Abstract
Background:
The aim of this study was to measure the changes in the bacterial bioburden in orthokeratology (OK) lens storage cases using the DNA dot hybridization assay (DHA) after forewarning patients about their bacterial contamination severity.
Methods:
Thirty-one OK lens wearers were prospectively enrolled in this study. Dot hybridization assay was used for serial measurements of bacterial bioburden in OK storage cases after lenses had been soaked for approximately 6 hr. After the first assessment, the lens wearers were informed of the extent of case contamination and the possible risk of microbial keratitis (MK), and best practices for lens care and lens case hygiene were reviewed and reinforced. A second assessment by the same DHA method was performed after approximately 6 months.
Results:
Two universal bacterial probes confirmed a significant decrease in bacterial bioburden at the second assessment (P<0.01 and P<0.001). Genus-specific probes showed significant reductions in Acinetobacter and Klebsiella (P=0.02 and P=0.01), but not in Pseudomonas (P=0.42).
Conclusions:
Making OK lens wearers aware of the bacterial bioburden in their lens cases resulted in improved quality of case care and reduced bioburden. Our results suggest that a strategy of bioburden assessment with forewarning could be a useful method to decrease the incidence of OK-related MK.
Key Words: Dot hybridization assay, Orthokeratology, Bacterial bioburden, Lens storage case, Forewarning strategy
Orthokeratology (OK) using reverse geometry rigid gas-permeable contact lenses (CLs) is designed to reduce myopia and astigmatism by temporarily flattening the central cornea.1,2 Wearers experience clear unaided vision in the daytime after wearing OK lenses overnight and removing them in the morning. Furthermore, several studies have shown that OK lenses retard ocular axial elongation and myopic progression in children,3–7 but do not influence ocular accommodative function.8 Orthokeratology lenses have gained increasing popularity for control of myopia, particularly among Asian populations with a high prevalence of myopia.3,9
Wearing OK CLs overnight is essential for epithelial molding sufficient to flatten the anterior corneal curvature, and safety is the major concern with this treatment modality. Complications associated with OK include corneal epithelial staining, corneal edema or abrasion, keratoconic change, induced astigmatism, and microbial keratitis (MK).1,2 Among these, MK is the most sight-threatening event, and several studies have found an association between OK lens wear and MK.10–13 In up to 90% of individuals with CL-related MK, testing of CL care accessories identifies the same pathogenic microorganism as the corneal culture.14,15 Lens storage cases receive the least amount of cleaning attention and are the most frequently contaminated lens care accessories.15,16 Poor adherence to the recommended disinfecting regimen is the main cause of lens case contamination. Therefore, improving compliance with the lens care regimen is important, especially for the growing population of pediatric OK lens wearers.
Many strategies have been proposed for decreasing rates of lens case contamination, including improvement of CL disinfecting systems,17–19 shortening case replacement schedules,20–22 special case designs,23–25 and others.26,27 The Feedback Intervention Trial28 showed that coupling feedback with personalized action planning improved hand-hygiene compliance among health care workers in the United Kingdom. We modified this concept and proposed a forewarning strategy to improve lens case hygiene among OK lens wearers by providing feedback in the form of an MK risk report based on objective measurement of OK storage case contamination. We hypothesized that providing wearers/parents with scientific evidence of lens case contamination would alert them to contamination sites, reinforce compliance with recommended lens cleaning and disinfecting regimens, and finally, decrease microbial contamination.
Our forewarning strategy is based on the dot hybridization assay (DHA) that we developed to assess microbial contamination in our previous study.29 The DHA model was verified as a reliable means for assessing bacterial bioburden, grading contamination severity, and tracing potentially hazardous bacterial contamination for lens storage cases using a single test. The aim of the present study was to evaluate the changes in the bacterial bioburden in lens storage cases using DHA after forewarning pediatric OK lens wearers and their parents/guardians about the risk of contamination.
METHODS
Subjects
This prospective study was performed at the Kaohsiung Chang Gung Memorial Hospital (CGMH) and included OK lens wearers (Euclid System Orthokeratology [oprifocon A] CLs for Overnight Wear; Euclid System Corporation, Herndon, VA) aged between 8 and 18 years who had worn the lenses for more than 1 year. Exclusion criteria were age below 8 or above 18 years, using new lens cases within 3 days before sample collection, or unable to attend follow-up visits as scheduled. The necessary sample size was estimated by the Altman normogram according to a 1.59 mean difference and 3.0 SD of mean difference based on standardized intensities of the probe PB2 for paired samples from our preliminary study (power=0.8 and P<0.05 as the level of statistical significance), and at least 28 participants were required. The purposes and procedures were explained to the study subjects and their responsible parents/guardians, and written informed consent was obtained from all participants. All procedures involving human subjects adhered to the tenets of the Declaration of Helsinki. Institutional review board/ethics committee approval was obtained from the Committee of Medical Ethics and Human Experiments at CGMH.
Bioclean (Ophtecs Corporation, Toyooka, Japan) multipurpose solution (MPS) was the disinfecting system recommended to these subjects. The standard lens/case hygiene recommendations for OK lens wearers were as follows: Proper hygiene and immediate consultation upon the first sign of a red painful eye are essential to avoid serious complications and achieve ongoing success. When inserting an OK lens: (1) Wash, rinse, and dry your hands thoroughly before handling the lenses. Do not use soaps that have lotions, oily cosmetics, or cold cream. Never touch your eyes or lenses without washing your hands. (2) Remove the lens from the storage case, hold the lens with your fingertips, and check to make sure that the lens is clean, moist, and free from cracks, scratches, or other debris. (3) Place two to three drops of the rewetting solution in each eye and several drops on the back of the lens before placing the lens on your eye. (4) Remove the lens immediately if you feel discomfort (pain, red eye, and/or tearing) after insertion. Check, clean, and rinse the lens again before reinsertion. If discomfort persists, remove the lens and consult your doctor. When removing an OK lens: (1) After washing and drying your hands, place a drop of the rewetting solution into each eye. (2) Check whether the lens is moving freely before removing it; remove the lens once it moves freely. (3) Clean the lens by rubbing it gently with the recommended MPS for approximately 20 sec, and then rinse it thoroughly immediately after removal. Make sure not to press or squeeze the lens excessively, as it is prone to breakage and distortion. (4) Place your lens into your dry lens case and cover it with the MPS. Make sure that the case is covered completely and tightly. Soak your lens in the MPS for at least 4 hr. (5) Clean your OK lens with an appropriate protein removal system at least once every 2 weeks to ensure that the lens is clean and sanitary. For lens case/lens care and follow-up visits: (1) Rinse the case with hot water, dry with a tissue, and leave to dry overnight in your bedroom, not the bathroom. Replace the CL case at least every 3 months. (2) Replace your OK lens every 2 years. (3) Visit your doctor regularly to check your lenses and ocular conditions.
Sample Collection
Orthokeratology storage case sampling for each participant was performed two times within a 6-month interval during the period from January 2013 to September 2013. Subjects were asked to perform their lens care according to their usual habits, to remove the OK lenses after overnight wear, and to soak them in their storage cases. The sampling procedure was described in our previous study.29 In brief, the OK lenses were soaked for approximately 6 hr before sampling. Using a sterile procedure, the lenses were then transferred into the new lens case with a fresh disinfectant. The inner surface of each original lens case was rubbed with an aseptic cotton swab. The resulting case fluid was collected with a pipette, and 1 mL of the fluid was transferred to a microcentrifuge tube. The original case and the cotton swab were then discarded. The sample (1 mL) was centrifuged at 12,000 rpm for 10 min in a microfuge, the precipitate was extracted using a commercial kit (DNeasy Blood & Tissue Kit; Qiagen, Valencia, CA), and 100 μL aliquots of the extracted microbial DNA product were stored at −70°C until DHA. The lens case for the second assessment was either the one provided at the first assessment or a newer one purchased by the subject based on our recommendation to replace the case within 3 months.
Dot Hybridization Assay
Bacterial DNA fragments were amplified using polymerase chain reaction (PCR) before DHA.29 The bacteria-specific universal primers 13BF (5′-digoxigenin-GTGAATACGTTCCCGGGCCT-3′) and 6R (5′-digoxigenin-GGGTTYCCCCRTTCRGAAAT-3′; Y=C or T; R=A or G) were used to acquire a DNA amplicon.30 Each primer was labeled with a digoxigenin molecule at its 5′ end. The PCR mixture (25 μL) consisted of 2.5 μL of template DNA, 0.4 μM of each primer, and other reagents obtained from the PCR kit (JMR-THS5; JMR Holdings, Inc., St Augustine, FL). Cycling conditions were set as follows: initial denaturation (95°C, 3 min), 35 cycles of denaturation (95°C, 30 sec), annealing (55°C, 45 sec), and extension (72°C, 45 sec), followed by final extension (72°C, 10 min). A negative control was included with each run by replacing the template DNA with sterile water.
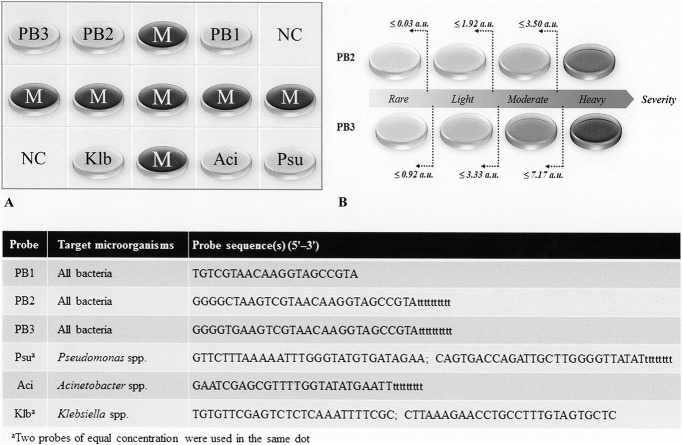
A 10-μL aliquot of the PCR product was used for DHA (Fig. 1) as described in our previous study.29 The reagents and procedures for prehybridization, hybridization (55°C for 90 min), reaction with alkaline phosphatase-conjugated antidigoxigenin antibodies, and phosphatase substrates were described previously.31 Images of hybridized spots (400 μm in diameter) were captured with a scanner (Image-Scanner III; GE Healthcare, United Kingdom), and the hybridization signal intensity was quantified using ImageJ software (http://imagej.nih.gov/ij/; National Institutes of Health, Bethesda, MD).29 Each image was adjusted to a fixed size (300 × 200 pixels), transformed to grayscale for detection and recording, and corrected according to the image background (mean gray level of the negative controls). After standardization of the intensities, two universal bacterial probes (PB2 and PB3) were used to measure the bacterial bioburden and to grade the severity of contamination of the storage case. As determined in our previous study,29 the PB1 probe had no role for this purpose. Three genus-specific probes, Psu, Aci, and Klb, were used to qualitatively monitor the presence of potentially hazardous Pseudomonas, Acinetobacter, and Klebsiella spp. in the lens storage cases.
FIG. 1.
(A) Layout of oligonucleotide probes on the array (size 0.6 × 0.4 cm). The universal bacteria probes PB1, PB2, and PB3 were designed from a conserved region at the 16S rRNA gene. Dots labeled Psu, Aci, and Klb are the genus-specific probes that are used to identify Pseudomonas, Acinetobacter, and Klebsiella spp., dots labeled M are position markers (an irrelevant digoxigenin-labeled oligonucleotide), and those labeled NC are negative controls composed of the tracking dye only. (B) The universal probes PB2 and PB3 can be used to assess the bacterial bioburden by standardized signal intensities (a.u., arbitrary unit) and to grade the contamination severity.29 Probe sequences for all dots are listed in the table (inset).
Feedback and Forewarning
A feedback report was prepared for the lens wearers/parents/guardians after the first DHA to inform them about the severity of contamination (rare/light/moderate/heavy)29 of their lens storage cases and to forewarn them about the associated risks (minimal/low/moderate/high) of MK. The standard lens/case hygiene recommendation was reiterated and reinforced by this feedback report. No new recommendations or reminders were given to the subjects during the review of any inappropriate lens/case hygiene practices. The second assessment was performed using the same DHA procedure approximately 6 months after feedback and forewarning were provided.
Statistical Analysis
The Wilcoxon signed-rank test and Fisher exact test were, respectively, used to test the statistical differences of bacterial bioburden (standardized intensities of PB2 and PB3) and potentially hazardous microorganisms (positive Psu, Aci, and Klb assay) between the first (preforewarning) and the second (postforewarning) assessment. In addition, chi-square statistics and radar plots were used to analyze the changes in contamination severity (as graded by PB2 and PB3) between the first and the second assessment. Statistical significance was accepted at P<0.05. Analyses were performed using Microsoft Excel 2010 and the Real Statistics Resource Pack (http://www.real-statistics.com/).
RESULTS
Participants
A total of 31 OK lens wearers, including 17 girls and 14 boys (54.8% vs. 45.2%, P=0.54 by the 1-sample Z-test) were included in the study. The mean age of all participants was 12.5 years (range 8–18 years), and there was no age difference between girls and boys (12.3±2.5 vs. 12.9±3.0, P=0.57 by the 2-tailed Student t test). The mean OK wearing experience was 3.6 years, and there was no difference between girls and boys (3.3±1.6 vs. 4.0±2.2, P=0.34 by the 2-tailed Student t test). Neither MK nor CL-related peripheral ulcer was identified in any participant during the study period.
Changes in Bacterial Bioburden and Contamination Severity After Forewarning
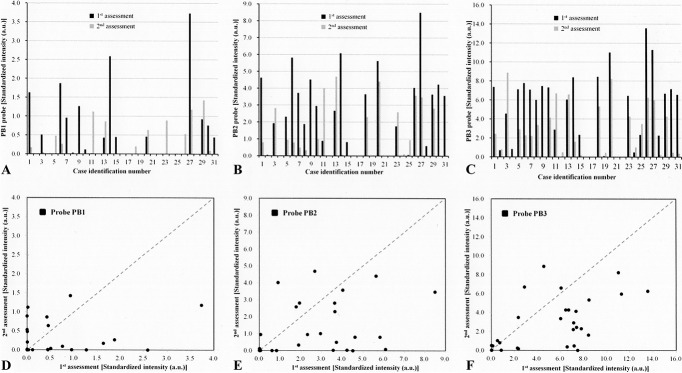
Pre- and post-forewarning DHA results determined by the standardized hybridization intensities of separate samples with each of the three universal bacteria probes (PB1, PB2, and PB3) are shown in Figure 2A–C. We included the PB1 probe here for completeness, although, as mentioned, we have previously shown that it does not have a role in assessing bacterial bioburden in the lens case.29 In comparison with the first assessment, there were more lens cases with reduced standardized hybridization intensities of probe PB2 at the second assessment (Fig. 2E), and there was a similar result for probe PB3 (Fig. 2F). The decreases in bacterial bioburden detected by probes PB2 and PB3 at the postforewarning DHA were significant (Fig. 3).
FIG. 2.
(A–C) Standardized hybridization intensities of individual samples with each of the three universal bacteria probes (PB1, PB2, and PB3) for preforewarning (first) assessment and postforewarning (second) assessment. (D–F) Changes in the bioburden before and after forewarning for each participant according to the signals of probes PB1, PB2, and PB3. Dots behind the diagonal dashed line suggest decreased bacterial contamination after forewarning, and those above the line suggest that contamination increased after forewarning.
FIG. 3.
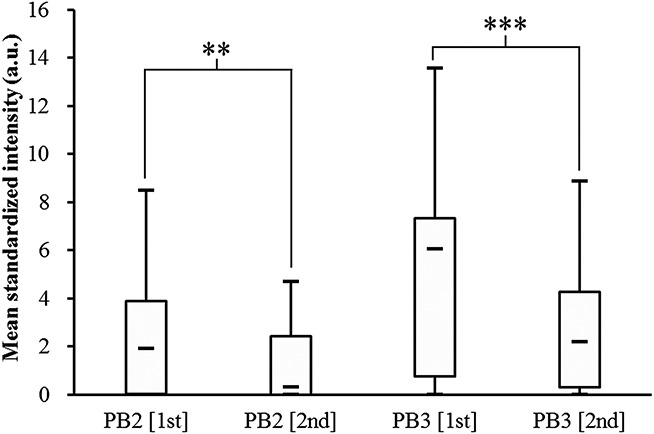
Boxplot comparison of pre- and post-forewarning standardized hybridization intensities of the two universal bacteria probes (PB2 and PB3) for all participants. Statistical significance is according to the Wilcoxon signed-rank test (**P<0.01 and ***P<0.001).
A verified grading system (Fig. 1B) for contamination severity based on the cutoff values of standardized intensities for probes PB2 and PB3 was used to assess the severity shift from the preforewarning to the postforewarning assessment.29 In Figure 4, the two radar plots based on the severity grading systems of probes PB2 and PB3 show that the population shifted to lower contamination severities after forewarning, and the results of both universal bacteria probes were statistically significant.
FIG. 4.
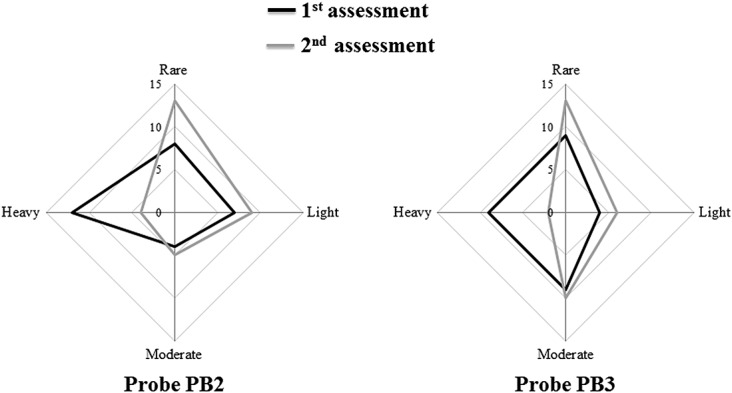
Severity of contamination determined by the standardized hybridization cutoff intensities of probes PB2 and PB3. Radar plots show the population shifts in contamination severities after forewarning. There were significant changes for both probes (P<0.001, PB2, and <0.0001, PB3) by the chi-square test.
Potentially Hazardous Microorganisms in Lens Storage Cases After Forewarning
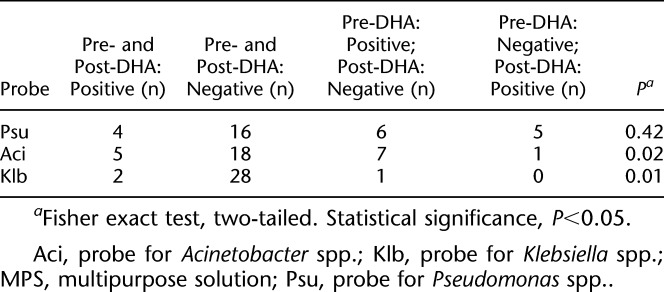
The three genus-specific probes (Fig. 1) were used to detect potentially hazardous bacterial contamination in pre- and post-forewarning samples. For Pseudomonas spp., four lens cases were positive at both evaluations, and 16 showed negative results in both the pre- and post-forewarning samples (Table 1). Six lens cases were positive for Pseudomonas spp. in the preforewarning DHA and negative in the postforewarning DHA, and five lens cases were negative for Pseudomonas spp. in the preforewarning analysis and positive in the postforewarning analysis. There were five lens cases that were positive for Acinetobacter spp. at both evaluations and 18 that had negative results in pre- and post-forewarning assays. Notably, there were seven lens wearers whose cases were positive for Acinetobacter spp. in preforewarning tests and negative postforewarning tests, whereas only 1 lens case was negative at preforewarning and positive at postforewarning. Similar results were found for Klebsiella spp. Significant decreases of potentially hazardous microorganisms after forewarning were noted in Acinetobacter spp. (P=0.02) and Klebsiella spp. (P=0.01), but not in Pseudomonas spp. (P=0.42) (Table 1).
TABLE 1.
Pre- and Post-Forewarning Results of Genus-Specific Probes
DISCUSSION
A contaminated lens storage case can act as a reservoir for microorganisms that can compromise CL wear and lead to sight-threatening adverse events.32 The lens storage case is the most frequently contaminated component of the lens care system. A variety of noncompliant lens care behaviors have been associated with significant increases in the risks of lens contamination,33 and inadequate case care is the most common noncompliant behavior. Awareness of the importance of proper lens care among lens wearers can be improved by reinforcement strategies.34 Therefore, we proposed an objective assessment of case care to provide feedback on severity of lens case contamination and to forewarn vulnerable OK lens wearers about their risk of infection posed by improper lens care. The DHA, a verified bacterial bioburden assessment model for case contamination,29 revealed a significant decrease in bacterial bioburden after forewarning. Besides that, the DHA also showed that more storage cases shifted to lower contamination severities and harbored lower apparent concentrations of potentially hazardous microorganisms after forewarning.
In our study design, we ignored the influence of case age because lens case contamination can develop rapidly even after 2 weeks of use.35 Cho et al.34 reported that the overall contamination rates for the OK lens case did not improve after monthly replacement. However, they noticed that some of the storage cases from the subjects in their study yielded no microbial growth at subsequent visits, possibly due to monthly replacement and warnings. In addition, Kuzman et al.33 found that bacterial contamination was associated with the case age, and less contamination was identified in lens cases used less than 3 months compared with those replaced after a longer time. According to our standard lens/case hygiene recommendations for OK lens wearers, the lens case should be replaced at least every 3 months. Therefore, if the wearers have good compliance, the age of the lens case should be less than 3 months at the first assessment or at the second assessment. However, no control of case age may be a confounding factor because some lens cases could have been much older than 6 months at the first sampling visit, but at the second sampling visit all lens cases were less than or equal to only 6 months of age. Therefore, some subjects' cases in the second visit might intrinsically have lower bioburden than did their cases in the first visit even without forewarning intervention. Additional study to minimize the impact of this confounding factor will be needed to determine the extent of the increase in efficiency that is attributable to this strategy.
Many strategies have been proposed for minimizing microbial contamination of lens storage cases.25 Multipurpose solutions may improve user compliance by simplifying lens care, although the contamination rate remains consistent.32 A lens case with an easily cleanable design may arrest biofilm formation, but this is still dependent on user compliance, and one study found no difference compared with standard lens cases.36 A decrease in contamination of approximately 40% has been reported with commercialized silver-impregnated lens cases compared with other standard lens cases as a result of the bactericidal activity of silver, but there are variations in in vitro antimicrobial activity among different cases.37 Selenium-impregnated cases can inhibit formation of S. aureus biofilm, but their effectiveness needs further investigation before commercialization.32 Some combination of all of these methods is anticipated because no single method can guarantee contamination-free CLs, and there are many portals for contamination throughout the lens care process.16
We modified the concept described in the Feedback Intervention Trial28 and proposed a feedback system that couples DHA for measurement of bacterial burden with forewarning about MK risk to promote best lens care practices among OK lens wearers. Our result proved the strategy effective for contributing to decreased bacterial contamination of lens cases. There are several areas of lens care that might be positively influenced by this feedback. OK lens wearers may reinforce their hand washing habits, shorten the case renewal period, rub the lenses sufficiently before soaking, ensure that the disinfecting solution is exchanged each day instead of simply topping off the solution, perform lens care in a dry location, etc. Additional studies with a larger sample size will be needed to clarify how these lens care behaviors are influenced by this novel strategy.
In our previous study focusing on the assessment before forewarning,29 we found that male OK lens wearers had a higher bioburden in their lens case than did female OK lens wearers. Both male and female OK lens wearers exhibited a decrease in the average bioburden, according to the results with probes PB2 and PB3. However, male OK lens wearers presented a significant decrease of the bioburden after forewarning (PB2, P=0.03; PB3, P=0.01), whereas female OK lens wearers had no significant decrease in the bioburden (PB2, P=0.25; PB3, P=0.27). Therefore, the impact of this strategy might be greater in male subjects than in female subjects.
Although most DHA indices of contamination showed improvement, the rate of Pseudomonas contamination was high and was without significant decrease (preforewarning vs. postforewarning, 32.3% vs. 29.0%). Pseudomonas spp. are ubiquitous, and they are natural residents of soil and water. Dot hybridization assay detects both viable and nonviable pathogens in lens cases, and it is not unexpected that there would be no decrease in contamination by Pseudomonas spp. Although a high rate of eradication (>90%) of Pseudomonas spp. by disinfecting solutions was found in our previous study,29 a strongly positive result of the Psu probe can alert wearers and the eye-care practitioner about Pseudomonas contamination during lens care. These wearers should be urged to perform their lens care in a dry area. Pseudomonas remains the dominant pathogen in CL-related MK38 because it has an intrinsic adhesive capacity through the type III secretory system and other adhesins39 and can forcefully invade the ocular surface once any miss occurs during lens care procedures.
Five subjects (three girls and two boys) had an increased bioburden (conversion to higher severity) at the second assessment identified by probes PB2 or PB3. Four of the five subjects had positive results for Pseudomonas contamination at the second assessment. Three of the four subjects with Pseudomonas contamination at the second assessment were negative for Pseudomonas contamination at the first assessment. One of these subjects was cocontaminated with Acinetobacter spp., and one of them was cocontaminated with Klebsiella spp. at both assessments. For these subjects, it is highly recommended that the lens/case hygiene practices be individually reviewed to identify the causal factors of the case contamination and additional specific forewarning be provided. Seven subjects (five girls and two boys) had low bioburden (rare severity) shown in assays with probe PB2 or PB3 at both assessments. The results of assessment with the genus probes were all negative at both assessments except in 1 subject (case 4) who was positive for Pseudomonas spp. at her first assessment. These seven subjects were older (13.1±2.3 vs. 12.0±2.7 years, P=0.68) and had a longer experience of wearing OK lenses (4.4±1.0 vs. 3.7±1.9 years, P=0.52) than that of the five subjects with an increased bioburden, but these two factors did not reach statistical significance (Mann–Whitney U test).
It is difficult to determine whether one disinfecting system performs better than the others because the antimicrobial efficiency of the active ingredients and the formulation of each system may be dependent on the microbial strains that are present.32 Boost et al.40 pointed out that the current formulations of MPSs for RGP lenses have an improved disinfecting capacity compared with the previously used unifunctional disinfecting solutions, which is particularly important for OK lens wearers. The appropriate inclusion of certain detergents into MPS formulas can improve the ability of the solutions to remove adhering bacteria. The agents used in MPSs for OK lenses are largely similar to those in MPSs for soft CLs, and the concentrations of agents in the former tend to be higher than those in the latter. Therefore, MPSs for OK lenses have a better disinfecting efficacy than that for soft lenses. In this study, we could not determine how the disinfectant system affected the case contamination because most subjects used the recommended MPS as their disinfecting system.
In this study, only three genus-specific probes were validated to identify the potentially hazardous microorganisms (Acinetobacter spp., Pseudomonas spp., and Klebsiella spp.) of OK storage case. There were several potentially hazardous microorganisms that could not be directly assessed using the current DHA. We plan to design and validate other important genus-specific probes in the future to improve the current DHA, particularly targeting those with a higher risk of causing MK.
In conclusion, DHA coupled with forewarning feedback is a valuable strategy for decreasing contamination of OK storage cases. This strategy might help wearers to identify inappropriate lens care habits, encourage them to change lens care environments, or promote lens care compliance, but further study is needed to determine which factors are most influenced by this strategy. However, lens care quality was better after forewarning, and we believe the risk of OK-related MK can be also decreased by this strategy.
ACKNOWLEDGMENTS
The authors acknowledge the Genomic & Proteomic Core Laboratory, Department of Medical Research, Chang Gung Memorial Hospital at Kaohsiung for supplying a spotting arrayer and an image scanner.
Footnotes
The authors have no conflicts of interest to disclose.
Cofunded by Chang Gung Research Proposal (CMRPG8C0761, CMRPG8E0341), and the Ministry of Science and Technology (MOST 103-2314-B-182A-044, 104-2314-B-182A-101-MY3). The funding organizations had no role in the design or conduct of this research.
REFERENCES
- 1.Woo GC, Wilson MA. Current methods of treating and preventing myopia. Optom Vis Sci 1990;67:719–727. [DOI] [PubMed] [Google Scholar]
- 2.Nichols JJ, Marsich MM, Nguyen M, et al. Overnight orthokeratology. Optom Vis Sci 2000;77:252–259. [DOI] [PubMed] [Google Scholar]
- 3.Cho P, Cheung SW, Edwards M. The longitudinal orthokeratology research in children (LORIC) in Hong Kong: A pilot study on refractive changes and myopic control. Curr Eye Res 2005;30:71–80. [DOI] [PubMed] [Google Scholar]
- 4.Walline JJ, Jones LA, Sinnott LT. Corneal reshaping and myopia progression. Br J Ophthalmol 2009;93:1181–1185. [DOI] [PubMed] [Google Scholar]
- 5.Hiraoka T, Kakita T, Okamoto F, et al. Long-term effect of overnight orthokeratology on axial length elongation in childhood myopia: A 5-year follow-up study. Invest Ophthalmol Vis Sci 2012;53:3913–3919. [DOI] [PubMed] [Google Scholar]
- 6.Cho P, Cheung SW. Retardation of myopia in Orthokeratology (ROMIO) study: A 2-year randomized clinical trial. Invest Ophthalmol Vis Sci 2012;53:7077–7085. [DOI] [PubMed] [Google Scholar]
- 7.Zhu MJ, Feng HY, He XG, et al. The control effect of orthokeratology on axial length elongation in Chinese children with myopia. BMC Ophthalmol 2014;14:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Felipe-Marquez G, Nombela-Palomo M, Cacho I, et al. Accommodative changes produced in response to overnight orthokeratology. Graefes Arch Clin Exp Ophthalmol 2015;253:619–626. [DOI] [PubMed] [Google Scholar]
- 9.Chan B, Cho P, Cheung SW. Orthokeratology practice in children in a university clinic in Hong Kong. Clin Exp Optom 2008;91:453–460. [DOI] [PubMed] [Google Scholar]
- 10.Tseng CH, Fong CF, Chen WL, et al. Overnight orthokeratology-associated microbial keratitis. Cornea 2005;24:778–782. [DOI] [PubMed] [Google Scholar]
- 11.Hsiao CH, Yeung L, Ma DH, et al. Pediatric microbial keratitis in Taiwanese children: A review of hospital cases. Arch Ophthalmol 2007;125:603–609. [DOI] [PubMed] [Google Scholar]
- 12.Young AL, Leung KS, Tsim N, et al. Risk factors, microbiological profile, and treatment outcomes of pediatric microbial keratitis in a tertiary care hospital in Hong Kong. Am J Ophthalmol 2013;156:1040–1044. [DOI] [PubMed] [Google Scholar]
- 13.Lee YS, Tan HY, Yeh LK, et al. Pediatric microbial keratitis in Taiwan: Clinical and microbiological profiles, 1998-2002 versus 2008-2012. Am J Ophthalmol 2014;157:1090–1096. [DOI] [PubMed] [Google Scholar]
- 14.Mayo MS, Schlitzer RL, Ward MA, et al. Association of Pseudomonas and Serratia corneal ulcers with use of contaminated solutions. J Clin Microbiol 1987;25:1398–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keay LJ, Gower EW, Iovieno A, et al. Clinical and microbiological characteristics of fungal keratitis in the United States, 2001-2007: A multicenter study. Ophthalmology 2011;118:920–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Szczotka-Flynn LB, Pearlman E, Ghannoum M. Microbial contamination of contact lenses, lens care solutions, and their accessories: A literature review. Eye Contact Lens 2010;36:116–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hughes R, Kilvington S. Comparison of hydrogen peroxide contact lens disinfection systems and solutions against Acanthamoeba polyphaga. Antimicrob Agents Chemother 2001;45:2038–2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Willcox MD, Carnt N, Diec J, et al. Contact lens case contamination during daily wear of silicone hydrogels. Optom Vis Sci 2010;87:456–464. [DOI] [PubMed] [Google Scholar]
- 19.Kilvington S, Huang L, Kao E, et al. Development of a new contact lens multipurpose solution: Comparative analysis of microbiological, biological and clinical performance. J Optom 2010;3:134–142. [Google Scholar]
- 20.Wilson LA, Sawant AD, Simmons RB, et al. Microbial contamination of contact lens storage cases and solutions. Am J Ophthalmol 1990;110:193–198. [DOI] [PubMed] [Google Scholar]
- 21.Food and Drug Administration USA. Focusing on Contact Lens Safety. Available at: http://www.fda.gov/ForConsumers/ConsumerUpdates/ucm048893.htm. Accessed December 10, 2015. [Google Scholar]
- 22.Hall B, Jones L. Countering noncompliance with lens care and case technology. Contact Lens Spectr 2010. Available at: http://www.clspectrum.com/articleviewer.aspx?articleID=105013. Accessed December 10, 2015. [Google Scholar]
- 23.Dantam J, Zhu H, Stapleton F. Biocidal efficacy of silver-impregnated contact lens storage cases in vitro. Invest Ophthalmol Vis Sci 2011;52:51–57. [DOI] [PubMed] [Google Scholar]
- 24.Dutta D, Cole N, Kumar N, et al. Broad spectrum antimicrobial activity of melimine covalently bound to contact lenses. Invest Ophthalmol Vis Sci 2013;54:175–182. [DOI] [PubMed] [Google Scholar]
- 25.Dutta D, Willcox MD. Antimicrobial contact lenses and lens cases: A review. Eye Contact Lens 2014;40:312–324. [DOI] [PubMed] [Google Scholar]
- 26.Yung AM, Boost MV, Cho P, et al. The effect of a compliance enhancement strategy (self-review) on the level of lens care compliance and contamination of contact lenses and lens care accessories. Clin Exp Optom 2007;90:190–202. [DOI] [PubMed] [Google Scholar]
- 27.Willcox M, Verma M. Contact lenses and microbes—latest innovations. Contact Lens 2015. Available at: http://contactlensupdate.com/2015/10/28/contact-lenses-and-microbes-latest-innovations/. Accessed December 10, 2015. [Google Scholar]
- 28.Fuller C, Michie S, Savage J, et al. The Feedback Intervention Trial (FIT)—improving hand-hygiene compliance in UK healthcare workers: A stepped wedge cluster randomised controlled trial. PLoS One 2012;7:e41617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuo MT, Chien CC, Lo J, et al. A DNA dot hybridization model for assessment of bacterial bioburden in orthokeratology lens storage cases. Invest Ophthalmol Vis Sci 2015;56:445–450. [DOI] [PubMed] [Google Scholar]
- 30.Gurtler V, Stanisich VA. New approaches to typing and identification of bacteria using the 16S-23S rDNA spacer region. Microbiology 1996;142:3–16. [DOI] [PubMed] [Google Scholar]
- 31.Su SC, Vaneechoutte M, Dijkshoorn L, et al. Identification of non-fermenting Gram-negative bacteria of clinical importance by an oligonucleotide array. J Med Microbiol 2009;58:596–605. [DOI] [PubMed] [Google Scholar]
- 32.Wu YT, Willcox M, Zhu H, et al. Contact lens hygiene compliance and lens case contamination: A review. Contact Lens Anterior Eye 2015;38:307–316. [DOI] [PubMed] [Google Scholar]
- 33.Kuzman T, Kutija MB, Juri J, et al. Lens wearers non-compliance - is there an association with lens case contamination? Contact Lens Anterior Eye 2014;37:99–105. [DOI] [PubMed] [Google Scholar]
- 34.Cho P, Boost M, Cheng R. Non-compliance and microbial contamination in orthokeratology. Optom Vis Sci 2009;86:1227–1234. [DOI] [PubMed] [Google Scholar]
- 35.Lakkis C, Anastasopoulos F, Terry C, et al. Time course of the development of contact lens case and contact lens contamination. ARVO Meeting Abstracts 2009;50. E-Abstract 6352. [Google Scholar]
- 36.Devonshire P, Munro FA, Abernethy C, et al. Microbial contamination of contact lens cases in the west of Scotland. Br J Ophthalmol 1993;77:41–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu YT, Zhu H, Willcox M, et al. Impact of cleaning regimens in silver-impregnated and hydrogen peroxide lens cases. Eye Contact Lens 2011;37:365–369. [DOI] [PubMed] [Google Scholar]
- 38.Watt K, Swarbrick HA. Microbial keratitis in overnight orthokeratology: Review of the first 50 cases. Eye Contact Lens 2005;31:201–208. [DOI] [PubMed] [Google Scholar]
- 39.Shen EP, Tsay RY, Chia JS, et al. The role of type III secretion system and lens material on adhesion of Pseudomonas aeruginosa to contact lenses. Invest Ophthalmol Vis Sci 2012;53:6416–6426. [DOI] [PubMed] [Google Scholar]
- 40.Boost M, Cho P, Lai S. Efficacy of multipurpose solutions for rigid gas permeable lenses. Ophthalmic Physiol Opt 2006;26:468–475. [DOI] [PubMed] [Google Scholar]