Abstract
Background
About 7% of US adults have severe hypercholesterolemia (untreated LDL cholesterol ≥190 mg/dl). Such high LDL levels may be due to familial hypercholesterolemia (FH), a condition caused by a single mutation in any of three genes. Lifelong elevations in LDL cholesterol in FH mutation carriers may confer CAD risk beyond that captured by a single LDL cholesterol measurement.
Objectives
Assess the prevalence of a FH mutation among those with severe hypercholesterolemia and determine whether CAD risk varies according to mutation status beyond the observed LDL cholesterol.
Methods
Three genes causative for FH (LDLR, APOB, PCSK9) were sequenced in 26,025 participants from 7 case-control studies (5,540 CAD cases, 8,577 CAD-free controls) and 5 prospective cohort studies (11,908 participants). FH mutations included loss-of-function variants in LDLR, missense mutations in LDLR predicted to be damaging, and variants linked to FH in ClinVar, a clinical genetics database.
Results
Among 8,577 CAD-free control participants, 430 had LDL cholesterol ≥190 mg/dl; of these, only eight (1.9%) carried a FH mutation. Similarly, among 11,908 participants from 5 prospective cohorts, 956 had LDL cholesterol ≥190 mg/dl and of these, only 16 (1.7%) carried a FH mutation. Within any stratum of observed LDL cholesterol, risk of CAD was higher among FH mutation carriers when compared with non-carriers. When compared to a reference group with LDL cholesterol <130 mg/dl and no mutation, participants with LDL cholesterol ≥190 mg/dl and no FH mutation had six-fold higher risk for CAD (OR 6.0; 95%CI 5.2–6.9) whereas those with LDL cholesterol ≥190 mg/dl as well as a FH mutation demonstrated twenty-two fold increased risk (OR 22.3; 95%CI 10.7–53.2).
Conclusions
Among individuals with LDL cholesterol ≥190 mg/dl, gene sequencing identified a FH mutation in <2%. However, for any given observed LDL cholesterol, FH mutation carriers are at substantially increased risk for CAD.
Keywords: familial hypercholesterolemia, low-density lipoprotein cholesterol, gene sequencing, coronary artery disease, genetics
Introduction
Primary, severe hypercholesterolemia, defined as having a low-density lipoprotein (LDL) cholesterol ≥ 190 mg/dl, is a treatable risk factor for coronary artery disease (CAD)1,2; current treatment guidelines place particular emphasis on intensive lifestyle and pharmacologic therapy in this population.3 One etiology of severely elevated LDL cholesterol is familial hypercholesterolemia (FH), an autosomal dominant monogenic disorder linked to impaired hepatic clearance of LDL cholesterol particles.4 It is often assumed that individuals with LDL cholesterol ≥ 190 mg/dl have FH but this may not be the case. Large-scale gene sequencing provides an opportunity to clarify the diagnostic yield and clinical impact of identifying a FH mutation in severely hypercholesterolemic patients.
Previous studies of the diagnostic yield of genetic testing in severe hypercholesterolemia have focused on individuals with clinically-suspected FH and in these samples, a FH mutation prevalence ranging from 20 to 80% has been reported.5–16 This variability is likely due to differing ascertainment schemes based on family history, physical exam features, elevated LDL cholesterol at a young age, or referral to specialized clinics, each of which may enrich for monogenic etiologies. In contrast, if ascertainment from the general population is based solely on elevated LDL cholesterol, the extent to which FH mutations contribute to severe hypercholesterolemia is unknown. Such knowledge may inform design and effectiveness of universal FH screening proposals.17–18
Knowledge of FH mutation status may also provide CAD risk information beyond that of a single LDL cholesterol measurement. 4,18 A FH mutation leads to cumulative exposure to higher LDL cholesterol levels over a lifetime and as such, within any stratum of LDL cholesterol, the risk of CAD may be greater if the LDL elevation is due to a monogenic mutation versus other causes. The extent to which CAD risk is modulated by the presence of a causal FH mutation is uncertain.
We analyzed gene sequences of three FH genes, low-density lipoprotein receptor (LDLR), apolipoprotein B (APOB), and proprotein convertase subtilisin/kexin type 9 (PCSK9), in twelve distinct cohorts including >26,000 participants to determine: 1) the diagnostic yield of gene sequencing to identify a FH mutation in severely hypercholesterolemic individuals; and 2) the clinical impact of a FH mutation with regard to CAD risk within any given stratum of LDL cholesterol levels.
Methods
Study Populations
Whole exome sequencing was performed in seven previously described CAD case-control cohorts of the Myocardial Infarction Genetics Consortium (Supplementary Table 1). Studies included the Italian Atherosclerosis Thrombosis and Vascular Biology study, 19 the Exome Sequencing Project Early-Onset Myocardial Infarction (ESP-EOMI) study,20 a nested case-control of the Jackson Heart Study (JHS),15 the Munich Myocardial Infarction study,22 the Ottawa Heart Study,23 the Precocious Coronary Artery Disease (PROCARDIS) study,24 and the Pakistan Risk of Myocardial Infarction Study (PROMIS).25 The effect of lipid-lowering therapy in those reporting use at the time of lipid measurement was taken into account by dividing the measured total cholesterol and LDL cholesterol by 0.8 and 0.7 respectively as has been implemented previously.26–28 Primary, severe LDL cholesterol elevation was defined as ≥ 190 mg/dl in accordance with current cholesterol treatment guidelines.3
The prevalence of a FH mutation in individuals with a LDL cholesterol > 190 mg/dl was additionally determined in 11,908 participants from five prospective cohort studies derived from the Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) Consortium:29 Atherosclerosis Risk in Communities Study (ARIC), Cardiovascular Health Study, Framingham Heart Study (FHS), Rotterdam Baseline Study, and Erasmus Rucphen Family Study (Supplementary Table 2).
In order to determine the cumulative exposure to LDL cholesterol according to FH mutation status, publically available data from the National Center for Biotechnology Information dbGAP database was analyzed. These data included 5,727 ARIC cohort participants and 2,714 FHS Offspring Study participants.
Gene Sequencing
The CAD case-control whole exome sequencing was performed as previously described at the Broad Institute (Cambridge, MA).20 The population-based cohort sequencing was performed at the Baylor College of Medicine (Houston, Texas) for the ARIC, CHS, and FHS cohorts and at Erasmus Medical Center (Rotterdam, Netherlands) for the RS and ERF cohorts respectively. Additional sequencing methodology details available in Supplementary Methods.
Genetic Variant Annotation
Three classes of genetic variants were aggregated with respect to association with FH: 1) loss of function variants in LDLR: single base changes that introduce a stop codon leading to premature truncation of a protein (nonsense), insertions or deletions (indels) of DNA that scramble the protein translation beyond the variant site (frameshift), or point mutations at sites of pre-mRNA splicing that alter the splicing process (splice-site); 2) missense variants in LDLR predicted to be deleterious by each of five in silico prediction algorithms (LRT score, MutationTaster, PolyPhen-2 HumDiv, PolyPhen-2 HumVar and Sorting Intolerant From Tolerant (SIFT)) as described previously; 20,30 and 3) Variants in LDLR, APOB, or PCSK9, annotated as “pathogenic” or “likely pathogenic” in ClinVar, a publically available archive of genetic variations associated with clinical phenotypes.31 Additional sensitivity analyses aggregated all rare (allele frequency < 0.01) missense mutations in LDLR, exon 26 of APOB which encodes key components of apolipoprotein B binding to the LDL receptor and harbor the majority of APOB variants linked to FH,32 and those that produce a change in PCSK9 at an amino acid associated with FH in ClinVar. Rare synonymous variants at these same locations were included as a negative control. Software used to annotate observed variants included Variant Effect Predictor (version 77) 33 and associated LOFTEE plugin,34 and the dbNSFP database (version 3.0b1).35
Longitudinal Analysis of LDL Cholesterol Exposure
Individuals with a FH mutation and LDL cholesterol ≥ 130 mg/dl were identified in ARIC and FHS Offspring Study cohorts. LDL cholesterol values were adjusted in those reporting lipid-lowering therapy by dividing measured value by 0.7. Mean LDL cholesterol exposure was calculated as cumulative exposure, determined via an area under the curve analysis, divided by length of follow-up. 27 FH mutation carriers met the above inclusion criteria and were matched 1:1 to a mutation negative control according to age (within 10 years), gender, statin use at time of last visit, and similar LDL cholesterol at last visit (within 10 mg/dl). A match was available in 25 of 27 (93%) individuals. Mean LDL cholesterol exposure was compared among those with and without FH mutation using a paired t-test.
Statistical Analysis
The impact of aggregations of genetic variants on levels of LDL cholesterol was assessed using linear regression with adjustment for age, age2, gender, cohort, and the first five principal components of ancestry. Odds ratios for CAD were calculated using logistic regression with adjustment for gender, cohort, and the first five principal components of ancestry. In analyses conducted on ordinal strata of LDL cholesterol, individuals with LDL cholesterol <130 mg/dl and no mutation linked to FH served as the reference group.
Analyses were performed using R version 3.2.2 software (The R Project for Statistical Computing, Vienna, Austria). Figures were creating using the “ggplot2” package within R.36
Results
Within the Myocardial Infarction Genetics Consortium CAD case-control cohorts, a total of 14,117 participants with both LDL cholesterol level and sequence data for FH genes were available for analysis – 8,577 CAD-free controls and 5,540 CAD cases (Supplementary Table 3). The study population included 10,421 (74%) males with mean age 53 years (SD 14). Proportions of self-identified race were 47%, 46%, and 7% for White, South Asian, and Black respectively. 47% of study participants had a history of hypertension, 27% had a history of diabetes, 34% were current smokers, and 14% were on lipid-lowering medications.
Sequencing identified 86 variants linked to FH on the basis of leading to loss of function in LDLR, missense mutations in LDLR predicted to be damaging by each of five computer prediction algorithms, or a variant in LDLR, APOB, or PCSK9 previously linked to FH in the ClinVar genetics database. These included 13 premature stop (“nonsense”) codons, 6 splice acceptor/donor variants, 4 frameshift mutations, and 63 missense mutations (Supplementary Table 4).
164 individuals harbored a mutation linked to FH, including 48 CAD-free controls (0.6%; 95%CI 0.4 – 0.7%) and 116 CAD cases (2.1%; 95%CI 1.7 – 2.5%) (Supplementary Table 5). The mutation was located in LDLR for 141 participants (86%), in APOB for 22 (13%), and in PCSK9 for 1 (0.6%) (Supplementary Table 4). Only one homozygote (or compound heterozygote) participant was identified; a 33-year old patient with LDL cholesterol of 539 mg/dl and CAD was homozygous for a p.Q33* premature stop codon in LDLR.
Diagnostic Yield of Gene Sequencing in Severe Hypercholesterolemia
Among 8,577 CAD-free control participants from the Myocardial Infarction Genetics Consortium cohorts, LDL cholesterol approximated a normal distribution (Supplementary Figure 1). The prevalence of a FH mutation increased across categories of LDL cholesterol levels (P < 0.001) (Supplementary Figure 2). Of 8,577 control participants, 430 participants (5% of control sample) had LDL cholesterol ≥ 190 mg/dl and of these 430, only 8 carried a FH mutation (1.9%; 95%CI 0.9 – 3.8%) (Table 1 & Central Illustration).
Table 1.
Prevalence of a Familial Hypercholesterolemia Mutation Among Participants with Severe Hypercholesterolemia (LDL Cholesterol ≥ 190 mg/dl)
| N with LDL Cholesterol ≥ 190 mg/dl (% of Cohort) | N with FH Mutation (% of Individuals with LDL Cholesterol ≥ 190) | |
|---|---|---|
| Controls of the Myocardial Infarction Genetics (MIGen) Consortium | ||
| Atherosclerosis, Thrombosis and Vascular Biology Italian Study (N = 1,050) | 44 (4%) | 1 (2.3%) |
| Exome Sequencing Project; Early-Onset Myocardial Infarction (N = 1,213) | 160 (13%) | 3 (1.9%) |
| Jackson Heart Study (N = 599) | 26 (4%) | 1 (3.8%) |
| Munich Myocardial Infarction Study (N = 272) | 15 (6%) | 0 (0%) |
| Ottowa Heart Study (N = 889) | 59 (7%) | 0 (0%) |
| Precocious Coronary Artery Disease (N = 870) | 36 (4%) | 1 (2.8%) |
| Pakistani Risk of Myocardial Infarction Study (N = 3,684) | 90 (2%) | 2 (2.2%) |
| Total (N = 8,577) | 430 (5%) | 8 (1.9%) |
| Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) Consortium | ||
| Atherosclerosis Risk in Communities Study (N = 7,959) | 657 (8%) | 12 (1.8%) |
| Cardiovascular Health Study (n = 732) | 47 (4%) | 1 (2.1%) |
| Framingham Heart Study (N = 1,175) | 38 (5%) | 2 (5.3%) |
| Rotterdam Baseline Study (N = 794) | 99 (12%) | 0 (0%) |
| Erasmus Rucphen Family Study (N = 1,248) | 115 (9%) | 1 (0.9%) |
| Total (N = 11,908) | 956 (8%) | 16 (1.7%) |
| Combined MIGen Controls + CHARGE (N = 20,485) | 1386 (7%) | 24 (1.7%) |
Central Illustration.
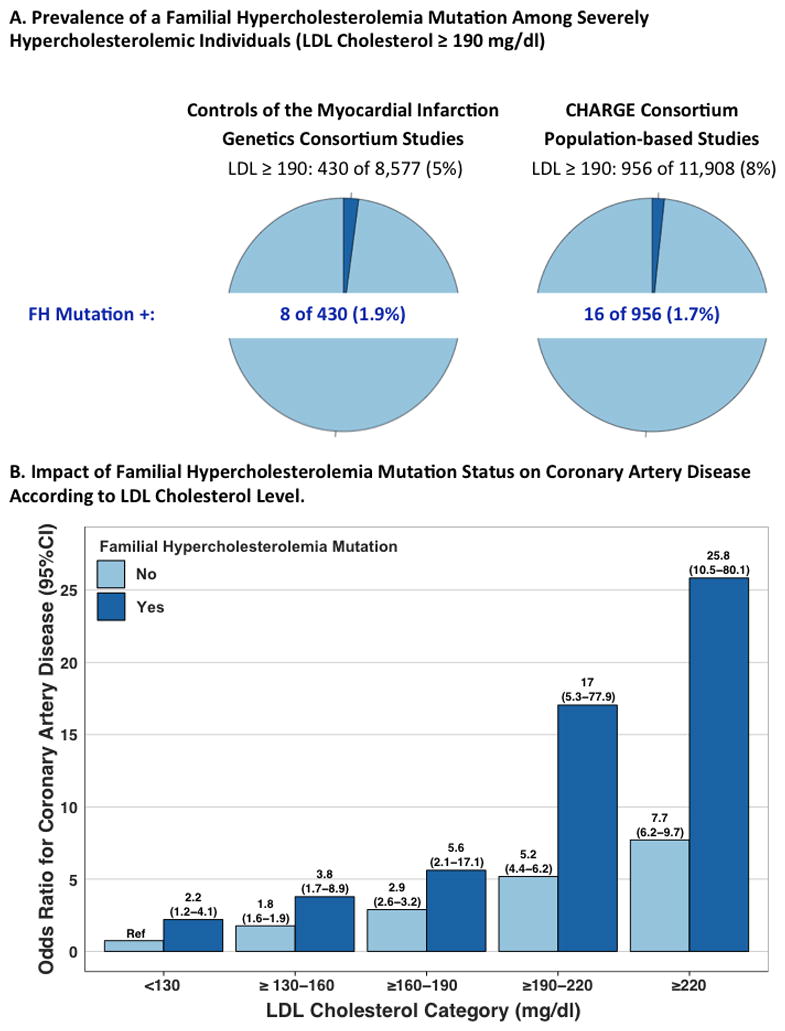
A. Prevalence of a FH mutation amongst severely hypercholesterolemic individuals. B. Risk of coronary artery disease across LDL cholesterol and familial hypercholesterolemia mutation status categories. Odds ratios for CAD were calculated via logistic regression with adjustment for gender, cohort, and principal components of ancestry relative to a reference category of LDL cholesterol <130 mg/dl without a familial hypercholesterolemia (FH) mutation. Counts of CAD-free controls vs. CAD cases in each category are provided in Supplementary Table 6. P-value for mutation carriers vs. noncarriers across strata of LDL cholesterol < 0.0001. P-interaction between LDL cholesterol category and mutation status = 0.51
This prevalence estimate was replicated in 11,908 participants from five prospective cohort studies of the CHARGE consortium; 956 (8%) had a LDL cholesterol >190 mg/dl and of these, 16 (1.7%; 95%CI 1.0 – 2.8%) harbored a FH mutation. Across the twelve studies combined (n=20,485), 1386 (7%) displayed LDL cholesterol ≥ 190 mg/dl and of these, 24 (1.7%) carried a FH mutation (Table 1).
Clinical Impact of FH Mutation Identification on CAD Risk
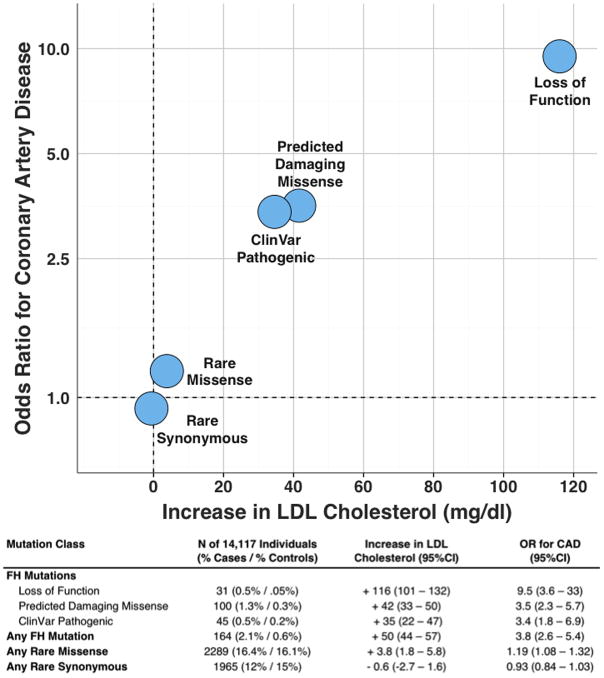
In the Myocardial Infarction Genetics Consortium case-control studies, the presence of a FH mutation was associated with a 50 mg/dl (95%CI 44– 57) increase in LDL cholesterol and a 3.8 fold (95%CI 2.6 – 5.4) increase in odds of CAD. These effects were most pronounced in those with loss of function mutations in LDLR (Figure 1). Average LDL cholesterol was 190 mg/dl in those with a FH mutation and 73/164 (45%) had a LDL cholesterol ≥ 190 mg/dl. However, despite the observed large effect on average levels, a wide spectrum of circulating LDL cholesterol concentrations was noted in those who were mutation positive (Figure 2). 44 of 164 (27%) mutation carriers had an observed LDL cholesterol less than 130 mg/dl; reflecting incomplete penetrance. An aggregation of all rare missense mutations had a modest impact on both LDL cholesterol and CAD risk. As expected, synonymous mutations, which do not lead to a change in amino acid sequence, had no effect on either parameter (Figure 1). Beyond LDL cholesterol levels, a FH mutation was associated with a nominally significant reduction in high-density lipoprotein cholesterol (−1.9 mg/dl; 95%CI −3.7 – −0.1; p = 0.04) but no association with circulating triglycerides (p = 0.36).
Figure 1. Impact of Familial Hypercholesterolemia, Rare Missense, and Rare Synonymous Mutations on LDL Cholesterol and Coronary Artery Disease.
For each class of variants, the number of individuals within the 14,117 participants of the Myocardial Infarction Genetics Consortium case-control studies and % of CAD cases and CAD-free controls is provided. Number of individuals within each mutation category sum to more than the overall familial hypercholesterolemia mutation numbers due to overlap across variant classification. Increase in LDL cholesterol values determined via linear regression with adjustment for age, age2, gender, cohort, and principal components of ancestry. Odds ratios for CAD were calculated via logistic regression with adjustment for gender, cohort, and principal components of ancestry.
Figure 2. LDL Cholesterol Values According to Familial Hypercholesterolemia Mutation Status.
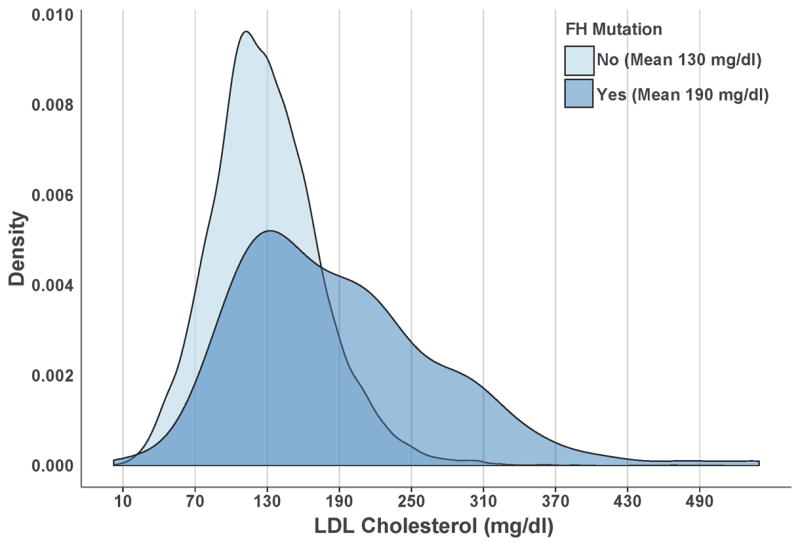
The distribution of low-density lipoprotein (LDL) cholesterol values according to familial hypercholesterolemia (FH mutation status) among the Myocardial Infarction Genetics Consortium studies is displayed. LDL cholesterol values were higher in FH mutation carriers (N = 164) as compared to noncarriers (N=13,954), p < 0.001.
Within the Myocardial Infarction Genetics Consortium case-control cohort populations, those with a FH mutation were at substantially higher risk compared to those without a mutation (Table 2, p-value for difference = 0.001). For participants with both LDL cholesterol ≥ 190 mg/dl and a FH mutation, the odds of coronary artery disease were increased twenty-two fold (OR 22.3; CI 10.7 – 53.2) when compared to those with LDL cholesterol < 130 mg/dl and no mutation. For participants with LDL cholesterol ≥ 190 mg/dl and no FH mutation, odds of coronary artery disease were increased six-fold (OR 6.0; CI 5.2 – 6.9) compared to the same reference group. This difference persisted after additional adjustment for measured LDL cholesterol level (p = 0.02).
Table 2.
Risk of Coronary Artery Disease in those with Elevated LDL cholesterol (≥190 mg/dl) According to Familial Hypercholesterolemia Mutation Status.
| N (N CAD-free Controls/N CAD Case) | OR for CAD (95%CI) P-value* |
P-value (FH Mutation + vs. −)y | LDL Cholesterol-Adjusted ORfor CAD (95%CI) P-value* |
P-value (FH Mutation + vs. −) y | |
|---|---|---|---|---|---|
| LDL Cholesterol ≥ 190 mg/dl | |||||
| Familial Hypercholesterolemia Mutation − | 1,264 (422/842) | 6.0 5.2 – 6.9) P < 0.001 |
0.001 | 1.6 (1.3 – 2.1) P < 0.001 |
0.02 |
| Familial Hypercholesterolemia Mutation + | 73 (8/65) | 22.3 (10.7 – 53.2) P < 0.001 |
4.2 (1.9 – 10.4) P < 0.001 |
||
| LDL Cholesterol < 130 mg/dl and Familial Hypercholesterolemia Mutation − | 7,485 (5,175/2,310) | Reference | Reference |
Odds ratios (OR) for coronary artery disease (CAD) calculated via logistic regression with adjustment for gender, cohort, and principal components of ancestry relative to a reference category of LDL cholesterol <130 mg/dl without a familial hypercholesterolemia (FH) mutation. Odds ratio values with and without additional adjustment for observed LDL cholesterol, expressed as a continuous variable, are provided.
P-value for difference in OR compared to reference category.
P-value for difference in OR between FH Mutation + vs. FH Mutation − among participants with LDL cholesterol (≥190 mg/dl)
Separation of the population into clinically relevant categories of LDL cholesterol levels demonstrated trends towards higher risk in those with a FH mutation within each stratum (Central Illustration; Supplementary Table 6). The impact of a FH mutation was similar across strata of LDL cholesterol levels (p-interaction = 0.51). Within the subgroup of participants with a LDL cholesterol in the ≥ 190 to 220 mg/dl range, those with a mutation had 17-fold increased CAD risk versus 5-fold for those without a mutation. This difference was noted despite similar observed LDL cholesterol levels in this stratum (mean LDL cholesterol in those with mutation=205 mg/dl versus mean LDL cholesterol in those without a FH mutation = 203 mg/dl; p-value for difference = 0.22).
Cumulative LDL Cholesterol Exposure According to FH Mutation Status
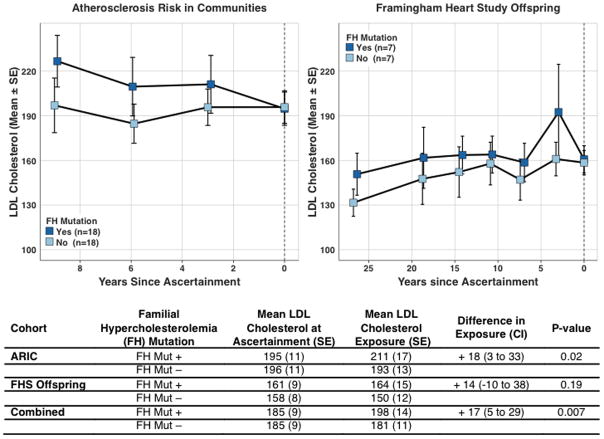
For any given observed LDL cholesterol, those harboring a mutation might have a higher average LDL cholesterol exposure over their lifetime compared to those who do not harbor a mutation and this could explain a higher CAD risk among mutation carriers. We tested this hypothesis using two prospective cohort studies – ARIC and the FHS Offspring Study – where sequencing data and serial measurements of LDL cholesterol were available. We identified 25 individuals with a FH mutation and LDL cholesterol ≥ 130 mg/dl. Mean LDL cholesterol at time of last study visit was 185 mg/dl. As compared to matched non-carriers with similar LDL cholesterol at the last visit, individuals with a FH mutation had a 17 mg/dl (95%CI 5 – 29; p = 0.007) higher average LDL cholesterol exposure in the years preceding the last visit (Figure 3; Supplementary Table 7).
Figure 3. Cumulative LDL cholesterol Exposure in Familial Hypercholesterolemia Mutation Carriers Compared on Non-carriers Matched on LDL cholesterol at Ascertainment.
Hypercholesterolemic [low-density lipoprotein (LDL) cholesterol ≥ 130 mg/dl] carriers of a familial hypercholesterolemia (FH) mutation were identified in the Atherosclerosis Risk in Communities (ARIC) and Framingham Heart Study (FHS) Offspring cohorts and matched 1:1 to a FH mutation non-carriers according to age, gender, statin use, and LDL cholesterol at time of ascertainment. Mean ± standard error (SE) LDL cholesterol values at each study visit are displayed in each cohort according to mutation status. A matched pairs t-test demonstrated higher cumulative exposure to LDL cholesterol in FH mutation carriers versus non-carriers.
Discussion
Among 20,485 multiethnic participants from 12 studies, we found that 1,386 (7%) had severe hypercholesterolemia (LDL cholesterol ≥ 190 mg/dl) and of those with severe hypercholesterolemia, only a small fraction (<2%) also carried a FH mutation. However, within any stratum of LDL cholesterol, those who carried a FH mutation were at substantially higher risk for CAD compared to those who did not. This increased CAD risk among mutation carriers was at least partially explained by a greater cumulative exposure to LDL cholesterol over a lifetime.
These results permit several conclusions. First, FH mutations explain only a small fraction of severe hypercholesterolemia in the population. Previous reports have noted a substantially higher rate of mutation detection in those with clinically-suspected FH, as ascertained on the basis of features (e.g. family history, physical exam, or severe hypercholesterolemia at a young age) that enrich for a monogenic etiology.5–16 Here, we address a scientific question – what fraction of severely hypercholesterolemic individuals carry a mutation in any of three high LDL genes – that is distinct from these earlier, seminal reports. When participants are ascertained solely on the basis of a single elevated LDL cholesterol level, we identify a FH mutation in fewer than 2% of severely hypercholesterolemic individuals. These sequencing results are broadly consistent with those of a recent study of 98,098 individuals from the Copenhagen General Population Study in which genotyping of the four most common FH mutations was used to extrapolate overall FH mutation prevalence. In this Danish study, of 5,332 individuals with LDL cholesterol ≥ 5 mmol/l (193 mg/dl), fewer than 5% were predicted to harbor a FH mutation.28
If not a monogenic mutation in the three FH genes, what might be the cause of elevated LDL cholesterol in the remaining >95% of participants with severe hypercholesterolemia? Possibilities include polygenic hypercholesterolemia, lifestyle factors, and/or a combination of these. For example, individuals in the top quartile of a polygenic LDL cholesterol gene score comprised of 95 common variants were 13 fold more likely to have high LDL cholesterol.37 Similarly, individuals in the top decile of a LDL cholesterol gene score comprised of 12 common variants were 4.2 fold more likely to have a LDL ≥ 190 mg/dl in the UK Whitehall II study.38 Future genetics studies may identify additional causal variants, genes beyond those considered in this study, or large-effect regulatory variants that underlie severe hypercholesterolemia. Other non-genetic explanations for severe elevations in LDL cholesterol include secondary causes (e.g. hypothyroidism or nephrotic syndrome), lifestyle factors such as dietary fat, or some combination of these.
Second, within any stratum of a single observed LDL cholesterol, CAD risk was higher in those with a FH mutation when compared to those without, reinforcing the potential utility of genetic testing. We analyzed 25 matched pairs of individuals with similarly elevated LDL cholesterol levels at time of ascertainment and found a higher cumulative exposure to LDL cholesterol in those with a FH mutation. These data support the hypothesis that a FH mutation, present since birth, increases CAD risk via lifelong exposure to high LDL cholesterol.39 By contrast, an isolated elevation in LDL cholesterol in those without a genetic predisposition may reflect a time-limited exposure related to a current environmental perturbation or a value that is more likely to regress towards the mean in the future. Future studies may identify additional metabolic parameters, such as increased lipoprotein(a) levels,40 that also contribute to the excess CAD risk noted in those with a FH mutation.
Finally, these data contribute to ongoing discussion regarding how to define FH. Classically, FH refers to elevated LDL cholesterol caused by a single mutation in any of several genes segregating in an autosomal dominant manner. Alternate approaches to two features – LDL cholesterol threshold and mutation definition – impact FH prevalence estimates (Table 3). An approach that includes all individuals with untreated LDL cholesterol ≥ 190 mg/dl (i.e., without a FH mutation requirement) would combine non-genetic and genetic causes and classify about 7% of the US adult population as having FH. An alternative possibility is to withhold an LDL cholesterol threshold and require only a stringent mutation definition; in such an analysis of 20,485 participants, we identified a FH mutation in 97 individuals (1 in 211). This estimate is nearly identical to a population-based analysis in the Copenhagen General Population Study (1 in 217).28 However, if one additionally requires that a FH mutation is accompanied by an elevated LDL cholesterol, FH prevalence in our study declines (1 in 301 with LDL threshold ≥130 mg/dl and 1 in 853 with LDL threshold ≥ 190 mg/dl).
Table 3.
Prevalence of Familial Hypercholesterolemia According to Different LDL Cholesterol Thresholds and Mutation Classification Schemes.
| LDL Cholesterol Criteria | Mutation Criterion | Prevalence of Familial Hypercholesterolemia |
|---|---|---|
| LDL Cholesterol ≥ 190 mg/dl | No mutation required | 1,386/20,485 (1 in 14) |
| No threshold requirement |
*LDLR loss of function variant; or *LDLR predicted damaging rare missense variant; or *LDLR, APOB, PCSK9 variant pathogenic in ClinVar |
97/20,485 (1 in 211) |
| LDL Cholesterol ≥ 190 mg/dl |
*LDLR loss of function variant; or *Any rare LDLR missense variant |
80/20,485 (1 in 256) |
| LDL Cholesterol ≥ 130 mg/dl |
*LDLR loss of function variant: or *LDLR predicted damaging rare, missense variant; or *LDLR, APOB, PCSK9 variant pathogenic in ClinVar |
68/20,485 (1 in 301) |
| No threshold requirement |
*LDLR loss of function variant; or *LDLR predicted damaging rare missense variant |
60/20,485 (1 in 341) |
| LDL Cholesterol ≥ 190 mg/dl |
*LDLR loss of function variant; or *LDLR predicted damaging rare missense variant; or *LDLR, APOB, PCSK9 variant pathogenic in ClinVar |
24/20,485 (1 in 853) |
For each classification scheme, we provide the number who meet the criteria out of a total 20,485 participants (CAD-free controls of the Myocardial Infarction Genetics Consortium combined with CHARGE Consortium participants). Loss of function variants defined as single base changes that introduce a stop codon leading to premature truncation of a protein (nonsense), insertions or deletions (indels) of DNA that scramble the protein translation beyond the variant site (frameshift), or point mutations at sites of pre-mRNA splicing that alter the splicing process (splice-site). Predicted damaging variants refer to those LDLR predicted to be deleterious by each of five in silico prediction algorithms (LRT score, MutationTaster, PolyPhen-2 HumDiv, PolyPhen-2 HumVar and Sorting Intolerant From Tolerant (SIFT)). Rare variants refers to those with minor allele frequency < 1% in the sequenced population.
With regard to defining a FH mutation, all schema agree on the inclusion of loss of function alleles in LDLR but differ on how to handle missense mutations. For missense mutations, we applied a rigorous threshold – requiring that the mutation be designated as damaging by each of five computer prediction algorithms or be previously annotated as pathogenic in the ClinVar clinical genetics database. A key advantage of this approach is that it ensures that classification is both fully reproducible and generalizable to genes beyond those related to FH.
When routine genetic testing is not available, clinical scoring systems such as the Dutch Lipid Clinical Network, Simon Broome, and MEDPED criteria have been developed to approximate FH status.4 Ongoing collaborative efforts on how to optimally incorporate population-based genetic sequencing data into existing frameworks for the clinical diagnosis of FH will be critically important.
Study Limitations
Our data did not permit stratifying individuals by family history or physical exam features, as suggested by the Dutch Lipid Clinic Network and Simon Broome criteria.41,42 Secondly, we accounted for an estimated 30% reduction in LDL cholesterol in those on lipid-lowering therapy as has been previously implemented.26–28 This approach may imperfectly estimate untreated LDL cholesterol given heterogeneity in drug selection, dosing, response, and variability across baseline LDL cholesterol levels or mutation status. However, a sensitivity analysis limited to Myocardial Infarction Genetics Consortium cohort participants not on lipid-lowering therapy similarly noted a pronounced difference in risk among severely hypercholesterolemic individuals when stratified by mutation status (Supplementary Table 8). Third, structural and copy number genetic variation are inadequately captured by current exome sequencing techniques and as such, some FH mutations may have been missed. Fourth, our approach to annotating missense variants using prediction algorithms and the ClinVar database may have led to misclassification in some cases. Additional studies that implement large-scale functional screens of identified variants or pool phenotypes across additional studies may provide additional refinement of pathogenicity annotations. Lastly, FH mutation prevalence was determined in CAD-free controls and population-based cohorts. These individuals survived to middle-age and few had clinically manifest CAD, raising the possibility of survivorship or selection bias. Our case-control population was enriched for individuals with premature CAD; effect estimates of mutations on coronary risk may be different in patients with later disease onset.
Conclusions
Genetic sequencing identified a FH mutation in only a small proportion of severely hypercholesterolemic individuals. For any given observed LDL cholesterol level, risk for CAD is substantially higher in carriers of a FH mutation versus non-carriers, likely related in large part to higher lifelong exposure to atherogenic LDL particles. A primary goal of precision medicine is to use molecular diagnostics to identify a small subset of the population at increased disease risk in which to deliver an intervention. Systematic efforts to identify and treat severely hypercholesterolemic individuals who carry a FH mutation may represent one such opportunity.
Supplementary Material
Perspectives.
Competency in Medical Knowledge 1: Sequencing of three genes causative for familial hypercholesterolemia identifies a mutation in only a small fraction of severely hypercholesterolemic individuals.
Competency in Medical Knowledge 2: For any given observed LDL cholesterol, those who harbor a familial hypercholesterolemia mutation are at substantially increased risk of coronary disease as compared to noncarriers, likely related to increased lifelong exposure to LDL cholesterol.
Translational Outlook 1: Future research will determine the relative contributions of novel genes, other common and rare genetic variants, and lifestyle factors to severe hypercholesterolemia.
Translational Outlook 2: Additional research is needed to understand if genetic testing may prove clinically useful in patients with severe hypercholesterolemia.
Acknowledgments
Funding/Support:
Field-work, genotyping, and standard clinical chemistry assays in PROMIS were principally supported by grants awarded to the University of Cambridge from the British Heart Foundation, UK Medical Research Council, Wellcome Trust, EU Framework 6–funded Bloodomics Integrated Project, Pfizer, Novartis, and Merck. Additional support for PROMIS was provided by by the UK Medical Research Council (MR/L003120/1), British Heart Foundation (RG/13/13/30194), UK National Institute for Health Research Cambridge Biomedical Research Centre, European Research Council (268834), and European Commission Framework Programme 7 (HEALTH-F2-2012-279233). The Jackson Heart Study is supported by contracts HHSN268201300046C, HHSN268201300047C, HHSN268201300048C, HHSN268201300049C, HHSN268201300050C from the National Heart, Lung, and Blood Institute and the National Institute on Minority Health and Health Disparities. The Munich Study is supported by the German Federal Ministry of Education and Research (BMBF) in the context of the e:Med program (e:AtheroSysMed) and the FP7 European Union project CVgenes@target (261123). Further grants were received by the Fondation Leducq (CADgenomics: Understanding Coronary Artery Disease Genes, 12CVD02). This study was also supported through the Deutsche Forschungsgemeinschaft (DFG) cluster of excellence ‘Inflammation at Interfaces and SFB 1123. Funding support for “Building on GWAS for NHLBI-diseases: the U.S. CHARGE Consortium” was provided by the NIH through the American Recovery and Reinvestment Act of 2009 (ARRA) (5RC2HL102419). Data for “Building on GWAS for NHLBI-diseases: the U.S. CHARGE Consortium” was provided by Eric Boerwinkle on behalf of the Atherosclerosis Risk in Communities Study, L. Adrienne Cupples, principal investigator for the Framingham Heart Study, and Bruce Psaty, principal investigator for the Cardiovascular Health Study (CHS). Sequencing was carried out at the Baylor Genome Center (U54 HG003273). The ARIC Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute (NHLBI) contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). The Framingham Heart Study is conducted and supported by the NHLBI in collaboration with Boston University (Contract No. N01-HC-25195), and its contract with Affymetrix, Inc., for genome-wide genotyping services (Contract No. N02-HL-6-4278), for quality control by Framingham Heart Study investigators using genotypes in the SNP Health Association Resource (SHARe) project. A portion of this research was conducted using the Linux Cluster for Genetic Analysis (LinGA-II) funded by the Robert Dawson Evans Endowment of the Department of Medicine at Boston University School of Medicine and Boston Medical Center. This CHS research was supported by NHLBI contracts HHSN268201200036C, HHSN268200800007C, N01HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, N01HC85086; and NHLBI grants U01HL080295, R01HL087652, R01HL105756, R01HL103612, and R01HL120393 with additional contribution from the National Institute of Neurological Disorders and Stroke (NINDS). Additional support was provided through R01AG023629 from the National Institute on Aging (NIA). A full list of principal CHS investigators and institutions can be found at CHS-NHLBI.org. The Italian ATVB Study was supported by a grant from RFPS-2007-3-644382 and Programma di ricerca Regione-Università 2010–2012 Area 1 - Strategic Programmes – Regione Emilia-Romagna. Funding for the exome sequencing project (ESP) was provided by RC2 HL103010 (HeartGO), RC2 HL102923 (LungGO) and RC2 HL102924 (WHISP). Exome sequencing was performed through RC2 HL102925 (BroadGO) and RC2 HL102926 (SeattleGO). Exome sequencing in ATVB, PROCARDIS, Ottawa, PROMIS, Munich Study, and Jackson Heart Study was supported by 5U54HG003067 (to E.S.L. and S.G.). Dr. Kathiresan is supported by a Research Scholar award from the Massachusetts General Hospital (MGH), the Donovan Family Foundation, and R01 HL127564. The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Institutes of Health; or the U.S. Department of Health and Human Services.
Role of the Funder/Sponsor: The sponsors had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Abbreviations
- APOB
apolipoprotein B
- CAD
coronary artery disease
- FH
familial hypercholesterolemia
- HDL
high-density lipoprotein
- LDL
low-density lipoprotein
- LDLR
low-density lipoprotein receptor
- PCSK9
proprotein convertase subtilisin/kexin type 9
Footnotes
Conflict of Interest Disclosures:
Dr Khera is supported by an ACC/Merck Fellowship award and reported consulting fees from Merck and Amarin. Dr Peloso is supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under Award Number K01HL125751. Dr Kessler is supported by a DZHK Rotation Grant. Dr Rader reported consulting fees from Aegerion, Alnylam, Eli Lilly, Pfizer, and Novartis, is an inventor on a patent related to lomitapide that is owned by the University of Pennsylvania and licensed to Aegerion, and is a co-founder of VascularStrategies and Staten Biotechnology. Dr Kathiresan has received grants from Bayer Healthcare, Aegerion, and Regeneron and reported consulting fees from Merck, Quest Diagnostics, Genomics PLC, and Eli Lilly.
References
- 1.Emerging Risk Factors Collaboration. Major lipids, apolipoproteins, and risk of vascular disease. JAMA. 2009;302(18):1993–2000. doi: 10.1001/jama.2009.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baigent C, Keech A, Kearney PM, et al. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366(9493):1267–1278. doi: 10.1016/S0140-6736(05)67394-1. [DOI] [PubMed] [Google Scholar]
- 3.Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 Suppl 2):S1–45. doi: 10.1161/01.cir.0000437738.63853.7a. [DOI] [PubMed] [Google Scholar]
- 4.Gidding SS, Ann Champagne M, de Ferranti SD, et al. The Agenda for Familial Hypercholesterolemia: A Scientific Statement From the American Heart Association. Circulation. 2015 Dec 1;132(22):2167–92. doi: 10.1161/CIR.0000000000000297. [DOI] [PubMed] [Google Scholar]
- 5.Fouchier SW, Defesche JC, Umans-Eckenhausen MW, Kastelein JP. The molecular basis of familial hypercholesterolemia in The Netherlands. Hum Genet. 2001;109(6):602–15. doi: 10.1007/s00439-001-0628-8. [DOI] [PubMed] [Google Scholar]
- 6.Graham CA, McIlhatton BP, Kirk CW, et al. Genetic screening protocol for familial hypercholesterolemia which includes splicing defects gives an improved mutation detection rate. Atherosclerosis. 2005 Oct;182(2):331–40. doi: 10.1016/j.atherosclerosis.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 7.Humphries SE, Whittall RA, Hubbart CS, et al. Genetic causes of familial hypercholesterolaemia in patients in the UK: relation to plasma lipid levels and coronary heart disease risk. J Med Genet. 2006;43:943–49. doi: 10.1136/jmg.2006.038356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lombardi MP, Redeker EJ, van Gent DH, et al. Molecular genetic testing for familial hypercholesterolemia in the Netherlands: a stepwise screening strategy enhances the mutation detection rate. Genet Test. 2006 Summer;10(2):77–84. doi: 10.1089/gte.2006.10.77. [DOI] [PubMed] [Google Scholar]
- 9.Civeira F, Ros E, Jarauta E, et al. Comparison of genetic versus clinical diagnosis in familial hypercholesterolemia. Am J Cardiol. 2008;102(9):1187–93. doi: 10.1016/j.amjcard.2008.06.056. [DOI] [PubMed] [Google Scholar]
- 10.Taylor A, Wang D, Patel K, et al. Mutation detection rate and spectrum in familial hypercholesterolaemia patients in the UK pilot cascade project. Clin Genet. 2010;77(6):572–80. doi: 10.1111/j.1399-0004.2009.01356.x. [DOI] [PubMed] [Google Scholar]
- 11.Medeiros AM, Alves AC, Francisco V, Bourbon M investigators of the Portuguese FH Study. Update of the Portuguese Familial Hypercholesterolaemia Study. Atherosclerosis. 2010;212(2):553–8. doi: 10.1016/j.atherosclerosis.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 12.Chmara M, Wasag B, Zuk M, et al. Molecular characterization of Polish patients with familial hypercholesterolemia: novel and recurrent LDLR mutations. J Appl Genet. 2010;51(1):95–106. doi: 10.1007/BF03195716. [DOI] [PubMed] [Google Scholar]
- 13.Marduel M, Carrié A, Sassolas A, et al. Molecular spectrum of autosomal dominant hypercholesterolemia in France. Hum Mutat. 2010;31(11):E1811–24. doi: 10.1002/humu.21348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van der Graaf A, Avis HJ, et al. Molecular basis of autosomal dominant hypercholesterolemia: assessment in a large cohort of hypercholesterolemic children. Circulation. 2011;123(11):1167–73. doi: 10.1161/CIRCULATIONAHA.110.979450. [DOI] [PubMed] [Google Scholar]
- 15.Ahmad Z, Adams-Huet B, Chen C, Garg A. Low prevalence of mutations in known loci for autosomal dominant hypercholesterolemia in a multiethnic patient cohort. Circ Cardiovasc Genet. 2012;5(6):666–75. doi: 10.1161/CIRCGENETICS.112.963587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klančar G, Grošelj U, Kovač J, et al. Universal Screening for Familial Hypercholesterolemia in Children. J Am Coll Cardiol. 2015;66(11):1250–7. doi: 10.1016/j.jacc.2015.07.017. [DOI] [PubMed] [Google Scholar]
- 17.Goldberg AC, Hopkins PN, Toth PP, et al. Familial hypercholesterolemia: screening, diagnosis and management of pediatric and adult patients: clinical guidance from the National Lipid Association Expert Panel on Familial Hypercholesterolemia. J Clin Lipidol. 2011;5:1–8. doi: 10.1016/j.jacl.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 18.Nordestgaard BG, Chapman MJ, Humphries SE, et al. Familial hypercholesterolaemia is underdiagnosed and undertreated in the general population: guidance for clinicians to prevent coronary heart disease: consensus statement of the European Atherosclerosis Society. Eur Heart J. 2013;34:3478–90a. doi: 10.1093/eurheartj/eht273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Atherosclerosis Thrombosis, and Vascular Biology Italian Study Group. No evidence of association between prothrombotic gene polymorphisms and the development of acute myocardial infarction at a young age. Circulation. 2003;107:1117–22. doi: 10.1161/01.cir.0000051465.94572.d0. [DOI] [PubMed] [Google Scholar]
- 20.Do R, Stitziel NO, Won H-H, et al. Exome sequencing identifies multiple rare alleles at LDLR and APOA5 that confer risk for myocardial infarction. Nature. 2015;519:102–106. doi: 10.1038/nature13917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taylor HA., Jr The Jackson Heart Study: an overview. Ethnicity & disease. 2005;15:S6–1-3. [PubMed] [Google Scholar]
- 22.Crosby J, Peloso GM, Auer PL, et al. Loss-of-function mutations in APOC3, triglycerides, and coronary disease. N Engl J Med. 2014;371:22–31. doi: 10.1056/NEJMoa1307095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McPherson R, Pertsemlidis A, Kavaslar N, et al. A common allele on chromosome 9 associated with coronary heart disease. Science. 2007;316:1488–91. doi: 10.1126/science.1142447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clarke R, Peden JF, Hopewell JC, et al. Genetic variants associated with Lp(a) lipoprotein level and coronary disease. N Engl J Med. 2009;361:2518–28. doi: 10.1056/NEJMoa0902604. [DOI] [PubMed] [Google Scholar]
- 25.Saleheen D, Zaidi M, Rasheed A, et al. The Pakistan Risk of Myocardial Infarction Study: a resource for the study of genetic, lifestyle and other determinants of myocardial infarction in South Asia. European journal of epidemiology. 2009;24:329–38. doi: 10.1007/s10654-009-9334-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baigent C, Keech A, Kearney PM, et al. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366(9493):1267–1278. doi: 10.1016/S0140-6736(05)67394-1. [DOI] [PubMed] [Google Scholar]
- 27.Myocardial Infarction Genetics Consortium Investigators. Inactivating mutations in NPC1L1 and protection from coronary heart disease. N Engl J Med. 2014;371(22):2072–82. doi: 10.1056/NEJMoa1405386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Benn M, Watts GF, Tybjærg-Hansen A, Nordestgaard BG. Mutations causative of familial hypercholesterolaemia: screening of 98 098 individuals from the Copenhagen General Population Study estimated a prevalence of 1 in 217. Eur Heart J. 2016 Feb 22; doi: 10.1093/eurheartj/ehw028. Epub ahead of print. [DOI] [PubMed]
- 29.Psaty BM, O’Donnell CJ, Gudnason V, et al. Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) Consortium: Design of prospective meta-analyses of genome-wide association studies from five cohorts. Circ Cardiovasc Genet. 2009;2:73–80. doi: 10.1161/CIRCGENETICS.108.829747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Purcell SM, Moran JL, Fromer M, et al. A polygenic burden of rare disruptive mutations in schizophrenia. Nature. 2014;506:185–90. doi: 10.1038/nature12975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Landrum MJ, Lee JM, Riley GR, Jang W, Rubinstein WS, Church DM, Maglott DR. ClinVar: public archive of relationships among sequence variation and human phenotype. Nucleic Acids Res. 2014;42:D980–5. doi: 10.1093/nar/gkt1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Borén J, Ekström U, Agren B, Nilsson-Ehle P, Innerarity TL. The molecular mechanism for the genetic disorder familial defective apolipoprotein B100. J Biol Chem. 2001;276:9214–9218. doi: 10.1074/jbc.M008890200. [DOI] [PubMed] [Google Scholar]
- 33.McLaren W, Pritchard B, Rios D, Chen Y, Flicek P, Cunningham F. Deriving the consequences of genomic variants with the Ensembl API and SNP Effect Predictor. Bioinformatics. 2010;26:2069–70. doi: 10.1093/bioinformatics/btq330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karczewski KJ. LOFTEE (Loss-Of-Function Transcript Effect Estimator) 2015 < https://github.com/konradjk/loftee>.
- 35.Liu X, Jian X, Boerwinkle E. dbNSFP v2.0: a database of human non-synonymous SNVs and their functional predictions and annotations. Hum Mutat. 2013 Sep;34(9):E2393–402. doi: 10.1002/humu.22376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wikham H. ggplot2: elegant graphics for data analysis. Springer; New York: 2009. [Google Scholar]
- 37.Teslovich TM, Musunuru K, Smith AV, et al. Biological, clinical and population relevance of 95 loci for blood lipids. Nature. 2010;466(7307):707–13. doi: 10.1038/nature09270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Talmud PJ, Shah S, Whittall R, et al. Use of low-density lipoprotein cholesterol gene score to distinguish patients with polygenic and monogenic familial hypercholesterolaemia: a case-control study. Lancet. 2013;381:1293–301. doi: 10.1016/S0140-6736(12)62127-8. [DOI] [PubMed] [Google Scholar]
- 39.Brown MS, Goldstein JL. Biomedicine: lowering LDL — not only how low, but how long? Science. 2006;311:1721–3. doi: 10.1126/science.1125884. [DOI] [PubMed] [Google Scholar]
- 40.Alonso R, Andres E, Mata N, et al. Lipoprotein(a) levels in familial hypercholesterolemia: an important predictor of cardiovascular disease independent of the type of LDL receptor mutation. J Am Coll Cardiol. 63(19):1982–9. doi: 10.1016/j.jacc.2014.01.063. [DOI] [PubMed] [Google Scholar]
- 41.World Health Organization. Report of a second WHO consultation. Geneva: World Health Organization; 1999. Familial hypercholesterolaemia (FH) [Google Scholar]
- 42.National Collaborating Centre for Primary Care. NICE clinical guideline 71. London: National Institute for Health and Clinical Excellence; 2008. Identification and management of familial hypercholesterolaemia. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.