Abstract
Background
Nosocomial pneumonias are common in trauma patients and so interventions to prevent and treat nosocomial pneumonia may improve outcomes. Our prior work strongly suggests that tissue injury predisposes to infections like nosocomial pneumonia because mitochondrial debris (MTD) originating from injured cells contains damage-associated molecular patterns (DAMPs) that can reduce neutrophil (PMN) migration into the airway and diminish PMN function in response to bacterial inoculation of the airway. This suggested that putting exogenous “normal” PMN into the airway might be beneficial.
Methods
Post-injury pneumonia (PNA) commonly arises in two groups, early, community-acquired PNA (CAP) and later hospital-acquired PNA (HAP). Post-traumatic Early onset CAP and Late onset HAP were modeled in CD-1 mice using S. aureus (SA) or P. aeruginosa (PA) instilled intra-tracheal (i.t.) at clinically relevant times with or without extrapulmonary injuries mimicked by an intraperitoneal application of mitochondrial DAMPs. We applied bone-marrow derived PMN intratracheally to access bacterial clearance in the lung.
Results
BM-PMN instillation i.t. had no untoward clinical effects on recipient animals. In both the Early/CAP and Late/HAP models, clearance of the inoculum from the lung was suppressed by MTD and restored to uninjured levels by i.t. instillation of exogenous BM-PMN. Furthermore PMN instillation cleared the inoculum of P. aeruginosa that could not be cleared by uninjured mice. The instillation of PMN into the lung, even across strains (CD-1 vs. C57BL/6) had no injurious effect.
Conclusion
These initial studies suggest PMN instillation (i.t.) is worthy of further study as a potential adjunctive therapy aimed at decreasing the morbidity of lung infections in trauma patients. Moreover, PMN instillation (i.t.) may represent a unique means of preventing or treating pneumonia after serious injury that is completely independent of the need for antibiotic use.
Keywords: Mitochondria, DAMPs, pneumonia, trauma, neutrophil, mice
Introduction
Trauma is the leading cause of death in the under-45 age group and the third-leading cause of death in all populations1. Nosocomial pneumonia is a common cause of morbidity and death in trauma patients2–5. Thus prevention of nosocomial pneumonia would contribute significantly to reducing the morbidity and cost of injury. Early after injury, trauma patients are susceptible to “community acquired” pneumonias (CAP), that are commonly thought to arise from the patient’s endogenous oropharyngeal flora that are inoculated into the airway due to early transient loss of airway protective reflexes. Early CAP is typically caused by Gram-positive organisms. Later, patients who remain ill or endotracheally intubated are at risk for “hospital-” or “healthcare-acquired” pneumonias (HAP). These are commonly caused by Gram-negative organisms or resistant Gram-positive organisms that colonize the patient over time, especially in the presence of antibiotics. Thus common HAP pathogens include pseudomonas aeruginosa (PA) and methicillin-resistant Staphylococcus aureus (MRSA). Although it has been widely assumed that pneumonia after injury is a function of increased airway inoculation and suppressed bacterial clearance due to poor cough, these suppositions are unsupported6 and it is now clear that suppression of innate immunity by injury is a critical and primary determinant of infection after trauma.
The immunologic links between injury itself and nosocomial pneumonia are still very poorly understood, but neutrophils (PMN) are the predominant effector cells protecting the airway from infection. Addressing that issue, we have clearly shown that damage-associated molecular patterns (DAMPs) emanating from injured tissue are capable of placing distant sites like the lung, at risk for infection by bacterial inocula that would otherwise be ineffective7. Mitochondria-derived DAMPs (MTD) released from injured tissues predispose to pneumonia, at least in part, by reducing PMN recruitment to the lungs7,8. But we suspect that PMN failure to protect the lung against inoculation may reflect suppressed PMN function as much as failure to marginate at the host-airway interface after trauma.
This novel mechanistic understanding may be important in several ways. First, it suggests that we should be looking for ways to augment innate immunity, thus decreasing the establishment of inoculated bacteria and preventing pneumonia. Second, it suggests augmenting innate immunity may allow us to treat pneumonias that are resistant to antibiotics. Third, non-antibiotic methods of decreasing the incidence of pneumonia after injury may allow us to decrease the emergence of antibiotic resistance in the first instance. To achieve our aims, we chose to both explore the molecular biology of suppressed inmate immunity and at the same time to look for novel therapies that reverse or bypass inmate immune failure. In this set of investigations, we explore the possibility that replacing endogenous suppressed neutrophils with exogenous normal neutrophils can prevent or reverse establishment of bacterial growth in the lung after injury. To do that we used our established models of injury combined with intra-tracheal inoculation of Staphylococcus aureus (SA) to model early, Gram-positive CAP, and Pseudomonas aeruginosa (PA) to model late, Gram-negative HAP. Cell-based therapies using PMN could also be totally independent of antibiotics and thus could become a new and highly desirable form of prevention and/or treatment for post-traumatic pneumonia.
METHODS
Animals
All animal experimental procedures were approved by Institutional Animal Care and Use Committee (IACUC) at Beth Israel Deaconess Medical Center. Seven to 9 week-old CD-1 mice were kept at our animal care facility with 12-hour light/dark cycles with free access to water and standard food. Animals were allowed to acclimatize for at least a day before being used for experimental procedures.
Bacterial strains
Staphylococcus aureus Rosenbach (S. aureus, gram-positive, ATCC 14458) and Pseudomonas aeruginosa (Schroeter) Migula (P. aeruginosa, gram-negative, ATCC 10145) and were purchased from American Type Culture Collection (ATCC, Manassas, VA) and used by following the supplier’s protocol. Briefly, bacteria were kept at −80°C. Prior to use, bacteria were grown in 100 mL of Bacto Agar medium (Becton Dickinson) overnight. Then 1 mL of bacteria containing medium was applied to 100 mL of fresh agar medium for 2 hrs. Bacteria were then spun down at 4,500 rpm for 10 min at 4°C. Finally, bacteria were resuspended in sterile PBS to OD600=0.1. This protocol results in an inoculum of 5×106 CFU in 50 μL (as confirmed by direct count).
Bone marrow derived PMN
Bone marrow derived PMN (BM-PMN) were freshly isolated from the femurs of donor animals such as CD-1 or C57BL/6 mice as described by Swamydas et al.9 Cells were suspended in sterile saline to a concentration of ~2×106 in 50 μL. We confirmed that BM-PMN were functionally active by showing that they increased their intracellular calcium levels and chemotaxis in response to fMLP. Also, we validated their functional activity by showing they respond to bacteria with phagocytosis and neutrophil extracellular trap (NET) formation (data not shown). Other groups have reported similar functions of BM-PMN10,11. It has also been shown that each BM-PMN can ingest up to 100 S. aureus or E. coli in 30 min in vitro12,13. Thus theoretically, 104 PMN should be able to kill 106 bacteria and we therefore elected to apply this higher number of BM-PMN per bacteria in in vivo experiments.
DAMPs preparation and injury modeling
In order to model systemic exposure to DAMPs similar to those seen after abdominal injury, we used intra-peritoneal (i.p.) injection of sonicated mitochondrial debris (MTD) isolated from 10% of a CD-1 mouse liver. We have previously shown that the DAMPs present in this amount of MT affect systemic neutrophil responses in a way that is indistinguishable from that caused by crushing 10% of the mouse’s liver at laparotomy or by injection of morcellated mouse liver (10% of the animal’s estimated liver weight) into the peritoneal cavity at laparotomy. Our methods for isolating MT and preparing MTD from mouse liver are published in detail elsewhere14.
Intratracheal instillation
Mice were anesthetized with a combination of Ketamine (100 mg/kg) and xylazine (20 mg/kg). Tracheal intubation and infusion was performed using the protocol described by Cai and Kimura15. The mice are placed on a vertical support. We then place a 22G plastic catheter (sterile, single use, Fisher Scientific) in the trachea. We apply experimental solutions to the top (hub) of the catheter and wait (typically 2–3 minutes) until the fluids are fully inhaled as a result of the animal’s own respiratory efforts. The total volume of each PMN and bacterial inoculum (and/or the saline vehicle controls) was 50 μL. As soon as the solutions are inhaled into the lung, the catheters are removed, the animals are returned to their cages and placed on heating pads until they are mobile. Critically, we apply all experimental solutions passively through inhalation via the catheter without using a syringe to force the materials into the lung. This method guarantees that all applied materials go intra-tracheal and is also less stressful to the animals15.
Bronchoalveolar lavage fluid (BALF) and lung homogenates
BALF was obtained after sacrifice by application of 1 mL sterile PBS through a 30G catheter secured into the trachea and used to gently irrigate the lungs. For whole lung homogenates, lungs were isolated, placed in 5 mL of ice-cold PBS and homogenized in a Stomacher 80 (Seward, UK). 20–100 μL of freshly isolated BALF or lung homogenates were then applied to tryptic soy agar plates to determine bacterial counts. Note that separate animals were used for BALF and lung homogenization experiments.
Experimental Protocols
For each experiment we randomly divided CD-1 mice into three experimental groups. Establishment of early onset and late onset pneumonia after injury (CAP and HAP respectively) were modeled by inoculation of S. aureus and P. aeruginosa in the presence of injury modeled by intraperitoneal injection of mitochodrial DAMPs (mtDAMPs). Protocols can be found in Figure 1.
Figure 1. Protocols for the study of early and late onset of pneumonia.
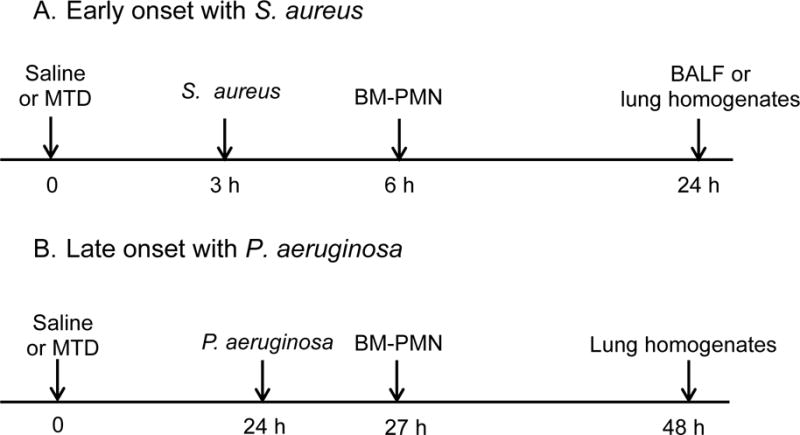
Early onset of pneumonia with S. aureus.
Group 1: saline at time 0. S. aureus (OD600=0.1, 50 μL) at 3 h.
Group 2: MTD (10% liver) at time 0 and S. aureus at 3 h.
Group 3: MTD at time 0. S. aureus at 3 h and BM-PMN (~ 2×106) at 6 h.
Animals were sacrificed at 24 h for BALF or lung homogenates preparation.
B. Late onset of pneumonia with P. aeruginosa.
Group 1: saline at time 0. P. aeruginosa (OD600=0.1, 50 μL) at 24 h.
Group 2: MTD (10% liver) at time 0 and P. aeruginosa at 24 h.
Group 3: MTD at time 0. P. aeruginosa at 3 h and BM-PMN (~ 2×106) at 6 h.
Animals were sacrificed at 48 h for lung homogenates preparation.
1. Prevention of early onset pneumonia (see Figure 1, Timeline A)
Group-1 (Bacteria-only) had 0.9% NaCl (saline) injected intra-peritoneal (i.p.) at T=0 and bacteria (S. aureus, SA, 1×106 CFU in 50μL saline) injected intra-tracheal (i.t.) at T=3 h. n=8 animals. Group-2 (DAMPs+bacteria) got MTD (isolated from 10% of a CD-1 mouse liver) i.p. at T=0 and then were inoculated with SA i.t. at T=3 h. n=8 animals. Group-3 (PMN rescue) got MTD i.p. at T=0, SA i.t. at T=3 h and BM-PMN (2×106 in 50μL saline from CD-1 mice) i.t. at T=6 h. n=9 animals. Animals were sacrificed at T=24 h. Bacterial clearance was assayed by culturing bronchoalveolar lavage fluid (BALF) or lung homogenates on Tryptic Soy Agar plates to evaluate bacterial clearance (CFU). (See Figure 2)
Figure 2. Effects of mtDAMPs and external PMN on S. aureus (SA) clearance in lung.
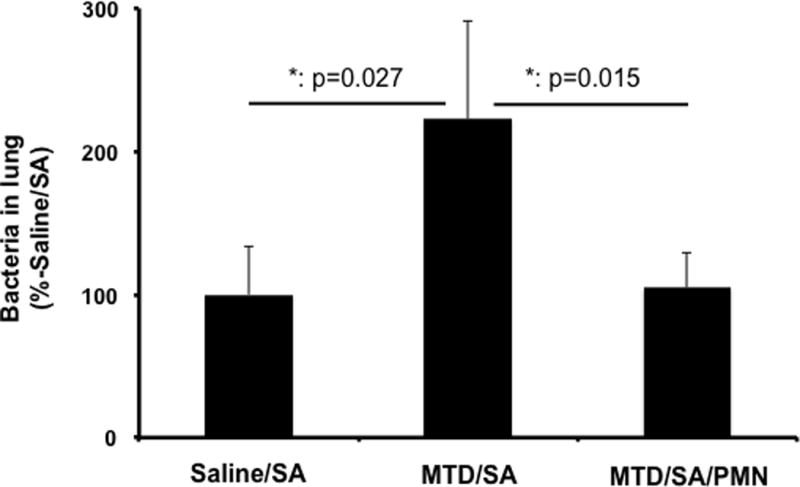
CD-1 mice were separated into three groups. 1. Saline i.p. injection (time 0) followed by S. aureus (3h) i.t. Saline/SA 2. MTD i.p. followed by S. aureus MTD/SA, 3. MTD i.p. followed by S. aureus and BM-PMN i.t. (6h) MTD/SA/PMN. Animals were sacrificed t=24 h. Lung homogenates were prepared to determine bacterial presence. MTD prepared from 10% of total liver in saline. 50 uL of OD600=0.1 S. aureus was injected intratracheally. BM-PMN were freshly prepared from donor CD-1 mice and ~2×106 cells were injected intratracheally.
Saline/SA: N=8, MTD/SA: N=8, MTD/SA/PMN: N=9.
Mean and SD values are shown. * denotes a significant difference by One-Way ANOVA, Tukey.
2. Prevention of late onset pneumonia (see Figure 1, Timeline B)
CD-1 mice were assigned to groups as in the early onset pneumonia experiments. Rather than staphylococcus (SA), in this case mice were inoculated with P. aeruginosa (PA). Also, we performed the bacterial inoculations somewhat later to reflect the later clinical timing of inoculation by Gram-negative hospital acquired bacteria. Thus here, Group-1 got saline injected intra-peritoneal (i.p.) at T=0 and bacteria (PA, 1×106 CFU in 50μL saline) injected intra-tracheal (i.t.) at T=24 h. n=10 animals. Group-2 got MTD i.p. at T=0 and PA i.t. at T=24 h. n=11 animals. Group-3 got MTD i.p. at T=0, bacteria i.t. at T=24 h and BM-PMN (2×106 in 50μL saline) i.t. at T=27 h. n=12 animals. All animals were sacrificed at T=48h and bacterial clearance was evaluated as above. Aliquots of lung homogenates were applied to Tryptic Soy Agar plates to evaluate bacterial clearance (CFU) as above. Similarly, we applied more bacteria (CFU, OD600=0.111) to evaluate the role of external PMN application on bacterial clearance. (See Figure 3)
Figure 3. Effects of mtDAMPs and external PMN on P. aeruginosa clearance (PA) in lung.
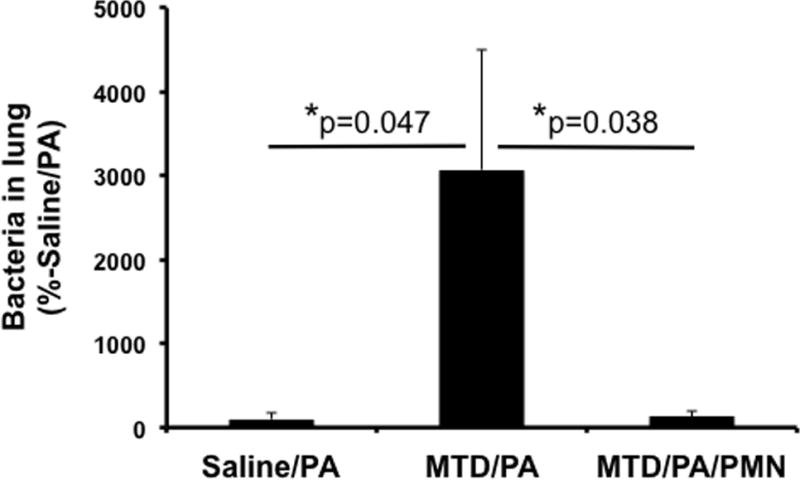
CD-1 mice were separated into three groups. 1. Saline/PA: Saline i.p. injection (time 0) followed by P. aeruginosa (24 h) i.t. 2. MTD/PA: MTD i.p. (time 0) followed by P. aeruginosa i.t. (24 h), 3. MTD/PA/PMN: MTD i.p. (time 0) followed by P. aeruginosa i.t. (24 h) and BM-PMN i.t. (27 h). Animals were sacrificed t=48 h. Lung homogenates were prepared to determine bacterial presence. MTD were prepared from 10% of total liver in saline. 50 μL of OD600=0.1, P. aeruginosa was injected intratracheally.
BM-PMN were freshly prepared from donor CD-1 mice and ~2×106 cells were injected intratracheally. Saline/Bac: N=10, MTD/Bac: N=11, MTD/Bac/PMN: N=12. Mean and SD values are shown.
* denotes a significant difference by One-Way ANOVA, Tukey.
3. Prevention of late onset of pneumonia with increased number of P. aeruginosa
Either saline (Group 1, Controls) or mtDAMPs (Groups 2 and 3) were given i.p. at T=0. At T=24 hours, all groups had P. aeruginosa (OD600=0.111, 5×106 CFU i.t. in 50μL) instilled intra-tracheal (note 5× higher PA dose than Experiments 1 and 2). Three hours later, BM-PMN (2×106 cells) were applied i.t. in Group 3 only. At 24 h after bacterial inoculation all animals were sacrificed to evaluate bacterial clearance in lung as discussed below. Number of animals used for Group 1, 2, and 3 were 4, 6, and 8, respectively. (See Figure 4)
Figure 4. Effects of exogenous PMN on clearance of Pseudomonas pneumonia.
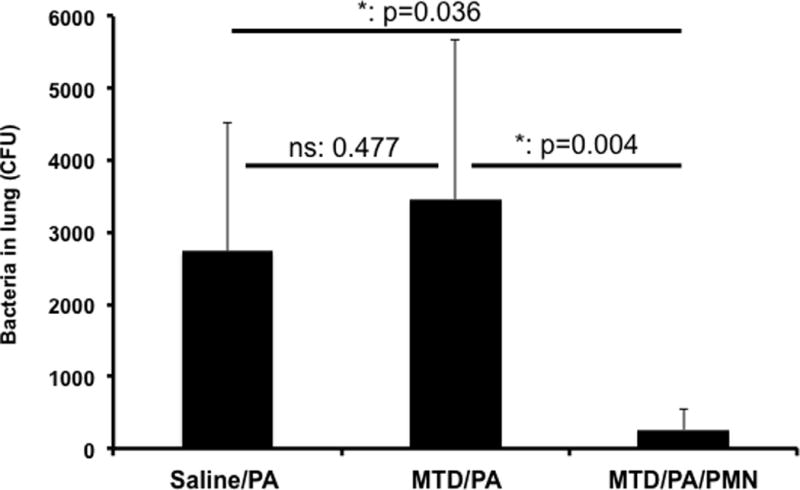
Protocols are similar to Figure 2, however an increased number of P. aeruginosa (OD600=0.111) was injected to the lungs.
Saline/PA: n=4, MTD/PA: n=6, MTD/PA/PMN: n=8. Mean and SD values are shown. * denotes a significant difference by One-Way ANOVA, Tukey.
4. Evaluation of potential lung injury due to PMN instillation
First, PMN from CD-1 mice (or saline) were instilled i.t. in CD-1 mice given i.p MTD plus i.t. p. Aeruginosa. Then, cross instillation of CD-1 PMN into BL6 mice and vice versa was performed. BALF samples were collected from the mice at 24 hours as described above. Protein concentrations in the BALF samples were examined as a marker for lung damage caused by the various treatments. Protein concentrations were determined by BCA protein assay (ThermoFisher) where each samples were evaluated in triplicates. (See Figure 5).
Figure 5. Exogenous PMN do not injure the lung.
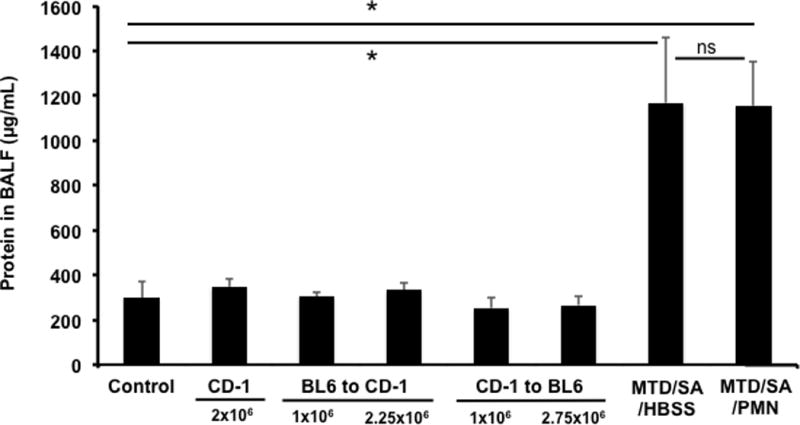
Lung injury was evaluated by assaying protein leak into BAL fluids. Control: saline i.t. (n=5), CD-1: BM-PMN from CD-1 to CD-1, 2×106 i.t. (n=5), BL6 to CD-1: BM-PMN from BL6 mice (n=2) were instilled i.t. into CD-1 mice. CD-1 to BL6: BM-PMN from CD-1 were instilled i.t. into BL6 mice (n=2). MTD/SA: MTD from 10% liver is given i.p. at t=0. S. aureus is given i.t. (8.6×106 CFU) at T=3 h (n=3). MTD/SA/PMN: As in MTD/SA but followed by PMN i.t. (1×106) at T=6 h (n=3). * denotes P<0.001 by One-Way ANOVA with Tukey’s test. ns denotes P=0.090. Sample collection: Control (saline) and CD-1 to CD-1 PMN i.t. 24 h, BL6 to CD-1 or CD-1 to BL6: 72 h, MTD/SA/HBSS and MTD/SA/PMN: 23 h. The number in bracket represents the number of animals used. Even though only two animals were used for some conditions, each BALF was evaluated for protein assay in triplicates. Thus statistical analysis is trustworthy since we are using at least 6 numbers to obtain “mean and SD values”.
Data Analysis and Power calculations and mice number estimation
Data were analyzed by One-Way ANOVA analysis with Tukey’s post hoc test utilizing SigmaPlot 13.1 statistical software (Systat Software, Inc.) to evaluate for significant differences. Our preliminary results suggest that mean bacterial numbers for mtDAMPs + bacteria and mtDAMPs + bacteria + PMN were 5600 and 1300, respectively. Also, standard deviations were 4000 and 700, respectively. Power analysis calculations using SigmaPlot 13.1 show that samples from 8 mice of each group would provide approximately 80% power to detect differences in true mean value of each treatment at the p=0.005 level of significance. Similarly, we used at least 8 mice per group for other experiments. Numbers of animals were modified depending on data we obtained. Mean and SD values were also shown.
RESULTS
1. Intratracheal Instillation of PMN prevents establishment of early Gram (+) pneumonia after injury
We tested whether the number of PMN and function in lungs at the time of infection might be crucial for Gram (+) bacterial clearance. We analyzed three mouse groups: 1. i.p. saline + i.t. bacteria (n=8); 2. i.p. mtDAMPs + i.t. bacteria (n=8); and 3. i.p. mtDAMPs + i.t. bacteria + i.t. PMN (n=9). The number in each bracket represents the number of animals used.
Three hours after i.p. injection of mtDAMPs or saline (vehicle), bacteria (S. aureus, 5×106 CFU/50μL) was applied. Another 3h later PMN (2×106 cells) were applied i.t. in Group 3. Twenty-four hours after bacteria infusion, we examined the lung for bacterial clearance.
In prior work8, we showed that exposure to mtDAMPs in fractures decreased clearance of S. aureus (SA) from the airspaces. We see again that systemic exposure to DAMPs (in this case MTD given i.p. to model abdominal injury) reduced SA clearance from whole lung homogenates (P=0.047, Figure 2 Bar 1 vs. 2). Essentially identical results were seen in culturing BALF samples (Supplemental Digital Content 1). Critically, we also now see that i.t. instillation of exogenous BM-PMN restored clearance of SA to the level of bacteria-only controls (p<0.038, Figure 2, Bar 2 vs. 3). This was again reproduced in BALF analysis. In each case, i.t.-PMN returned clearance of the SA inoculum after i.p. MTD administration to the level of controls (no MTD, P=NS). These data show that intra-tracheal PMN administration can restore normal pulmonary clearance of gram-positive bacteria, thus preventing establishment of early onset, post-traumatic pneumonia in an animal system where abdominal trauma has been modeled by administration of i.p mtDAMPs.
2. Intratracheal Instillation of PMN prevents establishment of late Gram (−) pneumonia after injury
We tested whether the number of PMN and function in lungs at the time of infection might be crucial for Gram (−) bacterial clearance. We analyzed three analogous mouse groups (Figure 3). These were different in that the time points used for experimentation were somewhat later so as to better reflect the evolution of late, hospital acquired pneumonia (HAP). The Gram (−) Pseudomonas aeruginosa (PA, OD600=0.1; 5×106 CFU in 50 μL) was instilled intra-tracheal 24 hrs after mtDAMPs were given i.p.. In Group 3 BM-PMN (2×106 cells) were applied i.t. 3h later. 24 h after bacterial inoculation animals were sacrificed to evaluate bacterial clearance from the lung as previously. Similar to early S. aureus infection, the saline/bacteria group (n=10) showed complete bacterial clearance from the lung (Bar 1). The group given MTD i.p. prior to bacteria group (n=11, Bar 2) showed very limited bacterial clearance. But the group given MTD and bacteria followed by PMN (n=12, Bar 3) showed near complete bacterial clearance very similar to that seen in Saline/Bac. Clearance was significantly different than the MTD/Bac group (P=0.038). The number in each bracket represents the number of animals used.
3. Intratracheal Instillation of PMN can treat Gram (−) pneumonia after injury
Since results one and two above showed that PMN could prevent establishment of CAP and HAP after injury, we next examined whether exogenous PMN could prevent pneumonia from becoming established after a dose of bacteria that was sufficient to cause pneumonia in the absence of injury. We thus applied more bacteria intratracheally (PA, 50μL of OD600=0.111) and as shown in Figure 4 P. aeruginosa at this higher dose is not cleared spontaneously (Bar 1, saline/Bac, n=4). In fact, bacterial growth after this inoculum is very similar to that seen in in the presence of DAMPs (MTD/Bac group, Bar 2, n=6). Nonetheless, even in the presence of DAMPs, instillation of external PMN (MTD/Bac/PMN, n=8) completely eliminates bacteria from the lungs. The number in each bracket represents the number of animals used.
4. Intratracheal Instillation of PMN between mouse strains does not increase lung leak
Infusion of BM-PMN was found to have no adverse effects on lung leak when using PMN from the same strain of mouse (Figures 2 and 3). Next, we evaluated whether instillation of BM-PMN between different strains of mice caused changes in permeability as measured by changes in BALF total protein levels. This is important for design of future mechanistic studies as well as for future treatment options since it infers that xenograft PMN might be used this way therapeutically. We therefore prepared BM-PMN from C57BL/6 and CD-1 mice and instilled them intratracheally into CD-1 and C57BL/6 mice, respectively. 72 hours after i.t. installation, BALF specimens were prepared to evaluate alveolar protein concentrations as a marker for lung injury. As controls, CD-1 mice were instilled with saline i.t. (n=5) or BM-PMN i.t. from CD-1 mice (n=5) and BALF specimens were collected 24 h after instillation. In addition, CD-1 mice were injected with MTD (10% liver, i.p.) followed by S. aureus i.t. and Hepes buffered saline solution (n=3) or BM-PMN (both i.t.) (n=3). As shown in Figure 5 not only saline and BM-PMN from CD-1 but an exchange of BM-PMN between strains (n=2 each) did not induce any lung injury (~300 μg/mL). As expected bacterial infection did cause lung injury (1,200 μg/mL) but instillation of exogenous PMN did not increase it (~1,200 μg/mL). BALF protein in controls and all PMN exchanges were all significantly less than that seen in bacterially infected mice. Note that even increasing the number of PMN infused (up to 2.75 million) between different strains did not cause any damage to the lungs. The number in each bracket represents the number of animals used. Note that BALF from each animal was evaluated for protein concentration in triplicates. Thus we compared “mean and SD data” from at least 6 individual results.
DISCUSSION
Trauma patients in intensive care units, and especially those with abdominal injuries are at high risk for developing infections and nosocomial pneumonias are common in this population17. Historically, this predilection to pneumonia has been attributed to contamination of the lung by loss of airway reflexes, to aspiration related to endotracheal intubation and to poor pulmonary toilet due to pain from injury or needed operations; but these assertions are unproven6. We show here that exogenous neutrophils delivered via the airway can protect against early community acquired pneumonia as well as late, hospital-acquired pneumonias after injury. We also find tantalizing early data suggesting that airway-delivered PMN may be used to attack established gram-negative pneumonias directly since PMN application effectively cleared increased number of Gram (−) bacterial infection that could not be cleared in uninjured animals. In addition, our data showed that PMN exchange between two different strains of mice does not induce any lung injury suggesting that PMN from animals such as pigs may be used to humans in the future.
Mitochondria (MT) originated as bacterial endosymbionts18, so when they are released from injured tissues they present specific N-formylated-peptides and mitochondrial DNA species to the innate immune system. These DAMPs modulate systemic immune function7,8,14,15,19,20. Mitochondria also contain n-formyl peptides that are powerful PMN chemoattractants via the formyl peptide receptors (FPRs)23. FPR signaling depends heavily upon intracellular calcium ([Ca2+]i) release and subsequent store-operated calcium entry (SOCE)22. Mitochondrial DNA (mtDNA) is another key component of MT that activates PMN via toll-like receptors (TLR-9), and induces IL-8 release by activating MAP-kinases23; mtDNA also stimulates formation of neutrophil extracellular traps (NETs)24,25,26. These responses all play important roles in bacteria killing14. Moreover, mtDAMPs activate monocytes/macrophages, leading to further production of chemoattractants at local injury sites. Thus mtDAMPs can elicit chemoattractant gradients to injury sites, causing PMN to migrate preferentially to sites of injury. We have shown that liver injuries, fractures (experimental pseudo fractures) and intraperitoneal (i.p.) injection of damaged MT (MTD), all act as injury models, attracting PMN to sites of injury8. Furthermore, we have shown that exposure to formyl peptides originating from clinical injury makes PMN less sensitive to lung-derived chemokines7. Accordingly, after injury we see decreased innate immune and PMN response to bacterial colonization of the lung27,28.
Gram-negative bacteria are increasingly resistant to available antibiotics and some strains are resistant to most or all available treatments. S. aureus is also a common cause of resistant bacterial infections after trauma29,30. These events increase morbidity and mortality from bacterial infections and thus to higher healthcare costs29. Moreover, no new antibiotics are expected in the near future31,32. Thus cell-based, non-antibiotic therapies may become vital for treatment of post-traumatic pneumonias. Also, if increased susceptibility to pneumonia after injury reflects the exhaustion of PMN function after partial activation, broad-based therapies to prevent or treat infection could be directed at recruiting functionally active PMN to the airway or blocking receptors (like FPR-1) that increase PMN migration to and activation at sites of injury.
In conclusion, we investigated the association between tissue injury and distant infections and established that when mtDAMPs are released from damaged tissues PMN activity in the lung is suppressed. This suppression can be overcome directly by instilling functional neutrophils into the airway in the mouse. Our results suggest PMN instillation prevents bacterial colonization but does not prove its effectiveness on established pneumonia. We will further investigate the effectiveness on established pneumonia by delaying PMN instillation. Large animal models including primate will be utilized before we can consider such therapies in humans, but the use of exogenous immune cells to bolster the innate immune resistance of the airway to infection after injury is an exciting possibility.
Supplementary Material
Supplemental Digital Content 1. Effects of mtDAMPs and external PMN on S. aureus (SA) clearance in lung. CD-1 mice were separated into three groups. 1. Saline i.p. injection (time 0) followed by S. aureus (3h) i.t. Saline/SA.
2. MTD i.p. followed by S. aureus MTD/SA, 3. MTD i.p. followed by S. aureus and BM-PMN i.t. (6h) MTD/SA/PMN. Animals were sacrificed t=24 h. BALF and lung homogenates were prepared to determine bacterial presence.
MTD prepared from 10% of total liver in saline. 50 uL of OD600=0.1 S. aureus was injected intratracheally. BM-PMN were freshly prepared from donor CD-1 mice and ~2×106 cells were injected intratracheally.
BALF: Saline/SA: N=16, MTD/SA: N=12, MTD/SA/PMN: N=16.
Mean and SD values are shown.
* denotes a significant difference by One-Way ANOVA, Tukey.
Acknowledgments
Funding
NIH/NIGMS 5 R01 GM089711 (C.J.H.)
CNPq, Conselho Nacional de Desenvolvimento Científico e Tecnológico - Brasil (I.R.)
Footnotes
Conflict of interest
Authors declare no conflict of interest
Meeting presentation
This study was presented at the 75th annual meeting of the American Association for the Surgery of Trauma, September 14–17, 2016, in Waikoloa, Hawaii.
Author contributions
Experiments were conceived and designed by KI and CJH. Experiments were performed by KI, IR, JZ, DG, and MDP. Data were analyzed by KI. The paper was written by KI, CJH, and LEO.
References
- 1.Ramaiah R, Grabinsky A, Williamson K, Bhanankar SM. Trauma care today, what’s new? Int J Crit Illn Inj Sci. 2011;1:22–26. doi: 10.4103/2229-5151.79278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Michetti CP, Fakhry SM, Ferguson PL, Cook A, Moore FO, Gross R, Investigators AV-AP Ventilator-associated pneumonia rates at major trauma centers compared with a national benchmark: a multi-institutional study of the AAST. J Trauma. 2012;72:1165–1173. doi: 10.1097/TA.0b013e31824d10fa. [DOI] [PubMed] [Google Scholar]
- 3.Papia G, McLellan BA, El-Helou P, Louie M, Rachlis A, Szalai JP, Simor AE. Infection in hospitalized trauma patients: incidence, risk factors, and complications. J Trauma. 1999;47:923–927. doi: 10.1097/00005373-199911000-00018. [DOI] [PubMed] [Google Scholar]
- 4.Chevret S, Hemmer M, Carlet J, Langer M. Incidence and risk factors of pneumonia acquired in intensive care units. Results from a multicenter prospective study on 996 patients. European Cooperative Group on Nosocomial Pneumonia. Intensive Care Med. 1993;19:256–264. doi: 10.1007/BF01690545. [DOI] [PubMed] [Google Scholar]
- 5.Wallace WC, Cinat M, Gornick WB, Lekawa ME, Wilson SE. Nosocomial infections in the surgical intensive care unit: a difference between trauma and surgical patients. Am Surg. 1999;65:987–990. [PubMed] [Google Scholar]
- 6.Guimaraes MM, El Dib R, Smith AF, Matos D. Incentive spirometry for prevention of postoperative pulmonary complications in upper abdominal surgery. Cochrane Database Syst Rev. 2009:CD006058. doi: 10.1002/14651858.CD006058.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li H, Itagaki K, Sandler N, Gallo D, Galenkamp A, Kaczmarek E, Livingston DH, Zeng Y, Lee YT, Tang IT, et al. Mitochondrial damage-associated molecular patterns from fractures suppress pulmonary immune responses via formyl peptide receptors 1 and 2. J Trauma Acute Care Surg. 2015;78:272–279. doi: 10.1097/TA.0000000000000509. discussion 279–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao C, Itagaki K, Gupta A, Odom S, Sandler N, Hauser CJ. Mitochondrial damage-associated molecular patterns released by abdominal trauma suppress pulmonary immune responses. J Trauma. 2014;76:1222–1227. doi: 10.1097/TA.0000000000000220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Swamydas M, Lionakis MS. Isolation, purification and labeling of mouse bone marrow neutrophils for functional studies and adoptive transfer experiments. J Vis Exp. 2013:e50586. doi: 10.3791/50586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gauthier JF, Fortin A, Bergeron Y, Dumas MC, Champagne ME, Bergeron MG. Differential contribution of bacterial N-formyl-methionyl-leucyl- phenylalanine and host-derived CXC chemokines to neutrophil infiltration into pulmonary alveoli during murine pneumococcal pneumonia. Infect Immun. 2007;75:5361–5367. doi: 10.1128/IAI.02008-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boxio R, Bossenmeyer-Pourie C, Steinckwich N, Dournon C, Nusse O. Mouse bone marrow contains large numbers of functionally competent neutrophils. J Leukoc Biol. 2004;75:604–611. doi: 10.1189/jlb.0703340. [DOI] [PubMed] [Google Scholar]
- 12.Hammer MC, Baltch AL, Sutphen NT, Smith RP, Conroy JV. Pseudomonas aeruginosa: quantitation of maximum phagocytic and bactericidal capabilities of normal human granulocytes. J Lab Clin Med. 1981;98:938–948. [PubMed] [Google Scholar]
- 13.Leijh PC, van den Barselaar MT, van Zwet TL, Dubbeldeman-Rempt I, van Furth R. Kinetics of phagocytosis of Staphylococcus aureus and Escherichia coli by human granulocytes. Immunology. 1979;37:453–465. [PMC free article] [PubMed] [Google Scholar]
- 14.Itagaki K, Kaczmarek E, Lee YT, Tang IT, Isal B, Adibnia Y, Sandler N, Grimm MJ, Segal BH, Otterbein LE, et al. Mitochondrial DNA released by trauma induces neutrophil extracellular traps. PLoS One. 2015;10:e0120549. doi: 10.1371/journal.pone.0120549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun S, Sursal T, Adibnia Y, Zhao C, Zheng Y, Li H, Otterbein LE, Hauser CJ, Itagaki K. Mitochondrial DAMPs Increase Endothelial Permeability through Neutrophil Dependent and Independent Pathways. PLoS One. 2013;8:e59989. doi: 10.1371/journal.pone.0059989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cai Y, Kimura S. Noninvasive intratracheal intubation to study the pathology and physiology of mouse lung. J Vis Exp. 2013:e50601. doi: 10.3791/50601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Antonelli M, Moro ML, Capelli O, De Blasi RA, D’Errico RR, Conti G, Bufi M, Gasparetto A. Risk factors for early onset pneumonia in trauma patients. Chest. 1994;105:224–228. doi: 10.1378/chest.105.1.224. [DOI] [PubMed] [Google Scholar]
- 18.Margulis L. Genetic and evolutionary consequences of symbiosis. Exp Parasitol. 1976;39:277–349. doi: 10.1016/0014-4894(76)90127-2. [DOI] [PubMed] [Google Scholar]
- 19.Hauser CJ, Sursal T, Rodriguez EK, Appleton PT, Zhang Q, Itagaki K. Mitochondrial damage associated molecular patterns from femoral reamings activate neutrophils through formyl peptide receptors and P44/42 MAP kinase. J Orthop Trauma. 2010;24:534–538. doi: 10.1097/BOT.0b013e3181ec4991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raoof M, Zhang Q, Itagaki K, Hauser CJ. Mitochondrial peptides are potent immune activators that activate human neutrophils via FPR-1. J Trauma. 2010;68:1328–1332. doi: 10.1097/TA.0b013e3181dcd28d. discussion 1332–1324. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Q, Itagaki K, Hauser CJ. Mitochondrial DNA is released by shock and activates neutrophils via p38 map kinase. Shock. 2010;34:55–59. doi: 10.1097/SHK.0b013e3181cd8c08. [DOI] [PubMed] [Google Scholar]
- 22.Putney JW., Jr A model for receptor-regulated calcium entry. Cell Calcium. 1986;7:1–12. doi: 10.1016/0143-4160(86)90026-6. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, Brohi K, Itagaki K, Hauser CJ. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464:104–107. doi: 10.1038/nature08780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brinkmann V, Goosmann C, Kuhn LI, Zychlinsky A. Automatic quantification of in vitro NET formation. Front Immunol. 2012;3:413. doi: 10.3389/fimmu.2012.00413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brinkmann V, Laube B, Abu Abed U, Goosmann C, Zychlinsky A. Neutrophil extracellular traps: how to generate and visualize them. J Vis Exp. 2010 doi: 10.3791/1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 27.Tarlowe MH, Duffy A, Kannan KB, Itagaki K, Lavery RF, Livingston DH, Bankey P, Hauser CJ. Prospective study of neutrophil chemokine responses in trauma patients at risk for pneumonia. Am J Respir Crit Care Med. 2005;171:753–759. doi: 10.1164/rccm.200307-917OC. [DOI] [PubMed] [Google Scholar]
- 28.Tarlowe MH, Kannan KB, Itagaki K, Adams JM, Livingston DH, Hauser CJ. Inflammatory chemoreceptor cross-talk suppresses leukotriene B4 receptor 1-mediated neutrophil calcium mobilization and chemotaxis after trauma. J Immunol. 2003;171:2066–2073. doi: 10.4049/jimmunol.171.4.2066. [DOI] [PubMed] [Google Scholar]
- 29.Bassetti M, De Waele JJ, Eggimann P, Garnacho-Montero J, Kahlmeter G, Menichetti F, Nicolau DP, Paiva JA, Tumbarello M, Welte T, et al. Preventive and therapeutic strategies in critically ill patients with highly resistant bacteria. Intensive Care Med. 2015;41:776–795. doi: 10.1007/s00134-015-3719-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lowy FD. Staphylococcus aureus infections. N Engl J Med. 1998;339:520–532. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- 31.Chambers HF, Deleo FR. Waves of resistance: Staphylococcus aureus in the antibiotic era. Nat Rev Microbiol. 2009;7:629–641. doi: 10.1038/nrmicro2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.DeLeo FR, Otto M, Kreiswirth BN, Chambers HF. Community-associated meticillin-resistant Staphylococcus aureus. Lancet. 2010;375:1557–1568. doi: 10.1016/S0140-6736(09)61999-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content 1. Effects of mtDAMPs and external PMN on S. aureus (SA) clearance in lung. CD-1 mice were separated into three groups. 1. Saline i.p. injection (time 0) followed by S. aureus (3h) i.t. Saline/SA.
2. MTD i.p. followed by S. aureus MTD/SA, 3. MTD i.p. followed by S. aureus and BM-PMN i.t. (6h) MTD/SA/PMN. Animals were sacrificed t=24 h. BALF and lung homogenates were prepared to determine bacterial presence.
MTD prepared from 10% of total liver in saline. 50 uL of OD600=0.1 S. aureus was injected intratracheally. BM-PMN were freshly prepared from donor CD-1 mice and ~2×106 cells were injected intratracheally.
BALF: Saline/SA: N=16, MTD/SA: N=12, MTD/SA/PMN: N=16.
Mean and SD values are shown.
* denotes a significant difference by One-Way ANOVA, Tukey.


