophorectomy, ovarian cystectomy, or fulguration, and the women with endometriosis were excluded from the study. The participants with polycystic ovary syndrome, diabetes mellitus, and thyroid disorders were also excluded from the study.
Written informed consent was obtained from all the participants, and institutional ethics review committee approval was obtained before commencing the study. The study participants were divided into two age groups. There were 97 participants in the below 30 years age group and 65 participants in the age group above 30 years. The participants of both the age groups of the study were further divided into physically active and inactive groups. There were 57 participants in the below 30 years age group who were physically active and 40 participants who were physically inactive. Similarly, in the above 30 years age group, there were 33 physically active and 32 physically inactive participants. Physical activity was assessed using the International Physical Activity Questionnaire and measured in Met minutes/week. On the days 3–6 of the menstrual cycle, 10 ml of blood was collected from the participants for the estimation of FSH and AMH levels. Serum FSH level was measured using a specific immunometric assay kit (Immulite; Diagnostic Products Corporation, Los Angeles, CA, USA). The measurement of serum AMH levels was performed using AMH/Müllerian-inhibiting substance Elisa kit (Diagnostic Systems Lab, Webster, TX, USA). On the same day, transvaginal ultrasound examination was performed to count the number of AFC between 2 and 6 mm in both the ovaries. FSH levels, AMH levels, and AFCs were compared in physically active and inactive participants in both the age groups. Statistical analyses were performed using the Statistical Package for the Social Sciences version 16 software (trial version, IBM SPSS Inc., Chicago, IL, USA). The analysis of variance was used for comparing the groups. For all statistical analyses, P < 0.05 was considered as statistically significant.
RESULTS
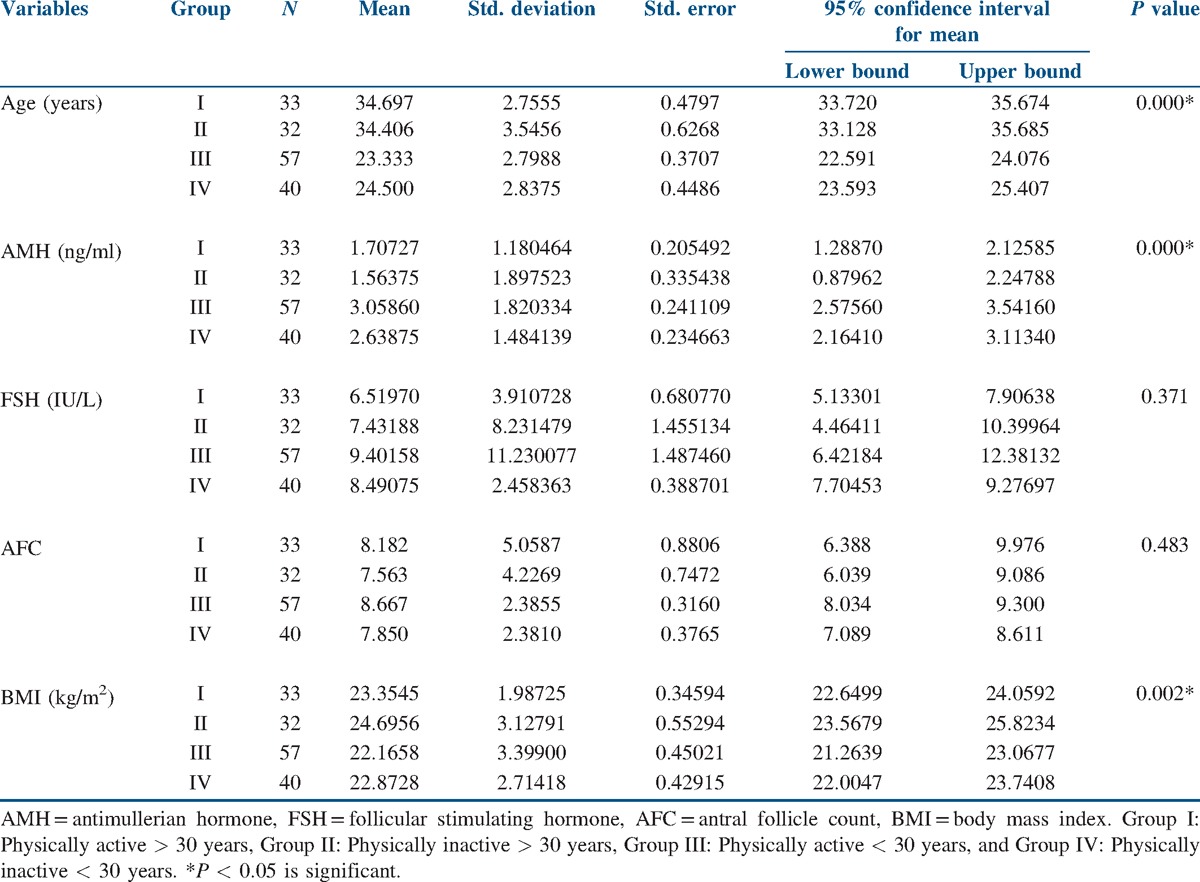
The data of all the 162 study participants were analyzed. The mean age of the physically active participants in the below 30 years age group was 23.33 ± 2.78 years, and the mean age of the physically inactive participants in the below 30 years age group was 24.5 ± 2.84 years. Similarly, the mean age of the physically active participants in the above 30 years age group was 34.7 ± 2.75 years and that of the physically inactive participants was 34.45 ± 3.55 years. The mean body mass index (BMI) in the below 30 years age group was 22.16 ± 3.34 in the physically active group and 22.87 ± 2.71 in the inactive group. The mean BMI in the above 30 years age group was 23.35 ± 1.99 in the physically active group and 24.69 ± 3.13 in the inactive group. The mean AMH level in the below 30 years age group was 3.06 ± 1.82 in the physically active group and 2.64 ± 1.48 in the inactive group. The mean AMH level in the above 30 years age group was 1.71 ± 1.18 in the physically active group and 1.56 ± 1.89 in the inactive group. The mean FSH level in the below 30 years age group was 6.51 ± 3.91 in the physically active group and 8.23 ± 1.45 in the inactive group. The mean FSH level in the above 30 years age group was 8.49 ± 2.46 in the physically active group and 9.40 ± 11.23 in the inactive group. The mean AFC in the below 30 years age group was 8.67 ± 2.39 in the physically active group and 7.85 ± 2.38 in the inactive group. The mean AFC in the above 30 years age group was 8.18 ± 5.06 in the physically active group and 7.56 ± 2.38 in the inactive group. When the study participants in the above and below 30 years age groups were compared for the effect of moderate exercise on ovarian reserve profile, better ovarian reserve profile was observed in the physically active participants in both the age groups [Figure 1]. Statistically significant differences were not seen with respect to FSH levels (P = 0.371) and AFC (P = 0.483) in both the age groups, but significant difference was observed with respect to AMH levels in the below 30 years age group compared to the above 30 years age group (P ≤ 0.001) [Table 1].
Figure 1.
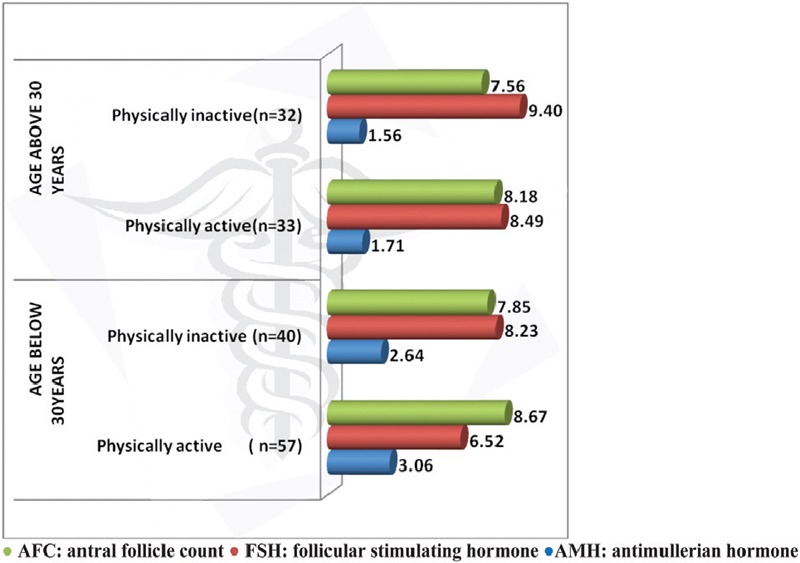
Effect of Physical activity on ovarian reserve profile in above and below 30 years age groups
Table 1.
Descriptive statistics of the study variables
DISCUSSION
Lifestyle changes and nutritional factors were found to be the most valuable and promising interventions in preserving human health and fertility and have been termed as the most fascinating area of medical research yet to be fully explored.[6] Many studies have demonstrated a link between physical activity and fertility with inconclusive results. Most of these studies concentrated around women participating in sports activities, and these studies projected on the negative impact of strenuous physical activity on female reproductive health such as by amenorrhea, osteoporosis, and eating disorder commonly known as female athlete triad.[16] Few recently conducted studies have demonstrated the beneficial effects of physical activity on fertility and the interrelationship between energy balance and the hypothalamic pituitary gonadal axis.[7,8] This study examined the effect of moderate exercise on ovarian reserve markers in the normal weight women of the two reproductive age groups (below and above 30 years). The observations made in this study reveal that moderate physical activity has a positive influence on the ovarian reserve profile in both the age groups of women examined [Figure 1]. When the study participants in both the age groups (below and above 30 years) were compared for the effect of physical activity on the ovarian reserve profile, a more beneficial effect of physical activity was observed in the participants of the below 30 years age group compared to the above 30 years age group, and this positive effect of physical activity on the ovarian reserve markers was not uniform on all the studied ovarian reserve markers. AMH levels were found to be significantly higher compared to FSH level and AFC in the below 30 years age group [Table 1]. Even though the BMI of the participants in the above 30 years age group was a little higher compared to the below 30 years age group, a positive influence of exercise on the ovarian reserve markers was more appreciated in the below 30 years age group. In the studies conducted by Orio et al.,[5] exercise training was associated with an improved reproductive hormonal profile compared to a diet-induced weight loss. In the Hunt 2 study conducted in premenopausal Norwegian women, moderate physical activity was associated with improved fertility rates compared to the women participating in <15 min duration of exercise per day. In the same study, high-intensity physical activity was associated with reduced fertility rates in the first conception, and the same was not seen in the later pregnancies. In this study, the effect of physical activity on fertility was independent of the changes in BMI, suggesting an involvement of other mechanisms.[17] In the study conducted by Chavarro et al.,[18] 30 min of vigorous exercise per day was associated with a reduced risk of infertility, even though the association did not reach statistical significance as in this study. In the studies conducted by Rich-Edwards et al.,[15] 1 h of vigorous exercise/week was associated with 7% lower risk of ovulatory infertility, and this association was found to be stronger in normal weight women as in this study. In the studies conducted by Wise et al.,[19] moderate physical activity was associated with a small increase in fecundability independent of BMI. In the studies conducted by Kucuk et al.,[20] physical activity during assisted reproduction was associated with better implantation rates and live births. Some recent studies revealed that the impact of physical activity on ovarian reserve parameters was more pronounced in overweight and obese reproductive age women compared to normal weight reproductive age women.[21] Irrespective of the mechanisms involved, all these studies postulated a beneficial effect of regular physical activity on fertility outcomes. The limitations of the study are the limited number of participants studied and the lack of similar studies in this region for comparison.
CONCLUSION
This study demonstrates that lifestyle factors such as physical activity are associated with improved age-specific levels of ovarian reserve markers.[22] The identification of potentially modifiable lifestyle factors such as diet, exercise, smoking, and alcohol will not only improve the general health but also the reproductive health and reduce the treatment costs of assisted reproduction and associated adverse events. Given the tight interaction between energy metabolism and reproduction, physical activity may influence the neurohumoral modulation of the metabolic pathways involved in energy metabolism and reproduction.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Das P, Horton R. Physical activity − Time to take it seriously and regularly. Lancet. 2016;388:1254–5. doi: 10.1016/S0140-6736(16)31070-4. [DOI] [PubMed] [Google Scholar]
- 2.Andersen LB, Mota J, Di Pietro L. Update on the global pandemic of physical inactivity. Lancet. 2016;388:1255–6. doi: 10.1016/S0140-6736(16)30960-6. [DOI] [PubMed] [Google Scholar]
- 3.Anderson K, Nisenblat V, Norman R. Lifestyle factors in people seeking infertility treatment − A review. Aust N Z J Obstet Gynaecol. 2010;50:8–20. doi: 10.1111/j.1479-828X.2009.01119.x. [DOI] [PubMed] [Google Scholar]
- 4.Saremi A, Shavandi N, Dezfolian M. The effect of physical activity on serum levels of anti-mullerian hormone and fertility parameters in reproductive age women. Arak Med Univ J. 2013;16:51–8. [Google Scholar]
- 5.Orio F, Muscogiuri G, Ascione A, Marciano F, Volpe A, La Sala G, et al. Effects of physical exercise on the female reproductive system. Minerva Endocrinol. 2013;38:305–19. [PubMed] [Google Scholar]
- 6.Fontana R, Torre SD. The deep correlation between energy metabolism and reproduction: A view on the effects of nutrition for women fertility. Nutrients. 2016;87:1–34. doi: 10.3390/nu8020087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gao Q, Horvath TL. Cross-talk between estrogen and leptin signaling in the hypothalamus. Am J Physiol Endocrinol Metab. 2008;294:E817–26. doi: 10.1152/ajpendo.00733.2007. [DOI] [PubMed] [Google Scholar]
- 8.Reseland JE, Anderssen SA, Solvoll K, Hjermann I, Urdal P, Holme I, et al. Effect of long-term changes in diet and exercise on plasma leptin concentrations. Am J Clin Nutr. 2001;73:240–5. doi: 10.1093/ajcn/73.2.240. [DOI] [PubMed] [Google Scholar]
- 9.Golbidi S, Laher I. Exercise induced adipokine changes and the metabolic syndrome. J Diabetes Res. 2014;2014:726861. doi: 10.1155/2014/726861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mitchell M, Armstrong DT, Robker RL, Norman RJ. Adipokines: Implications for female fertility and obesity. Reproduction. 2005;130:583–97. doi: 10.1530/rep.1.00521. [DOI] [PubMed] [Google Scholar]
- 11.Sharma RS, Bhargava PM, Nomita C, Saxena NC. ICMR/NAMS. National Guidelines for Accreditation, Supervision and Regulation of ART Clinics in India. New Delhi: ICMR/NAMS; 2005. Guidelines for ART Clinics in India. [Google Scholar]
- 12.Broer SL, Mol BW, Hendriks D, Broekmans FJ. The role of antimullerian hormone in prediction of outcome after IVF: Comparison with the antral follicle count. Fertil Steril. 2009;91:705–14. doi: 10.1016/j.fertnstert.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 13.Broekmans FJ, Kwee J, Hendriks DJ, Mol BW, Lambalk CB. A systematic review of tests predicting ovarian reserve and IVF outcome. Hum Reprod Update. 2006;12:685–718. doi: 10.1093/humupd/dml034. [DOI] [PubMed] [Google Scholar]
- 14.Nelson SM, Yates RW, Lyall H, Jamieson M, Traynor I, Gaudoin M, et al. Anti-Müllerian hormone based approach to controlled ovarian stimulation for assisted conception. Hum Reprod. 2009;24:867–75. doi: 10.1093/humrep/den480. [DOI] [PubMed] [Google Scholar]
- 15.Rich-Edwards JW, Spiegelman D, Garland M, Hertzmark E, Hunter DJ, Colditz GA, et al. Physical activity, body mass index, and ovulatory disorder infertility. Epidemiology. 2002;13:184–90. doi: 10.1097/00001648-200203000-00013. [DOI] [PubMed] [Google Scholar]
- 16.Warren MP, Perlroth NE. The effects of intense exercise on the female reproductive system. J Endocrinol. 2001;170:3–11. doi: 10.1677/joe.0.1700003. [DOI] [PubMed] [Google Scholar]
- 17.Gudmundsdottir SL, Flanders WD, Augestad LB. Physical activity and fertility in women: The North-Trondelag Health Study. Hum Reprod. 2009;24:3196–204. doi: 10.1093/humrep/dep337. [DOI] [PubMed] [Google Scholar]
- 18.Chavarro JE, Rich-Edwards JW, Rosner BA, Willett WC. Diet and lifestyle in the prevention of ovulatory disorder infertility. Obstet Gynecol. 2007;110:1050–8. doi: 10.1097/01.AOG.0000287293.25465.e1. [DOI] [PubMed] [Google Scholar]
- 19.Wise LA, Cramer DW, Hornstein MD, Ashby RK, Missmer SA. Physical activity and semen quality among men attending an infertility clinic. Fertil Steril. 2011;95:1025–30. doi: 10.1016/j.fertnstert.2010.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kucuk M, Doymaz F, Urman B. Effect of energy expenditure and physical activity on the outcomes of assisted reproduction treatment. Reprod Biomed. 2010;20:274–9. doi: 10.1016/j.rbmo.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 21.Surekha T, Himabindu Y, Sriharibabu M, Pandey AK. Impact of physical activity on ovarian reserve markers in normal, overweight and obese reproductive age women. Indian J Physiol Pharmacol. 2014;58:162–5. [PubMed] [Google Scholar]
- 22.Dólleman M, Verschuren WM, Eijkemans MJ, Dollé ME, Jansen EH, Broekmans FJ, et al. Reproductive and lifestyle determinants of anti-Müllerian hormone in a large population-based study. J Clin Endocrinol Metab. 2013;98:210615. doi: 10.1210/jc.2012-3995. [DOI] [PubMed] [Google Scholar]


