Abstract
Healthcare is expensive for a large proportion of the population in spite of high per capita income and good health insurance penetration. In an effort to reduce cost of the procedure, reprocessing of devices was started in the late 1970s. Reprocessing practice includes various measures such as proper cleaning, disinfection, and sterilization procedures. As reprocessing is aimed at reducing cost, there is a potential risk of compromising patient safety due to cross contamination after inadequate sterilization. There is also risk of performance alteration of urological reprocessed devices during sterilization/disinfection processing. Therefore, there is a need for formulating proper guidelines to decide methods of reprocessing for various urological equipment. There is also need to discuss the problematic areas that urologists face and to find their solutions. A PubMed search was made in September 2016, using key words “reprocessing of medical devices,” “Single Use Devices,” “methods of reprocessing of devices in clinical practice,” “use of formalin chamber,” “urological disposable sterilization,” etc., After excluding duplicates, all English articles were reviewed by title and abstract. Full texts of selected articles were obtained, and these articles were cross-referenced to find any other related articles. All the articles were reviewed. A product can be reused if it can be economically reprocessed with validated protocols with preservation of its function. There is no reason to discard it after one use. This practice is useful for controlling economics of a urological case and to reduce the financial burden. Current Food and Drug Administration guidelines are stringent. The contamination described to test the sterilization process in the suggested guidelines actually does never exist in clinical practice. Therefore, new guidelines considering the clinical practice scenario are desirable.
Key Words: Reprocessing, sterilization, urological devices
INTRODUCTION
In last two decades, there has been an increase in the number of urological endoscopic procedures because of increase in diagnostic facilities. There is also an increase in the number of operations being performed using minimally invasive techniques. A variety of endoscopes and accessories are now available with the advent of minimally invasive urology. However, these instruments are expensive. Some of them are designed for multiple uses after reprocessing while many of them are designed for single-use only. It is not possible to use one instrument for a patient and then dispose it off permanently due to the cost factor. This disturbs a whole economics of a surgical case.
The reuse of single-use medical devices began in the late 1970s.[1] Reuse of single-use devices (SUDs) increased as a cost-saving measure. Approximately, 20%–30% of the US hospitals reported that they reuse at least one type of SUD.[2] Reuse of SUDs involves regulatory, ethical, medical, legal and economic issues and has been extremely controversial for more than two decades. A reused SUD will have to comply with the same regulatory requirements of the device when it was originally manufactured.[3]
Currently, in health-care facilities, hospitals or urologists are using in-house facilities to reprocess reusable medical devices. In clinical practice, many of urologists are also reprocessing disposable medical devices that have been approved by manufacturers for single-use. They are reusing these devices several times on additional patients and in most of the times, without notifying patients that the device may have already been used.
On the other side, this practice can compromise patient safety. Patient to patient transmission of infection from cross contamination has been well documented after improper disinfection of urological equipment.[4] The transmission of infectious organisms is widespread within a hospital or clinical setting. Hospital-acquired infection with resistant bacteria and viruses are increasing due to irresponsible use of antibiotics.[5,6]
There has been extensive debate regarding the practice of reprocessing medical devices that have been designed, manufactured, and recommended for single-use only by manufacturers. There are studies showing pros and cons of using reprocessed SUDs.[7] This practice raises two patient safety concerns. One, whether the SUDs can be adequately cleaned and sterilized for use in other patients, and two, whether attempts to clean and sterilize these devices may affect their performance or may lead to product failure. With the reuse of reprocessed devices, important things to consider are providing equal protection to patients from infections, calculated risks to patients, need of informed consent, regulatory fairness, and proper monitoring of reprocessing units.
With increasing workloads in urologic endoscopy even developed countries which have high per capita income and good health insurance penetration are reusing the devices after reprocessing. There is a need for developing “guidelines for reprocessing” for developing countries with poor health insurance penetration where economics of a surgical case becomes very important. There is also need for the development of guidelines for the choice of appropriate methods of reprocessing with relate to the risks of infection associated with the particular procedure, the availability and affordability of the proper equipment and the time available for reprocessing.
Ahuja and Tandon did a survey regarding disinfection practice and reuse of single-use accessories by sending written questionnaire. They found that only 38.7% of those responded were carrying out reprocessing according to specified protocols. They also found that more than 90% of respondents were ready to reuse SUDs after reprocessing.[8]
Therefore, it is critical to formulate guidelines to achieve maximum cleaning, disinfection, and sterilization of equipment used in urological practice. It is also important that proper methods are used to process instruments and devices. Furthermore, staff responsible for it should be well-trained, skilled, and thorough. This ensures that each device used on every patient has been reliably sterilized. These processes should be monitored on a continual basis.
In this article, we will discuss a brief review of sterilization and disinfection processes used for single and multi-use medical devices and instruments employed in urological practice. Problematic areas faced by urologists in day-to-day clinical practice will also be discussed. Industry and hospital standards will be discussed with the emphasis on the outcome for the patient in developing countries.
SPAULDING CLASSIFICATION SCHEME
It is Food and Drug Administration (FDA) approved classification scheme to describe the items being used in the health-care facilities on the basis of the potential risk of infection caused by the use of the device. Critical items are the objects that enter sterile tissue or the vascular system. This category includes ureteric catheters, double lumen ureteric stents, and requires sterilization only. Semi-critical items are the objects that come in contact with mucous membranes or nonintact skin. This category includes cystoscopes, ureteroscopes, nephroscopes, and guide wires and they require either sterilization or high-level disinfection.[9]
PROCESS OVERVIEW
SUD is a device that is intended for one use or on a single patient during a single procedure. The reusable medical device is a device intended for repeated use either on the same or different patients, with appropriate cleaning and other reprocessing between uses.[10]
Reprocessing is defined as validated processes used to render a medical device, which has been previously used or contaminated, fit for a subsequent single-use.[10] These processes are designed to remove soil and contaminants by cleaning and to inactivate microorganisms by disinfection or sterilization. During reprocessing, it is always preferable to consider the worst-case scenario with contamination of devices by most resistant microbes during their clinical use. Another important thing to consider is the ability of the device material to withstand repeated disinfection/sterilization. Cleaning, disinfection and sterilization are the important steps of reprocessing.
Cleaning: The initial and most important step of reprocessing
Cleaning is the removal of visible soil (e.g., organic and inorganic material) from objects and surfaces and normally is accomplished manually or mechanically using water with detergents or enzymatic products.[11] Thorough cleaning is essential before high-level disinfection and sterilization because inorganic and organic materials that remain on the surfaces of instruments interfere with the effectiveness of these processes. It has been shown that cleaning reduces the level of microbial contamination by 4–6 log10.[12]
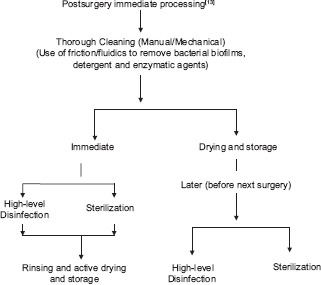
Organic matter in the form of serum, blood, pus, or lubricant material can interfere with the antimicrobial activity of disinfectants. This interference occurs first by a chemical reaction between the germicide and the organic matter resulting in a complex that is less germicidal or nongermicidal, leaving less of the active germicide available for disinfection/sterilization. Second, organic material can protect microorganisms from attack by acting as a physical barrier.[14,15] This emphasizes the importance of meticulous cleaning of medical devices before any sterilization or disinfection procedure because both organic and inorganic soils are easily removed by washing.
Mechanical cleaning process also removes the biofilms over the surface of devices. Biofilms are microbial communities that are tightly attached to surfaces and cannot be easily removed. Bacteria within the biofilm are up to 1000 times more resistant to antimicrobials than are the same bacteria in suspension.[16]
Therefore, reprocessing starts with prompt initial cleaning measures to prevent drying of soil and contaminants in and on the device. Immediate, thorough cleaning is the most important step in reprocessing. Any delay in reprocessing may increase the challenge to disinfection or sterilization.
Disassembly and reassembly
For devices which have removable parts, reprocessing must include prior disassembly and reassembly of the device to facilitate cleaning.[13] Reassembly should be done before or after the sterilization process as advised by manufacturer.
Methods of cleaning
Cleaning can be done manually or mechanically. Manual cleaning is done where either mechanical units are not available or for fragile instruments. The two essential components of manual cleaning are friction and fluidics. Friction is rubbing/scrubbing the soiled area with a brush while fluidics is used to remove soil and debris from internal channels after brushing and when the design does not allow passage of a brush through a channel. It requires minimum 60 s of rapid water flow through all the internal channels.[17] Automated/mechanical cleaning can be done with the help of dishwasher, utensil washer-sanitizer, ultrasonic cleaner, etc., Mechanical cleaning equipment may increase productivity, and effectiveness, and decrease worker exposure.[18,19]
The effectiveness of cleaning process can be increased with additional use of cleaning agents and enzymes. Cleaning agents (e.g., detergents such as quaternary ammonium compounds and enzymatic detergents) that have been demonstrated to be compatible with the device, and are effective in cleaning the device can be used.[20] A near-neutral pH detergent solution provides the best material compatibility profile and good soil removal. Enzymes (e.g., amylase, lipase, and proteases) can be added to cleaning agents to assist in removing organic material as they attack proteins of blood and pus.[21]
There is currently no standard to define when a device is “clean” and cleanliness is decided by visual inspection only. At a minimum, a cleaning process should reduce the natural bioburden and remove organic/inorganic contaminants. Thus devices, when sterilized, will have a good sterility assurance level (SAL 10−6).[22]
Rinsing, drying, and storage
Rinsing is to be done to remove chemical residues used during cleaning/reprocessing so that they no longer interfere with subsequent reprocessing steps.[13] Published guidelines allow tap water for rinsing rather than using only sterile water or filtered water.[23,24] Saline is not recommended for final rinsing as it leads to corrosion of certain devices.[13]
As the devices will be wet at the end of reprocessing, active device drying is advisable. Moisture remaining on devices after sterilization/disinfection procedures can compromise the integrity of packages and effectiveness of seals. Drying reduces or eliminates recontamination of unwrapped devices which have been reprocessed with high-level disinfection or sterilization.[25]
A contaminated endoscope should never be placed in the carrying case because the carrying case can also become contaminated. The carrying case used to transport clean and reprocessed endoscopes outside the health-care environment should be separate from a carrying case used to store and transport the instrument within the health-care facility [Figure 1].[26]
Figure 1.
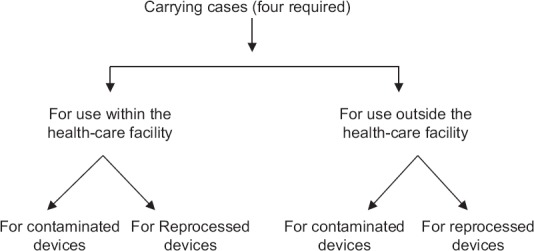
Flow chart showing how to handle instrument carrying cases
High-level disinfection
Disinfection describes a process that eliminates many or all pathogenic microorganisms, except bacterial spores, on inanimate objects.[11] In health-care settings, objects usually are disinfected by liquid chemicals. Unlike sterilization, disinfection is not sporicidal. A few disinfectants will kill spores with prolonged exposure times (3–12 h); these are called chemical sterilants. High-level disinfection is a lethal process utilizing a sterilant under less than sterilizing conditions. The process kills all forms of microbial life except for large numbers of bacterial spores.[11] Descending order of resistance of microorganism to germicide chemical from most resistant to least resistant is bacterial spores, mycobacteria, nonlipid viruses, fungi, vegetative bacteria, and lipid viruses.
Commonly used high-level disinfectant in urology practice is glutaraldehyde and ortho-phthalaldehyde (OPA). Rational use of disinfectants is recommended in an attempt to prevent the development of resistant microbes.[27] Ethyl alcohol is not recommended for sterilizing medical and surgical materials principally because it lacks sporicidal action. It also cannot penetrate protein-rich materials.[28]
Formaldehyde, when used as a water-based solution called formalin, (37% formaldehyde by weight), is a bactericide, tuberculocide, fungicide, virucide, and sporicide.[29,30] Paraformaldehyde is a solid polymer of formaldehyde. It can be vaporized by heat and is laminar flow can be used for the gaseous decontamination of biologic safety cabinets. In spite of this, its use is limited by its irritating fumes and its pungent odor even at very low levels (<1 ppm). It also causes skin irritation such as dermatitis and itching, respiratory problems such as asthma. It may have a role as a suspected human carcinogen linked to nasal cancer and lung cancer.[31]
Glutaraldehyde has gained wide acceptance as a high-level disinfectant for urological equipment because of its advantages such as excellent biocidal properties; activity in the presence of organic matter; and noncorrosive action to endoscopic equipment. It causes no residual damage to instruments with a lens, rubber, or plastics.[32]
When the solution is “activated” by use of alkalinating agents to pH 7.5–8.5, it effectively kills vegetative bacteria in <2 min and Mycobacterium tuberculosis, fungi, and viruses including HIV, hepatitis B and hepatitis C in <10 min. Spores of Bacillus species are rapidly killed by glutaraldehyde, however, spores of Bacillus and Clostridium species takes 3 h.[33] 20 min at room temperature is considered the minimum exposure time needed to reliably kill mycobacteria and other vegetative bacteria with >2% glutaraldehyde.[34] Once activated, these solutions have a shelf-life of minimally 14 days. Novel glutaraldehyde formulations have overcome the problem of rapid loss of activity, and they can be used up to 28–30 days while maintaining excellent microbicidal activity.[35,36]
Acute or chronic exposure of glutaraldehyde can result in skin irritation or dermatitis. Epistaxis, allergic contact dermatitis, asthma, and rhinitis also have been reported in health-care workers exposed to glutaraldehyde.[37,38,39]
OPA is a high-level disinfectant that has received FDA clearance in October 1999. It has an excellent microbicidal activity and a superior mycobactericidal activity with a required exposure time of 12 min only. It has excellent stability over a wide pH range (pH 3–9), is not a known irritant to the eyes and nasal passages, does not require exposure monitoring, has a barely perceptible odor, and requires no activation.[40] However, OPA is not recommended for reprocessing urologic instrumentation as cases of anaphylaxis-like reaction after cystoscopy has been reported where the scope was reprocessed using OPA.[41]
Sterilization
Sterilization describes a process that destroys or eliminates all forms of microbial life and is carried out in health-care facilities by physical or chemical methods.[11] Medical devices that have contact with sterile body tissues or fluids should be sterile because any microbial contamination could result in disease transmission. Steam under pressure, ethylene oxide (ETO) gas, hydrogen peroxide gas plasma, ozone gas, and liquid chemicals are the principal sterilizing agents used by urologists in health-care facilities.
SAL of the product is defined as the probability of a single viable microorganism occurring on a product after sterilization. SAL is normally expressed as 10−n. In short, a SAL is an estimate of lethality of the entire sterilization process. For reusable devices that are intended to be used sterile, they should attain a SAL of 10−6.[42]
Factors affecting the efficacy of sterilization of device are bioburden–number and location of microorganism, prior cleaning, pathogen type, presence of protein and salt, biofilm accumulation, lumen length and diameter.[43,44]
If equipment are heat resistant, the recommended sterilization process is steam sterilization, because it has the largest margin of safety due to its reliability, consistency, and lethality. However, reprocessing heat sensitive items requires the use of low-temperature sterilization technologies (LTSTs).[45]
Moist heat steam sterilization in the form of saturated steam under pressure is the most widely used and acceptable sterilization method. It is nontoxic, inexpensive, rapidly microbicidal, sporicidal, and rapidly penetrative.[46,47] It can be used on all critical and semicritical items that are heat and moisture resistant. Minimum exposure periods for sterilization are 30 min at 121°C in a gravity displacement sterilizer or 4 min at 132°C in a prevacuum sterilizer.
“Flash” steam sterilization is a sterilization of an object a 132°C for 3 min at 27–28 lbs of pressure. It can be used for processing cleaned patient care items and can also be used when there is insufficient time to sterilize an item by the preferred method. Because of the potential for serious infections, flash sterilization is not recommended for implantable devices (i.e., devices to be placed in the human body).[48]
Low-temperature sterilization technologies
This includes ETO gas sterilization (ETO-CFC, ETO-CO2, ETO-HCFC, and 100% ETO) and newer LTSTs such as hydrogen peroxide gas plasma, vaporized hydrogen peroxide sterilization, and ozone sterilization. All LTSTs have limitations. First of all, they demonstrate a significant number of failures in the presence of serum or salt as these agents provide protection for spores and bacteria. In addition, it has been also shown that the problem increases exponentially with a decrease in lumen diameter and increase in lumen length. When microorganisms are mixed with body fluid, they form physical crystals that protect the microorganisms. However, with exposure for 1 min to water, the salts dissolve, and the protective effect disappears. This shows the importance of meticulous cleaning before sterilization.[49,50,51]
ETO “Gas” Sterilization has excellent microbicidal activity, and it inactivates all microorganisms and most of the bacterial spores. It is commonly used in healthcare facilities to sterilize critical items as well as semi-critical items and items that are moisture or heat sensitive.[52]
The main advantage is that it can sterilize heat- or moisture-sensitive medical equipment without deleterious effects on the material used in the medical devices. Other advantages are that (1) it is very effective at killing microorganisms, (2) penetrates medical packaging and many plastics, (3) compatible with most medical materials, and (4) cycle is easy to control and monitor. Main disadvantages are the lengthy cycle time and aeration time, the cost per cycle, the possibility of damage to expensive equipment and its potential hazards to patients and staff.[49,50] Extended periods of aeration is required because of the ability of ETO to penetrate.
Acute exposure to ETO may result in irritation (e.g., to skin, eyes, gastrointestinal, or respiratory tracts) and central nervous system depression. Chronic inhalation has been linked to the formation of cataracts, cognitive impairment, neurologic dysfunction, and disabling polyneuropathies.[53,54,55]
Ozone sterilization
Ozone was cleared by FDA in August 2003 for processing reusable medical devices. It converts back to oxygen and water vapor at the end of the cycle before being exhausted into the room. The duration of the sterilization cycle is about 4 h and 15 m, and it occurs at 30°C–35°C. It has shown a good microbicidal activity against a variety of microorganisms. Disadvantages are small sized (4ft3) sterilization chamber, limited microbicidal efficacy data due to its limited use.[56]
PROBLEMATIC AREAS
High standards set by Food and Drug Administration
FDA has set very high criteria for standardization of disinfection and sterilization. FDA requires the presence of 5% fetal calf serum dried onto the devices inoculated with 106 colony forming units of most resistant test organisms to test the efficacy of disinfection and sterilization. Cleaning before sterilization is not allowed in the demonstration of sterilization efficacy.[57] In the presence of these criteria, almost all of the sterilization processes will fail to reliably inactivate the microbial load.[58]
While in actual clinical setting, such strict criteria are never seen. In general, used medical devices are contaminated with a relatively low bioburden of organisms. Nystrom evaluated medical instruments that are used in general surgical/urosurgical operations.[59] He found that 62% of the instruments were contaminated with <101 organisms after use, 82% with <102, and 91% with <103. After being washed in an instrument washer, more than 98% of the instruments had <101 organisms, and none >102 organisms. In another study of rigid-lumen medical devices, the bioburden on both the inner and outer surface of the lumen ranged from 101 to 104 organisms per device. After cleaning, 83% of the devices had a bioburden of ≤102 organisms.[59,60]
Role of manufacturers
Manufacturers of urology devices never recommend reuse of SUDs. FDA policy states that they can be reused after standard reprocessing techniques if the product following reprocessing techniques complies with the manufacturers' sterilization criteria. As we have already discussed, FDA has set very high criteria for standardization of reprocessing techniques. Such microbial load practically never exists in actual clinical settings to justify reuse policy. However, considering the stringent criteria set by FDA for manufacturers, it is practically difficult for manufacturers to recommend reprocessing and reuse. Therefore, no proper instructions are given on the labeling for proper disassembly, reassembly, cleaning, and reprocessing. Using a new device, for each new case, as per manufacturers' recommendation, increases final cost of surgery and it creates a huge economic load in health-care industry.
Medicolegal issues and fear psychosis of urologists
As no standard guidelines available regarding reprocessing of SUDs, there is high chance of developing fear psychosis among urologists due to associated medicolegal issues.
Sterilization/disinfection of devices with long and narrow lumen
Immediate cleaning of any narrow-lumen medical device used in patient care presents a major challenge to reprocessing.[61,62] It has been shown that retro-flushing with the narrow lumen provided adequate cleaning. If reprocessing was delayed for more than 24 h, retro-flush cleaning was no longer effective. Physical properties of the object such as presence of crevices, hinges, and lumens in the devices also affect the disinfection and sterilization processes.
Use of formalin chambers
Formaldehyde vapor cabinets have been in use since the end of 19th century. It was used regularly in the hospitals until the end of 1988. These cabinets operate according to Janet's principle of sterilization by formaldehyde gas at ambient temperature and at appropriate pressures over a period of 24 h.[63] However, the release of gas from paraformaldehyde tablets (placed on the lower tray) is slow and produces a low partial pressure of gas. Temperature and humidity within the cabinet could not be controlled, and there is no facility for effective circulation and extraction of formaldehyde gas. The cabinet contains perforated drawers, which may prevent diffusion of gas especially when they contain instruments. Hence, the microbicidal quality of this procedure is not known.[64]
No proper manufacturers' guidelines available for the use of these cabinets and for the effective production of gas inside it. The formaldehyde steam sterilization system has not been cleared by FDA for use and its use as a high-level disinfectant is not recommended at all.
High workload and rapid surgeries
In the centers with high number of urological cases per day with limited sets of instrument, it is not possible to sterilize each and every item in between two cases because of time factor. In such cases, high-level disinfection is recommended. If glutaraldehyde is used for disinfection, there should be minimum 30 min gap between two cases for effective high-level disinfection.
CONCLUSIONS
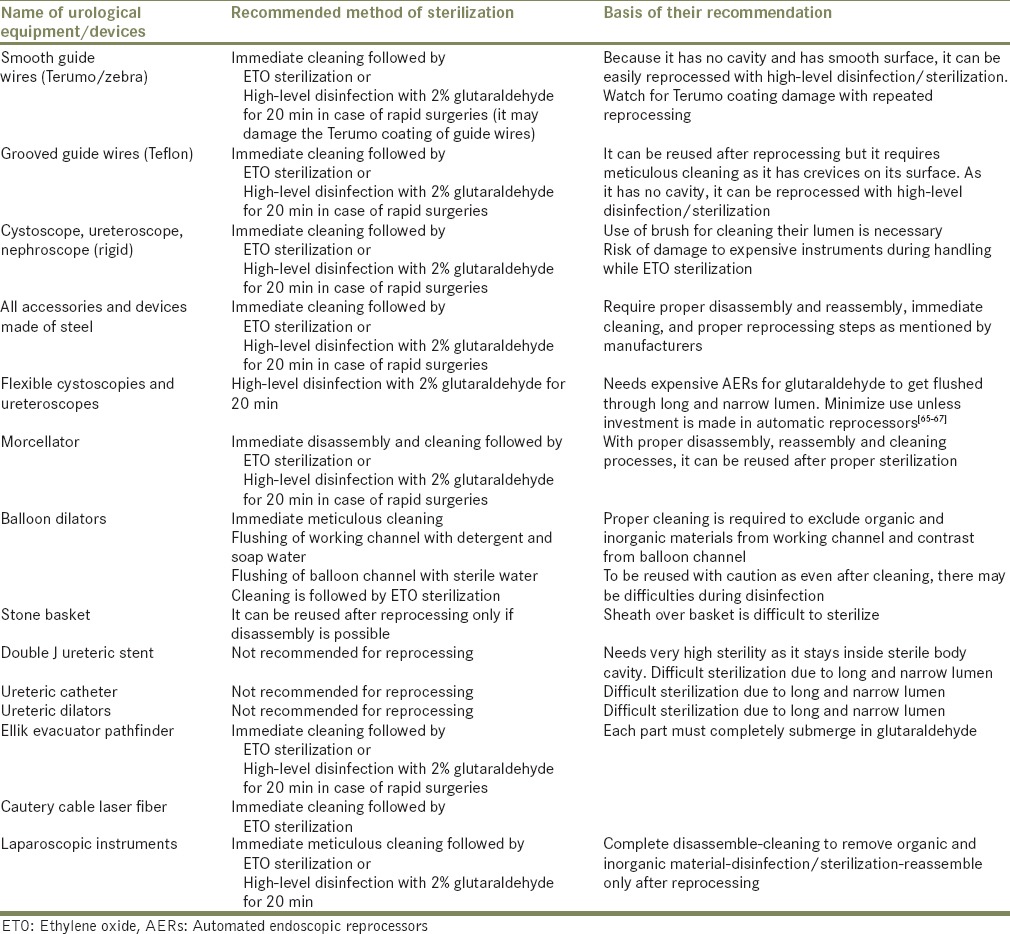
If FDA guidelines are strictly followed, disinfection and sterilization can ensure the safe reuse of reusable devices and many of the single-use urological devices [Table 1].
Table 1.
Urological equipment and recommended method of their reprocessing
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Dunnigan A, Roberts C, McNamara M, Benson DW, Jr, Benditt DG. Success of re-use of cardiac electrode catheters. Am J Cardiol. 1987;60:807–10. doi: 10.1016/0002-9149(87)91028-9. [DOI] [PubMed] [Google Scholar]
- 2.Greene VW. Reuse of disposable devices. In: Mayhall CG, editor. Infection Control and Hospital Epidemiology. Philadelphia: Lippincott Williams and Wilkins; 1999. pp. 1201–8. [Google Scholar]
- 3.Enforcement Priorities for Single-use Devices Reprocessed by Third Parties and Hospitals. Rockville, MD: Food and Drug Administration; 2000. Food and Drug Administration. [Google Scholar]
- 4.Schultz JK. Decontamination: Recommended practices. In: Reichert M, Young JH, editors. Sterilization Technology for the Health Care Facility. Gaithersburg, MD: Aspen Publication; 1997. pp. 10–20. [Google Scholar]
- 5.Singh J, Bhatia R, Gandhi JC, Kaswekar AP, Khare S, Patel SB, et al. Outbreak of viral hepatitis B in a rural community in India linked to inadequately sterilized needles and syringes. Bull World Health Organ. 1998;76:93–8. [PMC free article] [PubMed] [Google Scholar]
- 6.Eickhoff TC. An outbreak of surgical wound infections due to Clostridium perfringens. Surg Gynecol Obstet. 1962;114:102–8. [PubMed] [Google Scholar]
- 7.Avitall B, Khan M, Krum D, Jazayeri M, Hare J. Repeated use of ablation catheters: A prospective study. J Am Coll Cardiol. 1993;22:1367–72. doi: 10.1016/0735-1097(93)90544-b. [DOI] [PubMed] [Google Scholar]
- 8.Ahuja V, Tandon RK. Survey of gastrointestinal endoscope disinfection and accessory reprocessing practices in the Asia-Pacific region. J Gastroenterol Hepatol. 2000;15(Suppl S3):G78–81. doi: 10.1046/j.1440-1746.2000.02270.x. [DOI] [PubMed] [Google Scholar]
- 9.Spaulding EH. The role of chemical disinfection in the prevention of nosocomial infections. In: Brachman PS, Eickoff TC, editors. Proceedings of the International Conference on Nosocomial Infections, 1970. Chicago: American Hospital Association; 1971. pp. 254–74. [Google Scholar]
- 10.Spaulding EH. Chemical disinfection of medical and surgical materials. In: Lawrence C, Block SS, editors. Disinfection, Sterilization, and Preservation. Philadelphia: Lea and Febiger; 1968. pp. 517–31. [Google Scholar]
- 11.Pflug IJ. Ch. 1-3. 7th ed. Minneapolis: Environmental Sterilization Laboratory; 1990. Microbiology and Engineering of sterilization Processes. [Google Scholar]
- 12.Rutala WA, Weber DJ. FDA labeling requirements for disinfection of endoscopes: A counterpoint. Infect Control Hosp Epidemiol. 1995;16:231–5. doi: 10.1086/647095. [DOI] [PubMed] [Google Scholar]
- 13.Ulatowski TA. FDA: Reuse of single-use devices. In: Rutala WA, editor. Disinfection, Sterilization and Antisepsis: Principles, Practices, Challenges, and New Research. Washington, DC: Association for Professionals in Infection Control and Epidemiology; 2004. pp. 15–23. [Google Scholar]
- 14.Lewis DL, Arens M. Resistance of microorganisms to disinfection in dental and medical devices. Nat Med. 1995;1:956–8. doi: 10.1038/nm0995-956. [DOI] [PubMed] [Google Scholar]
- 15.Muscarella LF. Sterilizing dental equipment. Nat Med. 1995;1:1223–5. doi: 10.1038/nm1295-1223b. [DOI] [PubMed] [Google Scholar]
- 16.Vickery K, Pajkos A, Cossart Y. Removal of biofilm from endoscopes: Evaluation of detergent efficiency. Am J Infect Control. 2004;32:170–6. doi: 10.1016/j.ajic.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 17.Reichert M. Preparation of supplies for terminal sterilization. In: Reichert M, Young JH, editors. Sterilization Technology for the Health Care Facility. Gaithersburg, MD: Aspen Publication; 1997. pp. 36–50. [Google Scholar]
- 18.Miller CH, Riggen SD, Sheldrake MA, Neeb JM. Presence of microorganisms in used ultrasonic cleaning solutions. Am J Dent. 1993;6:27–31. [PubMed] [Google Scholar]
- 19.Schultz JK. Decontamination alternative. Infect Control Hosp Epidemiol. 1990;11:8–9. doi: 10.1086/646069. [DOI] [PubMed] [Google Scholar]
- 20.Lee CH, Cheng SM, Humar A. Acute febrile reactions with hypotension temporally associated with the introduction of a concentrated bioenzyme preparation in the cleaning and sterilization process of endomyocardial bioptones. Infect Control Hosp Epidemiol. 2000;21:102. [Google Scholar]
- 21.Hutchisson B, LeBlanc C. The truth and consequences of enzymatic detergents. Gastroenterol Nurs. 2005;28:372–6. doi: 10.1097/00001610-200509000-00003. [DOI] [PubMed] [Google Scholar]
- 22.Lipscomb IP, Sihota AK, Botham M, Harris KL, Keevil CW. Rapid method for the sensitive detection of protein contamination on surgical instruments. J Hosp Infect. 2006;62:141–8. doi: 10.1016/j.jhin.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 23.American Society for Gastrointestinal Endoscopy. Position statement: Reprocessing of flexible gastrointestinal endoscopes. Gastrointest Endosc. 1996;43:541–6. doi: 10.1016/s0016-5107(96)81581-1. [DOI] [PubMed] [Google Scholar]
- 24.Alvarado CJ, Reichelderfer M. APIC guideline for infection prevention and control in flexible endoscopy. Association for Professionals in Infection Control. Am J Infect Control. 2000;28:138–55. [PubMed] [Google Scholar]
- 25.Nelson DB, Jarvis WR, Rutala WA, Foxx-Orenstein AE, Isenberg G, Dash GR, et al. Multi-society guideline for reprocessing flexible gastrointestinal endoscopes. Society for Healthcare Epidemiology of America. Infect Control Hosp Epidemiol. 2003;24:532–7. doi: 10.1086/502237. [DOI] [PubMed] [Google Scholar]
- 26.Rutala WA, Weber DJ. Guidelines for Disinfection and Sterilization in Healthcare Facilities. 2008:16–7. [Google Scholar]
- 27.Murtough SM, Hiom SJ, Palmer M, Russell AD. A survey of rotational use of biocides in hospital pharmacy aseptic units. J Hosp Infect. 2002;50:228–31. doi: 10.1053/jhin.2001.1155. [DOI] [PubMed] [Google Scholar]
- 28.Nye RN, Mallory TB. A note on the fallacy of using alcohol for the sterilization of surgical instruments. Boston Med Surg J. 1923;189:561–3. [Google Scholar]
- 29.McCulloch EC, Costigan S. A comparison of the efficiency of phenol, liquor cresolis, formaldehyde, sodium hypochlorite and sodium hydroxide against Eberthella typhi at various temperatures. J Infect Dis. 1936;59:281–4. [Google Scholar]
- 30.Sagripanti JL, Eklund CA, Trost PA, Jinneman KC, Abeyta C, Jr, Kaysner CA, et al. Comparative sensitivity of 13 species of pathogenic bacteria to seven chemical germicides. Am J Infect Control. 1997;25:335–9. doi: 10.1016/s0196-6553(97)90026-2. [DOI] [PubMed] [Google Scholar]
- 31.Occupational Safety and Health Administration. Formaldehyde: OSHA Fact Sheet: Occupational Safety and Health Administration. 2002 [Google Scholar]
- 32.Cheung RJ, Ortiz D, DiMarino AJ., Jr GI endoscopic reprocessing practices in the United States. Gastrointest Endosc. 1999;50:362–8. doi: 10.1053/ge.1999.v50.99615. [DOI] [PubMed] [Google Scholar]
- 33.Babb JR, Bradley CR, Ayliffe GA. Sporicidal activity of glutaraldehydes and hypochlorites and other factors influencing their selection for the treatment of medical equipment. J Hosp Infect. 1980;1:63–75. doi: 10.1016/0195-6701(80)90033-x. [DOI] [PubMed] [Google Scholar]
- 34.Scott EM, Gorman SP. Glutaraldehyde. In: Block SS, editor. Disinfection, Sterilization, and Preservation. Philadelphia: Lippincott Williams and Wilkins; 2001. pp. 361–81. [Google Scholar]
- 35.Boucher RM. Potentiated acid 1,5 pentanedial solution – A new chemical sterilizing and disinfecting agent. Am J Hosp Pharm. 1974;31:546–57. [PubMed] [Google Scholar]
- 36.Miner NA, McDowell JW, Willcockson GW, Bruckner NI, Stark RL, Whitmore EJ. Antimicrobial and other properties of a new stabilized alkaline glutaraldehyde disinfectant/sterilizer. Am J Hosp Pharm. 1977;34:376–82. [PubMed] [Google Scholar]
- 37.Beauchamp RO, Jr, St Clair MB, Fennell TR, Clarke DO, Morgan KT, Kari FW. A critical review of the toxicology of glutaraldehyde. Crit Rev Toxicol. 1992;22:143–74. doi: 10.3109/10408449209145322. [DOI] [PubMed] [Google Scholar]
- 38.Corrado OJ, Osman J, Davies RJ. Asthma and rhinitis after exposure to glutaraldehyde in endoscopy units. Hum Toxicol. 1986;5:325–8. doi: 10.1177/096032718600500505. [DOI] [PubMed] [Google Scholar]
- 39.Norbäck D. Skin and respiratory symptoms from exposure to alkaline glutaraldehyde in medical services. Scand J Work Environ Health. 1988;14:366–71. [PubMed] [Google Scholar]
- 40.Hession SM. Endoscope disinfection by ortho-phthalaldehyde in a clinical setting: An evaluation of reprocessing time and costs compared with glutaraldehyde. Gastroenterol Nurs. 2003;26:110–4. doi: 10.1097/00001610-200305000-00005. [DOI] [PubMed] [Google Scholar]
- 41.Sokol WN. Nine episodes of anaphylaxis following cystoscopy caused by Cidex OPA (ortho-phthalaldehyde) high-level disinfectant in 4 patients after cytoscopy. J Allergy Clin Immunol. 2004;114:392–7. doi: 10.1016/j.jaci.2004.04.031. [DOI] [PubMed] [Google Scholar]
- 42.Favero MS. Sterility assurance: Concepts for patient safety. In: Rutala WA, editor. Disinfection, Sterilization and Antisepsis: Principles and Practices in Healthcare Facilities. Washington, DC: Association for Professional in Infection Control and Epidemiology; 2001. pp. 110–9. [Google Scholar]
- 43.Favero MS, Bond WW. Chemical disinfection of medical and surgical materials. In: Block SS, editor. Disinfection, Sterilization, and Preservation. Philadelphia: Lippincott Williams and Wilkins; 2001. pp. 881–917. [Google Scholar]
- 44.Block SS. Disinfection, Sterilization, and Preservation. Philadelphia: Lippincott Williams and Wilkins; 2001. [Google Scholar]
- 45.Rutala WA, Weber DJ. Clinical effectiveness of low-temperature sterilization technologies. Infect Control Hosp Epidemiol. 1998;19:798–804. doi: 10.1086/647730. [DOI] [PubMed] [Google Scholar]
- 46.Adler S, Scherrer M, Daschner FD. Costs of low-temperature plasma sterilization compared with other sterilization methods. J Hosp Infect. 1998;40:125–34. doi: 10.1016/s0195-6701(98)90091-3. [DOI] [PubMed] [Google Scholar]
- 47.Agalloco JP, Akers JE, Madsen RE. Moist heat sterilization – myths and realities. PDA J Pharm Sci Technol. 1998;52:346–50. [PubMed] [Google Scholar]
- 48.Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR. Guideline for prevention of surgical site infection, 1999.Hospital Infection Control Practices Advisory Committee. Infect Control Hosp Epidemiol. 1999;20:250–78. doi: 10.1086/501620. [DOI] [PubMed] [Google Scholar]
- 49.Hüller C, Martiny H, Christiansen B, Rüden H, Gundermann KO. The efficacy of low temperature plasma (LTP) sterilization, a new sterilization technique. Zentralbl Hyg Umweltmed. 1993;194:380–91. [PubMed] [Google Scholar]
- 50.Rutala WA, Gergen MF, Weber DJ. Comparative evaluation of the sporicidal activity of new low-temperature sterilization technologies: Ethylene oxide, 2 plasma sterilization systems, and liquid peracetic acid. Am J Infect Control. 1998;26:393–8. doi: 10.1016/s0196-6553(98)70034-3. [DOI] [PubMed] [Google Scholar]
- 51.Alfa MJ, Olson N, Degagne P, Hizon R. New low temperature sterilization technologies: Microbicidal activity and clinical efficacy. In: Rutala WA, editor. Disinfection, Sterilization, and Antisepsis in Healthcare. Champlain, New York: Polyscience Publications; 1998. pp. 67–78. [Google Scholar]
- 52.Ernst RR, Doyle JE. Sterilization with gaseous ethylene oxide: A review of chemical and physical factors. Biotech Bioeng. 1968;10:5–40. [Google Scholar]
- 53.Fisher AA. Ethylene oxide dermatitis. Cutis. 1984;34:20, 22, 24. [PubMed] [Google Scholar]
- 54.Jay WM, Swift TR, Hull DS. Possible relationship of ethylene oxide exposure to cataract formation. Am J Ophthalmol. 1982;93:727–32. doi: 10.1016/0002-9394(82)90468-8. [DOI] [PubMed] [Google Scholar]
- 55.Salinas E, Sasich L, Hall DH, Kennedy RM, Morriss H. Acute ethylene oxide intoxication. Drug Intell Clin Pharm. 1981;15:384–6. doi: 10.1177/106002808101500509. [DOI] [PubMed] [Google Scholar]
- 56.Berrington AW, Pedler SJ. Investigation of gaseous ozone for MRSA decontamination of hospital side-rooms. J Hosp Infect. 1998;40:61–5. doi: 10.1016/s0195-6701(98)90026-3. [DOI] [PubMed] [Google Scholar]
- 57.Guidance on Premarket Notification [510(k)] Submissions for Sterilizers Intended for Use in Health Care Facilities. Rockville, MD: Food and Drug Administration, Division of General and Restorative Devices; 1993. Food and Drug Administration, Division of General and Restorative Devices. [Google Scholar]
- 58.Graham GS, Riley R. Sterilization manufacturers: Interactions with regulatory agencies. In: Rutala WA, editor. Disinfection, Sterilization, and Antisepsis in Healthcare. Champlain, New York: Polyscience Publications; 1998. pp. 41–8. [Google Scholar]
- 59.Nystrüm B. Disinfection of surgical instruments. J Hosp Infect. 1981;2:363–8. doi: 10.1016/0195-6701(81)90069-4. [DOI] [PubMed] [Google Scholar]
- 60.Rutala WA, Gergen MF, Jones JF, Weber DJ. Levels of microbial contamination on surgical instruments. Am J Infect Control. 1998;26:143–5. doi: 10.1016/s0196-6553(98)80034-5. [DOI] [PubMed] [Google Scholar]
- 61.Alfa MJ, Nemes R. Inadequacy of manual cleaning for reprocessing single-use, triple-lumen sphinctertomes: Simulated-use testing comparing manual with automated cleaning methods. Am J Infect Control. 2003;31:193–207. doi: 10.1067/mic.2003.22. [DOI] [PubMed] [Google Scholar]
- 62.Alfa MJ, Nemes R. Reprocessing of lumened instruments. In: Rutala WA, editor. Disinfection, Sterilization and Antisepsis: Principles, Practices, Challenges, and New Research. Washington DC: Association for Professionals in Infection Control and Epidemiology; 2004. pp. 189–99. [Google Scholar]
- 63.Roncoroni AJ, Casewell MW, Phillips I. The disinfection of clinically contaminated Matburn suction pumps and baby incubators in an 'Aseptor' formalin cabinet. J Hosp Infect. 1980;1:251–9. doi: 10.1016/0195-6701(80)90063-8. [DOI] [PubMed] [Google Scholar]
- 64.Cumberland NS, Botting FG. Formaldehyde vapour cabinets. J Hosp Infect. 1991;19:67–70. doi: 10.1016/0195-6701(91)90130-z. [DOI] [PubMed] [Google Scholar]
- 65.Bradley CR, Babb JR. Endoscope decontamination: Automated vs. manual. J Hosp Infect. 1995;30(Suppl 1):537–42. doi: 10.1016/0195-6701(95)90060-8. [DOI] [PubMed] [Google Scholar]
- 66.Muscarella LF. Advantages and limitations of automatic flexible endoscope reprocessors. Am J Infect Control. 1996;24:304–9. doi: 10.1016/s0196-6553(96)90062-0. [DOI] [PubMed] [Google Scholar]
- 67.Muscarella LF. Automatic flexible endoscope reprocessors. Gastrointest Endosc Clin N Am. 2000;10:245–57. [PubMed] [Google Scholar]


