ABSTRACT
Porphyromonas endodontalis lipopolysaccharide (P.e LPS) is an important initiating factor for periapical inflammation and bone destruction. Matrix metalloproteinase-13 (MMP-13) has been shown to participate in the formation and diffusion of periapical bone lesion in chronic apical periodontitis. Sirtuin 1 (SIRT1) is a key regulator of inflammation in mammalian cells which suppresses the release of inflammatory mediators. This study aimed to explore the role of SIRT1 in regulating MMP-13 expression induced by P.e LPS in osteoblasts. P.e LPS stimulated MMP-13 expression in MC3T3-E1 cells. Knockdown of SIRT1 reinforced the increase of MMP-13mRNA expression induced by P.e LPS. SIRT1 activator resveratrol significantly reduced the expression of MMP-13 and SIRT1 inhibitor EX-527 enhanced the expression of MMP-13. Moreover, SIRT1 activation with resveratrol inhibited acetylation of NF-κB p65 and NF-κB transcriptional activity, which were enhanced by P.e LPS. In addition, NF-κB p65 was involved in P.e LPS-induced MMP-13 expression via directly binding to the MMP-13 promoter. However, SIRT1 activation significantly interfered with this binding. These findings strongly suggest that P.e LPS induces MMP-13 expression in osteoblasts, and SIRT1 suppresses this expression of MMP-13 through targeting NF-κB p65. This provides new insights into understanding the actions of SIRT1 on anti-inflammatory and anti-bone resorption activity.
KEYWORDS: Apical periodontitis, resveratrol, EX-527, histone deacetylase
Introduction
Matrix metalloproteinases (MMPs) are a family of host-derived enzymes responsible for degradation of most extracellular matrix proteins [1]. MMPs comprises 25 structurally and functionally related members that can be classified into five groups: collagenases, gelatinases, stromelysins, minimal MMPs, and membrane-type MMPs [2]. MMP-13 (collagenase-3) belongs to the collagenase subfamily, along with MMP-1 and MMP-8. It is secreted from osteoblasts and macrophages and initiates the degradation of native fibrillar collagen types I, II, and III in bone matrix. These play pivotal roles in bone remodeling activation and pathological bone resorption [3,4]. MMP-13 has been recognized as a potential target for bone resorption–related diseases such as osteoarthritis and periodontitis [5–7]. Recently, emerging evidence suggests that MMP-13 plays a key role in the formation and diffusion of periapical bone lesion in chronic apical periodontitis and the conversion from periapical granuloma to a radicular cyst [8–11].
Porphyromonas endodontalis (P.e) is a member of the gram-negative anaerobic microorganism, and it is considered to be one of the major pathogenic bacteria involved in chronic apical periodontitis [12]. Lipopolysaccharide (LPS) is the main constituent of the outer membrane of P.e; it is an important initiating factor for periapical inflammatory reaction and bone destruction [13]. A previous study in a mouse model indicated that P.e LPS is a pivotal inducer for bone resorption [14] and for the expression of numerous inflammatory mediators, including interleukin (IL)-6, macrophage colony stimulating factor (M-CSF), and Wnt5a in osteoblasts [15–17]. However, there has been little information about the role of P.e LPS in MMP-13 expression in osteoblasts.
Sirtuin 1 (SIRT1), nicotinamide adenine dinucleotide–dependent class III histone deacetylase, is a member of the sirtuins family (SIRT1–SIRT7). SIRT1 plays a fundamental role in various cellular processes, including gene expression, metabolism, stress resistance, and apoptotic cell death [18–22]. Knockdown of SIRT1 promoted tumor necrosis factor alpha (TNF-±;) release induced by LPS and metabolites of ethanol in macrophage cells [23]. SIRT1 overexpression or SIRT1 activation by its activator resveratrol could protect pancreatic ²-cells against cytokine toxicity [24]. These all suggest the anti-inflammatory effects of SIRT1. However, the role of SIRT1 in the MMP-13 expression induced by P.e LPS is still unknown.
Besides histones, SIRT1 can also deacetylate some important transcription factors such as nuclear factor (NF)-κB, p53, and activator protein (AP)-1 [21,25,26]. Among these transcription factors, NF-κB is in charge of the regulation of numerous gene transcriptions involved in inflammatory responses [27]. It was generally considered that the activation of NF-κB pathway depends on the degradation of inhibitor-κB (IκB) and the translocation of NF-κB dimers from cytoplasm to nucleus. However, these alone are not enough to ensure transcriptional initiation. Post-translational modifications of NF-κB subunits are also required [28]. For instance, the acetylation of NF-κB p65 catalyzed by co-activator proteins CBP/p300 is necessary for NF-κB to initiate transcription [29,30]. Previous researches have demonstrated that SIRT1 could deacetylate NF-κB p65 at lysine 310, thus disrupting the balance between p65 acetylation and deacetylation [31]. Therefore, SIRT1 is a possible candidate for the regulation of NF-κB-dependent gene transcription.
The present study examined the level of MMP-13 expression in MC3T3-E1 cells after P.e LPS stimulation. It also investigated the role of SIRT1 on MMP-13 expression and NF-κB signaling pathway in P.e LPS–treated MC3T3-E1 cells. The results showed that P.e LPS stimulation induced a dramatic increase in MMP-13 expression in osteoblasts. Importantly, the findings indicate that SIRT1 suppressed P.e LPS–stimulated MMP-13 expression through the inhibition of NF-κB activity in osteoblasts.
Methods
Bacterial culture and LPS extraction
P.e (ATCC35406) was obtained from the Central Laboratory of Capital Medical University (Beijing, China). The cultures were anerobically grown at 37°C, and LPS preparation was established by the hot phenol–water method, as described previously [32,33]. Finally, isolated P.e LPS was qualitatively analyzed with a limulus amebocyte lysate test, as described in a previous study [14].
Cell culture
Mouse osteoblast-like cells MC3T3-E1 were obtained from the Cell Bank of Chinese Academy of Sciences (Shanghai, China) and were cultured in α-minimum essential medium (α-MEM; Invitrogen, Carlsbad, CA) containing 10% fetal bovine serum (Invitrogen) at 37°C in a humidified atmosphere of 5% CO2/95% air. The cells were subcultured every 3 days by treating the cells with 0.25% trypsin together with 1 mM EDTA in Ca2+, Mg2+ free phosphate-buffered saline (PBS).
SIRT1 small interfering RNA transfection
MC3T3-E1 cells were seeded into six-well plates. The cells were grown until 70–80% confluent and were transiently transfected with SIRT1 siRNA (100 nM; GenePharma, Shanghai, China) using Lipofectamine 2000 transfection reagent (Invitrogen) according to the manufacturer’s instructions. Non-specific siRNA was transfected as a negative control (NC siRNA). After 48 h, the silencing effect of SIRT1 was confirmed by real-time polymerase chain reaction (PCR). The sequences of mouse SIRT1 siRNA and NC siRNA were as follows: SIRT1 siRNA, 5ʹ-GCG GAU AGG UCC AUA UAC UTT-3ʹ (sense) and 5ʹ-AGU AUA UGG ACC UAU CCG CTT-3ʹ (antisense); NC siRNA, 5ʹ-UUC UCC GAA CGU GUC ACG UTT-3ʹ (sense) and 5ʹ-ACG UGA CAC GUU CGG AGA ATT-3ʹ (antisense).
Real-time PCR analysis
Total RNA was isolated and transcribed into complementary DNA using the RNAiso Plus (Takara, Kyoto, Japan) and ReverTra Ace® qPCR RT Master Mix (Toyobo, Tokyo, Japan). Real-time PCR was performed using SYBR® select Master Mix (Applied Biosystems, Foster City, CA). Amplified reactions were quantified on an ABI 7500 real-time PCR system (Applied Biosystems). Sense and antisense primers for mouse MMP-13 or β-actin mRNA were as follows [34]: mouse MMP-13 (forward), 5ʹ-TGG AGT GCC TGA TGT GGG TGA ATA-3ʹ; mouse MMP-13 (reverse), 5ʹ-TGG TGT CAC ATC AGA CCA GAC CTT-3ʹ; mouse β-actin (forward), 5ʹ-CAA TAG TGA TGA CCT GGC CGT-3ʹ; mouse β-actin (reverse), 5ʹ-AGA GGG AAA TCG TGC GTG AC-3ʹ.
Enzyme-linked immunsorbent assay
The conditioned medium was collected, and then measured for MMP-13 concentration by a mouse MMP-13 enzyme-linked immunosorbent assay (ELISA) kit (Boster, Wuhan, China) according to the manufacturer’s protocol.
Western blot analysis
The cells were washed twice with PBS and then lysed, homogenized, sonicated, and centrifuged. The protein concentration in the supernatant was determined by using a protein assay reagent (Bio-Rad, Hercules, CA). Samples and prestained molecular weight markers were separated by SDS-PAGE and transferred to polyvinylidene fluoride (PVDF) membranes (Millipore, Bedford, MA). The membranes were blocked in 5% skim milk in PBS containing 0.05% Tween-20 (PBS-Tween) for 2 h. The membranes were incubated in PBS-Tween containing rabbit anti-MMP-13 antibody (Abcam, Cambridge, MA; diluted at 1:400), rabbit anti-SIRT1 antibody (Abcam; diluted at 1:1,000), rabbit anti-acetyl-p65 antibody (Abcam; diluted at 1:500), or rabbit anti-β-actin antibody (Santa Cruz Biotechnology, Santa Cruz, CA; diluted at 1:1,000) for 1 h at ambient temperature and then incubated overnight at 4°C. The following day, it was incubated for another 2 h at ambient temperature with anti-rabbit secondary horseradish peroxidase-linked antibody (CST, Danvers, MA; diluted at 1:2,000). The peroxidase activity on the PVDF membrane was visualized on X-ray films by using an ECL detection kit (Amersham Pharmacia Biotech, Chalfont St Giles, UK), according to the manufacturer’s protocol.
Dual-luciferase reporter assay
The luciferase plasmid p NF-κB-Luc was obtained from Stratagene (La Jolla, CA). MC3T3-E1 cells were seeded into 35 mm plates at a density of 2.0 × 105 cells/well. After 24 h, the cells were co-transfected with 1 μg of NF-κB-Luc and 0.05 μg of pRL-TK renilla luciferase vector (Promega, Madison, WI) with the aid of LipofectamineTM Reagent (Invitrogen). After 24 h, the cells were pretreated with resveratrol, EX-527, or vehicle for 1 h and then stimulated by 20 μg/mL of P.e LPS or vehicle for 30 min. The cells were harvested and treated with passive lysis buffer according to the dual-luciferase assay manual (Promega). The relative luciferase activity was obtained by normalizing with pRL-TK renilla luciferase signals for individual analysis to eliminate the variations of transfection efficiencies.
Chromatin immunoprecipitation assay
Subconfluent MC3T3-E1 cells were pretreated with resveratrol, EX-527, or vehicle for 1 h followed by 20 μg/mL of P.e LPS or vehicle stimulating for 1 h. Cells were cross-linked with 1% formaldehyde at 37°C for 10 min. The chromatin immunoprecipitation (ChIP) assay was performed with a ChIP assay kit (Millipore), according to the manufacturer’s instructions. Briefly, collected cells were resuspended in lysis buffer (50 mM of Tris-HCl [pH = 8.1], 10 mM of EDTA, 1% SDS and protease inhibitor cocktail) and sonicated six times for 10 s each, followed by centrifugation for 10 min. The cells were then precleared with protein A-agarose. IPs were performed overnight at 4°C with antibodies. Protein A-Sepharose beads were added and then washed sequentially with low salt, high salt, LiCl buffer, and Tris-EDTA buffer. After elution and reverse cross-linking, the purified DNA was resuspended in TE buffer. Real-time PCR analysis was performed, as previously described. Primers were designed to amplify the sequence including NF-κB p65 binding sites (TGA GTT TTT CA) on the MMP-13 promoter (−338/−348 bp). The primers used were listed in the follows: forward, 5ʹ-AGT ACT AAG TTT CTC TTT-3 (−400/−383 bp); reverse, 5ʹ-TCA AAA CCC ATC TGG CAA-3ʹ (−217/−200 bp). The region that has no NF-κB binding sites served as a negative control, and was amplified using the following primer pair: forward, 5ʹ-ATG CCT TTT AAA CCT AGA-3ʹ (−721/−704 bp); reverse, 5ʹ-ACT TGG AGG TGC TAC GGC-3ʹ (−538/−521 bp). ChIP data were presented as percentage of input normalized to control purifications.
Analysis
Each series of experiments was repeated at least three times, and the data were presented as means ± standard error of mean (SEM). Statistical analysis was performed by analysis of variance. A p-value of <0.05 was considered statistically significant.
Results
P.e LPS–induced MMP-13 expression in MC3T3-E1 cells
First, this study investigated whether P.e LPS stimulates MMP-13 synthesis in MC3T3-E1 cells. Cells were treated with the indicated concentration of P.e LPS for 24 h or 20 μg/mL of P.e LPS for indicated periods, and the expression of MMP-13 was examined. P.e LPS significantly increased the expression of MMP-13 mRNA in a dose-dependent and time-dependent manner, as determined by real-time PCR (Figures 1(a) and (b)). The expression and release of MMP-13 protein were increased by P.e LPS treatment following the increase of time within 24 h, and at 48 h, the expression and release of MMP-13 protein remained at a similar level to the 24 h group, as determined by Western blot (Figure 1(c)) and ELISA (Figure 1(d)).
Figure 1.
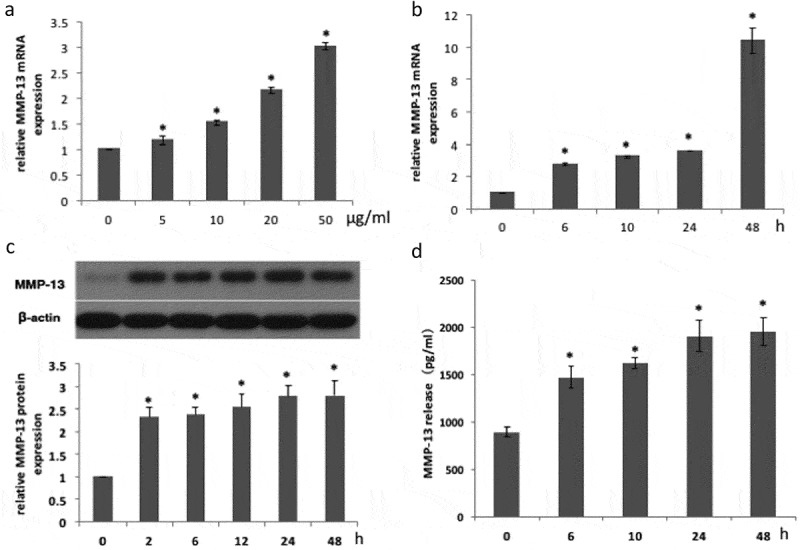
Matrix metalloproteinase-13 (MMP-13) expression is increased in MC3T3-E1 cells induced by Porphyromonas endodontalis lipopolysaccharide (P.e LPS). MCET3-E1 cells were stimulated with P.e LPS (the indicated concentration) for 24 h. (a) MMP-13 mRNA expression was determined by real-time polymerase chain reaction (PCR). MC3T3-E1 cells were treated with 20 μg/mL of P.e LPS for the indicated periods. (b) MMP-13 mRNA expression was determined by real-time PCR. (c) MMP-13 protein expression was determined by Western blot). (d) MMP-13 release was determined by enzyme-linked immunsorbent assay (ELISA). *p < 0.05, compared with the control group.
SIRT1 was involved in P.e LPS–induced MMP-13 expression in MC3T3-E1 cells
To examine whether SIRT1 is involved in P.e LPS–induced MMP-13 expression, first the study evaluated the effects of P.e LPS on the expression of SIRT1 protein in MC3T3-E1 cells. As shown in Figure 2(a), SIRT1 expression decreased in a time-dependent manner following P.e LPS stimulation. Next, an RNA interference technique was used to knock down SIRT1 expression. Expression of SIRT1 mRNA was significantly reduced after 48 h of siRNA transfection (Figure 2(b)). The increased expression of MMP-13 mRNA induced by P.e LPS was augmented by SIRT1 siRNA transfection (Figure 2(c)). The study further investigated the effects of resveratrol (a SIRT1 activator) and EX-527 (a SIRT1 inhibitor) on P.e LPS–induced MMP-13 synthesis. Resveratrol significantly suppressed MMP-13 mRNA expression, MMP-13 protein expression, and MMP-13 release induced by P.e LPS; on the contrary, EX-527 significantly increased the MMP-13 mRNA level, MMP-13 protein level, and MMP-13 release, as determined by real-time PCR (Figure 2(d)), Western blot (Figure 2(e)), and ELISA (Figure 2(f)). These data suggest a negative regulatory role of SIRT1 in P.e LPS–induced MMP-13 expression in MC3T3-E1 cells.
Figure 2.
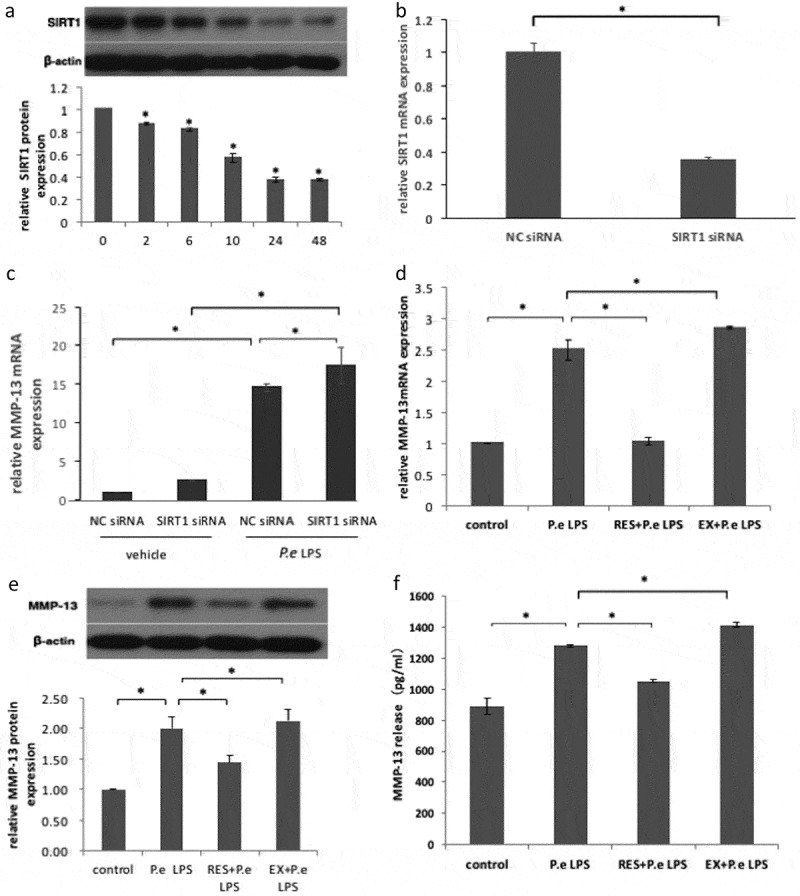
Sirtuin 1 (SIRT1) downregulates P.e LPS–induced MMP-13 expression in MC3T3-E1 cells. MC3T3-E1 cells were treated with 20 μg/mL of P.e LPS for the indicated time periods. (a) SIRT1 expression was determined by Western blot. (b) MC3T3-E1 cells were transiently transfected with SIRT1 siRNA or negative control (NC) siRNA for 48 h, and then the mRNA expression of SIRT1 was determined by real-time PCR. (c) The cells were stimulated with 20 μg/mL of P.e LPS or vehicle for 24 h after transfecting with SIRT1 siRNA or NC siRNA for 48 h, and then MMP-13 mRNA expression was determined by real-time PCR. MC3T3-E1 cells were left untreated (control), stimulated with 20 μg/mL of P.e LPS for 24 h only (P.e LPS), pretreated with 50 μM of resveratrol for 1 h followed by P.e LPS stimulation (RES + P.e LPS), or pretreated with 10 μM of EX-527 for 1 h followed by P.e LPS stimulation (EX + P.e LPS). MMP-13 expression was determined by (d) real-time PCR and (e) Western blot, and MMP-13 release was determined by (f) ELISA. *p < 0.05.
SIRT1 inhibited NF-κB p65 acetylation and NF-κB transcriptional activity induced by P.e LPS in MC3T3-E1 cells
To explore whether SIRT1 plays a role in regulating the NF-κB pathway in P.e LPS–treated osteoblasts, the effects of SIRT1 activator and SIRT1 inhibitor on NF-κB p65 acetylation (acetyl-p65) and NF-κB transcriptional activity were examined. Western blot analysis (Figure 3(a)) revealed that after P.e LPS stimulation, the level of acetyl-p65 markedly increased. Resveratrol pretreatment significantly counteracted the increase of acetyl-p65 induced by P.e LPS; conversely, EX-527 pretreatment further enhanced the acetyl-p65 level. Moreover, as shown in Figure 3(b), P.e LPS highly stimulated the increase in NF-κB transcriptional activity in MC3T3-E1 cells. Resveratrol treatment for 1 h significantly reduced the increase of NF-κB transcriptional activity induced by P.e LPS, whereas EX-527 exhibited the opposite effect. These data demonstrated that SIRT1 could suppress the increases of NF-κB p65 acetylation and NF-κB transcriptional activity, which were induced by P.e LPS in osteoblasts.
Figure 3.

SIRT1 downregulates NF-κB p65 acetylation and NF-κB transcriptional activity induced by P.e LPS in MC3T3-E1 cells. (a) MC3T3-E1 cells were left untreated (control), stimulated by 20 μg/mL of P.e LPS for 24 h only (P.e LPS), or pretreated with 50 μM of resveratrol (RES + P.e LPS) or 10 μM of EX-527 (EX+P.e LPS) for 1 h before P.e LPS stimulation. Acetyl-p65 expressions were determined by Western blot. (b) MC3T3-E1 cells were transfected with NF-κB-Luc or pGL3 (empty vector). After 24 h, the cells were treated with vehicle only (control), pretreated with vehicle for 1 h, and then stimulated by 20 μg/mL of P.e LPS for 30 min (P.e LPS), or pretreated with 50 μM of resveratrol (RES + P.e LPS) or 10 μM of EX-527 (EX + P.e LPS) for 1 h before P.e LPS stimulation. Cells were lysed with passive lysis buffer, and the luciferase activity was measured. The results were normalized with pRL-TK activities. Each column represents the mean ± standard deviation. *p < 0.05.
SIRT1 suppressed MMP-13 expression in P.e LPS–treated MC3T3-E1 cells through NF-κB pathway
To study whether SIRT1 suppressed MMP-13 expression through NF-κB, first, this study examined the role of NF-κB in P.e LPS–induced MMP-13 expression in osteoblasts. Cells were pretreated with 10 μM of NF-κB inhibitor Bay 11-7082. Bay 11-7082 significantly reduced P.e LPS–induced MMP-13 mRNA and protein expression and MMP-13 release, as determined by real-time PCR (Figure 4(a)), Western blot (Figure 4(b)), and ELISA (Figure 4(c)), respectively. Moreover, to assess whether SIRT1 has any effect on the binding of NF-κB p65 on the MMP-13 promoter, ChIP assay was performed using anti-NF-κB p65 antibody. After 20 μg/mL of P.e LPS stimulation of MC3T3-E1 cells for 1 h, the enrichment level of NF-κB p65 on the MMP-13 promoter significantly increased. This increased level was markedly attenuated by the pretreatment with resveratrol but enhanced by that with EX-527 (Figure 4(d)). These data suggest that SIRT1 suppresses MMP-13 expression through inhibiting the binding of NF-κB p65 to the promoter region of MMP-13 in osteoblasts.
Figure 4.

SIRT1 inhibited the binding of NF-κB p65 to the promoter region of MMP-13 in P.e LPS–treated osteoblasts. MC3T3-E1 cells were left untreated (control), stimulated by 20 μg/mL of P.e LPS for 24 h only (P.e LPS), or pretreated with 10 μM of Bay 11-7082 for 1 h followed by P.e LPS stimulation for 24 h (Bay + P.e LPS). MMP-13 expression was determined by (a) real-time PCR and (b) Western blot, and MMP-13 release was determined by (c) ELISA. MC3T3-E1 cells were treated with vehicle (control), or 20 μg/mL of P.e LPS for 1 h (P.e LPS), or pretreated with 50 μM of resveratrol (RES + P.e LPS) or 10 μM of EX-527 (EX + P.e LPS) for 1 h before P.e LPS stimulation. Chromatin immunoprecipitation assay was performed with antibodies against NF-κB p65 and immunoglobulin G. Real-time PCR was used to measure the relative enrichment of NF-κB p65 on MMP-13 promoter region (−400/–200). The region (−721/–521) without p65 binding sites was amplified and used as a negative control (Dd). *p < 0.05.
Discussion
The present study demonstrated that P.e LPS stimulated MMP-13 expression in mouse osteoblasts. SIRT1 suppressed the expression of MMP-13 induced by P.e LPS via deacetylating NF-κB, which further inhibited NF-κB transcriptional activity and the binding of NF-κB subunit p65 to the promoter region of MMP-13.
It has been reported previously that MMP-13 expression could be induced by parathyroid hormone (PTH) and some inflammatory factors such as IL-1²) and TNF-±; [35–37]. Gao et al. [34] used Escherichia coli LPS to stimulate osteoblasts. They found that LPS is another stimulator inducing the transcriptional activation of MMP-13. This study used LPS extracted from P.e as a stimulator to study the role of MMP-13 in the context of chronic apical periodontitis. The results showed for the first time that P.e LPS induced MMP-13 expression in a dose- and time-dependent fashion in mouse osteoblasts. MMP-13 has proven to be a key bone-destruction perpetrator in bone resorption–related diseases, including chronic apical periodontitis [3,4,8]. In a previous study, it was observed with micro-computed tomography (CT) that P.e LPS injection caused bone resorptions in a mouse model [14]. Based on these findings, it is probable that the promotion effect of P.e LPS on periapical bone resorption is mediated, at least in part, by the release of MMP-13 in osteoblasts.
SIRT1 is known to regulate inflammation-immune function via suppressing production of inflammatory cytokines [23]. It has been shown that the expression of CD40 induced by TNF-±; in endothelial cells is downregulated by resveratrol, a SIRT1 activator [38]. Another study has suggested that following intro-articular resveratrol injection, the expression of MMP-13 in cartilage was reduced in a mouse model of osteoarthritis [39]. In line with these reports, the present study showed in vitro that resveratrol significantly attenuated MMP-13 expression induced by P.e LPS in MC3T3-E1 cells. On the contrary, it also showed that EX-527 (SIRT1-specific inhibitor) markedly increased the expression of MMP-13. The study also found that knockdown of SIRT1 by SIRT1 siRNA transfection significantly augmented P.e LPS–induced MMP-13 expression. Moreover, it was found that unstimulated MC3T3-E1 cells showed a relatively high SIRT1 expression, but following treatment with P.e LPS, the level of SIRT1 expression significantly decreased. Thus, the decrease in SIRT1 expression in osteoblasts after P.e LPS stimulation could be responsible for the increased MMP-13 expression. It has been previously known that LPS could stimulate the release of pro-inflammatory cytokines such as IL-1 and TNF-±;, and these pro-inflammatory cytokines would initiate and augment subsequent inflammatory cascades leading to tissue destruction [40,41]. In another study, TNF-±; was found to induce MMP-13 expression and meanwhile decrease the SIRT1 level in MC3T3-E1 cells. In addition, SIRT1 inhibitor EX-527 significantly enhanced TNF-±;-induced MMP-13 expression (data not shown). Based on the above findings as a whole, SIRT1 might act as a target point to slow down apical bone resorption through negatively regulating the expressions of MMP-13 induced by P.e LPS and pro-inflammatory factors such as TNF-±;.
As a deacetylase, SIRT1 regulates gene expression through modulating the acetylation status of its targets, including NF-κB. It was demonstrated that SIRT1 physically associates with the p65 subunit of NF-κB and deacetylase p65 at Lys310 residue to reduce the transcriptional activity of NF-κB [30,31]. The results also indicate that the acetylation level of p65 and NF-κB transcriptional activity were markedly attenuated by SIRT1 activation and promoted by SIRT1 inhibition in P.e LPS–stimulated osteoblasts. Of course, deacetylating p65 might not be the only mechanism of SIRT1 inhibiting NF-κB transcriptional activity. Huang et al. indicated that overexpression of SIRT1 suppressed NF-κB transcriptional activity in TNF-±;-treated osteoblasts through the inhibition of the degradation of IκB [42]. In addition, Pan et al. found that SIRT1 repressed the NF-κB pathway through downregulating the protein levels of p65 in human umbilical endothelial cells [38]. Taken together, these data indicate that SIRT1 is a negative regulator for NF-κB transcriptional activity. A previous study indicated that P.e LPS induces the expressions of M-CSF, Wnt5a, and IL-6 through promoting the transcriptional activity of NF-κB in MCET3-E1 cells [16,17,43]. In order to study whether NF-κB is also involved in the expression of MMP-13 in P.e LPS–induced osteoblasts, the cells were pretreated with NF-κB inhibitor Bay 11-7082. Bay 11-7082 significantly suppressed the production of MMP-13 induced by P.e LPS in MC3T3-E1 cells. This suggests a link between NF-κB and MMP-13 expression induced by P.e LPS. To explore further the mechanism responsible for the increased MMP-13 expression in P.e LPS–induced osteoblasts, ChIP analysis was performed to identify the recruitment of p65 to the MMP-13 promoter. The increased level of p65 was found on the MMP-13 promoter, which further supports that P.e LPS regulates MMP-13 expression through NF-κB. These results are consistent with an earlier study on MMP-13 expression in CCN3-stimulated human chondrosarcoma cells [44]. However, Imagawa et al. [45] reported that NF-κB does not play a regulatory role in the MMP-13 expression in human chondrocytes. Thus, it is likely that the mechanisms responsible for MMP-13 expression in various cells are quite different. Since the deacetylation of NF-κB p65 could lower its binding to the promoter of the target gene [29], it was hypothesized that the negative regulation of SIRT1 on MMP-13 expression in osteoblasts might be exerted by directly blocking the NF-κB binding to the MMP-13 promoter. The results showed that resveratrol, the activator of SIRT1, inhibited the binding of NF-κB p65 to the MMP-13 promoter, and the situation was the opposite after being treated with the inhibitor of SIRT1. Taken together, SIRT1 suppressed the MMP-13 expression through blocking the binding of NF-κB to the MMP-13 promoter in P.e LPS–stimulated osteoblasts.
Interestingly, the present results showed that NF-κB inhibitor decreased P.e LPS–induced MMP-13 to a lesser extent compared with that of SIRT1 activator. It prompts that NF-κB is not the only target in the regulation of SIRT1 on MMP-13 expression. In addition to NF-κB, activator protein-1 (AP-1) and p38 MAP kinase have also been shown to be involved in regulating MMP-13 expression in osteoblasts [34,46]. SIRT1 has been shown to inhibit PTH stimulation of MMP-13 expression by association with c-Jun at the AP-1 site of the MMP-13 promoter [46]. It would be interesting to investigate whether inhibition of SIRT1-AP-1 signaling and SIRT1-p38MAPK signaling also contribute to increased MMP-13 production by P.e LPS in osteoblasts.
In summary, the current data provide evidence that SIRT1 inhibits P.e LPS–induced MMP-13 expression in osteoblasts by suppressing NF-κB transcriptional activity. These findings might be pivotal for understanding the potential role of SIRT1 in modulating inflammatory events in chronic apical periodontitis.
Funding Statement
This work was supported by Shenyang Science and Technology Bureau Project (No. F15-199-1-56).
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Uusitalo H, Hiltunen A, Söderström M. Expression of cathepsins B, H, K, L, and S and matrix metalloproteinases 9 and 13 during chondrocyte hypertrophy and endochondral ossification in mouse fracture callus. Calcif Tissue Int. 2000;67:382–9. doi: 10.1007/s002230001152. [DOI] [PubMed] [Google Scholar]
- Lenglet S, Mach F, Montecucco F. Role of matrix metalloproteinase-8 in atherosclerosis. Mediators Inflamm. 2013;2013:659282. doi: 10.1155/2013/659282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardif G, Reboul P, Pelletier J-P. Ten years in the life of an enzyme: the story of the human MMP-13 (collagenase-3) Mod Rheumatol. 2004;14:197–204. doi: 10.1007/s10165-004-0292-7. [DOI] [PubMed] [Google Scholar]
- Vandenbroucke RE, Dejonckheere E, Van Hauwermeiren F. Matrix metalloproteinase 13 modulates intestinal epithelial barrier integrity in inflammatory diseases by activating TNF. EMBO Mol Med. 2013;5:932–948. doi: 10.1002/emmm.201202100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlgren J, Maisi P, Sorsa T. Expression and induction of collagenases (MMP-8 and −13) in plasma cells associated with bone-destructive lesions. J Pathol. 2001;194:217–224. doi: 10.1002/path.854. [DOI] [PubMed] [Google Scholar]
- Stickens D, Behonick DJ, Ortega N. Altered endochondral bone development in matrix metalloproteinase 13-deficient mice. Development. 2004;131:5883–5895. doi: 10.1242/dev.01461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Aquino SG, Guimaraes MR, Stach-Machado DR. Differential regulation of MMP-13 expression in two models of experimentally induced periodontal disease in rats. Arch Oral Biol. 2009;54:609–617. doi: 10.1016/j.archoralbio.2009.03.007. [DOI] [PubMed] [Google Scholar]
- Bhalla G, Astekar MS, Ramesh G. Collagenase-3 expression in periapical lesions: an immunohistochemical study. Biotech Histochem. 2014;89:457–463. doi: 10.3109/10520295.2014.893015. [DOI] [PubMed] [Google Scholar]
- Matsui H, Yamasaki M, Nakata K. Expression of MMP-8 and MMP-13 in the development of periradicular lesions. Int Endod J. 2011;44:739–745. doi: 10.1111/j.1365-2591.2011.01880.x. [DOI] [PubMed] [Google Scholar]
- Leonardi R, Caltabiano R, Loreto C. Collagenase-3 (MMP-13) is expressed in periapical lesions: an immunohistochemical study. Int Endod J. 2005;38:297–301. doi: 10.1111/j.1365-2591.2005.00943.x. [DOI] [PubMed] [Google Scholar]
- Andonovska B, Dimova C, Panov S. Matrix metalloproteinases (MMP-1, −8, −13) in chronic periapical lesions. Vojnosanit Pregl. 2008;65:882–886. doi: 10.2298/vsp0812882a. [DOI] [PubMed] [Google Scholar]
- Tomazinho LF, Avila-Campos MJ. Detection of Porphyromonas gingivalis, Porphyromonas endodontalis, Prevotella intermedia, and Prevotella nigrescens in chronic endodontic infection. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103:285–288. doi: 10.1016/j.tripleo.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Murakami Y, Hanazawa S, Tanaka S. A possible mechanism of maxillofacial abscess formation: involvement of Porphyromonas endodontalis lipopolysaccharide via the expression of inflammatory cytokines. Oral Microbiol Immunol. 2001;16:321–325. doi: 10.1034/j.1399-302x.2001.160601.x. [DOI] [PubMed] [Google Scholar]
- Guo J, Yang D, Okamura H. Calcium hydroxide suppresses Porphyromonas endodontalis lipopolysaccharide-induced bone destruction. J Dent Res. 2014;93:508–513. doi: 10.1177/0022034514526886. [DOI] [PubMed] [Google Scholar]
- Yang D, Li R, Qiu LH. Effects of lipopolysaccharides extracted from Porphyromonas endodontalis on the expression of IL-1beta mRNA and IL-6 mRNA in osteoblasts. Shanghai Kou Qiang Yi Xue. 2009;18:194–197. [PubMed] [Google Scholar]
- Yu Y, Qiu L, Guo J. Effect of lipopolysaccharides from Porphyromonas endodontalis on the expression of macrophage colony stimulating factor in mouse osteoblasts. Zhonghua Kou Qiang Yi Xue Za Zhi. 2014;49:535–539. [PubMed] [Google Scholar]
- Yu Y, Qiu L, Guo J. TRIB3 mediates the expression of Wnt5a and activation of nuclear factor-kappaB in Porphyromonas endodontalis lipopolysaccharide-treated osteoblasts. Mol Oral Microbiol. 2015;30:295–306. doi: 10.1111/omi.12094. [DOI] [PubMed] [Google Scholar]
- Edwards JR, Perrien DS, Fleming N. Silent information regulator (Sir)T1 inhibits NF-κB signaling to maintain normal skeletal remodeling. J Bone Miner Res. 2013;28:960–969. doi: 10.1002/jbmr.1824. [DOI] [PubMed] [Google Scholar]
- Zhang QB, Cao W, Liu YR. Effects of Sirtuin 1 on the proliferation and osteoblastic differentiation of periodontal ligament stem cells and stem cells from apical papilla. Genet Mol Res. 2016;15:gmr.15015234 doi: 10.4238/gmr.15015234. [DOI] [PubMed] [Google Scholar]
- Herranz D, Munoz-Martin M, Canamero M. Sirt1 improves healthy ageing and protects from metabolic syndrome-associated cancer. Nat Commun. 2010;1:3. doi: 10.1038/ncomms1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S-I, Min K-S, Bae W-J. Role of SIRT1 in heat stress- and lipopolysaccharide-induced immune and defense gene expression in human dental pulp cells. J Endod. 2011;37:1525–1530. doi: 10.1016/j.joen.2011.07.006. [DOI] [PubMed] [Google Scholar]
- Singh NP, Singh UP, Hegde VL. Resveratrol (trans-3,5,4ʹ-trihydroxystilbene) suppresses EL4 tumor growth by induction of apoptosis involving reciprocal regulation of SIRT1 and NF-kappaB. Mol Nutr Food Res. 2011;55:1207–1218. doi: 10.1002/mnfr.201000576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Z, Ajmo JM, Rogers CQ. Role of SIRT1 in regulation of LPS- or two ethanol metabolites-induced TNF-alpha production in cultured macrophage cell lines. Am J Physiol Gastrointest Liver Physiol. 2009;296:G1047–G1053. doi: 10.1152/ajpgi.00016.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J-H, Song M-Y, Song E-K. Overexpression of SIRT1 protects pancreatic beta-cells against cytokine toxicity by suppressing the nuclear factor-kappaB signaling pathway. Diabetes. 2009;58:344–351. doi: 10.2337/db07-1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J, Nikolaev AY, Imai S. Negative control of p53 by Sir2alpha promotes cell survival under stress. Cell. 2001;107:137–148. doi: 10.1016/s0092-8674(01)00524-4. [DOI] [PubMed] [Google Scholar]
- Jeong YJ, Shin JM, Bae YS. Melittin has a chondroprotective effect by inhibiting MMP-1 and MMP-8 expressions via blocking NF-kappaB and AP-1 signaling pathway in chondrocytes. Int Immunopharmacol. 2015;25:400–405. doi: 10.1016/j.intimp.2015.02.021. [DOI] [PubMed] [Google Scholar]
- Zheng C, Yin Q, Wu H. Structural studies of NF-κB signaling. Cell Res. 2011;21:183–195. doi: 10.1038/cr.2010.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghizzoni M, Haisma HJ, Maarsingh H. Histone acetyltransferases are crucial regulators in NF-κB mediated inflammation. Drug Discov Today. 2011;16:504–511. doi: 10.1016/j.drudis.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiernan R, Bres V, Ng RW. Post-activation turn-off of NF-kappa B-dependent transcription is regulated by acetylation of p65. J Biol Chem. 2003;278:2758–2766. doi: 10.1074/jbc.M209572200. [DOI] [PubMed] [Google Scholar]
- Chen L, Fischle W, Verdin E. Duration of nuclear NF-kappaB action regulated by reversible acetylation. Science. 2001;293:1653–1657. doi: 10.1126/science.1062374. [DOI] [PubMed] [Google Scholar]
- Yeung F, Hoberg JE, Ramsey CS. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. Embo J. 2004;23:2369–2380. doi: 10.1038/sj.emboj.7600244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga T, Nishihara T, Fujiwara T. Biochemical and immunobiological properties of lipopolysaccharide (LPS) from Bacteroides gingivalis and comparison with LPS from Escherichia coli. Infect Immun. 1985;47:638–647. doi: 10.1128/iai.47.3.638-647.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts HC, Moseley R, Sloan AJ. Lipopolysaccharide alters decorin and biglycan synthesis in rat alveolar bone osteoblasts: consequences for bone repair during periodontal disease. Eur J Oral Sci. 2008;116:207–216. doi: 10.1111/j.1600-0722.2008.00535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao A, Kantarci A, Herrera BS. A critical role for suppressors of cytokine signaling 3 in regulating LPS-induced transcriptional activation of matrix metalloproteinase-13 in osteoblasts. PeerJ. 2013;1:e51. doi: 10.7717/peerj.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess J, Porte D, Munz C. AP-1 and Cbfa/runt physically interact and regulate parathyroid hormone-dependent MMP13 expression in osteoblasts through a new osteoblast-specific element 2/AP-1 composite element. J Biol Chem. 2001;276:20029–20038. doi: 10.1074/jbc.M010601200. [DOI] [PubMed] [Google Scholar]
- Wu G, Wang L, Li H. Function of sustained released resveratrol on IL-1beta-induced hBMSC MMP13 secretion inhibition and chondrogenic differentiation promotion. J Biomater Appl. 2016;30:930–939. doi: 10.1177/0885328215614425. [DOI] [PubMed] [Google Scholar]
- Lin TH, Tang CH, Wu K. 15-deoxy-Delta(12,14) -prostaglandin-J2 and ciglitazone inhibit TNF-alpha-induced matrix metalloproteinase 13 production via the antagonism of NF-kappaB activation in human synovial fibroblasts. J Cell Physiol. 2011;226:3242–3250. doi: 10.1002/jcp.22685. [DOI] [PubMed] [Google Scholar]
- Pan W, Yu H, Huang S. Resveratrol protects against TNF-α-induced injury in human umbilical endothelial cells through promoting Sirtuin-1-induced repression of NF-KB and p38 MAPK. Plos One. 2016;11:e0147034. doi: 10.1371/journal.pone.0147034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Cai L, Zhang Y. Intra-articular resveratrol injection prevents osteoarthritis progression in a mouse model by activating SIRT1 and thereby silencing HIF-2α. J Orthop Res. 2015;33:1061–1070. doi: 10.1002/jor.22859. [DOI] [PubMed] [Google Scholar]
- Hong C-Y, Lin S-K, Kok S-H. The role of lipopolysaccharide in infectious bone resorption of periapical lesion. J Oral Pathol Med. 2004;33:162–169. doi: 10.1111/j.0904-2512.2004.00045.x. [DOI] [PubMed] [Google Scholar]
- Wilson M, Reddi K, Henderson B. Cytokine-inducing components of periodontopathogenic bacteria. J Periodontal Res. 1996;31:393–407. doi: 10.1111/j.1600-0765.1996.tb00508.x. [DOI] [PubMed] [Google Scholar]
- Huang W, Shang WL, Wang HD. Sirt1 overexpression protects murine osteoblasts against TNF-alpha-induced injury in vitro by suppressing the NF-kappaB signaling pathway. Acta Pharmacol Sin. 2012;33:668–674. doi: 10.1038/aps.2011.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu YQ, Guo JJ, Qiu LH. Effect of NF-kappaB on the expression of interleukin-6 induced by lipopolysaccharides of Porphyromonas endodontalis in MC3T3-E1 cells. Shanghai Kou Qiang Yi Xue. 2013;22:378–383. [PubMed] [Google Scholar]
- Tzeng H-E, Chen J-C, Tsai C-H. CCN3 increases cell motility and MMP-13 expression in human chondrosarcoma through integrin-dependent pathway. J Cell Physiol. 2011;226:3181–3189. doi: 10.1002/jcp.22672. [DOI] [PubMed] [Google Scholar]
- Imagawa K, De Andrés MC, Hashimoto K. The epigenetic effect of glucosamine and a nuclear factor-kappa B (NF-kB) inhibitor on primary human chondrocytes–implications for osteoarthritis. Biochem Biophys Res Commun. 2011;405:362–367. doi: 10.1016/j.bbrc.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fei Y, Shimizu E, McBurney MW. Sirtuin 1 is a negative regulator of parathyroid hormone stimulation of matrix metalloproteinase 13 expression in osteoblastic cells: role of sirtuin 1 in the action of PTH on osteoblasts. J Biol Chem. 2015;290:8373–8382. doi: 10.1074/jbc.M114.602763. [DOI] [PMC free article] [PubMed] [Google Scholar]


