Abstract
After myocardial infarction (MI), the heart undergoes fibrotic pathological remodeling instead of repair and regeneration. With multiple pathologies developing after MI, treatment using several proteins is expected to address this range of pathologies more effectively than a single-agent therapy. A factorial design of experiments study guided us to combine three complementary factors in one injection: tissue inhibitor of metalloproteinases-3 (TIMP-3) was embedded in a fibrin gel for signaling in the initial phase of the treatment, while basic fibroblast growth factor (FGF-2) and stromal cell-derived factor 1-alpha (SDF-1α) were embedded in heparin-based coacervates for sustained release and distributed within the same fibrin gel to exert their effects over a longer period. The gel was then tested in a rat model of myocardial infarction. Contractility of rat hearts treated with the protein coacervate-gel composite stabilized and slightly improved after the first week while contractility continued to decrease in rats treated with free proteins or saline over the 8 week study period. Hearts receiving the protein coacervate-gel composite treatment also exhibited reduced ventricular dilation, inflammation, fibrosis, and extracellular matrix (ECM) degradation. Revascularization, cardiomyocyte preservation, stem cell homing, and increased myocardial strain likely all contributed to the repair. This study demonstrates the potential of a multifactorial therapeutic approach in MI, using three complementary proteins delivered sequentially for comprehensive healing. The study also shows the necessity of controlled delivery for growth factors and cytokines to be an effective treatment.
Keywords: Myocardial infarction, controlled release, proteins, cardiac repair, coacervate, fibrin gel
Introduction
Myocardial infarction (MI) affects 7.6 million Americans with approximately 720,000 experiencing a heart attack each year [1]. MI leads to defects in the contractile function of cardiomyocytes and alterations in the extracellular matrix (ECM) and ventricle geometry. As a consequence of the maladaptation, a non-contracting scar tissue forms, a significant portion of which results in congestive heart failure. Current treatments such as reperfusion, β-blockers, and angiotensin converting enzyme inhibitors, reduce damage but do not restore function. Therefore, therapies that can prevent or reverse the multiple pathologies caused by MI, regenerate the myocardium, and restore cardiac function are urgently needed [2].
To treat multiple pathologies resulting from MI, we set out to explore the use of multiple therapeutic proteins [2]. Our recent study using a statistical fractional factorial design of experiment focused our effort into the controlled and timed release of a combination of complementary proteins that are relatively distinct in their roles in cardiac function: tissue inhibitor of metalloproteinases 3 (TIMP-3), basic fibroblast growth factor (FGF-2), and stromal cell-derived factor 1 alpha (SDF-1α) [3]. TIMP-3 inhibits the activity of matrix metalloproteinases (MMPs) which cleave ECM components [4]. FGF-2 plays a chief role in formation of neovasculature [5]. SDF-1α is a potent chemotactic factor that can recruit stem cells to the infarct region [6, 7]. In the recent study, we demonstrated that optimized dosages of each protein contribute individually to improved functionality [3].
TIMP-3 reduces ECM degradation soon after MI. FGF-2 and SDF-1α promote angiogenesis and recruit progenitor cells to the infarct region, which are events that require prolonged signaling. Therefore, we designed a composite hydrogel comprised of fibrin gel and heparin-based coacervates to achieve the sequential release of TIMP-3 followed by FGF-2 and SDF-1α. To achieve temporal control of the release, TIMP-3 was encapsulated in fibrin gel so it will dominate the early signaling, while FGF-2 and SDF-1α were encapsulated in heparin-based coacervates and distributed in the same fibrin gel to dominate signaling at the later stage (Fig. 1A). Complex coacervates form spontaneously by electrostatic interactions between the aqueous solutions of a polycation and a polyanion [8]. We utilized a synthetic polycation poly(ethylene argininylaspartate diglyceride) (PEAD), the natural polyanion heparin, and heparin-binding proteins to form protein-loaded coacervates. This design allows encapsulation of proteins with high efficiency and enables their controlled release [8, 9].
Figure 1. Design and protein release kinetics of coacervate-fibrin gel composite.
(A) The release system was comprised of a fibrin gel embedding TIMP-3 aimed for early release; and FGF-2/SDF-1α-loaded coacervates distributed within the same gel aimed for late release. The coacervate was formed through electrostatic interactions by combining FGF-2 and SDF-1α with heparin then with PEAD polycation. (B) The cumulative release plot of the proteins shows the total percentage amount of each protein released with time. The plot shows quick release of TIMP-3 by 1 week followed by a sustained release of FGF-2 and SDF-1α up to 6 weeks. Data are presented as means ± SD (n=3).
In this study, we thoroughly tested the efficacy of the spatiotemporal delivery of TIMP-3, FGF-2, and SDF-1α on cardiac function, ventricular dilation and wall thinning, myocardial strain levels, MMP activity, fibrosis, inflammation, cardiomyocyte survival, angiogenesis, stem cell homing, protein signaling, and cell apoptosis at 2 and 8 weeks after MI. This controlled release of three therapeutic proteins can mitigate MI-induced injuries and initiate a robust cardiac repair process, giving hope of a higher level of functional and structural recovery of the infarcted heart.
Materials and Methods
Coacervate formation and release assay of complementary proteins
PEAD was synthesized as previously described [10], yielding the polycation. To form the coacervate, PEAD (25mg/ml) and heparin (5mg/ml, Scientific Protein Labs, Waunakee, WI) were first separately dissolved in 0.9% saline. 100ng of each FGF-2 and SDF-1α were then added to 2μl heparin solution followed by PEAD forming a turbid coacervate. To form the coacervate-gel composite, fibrinogen solution (20mg/ml) containing 100ng TIMP-3 was combined with the coacervate, followed by gelation by adding thrombin (1mg/ml). This is detailed further in Supplementary Methods.
The release assay was performed as previously described [11]. Briefly, each coacervate-gel composite was submerged in 100 μl 0.9% saline (n=3). At each specified timepoint (1h, 6h, 1, 4, 7, 14, 28, and 42 days), the gel was centrifuged at 12,100g for 10min and the supernatant was collected and stored at −80ºC. Fresh saline was then added atop each gel. Protein concentrations in the releasate were calculated by sandwich enzyme-linked immunosorbent assay (ELISA) kits (Peprotech, Rocky Hill, NJ) according to manufacturer instructions. Standard solutions (n=3) containing 100ng of each protein in 100μl saline were prepared to create standard curves and calculate protein release.
Rat acute MI model
University of Pittsburgh Institutional Animal Care and Use Committee (IACUC) approval was obtained prior to beginning all animal studies. The induction of MI was performed by permanent ligation of the left anterior descending coronary artery as previously described [3, 11] and further detailed in Supplementary Methods. A total of 56 rats were used in this study across 4 groups: shame, saline, free proteins, and controlled release with evaluations performed at 2 weeks (n=17, 4–5 per group) and 8 weeks (n=39, 9–10 per group). Empty vehicle (empty coacervate-gel composite) was not tested as a control in this study as it has shown no difference to saline in our previous work [11].
Echocardiography and cardiac MRI
Echocardiography and cardiac MRI were performed to compute end systolic area (ESA), end diastolic area (EDA), fractional area change (FAC), end systolic volume (ESV), end diastolic volume (EDV), and ejection fraction (EF) as previously described [3, 11] and further detailed in Supplementary Methods. Echocardiography measurements were made pre-MI, 1, 2, 5, and 8 weeks post-MI using B-mode short axis imaging. Cardiac MRI measurements were made only at the 8 week timepoint. NIH ImageJ software was used to make image-based mesurements.
Myocardial strain level measurements
The B-mode frames of left ventricle (LV) short-axis view acquired at 8 weeks post-MI were analyzed (n=5 rats per group) using a strain analysis algorithm (VevoStrainTM, Vevo2100). Five regions of interest (ROI) were selected along the LV mid-wall including one ROI in the anterior lateral (infarcted area) and four ROIs in the anterior medial, septal, posterior, posterior lateral (unaffected areas) walls of the LV. The peak strain in the infarcted area was normalized to the average peak strains of the four ROIs in unaffected LV walls during full cardiac cycles. The radial strain, defined as the percent change in myocardial wall thickness, was computed.
Histology
At 2 weeks (n=4–5 per group) and 8 weeks (n=5–7 per group) post-infarction, rats were sacrificed by injecting 2ml of saturated potassium chloride (KCl) solution (Sigma Aldrich, St. Louis, MO) in the LV to arrest the heart in diastole. Hearts were harvested, fixed in 2% paraformaldehyde (Fisher Scientific, Fair Lawn, NJ) for 1–2 hours, deposited in 30% sucrose solution (w/v) overnight, frozen in O.C.T compound (Fisher Healthcare, Houston, TX), and stored at −20°C. Specimens were cryosectioned at 8 μm thickness from apex to the ligation level with 500 μm intervals.
Hematoxylin and eosin (H&E) staining was performed for general evaluation. H&E stained slides were selected and the ventricular wall thickness in the infarct zone (n=3–4 per group at 2 wks, n=4–6 at 8 wks) was measured near the mid-section level of the infarct tissue using NIS Elements AR imaging software (Nikon Instruments, Japan).
For assessment of interstitial fibrosis, picrosirius red staining was used to stain collagen fibers and image under polarized light. The fraction area of collagen deposition in the cross-sectional area of the whole heart was measured by NIS software near the mid-section level of the infarct tissue (n=3–5 per group at 2 wks, n=4–7 at 8 wks). An object count tool was used to include RGB pixels specific to the stained collagen fibers in the heart area by defining a proper threshold value.
Mast cells are known to infiltrate the heart following MI and are key regulators in the activation of MMPs and inflammation [12]. To assess their infiltration, toluidine blue staining was performed, which stains mast cells a deep violet color. The density of mast cells was quantified at the mid-level of infarct by manually counting the number of cells present at two opposite regions of the infarct border zone and reporting the average as cells/mm2 (n=5 rats per group at 8 wks).
Immunohistochemistry
For evaluation of inflammation, a rabbit polyclonal antibody F4/80 (1:100, Santa Cruz Biotechnology, Dallas, TX), a pan-macrophage surface marker, was used followed by an Alexa fluor 594 goat anti-rabbit antibody (1:200, Invitrogen, Carlsbad, CA). Slides were also co-stained by a mouse anti-rat CD163 (1:150, Bio-Rad Laboratories, Hercules, CA), an M2 macrophage phenotype marker, followed by an Alexa fluor 488 goat anti-mouse antibody (1:200, Invitrogen, Carlsbad, CA). Slides were last counterstained with 4′,6-diamidino-2-phenylindole (DAPI) (Invitrogen, Carlsbad, CA). For quantification near the mid-section level of the infarct tissue, F4/80+ and CD163+ cells were counted in two opposite regions of the infarct border zone, averaged, and reported per mm2 areas (n=3–4 rats per group at 2 wks).
For evaluation of cardiac muscle viability, a rabbit polyclonal cardiac troponin I (cTnI) antibody (1:200, US Abcam, Cambridge, MA) was used followed by an Alexa fluor 488 goat anti-rabbit antibody (1:200, Invitrogen, Carlsbad, CA). Slides were counterstained with DAPI. The fraction area of viable cardiac muscle in the cross-sectional area of the whole heart was measured by NIS Elements AR software near the mid-section level of the infarct tissue (n=3–5 per group at 2 wks, n=5–6 at 8 wks). An object count tool was used to include RGB pixels specific to the stained viable cardiac muscle in the heart area by defining a proper threshold value.
For evaluation of angiogenesis, endothelial cells (ECs) were detected by a rabbit polyclonal von Willebrand factor (vWF) antibody (1:200, US Abcam, Cambridge, MA) followed by an Alexa fluor 594 goat anti-rabbit antibody (1:200). Mural cells were detected by a FITC-conjugated anti-α-smooth muscle actin (α-SMA) monoclonal antibody (1:500, Sigma Aldrich, St. Louis, MO). Slides were last counterstained with DAPI. For quantification near the midsection level of the infarct tissue, vWF+ vessels (defined as those with lumen) and α-SMA+ vessels were counted in two opposite regions of the infarct border zone, averaged, and reported per mm2 areas (n=3–4 rats per group at 2 wks, n=5–6 per group at 8 wks).
For evaluation of stem cell homing, stem/progenitor cells were detected by a rabbit polyclonal c-Kit antibody (1:100, Santa Cruz Biotechnology, Dallas, TX) followed by an Alexa fluor 488 goat anti-rabbit antibody (1:200). Slides were counterstained with DAPI. For quantification near the mid-section level of the infarct tissue, c-Kit+ cells were counted in two opposite regions of the infarct border zone, averaged, and reported per mm2 areas (n=5 rats per group at 8 wks).
Molecular markers expression by western blot
Rat hearts (n=15) were harvested and rapidly stored at −80°C for western blotting. For protein extraction, myocardial specimens weighing approximately 100 mg were excised from the LV generating a composite material comprising a spectrum between normal, infarct, and borderzone tissue. The tissues were then homogenized at 0.2 μg/ml in a modified lysis RIPA buffer (50mM Tris–HCl, 1% NP-40, 20 mM DTT, 150 mM NaCl, pH=7.4) with protease and phosphatase inhibitors. The complex was then centrifuged at 12,100 g for 10 min, and the supernatant was collected and stored at −80°C until use.
For total protein content, the extracts above were quantified with Pierce 660 nm Protein Assay (Thermo Fisher Scientific, Waltham, MA). The equivalent of 100 μg protein was separated using 11.5% gel and then transferred onto a PVDF membrane (Bio-Rad Laboratories, Hercules, CA). The membrane was blocked with 5% BSA in TBS with 0.05% Tween 20 for 1h, then incubated with following antibody solutions: AKT, p-AKT, ERK1/2, p-ERK1/2 (all at 1:300, Santa Cruz Biotechnology, Dallas, TX), cleaved caspase-3 (1:1,000, Cell Signaling Technology, Boston, MA), and GAPDH (1:5000, US Abcam, Cambridge, MA). The membranes were washed with TBS 3 times and incubated with secondary antibodies for 2h at room temperature. Signals were visualized using the ChemiDocTM XRS + Imaging System (Bio-Rad Laboratories, Hercules, CA), and band densities were quantified using NIH ImageJ (n=3 per group).
Myocardial protein secretion levels by ELISA
The tissue lysates acquired in the western blot section (n=3–4 rats per group) were used for detecting the levels of insulin-like growth factor-I (IGF-I), vascular endothelial growth factor (VEGF), sonic hedgehog (Shh), transforming growth factor-β1 (TGF-β1), interleukin 1β (IL-1β), interleukin 6 (IL-6), and tissue necrosis factor-α (TNF-α) in the LV myocardium. Sandwich ELISA kits (PeproTech, Rocky Hill, NJ) were used per the manufacturer’s instructions with lysate dilutions for VEGF (1:20), IGF-I (1:50), IL-1β (1:15), IL-6 (1:15), and TNF-α (1:15). For Shh and TGF-β1, indirect ELISAs were run using rabbit polyclonal antibodies against Shh and TGF-β1 (both at 1:30, Santa Cruz Biotechnology, Dallas, TX) followed by a secondary biotinylated goat anti-rabbit IgG (1:100, Santa Cruz Biotechnology, Dallas, TX). Lysates were diluted 1:15 for Shh and 1:25 for TGF-β1. The absorbance at 450/540nm was measured by a SynergyMX plate reader. Results were corrected to account for differences in total protein content of samples.
MMP-2/9 activity assay
The tissue lysates acquired in the western blot section (n=3–4 rats per group) were used for detecting the activity of MMP-2/9 in the LV myocardium. The Calbiochem InnoZymeTM Gelatinase activity assay fluorogenic kit (EMD Millipore, Bellerica, MA) was followed per the manufacturer’s instructions. Briefly, lysate samples (diluted 1:2 in activation buffer) were incubated with a fluorogenic substrate solution that is highly selective for MMP-2 and MMP-9. Gelatinases in the sample lysates of the myocardium cleave the substrate, resulting in an increase in fluorescent signal measured at an excitation wavelength of 320nm and an emission wavelength of 405nm by a SynergyMX plate reader. The gelatinase control, activated similarly, was used at serial dilutions to create a standard curve for converting the fluorescence values of MMP activity to concentrations (ng/ml).
Statistical analysis
Results are presented as means ± standard deviations (SD). GraphPad Prism 5.0 software (La Jolla, CA) was used for statistical analysis. Statistical differences between groups were analyzed by one-way independent ANOVA (multiple groups) or two-way mixed ANOVA (repeated echocardiographic measurements) with 95% confidence interval. Bonferroni multiple comparison test was performed for ANOVA post-hoc analysis. Statistical significance was set at p<0.05.
Results
Temporal control of protein release
We tested the coacervate-gel composite’s ability to release TIMP-3 early followed by a sustained release of FGF-2 and SDF-1α by an in vitro release assay (Fig. 1A). The loading efficiencies were 85% for TIMP-3, 97% for FGF-2, and 98% for SDF-1α (Fig. 1B). By day 1, approximately 40% of loaded TIMP-3 was released, reaching 90% total release by one week (Fig. 1B) translating into relatively higher concentrations of TIMP-3 reaching a maximum of 1 ng/ml during the first week and decreasing thereafter (Fig. S1). We observed a longer sustained release for FGF-2 and SDF-1α with concentrations between 0.25–0.5 ng/ml that lasted for >6 weeks due to their affinity to heparin within the coacervates inside the gel (Fig. S1). By one week, only 21% of FGF-2 and 28% of SDF-1α were released, reaching 55% and 48% total release respectively by 6 weeks (Fig. 1B). Thus the coacervate-gel achieved quick release of TIMP-3 after MI to reduce ECM degradation and inflammation, while providing FGF-2 and SDF-1α in a sustained manner for triggering a robust neovasculature formation process and progenitor cell recruitment.
Improved cardiac function and reduced ventricular dilation
We evaluated the effect of spatiotemporal delivery of TIMP-3, FGF-2, and SDF-1α in a rat MI model using sham, saline, and free proteins as controls. We evaluated changes in left ventricle (LV) contractility as a measure of heart function. Using echocardiography, fractional area change (FAC) was computed from end-systolic area (ESA) and end diastolic area (EDA) values (Fig. 2A). Sham group maintained an FAC value of approximately 55% at all time points post-MI, significantly higher than all infarct groups (Fig. 2B). One week post-infarction, FAC values of saline, free protein, and controlled release groups dropped significantly, however, both controlled release and free proteins had significantly higher FAC than saline (p<0.01). This suggests that the 3 proteins significantly improved cardiac function within 1 week after MI. At 2 weeks, the controlled release group diverged from the negative controls and improved function significantly (p<0.001). Although free proteins performed significantly better than saline (p<0.001) up to 5 weeks, function in both groups kept dropping. In contrast, functional improvement in the controlled release group continued and displayed increasingly larger differences relative to the controls. At 8 weeks, the last time point of the study, controlled release led to a 48% FAC, which was 87% that of the normal FAC value and represented a 74% improvement over saline. The two control groups, saline and free proteins, no longer showed any statistical difference at 8 weeks (p>0.05) (Fig. 2B).
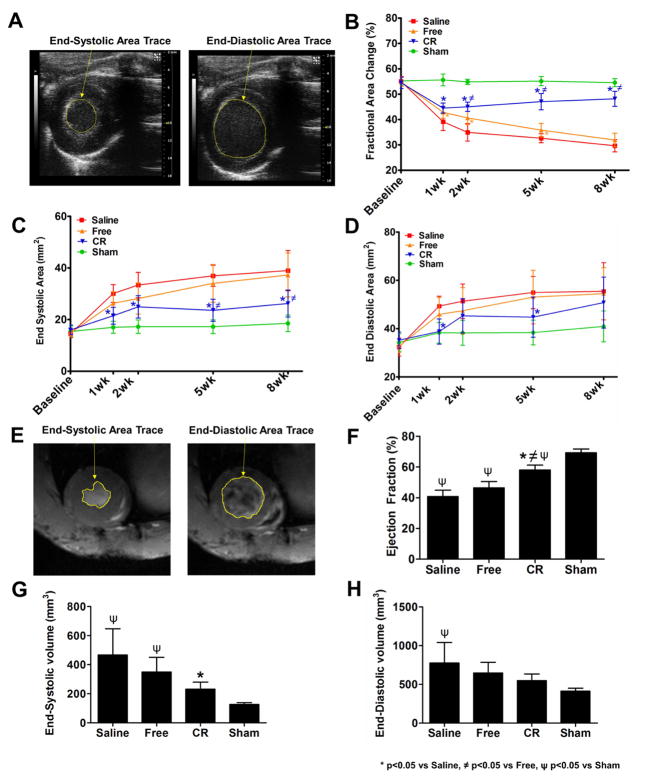
Figure 2. Effect of controlled protein release on cardiac function and LV dilation.
(A) Traces of ESA and EDA areas from short-axis B-mode images of the left ventricle using echocardiography. (B) FAC values show differences between groups after MI at multiple time points, with significantly higher FAC value of controlled release (CR) compared to saline and free proteins from 2 weeks onward. (C) Saline and free proteins groups show increasing ESA values, which were reduced in CR group. (D) Saline and free proteins groups show increasing EDA values, which were reduced in CR group. Data are presented as means ± SD (n=9–10 per group). (E) Traces of ESA and EDA areas from short-axis view images of the LV using cardiac MRI. (F) EF values show differences between groups after MI at 8 weeks, with significantly higher EF% of the CR group compared to saline and free proteins. (G) Saline and free proteins groups show increasing ESV value at 8 weeks, which was significantly reduced in CR. (H) Saline and free proteins groups show increasing EDV value at 8 weeks, which was reduced in CR. Data are presented as means ± SD (n=5–8 per group). * p<0.05 vs saline, ψ p<0.05 vs free proteins, ≠ p<0.05 vs sham.
To evaluate the therapy’s effect on ventricular dilation, we assessed the changes in EDA and ESA values. The controlled release group showed significant reduction or trend towards lower EDA and ESA after MI compared to saline (Fig. 2C,D). On the other hand, saline and free protein groups showed progressively higher ESA and EDA at all time points after MI, with no statistical differences between them (p>0.05) (Fig. 2C,D).
The echocardiography results were consistent with MRI measurement at 8 weeks. End-systolic volume (ESV) and end-diastolic volume (EDV) were computed and ejection fraction (EF) was calculated (Fig. 2E). EF in the controlled release group was 58%, which was at 84% of the sham group (69%) and significantly higher (p<0.001) than saline (41%) and free proteins (46%) (Supplementary videos). The two negative controls showed no difference between each other (p>0.05) (Fig. 2F). Correspondingly, the left ventricle of the controlled release group was less dilated with a significantly smaller ESV than saline (p<0.01) and not significantly different from sham (Fig. 2G). The controlled release group also showed a trend towards lower EDV compared with saline and free protein groups (Fig. 2H).
Preserved myocardial elasticity
We performed myocardial strain analysis at 8 weeks post-MI to evaluate the changes in the radial strain levels of the myocardium with respect to the various treatments by normalizing the peak strain of the infarcted region to the average peak strain in 4 non-infarct regions (Fig. 3A). The radial strain, defined as the percent change in myocardial wall thickness, was measured. The controlled release group exhibited a radial myocardial strain similar to that of sham control (p>0.05), and was significantly higher than the saline group (p<0.01) (Fig. 3B). The free proteins group is similar to saline (p>0.05) and significantly less than sham (p<0.05) (Fig. 3B). This result suggests that controlled delivery of TIMP-3, FGF-2, and SDF-1α protects myocardial elasticity after MI. The prevention of ventricular wall stiffening helps to maintain the heart’s ability to contract and dilate properly.
Figure 3. Effect of controlled protein release on myocardial strain levels.
(A) Strain of an infarcted sample was estimated by normalizing the estimated peak radial strain in the infarcted area to that of the average of 4 non-infarct areas in LV walls during a cardiac cycle. (B) Saline and free proteins groups show decreasing radial strain at 8 weeks, which was significantly higher in the controlled release (CR) group. Data are presented as means ± SD (n=5 per group). * p<0.05 vs saline, ψ p<0.05 vs sham.
Reduced left ventricle wall thinning, MMP activity, and fibrosis
In order to understand tissue level changes that contributed to the functional improvement, we investigated ventricular wall thickness, MMP activity, and fibrosis at 2 and 8 weeks. H&E stained hearts showed increased granulated scar tissue areas with thinner left ventricle walls in the infarct zone and border zone that exacerbated with time in saline and free proteins groups but to a less extent in the controlled release group (Fig. 4A). Controlled protein release significantly prevented ventricular wall thinning at 2 weeks compared to saline (p<0.05) (Fig. 4B). In contrast, LV wall thickness decreased considerably in saline and free proteins groups as early as 2 weeks. At 8 weeks, there were no statistical differences in wall thickness between saline, free proteins, and controlled release although the controlled release group clearly maintained a thicker wall average (Fig. 4B). Wall thickness in the controlled release group was not statistically indifferent to sham at both time points (p>0.05).
Figure 4. Effect of controlled protein release on left ventricle wall thinning, MMP activity, and fibrosis.
(A) Representative H&E images showed left ventricle (LV) wall thinning with damaged cardiac muscle surrounded by scar tissue in saline and free proteins groups at 2 and 8 weeks. However, these damages were apparently alleviated in the controlled release (CR) group. Scale bar=1000μm. (B) Quantitative analysis shows reduced ventricular wall thinning by CR at 2 and 8 weeks over saline and free proteins groups. Data are presented as means ± SD (n=3–4/group at 2 wks, n=4–6 at 8 wks). (C) MMP-2/9 activity assay showed high levels of activity in infarct groups at 8 weeks, but was significantly reduced in CR compared to saline. Data are presented as means ± SD (n=3–4 per group). (D) Representative picrosirius red staining images show the dense collagen deposition along the left ventricle wall and infarct zone in saline, followed by the free proteins group, whereas it was limited to the infarct region in CR at 8 weeks. (E) Quantitative analysis shows that collagen deposition was not different in infarct groups at 2 weeks but was significantly less in CR compared to saline and free groups at 8 weeks. Data are presented as means ± SD (n=3–5/group at 2 wks, n=4–7 at 8 wks). * p<0.05 vs saline, ≠ p<0.05 vs free proteins, ψ p<0.05 vs sham.
At 8 weeks, we evaluated the activity of MMPs in the heart samples. MMP-2 and MMP-9 are important players implicated in many cardiovascular diseases and ECM degradation [13]. They can exacerbate contractile dysfunction by degrading cardiac proteins [2]. All infarct groups showed a high level of MMP-2/9 activity (Fig. 4C). However, controlled release showed significantly lower MMP activity compared to saline (p<0.01) and also lower activity than free proteins group but not to a significant level (p>0.05) (Fig. 4C). MMP activity in the controlled release group was not statistically different from sham (p>0.05). The enhanced reduction of MMP activity by the controlled release group is likely due to the controlled delivery of TIMP-3 within the coacervate-gel, where TIMP-3 can form tight complexes with MMP-2 and MMP-9 to prevent their activation, and thereby reducing ECM degradation and ventricular dilation and remodeling.
Interstitial fibrosis develops at the infarct region and extends to non-infarct areas due to the excessive and uncontrollable collagen deposition that takes place in later stages after MI. This increased collagen deposition leads to increased stiffness in the myocardium, leading to contractile dysfunction. The extent of fibrosis was assessed using picrosirius red staining which stains collagen fibers (Fig. 4D). The saline group, and to a lesser degree the free proteins group, showed extensive amount of fibrosis that extended from the infarct to non-infarct regions, while controlled release showed far less fibrosis that seemed limited to the infarct area at 2 weeks (Fig. S2) and at 8 weeks (Fig. 4D). Collagen deposition was quantified as a positive fraction of the heart area and no statistical differences were found between the infarct groups at 2 weeks despite a visible reduction in the controlled release group (p>0.05) (Fig. 4E). At 8 weeks, collagen deposition increased in all infarct groups, but it was found to be significantly less in the controlled release group (11%) compared to both saline (23%) (p<0.01) and free proteins (18%) groups (p<0.01) (Fig. 4E). Sham had significantly less collagen than all groups at both time points.
Reduced inflammation
Modulating the inflammatory response after MI can be very beneficial for the treatment of the infarcted myocardium. In this study, we assessed inflammation by co-staining for F4/80, a pan-macrophage cell surface marker, and CD163, an M2 macrophage marker (Fig. 5A). Non-M2 macrophages, namely M1, promote inflammation, whereas M2 macrophages contribute to tissue repair and anti-inflammation [14]. At 2 weeks post-MI, controlled release showed a trend towards decreasing the presence of non-M2 macrophages, while they were present in high numbers in saline and free proteins groups (p>0.05) (Fig. 5A,B). On the other hand, controlled release of proteins significantly increased the presence of the beneficial M2 macrophages compared to saline (p<0.01) (Fig. 5A,B). Saline and free proteins showed no statistical differences in their M2 macrophage numbers (p>0.05) (Fig. 5A,B). The sham control showed minimal presence of macrophages.
Figure 5. Effect of controlled protein release on inflammation.
(A) Representative images of the different groups showing co-staining of F4/80 (red), a pan-macrophage marker, and CD163, an M2 macrophage marker (green) at 2 weeks. Co-localization of the 2 markers shows the color as yellow. (B) The controlled release (CR) group shows a reduced number of non-M2 macrophages compared to saline and free proteins, but not statistically significant. (C) CR shows a significantly increased presence of M2 macrophages compared to saline. Data are presented as means ± SD (n=3–4 per group at 2 wks). * p<0.05 vs saline, ψ p<0.05 vs sham.
We further investigated the effect of the treatment on the secretion of pro-inflammatory cytokines. We tested tissue lysates at 8 weeks for IL-1β, IL-6, and TNF-α (Fig. 6). Quantitative analysis by ELISA showed significantly lower levels of IL-1β in controlled release and free proteins groups (p<0.05) compared to saline (Fig. 6A). There were no statistical differences in IL-6 levels between groups (p>0.05) (Fig. 6B). Finally, controlled release significantly reduced the levels of TNF-α compared to saline and free proteins (p<0.01), while the free proteins group was statistically indifferent to saline (p>0.05) (Fig. 6C). These results indicate the efficacy of the controlled release of TIMP-3, FGF-2, and SDF-1α at reducing the detrimental effects of an excessive inflammatory environment post-MI and at promoting tissue healing through polarization toward M2 macrophages.
Fig. 6. Effect of controlled protein release on cytokine secretion levels at 8 weeks.
Quantitative ELISA analysis shows (A) significant reduction in IL-1β levels in delivery and free proteins groups compared to saline, (B) no difference in IL-6 levels among the infarct groups, and (C) significant reduction in TNF-α level in delivery group compared to both saline and free proteins. Data are presented as means ± SD (n=3–4/group at 8 wks). * p<0.05 vs saline, ≠ p<0.05 vs free proteins, ψ p<0.05 vs sham.
Increased cardiomyocyte survival and reduced apoptosis
The viability of the cardiac muscle is crucial for the proper function of the heart. Cardiomyocytes are responsible for imparting proper and synchronized contraction of the heart. As MI and the pathologies developed afterward trigger massive death of cardiomyocytes, it is beneficial to promote their survival, prevent their apoptosis, and trigger the regeneration of a viable myocardium. We observed a major loss of viable myocardium in the saline group followed by the free proteins group, then by the controlled release group that apparently preserved the live cardiomyocytes to a larger extent at 2 weeks (Fig. S3) and at 8 weeks (Fig. 7A). Quantitative analysis of the area fraction of the viable cardiac muscle demonstrated a reduction in the density of survived cardiomyocytes in all infarct groups at 2 weeks, with no statistical differences between them (p>0.05) (Fig. 7B). At 8 weeks, the viability of the cardiac muscle was reduced more in the saline group (64% viable muscle), followed by the free proteins group (75%) with no significant differences between them (p>0.05). In contrast, controlled release of proteins was able to maintain the survival of the cardiac muscle (83%) significantly better than saline at 8 weeks (p<0.01) (Fig. 7B).
Figure 7. Effect of controlled protein release on cardiomyocyte survival and apoptosis.
(A) Representative images of the different groups showing staining of viable cardiac muscle by cardiac troponin I (cTnI) (green). Reduced viable muscle can be observed in all infarct groups, with better preservation of the muscle in the controlled release (CR) group at 8 weeks. (B) Quantitative analysis shows no differences between infarct groups at 2 weeks, but demonstrates the controlled release group’s significant preservation of cardiac muscle viability at 8 weeks compared to saline. Data are presented as means ± SD (n=3–5/group at 2 wks, n=5–6 at 8 wks). (C) Representative western blot images of the expression levels of p-ERK, p-Akt and cleaved caspase-3 in study groups at 8 weeks. (D) Intensity band analysis of cleaved caspase-3 shows significant reduction of expression level in CR compared to saline and free proteins groups. (E) Analysis of p-ERK1/2 shows significant increase of expression level in CR compared to saline and free proteins groups, with free showing significance over saline as well. (F) Analysis of p-Akt shows significant of expression level in CR compared to saline and free groups. Data are presented as means ± SD (n=3/group at 8 wks). * p<0.05 vs saline, ≠ p<0.05 vs free proteins, ψ p<0.05 vs sham.
A number of molecular pathways play important roles in promoting survival or inducing apoptosis of cells. The activated (phosphorylated) MAPK/ERK and Akt pathways have been shown to be cardioprotective after ischemia and preventive of apoptosis [15–17]. To analyze the effect of our treatment, we quantified the expression levels of cleaved caspase-3, a pro-apoptosis mediator, and pro-survival markers p-ERK1/2 and p-Akt at 8 weeks by western blotting (Fig. 7C). Among infarcted animals, the controlled release group had the lowest level of cleaved caspase-3 and the highest levels of p-ERK1/2 and p-Akt (Fig. 7C). The free proteins group displayed significantly higher p-ERK1/2 expression than saline (p<0.01) (Fig. 7D). However, the controlled release group exhibited significantly reduced the expression of cleaved caspase-3 and increased the expression of p-ERK1/2 and p-Akt compared to both saline (p<0.001) and free proteins (p<0.01) groups (Fig. 7D,E,F). The controlled release group was statistically indifferent to sham in all 3 cases. Taken together, these results demonstrate the effectiveness of our approach at supporting the long-term survival of cardiomyocytes, preventing their apoptosis, and providing overall cardioprotection after MI through activation of the Akt and ERK1/2 signaling pathways and the suppression of caspase-3 mediated apoptosis.
Enhanced angiogenesis
The revascularization of the ischemic myocardium is key to tissue regeneration and functional recovery. New blood vessel formation can help restore the blood, nutrient, and oxygen flow to the damaged myocardial regions, and thereby enhance the survival of cardiomyocytes, and reduce the risk of chronic heart failure. To investigate the process of angiogenesis, we co-stained tissue slices for vWF and α-SMA at 2 weeks (Fig. S4) and 8 weeks (Fig. 8A). We evaluated angiogenesis only in the infarct groups and not in sham since angiogenesis happens after infarction and not in healthy hearts. We observed a higher number of neovessels in the controlled release group compared to saline and free proteins (Fig. 8A). Quantitative analysis of infarct groups showed significantly higher number of vWF+ vessels in the controlled release group compared to saline at 2 weeks (p<0.05) (Fig. 8B). At 8 weeks, the controlled release group showed a significantly higher number of vWF+ vessels than both saline and free proteins groups (p<0.01) (Fig. 8B).
Figure 8. Effect of controlled protein release on angiogenesis.
(A) Representative images of the different groups showing co-staining of vWF (red), an endothelial marker, and α-SMA (green), a pericyte marker at 8 weeks. (B) The controlled release (CR) group shows a significantly greater number of vWF+ vessels compared to saline at 2 weeks and compared to saline and free proteins at 8 weeks. (C) CR shows a significantly greater number of vWF+ α-SMA+ vessels than saline and free proteins groups at 8 weeks but not at 2 weeks. Data are presented as means ± SD (n=3–4/group at 2 wks, n=5–6 at 8 wks). * p<0.05 vs saline, ≠ p<0.05 vs free proteins.
We used co-localization of vWF and α-SMA as markers of mature neovessels, and found no significant differences among the infarct groups at 2 weeks (p>0.05) (Fig. 8C). However, at 8 weeks, controlled release showed significantly higher presence of mature neovessels than saline and free proteins groups (p<0.001) (Fig. 8C). Our results demonstrate the ability of our treatment to induce robust angiogenesis with stable and mature neovasculature. This enhanced revascularization in the controlled release group is likely due to the sustained presence of the potent angiogenic factor FGF-2 being provided by the heparin-based coacervate within our composite gel.
Greater progenitor cell homing to the myocardium
Progenitor cells recruited to the infarcted myocardium have the potential to differentiate into functional cells of cardiac lineages such as vascular endothelial, and mural cells. Progenitor cells can also impart beneficial paracrine effects that activate repair and regeneration signaling [18]. To examine the homing of these progenitor cells to the infarcted myocardium, we stained for c-Kit, a progenitor cell marker (Fig. 9A). At 8 weeks after MI, saline and free proteins groups showed no significant differences in the number of c-Kit+ cells present at the borderzone (p>0.05) (Fig. 9B). In contrast, the controlled release group showed a significantly greater presence of c-Kit+ cells at the border zone compared to both saline and free proteins groups (p<0.01) (Fig. 9B). The sham control showed very few progenitor cells in the area where an infarct would have been induced, suggesting their limited presence in absence of an MI injury. Mast cells also express c-Kit [12], and their density in response to treatment was quantified using toluidine blue staining. The density of mast cells within the border zone were similar irrespective of treatment (Fig. S5), and these account for only a fraction of c-Kit+ cells reported. The sham control showed very few mast cells within the myocardium. These data indicate that a controlled release of proteins stimulates the infiltration of c-Kit+ progenitor cells independent of mast cell recruitment. These results indicate the efficacy of the controlled release approach at recruiting progenitor cells to the infarct region to potentially contribute in the repair and regeneration of the myocardium. The enhanced and long-term presence of progenitor cells in the controlled release group is likely due to the sustained availability of the powerful chemoattractant SDF-1α within our composite gel.
Figure 9. Effect of controlled protein release on progenitor cell homing.
(A) Representative images of the different groups showing staining of c-Kit+ cardiac cells (green) at 8 weeks. (B) Quantitative analysis shows significantly greater number of c-Kit+ cells in the controlled release (CR) compared to saline and free proteins groups. Data are presented as means ± SD (n=5/group at 8 wks). * p<0.05 vs saline, ≠ p<0.05 vs free proteins, ψ p<0.05 vs sham.
Secretion of key signaling proteins
Certain proteins are involved in triggering cardiac repair mechanisms and others are implicated in advancing pathological changes post infarction. Therefore, regulation of the secretion levels of such proteins represents an important aspect of effective therapies. The presence of proteins such as the ones in our complementary combination, TIMP-3, FGF-2, and SDF-1α, likely affect the signaling and secretions levels of other proteins in the heart after MI. To investigate the effect of our treatment on the levels of relevant proteins, we tested tissue lysates for the levels of IGF-I, VEGF, Shh, and TGF-β1 at 8 weeks (Fig. S6). Quantitative analysis by ELISA showed significantly higher levels of IGF-I, an anti-apoptotic factor, in controlled release (p<0.001) and free proteins (p<0.01) groups compared to saline (Fig. S6A). Moreover, controlled release of proteins significantly increased the levels of VEGF, a potent angiogenic factor, and Shh, a master cardiac morphogen, over saline (p<0.05), while the free proteins group was statistically indifferent to saline (p>0.05) (Fig. S6B,C). Lastly, controlled release significantly decreased the levels of TGF-β1, a pro-fibrotic factor, compared to saline (p<0.001) and free proteins (p<0.05) groups (Fig. S6D). The free proteins group also significantly decreased the levels of TGF-β (p<0.05) (Fig. S6D).
Discussion
MI results in multiple pathologies and maladaptive remodeling of the heart. Numerous efforts toward cardiac repair and regeneration are underway. Stem cell-related technology can provide new cardiomyocytes via direct reprogramming and paracrine signaling via cell injection. Proteins and nucleic acids can alter the composition of local signaling molecules and enhance repair and regeneration [2, 19, 20]. Tissue-engineered patches can combine cells, growth factors, and mechanical signal to provide comprehensive cues to restore structure and functions of the heart [21]. Proper spatial and temporal signals of proteins can benefit all 3 approaches [21]. Here we explored the concept of sequential delivery of TIMP-3, FGF-2, and SDF-1α on countering the multitude of pathologies post-MI in a rat model. These 3 proteins can impart significant benefit on cardiac function after MI as we demonstrated in a recent study [3]. We found out, using a statistical fractional factorial design of experiment, that TIMP-3, FGF-2, and SDF-1α, combined at optimized doses, contribute individually to improved functionality. It predicted the need for each one of these proteins for the optimal cardiac benefit in comparison to single proteins or two combined. This allowed us to bypass the need to test single proteins or smaller combinations.
To reduce ECM degradation early after MI, TIMP-3 was encapsulated within a fibrin gel. To trigger a robust angiogenesis process and progenitor cell recruitment, FGF-2 and SDF-1α were encapsulated within heparin-based coacervates and distributed in the same fibrin gel. We have shown the efficacy of this coacervate-gel system for sequential release of VEGF and platelet-derived growth factors (PDGF) previously [11]. We have demonstrated in a number of studies the advantage of coacervate-delivered proteins for many biomedical applications, including cardiac repair [9, 11, 22–26]. The complex coacervate is comprised of a synthetic polycation PEAD combined with heparin and a heparin-binding protein, enabling its sustained release over time due to PEAD degradation, changes in ionic strength, presence of enzymes, and other factors [8, 10]. Our results demonstrated the ability of the coacervate-gel composite to provide early release of TIMP-3 by one week, followed by a sustained release of FGF-2 and SDF-1α that lasted at least six weeks in vitro. Our pervious study indicated that the heparin-binding protein was present 4 weeks after injection into the infarcted mouse heart when injected as a coacervate [22].
We tested the efficacy of spatiotemporal release of TIMP-3, FGF-2, and SDF-1α from the coacervate-gel composite in a rat MI model and compared it to sham, saline, and free proteins groups. We demonstrated the controlled release group’s significant potential to improve cardiac function and trigger repair mechanisms after infarction bringing it close to the normal case of the sham control in many evaluations. In most cases, the controlled release group showed significant differences compared to saline group, and to free proteins group in many cases. The free proteins group, although showing some potential and trends of improvement in different evaluations, was not able to induce significant repair as controlled release did compared to saline. This was indicative of the importance of controlled and timed release of TIMP-3, FGF-2, and SDF-1α. Many protein therapies fail to prove long-term efficacy for MI treatment because of the shortcomings of proteins applied in free form, including very short-half lives, low retention at the target site, high doses required, and lack of spatiotemporal cues [27]. A recent study concluded that bolus injections of a cocktail of four important proteins: FGF-2, SDF-1α, IGF-I, and hepatocyte growth factor, did not improve cardiac function, reduce infarct size, or promote stable microvasculature [28]. The study’s results might be attributed to the absence of controlled release because without properly protecting the therapeutic proteins and delivering them spatiotemporally, a therapy might prove ineffective at cardiac repair. Our delivery approach offers a solution to these challenges, by protecting the proteins within the coacervate-gel, localizing their presence at target tissue, and releasing them spatiotemporally.
The controlled release group significantly improved the heart contractile function as early as 1 week after MI and lasted up to 8 weeks in comparison to saline and free proteins groups, which had the cardiac function continuously drop over the period tested, measured by echocardiography and further confirmed by cardiac MRI at 8 weeks. We reported a cardiac function improvement of 60–75% above non-treated infarcted hearts. This effectively reduced the risk of MI progressing to heart failure. We also demonstrated significant reductions in ventricular dilation, ventricular wall thinning, myocardial stiffness, and MMP activity. These assessments are interrelated and linked to adverse remodeling and early ECM degradation. The reductions we show in these evaluations might be highly attributed to the vital role of early TIMP-3 release from our delivery system. TIMP-3 is an ECM-bound enzyme that forms tight non-covalent and stable complexes with the non-activated latent form of MMPs (pro-MMP), blocking the MMP’s catalytic domain and preventing its access to substrates [4, 29, 30]. This effectively inhibits activation of MMPs, responsible for cleaving and hydrolyzing many components of the ECM including elastin, fibronectin, collagen, and proteoglycans [4, 29, 31]. This feature of TIMP-3, being able to reduce ECM degradation, likely contributed to mitigating LV adverse remodeling, wall thinning, and dilation; thereby reducing the risk of cardiac rupture and contractile dysfunction. Other studies have shown the importance of TIMP-3 in cardiac diseases. Deficiency in TIMP-3 has been reported to lead to cardiac dilation, dysfunction, rupture, and mortality [32–34]. Cell-based TIMP-3 gene delivery improved heart function and reduced cardiac expression and activity of MMP-2 and -9 [35]. TIMP-3 delivered by collagen or hyaluronic acid gels was able to improve ejection fraction and reduce ventricular dilation and infarct size in rat and pig models [36, 37].
The unregulated and excessive collagen deposition in the infarct, and later non-infarct regions, leads to interstitial fibrosis that increases myocardial stiffness and risk of contractile dysfunction. Fibrosis arises as a result of an imbalance in ECM structure and increased production of collagen by different cells, mainly myofibroblasts. Myofibroblasts contribute to adverse remodeling and are heavily influenced by the signaling of pro-fibrotic factors such as TGF-β [38, 39]. In this study, our findings demonstrated that the spatiotemporal delivery of TIMP-3, FGF-2, and SDF-1α prevented the development of interstitial fibrosis and the expansion of scar and granulation tissue to a large extent. All of these 3 complementary proteins play important roles in reducing fibrosis in the heart after MI injury [23, 37, 40–44]. Our controlled release group proved very effective at decreasing the levels of TGF-β1, a main promoter of fibrosis post-infarction [38]. Therapies that aimed to antagonize TGF-β and reduce fibrosis proved beneficial for the heart recovery [45–48]. These results likely helped in the preservation of myocardial elasticity as witnessed in the controlled release group. Therefore, the efficacy of our spatiotemporal delivery approach at preventing the excessive deposition of fibrillary collagen reduced the risk of stiffening the ventricular wall, its loss of contractile ability, and progression to heart failure.
An excessive inflammatory response can have detrimental effects after MI. Large amounts of reactive oxygen species produced by inflammatory cells invading the infarcted myocardium can cause massive cell death [49]. The spatiotemporal delivery approach employed in our study proved effective at reducing inflammation and promoting tissue repair. Our results revealed a reduced presence of non-M2 macrophages in the controlled release group, which contain M1 macrophages that exacerbate inflammation and ECM degradation [14]. We also reported an increase in M2 macrophages which contribute to reconstruction of the ECM and anti-inflammatory effects [14]. TIMP-3, provided by our delivery approach, can exert anti-inflammatory effects by inhibiting TNF-α-converting enzyme, the enzyme activator of TNF-α [50, 51]. TNF-α is a pro-inflammatory factor which increases in heart failure and is involved in inducing inflammatory cell invasion of the infarcted myocardium, MMP production, and cell apoptosis [36, 50, 51]. We demonstrated that the controlled release group helps reduce the levels of pro-inflammatory cytokines IL-1β and and TNF-α. Our strategy reduced the potentially deleterious impact of excessive inflammation by preventing the infiltration of harmful macrophages into the infarcted myocardium or possibly forcing a change in the phenotype of present ones to become of M2 phenotype involved in tissue repair.
As MI causes the death of millions of cardiomyocytes and puts millions more at risk, it is an indispensable task to support the survival of cardiomyocytes after MI and prevent their apoptosis. Controlled release of the proteins preserves the viability of the cardiac muscle, activates pro-survival molecular pathways ERK1/2 and Akt, inhibit apoptosis mediated by caspase-3, and increase expression of anti-apoptotic factor IGF-I. Many studies have proved the important role of activating the PI3K/Akt and Ras-Raf-MEK-ERK pathways to inhibit apoptosis and provide cardioprotection [15–17]. The complementary proteins in our system TIMP-3, FGF-2, and SDF-1α have all been reported to prevent cardiomyocyte apoptosis [35, 37, 52–57]. FGF-2 and SDF-1α specifically activate these pathways upon binding with their surface receptors FGFR1 and CRCX4/CRCX7, respectively, improving cell survival and proliferation [52, 58, 59]. Moreover, controlled protein release induced higher secretions levels of IGF-I and Shh. IGF-I is a well-studied potent cardio-protective and anti-apoptotic factor that activates the PI3K/Akt pathway and prevents cardiomyocyte apoptosis [60–62]. Shh also reduces cardiomyocyte apoptosis through increased expression of pro-survival markers and reduced expression of apoptotic markers, as we and other groups have shown [24, 63, 64].
In addition, the controlled release group improved revascularization of the infarcted myocardium, triggering a robust angiogenesis process that led to the formation of mature neovessels with potential of participating in blood flow and perfusion. The triggers behind the formation of mature neovasculature in the borderzones of the infarct region can be linked mainly to FGF-2 and, to a lesser degree SDF-1α, present in our protein combination and delivered in a sustained manner by the coacervate. As a strong angiogenic factor, FGF-2 induces endothelial cell proliferation and sprouting leading to the formation of tube-like structures that evolve into neovessels with lumens [5]. We have shown in our previous studies the ability of FGF-2 coacervate to induce persistent angiogenesis and cardiac repair [22, 23, 65]. Our protein signaling results show that the delivery group upregulates VEGF expression, which is an endothelial-specific factor that is important for angiogenesis and vasodilation [66, 67]. This result concurs with previous studies which demonstrated that FGF-2 upregulates VEGF and vice versa [68, 69]. FGF-2 also acts in synergy with PDGF signaling to improve neovessels’ maturation and stability by pericytes [70]. In addition, SDF-1α can increase angiogenesis and myocardial repair by recruiting endothelial progenitor cells to the ischemic tissue and in response to VEGF signaling [71, 72]. TIMP-3 was also reported to increase VEGF levels from cardiac fibroblasts within the infarct area [37]. Moreover, increased expression of Shh, as found in this study due to controlled protein release, has been shown to activate several signaling pathways and contribute to angiogenesis [63, 64, 73]. All these indications support our finding that spatiotemporal delivery of TIMP-3, FGF-2, and SDF-1α leads to the formation of new, mature, and stable blood vessels, necessary to the repair of MI injury.
Progenitor cell recruitment to the infarct region is another important aspect of an effective therapy because of the potential of progenitor cells to ultimately differentiate into functional cells and/or support the repair by paracrine signaling, thereby supporting the survival of remaining cardiomyocytes and regeneration of a viable myocardium that replaces the lost damaged one. The controlled release group showed significant ability at homing progenitor cells to the border zones of the infarct. This is likely due to the sustained bioavailability of SDF-1α provided by the coacervate within our composite. SDF-1α is a powerful chemoattractant that can mobilize different types of progenitor cells such as endothelial progenitor cells (EPCs), hematopoietic stem cells, mesenchymal stem cells (MSCs), and cardiac stem cells (CSCs) to the infarcted myocardium [52]. Our group has shown that tissue-engineered scaffolds coated with SDF-1α coacervates improved migration and infiltration of EPCs and MSCs [74]. The stem/progenitor cells, when recruited by SDF-1α to the heart, can enhance angiogenesis and cardiomyocyte survival and differentiation [6, 7, 75, 76]. FGF-2, present within our delivery system, and IGF-I, induced by the controlled protein release, have been suggested to trigger differentiation of CSCs into functional cardiomyocytes [77, 78].
There are several limitations to this study: (1) Injections were performed immediately post-MI, which is not clinically-relevant. However, they were performed in this manner so that repeat surgery for injections and post-surgery complications can be avoided, leading to a decrease in mortality rate. (2) an ischemia-reperfusion model would have been more clinically-relevant, but a permanent ligation MI model was used in order to induce more damage to the myocardium, thus enabling the detection of finer differences between comparison groups. (3) Some evaluations were performed with a relatively low number of animals, because of their limit. This limitation may reduce statistical power. In future experiments, we will ensure every evaluation is sufficiently powered. (4) Due to limited number of animals at the 2-week time point, only histology experiments were performed. Important assessments such as MMP activity and cytokine release, although performed at 8 weeks, would be evaluated at earlier time points in future experiments. (5) The controlled release experiment was performed in vitro. In vitro experiments have the limitation of not accounting for some in vivo factors that could affect the release kinetics. However, we speculate that given trends of release will remain the same for both in vitro and in vivo conditions. Future experiments will characterize in-depth the properties of the coacervate-gel in vivo in terms of degradibility, release kinetics, composition parameters, and other relevant factors. (6) Another area of improvement is to achieve catheter delivery to reduce surgical invasiveness. We need to optimize viscosity and gelation parameters so that gelation doesn’t occur while the injectable material is still in the catheter or too late after injection where the therapeutic cargo would diffuse away from the target site. Alternatives to fibrin under investigation now include shear-thinning and stimuli-responsive gels [79–81]. Future refinements would address these limitations and position the reported technology as a way to set the infarcted myocardium on a path to repair, regeneration, and functional recovery.
Conclusions
This study investigated the use of 3 complementary proteins released in a spatiotemporal manner to improve heart function and repair following MI. We have shown that an early release of TIMP-3 reduces matrix degradation and scar expansion while an extended release of FGF-2 and SDF-1α promote long-term repair through angiogenesis, protection of cardiomyocytes, and homing of progenitor cells to the infarct. These same proteins delivered as a bolus injection show none of these functional benefits at long-term timepoints. Our results suggest that the controlled delivery of these 3 distinct but complementary proteins encourage healing of the heart post-MI. Their combination is able to address multiple adverse reactions seen after MI beyond that of a single protein therapy. This novel use of short and long-term protein delivery emphasizes the impact of spatiotemporal release for clinical translation of protein therapies.
Supplementary Material
Acknowledgments
The authors would like to thank Dr. Kevin Hitchens and Lesley Foley for the acquisition of cardiac MRI, Nadim Farhat for providing guidance on myocardial strain analysis, and Scientific Protein Labs for donating heparin. This research was supported by the biomechanics in regenerative medicine (BiRM) T32 training program [grant # 5T32EB003392-09] of the National Institutes of Health, and the American Heart Association [grant # 12EIA9020016].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, et al. Heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation. 2014;129:e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Awada HK, Hwang MP, Wang Y. Towards comprehensive cardiac repair and regeneration after myocardial infarction: Aspects to consider and proteins to deliver. Biomaterials. 2016;82:94–112. doi: 10.1016/j.biomaterials.2015.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Awada HK, Johnson LA, Hitchens TK, Foley LM, Wang Y. Factorial Design of Experiments to Optimize Multiple Protein Delivery for Cardiac Repair. ACS Biomaterials Science & Engineering. 2016;2:879–86. doi: 10.1021/acsbiomaterials.6b00146. [DOI] [PubMed] [Google Scholar]
- 4.Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circulation research. 2003;92:827–39. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- 5.Cochain C, Channon KM, Silvestre JS. Angiogenesis in the infarcted myocardium. Antioxidants & redox signaling. 2013;18:1100–13. doi: 10.1089/ars.2012.4849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saxena A, Fish JE, White MD, Yu S, Smyth JW, Shaw RM, et al. Stromal cell-derived factor-1alpha is cardioprotective after myocardial infarction. Circulation. 2008;117:2224–31. doi: 10.1161/CIRCULATIONAHA.107.694992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Segers VF, Tokunou T, Higgins LJ, MacGillivray C, Gannon J, Lee RT. Local delivery of protease-resistant stromal cell derived factor-1 for stem cell recruitment after myocardial infarction. Circulation. 2007;116:1683–92. doi: 10.1161/CIRCULATIONAHA.107.718718. [DOI] [PubMed] [Google Scholar]
- 8.Chu H, Johnson NR, Mason NS, Wang Y. A [polycation:heparin] complex releases growth factors with enhanced bioactivity. Journal of controlled release: official journal of the Controlled Release Society. 2011;150:157–63. doi: 10.1016/j.jconrel.2010.11.025. [DOI] [PubMed] [Google Scholar]
- 9.Awada HK, Johnson NR, Wang Y. Dual delivery of vascular endothelial growth factor and hepatocyte growth factor coacervate displays strong angiogenic effects. Macromolecular bioscience. 2014;14:679–86. doi: 10.1002/mabi.201300486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chu H, Gao J, Wang Y. Design, synthesis, and biocompatibility of an arginine-based polyester. Biotechnology progress. 2012;28:257–64. doi: 10.1002/btpr.728. [DOI] [PubMed] [Google Scholar]
- 11.Awada HK, Johnson NR, Wang Y. Sequential delivery of angiogenic growth factors improves revascularization and heart function after myocardial infarction. Journal of controlled release: official journal of the Controlled Release Society. 2015;207:7–17. doi: 10.1016/j.jconrel.2015.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levick SP, Meléndez GC, Plante E, McLarty JL, Brower GL, Janicki JS. Cardiac mast cells: the centrepiece in adverse myocardial remodelling. Cardiovascular Research. 2010;89:12–9. doi: 10.1093/cvr/cvq272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Etoh T, Joffs C, Deschamps AM, Davis J, Dowdy K, Hendrick J, et al. Myocardial and interstitial matrix metalloproteinase activity after acute myocardial infarction in pigs. American journal of physiology Heart and circulatory physiology. 2001;281:H987–94. doi: 10.1152/ajpheart.2001.281.3.H987. [DOI] [PubMed] [Google Scholar]
- 14.Lambert JM, Lopez EF, Lindsey ML. Macrophage roles following myocardial infarction. International journal of cardiology. 2008;130:147–58. doi: 10.1016/j.ijcard.2008.04.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kis A, Yellon DM, Baxter GF. Second window of protection following myocardial preconditioning: an essential role for PI3 kinase and p70S6 kinase. Journal of molecular and cellular cardiology. 2003;35:1063–71. doi: 10.1016/s0022-2828(03)00208-6. [DOI] [PubMed] [Google Scholar]
- 16.Tsang A, Hausenloy DJ, Mocanu MM, Yellon DM. Postconditioning: a form of “modified reperfusion” protects the myocardium by activating the phosphatidylinositol 3-kinase-Akt pathway. Circulation research. 2004;95:230–2. doi: 10.1161/01.RES.0000138303.76488.fe. [DOI] [PubMed] [Google Scholar]
- 17.Wang Y. Mitogen-activated protein kinases in heart development and diseases. Circulation. 2007;116:1413–23. doi: 10.1161/CIRCULATIONAHA.106.679589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Malliaras K, Terrovitis J. Cardiomyocyte proliferation vs progenitor cells in myocardial regeneration: The debate continues. Global cardiology science & practice. 2013;2013:303–15. doi: 10.5339/gcsp.2013.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sager HB, Dutta P, Dahlman JE, Hulsmans M, Courties G, Sun Y, et al. RNAi targeting multiple cell adhesion molecules reduces immune cell recruitment and vascular inflammation after myocardial infarction. Science Translational Medicine. 2016;8:342ra80–ra80. doi: 10.1126/scitranslmed.aaf1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jay SM, Lee RT. Protein engineering for cardiovascular therapeutics: untapped potential for cardiac repair. Circulation research. 2013;113:933–43. doi: 10.1161/CIRCRESAHA.113.300215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ogle BM, Bursac N, Domian I, Huang NF, Menasché P, Murry CE, et al. Distilling complexity to advance cardiac tissue engineering. Science Translational Medicine. 2016;8:342ps13–ps13. doi: 10.1126/scitranslmed.aad2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen WC, Lee BG, Park DW, Kim K, Chu H, Kim K, et al. Controlled dual delivery of fibroblast growth factor-2 and Interleukin-10 by heparin-based coacervate synergistically enhances ischemic heart repair. Biomaterials. 2015;72:138–51. doi: 10.1016/j.biomaterials.2015.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chu H, Chen CW, Huard J, Wang Y. The effect of a heparin-based coacervate of fibroblast growth factor-2 on scarring in the infarcted myocardium. Biomaterials. 2013;34:1747–56. doi: 10.1016/j.biomaterials.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 24.Johnson NR, Wang Y. Controlled delivery of sonic hedgehog morphogen and its potential for cardiac repair. PloS one. 2013;8:e63075. doi: 10.1371/journal.pone.0063075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson NR, Kruger M, Goetsch KP, Zilla P, Bezuidenhout D, Wang Y, et al. Coacervate Delivery of Growth Factors Combined with a Degradable Hydrogel Preserves Heart Function after Myocardial Infarction. ACS Biomaterials Science & Engineering. 2015 doi: 10.1021/acsbiomaterials.5b00077. [DOI] [PubMed] [Google Scholar]
- 26.Lee MS, Ahmad T, Lee J, Awada HK, Wang Y, Kim K, et al. Dual delivery of growth factors with coacervate-coated poly(lactic-co-glycolic acid) nanofiber improves neovascularization in a mouse skin flap model. Biomaterials. doi: 10.1016/j.biomaterials.2017.01.036. [DOI] [PubMed] [Google Scholar]
- 27.Tayalia P, Mooney DJ. Controlled growth factor delivery for tissue engineering. Advanced materials. 2009;21:3269–85. doi: 10.1002/adma.200900241. [DOI] [PubMed] [Google Scholar]
- 28.Hwang H, Kloner RA. The combined administration of multiple soluble factors in the repair of chronically infarcted rat myocardium. Journal of cardiovascular pharmacology. 2011;57:282–6. doi: 10.1097/FJC.0b013e3182058717. [DOI] [PubMed] [Google Scholar]
- 29.Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annual review of cell and developmental biology. 2001;17:463–516. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu WH, Yu S, Meng Q, Brew K, Woessner JF., Jr TIMP-3 binds to sulfated glycosaminoglycans of the extracellular matrix. The Journal of biological chemistry. 2000;275:31226–32. doi: 10.1074/jbc.M000907200. [DOI] [PubMed] [Google Scholar]
- 31.Vu TH, Werb Z. Matrix metalloproteinases: effectors of development and normal physiology. Genes & development. 2000;14:2123–33. doi: 10.1101/gad.815400. [DOI] [PubMed] [Google Scholar]
- 32.Fan D, Takawale A, Basu R, Patel V, Lee J, Kandalam V, et al. Differential role of TIMP2 and TIMP3 in cardiac hypertrophy, fibrosis, and diastolic dysfunction. Cardiovascular research. 2014 doi: 10.1093/cvr/cvu072. [DOI] [PubMed] [Google Scholar]
- 33.Fedak PW, Smookler DS, Kassiri Z, Ohno N, Leco KJ, Verma S, et al. TIMP-3 deficiency leads to dilated cardiomyopathy. Circulation. 2004;110:2401–9. doi: 10.1161/01.CIR.0000134959.83967.2D. [DOI] [PubMed] [Google Scholar]
- 34.Hammoud L, Lu X, Lei M, Feng Q. Deficiency in TIMP-3 increases cardiac rupture and mortality post-myocardial infarction via EGFR signaling: beneficial effects of cetuximab. Basic research in cardiology. 2011;106:459–71. doi: 10.1007/s00395-010-0147-7. [DOI] [PubMed] [Google Scholar]
- 35.Tian H, Huang ML, Liu KY, Jia ZB, Sun L, Jiang SL, et al. Inhibiting matrix metalloproteinase by cell-based timp-3 gene transfer effectively treats acute and chronic ischemic cardiomyopathy. Cell transplantation. 2012;21:1039–53. doi: 10.3727/096368911X601000. [DOI] [PubMed] [Google Scholar]
- 36.Eckhouse SR, Purcell BP, McGarvey JR, Lobb D, Logdon CB, Doviak H, et al. Local hydrogel release of recombinant TIMP-3 attenuates adverse left ventricular remodeling after experimental myocardial infarction. Science translational medicine. 2014;6:223ra21. doi: 10.1126/scitranslmed.3007244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Uchinaka A, Kawaguchi N, Mori S, Hamada Y, Miyagawa S, Saito A, et al. Tissue inhibitor of metalloproteinase-1 and -3 improves cardiac function in an ischemic cardiomyopathy model rat. Tissue engineering Part A. 2014;20:3073–84. doi: 10.1089/ten.tea.2013.0763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Daskalopoulos EP, Janssen BJ, Blankesteijn WM. Myofibroblasts in the infarct area: concepts and challenges. Microscopy and microanalysis: the official journal of Microscopy Society of America, Microbeam Analysis Society, Microscopical Society of Canada. 2012;18:35–49. doi: 10.1017/S143192761101227X. [DOI] [PubMed] [Google Scholar]
- 39.van den Borne SW, Diez J, Blankesteijn WM, Verjans J, Hofstra L, Narula J. Myocardial remodeling after infarction: the role of myofibroblasts. Nature reviews Cardiology. 2010;7:30–7. doi: 10.1038/nrcardio.2009.199. [DOI] [PubMed] [Google Scholar]
- 40.Cheng Z, Ou L, Zhou X, Li F, Jia X, Zhang Y, et al. Targeted migration of mesenchymal stem cells modified with CXCR4 gene to infarcted myocardium improves cardiac performance. Mol Ther. 2008;16:571–9. doi: 10.1038/sj.mt.6300374. [DOI] [PubMed] [Google Scholar]
- 41.Fujita M, Ishihara M, Morimoto Y, Simizu M, Saito Y, Yura H, et al. Efficacy of photocrosslinkable chitosan hydrogel containing fibroblast growth factor-2 in a rabbit model of chronic myocardial infarction. The Journal of surgical research. 2005;126:27–33. doi: 10.1016/j.jss.2004.12.025. [DOI] [PubMed] [Google Scholar]
- 42.Kwon JS, Kim YS, Cho AS, Cho HH, Kim JS, Hong MH, et al. Regulation of MMP/TIMP by HUVEC transplantation attenuates ventricular remodeling in response to myocardial infarction. Life sciences. 2014;101:15–26. doi: 10.1016/j.lfs.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 43.Tang J, Wang J, Song H, Huang Y, Yang J, Kong X, et al. Adenovirus-mediated stromal cell-derived factor-1 alpha gene transfer improves cardiac structure and function after experimental myocardial infarction through angiogenic and antifibrotic actions. Mol Biol Rep. 2010;37:1957–69. doi: 10.1007/s11033-009-9642-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yan P, Chen KJ, Wu J, Sun L, Sung HW, Weisel RD, et al. The use of MMP2 antibody-conjugated cationic microbubble to target the ischemic myocardium, enhance Timp3 gene transfection and improve cardiac function. Biomaterials. 2014;35:1063–73. doi: 10.1016/j.biomaterials.2013.10.043. [DOI] [PubMed] [Google Scholar]
- 45.Porter KE, Turner NA, O’Regan DJ, Balmforth AJ, Ball SG. Simvastatin reduces human atrial myofibroblast proliferation independently of cholesterol lowering via inhibition of RhoA. Cardiovascular research. 2004;61:745–55. doi: 10.1016/j.cardiores.2003.11.032. [DOI] [PubMed] [Google Scholar]
- 46.Sun Y, Zhang JQ, Zhang J, Ramires FJ. Angiotensin II, transforming growth factor-beta1 and repair in the infarcted heart. Journal of molecular and cellular cardiology. 1998;30:1559–69. doi: 10.1006/jmcc.1998.0721. [DOI] [PubMed] [Google Scholar]
- 47.Turner NA, Porter KE, Smith WH, White HL, Ball SG, Balmforth AJ. Chronic beta2-adrenergic receptor stimulation increases proliferation of human cardiac fibroblasts via an autocrine mechanism. Cardiovascular research. 2003;57:784–92. doi: 10.1016/s0008-6363(02)00729-0. [DOI] [PubMed] [Google Scholar]
- 48.Yu CM, Tipoe GL, Wing-Hon Lai K, Lau CP. Effects of combination of angiotensin-converting enzyme inhibitor and angiotensin receptor antagonist on inflammatory cellular infiltration and myocardial interstitial fibrosis after acute myocardial infarction. Journal of the American College of Cardiology. 2001;38:1207–15. doi: 10.1016/s0735-1097(01)01518-2. [DOI] [PubMed] [Google Scholar]
- 49.Saparov A, Chen CW, Beckman SA, Wang Y, Huard J. The role of antioxidation and immunomodulation in postnatal multipotent stem cell-mediated cardiac repair. International journal of molecular sciences. 2013;14:16258–79. doi: 10.3390/ijms140816258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bradham WS, Moe G, Wendt KA, Scott AA, Konig A, Romanova M, et al. TNF-alpha and myocardial matrix metalloproteinases in heart failure: relationship to LV remodeling. American journal of physiology Heart and circulatory physiology. 2002;282:H1288–95. doi: 10.1152/ajpheart.00526.2001. [DOI] [PubMed] [Google Scholar]
- 51.Wisniewska M, Goettig P, Maskos K, Belouski E, Winters D, Hecht R, et al. Structural determinants of the ADAM inhibition by TIMP-3: crystal structure of the TACE-N-TIMP-3 complex. Journal of molecular biology. 2008;381:1307–19. doi: 10.1016/j.jmb.2008.06.088. [DOI] [PubMed] [Google Scholar]
- 52.Bromage DI, Davidson SM, Yellon DM. Stromal derived factor 1alpha: a chemokine that delivers a two-pronged defence of the myocardium. Pharmacol Ther. 2014;143:305–15. doi: 10.1016/j.pharmthera.2014.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cao R, Eriksson A, Kubo H, Alitalo K, Cao Y, Thyberg J. Comparative evaluation of FGF-2-, VEGF-A-, and VEGF-C-induced angiogenesis, lymphangiogenesis, vascular fenestrations, and permeability. Circulation research. 2004;94:664–70. doi: 10.1161/01.RES.0000118600.91698.BB. [DOI] [PubMed] [Google Scholar]
- 54.Karsan A, Yee E, Poirier GG, Zhou P, Craig R, Harlan JM. Fibroblast growth factor-2 inhibits endothelial cell apoptosis by Bcl-2-dependent and independent mechanisms. The American journal of pathology. 1997;151:1775–84. [PMC free article] [PubMed] [Google Scholar]
- 55.Tian H, Cimini M, Fedak PW, Altamentova S, Fazel S, Huang ML, et al. TIMP-3 deficiency accelerates cardiac remodeling after myocardial infarction. Journal of molecular and cellular cardiology. 2007;43:733–43. doi: 10.1016/j.yjmcc.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 56.Hu X, Dai S, Wu WJ, Tan W, Zhu X, Mu J, et al. Stromal cell derived factor-1 alpha confers protection against myocardial ischemia/reperfusion injury: role of the cardiac stromal cell derived factor-1 alpha CXCR4 axis. Circulation. 2007;116:654–63. doi: 10.1161/CIRCULATIONAHA.106.672451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huang C, Gu H, Zhang W, Manukyan MC, Shou W, Wang M. SDF-1/CXCR4 mediates acute protection of cardiac function through myocardial STAT3 signaling following global ischemia/reperfusion injury. American journal of physiology Heart and circulatory physiology. 2011;301:H1496–505. doi: 10.1152/ajpheart.00365.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ornitz DM, Itoh N. The Fibroblast Growth Factor signaling pathway. Wiley interdisciplinary reviews Developmental biology. 2015;4:215–66. doi: 10.1002/wdev.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen D, Xia Y, Zuo K, Wang Y, Zhang S, Kuang D, et al. Crosstalk between SDF-1/CXCR4 and SDF-1/CXCR7 in cardiac stem cell migration. Scientific reports. 2015;5:16813. doi: 10.1038/srep16813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chao W, Matsui T, Novikov MS, Tao J, Li L, Liu H, et al. Strategic advantages of insulin-like growth factor-I expression for cardioprotection. J Gene Med. 2003;5:277–86. doi: 10.1002/jgm.347. [DOI] [PubMed] [Google Scholar]
- 61.Li Q, Li B, Wang X, Leri A, Jana KP, Liu Y, et al. Overexpression of insulin-like growth factor-1 in mice protects from myocyte death after infarction, attenuating ventricular dilation, wall stress, and cardiac hypertrophy. The Journal of clinical investigation. 1997;100:1991–9. doi: 10.1172/JCI119730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Suleiman MS, Singh RJ, Stewart CE. Apoptosis and the cardiac action of insulin-like growth factor I. Pharmacology & therapeutics. 2007;114:278–94. doi: 10.1016/j.pharmthera.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 63.Ahmed RP, Haider KH, Shujia J, Afzal MR, Ashraf M. Sonic Hedgehog gene delivery to the rodent heart promotes angiogenesis via iNOS/netrin-1/PKC pathway. PloS one. 2010;5:e8576. doi: 10.1371/journal.pone.0008576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kusano KF, Pola R, Murayama T, Curry C, Kawamoto A, Iwakura A, et al. Sonic hedgehog myocardial gene therapy: tissue repair through transient reconstitution of embryonic signaling. Nature medicine. 2005;11:1197–204. doi: 10.1038/nm1313. [DOI] [PubMed] [Google Scholar]
- 65.Chu H, Gao J, Chen CW, Huard J, Wang Y. Injectable fibroblast growth factor-2 coacervate for persistent angiogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:13444–9. doi: 10.1073/pnas.1110121108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nature medicine. 2003;9:669–76. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 67.Zachary I, Gliki G. Signaling transduction mechanisms mediating biological actions of the vascular endothelial growth factor family. Cardiovascular research. 2001;49:568–81. doi: 10.1016/s0008-6363(00)00268-6. [DOI] [PubMed] [Google Scholar]
- 68.Seghezzi G, Patel S, Ren CJ, Gualandris A, Pintucci G, Robbins ES, et al. Fibroblast growth factor-2 (FGF-2) induces vascular endothelial growth factor (VEGF) expression in the endothelial cells of forming capillaries: an autocrine mechanism contributing to angiogenesis. The Journal of cell biology. 1998;141:1659–73. doi: 10.1083/jcb.141.7.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tomanek RJ, Holifield JS, Reiter RS, Sandra A, Lin JJ. Role of VEGF family members and receptors in coronary vessel formation. Developmental dynamics: an official publication of the American Association of Anatomists. 2002;225:233–40. doi: 10.1002/dvdy.10158. [DOI] [PubMed] [Google Scholar]
- 70.Cao R, Brakenhielm E, Pawliuk R, Wariaro D, Post MJ, Wahlberg E, et al. Angiogenic synergism, vascular stability and improvement of hind-limb ischemia by a combination of PDGF-BB and FGF-2. Nature medicine. 2003;9:604–13. doi: 10.1038/nm848. [DOI] [PubMed] [Google Scholar]
- 71.Yamaguchi J, Kusano KF, Masuo O, Kawamoto A, Silver M, Murasawa S, et al. Stromal cell-derived factor-1 effects on ex vivo expanded endothelial progenitor cell recruitment for ischemic neovascularization. Circulation. 2003;107:1322–8. doi: 10.1161/01.cir.0000055313.77510.22. [DOI] [PubMed] [Google Scholar]
- 72.Tang JM, Wang JN, Zhang L, Zheng F, Yang JY, Kong X, et al. VEGF/SDF-1 promotes cardiac stem cell mobilization and myocardial repair in the infarcted heart. Cardiovascular research. 2011;91:402–11. doi: 10.1093/cvr/cvr053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pola R, Ling LE, Silver M, Corbley MJ, Kearney M, Blake Pepinsky R, et al. The morphogen Sonic hedgehog is an indirect angiogenic agent upregulating two families of angiogenic growth factors. Nature medicine. 2001;7:706–11. doi: 10.1038/89083. [DOI] [PubMed] [Google Scholar]
- 74.Lee KW, Johnson NR, Gao J, Wang Y. Human progenitor cell recruitment via SDF-1alpha coacervate-laden PGS vascular grafts. Biomaterials. 2013;34:9877–85. doi: 10.1016/j.biomaterials.2013.08.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kanki S, Segers VF, Wu W, Kakkar R, Gannon J, Sys SU, et al. Stromal cell-derived factor-1 retention and cardioprotection for ischemic myocardium. Circulation Heart failure. 2011;4:509–18. doi: 10.1161/CIRCHEARTFAILURE.110.960302. [DOI] [PubMed] [Google Scholar]
- 76.Penn MS. Importance of the SDF-1:CXCR4 axis in myocardial repair. Circulation research. 2009;104:1133–5. doi: 10.1161/CIRCRESAHA.109.198929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Padin-Iruegas ME, Misao Y, Davis ME, Segers VF, Esposito G, Tokunou T, et al. Cardiac progenitor cells and biotinylated insulin-like growth factor-1 nanofibers improve endogenous and exogenous myocardial regeneration after infarction. Circulation. 2009;120:876–87. doi: 10.1161/CIRCULATIONAHA.109.852285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rosenblatt-Velin N, Lepore MG, Cartoni C, Beermann F, Pedrazzini T. FGF-2 controls the differentiation of resident cardiac precursors into functional cardiomyocytes. The Journal of clinical investigation. 2005;115:1724–33. doi: 10.1172/JCI23418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ding X, Gao J, Awada H, Wang Y. Dual physical dynamic bond-based injectable and biodegradable hydrogel for tissue regeneration. Journal of Materials Chemistry B. 2016;4:1175–85. doi: 10.1039/c5tb02254a. [DOI] [PubMed] [Google Scholar]
- 80.Ding X, Gao J, Wang Z, Awada H, Wang Y. A shear-thinning hydrogel that extends in vivo bioactivity of FGF2. Biomaterials. 2016;111:80–9. doi: 10.1016/j.biomaterials.2016.09.026. [DOI] [PubMed] [Google Scholar]
- 81.Friberg TR, Sinha M, Awada H, Ding X. An Injectable, Biodegradable, And Biocompatible Reverse Thermal Gel Designed for Controlled, Intra-vitreal Drug Delivery. Investigative Ophthalmology & Visual Science. 2016:57. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.