Abstract
Background
Indication to implantable cardioverter defibrillator (ICD) for primary prevention of sudden death relies on left ventricular ejection fraction (LVEF). We measured the proportion of patients in whom indication to ICD persisted at the time of generator replacement (GR) and searched for predictors of appropriate therapies after GR.
Methods
We identified all consecutive patients who had received an ICD at our hospital, for LVEF ≤35% and no previous arrhythmias or unexplained syncope. Then, we included the 166 patients who outlived their first device and underwent GR.
Results
At the time of GR (mean follow-up 59 ± 20 months), ICD indication (i.e. LVEF ≤35% or previously treated ventricular arrhythmias) persisted in 114 (69%) patients. After GR, appropriate ICD therapies were delivered in 30 (26%) patients with persistent ICD indication and in 12 (23%) of the remaining patients (p = 0.656). Nonetheless, the annual rate of therapies was higher in the first group (1.08 versus 0.53 events/year; p < 0.001), as well as the rate of inappropriate therapies (0.03 versus 0 events/year; p = 0.031). The only independent predictor of appropriate ICD therapies after GR was the rate of shocks received before replacement (Hazard Ratio: 1.41; 95% confidence interval: 1.01–1.96; p = 0.041).
Conclusion
In heart failure with reduced LVEF, ICD indication persisted at the time of GR in 69% of patients. However, even in the absence of persistent ICD indication at GR, the risk of recurrence of arrhythmic events was not null.
Keywords: ICD, Sudden cardiac death, Left ventricular ejection fraction, Replacement
1. Introduction
Currently, the selection of candidates for implantable cardioverter defibrillator (ICD) implantation for primary prevention of sudden cardiac death relies mainly on left ventricular ejection fraction (LVEF) evaluation at the time of pre-implantation screening [1].
Previous studies have shown that a proportion of patients implanted for primary prevention of sudden death improve their LVEF after ICD implantation [2], [3], [4], [5], [6], especially if treated with cardiac resynchronization therapy ICD (CRT-D).
As a substantial proportion of ICD recipients outlive their first device and must undergo generator replacement, some authors questioned the appropriateness of ICD replacement when primary prevention indication does not persist and no ventricular arrhythmias were documented during follow-up [7]. We hypothesized that patients without persistent ICD indication at device replacement could be at lower risk of ICD therapies after replacement.
We sought to determine the persistence of ICD indication at the time of generator replacement in patients who had received an ICD for primary prevention of sudden cardiac death, and to measure the rate of ICD therapies after replacement. In addition, we investigated the existence of predictors of appropriate therapies after generator replacement.
2. Materials and methods
The study was approved by our internal committee for Human Research. At the time of implantation, patients gave informed consent for future use of de-identified data.
2.1. Patient selection, pacemaker implantation and follow-up
We prospectively collected data about all consecutive adult patients who underwent ICD implantation from 2000 to 2015 at our hospital. For the aim of present analysis, we retrospectively evaluated patients who received ICD for primary prevention of sudden cardiac death. All patients were implanted according to the then contemporary international guidelines and underwent a trial of optimized medical therapy and revascularization before receiving ad ICD. Thus, we identified patients with LVEF ≤35% and no documentation of previous arrhythmias or unexplained syncope at the time of implantation. Of them, we included in analysis all patients who outlived their first device and underwent generator replacement. Data collection included patient characteristics and LVEF value at baseline and at replacement. LVEF was assessed by Simpson's equation using the apical four-chamber view. Data about delivery of appropriate therapies for ventricular arrhythmia were collected from device interrogation records. Spontaneous arrhythmic episodes detected by the device were validated by 2 independent electrophysiologists blinded to the patient outcome. If a consensus could not be reached, a third electrophysiologist was involved in episode review. Device implantation and replacement were performed according to standard clinical practice and optimization of ICD parameters and pharmacological treatments were based on clinical evaluation by the attending physicians. During follow-up, patients underwent standard transthoracic echocardiographic examination every 6 months and before device replacement.
We measured the proportion of patients with persistent ICD indication at the time of generator replacement. In particular, we identified those patients with LVEF ≤35% or who received appropriate ICD therapies before replacement. Moreover, we investigated the existence of predictors of appropriate therapies after generator replacement.
2.2. Statistical analysis
Continuous data were expressed as means ± standard deviation. Categorical data were expressed as percentages. Differences between mean data were compared by a t-test for Gaussian variables and by the Mann-Whitney-Wilcoxon nonparametric test for non-Gaussian variables. Differences in proportions were compared by a chi-square analysis. Event rates were summarized by constructing Kaplan–Meier curves. The log-rank test was applied to evaluate differences between trends. Cox regression was used to analyze possible predictors of appropriate ICD therapies or shocks after replacement. The rates of events were analyzed by using the Comparison of Incidence Rates (Large Sample) Test. A P value <0.05 was considered significant for all tests. All statistical analyses were performed by means of STATISTICA software, version 7.1 (StatSoft, Inc.).
3. Results
3.1. Study population and baseline evaluation
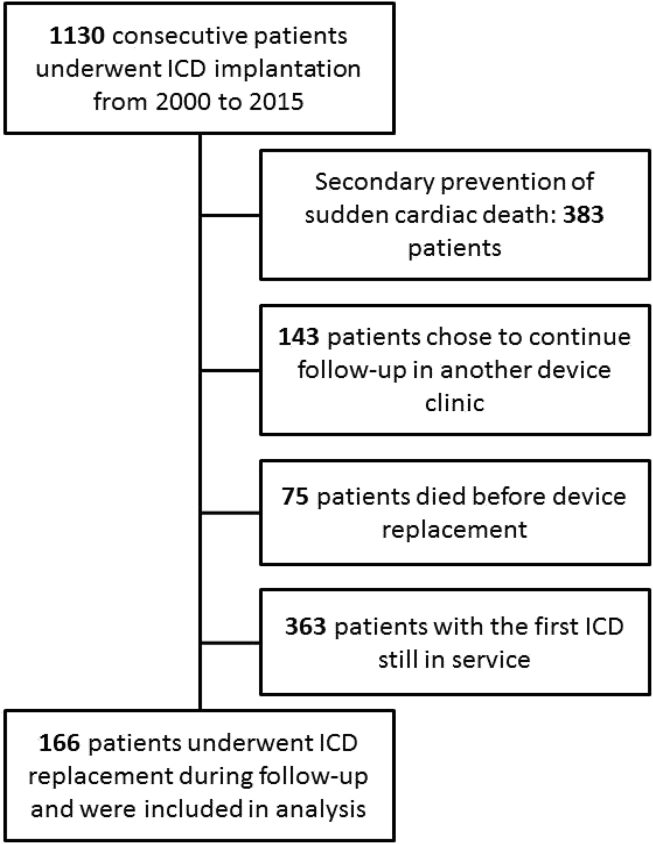
From 2000 to 2015, 1130 consecutive patients underwent ICD implantation at our hospital. Of them, 166 underwent one or more generator replacements during follow-up and had LVEF ≤35% and no documentation of previous arrhythmias or unexplained syncope at the time of first implantation (Fig. 1). Table 1 shows baseline clinical variables at the time of first implantation.
Fig. 1.
Study flow diagram.
Table 1.
Demographics and clinical parameters at the time of first implantation.
| Baseline | N = 166 |
|---|---|
| Male gender, n (%) | 135 (81) |
| Age, years | 66 ± 10 |
| Ischemic heart disease, n (%) | 90 (54) |
| NYHA class | |
| Class I, n (%) | 6 (3) |
| Class II, n (%) | 81 (49) |
| Class III, n (%) | 76 (46) |
| Class IV, n (%) | 3 (2) |
| CRT defibrillator, n (%) | 95 (57) |
| LV ejection fraction, % | 25 ± 6 |
| History of atrial fibrillation | |
| Paroxysmal, n (%) | 36 (22) |
| Persistent, n (%) | 26 (16) |
| Permanent, n (%) | 30 (18) |
| Class III antiarrhythmic use, n (%) | 26 (16) |
NYHA: New York Heart Association; LV: left ventricular.
3.2. Follow-up (from ICD implantation to first replacement)
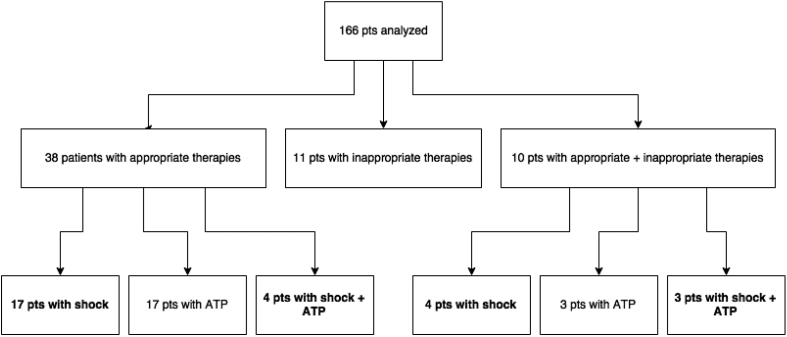
The patients in analysis underwent generator replacement for battery depletion after a mean follow-up of 59 ± 20 months. Twenty patients previously implanted with single- or dual-chamber ICD developed CRT indications and received a CRT-D device at replacement. At the time of device removal, ICD interrogations revealed appropriate therapies for ventricular arrhythmia in 48 patients (0.74 events/year in the overall group). In particular, 28 patients had received appropriate shock therapies (0.13 events/year). Moreover, 21 patients had received inappropriate therapies (0.03 events/year) (Fig. 2).
Fig. 2.
Number of patients (pts) with therapies before first ICD replacement. Number of patients with at least one appropriate shock is marked in bold in different subgroups (total: 28 patients; see text for details).
At the time of replacement, the mean LVEF was 33 ± 11% and 64 patients showed LVEF >35% (mean 44 ± 7% in this subgroup). In particular, 9 patients showed LVEF >50%, and 20% of patients with ICD alone (no CRT) showed a marked increase in EF (from 27 ± 5% to 43 ± 6%, p < 0.001). We therefore identified a persistent ICD indication for prevention of sudden death in 114 (69%) patients. The clinical parameters of patients with persistent and non-persistent ICD indication are reported in Table 2. More frequently, patients with persistent ICD indication were male, had ischemic disease, and did not receive CRT-D. Persistent ICD indications were reported in 57 (60%) patients initially implanted with CRT-D devices, and in 57 (80%) patients initially implanted with single- or dual-chamber ICDs (p = 0.005).
Table 2.
Clinical parameters and therapy at the time of first implantation and replacement.
| Persistent ICD indication (114) |
Non-persistent ICD indication (52) |
|||
|---|---|---|---|---|
| First Implantation | Replacement | First Implantation | Replacement | |
| Male gender, n (%) | 98 (86) | 37 (71)* | ||
| Age, years | 67 ± 9 | 66 ± 11 | ||
| Ischemic heart disease, n (%) | 71 (62) | 19 (37)* | ||
| NYHA class III-IV, n (%) | 51 (45) | 25 (22) | 27 (52) | 7 (13) |
| CRT defibrillator, n (%) | 57 (50) | 76 (67) | 38 (73)* | 39 (75) |
| LV ejection fraction, % | 25 ± 6 | 28 ± 7 | 27 ± 5 | 45 ± 7* |
| History of atrial fibrillation | ||||
| Paroxysmal, n (%) | 26 (23) | 10 (19) | ||
| Persistent, n (%) | 18 (16) | 8 (15) | ||
| Permanent, n (%) | 19 (17) | 11 (21) | ||
| Class III antiarrhythmic use, n (%) | 15 (13) | 45 (39) | 11 (21) | 14 (27) |
*: p < 0.05 versus Persistent.
3.3. Follow-up (after ICD replacement)
After ICD replacement, patients were followed for a mean period of 25 ± 23 months. In this second phase, appropriate therapies for ventricular arrhythmia were delivered in 42 patients (0.86 events/year in the overall group), and 21 patients received appropriate shock therapies (0.17 events/year). Moreover, 7 patients received inappropriate therapies (0.02 events/year). In particular, appropriate therapies were delivered in 30 (26%) patients with persistent ICD indication and in 12 (23%) of the remaining patients (p = 0.656). Nonetheless, the annual rate of therapies was higher in the first group: 1.08 events/year versus 0.53 events/year (p < 0.001). Appropriate shock therapies were delivered in 14 (12%) patients with persistent ICD indication and in 7 (13%) of the remaining patients (p = 0.832). The annual rate of shock was comparable between groups (0.17 events/year versus 0.17 events/year; p = 0.971), while the rate of inappropriate therapies was higher in the first group (0.03 events/year versus 0 events/year; p = 0.031). In particular, appropriate ICD therapies were reported in 3 patients with LVEF >50% at replacement.
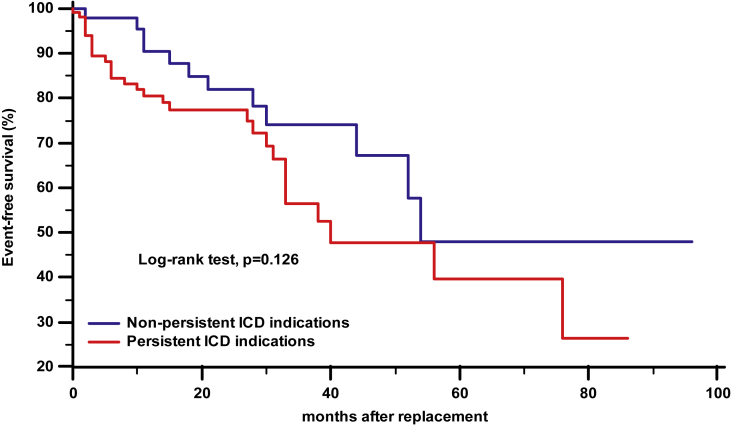
The Kaplan–Meier analysis of time from generator replacement to first appropriate therapy, stratified by persistence or non-persistence of indication to ICD, showed no differences between groups (Fig. 3).
Fig. 3.
Kaplan–Meier estimates of time from generator replacement to first appropriate therapy, stratified by persistence or non-persistence of primary prevention indication to ICD.
At Cox regression analysis (Table 3), the only independent predictor of appropriate ICD therapies after replacement was the rate of shocks received before replacement (Hazard Ratio: 1.41; 95% confidence interval: 1.01–1.96; p = 0.041).
Table 3.
Predictors of appropriate therapies and shocks after generator replacement (p for univariate analysis).
| All therapies |
Shocks |
|||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | |
| Male gender | 1.50 | 0.63–3.55 | 0.363 | 2.60 | 0.61–11.10 | 0.199 |
| Age | 1.00 | 0.97–1.04 | 0.755 | 0.99 | 0.95–1.03 | 0.647 |
| Ischemic etiology | 1.22 | 0.66–2.25 | 0.533 | 1.42 | 0.59–3.41 | 0.442 |
| CRT defibrillator (first implantation) | 1.18 | 0.63–2.19 | 0.605 | 0.86 | 0.37–2.04 | 0.740 |
| CRT defibrillator (after replacement) | 1.09 | 0.57–2.10 | 0.801 | 1.21 | 0.47–3.11 | 0.694 |
| LV ejection fraction (replacement) | 0.99 | 0.97–1.02 | 0.764 | 0.99 | 0.96–1.04 | 0.940 |
| NYHA class | 0.93 | 0.56–1.55 | 0.777 | 1.04 | 0.50–2.17 | 0.913 |
| History of paroxysmal atrial fibrillation | 2.01 | 0.98–3.94 | 0.081 | 2.09 | 0.85–5.17 | 0.111 |
| History of persistent atrial fibrillation | 1.07 | 0.48–2.141 | 0.868 | 0.88 | 0.26–2.97 | 0.880 |
| History of permanent atrial fibrillation | 0.92 | 0.42–1.98 | 0.827 | 1.53 | 0.60–3.94 | 0.377 |
| Class III antiarrhythmic use | 1.35 | 0.65–2.81 | 0.429 | 1.37 | 0.50–3.73 | 0.538 |
| Appropriate therapies before replacement or LVEF ≤35% | 1.68 | 0.86–3.28 | 0.133 | 1.31 | 0.53–3.25 | 0.563 |
| Rate of appropriate therapies before replacement | 1.03 | 0.99–1.06 | 0.057 | 1.02 | 0.98–1.07 | 0.263 |
| Rate of shocks before replacement | 1.41 | 1.01–1.96 | 0.041 | 1.31 | 0.83–2.06 | 0.256 |
4. Discussion
In the present study we demonstrated that the ICD indication for prevention of sudden cardiac death persisted at the time of generator replacement in 69% of patients who had received a primary prevention ICD in the setting of heart failure with reduced LVEF. Nonetheless, the persistence of indication was not associated with a higher risk of further appropriate ICD therapies. Indeed, the recurrence of arrhythmic events was only associated with a higher rate of shocks delivered before replacement.
Currently, the selection of ICD candidates relies mainly on LVEF evaluation at the time of pre-implantation screening [1], as reduced LVEF was the principal inclusion criterion in trials for primary prevention of sudden cardiac death. Nonetheless, although no other strong and reliable tools are available at the moment, in the last ten years growing evidence is accumulating that LVEF is not an optimal risk discriminator [8]. Indeed, LVEF has a limited specificity for the underlying risk, meaning that a reduced LVEF is a risk factor not only for sudden but also for non-sudden death [9], [10]. Moreover, only relatively few patients with reduced LVEF benefit from an ICD, and indeed the number of ICDs needed to prevent a death over 45 months of observation was estimated to be approximately 14 [11]. In addition, strong evidences are missing about patients with EF recovery after optimal therapy; in this setting ICD implantation is not usually recommended (a waiting time of 1–3months is usually warranted before SCD prophylaxis [1]), because early ICD implantation showed no benefit on overall mortality. However, some Authors proposed early implantation or bridge therapy with wearable defibrillator in patients at “high risk” (a poorly defined category) because SCD is not null and, after EF recovery or when a borderline EF is observed, a residual risk may persist in selected patients (e.g. the ones with anatomical substrate for ventricular arrhythmias) [12].
Our analysis confirmed that LVEF alone is a poor risk stratifier and that, even in the absence of documented ventricular arrhythmias, a subsequent life threatening arrhythmic event cannot be excluded in patients with improved LVEF at follow-up (and, maybe, even before primary prevention implant). We identified a large proportion of patients implanted for primary prevention of sudden cardiac death who had no ICD interventions and who improved their LVEF to more than 35% at the time of generator replacement. Although we noticed a lower rate of appropriate ICD therapies in this group after replacement, the proportion of patients with events was comparable in the two groups, and we also reported arrhythmic events among patients who showed full recovery of LVEF (i.e. >50%). Therefore, although the discontinuation of ICD therapy in these patients may be tempting from an economic point of view, as well as the possibility of downgrading a CRT-D to a CRT pacemaker in patients requiring biventricular pacing, the risk of sudden cardiac death remains not null. Nonetheless, although the potential complications associated with the replacement procedure are not negligible [13], we showed a lower rate of inappropriate therapies in patients with no persistent ICD indication, and thus a potentially lower negative impact on the outcome and the quality of life.
In the perspective of discontinuing ICD therapy, one additional open question concerns the management of the depleted device: leaving it in place, removing the generator or the complete system, using the ICD lead for right ventricular pacing in case of concomitant CRT or pacing indications. Every option is associated with different potential risks and should be carefully evaluated. Of course, the adoption of modern technologies, such as the totally subcutaneous ICD, would allow an easier management of possible system removal.
Looking at the question from a different perspective, the problem of changes in indication at the time of ICD replacement can be solved by decreasing the proportion of ICD recipients who outlive their first device. In our study, the mean longevity of ICDs implanted over the last 15 years was approximately 5 years. As Hauser suggested in 2005 [14], a 10-years battery would allow to provide an ICD that lasts a lifetime in the majority of patients, and recent data seem to show that modern battery technology (e.g. high capacity cells, highly efficient chemistry) has finally reached this goal [15].
4.1. Limitations
The first limitation of the present study is the retrospective design of the analysis. However, all the patients included were consecutive. Secondly, the number of patients in analysis was relatively small. Thirdly, several clinical variables or comorbid conditions at baseline and at replacement were not considered in our analysis of potential predictors. Fourthly, the long duration of the enrollment period may have impacted the results, as the implantation criteria and the ICD programming may have changed over time. However, the study was carried out in a single center, the operators in charge of patient selection, device implantation and clinical management did not change during the study period. Lastly, the therapies delivered by the ICD are only a surrogate endpoint and may not be necessarily considered as prevented sudden cardiac death, as episodes of nonsustained ventricular tachycardia frequently terminate spontaneously [16]. However, this approach was previously adopted by other Authors [5], [6] and, in our opinion, is the only feasible in a retrospective setting.
5. Conclusions
In our study we did not find sensitive predictors of recurrent arrhythmias after generator replacement, in patients who had received an ICD for primary prevention of sudden death in the setting of heart failure with reduced LVEF. Therefore, although current criteria for ICD indication did not persist at the time of replacement in about 30% of patients, in our opinion ICD therapy discontinuation seems not perfectly safe. Larger prospective trials are needed to identify reliable risk factors at the time of first ICD implantation and replacement.
Conflict of interest
Sergio Valsecchi is employee of Boston Scientific, Inc. No other conflicts of interest exist.
Footnotes
Peer review under responsibility of Indian Heart Rhythm Society.
References
- 1.Priori S.G., Blomström-Lundqvist C., Mazzanti A. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur Heart J. 2015 Nov 1;36(41):2793–2867. doi: 10.1093/eurheartj/ehv445. [DOI] [PubMed] [Google Scholar]
- 2.Schaer B., Theuns D.A., Sticherling C., Szili-Torok T., Osswald S., Jordaens L. Effect of implantable cardioverter-defibrillator on left ventricular ejection fraction in patients with idiopathic dilated cardiomyopathy. Am J Cardiol. 2010;106:1640–1645. doi: 10.1016/j.amjcard.2010.07.024. [DOI] [PubMed] [Google Scholar]
- 3.JE1 Schliamser, Kadish A.H., Subacius H. Significance of follow-up left ventricular ejection fraction measurements in the Defibrillators in Non-Ischemic Cardiomyopathy Treatment Evaluation trial (DEFINITE) Heart rhythm. 2013 Jun;10(6):838–846. doi: 10.1016/j.hrthm.2013.02.017. [DOI] [PubMed] [Google Scholar]
- 4.Ruwald M.H., Solomon S.D., Foster E. Left ventricular ejection fraction normalization in cardiac resynchronization therapy and risk of ventricular arrhythmias and clinical outcomes: results from the Multicenter Automatic Defibrillator Implantation Trial with Cardiac Resynchronization Therapy (MADIT-CRT) trial. Circulation. 2014 Dec 23;130(25):2278–2286. doi: 10.1161/CIRCULATIONAHA.114.011283. [DOI] [PubMed] [Google Scholar]
- 5.House C.M., Nguyen D., Thomas A.J., Nelson W.B., Zhu D.W. Normalization of left ventricular ejection fraction and incidence of appropriate antitachycardia therapy in patients with implantable cardioverter defibrillator for primary prevention of sudden death. J Card Fail. 2016 Feb;22(2):125–132. doi: 10.1016/j.cardfail.2015.10.015. [DOI] [PubMed] [Google Scholar]
- 6.Manfredi J.A., Al-Khatib S.M., Shaw L.K. Association between left ventricular ejection fraction post-cardiac resynchronization treatment and subsequent implantable cardioverter defibrillator therapy for sustained ventricular tachyarrhythmias. Circ Arrhythm Electrophysiol. 2013 Apr;6(2):257–264. doi: 10.1161/CIRCEP.112.000214. [DOI] [PubMed] [Google Scholar]
- 7.Kini V., Soufi M.K., Deo R. Appropriateness of primary prevention implantable cardioverter-defibrillators at the time of generator replacement. J Am Coll Cardiol. 2014;63:2388–2394. doi: 10.1016/j.jacc.2014.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zipes D.P., Camm A.J., Borggrefe M. ACC/AHA/ESC 2006 guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death—executive summary: a report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for practice guidelines (writing Committee to develop guidelines for management of patients with ventricular Arrhythmias and the prevention of sudden Cardiac death) J Am Coll Cardiol. 2006;48(5):1064–1108. doi: 10.1016/j.jacc.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 9.Mäkikallio T.H., Barthel P., Schneider R. Prediction of sudden cardiac death after acute myocardial infarction: role of Holter monitoring in the modern treatment era. Eur Heart J. 2005 Apr;26(8):762–769. doi: 10.1093/eurheartj/ehi188. [DOI] [PubMed] [Google Scholar]
- 10.JE1 Hartikainen, Malik M., Staunton A., Poloniecki J., Camm A.J. Distinction between arrhythmic and nonarrhythmic death after acute myocardial infarction based on heart rate variability, signal-averaged electrocardiogram, ventricular arrhythmias and left ventricular ejection fraction. J Am Coll Cardiol. 1996 Aug;28(2):296–304. doi: 10.1016/0735-1097(96)00169-6. [DOI] [PubMed] [Google Scholar]
- 11.Tung R., Zimetbaum P., Josephson M.E. A critical appraisal of implantable cardioverter-defibrillator therapy for the prevention of sudden cardiac death. J Am Coll Cardiol. 2008;52(14):1111–1121. doi: 10.1016/j.jacc.2008.05.058. [DOI] [PubMed] [Google Scholar]
- 12.Zaman S., Taylor A.J., Stiles M. Programmed ventricular stimulation to risk stratify for early cardioverter-defibrillator implantation to prevent tachyarrhythmias following acute myocardial infarction (PROTECT-ICD): trial protocol, background and significance. Heart Lung Circ. 2016;25(11):1055–1062. doi: 10.1016/j.hlc.2016.04.007. [DOI] [PubMed] [Google Scholar]
- 13.Poole J.E., Gleva M.J., Mela T. Complications associated with pacemaker and ICD generator replacements and upgrade procedures: results from the REPLACE registry. Circulation. 2010;122(16):1553–1561. doi: 10.1161/CIRCULATIONAHA.110.976076. [DOI] [PubMed] [Google Scholar]
- 14.Hauser R.G. The growing mismatch between patient longevity and the service life of implantable cardioverter-defibrillators. J Am Coll Cardiol. 2005 Jun 21;45(12):2022–2025. doi: 10.1016/j.jacc.2005.02.077. [DOI] [PubMed] [Google Scholar]
- 15.Landolina M., Curnis A., Morani G. Longevity of implantable cardioverter-defibrillators for cardiac resynchronization therapy in current clinical practice: an analysis according to influencing factors, device generation, and manufacturer. Europace. 2015 Aug;17(8):1251–1258. doi: 10.1093/europace/euv109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ellenbogen K.A., Levine J.H., Berger R.D. Are implantable cardioverter defibrillator shocks a surrogate for sudden cardiac death in patients with nonischemic cardiomyopathy? Circulation. 2006 Feb 14;113(6):776–782. doi: 10.1161/CIRCULATIONAHA.105.561571. [DOI] [PubMed] [Google Scholar]